Purpose of review
The circadian rhythms have a systemic impact on all aspects of physiology. Kidney diseases are associated with extremely high-cardiovascular mortality, related to chronic kidney disease–mineral bone disorder (CKD–MBD), involving bone, parathyroids and vascular calcification. Disruption of circadian rhythms may cause serious health problems, contributing to development of cardiovascular diseases, metabolic syndrome, cancer, organ fibrosis, osteopenia and aging. Evidence of disturbed circadian rhythms in CKD–MBD parameters and organs involved is emerging and will be discussed in this review.
Recent findings
Kidney injury induces unstable behavioral circadian rhythm. Potentially, uremic toxins may affect the master-pacemaker of circadian rhythm in hypothalamus. In CKD disturbances in the circadian rhythms of CKD–MBD plasma-parameters, activin A, fibroblast growth factor 23, parathyroid hormone, phosphate have been demonstrated. A molecular circadian clock is also expressed in peripheral tissues, involved in CKD–MBD; vasculature, parathyroids and bone. Expression of the core circadian clock genes in the different tissues is disrupted in CKD–MBD.
Summary
Disturbed circadian rhythms is a novel feature of CKD–MBD. There is a need to establish which specific input determines the phase of the local molecular clock and to characterize its regulation and deregulation in tissues involved in CKD–MBD. Finally, it is important to establish what are the implications for treatment including the potential applications for chronotherapy.
Keywords: activin A, fibroblast growth factor 23, klotho, parathyroid, renal osteodystrophy, vascular calcification
INTRODUCTION
Chronic kidney disease–mineral bone disorder CKD–MBD is a major cause of the excess mortality associated with uremia. CKD–MBD begins early in the course of kidney disease and it is characterized by renal osteodystrophy, increased fracture rates, vascular calcifications and cardiac diseases together with elevations of plasma phosphate, fibroblast growth factor 23 (FGF23), decrease of α-Klotho and an increase in activin A [1–4,5▪]. FGF23 is a hormone secreted by osteocytes which increases renal phosphate excretion [6]. The phosphaturic action of FGF23 requires α-Klotho, an antiaging protein that functions as coreceptor for signal transduction [6]. Activin A is another interesting new factor, a potential biomarker and therapeutic target in CKD–MBD [7,8,9▪]. It is a member of the TGF-β family, essential for kidney development and repair. It is not expressed in the normal kidney but induced in injured kidneys [2,10]. In bone, activin A is secreted by osteoblasts and during bone matrix resorption by osteoclasts. However, its role in bone metabolism is not fully clarified [10,11]. In advanced CKD–MBD secondary hyperparathyroidism (sHPT), calcitriol deficiency and hyperphosphatemia develop.
Proper rhythms in metabolism, hormone secretion, cell cycle and behavior are maintained by a circadian clock, an endogenous, self-sustaining pacemaker that operates with a periodicity of 24 h [12▪,13]. This is to anticipate predictable changes in environment following Earth rotation, day and night and especially changes in light. In mammals the master pacemaker of circadian rhythmicity is in the hypothalamic suprachiasmatic nucleus (SCN). In addition to this central pacemaker a molecular clock has been found in central nervous system (CNS) and peripheral tissues. The SCN is receiving light cues via intrinsically photosensitive retinal ganglion cells and is accordingly coordinating the peripheral and central clocks via neuronal, hormonal and metabolic signaling pathways [14,15]. The circadian rhythms have a systemic impact on all aspects of physiology including metabolism, immunity, cognition, organ function [16]. The hierarchical view on the circadian system level organization has been challenged by the discovery that the hepatic circadian clock was independently entrained by feeding rhythm [17,18]. Kidneys have a robust molecular circadian clock and many genes that determine renal function are expressed in a circadian manner [19,20▪]. Recently, it was shown that in kidney injury induced by adenine diet, CKD mice developed unstable behavioral circadian rhythm and a kidney-to-CNS feedback was proposed [21▪,22▪]. Potentially, uremic toxins can affect SCN [23▪]. Epidemiological studies in humans showed that a disturbed circadian rhythm is associated with an increased risk of osteopenia, metabolic syndrome and cancer. The understanding of the importance of disruption of circadian rhythmicity for the pathophysiology of uremic symptoms is at its beginning [24,25]. Recent results indicate existence of severe disturbances in the circadian rhythm of the CKD–MBD parameters, activin A, FGF23, parathyroid hormone (PTH) and phosphate [12▪]. Here we address the emerging impact of disruption of circadian rhythmicity in mineral homeostasis and organs involved in CKD–MBD.
Box 1.
no caption available
The molecular circadian clock
The biology of circadian rhythms is complex and understanding the mechanisms in chronobiology is still expanding. In 1971, Konopka and Benzer [26] discovered that the circadian rhythms have a genetic determinant. In 2017, Hall, Rosbash and Young were awarded the Nobel Prize in Physiology and Medicine for their discovery of the molecular clock machinery [27].
Both central and peripheral clocks involve the same set of genes and are regulated by an interplay of positive and negative feedback-loops. In addition, regulation of circadian transcription is also subject to modifications in the epigenetic state that change dynamically over day-night [28].
In the core of the circadian machinery is the complex of Bmal1 (brain-muscle Arnt-like protein 1) and Clock (circadian locomotor output cycles kaput) DNA-binding to E-box and E-box like sequences, which regulate the expression of approximately 10% of the transcripts in the genome in a tissue-specific manner (Fig. 1). Bmal1/Clock – dependent clock-controlled genes peak during the day, whereas the transcription is inhibited by the circadian repressors, Per (Period) and Cry (Cryptochrome) at night. Clock and Bmal1 represent major components of the clock's positive limb. They induce, among others expression of the proteins, Per and Cry, which constitute the major arm of the negative limb. Per accumulates in the cytoplasm, forms a complex with Cry as well as other modulator proteins and acts as repressor of Clock/Bmal1 and subsequently inhibits its own expression, resulting in the oscillation of gene expression in a circadian manner. This main loop is interplaying with other feedback-loops, including those of Rev-erbα or the retinoid acid related orphan receptor mediating opposing actions, repressing or activating Bmal1 gene expression. The preferential feedback-loop structures vary across tissues and peripheral organs, contributing to tissue-specific circadian rhythms [29].
FIGURE 1.
