Here, we demonstrate how microbial storage metabolism can adjust to a wide range of environmental conditions. Such flexibility generates a selective advantage under fluctuating environmental conditions. It can also explain the different observations reported in PAO literature, including the capacity of “Ca. Accumulibacter phosphatis” to act like glycogen-accumulating organisms (GAOs). These observations stem from slightly different experimental conditions, and controversy arises only when one assumes that metabolism can operate only in a single mode. Furthermore, we also show how the study of metabolic strategies is possible when combining omics data with functional cofactor assays and modeling. Genomic information can only provide the potential of a microorganism. The environmental context and other complementary approaches are still needed to study and predict the functional expression of such metabolic potential.
KEYWORDS: polyphosphate-accumulating organisms, enhanced biological phosphorus removal, central carbon metabolism, redox cofactors, flux balance analysis, enzymatic assays, “Candidatus Accumulibacter phosphatis”
ABSTRACT
Environmental fluctuations in the availability of nutrients lead to intricate metabolic strategies. “Candidatus Accumulibacter phosphatis,” a polyphosphate-accumulating organism (PAO) responsible for enhanced biological phosphorus removal (EBPR) from wastewater treatment systems, is prevalent in aerobic/anaerobic environments. While the overall metabolic traits of these bacteria are well described, the nonavailability of isolates has led to controversial conclusions on the metabolic pathways used. In this study, we experimentally determined the redox cofactor preferences of different oxidoreductases in the central carbon metabolism of a highly enriched “Ca. Accumulibacter phosphatis” culture. Remarkably, we observed that the acetoacetyl coenzyme A reductase engaged in polyhydroxyalkanoate (PHA) synthesis is NADH preferring instead of showing the generally assumed NADPH dependency. This allows rethinking of the ecological role of PHA accumulation as a fermentation product under anaerobic conditions and not just a stress response. Based on previously published metaomics data and the results of enzymatic assays, a reduced central carbon metabolic network was constructed and used for simulating different metabolic operating modes. In particular, scenarios with different acetate-to-glycogen consumption ratios were simulated, which demonstrated optima using different combinations of glycolysis, glyoxylate shunt, or branches of the tricarboxylic acid (TCA) cycle. Thus, optimal metabolic flux strategies will depend on the environment (acetate uptake) and on intracellular storage compound availability (polyphosphate/glycogen). This NADH-related metabolic flexibility is enabled by the NADH-driven PHA synthesis. It allows for maintaining metabolic activity under various environmental substrate conditions, with high carbon conservation and lower energetic costs than for NADPH-dependent PHA synthesis. Such (flexible) metabolic redox coupling can explain the competitiveness of PAOs under oxygen-fluctuating environments.
IMPORTANCE Here, we demonstrate how microbial storage metabolism can adjust to a wide range of environmental conditions. Such flexibility generates a selective advantage under fluctuating environmental conditions. It can also explain the different observations reported in PAO literature, including the capacity of “Ca. Accumulibacter phosphatis” to act like glycogen-accumulating organisms (GAOs). These observations stem from slightly different experimental conditions, and controversy arises only when one assumes that metabolism can operate only in a single mode. Furthermore, we also show how the study of metabolic strategies is possible when combining omics data with functional cofactor assays and modeling. Genomic information can only provide the potential of a microorganism. The environmental context and other complementary approaches are still needed to study and predict the functional expression of such metabolic potential.
INTRODUCTION
Natural habitats of microorganisms are dynamic environments with intermittent supplies of energy-generating nutrients. Under these dynamic conditions, organisms are selected that can accumulate growth substrates when these are abundant to compensate for periods when these are exhausted (1).
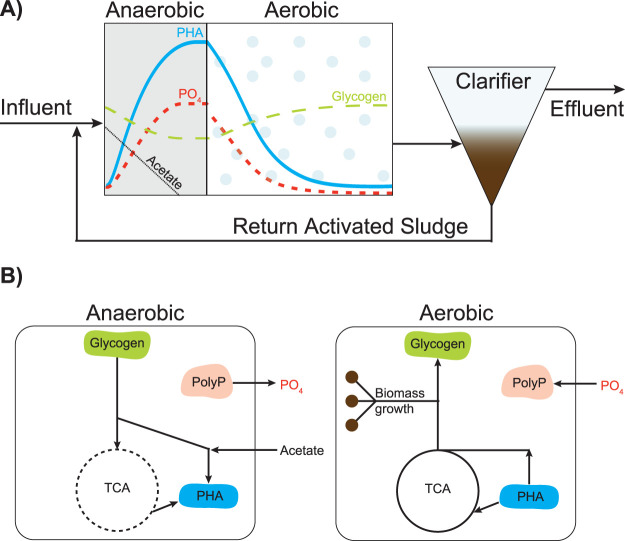
Enhanced biological phosphorus removal (EBPR) from wastewater is designed to make use of such physiological features by circulating activated sludge through alternating zones with and without an external electron acceptor, here defined as aerobic and anaerobic, respectively (Fig. 1A) (2, 3). This environment selects for polyphosphate-accumulating organisms (PAOs) like “Candidatus Accumulibacter phosphatis” (here referred to as Accumulibacter). These bacteria thrive under these dynamic conditions thanks to a complex metabolic strategy encompassing the cycling of three common storage polymers: polyphosphate, glycogen, and polyhydroxyalkanoates (PHAs). Of these, polyphosphate stands out for allowing ATP generation for fast and competitive sequestration of organic matter (e.g., acetate) in the absence of an external electron acceptor (Fig. 1B).
FIG 1.
Schematic diagram of an EBPR process with nutrient/polymer profiles (A) and corresponding metabolic strategy (B). Recirculated activated sludge containing PAOs is mixed with influent wastewater in an anaerobic reactor. To compensate for the absence of an external electron acceptor, Accumulibacter use intracellular polyphosphate (PolyP) and glycogen reserves to generate ATP to take up organic carbon sources (e.g., acetate) and accumulate them in the form of polyhydroxyalkanoates (PHA). Phosphate (PO4) is released at this stage. The tricarboxylic acid cycle (TCA) cycle is represented by a dashed line, as it can have different operating modes depending on the NADH level. When oxygen becomes available, Accumulibacter make use of their PHA storage to grow, to replenish glycogen and polyphosphate. The reaccumulation of polyphosphate in growing biomass and subsequent purge of this biomass leads to the net removal of phosphate from wastewater. Adapted from references 36 and 60.
In the past decades, a number of researchers have derived hypotheses about Accumulibacter’s (anaerobic) physiology (2, 4–6). One of the most important inconclusive discussions is about the source of reducing power for the anaerobic accumulation of PHAs from volatile fatty acids (e.g., acetate and propionate), as reviewed by Zhou et al. (7). Most enrichment culture-based experimental approaches were adequate to study the general physiology of Accumulibacter species; however, the inherent population heterogeneity has not yet been sufficiently addressed. For example, only recently have researchers reported different Accumulibacter clades showing different kinetic characteristics leading to different metabolic operations (8). Furthermore, novel metaomics approaches were used, which allowed for Accumulibacter-targeted (culture-independent) analysis without the interference of other, non-Accumulibacter, subpopulations. A comprehensive overview of the physiological studies published on Accumulibacter can be found in Document S1 in the supplemental material. In one of the metatranscriptomic studies (9), Oyserman and colleagues highlighted the need for validating assumptions often made in metabolic models of Accumulibacter. In particular, they refined the discussion on reducing power sources and requirements by making a distinction between the different redox cofactors, NADH and NADPH. This distinction imposes a constraint between sources and sinks for each redox cofactor, which can be alleviated only by energy-consuming transhydrogenase(-like) mechanisms or using electrons for hydrogen production. However, in their analysis, the specificity of the involved oxidoreductases was postulated from analogy with other organisms, without experimental proof.
In Accumulibacter, reducing power is required for the anaerobic conversion of acetate to 3-hydroxybutyrate (3HB), the monomer of the reserve polymer poly-3-hydroxybutyrate (PHB). The intermediate reduction step is catalyzed by the enzyme acetoacetyl coenzyme A (acetoacetyl-CoA) reductase (AAR). In aerobic PHA-accumulating bacteria such as Cupriavidus necator and Zoogloea ramigera, the preferred electron donor cofactor of the AAR is NADPH (10), which was the cofactor assumed by analogy for Accumulibacter (9). However, AARs accepting both NADH and NADPH have been reported for Azotobacter beijerinckii (11, 12) and Allochromatium vinosum (13). A brief thermodynamic feasibility analysis of the coupling between glycolysis and either NADPH- or NADH-preferring AAR is presented in Document S2 in the supplemental material.
So far and to our knowledge, the nature of the cofactor accepted by Accumulibacter’s acetoacetyl-CoA reductase has not yet been experimentally established. In this study, we addressed this knowledge gap by measuring the NADH- and NADPH-dependent acetoacetyl-CoA reductase activities in a cell extract from a highly enriched culture of Accumulibacter. We further extended the analysis to other key oxidoreductases involved in the metabolism of Accumulibacter. These results were then used to update Accumulibacter’s anaerobic biochemical model and as constraints in a flux balance analysis (FBA) framework. Our simulations show how the use of different pathways and storage compounds leads to metabolic flexibility. Without this flexibility, Accumulibacter’s anaerobic metabolism would become very restrictive, and in unpredictable environments, it could lead to situations in which only a limited fraction of the externally available substrate could be consumed.
RESULTS
Characterization of Accumulibacter enrichments.
This study was carried out using two independent Accumulibacter enrichment cultures. Sequencing batch reactor 1 (SBR-1) contained the highest enrichment of Accumulibacter observed in our lab. However, this cultivation was operating close to a critical dilution rate which, unfortunately, resulted in washout (i.e., enrichment deterioration) after sampling. In SBR-2, conditions were adjusted to reduce the risk of washout, i.e., higher carbon source load (i.e., higher chemical oxygen demand [COD]) and higher solids retention time (SRT) were used.