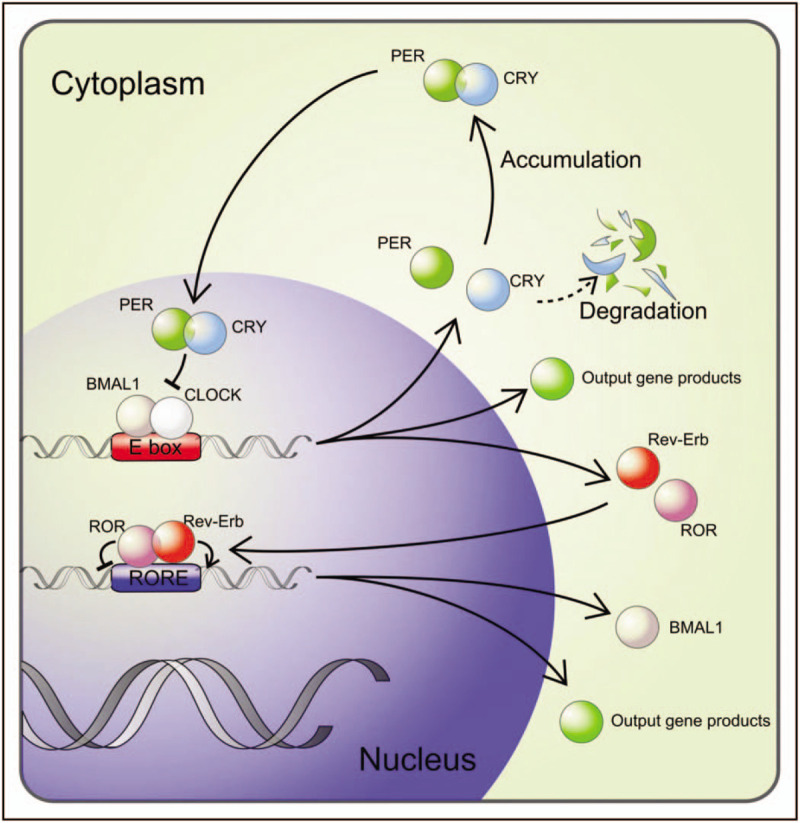
The molecular circadian clock. The transcription factors, circadian locomotor output cycles kaput (CLOCK) and brain-muscle Arnt-like protein 1 (BMAL1), are major components of the molecular circadian clock positive limb. CLOCK and BMAL1 heterodimerize, bind to E-box elements in the promoters of period (PER) and cryptochrome (CRY) and drive the negative limb in the feedback loop. PER and CRY proteins translocate back into the nucleus, hindering CLOCK and BMAL1 transcriptional activity, resulting in oscillation of the gene expressions in a circadian manner. This main loop is interplaying with a feedback loop driven by Rev-erb and receptor tyrosine kinase-like orphan receptor (ROR) mediating opposing actions, repressing or activating BMAL1 gene expression. The molecular circadian clock drives the expression of the clock-controlled tissue specific output genes and hereby about 10% of the transcriptome show circadian rhythmicity.
At the cellular level, regulation of pathways of autophagy, 5’ adenosine monophosphate-activated protein kinase, Sirtuin-1, NADP(H) as well as proliferative mechanisms that involve Wnt and mammalian target of rapamycin (mTOR) are connected to the physiological regulation of the body's circadian rhythm [30–34]. For instance, numerous genes, approximately 50, that are involved in Wnt signaling are under circadian regulation and have rhythmic expression profiles [30,31], impacting cell proliferation, renewal and differentiation of stem-cells and tissue patterning. Studies support a cross-talk between the circadian clock and the hypoxia signaling pathway. Cry1 acts as repressor of hypoxia-inducible factors (HIF's) via a specific protein–protein interaction that reduces binding of HIF's to target genes and by altering HIF's half-life [35].
The understanding of the interaction between circadian rhythms and the environment is expanding as well. Sunrise and sunset have the primary influence on the circadian rhythms. Interactions with light–dark cycle have now been expanded to include artificial light and blue light from computer screens [36]. Diet, including time restricted feeding and fasting are other key extrinsic cues, interacting with the intrinsic clock [37,38▪]. There are probably other cues such as activity, temperature, oxygen or social cues, all important for CKD patients. As such while operating by the same mechanisms the circadian rhythms may vary between individuals due to environmental differences, age and genetics [39].
Disturbances of the circadian clock
Evidence suggests that disruption of the circadian rhythm may cause serious health problems, contributing to development of cardiovascular diseases, metabolic syndrome, cancer, pulmonary fibrosis, osteopenia and aging [24,40–43,44▪,45]. Epidemiological studies indicate that disturbances of the circadian rhythm are associated with carcinogenesis [46], and shift work is classified as a group 2A carcinogenic factor. Studies have linked molecular clock with cell cycle and proliferation [30,47]. Certain types of cancer have an altered expression of circadian clock genes [47]. Clock components such as Per1 and Per2, Bmal1 and Cry decrease cell proliferation [48]. Disturbances in circadian rhythms affect the Wnt and HIFs signaling, which is of importance for cancer progression [35,47], and potentially also for the pathogenesis of CKD–MBD, as Wnt and HIFs have fundamental roles in bone metabolism, FGF23 regulation, renal fibrosis and vascular calcification [49,50].
Night shift work alters sleep timing and duration and is associated with low-bone mineral density and an increased risk of fractures [51]. It is however unclear if sleep restriction itself or circadian misalignment cause the changes in bone formation. The perturbation in circadian rhythmicity, but also timing of food intake may alter bone metabolism.
Disturbances of the circadian clock and chronic kidney disease
Daily oscillations in volume of urine production, renal blood flow, glomerular filtration rate (GFR) and electrolyte excretion and their impact on blood pressure (BP) regulation has been known for decades. Disruption in the circadian molecular clock, circadian rhythmicity in organ function and in circulating signals is an emerging concept in the systems biology of CKD. Kidneys have well documented expression of circadian clock genes and many renal transporter genes in the different part of the nephron are clock-controlled [19,20▪]. Recently, it was shown that the intrinsic glomerular circadian clock is regulating GFR [20▪]. In mice lacking Bmal1 in podocytes the circadian rhythm of GFR was lost together with alteration in the diurnal pattern in plasma aldosterone levels. Aldosterone is a major factor in deregulated sodium balance and BP in uremia. Potentially, a disturbance of the circadian molecular clocks in the individual peripheral tissues in CKD might contribute to uremic symptoms. The impact of kidney insufficiency on the CNS might also include disruption of the molecular clock system. Recently, it was shown that in kidney injury induced by adenine diet, CKD mice developed unstable behavioral circadian rhythm with fragmented sleep and lower locomotor activity, associated with lower amplitude in circadian rhythm of Period 2 expression in the SCN [21▪,22▪]. Uremic toxins can potentially affect the central circadian pacemaker in hypothalamus [23▪]. Misalignment of sleep-wake and fasting-feeding cycles with the endogenous circadian clock as a consequence of night shift work is associated with adverse metabolic effects. Sleep disorders are prevalent in patients with CKD. Melatonin is a hormone primarily released from the pineal gland, involved in sleep-wake timing, BP regulation and in synchronizing circadian rhythms. In CKD the amplitude of the melatonin rhythm decreases as renal function declines and disturbed melatonin rhythm in CKD patients is associated with sleep disorders [52–55]. Furthermore, restless legs syndrome, a sensorimotor disorder with circadian rhythmicity is common in patients with CKD [56]. The prevalence of increased BP during sleep and nondipper pattern is high in CKD population [57▪,58]. CKD patients have higher vagal activity during the day with lower sympathovagal balance at night [59]. Thus, several observations indicate an unbalanced circadian system in uremia, which potentially is representing a previously unrecognized risk factor. In the following the concept of disruption of the circadian clock system in organs involved in CKD–MBD, the parathyroid gland, vasculature and bone is discussed (Fig. 2).
FIGURE 2.
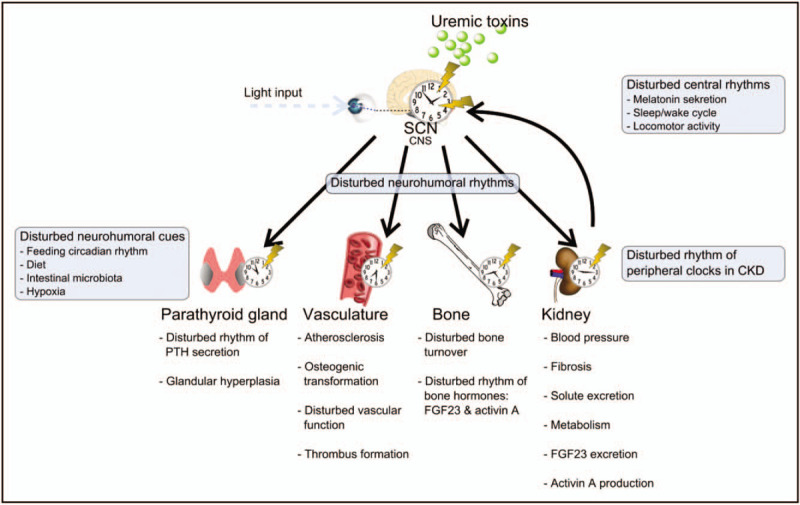
A simplified model of disturbed circadian rhythm in chronic kidney disease–mineral bone disorder. The circadian system involves the environmental cues that entrain the central pacemaker, a molecular circadian clock, located in the suprachiasmatic nucleus (SCN) and peripheral molecular circadian clocks, located in the peripheral cells, but being under control of the central pacemaker via neurohumoral signals. The kidney provides feedback input to the central clock as well. In chronic kidney disease the clock in central nervous system is potentially deregulated by uremic toxins, feedback input from the injured kidney as well as by disturbed response to environmental cues. The molecular clocks in organs involved in chronic kidney disease–mineral bone disorder are desynchronized from the central clock. The expressions of the peripheral clock genes in the parathyroid gland, vasculature, bone and kidney are disturbed, contributing to the chronic kidney disease–mineral bone disorder symptoms of secondary hyperparathyroidism, vascular calcifications, renal osteodystrophy, kidney fibrosis and disturbed circadian rhythmicity of the plasma parameters related to chronic kidney disease–mineral bone disorder, activin A, fibroblast growth factor 23, parathyroid hormone and phosphate.
Parathyroid hyperplasia and circadian clock-regulated cell proliferation in secondary hyperparathyroidism
The circadian rhythm of PTH secretion is well described and it is considered truly endogenous [12▪,60]. The parathyroid glands are not controlled by a superior hypothalamic-pituitary axis as many other endocrine glands and are likely to use other hitherto unknown regulatory mechanisms.
In CKD, sHPT is characterized by extensive growth of the glandular size, increasing up to 100-fold and disrupted circadian rhythmicity of PTH secretion [12▪]. Deregulation of the circadian clock might theoretically be of importance for parathyroid hyperplasia in CKD. The cell cycle regulators involved in parathyroid growth are known to be under control of the clock. c-Myc, p20, p21 and cyclin D1 all exhibit circadian pattern of gene expression and TGF-α/epidermal growth factor receptor signaling is known to regulate circadian rhythms within the CNS [61]. In sHPT, c-Myc is overexpressed in a substantial fraction of the parathyroid tumors [62]. In uremic rats, dietary phosphate restriction, high dietary calcium or administration of calcitriol prevented parathyroid hyperplasia by inducing the cell cycle inhibitor, p21, and decreasing TGF-α. An enhanced expression of TGF-α is known to promote cell growth and has been found in parathyroid hyperplasia in uremia. In hyperplastic parathyroid glands from uremic patients the expression of the cyclin-dependent kinase inhibitors, p21 and p27, was reduced in a manner, which depended upon the expression of the vitamin D receptor (VDR) [63,64]. Transgenic mice overexpressing parathyroid cyclin D1 developed parathyroid hyperplasia and hyperparathyroidism. Circadian pathways are intimately linked to the mTOR pathway [65]. Melatonin, that is controlling circadian rhythm regulates differential processes including cancer growth via mTOR [66], and the mTORC1 pathway is essential for parathyroid cell proliferation in sHPT [67].
In a translational model of CKD–MBD, we examined whether an internal molecular circadian clock was present in the rat parathyroid gland and found a strong expression of core molecular clock genes: Bmal1, Clock, Per1–3, Cry1–2 and Rev-Erbα, all having significant circadian rhythmicity. Furthermore, we found significant rhythmicity of the cell cycle gene Cyclin D1 in normal rats. In parathyroids from CKD rats Cyclin D1 expression was deregulated (unpublished data). As such, the demonstrated internal parathyroid circadian clock might be disturbed in uremia and might potentially contribute to the glandular hyperplasia. Parathyroid molecular circadian clock and its regulation by VDR, calcium-sensing receptor (CaR) and phosphate need to be examined in detail.
The vascular circadian clock is disrupted in chronic kidney disease–mineral bone disorder
The cardiovascular system, BP and heart rate and the endothelial function and thrombus formation are regulated by the circadian clock [68,69]. Disruption of 24-h rhythms may lead to cardiovascular disease, including heart failure, fibrosis, myocardial infarction (MI) and arrhythmias [24,25]. In addition, the onset of cardiovascular events such as acute MI, arrhythmias and stroke shows circadian pattern [40,70,71] and patients with hypertension benefits from antihypertensive chronotherapy [57▪,72].
Arterial calcification can be classified into tunica intima calcification, related to atherosclerosis, and tunica media calcification. Media calcification is predominant in systemic metabolic disorders, such as CKD, diabetes mellitus and aging. Cells with multilineage potential in the arterial wall, pericytes, smooth muscle cells and adventitial myofibroblasts may all contribute to the development of vascular calcifying diseases. In the context of chronic uremia, vascular smooth muscle cell can undergo maladaptive osteochondrocytic differentiation [73]. A functional circadian clock has been demonstrated in the different cell types of the blood vessel layers. The circadian clock components have been found in endothelial cells [74], in vascular smooth muscle cell of lamina media [75] and in cultured fibroblasts from the outer wall of the vessels [40]. An interesting study demonstrated development of atherosclerosis in mice with a disrupted circadian clock (Bmal1 or Per2,3 double-KO). Transplantation of blood vessels from these animals into wild-type littermates did not prevent this pathologic development, but still resulted in atherosclerosis [76]. This indicates that the intrinsic vascular tissue clock has an autonomous impact on atherosclerotic disease.
In CKD–MBD patients, a lack of the diurnal variation in arterial stiffness and a loss in nocturnal BP dipping have been demonstrated [58].
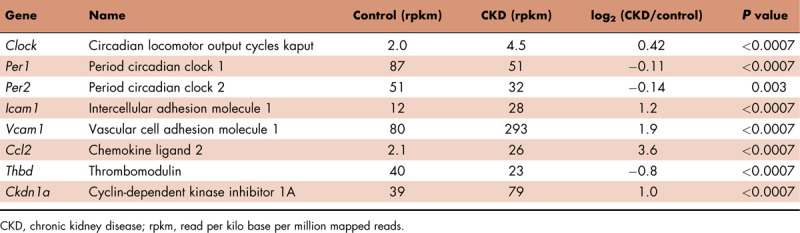
Our laboratory examined by RNAseq analysis the transcriptional profile of severely calcified aortas in a CKD–MBD rat model [73]. Several genes related to the circadian clock were expressed in the normal aorta and were significantly deregulated in the calcified uremic aorta. The expression of Per genes was significantly downregulated in the calcified aorta, whereas the expression of Cry genes was unaffected. The transcriptional activators Clock and Bmal1 were significantly increased in the calcified uremic aorta. Genes reported to be controlled by the clock system were significantly deregulated, as presented in Table 1.