For both cultures, anaerobic phosphorus (P) released per carbon (C) fed indicated that the PAOs present in the sludge were likely saturated with polyphosphate and that glycogen-accumulating organisms (GAOs) were not present (14). Furthermore, microbial characterization by fluorescence in situ hybridization (FISH) showed that Accumulibacter from type I constituted the majority of the microorganisms across all biomass samples, as can be seen by the high overlap between the Accumulibacter-specific probe and the general Bacteria probe (micrographs available in Document S3). The ppk1 gene analysis further specified that Accumulibacter clades IC and IA were dominant in SBR-1 and SBR-2, respectively. The 16S rRNA gene analysis provided information on the genus of the most abundant subpopulations next to Accumulibacter (Table 1 and Document S3).
TABLE 1.
Process parameters and key performance indicators (KPIs) of the two independent enrichments of Accumulibactera
| Parameter/KPI | SBR-1 | SBR-2 |
|---|---|---|
| pH | 7.6 | 7.6 |
| Temp | 20°C | 20°C |
| Carbon source | 210 mg COD/liter (63:37 acetateCOD:propionateCOD) | 400 mg COD/liter (75:25 acetateCOD:propionateCOD) |
| Phosphate load per C fed | 0.1 Pmol/Cmol | 0.1 Pmol/Cmol |
| Broth vol | 1.5 liters | 1.5 liters |
| Cycle time | 6 h: settling, 76 min; anaerobic, 135 min; aerobic, 135 min | 6 h: settling, 30 min; anaerobic, 112 min; aerobic, 200 min |
| Hydraulic retention time (HRT) | 12 h | 12 h |
| Solids retention time (SRT) | 4 days | 5.6 days |
| Aerobic SRT | 1.5 days | 3.1 days |
| Anaerobic P release per C fed | 0.58 Pmol/Cmol | ∼0.75 Pmol/Cmol |
| Anaerobic-feast length | ∼41 min | ∼42 min |
| FISH | PAO Acc I | PAO Acc I |
| 16S rRNA gene amplicon sequencing (dominant OTUs) | Ca_Accumulibacter, Rhodopseudomonas, Blastochloris | Start: Dechloromonas, Ca_Accumulibacter, genus from f__Hyphomonadaceae; end: Ca_Accumulibacter, Chryseobacterium, genus from o__Sphingobacteriales |
| ppk1 analysis | Dominant: clade IC | Dominant: clade IA |
Pmol, moles of phosphorus; Cmol, moles of carbon.
Redox cofactor preferences of key oxidoreductases.
For a better understanding of the metabolic mechanisms during the EBPR process, understanding the redox couplings is essential and has been a controversy in literature while direct biochemical evidence was missing. Therefore, it was essential to identify the cofactor specificity of relevant reactions and enable a validated model simulation. Enzymatic assays were performed using cell extracts. While these assays cannot discriminate which organism has the respective activity, the performed assays still showed that (i) if an activity is not observed for the whole community, it is also not present in Accumulibacter, and (ii) if the cell extract shows a clear preference for NAD(H) or NADP(H), this is most probably also the preferred cofactor of Accumulibacter, which is highly enriched in the culture.
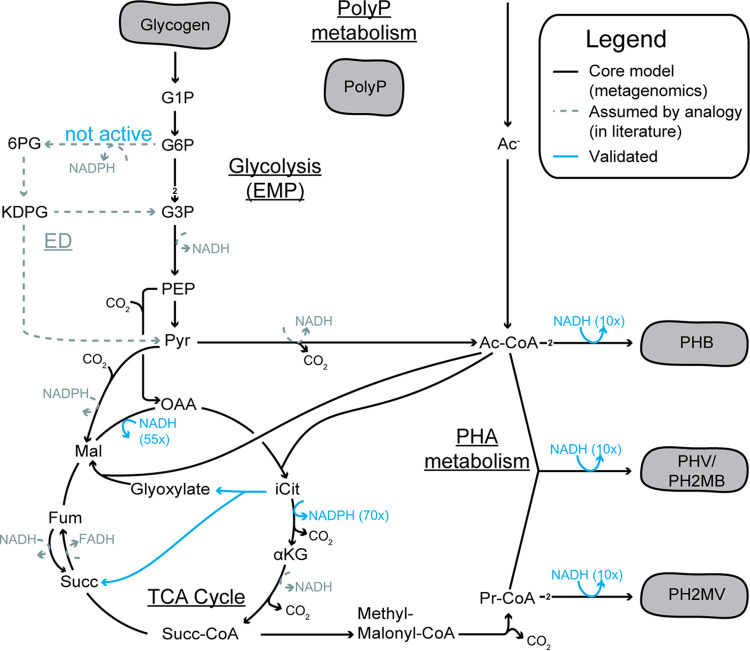
The enzymatic activity assay results display a clear preference which is sufficient to annotate a specific cofactor. These are shown in blue in the metabolic network in Fig. 2. Further details can be found in Document S4. The different assay results will be presented from the sinks (PHAs) to the putative sources of reducing power:
FIG 2.
Central carbon metabolic network of Accumulibacter, including redox cofactor preference of oxidoreductases assayed in this study. A simplified version of this network (Table 2) was used for the simulations. The values shown correspond to the factor difference between the preferred cofactor and the alternative one. This metabolic network is based on the ancestral genome reconstruction done previously (36). The arrows show the expected flux direction under anaerobic conditions. The untested cofactor preferences (in gray) remain as assumed by analogy with other microorganisms. Despite not being annotated in the genome, the ED pathway has been suggested as the route for glycogen degradation (32, 33). A more extensive overview of evidence for each pathway can be found in Document S1. G1P, glucose-1-phosphate; G6P, glucose-6-phosphate; 6PG, 6-phosphogluconate; KDPG, 2-keto-3-deoxy-6-phosphogluconate; G3P, glyceraldehyde-3-phosphate; PEP, phosphoenolpyruvate; Pyr, pyruvate; OAA, oxaloacetate; Ac-CoA, acetyl-CoA; iCit, isocitrate; αKG, α-ketoglutarate; Succ-CoA, succinyl CoA; Succ, succinate; Fum, fumarate; Mal, malate; Pr-CoA, propionyl-CoA; Ac-, acetate; EMP, Embden-Meyerhof-Parnas pathway; TCA, tricarboxylic acid.
-
1.
Redox cofactor preference (NADH or NADPH) of the PHA synthesis. The cell-free extract (CFE) from SBR-1 had an NADH-preferring acetoacetyl-CoA reductase. The activity was 10× higher with NADH than with NADPH as a substrate. To further confirm this cofactor preference, the acetoacetyl-CoA reductase activity was assayed using CFEs from a second enrichment (SBR-2), and both directions of the reaction were monitored [i.e., NAD(P)H + acetoacetyl-CoA↔NAD(P)+ + 3-hydroxybutyryl-CoA].
-
2.
Stoichiometry of glycolysis—Embden-Meyerhof-Parnas (EMP) or Entner-Doudoroff (ED)? To discriminate between the EMP and ED pathways, the presence of glucose-6-phosphate dehydrogenase (G6PDH), the enzyme that catalyzes the first step of the ED pathway, was measured. This reaction is also common to the oxidative branch of the pentose phosphate pathway (oxPP). No activity was found in the CFEs from both SBRs. The biological positive control, a CFE from Pseudomonas putida KT2440, showed activity with both NAD+ and NADP+, as expected (15). Therefore, neither Accumulibacter nor the community has an active ED or oxPP pathway, leaving the EMP pathway as the glycolytic route. This raises the following question: what is now the source of NADPH?
-
3.
Alternative NADPH sources. For many organisms, oxPP is an important source of NADPH for biomass formation (16). This activity was not found in Accumulibacter, raising the question of whether NADPH could be provided by other reaction, like the dehydrogenation of isocitrate by the isocitrate dehydrogenase (ICDH). This reaction was tested using either NAD+ or NADP+. The activity with NADP+ was more than 70 times higher, indicating that isocitrate dehydrogenation is a relevant source of NADPH in Accumulibacter (during the aerobic phase).
Additionally, the activity of other oxidoreductases and anaplerotic routes was measured. (i) The reduction of oxaloacetate to malate (catalyzed by malate dehydrogenase) had at least 55-times-higher activity when using NADH than when using NADPH. Because of the small activity observed when using NADPH, it was not possible to independently study the cofactor preference of the malic enzyme (oxidation of malate to pyruvate). (ii) The first step of the glyoxylate shunt, catalyzed by the isocitrate lyase (ICL), was also found to be active and to have an activity comparable to that of the positive biological control Escherichia coli grown in acetate (well known to make use of the glyoxylate shunt [17]). (iii) Lastly, fumarate reductase (FR) and α-ketoglutarate dehydrogenase (AKGDH) were also tested, but their activities were very low compared to those of all other enzymes tested in our study.
Anaerobic stoichiometric model construction.
Based on the defined reaction network (Fig. 2 and Table 2), the balance of reducing equivalents by Accumulibacter during anaerobic acetate conversion to PHAs was studied quantitatively. The cofactors NADPH and ferredoxin can be regenerated by donating their electrons to NADH using transhydrogenases without any energetic cost for the cell (16). This is not the case for reduced flavin adenine dinucleotide (FADH2), which would require an input of metabolic energy to be reoxidized using NAD+. Currently, there is still no experimentally validated mechanism on how Accumulibacter could reoxidize FADH2 in the absence of an external electron acceptor (e.g., oxygen or nitrate). Therefore, for this simulation, we blocked this FADH2-producing step in the tricarboxylic acid (TCA) cycle (i.e., succinyl CoA [Succ-CoA] to fumarate) and for the remaining reactions we neglected the different types of redox cofactors by simply balancing “electrons” (note that each redox cofactor carries 2 electrons, and in some publications, these are simply referred to as [H]). Since the FADH2-producing step in the TCA cycle is blocked, only the oxidative branch (oxaloacetate [OAA] to Succ-CoA via ICDH and AKGDH) and/or the reductive branch (OAA to Succ-CoA via malate dehydrogenase [MDH] and FR) are possible.