Table 1.
Disrupted expression of circadian clock genes and clock-controlled genes in the calcified aorta of uremic rats analyzed by RNAseq
Renal osteodystrophy and circadian clock
Bone remodeling is a complex process by which old bone is removed and replaced by new bone, requiring coordinated interaction between different bone cells. The diurnal variation in bone turnover markers and experimental circadian clock knockout models suggest that circadian rhythmicity is important for bone health [77].
Global deletion of murine Bmal1 led to a low bone mass, associated with increased bone resorption. Osteoclast-specific Bmal1-knockout mice showed a high bone mass phenotype due to reduced osteoclast differentiation [78]. It has been suggested that bone resorption is controlled by osteoclastic Bmal1 through interactions with the steroid receptor coactivator family and upregulation of nuclear factor (NF) of activated T cells, cytoplasmic 1, calcineurin-dependent 1 (Nfatc1) transcription through its binding to an E-box element located on the Nfatc1 promoter [78]. Studies indicate that the clock system is present in osteoblasts as well [79,80]. Coculture experiments revealed that Bmal1-deficient osteoblasts have a higher ability to support osteoclastogenesis, whereas overexpression of Bmal1/Clock inhibited calcitriol-induced receptor activator of NF κB ligand (Rankl) in osteoblasts [81].
Bone turnover has a circadian pattern with bone resorption and to a less extent bone formation increasing at night. A robust diurnal variation of the bone resorption markers N-terminal or C-terminal telopeptide of type I collagen has been shown [82,83]. It was not affected by bedrest, cortisol level, blindness (indicating independence from the light/dark cycle) or administration of salmon calcitonin. Fasting had a pronounced influence on the circadian variation of bone turnover [84] reducing the amplitude [82]. It is well known that the gut is an important regulator of bone homeostasis with gut-derived factors, including glucose-dependent insulinotropic polypeptide and peptide YY, controlling bone resorption and formation [85]. Recently it has been demonstrated that the intestinal circadian system regulates skeletal homeostasis [86]. The lack of the Bmal1 gene in the intestine (Bmal1Int−/− mice) caused bone loss, with bone resorption being activated and bone formation suppressed [86]. Mechanistically, Clock protein interaction with VDR accelerates its binding to the VDR response element by enhancing histone acetylation in a circadian-dependent manner, which was lost in Bmal1Int−/− mice. As a result, the rhythmic expression of VDR target genes involved in transcellular Ca absorption was abolished, Ca absorption impaired, and bone resorption activated [86].
The intestinal bacterial composition and function feature daily rhythmicity depending on the cues from the host circadian clock [87]. In a mice model of hyperparathyroidism, impacting the gut microbiome by antibiotics or using germ-free animals PTH-induced bone loss was ameliorated via a T-cell-related mechanism [88▪]. These observations indicate that metabolic bone disorder induced by circadian clock disruption can be affected by aberrations in the intestinal microbiome.
In CKD–MBD, the spectrum of renal osteodystrophy is related to plasma PTH levels and skeletal responsiveness to PTH. It is potentially affected by diurnal variation in circulating PTH and phosphate, and by the internal circadian clock in bone cells as well as the gut–bone crosstalk. Indeed CKD leads to alterations in the intestinal flora [89]. More research is necessary to unravel the relationship between microbiome composition, rhythmicity and renal osteodystrophy, including skeletal response to PTH.
Melatonin suppresses a microgravity stimulated osteoclast activity in the Goldfish Scales Model and is proposed potentially to prevent bone loss during space flight [90]. The circadian rhythm of melatonin is disrupted in CKD. Activin A and FGF23 are primarily bone-derived factors having circadian rhythmicity, and their levels are increased, and rhythmicity abolished in CKD [12▪]. This might potentially indicate a link between circadian clock regulation in bone and development of renal osteodystrophy. The importance of the molecular circadian clock in bone cells for the secretion of activin A and FGF23 in normal and CKD conditions needs however further investigation.
Circadian rhythms in plasma parameters of mineral metabolism in chronic kidney disease–mineral bone disorder
In uremia, disturbances in the circadian rhythms of activin A, FGF23, PTH and phosphate have been demonstrated (Fig. 3). Results from our laboratory showed for the first time existence of a circadian rhythm of plasma activin A, which was disturbed in CKD (Fig. 4) [12▪]. Plasma-phosphate and PTH have circadian rhythms, which should be taken into consideration when interpreting the circulating levels [12▪,91]. Similarly, p-activin A levels need to be related to the time of the day, they are obtained, as four-fold higher values are found at acrophase, compared with nadir (Fig. 4) [12▪].
FIGURE 3.
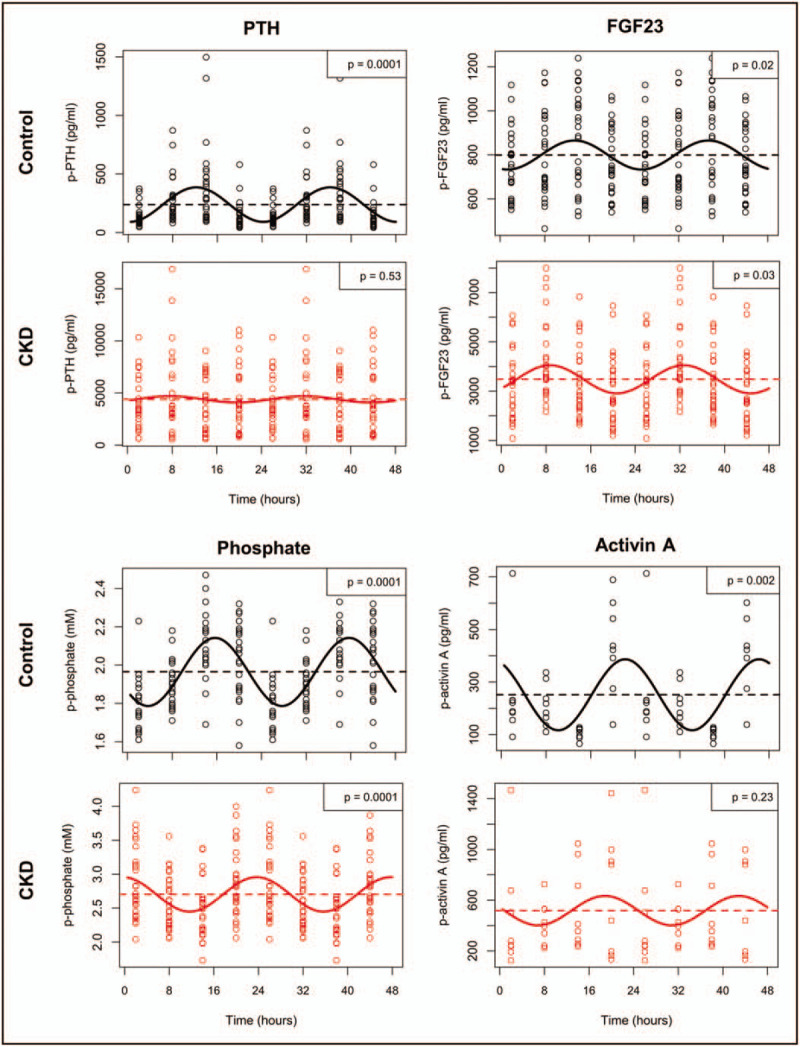
Disturbed circadian rhythms of chronic kidney disease–mineral bone disorder parameters in uremia. Circadian rhythms of circulating levels of plasma parathyroid hormone, fibroblast growth factor 23, phosphate, and activin A in chronic kidney disease and age-matched normal control rats are shown. Wistar rats were allocated to control or chronic kidney disease (5/6 nephrectomy and high phosphate diet for 24 weeks). Control rats exhibited circadian rhythm of all parameters. Significant rhythmicity was confirmed by cosinor analysis: parathyroid hormone (P < 0.0001), fibroblast growth factor 23 (P < 0.05), phosphate (P < 0.0001) and activin A (P < 0.01). Chronic kidney disease completely obliterated the circadian rhythm of parathyroid hormone and activin A. The circadian rhythms of fibroblast growth factor 23 and phosphate were maintained in chronic kidney disease rats (fibroblast growth factor 23: P < 0.05, phosphate: P < 0.0001), however, both rhythms were severely disturbed. As such, the acrophase of fibroblast growth factor 23 shifted from 13:00 in control to 09:00 in chronic kidney disease rats, whereas the acrophase of phosphate shifted from 16:00 in controls to 00:00 in chronic kidney disease rats [12▪].
FIGURE 4.
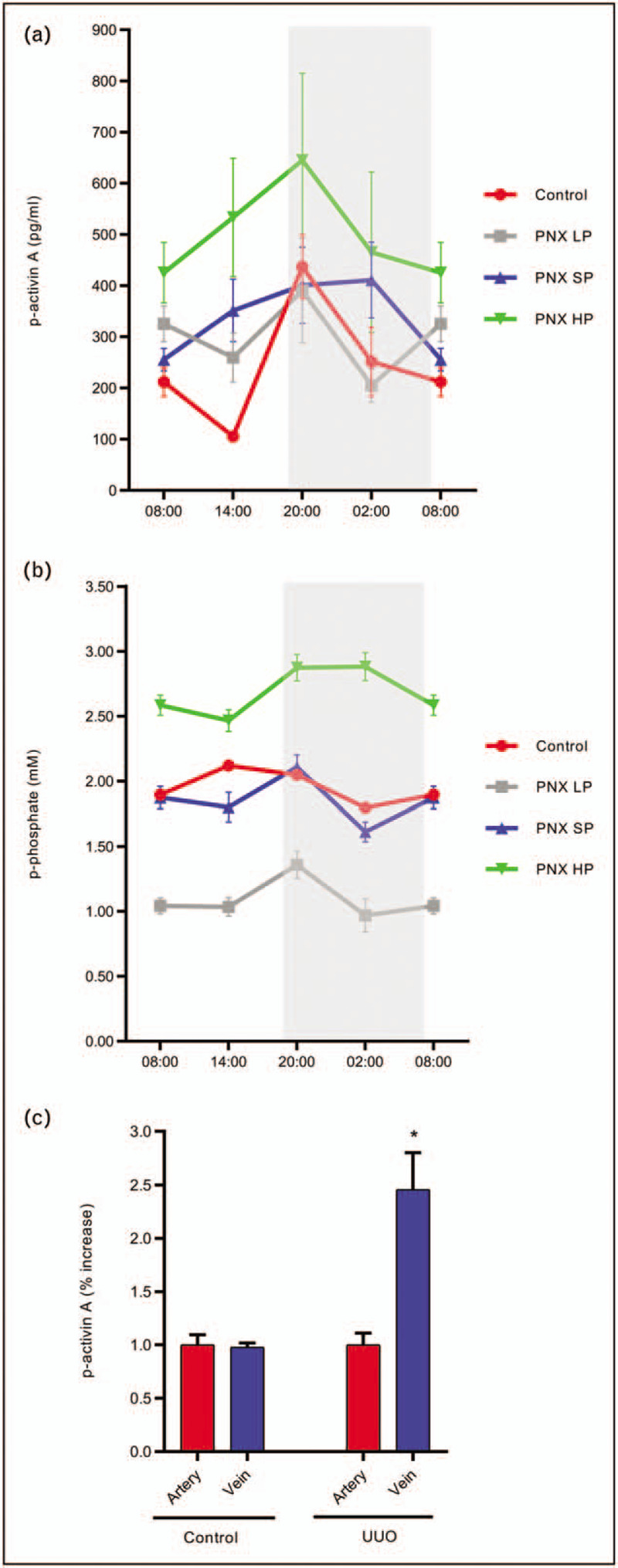
Circadian rhythm of plasma activin A and phosphate in normal and uremic rats on different phosphate diets. Induction and secretion of activin A from the injured kidney. Plasma activin A exhibits circadian rhythmicity in control rats, whereas the rhythm is obliterated by chronic kidney disease. An increase in plasma activin A levels was observed in chronic kidney disease rats, but depending upon the time of the day. In chronic kidney disease rats on a low phosphate diet the increase in plasma activin A was inhibited. However, the circadian rhythm was not restored (a). Similarly, circadian rhythmicity of plasma phosphate was disturbed in chronic kidney disease rats. Furthermore, chronic kidney disease rats on a high phosphate diet developed hyperphosphatemia. This was prevented by the low phosphate diet, which however did not restore the circadian rhythmicity of plasma phosphate in chronic kidney disease (b) [12▪]. Kidney injury was induced by unilateral ureter obstruction for 15 days and blood sampling from the isolated renal artery and vein was performed. Activin A was induced and secreted of from the injured kidney (c). partly nephrectomized: 5/6 partial nephrectomy. HP, high-phosphate diet; LP, low-phosphate diet; SP, standard-phosphate diet. Mean ± SEM.
Recent fascinating studies proposed that the CaR [92], FGFR1 [93,94] and sodium-phosphate cotransporter, PiT2 [95,96] are involved in extracellular sensing of phosphate. Changes in the expression and activity of the phosphate sensing receptors in CKD might contribute to the disturbed circadian rhythm of plasma phosphate and the phosphate regulating hormones, PTH and FGF23 in CKD [97]. Plasma Klotho expresses no circadian variation [12▪]. In addition to, disturbed circadian rhythm in plasma levels in CKD might be due to altered metabolism or the extra-skeletal secretion of FGF23 and activin A. Thus, FGF23 is excreted by the kidney [98] and both induction and secretion of FGF23 from the heart and bone marrow have been observed in CKD [49,99]. In our lab we found significant induction and secretion of activin A from injured kidneys [2] (Fig. 4).