TABLE 2.
Stoichiometry used for flux balance analysis (in mol basis) to simulate Accumulibacter’s anaerobic metabolisma
| Pathway | Stoichiometry | Enzyme(s) assayed in this study |
|---|---|---|
| Glycolysis (EMP) | (Glycogen)1 → 2 Pyr + 4 electrons | G6PDH (ED pathway) |
| Pyruvate to acetyl-CoA | Pyr → Ac-CoA + 2 electrons + CO2 | |
| Pyruvate to oxaloacetate | Pyr + CO2 ↔ OAA | |
| Oxidative TCA cycle branch |
OAA + Ac-CoA → Succ-CoA + 2 CO2 + 4 electrons |
ICDH, AKGDH |
| Reductive TCA cycle branch (reverse TCA cycle) | OAA + 4 electrons → Succ-CoA | MDH, FR |
| Glyoxylate shunt | 2 Ac-CoA → Succ-CoA + 2 electrons | ICL |
| Succinate-propionate shunt | Succ-CoA ↔ Pr-CoA + CO2 | |
| Ac-CoA* production (precursor for 3HB, 3HV and 3HMB) | Ac-CoA + electron → Ac-CoA* | AAR |
| Pr-CoA* production (precursor for 3H2MV, 3HV, and 3HMB) | Pr-CoA + electron → Pr-CoA* | AAR |
↔, reversible reaction; →, irreversible reaction. Note that these are lumped reactions, and each may involve several enzymatic steps. Please refer to Materials and Methods for a definition of Ac-CoA* and Pr-CoA*. A comprehensive overview of the physiological studies published on Accumulibacter supporting this stoichiometric model can be found in Document S1. “*” means plus 1 electron.
In contrast to electron balances, the ATP balance cannot be used, as there are still too many unknowns: (i) how much ATP can be generated/consumed from efflux of ions (potassium, magnesium, and phosphate) associated with polyphosphate hydrolysis, (ii) how much ATP is required for acetate uptake (i.e., to export the protons imported during acetate symport), (iii) how much is needed to upgrade redox cofactors (i.e., transfer electrons from NADH to NADP+, for example), and (iv) how much ATP is used for cellular maintenance. Therefore, ATP is not balanced in this simulation. Nevertheless, it is important to note that the different pathways used for redox balancing will lead to different levels of ATP generation. Thus, in cases of polyphosphate limitation, the metabolism could prefer pathways with higher ATP yield.
Experiments show that the ratio between glycogen and acetate consumption by PAOs is variable, e.g., between high and low temperatures (18) or polyphosphate and glycogen availabilities (14, 19, 20). To reflect this variability, simulations were performed for acetate and glycogen mixtures ranging from only acetate to only glycogen and results were compared to experimental data reported in the literature.
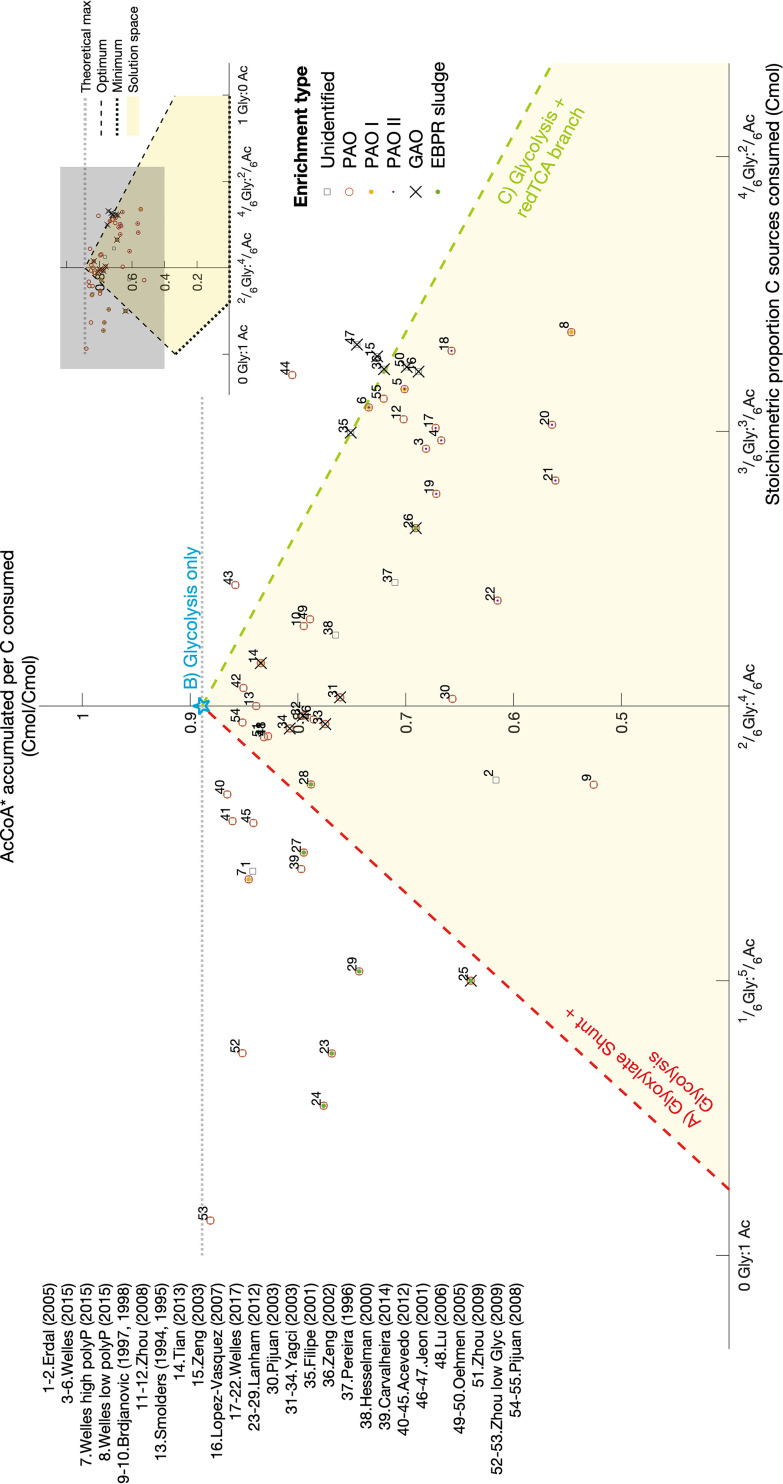
For the anaerobic conversion of acetate to PHA, acetate needs to be reduced (please refer to Materials and Methods for a definition of Ac-CoA*). The Ac-CoA* optimum in Fig. 3 corresponds to the situation in which most carbon is kept inside the cell. The required reducing power can come from either glycolysis (glycogen breakdown) or glyoxylate shunt (acetate oxidation) operation. The different modes are presented in Fig. 4. These modes respectively form the extreme left and right values of the optimum line in Fig. 3. If glycogen degradation can supply exactly the amount of electrons needed for the uptake and conversion of acetate, then only Ac-CoA* (as PHB) is produced and there is no need to use any part of the TCA cycle (Fig. 4B and the highest PHB yield in Fig. 3). The optimum can probably only be achieved with polyphosphate as the ATP source. In a typical GAO-like metabolism, an excess of glycogen is degraded compared to the acetate consumed to supply all ATP needed. This leads to the generation of an excess of reducing power, which is used to produce Pr-CoA* (see Materials and Methods for a definition of Pr-CoA*), leading to PHAs more reduced than PHB (e.g., poly-3-hydroxyvalerate [PHV] or poly-3-hydroxy-2-methylvalerate [PH2MV]), using the reductive TCA branch (Fig. 4C). On the other hand, in a PAO-like metabolism the amount of reducing equivalents generated by glycolysis is usually lower than required to reduce all consumed acetate. In this situation, part of the acetate is oxidized to produce reducing equivalents. Such oxidation is possible via an active glyoxylate shunt, which was also found from the enzymatic assays. The simulations show this as the optimal pathway to produce PHAs when glycogen is limiting (Fig. 4A); i.e., it allows for higher carbon conservation (i.e., higher PHA yield) than the “horseshoe” TCA operation.
FIG 3.
The amount of Ac-CoA* accumulated (PHB and PHV precursor) depends on the different proportion of glycogen to acetate consumed and on the available pathways (please refer to Materials and Methods for a definition of Ac-CoA*). The three optimum redox balancing strategies are shown in Fig. 4. From 0 to approximately 2/6 Cmolglycogen/Cmolconsumed, it can be considered that bacteria are performing a polyphosphate-based metabolism (PAM; optimum line in red), and from approximately 3/6 to 1 Cmolglycogen/Cmolconsumed, a glycogen-based metabolism (GAM; optimum line in green) is used. The optimum and minimum lines were obtained by minimizing and maximizing CO2 production, respectively. The feasible solution space (in yellow) lies in between these two cellular objectives, in which a mixture of different redox balancing strategies is used. The minimum carbon-conserving strategy is the one that uses only the oxidative TCA branch. Experimental data sets were retrieved from references 8, 14, 18 to 20, 23, 30, 33, 41, 44, and 61 to 75) and normalized to respect carbon and energy conservation principles as described in Materials and Methods. Similar plots for Pr-CoA*, CO2, alternative simulations, and including error bars on the experimental data points can be found in Document S5.