Chronotherapy
Chronotherapy or chronotherapeutic treatment is treatment scheduling according to circadian cycle utilizing time or delivery of medication to affect its efficacy [100,101]. The classical example of benefit from chronotherapy is a more efficient reduction in plasma cholesterol when simvastatin is administrated in the evening. That's because the levels of the 3-hydroxy-3-methylglutaryl CoA reductase, which are reduced by statins, are known to peak in the nighttime hours. Recent evidence has also shown that bedtime administration of antihypertensive medication, compared with the usual intake in the morning may provide a significantly better controlled hypertension and diminished occurrence of major cardiovascular events [57▪]. The mechanism by which this effect takes place is still speculative. Given a potential role of the circadian clock in CKD–MBD, chronotherapy might become an important part of the future treatment of this serious condition [57▪,72,100]. Thus, targeting circadian mechanisms by VDR or CaR activators given as chronotherapy might potentially result in amelioration of the parathyroid hyperplasia in CKD. Melatonin administration can potentially synchronize the internal circadian clocks in organs involved in CKD–MBD and improve bone remodeling. This needs to be examined in translational models. New compounds targeting the circadian clock components of relevance for bone remodeling, inflammation and organ fibrosis are emerging and deserve future investigations [47,80,100]. Finally, physiological and behavioral chrono-enhancement based on enhancement of input to the circadian system such as increasing day-night contrast, regular exercises and regular meal schedule might synchronize the circadian rhythms and have an impact on CKD–MBD.
CONCLUSION
The proper rhythms in metabolism, hormonal secretion, cardiovascular function and bone remodeling are controlled by a molecular circadian clock. Evidence is emerging on the disturbances in the circadian rhythms in CKD–MBD. There is an urgent need to characterize the impact of this malfunction on the parathyroid gland, bone and vascular system in CKD. Thus, what is the specific input that determines the phase of the molecular circadian clock in the peripheral tissues, involved in CKD–MBD, and how is the clock deregulated in CKD. Furthermore, translational studies are warranted to examine the applications for chronotherapy.
Acknowledgements
The authors thank Anders Nordholm, Eva Gravesen and Jakob L. Rukov for conducting many of the experiments referred to in the article.
Financial support and sponsorship
The Kirsten and Freddy Johannsen's Foundation.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Nordholm A, Mace ML, Gravesen E, et al. A potential kidney–bone axis involved in the rapid minute-to-minute regulation of plasma Ca2+. BMC Nephrol 2015; 16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordholm A, Mace ML, Gravesen E, et al. Klotho and activin A in kidney injury: plasma klotho is maintained in unilateral obstruction despite no upregulation of Klotho biosynthesis in the contralateral kidney. Am J Physiol Renal Physiol 2018; 314:F753–F762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69:1945–1953. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro AL, Mendes F, Carias E, et al. FGF23-klotho axis as predictive factors of fractures in type 2 diabetics with early chronic kidney disease. J Diabetes Complications 2020; 34:107476. [DOI] [PubMed] [Google Scholar]
- 5▪.Lima F, Mawad H, El-Husseini AA, et al. Serum bone markers in ROD patients across the spectrum of decreases in GFR: activin A increases before all other markers? Clin Nephrol 2019; 91:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]; Investigation of serum activin A, bone biomarkers, and bone histomorphometric parameters in chronic kidney disease (CKD) patients stages 2–5D. Serum activin A levels increase early in development of renal osteodystrophy.
- 6.Chen G, Liu Y, Goetz R, et al. α-Klotho is a nonenzymatic molecular scaffold for FGF23 hormone signalling. Nature 2018; 553:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugatani T, Agapova OA, Fang Y, et al. Ligand trap of the activin receptor type IIA inhibits osteoclast stimulation of bone remodeling in diabetic mice with chronic kidney disease. Kidney Int 2017; 91:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MJ, Sugatani T, Agapova OA, et al. The activin receptor is stimulated in the skeleton, vasculature, heart, and kidney during chronic kidney disease. Kidney Int 2017; 93:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Coyne DW, Singh HN, Smith WT, et al. Sotatercept safety and effects on hemoglobin, bone, and vascular calcification. Kidney Int Rep 2019; 4:1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]; Very interesting, small, phase II, randomized study examining safety, tolerability, and effects of an ActRIIA-IgG1 fusion protein trap (which binds with high affinity to activin A), on hemoglobin concentration, bone mineral density and abdominal aortic vascular calcification. A trend in slowing of vascular calcification was shown.
- 10.Bloise E, Ciarmela P, Dela Cruz C, et al. Activin A in mammalian physiology. Physiol Rev 2019; 99:739–780. [DOI] [PubMed] [Google Scholar]
- 11.Baroncelli M, Drabek K, Eijken M, et al. Two-day-treatment of activin-A leads to transient change in SV-HFO osteoblast gene expression and reduction in matrix mineralization. J Cell Physiol 2019; 235:4865–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Nordholm A, Egstrand S, Gravesen E, et al. Circadian rhythm of activin A and related parameters of mineral metabolism in normal and uremic rats. Pflugers Arch 2019; 471:1079–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence of the circadian rhythm of plasma activin A. The rhythmicity is severely disturbed by CKD and is associated with disturbed rhythms of phosphate and phosphate-regulating hormones parathyroid hormone (PTH) and fibroblast growth factor 23. Plasma Klotho has no circadian rhythm.
- 13.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002; 418:935–941. [DOI] [PubMed] [Google Scholar]
- 14.Rahman SA, Wright KP, Jr, Lockley SW, et al. Characterizing the temporal dynamics of melatonin and cortisol changes in response to nocturnal light exposure. Sci Rep 2019; 9:19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koskela S, Turunen T, Ala-Laurila P. Mice reach higher visual sensitivity at night by using a more efficient behavioral strategy. Curr Biol 2019; 30:42–53.e4. [DOI] [PubMed] [Google Scholar]
- 16.Del Rio-Martin A, Perez-Taboada I, Fernandez-Perez A, et al. Hypomorphic expression of pitx3 disrupts circadian clocks and prevents metabolic entrainment of energy expenditure. Cell Rep 2019; 29:3678–3692.e4. [DOI] [PubMed] [Google Scholar]
- 17.Stokkan KA, Yamazaki S, Tei H, et al. Entrainment of the circadian clock in the liver by feeding. Science 2001; 291:490–493. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Feng M, Zhang S, et al. Angptl8 mediates food-driven resetting of hepatic circadian clock in mice. Nat Commun 2019; 10:3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuber AM, Centeno G, Pradervand S, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 2009; 106:16523–16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Ansermet C, Centeno G, Nikolaeva S, et al. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Sci Rep 2019; 9:16089. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors tested a hypothesis that the circadian rhythm of glomerular filtration rate (GFR) is driven by intrinsic glomerular circadian clock. In mice lacking the circadian clock protein BMAL1 specifically in podocytes, the circadian rhythmicity in GFR was lost together with alteration in the diurnal pattern of plasma aldosterone levels.
- 21▪.Myung J, Wu MY, Lee CY, et al. The kidney clock contributes to timekeeping by the master circadian clock. Int J Mol Sci 2019; 20:2765. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors hypothesize that sleep disturbances in CKD originate from aberrant circadian rhythms in kidney. CKD mice developed unstable behavioral circadian rhythms, and the circadian clock in the kidney became less robust, with a longer period.
- 22▪.Motohashi H, Tahara Y, Whittaker DS, et al. The circadian clock is disrupted in mice with adenine-induced tubulointerstitial nephropathy. Kidney Int 2020; 97:728–740. [DOI] [PubMed] [Google Scholar]; Adenine induced tubulointerstitial nephropathy disrupted the circadian system both in central and peripheral organs. Clock mutant mice were more vulnerable to the effects of adenine.
- 23▪.Loganathan N, Salehi A, Chalmers JA, et al. Alters Bmal1, Per2, and Rev-Erba mRNA and requires Bmal1 to increase neuropeptide Y expression in hypothalamic neurons. Endocrinology 2019; 160:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bisphenol A, a potential uremic toxin, alters circadian clock in hypothalamus.
- 24.Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 2008; 294:R1675–R1683. [DOI] [PubMed] [Google Scholar]
- 25.Alibhai FJ, LaMarre J, Reitz CJ, et al. Disrupting the key circadian regulator CLOCK leads to age-dependent cardiovascular disease. J Mol Cell Cardiol 2017; 105:24–37. [DOI] [PubMed] [Google Scholar]
- 26.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 1971; 68:2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callaway E, Ledford H. Medicine Nobel awarded for work on circadian clocks. Nature 2017; 550:18. [DOI] [PubMed] [Google Scholar]
- 28.Gibo S, Kurosawa G. Theoretical study on the regulation of circadian rhythms by RNA methylation. J The Biol 2019; 490:110140. [DOI] [PubMed] [Google Scholar]
- 29.Pett JP, Kondoff M, Bordyugov G, et al. Co-existing feedback loops generate tissue-specific circadian rhythms. Life Sci Alliance 2018; 1:e201800078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsu-Ura T, Moore SR, Hong CI. WNT takes two to tango: molecular links between the circadian clock and the cell cycle in adult stem cells. J Biol Rhythms 2018; 33:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotak M, Sumova A, Pacha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med 2014; 46:221–232. [DOI] [PubMed] [Google Scholar]
- 32.Maiese K. Moving to the rhythm with clock (circadian) genes, autophagy, mTOR, and SIRT1 in degenerative disease and cancer. Curr Neurovasc Res 2017; 14:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiese K. Novel treatment strategies for the nervous system: circadian clock genes, non-coding rnas, and forkhead transcription factors. Curr Neurovasc Res 2018; 15:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laothamatas I, Gao P, Wickramaratne A, et al. Spatiotemporal regulation of NADP(H) phosphatase Nocturnin and its role in oxidative stress response. Proc Natl Acad Sci U S A 2019; 117:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimova EY, Jakupovic M, Kubaichuk K, et al. The circadian clock protein CRY1 is a negative regulator of HIF-1α. iScience 2019; 13:284–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahkamo L, Partonen T, Pesonen AK. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int 2019; 36:151–170. [DOI] [PubMed] [Google Scholar]
- 37.Waldman HS, Renteria LI, McAllister MJ. Time7-restricted feeding for the prevention of cardiometabolic diseases in high-stress occupations: a mechanistic review. Nutr Rev 2020; 78:459–464. [DOI] [PubMed] [Google Scholar]
- 38▪.Heyde I, Oster H. Differentiating external zeitgeber impact on peripheral circadian clock resetting. Sci Rep 2019; 9:20114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic investigation of relative contribution of two major zeitgebers light and food on central and peripheral tissue circadian clock resetting.
- 39.Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest 2017; 127:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 2019; 16:437–447. [DOI] [PubMed] [Google Scholar]
- 41.Welz PS, Benitah SA. Molecular connections between circadian clocks and aging. J Mol Biol 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Zhong LX, Li XN, Yang GY, et al. Circadian misalignment alters insulin sensitivity during the light phase and shifts glucose tolerance rhythms in female mice. PLoS One 2019; 14:e0225813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolbe I, Leinweber B, Brandenburger M, Oster H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol Metab 2019; 30:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Cunningham PS, Meijer P, Nazgiewicz A, et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development. Proc Natl Acad Sci U S A 2019; 117:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a mechanistic study linking tissue fibrosis and circadian clock in a mouse model of pulmonary fibrosis. Fibrotic mouse lungs exhibited asynchronous circadian rhythm. Disruption of fibroblast cells REVERBα exaggerated pulmonary fibrosis. Targeting of REVERBα by a synthetic ligand repressed myofibroblast differentiation and collagen synthesis. Targeting the core clock protein REVERBα could be therapeutic approach in fibrosis.
- 45.Clarkson-Townsend DA, Everson TM, Deyssenroth MA, et al. Maternal circadian disruption is associated with variation in placental DNA methylation. PLoS One 2019; 14:e0215745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulli G, Lam MTY, Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer 2019; 5:475–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulli G, Rommel A, Wang X, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018; 553:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katamune C, Koyanagi S, Hashikawa KI, et al. Mutation of the gene encoding the circadian clock component PERIOD2 in oncogenic cells confers chemoresistance by up-regulating the Aldh3a1 gene. J Biol Chem 2019; 294:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edmonston D, Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol 2020; 16:7–19. [DOI] [PubMed] [Google Scholar]
- 50.Hruska KA, Seifert M, Sugatani T. Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens 2015; 24:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feskanich D, Hankinson SE, Schernhammer ES. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int 2009; 20:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koch BC, Hagen EC, Nagtegaal JE, et al. Effects of nocturnal hemodialysis on melatonin rhythm and sleep-wake behavior: an uncontrolled trial. Am J Kidney Dis 2009; 53:658–664. [DOI] [PubMed] [Google Scholar]
- 53.Koch BC, Nagtegaal JE, Hagen EC, et al. The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: a randomized, placebo-controlled, cross-over study (EMSCAP study). Br J Clin Pharmacol 2009; 67:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koch BC, van der Putten K, Van Someren EJ, et al. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol Dial Transplant 2010; 25:513–519. [DOI] [PubMed] [Google Scholar]
- 55.Russcher M, Chaves I, Lech K, et al. An observational study on disturbed peripheral circadian rhythms in hemodialysis patients. Chronobiol Int 2015; 32:848–857. [DOI] [PubMed] [Google Scholar]
- 56.Novak M, Winkelman JW, Unruh M. Restless legs syndrome in patients with chronic kidney disease. Semin Nephrol 2015; 35:347–358. [DOI] [PubMed] [Google Scholar]
- 57▪.Hermida RC, Crespo JJ, Dominguez-Sardina M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; Clinical prospective chronotherapy trial showing that bedtime administration of hypertension therapy, in comparison to usual upon awakening, diminished occurrence of major cardiovascular events.