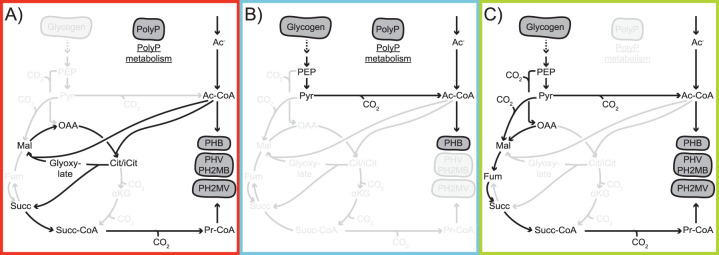
FIG 4.
Different optimum redox balancing strategies for Accumulibacter under anaerobic conditions. (A) When little glycogen is degraded and is not enough to reduce all acetate to PHB, the glyoxylate shunt as recommended previously (41) is the most optimal way to provide electrons required for the PHB pathway. (B) When the stoichiometric amount of glycogen is degraded for acetate reduction to PHB, there is no need to operate any part of the TCA cycle to balance redox and only PHB is then produced, as proposed previously (42). (C) When more glycogen than needed for acetate reduction is degraded, the reductive TCA branch can be used as proposed previously (41) for GAOs to sink electrons in more reduced PHAs (e.g., PHV and PH2MV). These flux distributions are unique; at the optimum (minimal CO2 production), no flux variability is observed (see Document S5).
DISCUSSION
Characterization of Accumulibacter enrichments.
The discussion is based on the observed high PAO enrichment of mainly “Ca. Accumulibacter phosphatis” type I. Consequently, extrapolations of these results to Accumulibacter type II or GAOs are speculative until further validation using respective environments for enrichments (8, 21–25).
Updated biochemical (stoichiometric) model.
The present study shows that the metabolic traits of Accumulibacter enable a flexible metabolic operation under anaerobic conditions. This flexibility is made possible by their energy storage in polyphosphate and reducing power balancing, enabling the observed phenotypes: fast anaerobic acetate uptake and anaerobic PHB synthesis (decoupled from growth). The flexible reducing power balancing by Accumulibacter depends on the nature of the redox cofactors used; in this study, enzymatic assays were performed to define the redox cofactor preferences of the main oxidoreductases in the central metabolic pathways of Accumulibacter.
The key finding from these assays is the NADH-preferring PHA synthesis in Accumulibacter. This NADH preference allows for a direct consumption of the NADH produced in most of Accumulibacter’s reducing power sources. This also eliminates the need for conversion of NADH into NADPH using the membrane-bound transhydrogenase (PntAB) driven by proton motive force, which was suggested in an earlier study (9). Although there are previous reports showing NADH-driven PHB accumulation (11, 26–29), the level of NADH preference of the acetoacetyl-CoA reductase from Accumulibacter measured in this study is striking. Further characterization of this enzyme was undertaken by Olavarria and colleagues (85). This observation allows us to rethink the role of PHAs: we hypothesize that depending on the environment where microorganisms thrive, aerobic PHA accumulation will play a role as carbon reservoir during metabolic overflow (NADPH-driven accumulation) or as an anaerobic electron reservoir during scarcity of external electron acceptors (NADH-driven accumulation). Thus, for Accumulibacter, anaerobically, PHA is essentially a fermentation product.
A related finding was the absence of activity of the first step of the sometimes-implicated ED glycolytic pathway. These results match those observed previously for PAOs (18) and GAOs (30), where no NADP+ dependent glucose-6-phosphate dehydrogenase activity was found. The experimental findings are in line with the absence of key ED pathway genes in the genome annotations of Accumulibacter and its closest relatives Dechloromonas aromatica and Azoarcus sp. strain EbN1 (25, 31). Furthermore, no enzymatic activity of the ED pathway with NAD+ as electron acceptor (15) was observed.
These findings are in contradiction with earlier 13C nuclear magnetic resonance (NMR) studies (32, 33), which indicated that the ED pathway was more likely the route for glycolysis than the EMP pathway. Nevertheless, it has to be noted that these early studies comprise interpretations of 13C patterns using simplified metabolic models, with limited information on the reversibility of each reaction and potentially with reactions missing; these are common pitfalls of the 13C-labeling method (34). Also note that these 13C NMR studies were performed before the first draft genome of Accumulibacter was available (31), and only the 13C pattern in the different PHAs was measured and not in the metabolic intermediates of each pathway used.
While NADPH is not required anaerobically by Accumulibacter, this reducing cofactor must be produced aerobically to drive biomass synthesis. In this study, isocitrate dehydrogenase was found to be NADP+ dependent. This is consistent with the observation that most acetate consumers use this conversion to produce NADPH for their anabolism (17) and confirms the protein annotation found previously (25). Since PHA accumulation is now known to be NADH preferring and without another sink of NADPH, the latter might accumulate anaerobically and thereby inhibit the isocitrate dehydrogenase reaction. Alternatively, a soluble transhydrogenase could convert this NADPH into NADH and allow the oxidative branch of the TCA cycle to be operational under anaerobic conditions.
Regarding the activity of other TCA cycle oxidoreductases and anaplerotic routes, (i) the oxidation of malate using NAD+ was also found in a previous study (18), (ii) the glyoxylate shunt was also found to be active as observed in previous assays (18, 33, 35) and metatranscriptomics/proteomics studies (24, 36–39), which is expected, as this is the anaplerotic route that allows for microorganisms to convert C2 sources like acetate into C4 building blocks for anabolism (17), and (iii) fumarate reductase and α-ketoglutarate dehydrogenase activities were very low compared to those of all other enzymes tested in our study and alike in the previous study (18). It has to be noted that information on the Accumulibacter type was not found for all studies and there can be differences depending on the enrichment cultivation parameters.
Additionally, there is one study suggesting the activity of an α-ketoglutarate:ferredoxin oxidoreductase: the enzyme was found in Accumulibacter’s genome and proteome (25). This membrane-bound enzyme has not been assayed in this study, as the simulations suggest an anaerobic operation mode via the glyoxylate shunt rather than a full or partial oxidative TCA cycle.
Flexible anaerobic metabolism: adjustments depending on the environment and intracellular storage compounds.
As suggested in a previous review (7), the flexibility of Accumulibacter’s metabolism is likely the major reason why there is controversy in literature regarding how reducing power is balanced under anaerobic conditions. Long-term exposure to constant conditions (e.g., pH, temperature, oxidation level of the substrate, nutrient availability, counterions, SRT, and settling time) will select for the best strategy for those conditions, but short-term perturbations of those set conditions have shown that Accumulibacter seems to still be able to solve the redox balancing problem even if suboptimally regarding carbon conservation (8, 19, 20, 40). Thus, based on our analysis, we observe that there is not one fixed stoichiometry but a range of possible stoichiometries that in the end are defined by the relative proportions of each of the substrates, supplied to the system or produced by a side population (e.g., acetate, glycogen, polyphosphate, other volatile fatty acids [VFAs], oxygen, nitrate, and H2).
The simulations showed Accumulibacter can operate in three distinct modes (and combinations in between): (i) when reducing equivalents from glycolysis (glycogen) are limiting compared to the acetate imported (Yagci’s model for PAOs [41]), (ii) when glycolysis (glycogen) supplies exactly enough reducing equivalents to convert all imported acetate into PHB (Mino’s model [42]), and, lastly, (iii) when glycolysis (glycogen) is producing an excess of reducing equivalents (like Yagci’s model for GAOs [41]).
The anaerobic use of the glyoxylate shunt in a PAO-like metabolism to provide for the reducing equivalents needed for PHA accumulation is supported by previous studies (35, 41); however, as seen in Fig. 3, it does not explain the higher levels of Ac-CoA* found (points above the red optimum line) when a shift to more reduced PHAs (Pr-CoA*) was expected. This indicates that there might be an alternative process that allows the cell to conserve extra carbon, in other words, accumulate more Ac-CoA* and less Pr-CoA*. It could be that (i) fully anaerobic conditions were not attained when performing the experiments, (ii) another side population provides electrons in the form of a more reduced organic substrate or even hydrogen gas (43), or (iii) Accumulibacter has yet another, alternative way of balancing redox which has yet to be described and demonstrated.
Also, for the scenario where glycolysis produces an excess of reduced cofactors as in a GAO-like metabolism, a few experimental data points fall outside the feasible solution space (points above the green optimum line in Fig. 3); these could be explained in the case that Accumulibacter produces H2 to solve an excess of NADH as described previously (9). While H2 production extends the metabolic flexibility, i.e., releases the redox constraints, H2 production leads to the loss of electrons that cannot be used for energy production during the aerobic phase. Therefore, we expect H2 formation only as a specific mechanism when redox cannot be balanced otherwise.
The multilayered complexity of a physiological analysis of Accumulibacter.
The experimental data gathered are likely also influenced by the intrinsic heterogeneity of Accumulibacter enrichments: clade differences, population heterogeneity, or even different environments (e.g., depending on the position of the cell in a floc/granule). Therefore, experimental data points under the optimum line in Fig. 3 likely represent mixtures of cells, each with a different metabolic mode (Fig. 4). Furthermore, any kinetic limitation (i.e., pathway capacity) may also explain a suboptimal, mixed phenotype.
Additionally, if all enzymatic machinery is available, it seems possible that the same cell might change modes depending on the environment and its intracellular storage dynamics. Particularly, availability of acetate (e.g., transition feast/famine) and/or polyphosphate storage as well as glycogen can trigger shifts in metabolic mode.