- 58.Rahman A, Hasan AU, Nishiyama A, Kobori H. Altered circadian timing system-mediated non-dipping pattern of blood pressure and associated cardiovascular disorders in metabolic and kidney diseases. Int J Mol Sci 2018; 19:E400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makimoto H, Shimizu K, Fujiu K, et al. Effect of sympatholytic therapy on circadian cardiac autonomic activity in non-diabetic chronic kidney disease. Int Heart J 2018; 59:1352–1358. [DOI] [PubMed] [Google Scholar]
- 60.el-Hajj Fuleihan G, Klerman EB, Brown EN, et al. The parathyroid hormone circadian rhythm is truly endogenous–a general clinical research center study. J Clin Endocrinol Metab 1997; 82:281–286. [DOI] [PubMed] [Google Scholar]
- 61.Mendoza J, Revel FG, Pevet P, Challet E. Shedding light on circadian clock resetting by dark exposure: differential effects between diurnal and nocturnal rodents. Eur J Neurosci 2007; 25:3080–3090. [DOI] [PubMed] [Google Scholar]
- 62.Bjorklund P, Akerstrom G, Westin G. Accumulation of nonphosphorylated beta-catenin and c-myc in primary and uremic secondary hyperparathyroid tumors. J Clin Endocrinol Metab 2007; 92:338–344. [DOI] [PubMed] [Google Scholar]
- 63.Lewin E, Rodriguez M. Olgaard K, Salusky IB, Silver J. Abnormal parathyroid gland function in CKD. The spectrum of mineral and bone disorders in chronic kidney disease 2nd ed.Oxford: Oxford University Press; 2010. 77–107. [Google Scholar]
- 64.Cozzolino M, Lu Y, Finch J, et al. p21WAF1 and TGF-alpha mediate parathyroid growth arrest by vitamin D and high calcium. Kidney Int 2001; 60:2109–2117. [DOI] [PubMed] [Google Scholar]
- 65.Wu R, Dang F, Li P, et al. The circadian protein period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 complex. Cell Metab 2019; 29:653–667.e6. [DOI] [PubMed] [Google Scholar]
- 66.Beker MC, Caglayan B, Caglayan AB, et al. Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Sci Rep 2019; 9:19082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volovelsky O, Cohen G, Kenig A, et al. Phosphorylation of ribosomal protein S6 mediates mammalian target of rapamycin complex 1-induced parathyroid cell proliferation in secondary hyperparathyroidism. J Am Soc Nephrol 2016; 27:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu K, Ivanov P, Hilton MF, et al. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A 2004; 101:18223–18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abbott AL, Merican J, Pearce DC, et al. Asymptomatic carotid stenosis is associated with circadian and other variability in embolus detection. Front Neurol 2019; 10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mistry P, Duong A, Kirshenbaum L, Martino TA. Cardiac clocks and preclinical translation. Heart Fail Clin 2017; 13:657–672. [DOI] [PubMed] [Google Scholar]
- 71.Nordenskjold AM, Eggers KM, Jernberg T, et al. Circadian onset and prognosis of myocardial infarction with non-obstructive coronary arteries (MINOCA). PLoS One 2019; 14:e0216073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slomski A. Circadian timing of medications affects CVD outcomes. JAMA 2019; 322:2375. [DOI] [PubMed] [Google Scholar]
- 73.Rukov JL, Gravesen E, Mace ML, et al. Effect of chronic uremia on the transcriptional profile of the calcified aorta analyzed by RNA sequencing. Am J Physiol Renal Physiol 2016; 310:F477–F491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takeda N, Maemura K, Horie S, et al. Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J Biol Chem 2007; 282:32561–32567. [DOI] [PubMed] [Google Scholar]
- 75.Chalmers JA, Martino TA, Tata N, et al. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (Movas-1). Am J Physiol Regul Integr Comp Physiol 2008; 295:R1529–R1538. [DOI] [PubMed] [Google Scholar]
- 76.Cheng B, Anea CB, Yao L, et al. Tissue-intrinsic dysfunction of circadian clock confers transplant arteriosclerosis. Proc Natl Acad Sci U S A 2011; 108:17147–17152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swanson CM, Kohrt WM, Buxton OM, et al. The importance of the circadian system & sleep for bone health. Metabolism 2018; 84:28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu C, Ochi H, Fukuda T, et al. Circadian clock regulates bone resorption in mice. J Bone Miner Res 2016; 31:1344–1355. [DOI] [PubMed] [Google Scholar]
- 79.Yuan G, Hua B, Yang Y, et al. The circadian gene clock regulates bone formation via PDIA3. J Bone Miner Res 2017; 32:861–871. [DOI] [PubMed] [Google Scholar]
- 80.Kim K, Kim JH, Kim I, et al. Rev-erbα negatively regulates osteoclast and osteoblast differentiation through p38 mapk signaling pathway. Mol Cells 2020; 43:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takarada T, Xu C, Ochi H, et al. Bone resorption is regulated by circadian clock in osteoblasts. J Bone Miner Res 2017; 32:872–881. [DOI] [PubMed] [Google Scholar]
- 82.Qvist P, Christgau S, Pedersen BJ, et al. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 2002; 31:57–61. [DOI] [PubMed] [Google Scholar]
- 83.van der Spoel E, Oei N, Cachucho R, et al. The 24-hour serum profiles of bone markers in healthy older men and women. Bone 2019; 120:61–69. [DOI] [PubMed] [Google Scholar]
- 84.St Hilaire MA, Rahman SA, Gooley JJ, et al. Relationship between melatonin and bone resorption rhythms in premenopausal women. J Bone Miner Metab 2019; 37:60–71. [DOI] [PubMed] [Google Scholar]
- 85.Schiellerup SP, Skov-Jeppesen K, Windelov JA, et al. Gut hormones and their effect on bone metabolism. potential drug therapies in future osteoporosis treatment. Front Endocrinol (Lausanne) 2019; 10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawai M, Kinoshita S, Yamazaki M, et al. Intestinal clock system regulates skeletal homeostasis. JCI insight 2019; 4:121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nobs SP, Tuganbaev T, Elinav E. Microbiome diurnal rhythmicity and its impact on host physiology and disease risk. EMBO Rep 2019; 20:e47129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88▪.Yu M, Malik Tyagi A, Li JY, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF(+) T cells and Th17 cells. Nat Commun 2020; 11:468. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study reveals mechanisms for microbiota-mediated gut-bone crosstalk in mice models of hyperparathyroidism. The gut microbiome is a required determinant of the skeletal effects of PTH.
- 89.Hobby GP, Karaduta O, Dusio GF, et al. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol 2019; 316:F1211–F1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ikegame M, Hattori A, Tabata MJ, et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J Pineal Res 2019; 67:e12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isakova T, Xie H, Barchi-Chung A, et al. Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol 2012; 7:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Centeno PP, Herberger A, Mun HC, et al. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun 2019; 10:4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takashi Y, Kosako H, Sawatsubashi S, et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc Natl Acad Sci U S A 2019; 116:11418–11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukumoto S. FGF23 and bone and mineral metabolism. Handb Exp Pharmacol 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 95.Bon N, Couasnay G, Bourgine A, et al. Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J Biol Chem 2018; 293:2102–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bon N, Frangi G, Sourice S, et al. Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab 2018; 11:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tatsumi S, Katai K, Kaneko I, et al. NAD metabolism and the SLC34 family: evidence for a liver–kidney axis regulating inorganic phosphate. Pflugers Arch 2018; 471:109–122. [DOI] [PubMed] [Google Scholar]
- 98.Mace ML, Gravesen E, Hofman-Bang J, et al. Key role of the kidney in the regulation of fibroblast growth factor 23. Kidney Int 2015; 88:1304–1313. [DOI] [PubMed] [Google Scholar]
- 99.Egli-Spichtig D, Zhang MYH, Perwad F. Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid-induced acute kidney injury. Front Physiol 2018; 9:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou Z, Lin Y, Gao L, et al. Circadian pharmacological effects of berberine on chronic colitis in mice: role of the clock component Rev-erbα. Biochem Pharmacol 2019; 172:113773. [DOI] [PubMed] [Google Scholar]
- 101.Thakur S, Singh B, Mishra V, et al. Bilayer tablet based chronotherapeutics in the management of nocturnal asthma: an overview. Recent Pat Drug Deliv Formul 2019; 13:74–82. [DOI] [PubMed] [Google Scholar]