Despite the heterogeneity and noise, the experimental data outside the solution space in Fig. 3 suggest that this stoichiometric model is still incomplete to represent all experimental conditions. The solution space expands, for example, when (i) allowing for hydrogen production under conditions of excess glycogen to acetate or (ii) oxidation of acetate in a fully operational TCA cycle in a glycolysis-limiting scenario. With these reactions, all experimental data can be explained (see Document S5). The first scenario is supported by the detection of hydrogen gas produced by an Accumulibacter enrichment (9). For the second scenario, a full TCA cycle has been previously suggested based on 13C NMR observations (44) and stoichiometric analyses (19). Additionally, a novel protein has been proposed based on metagenomic analysis that could regenerate FADH2 without an external electron acceptor, which allows for full TCA cycle operation under anaerobic conditions (31); however, there is still no direct biochemical experimental evidence that indeed validates a full anaerobic TCA operation. Some indirect observation is presented in reference 45. In that study, labeled propionate was used as the substrate and 13C enrichment was found in the PHV fragments that came from acetyl-CoA. This labeling pattern can be explained by a full TCA cycle, but this is not the only metabolic route that could explain this observation.
Alternatively, it should also be considered that, like many (strict) anaerobes, Accumulibacter may use electron bifurcation mechanisms (46) via ferredoxin oxidoreductases to allow for alternative pathways in the central carbon metabolism.
Conclusion.
In this study, a network-based modeling approach was used to unravel the metabolic flexibility of Accumulibacter under dynamic conditions. The approach integrates findings and hypotheses derived from previous metaomics studies as well as different physiological data sets and biochemical assays.
The developed metabolic model demonstrates the flexibility in metabolic function and could explain previous controversy in PAO literature. The NADH-dependent PHA synthesis is both an efficient link to catabolic pathways and key for enabling metabolic flexibility. Depending on the exact history and cultivation conditions, Accumulibacter can exhibit different metabolic phenotypes; the metabolic network can handle different combinations of carbon sources (i.e., acetate and glycogen) adjusting the use of glycolysis (EMP pathway), different branches of the TCA cycle (including glyoxylate shunt), and potentially other pathways not yet considered. This poses a challenge to predictive EBPR modeling, as this implies that stoichiometry is not fixed but variable, spanning continuously from polyphosphate to glycogen-based phenotypes.
This metabolic versatility is likely what allows Accumulibacter to be, in most situations, very close to a carbon conservation optimum, which is key to ensure its competitiveness in the dynamic environments of EBPR systems.
MATERIALS AND METHODS
Reactor operation.
Two independent Accumulibacter enrichments were obtained in sequencing batch reactors (SBRs). The main operational conditions are described in Table 1 and were adapted from the SBR-S as described by Welles et al. (8).
Cell-free extract preparation.
Broth samples (10 ml) collected from the bioreactor during both anaerobic and aerobic phases were centrifuged (2,500 × g, 10 min, 4°C), and the pellet was washed using 10 ml of buffer (here named buffer 1X) containing 50 mM Tris at pH 8, 5 mM MgCl2, 5 mM NaCl, and 5% (vol/vol) glycerol, and the obtained suspension was centrifuged again (2,500 × g, 10 min, 4°C). After centrifugation, pellets were kept at −20°C for no longer than 4 days until further analysis. For the enzymatic assay, cellular pellets were suspended in 10 ml of buffer 1X supplemented with 2 mM (L+D) 1,4-dithiothreitol (DTT) and cOmplete miniprotease inhibitor cocktail (Roche). To avoid overheating and protein denaturation, cells were kept on ice while being sonicated until the cell suspension was homogenized (i.e., no granules visible). The resulting suspension was centrifuged (15,000 × g for at least 45 min, 4°C). The obtained supernatant, a cell-free extract (CFE), was used for the enzymatic assays.
Purification of Accumulibacter’s acetoacetyl-CoA reductase.
A gene with high degree of similarity to phaB from Accumulibacter was cloned as described by Olavarria et al. (85). Total metagenomic DNA was isolated from a lab-scale reactor of freshwater aerobic granular sludge operated as described by de Graaff et al. (47). This DNA was used as a template in a PCR with primers designed according to the reference sequence of the phaB gene from Accumulibacter type II (locus CAP2UW1_3919). The sequences of the primers used can be found in Document S4.
After constructing several plasmids with the amplified gene, cloning, and sequencing, the clone with the highest identity with respect to the reference sequence (colony number 6; 89% DNA identity and 95% amino acid identity) was subcloned into another vector for overexpression in cells of E. coli BL21(DE3), purification using His tagging, and subsequent characterization (protein sequences available in Document S4). The purity of the obtained acetoacetyl-CoA reductase was confirmed by SDS-PAGE, and this purified enzyme was used for the enzymatic assays as a control.
Enzymatic assays.
The reaction mixtures used for each enzymatic assay are described in Table 3. Activities were calculated using the initial rate of reduction of NAD(P)+ or oxidation of NAD(P)H, which was obtained by following the changes in the absorbance at 340 nm and 30°C. To control the contribution of putative background reactions, reaction mixtures that contained all the components except one substrate or without addition of CFE were monitored (Table 3). The measured rates were normalized to the total protein concentration in the respective CFE. The protein concentration was measured using the Bradford dye-binding protein assay (Bio-Rad), using known concentrations of bovine serum albumin as external standards.
TABLE 3.
Origin of the CFE, buffer, substrates, controls, and references used for each enzymatic assay
| CFE | Tested enzyme | Buffer | Substrates | Controls | Reference |
|---|---|---|---|---|---|
| SBR-1 | Acetoacetyl-CoA reductase | 1X, supplemented | 200 μM acetoacetyl-CoA, 200 μM NADH, or 200 μM NADPH | No acetoacetyl-CoA; E. coli MG1655; E. coli MG1655 + p-phaCABa | 76 |
| SBR-2 | Acetoacetyl-CoA reductase | 1X, supplemented | 40 μM acetoacetyl-CoA, 100 μM NADH, or 200 μM NADPH | No acetoacetyl-CoA; isolated/purified enzyme | 77 |
| SBR-2 | 3-Hydroxybutyryl-CoA dehydrogenase | 1X, supplemented | 100 μM 3-hydroxybutyryl-CoA, 1,000 μM NAD(P)+ | No hydroxybutyryl-CoA | 77 |
| SBR-1 | Glucose-6-phosphate dehydrogenase | 1X, supplemented | 2 mM glucose-6-phosphate, 2,000 μM NAD+, or 200 μM NADP+ | No glucose-6-phosphate; no CFE | 78 |
| SBR-2 | Glucose-6-phosphate dehydrogenase | 1X, supplemented | 4 mM glucose-6-phosphate, 400 μM NAD(P)+ | No glucose-6-phosphate; no CFE | 78 |
| SBR-2 | Isocitrate dehydrogenase | 1X, supplemented | 400 μM NAD(P)+, 400 μM isocitrate | No substrate; no CFE | 79 |
| Malate dehydrogenase | 1X, supplemented | 200 μM NAD(P)H, 200 μM oxaloacetate | No substrate; no CFE | 16 | |
| Malic enzyme | 1X, supplemented | 200 μM NADP, 200 μM l-malate | No substrate; no CFE | 80 | |
| Isocitrate lyase | 40 mM HEPES (pH 7), 6 mM MgCl2 | 4 mM isocitrate, 280 μM NADH, 45 U of lactate dehydrogenase from rabbit muscle (Roche) | No isocitrate; no CFE; E. coli MG1655 grown on acetate, E. coli JW3975b grown on glucose | 81 | |
| Fumarate reductase | 50 mM HEPES (pH 7) | 15 mM fumarate, 250 μM NADH | No substrate; no CFE | 82 | |
| α-Ketoglutarate dehydrogenase | 50 mM HEPES (pH 7), 1 mM MgCl2, 1 mM thiamine pyrophosphate, 2.5 mM DTT | 2 mM α-ketoglutaric acid, 100 μM coenzyme A, 2.5 mM NAD(P)+ | No substrate; no CFE | 83 |
Plasmid bearing the genes phaCAB from C. necator under the control of its native promoter. These genes encode the enzymes of the pathway leading to polyhydroxybutyrate (PHB) synthesis, and the plasmid was kindly donated by J. G. Cabrera Gomez from Universidade de Sao Paulo, Brazil.
Strain without isocitrate lyase from the Keio collection (84): F− Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) λ− rph-1 Δ(rhaD-rhaB)568 ΔaceA782::kan hsdR514.
Microbial community characterization.
The microbial community present in our enrichments has been characterized by three orthogonal approaches as described below, and the detailed results can be found in Document S3.
Fluorescence in situ hybridization (FISH) was used to qualitatively assess the presence of Accumulibacter in the enrichment cultures. All bacteria were targeted by the EUB338 mix (general bacterial probe) (48–50). Accumulibacter types I and II were targeted by the probes Acc-1-444 and Acc-2-444 (51), respectively. Hybridized samples were examined with a Zeiss Axioplan-2 epifluorescence microscope.
To further confirm the specific Accumulibacter clade, the presence of the gene encoding the polyphosphate kinase I (ppk1) in Accumulibacter was tested as described previously (52, 53) using primers ACCppk1-254F and ACCppk1-1376R targeting the ppk1 gene from Accumulibacter-like bacteria (54).
To identify putative side populations, 16S rRNA gene amplicon sequencing was applied. DNA samples from cell pellets were extracted using the DNeasy UltraClean microbial kit (Qiagen, the Netherlands). Approximately 250 mg (wet biomass) was treated according to the standard protocol except that an alternative lysis method was implemented. This included a combination of 5 min of heat (65°C) followed by 5 min of bead-beating for cell disruption on a Mini-Beadbeater-24 (Biospec, USA). After extraction, the DNA was checked for quality by gel electrophoresis and quantified using a Qubit 4 (Thermo Fisher Scientific, USA).
After quality control, samples were sent to Novogene Ltd. (Hong Kong, China) for amplicon sequencing of the V3-V4 region of the 16S rRNA gene (positions 341 to 806) on an Illumina paired-end platform. After sequencing, the raw reads were quality filtered, chimeric sequences were removed, and operational taxonomic units (OTUs) were generated on the base of ≥97% identity. Subsequently, microbial community analysis was performed by Novogene using Mothur and Qiime software (v1.7.0) (55, 56). For phylogenetical determination, the most recent SSURef database from SILVA (57) was used. The microbial communities in each enrichment were compared based on the 10 most abundant OTUs with a distinctive genus (i.e., with most reads assigned to it).
Anaerobic biochemical model of Accumulibacter.
The metabolic network shown in Fig. 2 is based on the ancestral genome reconstruction of Accumulibacter proposed previously (36) as well as experimental observations obtained in this study. An extensive literature overview supporting this biochemical model of Accumulibacter can be found in Document S1. There are more complex metabolic models, including genome-scale metabolic models, available. For the purpose of this study, we focused on central carbon metabolism and lumped reactions capturing main functionalities.
Flux balance analysis (stoichiometric modeling).
To estimate the possible metabolic flux distributions, flux balance analysis (FBA) was applied (58). The flux distribution vopt can be obtained by optimization:
where N is the stoichiometry matrix containing the balanced metabolites and reactions (Table 2) and is the vector containing all reaction fluxes. The simulation assumes that none of the balanced intermediates accumulates inside the cell (steady-state assumption). This is justified by the short turnover time of the intracellular metabolites compared to the timespan of the experiment that is simulated. Inequality constraints were introduced for physiologically irreversible fluxes (virr) (these should always be positive). The consumption of acetate (vAcupt) and glycogen vGlycdeg is varied between only acetate to only glycogen . The respective experimental data are normalized to a summed consumption of 1 Cmol of C source.
Commonly, maximization of biomass synthesis is used in FBA as an optimization objective. However, Accumulibacter only produces biomass aerobically and using the intracellular carbon reserves (PHAs) accumulated during substrate uptake in the anaerobic period. Biomass synthesis was thus assumed to be proportional to the carbon stored as PHA. The objective of maximal C storage was “inverted” to minimal loss of carbon to the extracellular space, which for the used network is only CO2 production (vCO2prod). Using this objective, no sum of carbon in the different PHA pools is required, avoiding a putative bias toward one of those.
Furthermore, to prevent adding a stoichiometric constraint due to an assumption on the proportion of the different PHA polymers possible, i.e., poly-3-hydroxybutyrate (PHB), poly-3-hydroxyvalerate (PHV), poly-3-hydroxy-2-methylbutyrate (PH2MB), or poly-3-hydroxy-2-methylvalerate (PH2MV), the respective monomer amount is introduced, i.e., the reduced precursors Ac-CoA* and Pr-CoA*, such that:
The stoichiometric model was checked for conserved moieties, blocked reactions, and nonidentifiable reactions that form internal cycles. No conserved moieties or blocked reactions were found, and the internal cycles found did not respect the reversibility constraints. After analysis, the network contained 9 independently balanced intermediates, 13 (lumped) reactions, and, thus, 4 degrees of freedom. ATP was included in the analysis but was not balanced (i.e., not a constraint).
To identify whether the solutions obtained from linear programming for different glycogen/acetate ratios (see equations above) are unique, a null space analysis was performed. For this optimization, three extracellular rates are fixed: uptake of glycogen and acetate and the objective, production of CO2. When these rates are fixed, 1 degree of freedom remains, formed by one putative cycle: the reactions PDH, (−)PEPC, (−)oxTCA, TCAGOX, and ATPnet, which is an infeasible cycle given the thermodynamic (reversibility) constraints. Therefore, the flux solution for the optimum is unique.
Compilation and normalization of stoichiometric data.
The results of the aforementioned simulations were compared to stoichiometric data reported in the literature for several different Accumulibacter enrichments. Anaerobic-feast yields were used when available; otherwise, the yields of the whole anaerobic phase were used. In the case of missing compound rates (usually CO2 and PH2MV), these were estimated using electron and carbon balancing. For data sets with redundant measurements, a data reconciliation method was applied (59). For all calculations, we assumed that acetate and glycogen monomers were the only substrates and CO2, PHB, PHV, and PH2MV were the only products. In case yields were reported without the respective error, the error was calculated using error propagation. Here, the relative errors were assumed as follows: 5% for acetate and PHB measurements and 10% for PHV, PH2MV, and glycogen measurements. From the (reconciled) rates for PHB, PHV, and PH2MV, the respective Ac-CoA* and Pr-CoA* rates were determined and normalized to the amount of consumed substrates, in Cmol.
Data availability.
FISH micrographs, a phylogenetic tree based on Accumulibacter’s ppk1 gene analysis, and a taxonomic distribution of microbial community based on 16S rRNA gene copy numbers can be found in Document S3. The ppk1 gene sequences obtained in this study have been deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/) under accession numbers MH899084 to MH899086. The 16S rRNA gene amplicon data have been deposited in GenBank under BioProject accession number PRJNA490689. The AAR protein sequences, and enzymatic assays data are available in Document S4. The MATLAB (MathWorks, Natick, MA) code and data used for the simulations are available in a repository in GitHub (https://github.com/cell-systems-engineering-tud/anaerobic-flex-pao). All simulations were performed using MATLAB vR2018a (9.4.0.813654).
Supplementary Material
ACKNOWLEDGMENTS
These investigations were supported by SIAM Gravitation Grant 024.002.002, the Netherlands Organization for Scientific Research (NWO).
We thank Roel van de Wijgaart, Alexandre Carnet, Hein van der Wall, Koen Verhagen, David Weissbrodt, Alex Salazar, and Thomas Abeel for their collaboration in this project. We also thank to Sergio Tomás Martínez and Eleni Vasilakou for proofreading and Ben Oyserman and Sef Heijnen for their invaluable advice on the manuscript.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Van Loosdrecht MCM, Pot MA, Heijnen JJ. 1997. Importance of bacterial storage polymers in bioprocesses. Water Sci Technol 35:41–47. doi: 10.2166/wst.1997.0008. [DOI] [Google Scholar]
- 2.Seviour RJ, Mino T, Onuki M. 2003. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol Rev 27:99–127. doi: 10.1016/S0168-6445(03)00021-4. [DOI] [PubMed] [Google Scholar]
- 3.Barnard JL. 1976. A review of biological phosphorus removal in the activated sludge process. Water SA 2:136–144. [Google Scholar]
- 4.Seviour RJ, McIlroy S. 2008. The microbiology of phosphorus removal in activated sludge processes—the current state of play. J Microbiol 46:115–124. doi: 10.1007/s12275-008-0051-0. [DOI] [PubMed] [Google Scholar]
- 5.Mino T, van Loosdrecht MCM, Heijnen JJ. 1998. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res 32:3193–3207. doi: 10.1016/S0043-1354(98)00129-8. [DOI] [Google Scholar]
- 6.Oehmen A, Lemos PC, Carvalho G, Yuan Z, Keller J, Blackall LL, Reis MAM. 2007. Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res 41:2271–2300. doi: 10.1016/j.watres.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Pijuan M, Oehmen A, Yuan Z. 2010. The source of reducing power in the anaerobic metabolism of polyphosphate accumulating organisms (PAOs)—a mini-review. Water Sci Technol 61:1653–1662. doi: 10.2166/wst.2010.983. [DOI] [PubMed] [Google Scholar]
- 8.Welles L, Tian WD, Saad S, Abbas B, Lopez-Vazquez CM, Hooijmans CM, van Loosdrecht MCM, Brdjanovic D. 2015. Accumulibacter clades type I and II performing kinetically different glycogen-accumulating organisms metabolisms for anaerobic substrate uptake. Water Res 83:354–366. doi: 10.1016/j.watres.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Oyserman BO, Noguera DR, del Rio TG, Tringe SG, McMahon KD. 2016. Metatranscriptomic insights on gene expression and regulatory controls in Candidatus Accumulibacter phosphatis. ISME J 10:810–822. doi: 10.1038/ismej.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madison LL, Huisman GW. 1999. Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53. doi: 10.1128/MMBR.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie GAF, Senior PJ, Dawes EA. 1971. The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem J 121:309–316. doi: 10.1042/bj1210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senior PJ, Beech GA, Ritchie GA, Dawes EA. 1972. The role of oxygen limitation in the formation of poly-β-hydroxybutyrate during batch and continuous culture of Azotobacter beijerinckii. Biochem J 128:1193–1201. doi: 10.1042/bj1281193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebergesell M, Steinbüchel A. 1992. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem 209:135–150. doi: 10.1111/j.1432-1033.1992.tb17270.x. [DOI] [PubMed] [Google Scholar]
- 14.Welles L, Abbas B, Sorokin DY, Lopez-Vazquez CM, Hooijmans CM, van Loosdrecht MCM, Brdjanovic D. 2017. Metabolic response of “Candidatus Accumulibacter Phosphatis” clade II C to changes in influent P/C ratio. Front Microbiol 7:2121. doi: 10.3389/fmicb.2016.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olavarría K, Valdés D, Cabrera R. 2012. The cofactor preference of glucose-6-phosphate dehydrogenase from Escherichia coli—modeling the physiological production of reduced cofactors. FEBS J 279:2296–2309. doi: 10.1111/j.1742-4658.2012.08610.x. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrer T, Sauer U. 2009. Different biochemical mechanisms ensure network-wide balancing of reducing equivalents in microbial metabolism. J Bacteriol 191:2112–2121. doi: 10.1128/JB.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk G. 1986. Bacterial metabolism. Springer New York, New York, NY. [Google Scholar]
- 18.Erdal ZK, Erdal UG, Randall CW. 2005. Biochemistry of enhanced biological phosphorus removal and anaerobic COD stabilization. Water Sci Technol 52:557–567. doi: 10.2166/wst.2005.0736. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Pijuan M, Zeng RJ, Yuan Z. 2009. Involvement of the TCA cycle in the anaerobic metabolism of polyphosphate accumulating organisms (PAOs). Water Res 43:1330–1340. doi: 10.1016/j.watres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Pijuan M, Zeng RJ, Lu H, Yuan Z. 2008. Could polyphosphate-accumulating organisms (PAOs) be glycogen-accumulating organisms (GAOs)? Water Res 42:2361–2368. doi: 10.1016/j.watres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Flowers JJ, He S, Malfatti S, del Rio TG, Tringe SG, Hugenholtz P, McMahon KD. 2013. Comparative genomics of two “Candidatus Accumulibacter” clades performing biological phosphorus removal. ISME J 7:2301–2314. doi: 10.1038/ismej.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welles L, Lopez-Vazquez CM, Hooijmans CM, van Loosdrecht MCM, Brdjanovic D. 2016. Prevalence of ‘Candidatus Accumulibacter phosphatis’ type II under phosphate limiting conditions. AMB Express 6:44. doi: 10.1186/s13568-016-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acevedo B, Oehmen A, Carvalho G, Seco A, Borrás L, Barat R. 2012. Metabolic shift of polyphosphate-accumulating organisms with different levels of polyphosphate storage. Water Res 46:1889–1900. doi: 10.1016/j.watres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Wexler M, Richardson DJ, Bond PL. 2009. Radiolabelled proteomics to determine differential functioning of Accumulibacter during the anaerobic and aerobic phases of a bioreactor operating for enhanced biological phosphorus removal. Environ Microbiol 11:3029–3044. doi: 10.1111/j.1462-2920.2009.02007.x. [DOI] [PubMed] [Google Scholar]
- 25.Barr JJ, Dutilh BE, Skennerton CT, Fukushima T, Hastie ML, Gorman JJ, Tyson GW, Bond PL. 2016. Metagenomic and metaproteomic analyses of Accumulibacter phosphatis-enriched floccular and granular biofilm. Environ Microbiol 18:273–287. doi: 10.1111/1462-2920.13019. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Chang JH, Kim K-J. 2014. Crystal structure and biochemical properties of the (S)-3- hydroxybutyryl-CoA dehydrogenase PaaH1 from Ralstonia eutropha. Biochem Biophys Res Commun 448:163–168. doi: 10.1016/j.bbrc.2014.04.101. [DOI] [PubMed] [Google Scholar]
- 27.Barycki JJ, O’Brien LK, Strauss AW, Banaszak LJ. 2000. Sequestration of the active site by interdomain shifting: crystallographic and spectroscopic evidence for distinct conformations of L-3-hydroxyacyl-CoA dehydrogenase. J Biol Chem 275:27186–27196. doi: 10.1074/jbc.M004669200. [DOI] [PubMed] [Google Scholar]
- 28.Haywood GW, Anderson AJ, Chu L, Dawes EA. 1988. The role of NADH- and NADPH-linked acetoacetyl-CoA reductases in the poly-3-hydroxybutyrate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol Lett 52:259. doi: 10.1111/j.1574-6968.1988.tb02607.x. [DOI] [Google Scholar]
- 29.Ling C, Qiao G-Q, Shuai B-W, Olavarria K, Yin J, Xiang R-J, Song K-N, Shen Y-H, Guo Y, Chen G-Q. 2018. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA). Metab Eng 49:275–286. doi: 10.1016/j.ymben.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Filipe CDM, Daigger GT, Grady CPL. 2001. A metabolic model for acetate uptake under anaerobic conditions by glycogen accumulating organisms: stoichiometry, kinetics, and the effect of pH. Biotechnol Bioeng 76:17–31. doi: 10.1002/bit.1022. [DOI] [PubMed] [Google Scholar]
- 31.García Martín H, Ivanova N, Kunin V, Warnecke F, Barry KW, McHardy AC, Yeates C, He S, Salamov A. a, Szeto E, Dalin E, Putnam NH, Shapiro HJ, Pangilinan JL, Rigoutsos I, Kyrpides NC, Blackall LL, McMahon KD, Hugenholtz P. 2006. Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities. Nat Biotechnol 24:1263–1269. doi: 10.1038/nbt1247. [DOI] [PubMed] [Google Scholar]
- 32.Maurer M, Gujer W, Hany R, Bachmann S. 1997. Intracellular carbon flow in phosphorus accumulating organisms from activated sludge systems. Water Res 31:907–917. doi: 10.1016/S0043-1354(96)00369-7. [DOI] [Google Scholar]
- 33.Hesselmann RPX, Von RR, Resnick SM, Hany R, Zehnder AJ. 2000. Anaerobic metabolism of bacteria performing enhanced biological phosphate removal. Water Res 34:3487–3494. doi: 10.1016/S0043-1354(00)00092-0. [DOI] [Google Scholar]
- 34.van Winden WA, Verheijen P, Heijnen S. 2001. Possible pitfalls of flux calculations based on 13C-labeling. Metab Eng 3:151–162. doi: 10.1006/mben.2000.0174. [DOI] [PubMed] [Google Scholar]
- 35.Burow LC, Mabbett AN, Blackall LL. 2008. Anaerobic glyoxylate cycle activity during simultaneous utilization of glycogen and acetate in uncultured Accumulibacter enriched in enhanced biological phosphorus removal communities. ISME J 2:1040–1051. doi: 10.1038/ismej.2008.45. [DOI] [PubMed] [Google Scholar]
- 36.Oyserman BO, Moya F, Lawson CE, Garcia AL, Vogt M, Heffernen M, Noguera DR, McMahon KD. 2016. Ancestral genome reconstruction identifies the evolutionary basis for trait acquisition in polyphosphate accumulating bacteria. ISME J 10:2931–2945. doi: 10.1038/ismej.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skennerton CT, Barr JJ, Slater FR, Bond PL, Tyson GW. 2015. Expanding our view of genomic diversity in Candidatus Accumulibacter clades. Environ Microbiol 17:1574–1585. doi: 10.1111/1462-2920.12582. [DOI] [PubMed] [Google Scholar]
- 38.Wilmes P, Andersson AF, Lefsrud MG, Wexler M, Shah M, Zhang B, Hettich RL, Bond PL, VerBerkmoes NC, Banfield JF. 2008. Community proteogenomics highlights microbial strain-variant protein expression within activated sludge performing enhanced biological phosphorus removal. ISME J 2:853–864. doi: 10.1038/ismej.2008.38. [DOI] [PubMed] [Google Scholar]
- 39.He S, McMahon KD. 2011. ‘Candidatus Accumulibacter’ gene expression in response to dynamic EBPR conditions. ISME J 5:329–340. doi: 10.1038/ismej.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erdal UG, Erdal ZK, Daigger GT, Randall CW. 2008. Is it PAO-GAO competition or metabolic shift in EBPR system? Evidence from an experimental study. Water Sci Technol 58:1329–1334. doi: 10.2166/wst.2008.734. [DOI] [PubMed] [Google Scholar]
- 41.Yagci N, Artan N, Çokgör EU, Randall CW, Orhon D. 2003. Metabolic model for acetate uptake by a mixed culture of phosphate- and glycogen-accumulating organisms under anaerobic conditions. Biotechnol Bioeng 84:359–373. doi: 10.1002/bit.10765. [DOI] [PubMed] [Google Scholar]
- 42.Mino T, Tsuzuki Y, Matsuo T. 1987. Effect of phosphorus accumulation on acetate metabolism in the biological phosphorus removal process, p 27–38. In Ramadori R. (ed), Biological phosphate removal from wastewaters: proceedings from the International Conference of Advanced Water Pollution Control (IAWPRC). Pergamon Press, Oxford, England. [Google Scholar]
- 43.Lawson CE, Strachan BJ, Hanson NW, Hahn AS, Hall ER, Rabinowitz B, Mavinic DS, Ramey WD, Hallam SJ. 2015. Rare taxa have potential to make metabolic contributions in enhanced biological phosphorus removal ecosystems. Environ Microbiol 17:4979–4993. doi: 10.1111/1462-2920.12875. [DOI] [PubMed] [Google Scholar]
- 44.Pereira H, Lemos PC, Reis MAM, Crespo JPSG, Carrondo MJT, Santos H. 1996. Model for carbon metabolism in biological phosphorus removal processes based on in vivo 13C-NMR labelling experiments. Water Res 30:2128–2138. doi: 10.1016/0043-1354(96)00035-8. [DOI] [Google Scholar]
- 45.Lemos PC, Serafim LS, Santos MM, Reis MAM, Santos H. 2003. Metabolic pathway for propionate utilization by phosphorus-accumulating organisms in activated sludge: 13C labeling and in vivo nuclear magnetic resonance. Appl Environ Microbiol 69:241–251. doi: 10.1128/aem.69.1.241-251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckel W, Thauer RK. 2013. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation. Biochim Biophys Acta 1827:94–113. doi: 10.1016/j.bbabio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 47.de Graaff DR, van Dijk EJH, van Loosdrecht MCM, Pronk M. 2020. Strength characterization of full-scale aerobic granular sludge. Environ Technol 41:1637–1647. doi: 10.1080/09593330.2018.1543357. [DOI] [PubMed] [Google Scholar]
- 48.Daims H, Brühl A, Amann R, Schleifer KH, Wagner M. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 49.Amann RI. 1995. In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes, p 331–345. In Akkermans ADL, Van Elsas JD, De Bruijn FJ (ed), Molecular microbial ecology manual. Springer Netherlands, Dordrecht, Netherlands. [Google Scholar]
- 50.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925. doi: 10.1128/AEM.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flowers JJ, He S, Yilmaz S, Noguera DR, McMahon KD. 2009. Denitrification capabilities of two biological phosphorus removal sludges dominated by different “Candidatus Accumulibacter” clades. Environ Microbiol Rep 1:583–588. doi: 10.1111/j.1758-2229.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubio-Rincón FJ, Welles L, Lopez-Vazquez CM, Nierychlo M, Abbas B, Geleijnse M, Nielsen PH, van Loosdrecht MCM, Brdjanovic D. 2017. Long-term effects of sulphide on the enhanced biological removal of phosphorus: the symbiotic role of Thiothrix caldifontis. Water Res 116:53–64. doi: 10.1016/j.watres.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Saad SA, Welles L, Abbas B, Lopez-Vazquez CM, van Loosdrecht MCM, Brdjanovic D. 2016. Denitrification of nitrate and nitrite by ‘Candidatus Accumulibacter phosphatis’ clade IC. Water Res 105:97–109. doi: 10.1016/j.watres.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 54.McMahon KD, Yilmaz S, He S, Gall DL, Jenkins D, Keasling JD. 2007. Polyphosphate kinase genes from full-scale activated sludge plants. Appl Microbiol Biotechnol 77:167–173. doi: 10.1007/s00253-007-1122-6. [DOI] [PubMed] [Google Scholar]
- 55.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Horn DV, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orth JD, Thiele I, Palsson BØ. 2010. What is flux balance analysis? Nat Biotechnol 28:245–248. doi: 10.1038/nbt.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Heijden RTJM, Heijnen JJ, Hellinga C, Romein B, Luyben KCAM. 1994. Linear constraint relations in biochemical reaction systems: I. Classification of the calculability and the balanceability of conversion rates. Biotechnol Bioeng 43:3–10. doi: 10.1002/bit.260430103. [DOI] [PubMed] [Google Scholar]
- 60.McMahon KD, Read EK. 2013. Microbial contributions to phosphorus cycling in eutrophic lakes and wastewater. Annu Rev Microbiol 67:199–219. doi: 10.1146/annurev-micro-092412-155713. [DOI] [PubMed] [Google Scholar]
- 61.Brdjanovic D, Van Loosdrecht MCM, Hooijmans CM, Mino T, Alaerts GJ, Heijnen JJ. 1998. Effect of polyphospbate limitation on the anaerobic metabolism of phosphorus-accumulating microorganisms. Appl Microbiol Biotechnol 50:273–276. doi: 10.1007/s002530051289. [DOI] [Google Scholar]
- 62.Smolders GJF, van der Meij J, van Loosdrecht MCM, Heijnen JJ. 1994. Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. Biotechnol Bioeng 44:837–848. doi: 10.1002/bit.260440709. [DOI] [PubMed] [Google Scholar]
- 63.Smolders GJF, Klop JM, van Loosdrecht MCM, Heijnen JJ. 1995. A metabolic model of the biological phosphorus removal process: I. Effect of the sludge retention time. Biotechnol Bioeng 48:222–233. doi: 10.1002/bit.260480309. [DOI] [PubMed] [Google Scholar]
- 64.Tian W-D, Lopez-Vazquez CM, Li W-G, Brdjanovic D, van Loosdrecht MCM. 2013. Occurrence of PAOI in a low temperature EBPR system. Chemosphere 92:1314–1320. doi: 10.1016/j.chemosphere.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Zeng RJ, Yuan Z, Keller J. 2003. Model-based analysis of anaerobic acetate uptake by a mixed culture of polyphosphate-accumulating and glycogen-accumulating organisms. Biotechnol Bioeng 83:293–302. doi: 10.1002/bit.10671. [DOI] [PubMed] [Google Scholar]
- 66.López-Vázquez CM, Hooijmans CM, Brdjanovic D, Gijzen HJ, van Loosdrecht MCM. 2007. A practical method for quantification of phosphorus- and glycogen-accumulating organism populations in activated sludge systems. Water Environ Res 79:2487–2498. doi: 10.2175/106143007x220798. [DOI] [PubMed] [Google Scholar]
- 67.Lanham AB. 2012. Full-scale biological phosphorus removal: quantification of storage polymers, microbial performance and metabolic modelling. PhD dissertation Universidade Nova de Lisboa, Lisbon, Portugal. [Google Scholar]
- 68.Pijuan M, Saunders AM, Guisasola A, Baeza JA, Casas C, Blackall LL. 2004. Enhanced biological phosphorus removal in a sequencing batch reactor using propionate as the sole carbon source. Biotechnol Bioeng 85:56–67. doi: 10.1002/bit.10813. [DOI] [PubMed] [Google Scholar]
- 69.Zeng R, Yuan Z, van Loosdrecht MCM, Keller J. 2002. Proposed modifications to metabolic model for glycogen-accumulating organisms under anaerobic conditions. Biotechnol Bioeng 80:277–279. doi: 10.1002/bit.10370. [DOI] [PubMed] [Google Scholar]
- 70.Carvalheira M. 2014. The effect of key process operational conditions on enhanced biological phosphorus removal from wastewater. PhD dissertation Universidade Nova de Lisboa, Lisbon, Portugal. [Google Scholar]
- 71.Lu H, Oehmen A, Virdis B, Keller J, Yuan Z. 2006. Obtaining highly enriched cultures of Candidatus Accumulibacter phosphates through alternating carbon sources. Water Res 40:3838–3848. doi: 10.1016/j.watres.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Pijuan M, Oehmen A, Baeza JA, Casas C, Yuan Z. 2008. Characterizing the biochemical activity of full-scale enhanced biological phosphorus removal systems: a comparison with metabolic models. Biotechnol Bioeng 99:170–179. doi: 10.1002/bit.21502. [DOI] [PubMed] [Google Scholar]
- 73.Jeon CO, Lee DS, Lee MW, Park JM. 2001. Enhanced biological phosphorus removal in an anaerobic-aerobic sequencing batch reactor: effect of pH. Water Environ Res 73:301–306. doi: 10.2175/106143001x139407. [DOI] [PubMed] [Google Scholar]
- 74.Oehmen A, Yuan Z, Blackall LL, Keller J. 2005. Comparison of acetate and propionate uptake by polyphosphate accumulating organisms and glycogen accumulating organisms. Biotechnol Bioeng 91:162–168. doi: 10.1002/bit.20500. [DOI] [PubMed] [Google Scholar]
- 75.Brdjanovic D, van Loosdrecht MCM, Hooijmans CM, Alaerts GJ, Heijnen JJ. 1997. Temperature effects on physiology of biological phosphorus removal. J Environ Eng 123:144–153. doi: 10.1061/(ASCE)0733-9372(1997)123:2(144). [DOI] [Google Scholar]
- 76.Satoh Y, Tajima K, Tannai H, Munekata M. 2003. Enzyme-catalyzed poly(3-hydroxybutyrate) synthesis from acetate with CoA recycling and NADPH regeneration in vitro. J Biosci Bioeng 95:335–341. doi: 10.1016/s1389-1723(03)80064-6. [DOI] [PubMed] [Google Scholar]
- 77.Chohan SN, Copeland L. 1998. Acetoacetyl coenzyme A reductase and polyhydroxybutyrate synthesis in Rhizobium (Cicer) sp. strain CC 1192. Appl Environ Microbiol 64:2859–2863. doi: 10.1128/AEM.64.8.2859-2863.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olavarria K, Marone MP, da Costa Oliveira H, Roncallo JC, da Costa Vasconcelos FN, da Silva LF, Gomez JGC. 2015. Quantifying NAD(P)H production in the upper Entner-Doudoroff pathway from Pseudomonas putida KT2440. FEBS Open Bio 5:908–915. doi: 10.1016/j.fob.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dean AM, Golding GB. 1997. Protein engineering reveals ancient adaptive replacements in isocitrate dehydrogenase. Proc Natl Acad Sci U S A 94:3104–3109. doi: 10.1073/pnas.94.7.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bologna FP, Andreo CS, Drincovich MF. 2007. Escherichia coli malic enzymes: two isoforms with substantial differences in kinetic properties, metabolic regulation, and structure. J Bacteriol 189:5937–5946. doi: 10.1128/JB.00428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giachetti E, Pinzauti G, Vanni P. 1984. A new continuous optical assay for isocitrate lyase. Experientia 40:227–228. doi: 10.1007/BF01963614. [DOI] [Google Scholar]
- 82.Hillier AJ, Jericho RE, Green SM, Jago GR. 1979. Properties and function of fumarate reductase (NADH) in Streptococcus lactis. Aust J Biol Sci 32:625–635. doi: 10.1071/bi9790625. [DOI] [PubMed] [Google Scholar]
- 83.Bunik VI, Denton TT, Xu H, Thompson CM, Cooper AJL, Gibson GE. 2005. Phosphonate analogues of α-ketoglutarate inhibit the activity of the α-ketoglutarate dehydrogenase complex isolated from brain and in cultured cells. Biochemistry 44:10552–10561. doi: 10.1021/bi0503100. [DOI] [PubMed] [Google Scholar]
- 84.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olavarria K, Carnet A, van Ranselaar J, Quakkelaar C, Cabrera R, Guedes da Silva L, Smids AL, Villalobos P, van Loosdrecht MCM, Wahl SA. 2020. An NADH preferring acetoacetyl-CoA reductase is engaged in poly-3-hydroxybutyrate accumulation in Escherichia coli. J Biotechnol doi: 10.1016/j.jbiotec.2020.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
FISH micrographs, a phylogenetic tree based on Accumulibacter’s ppk1 gene analysis, and a taxonomic distribution of microbial community based on 16S rRNA gene copy numbers can be found in Document S3. The ppk1 gene sequences obtained in this study have been deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/) under accession numbers MH899084 to MH899086. The 16S rRNA gene amplicon data have been deposited in GenBank under BioProject accession number PRJNA490689. The AAR protein sequences, and enzymatic assays data are available in Document S4. The MATLAB (MathWorks, Natick, MA) code and data used for the simulations are available in a repository in GitHub (https://github.com/cell-systems-engineering-tud/anaerobic-flex-pao). All simulations were performed using MATLAB vR2018a (9.4.0.813654).