In various bacteria, the bacterial second messenger c-di-GMP influences a wide range of cellular processes. However, the function of c-di-GMP on biocontrol traits in the plant-beneficial rhizobacteria remains largely unclear. The present work shows that the QS system and polyketide antibiotic 2,4-DAPG production are regulated by c-di-GMP through RsmA and RsmE proteins in P. fluorescens 2P24. The diguanylate cyclases (DGCs) C0J56_12915, C0J56_13325, and C0J56_27925 are especially involved in regulating the biocontrol traits of 2P24. Our work also demonstrated a connection between the Gac/Rsm cascade and the c-di-GMP signaling pathway in P. fluorescens.
KEYWORDS: Pseudomonas fluorescens; c-di-GMP; RsmA/RsmE; quorum sensing; 2,4-DAPG
ABSTRACT
Pseudomonas fluorescens 2P24 is a rhizosphere bacterium that protects many crop plants against soilborne diseases caused by phytopathogens. The PcoI/PcoR quorum-sensing (QS) system and polyketide antibiotic 2,4-diacetylphloroglucinol (2,4-DAPG) are particularly relevant to the strain’s biocontrol potential. In this study, we investigated the effects of c-di-GMP on the biocontrol activity of strain 2P24. The expression of the Escherichia coli diguanylate cyclase (YedQ) and phosphodiesterase (YhjH) in P. fluorescens 2P24 significantly increased and decreased the cellular concentration of c-di-GMP, respectively. The production of the QS signals N-acyl homoserine lactones (AHLs) and 2,4-DAPG was negatively regulated by c-di-GMP in 2P24. The regulatory proteins RsmA and RsmE were positively regulated by c-di-GMP. Genomic analysis revealed that 2P24 has 23 predicted proteins that contain c-di-GMP-synthesizing or -degrading domains. Among these proteins, C0J56_12915, C0J56_13325, and C0J56_27925 contributed to the production of c-di-GMP and were also involved in the regulation of the QS signal and antibiotic 2,4-DAPG production in P. fluorescens. Overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 in 2P24 impaired its root colonization and biocontrol activities. Taken together, these results demonstrated that c-di-GMP played an important role in fine-tuning the biocontrol traits of P. fluorescens.
IMPORTANCE In various bacteria, the bacterial second messenger c-di-GMP influences a wide range of cellular processes. However, the function of c-di-GMP on biocontrol traits in the plant-beneficial rhizobacteria remains largely unclear. The present work shows that the QS system and polyketide antibiotic 2,4-DAPG production are regulated by c-di-GMP through RsmA and RsmE proteins in P. fluorescens 2P24. The diguanylate cyclases (DGCs) C0J56_12915, C0J56_13325, and C0J56_27925 are especially involved in regulating the biocontrol traits of 2P24. Our work also demonstrated a connection between the Gac/Rsm cascade and the c-di-GMP signaling pathway in P. fluorescens.
INTRODUCTION
The bacterial secondary messenger cyclic-di-GMP (c-di-GMP) is a broadly conserved, intracellular signal molecule that regulates multiple important phenotypes in many bacteria (1). In general, high levels of intracellular c-di-GMP induce biofilm formation, extracellular polysaccharide production, and cell cycle progression, whereas low levels are associated with high motility and the expression of virulence factors (1, 2). GGDEF, EAL, and HD-GYP domain proteins are involved in c-di-GMP turnover. The cellular c-di-GMP level is synthesized by diguanylate cyclases (DGCs) with the GGDEF domain and degraded by phosphodiesterases (PDEs) with the EAL or HD-GYP domain (3). These c-di-GMP-synthesizing and -degrading domain proteins are usually linked with sensory and signal transduction domains; therefore, the process of c-di-GMP synthesis and degradation is highly sensitive to differential environmental cues or cellular signaling pathways (1, 3). To exert its function, c-di-GMP binds to c-di-GMP effectors and subsequently affects the regulatory output (4, 5). A range of c-di-GMP effectors, including various transcriptional factors, PilZ domain proteins, GIL domain proteins, enzymatically inactive GGDEF and/or EAL domain proteins, RNA riboswitches, and ATPase, associated with the type II secretion system, have been identified thus far (6–9).
Recent studies have highlighted c-di-GMP as a central player in the pathogenesis of many plant and animal bacterial pathogens, including Pseudomonas aeruginosa (10), Salmonella enterica (11), Vibrio cholerae (12), Yersinia pestis (13), Xanthomonas campestris pv. campestris (14), and Dickeya dadantii (15). Genetic evidence has revealed a sophisticated c-di-GMP signaling network that regulates bacterial virulence. c-di-GMP was found to inhibit the virulence of the opportunistic pathogen P. aeruginosa in the murine model of burn wound infection, whereas virulence was decreased in several deletion mutants of PDEs and a mutant of the DGC SadC (10). These results suggested the effect of c-di-GMP on virulence in various pathogenic bacteria is complicated.
Although the role of c-di-GMP in the production of virulence factors has been extensively investigated, little is known about its function in biocontrol traits of plant growth-promoting rhizobacteria (PGPR). Pseudomonas fluorescens 2P24, a plant-beneficial root-colonizing bacterium originally isolated from the take-all decline soil of wheat, has been investigated for its ability to produce the secondary metabolite 2,4-diacetylphloroglucinol (2,4-DAPG), which contributes to the protection of many crops against soilborne disease caused by plant pathogens (16). The PcoI/PcoR quorum-sensing (QS) system is essential for rhizosphere colonization and involved in the regulation of biocontrol activity in P. fluorescens 2P24 (17). Previous study showed that the GacS/GacA two-component system is required for the production of 2,4-DAPG and the expression of the PcoI/PcoR QS system via a regulatory cascade involving small noncoding RNAs (RsmX, RsmX1, RsmY, RsmZ, and RgsA) and CsrA/RsmA family proteins RsmA and RsmE (18) (Fig. 1).
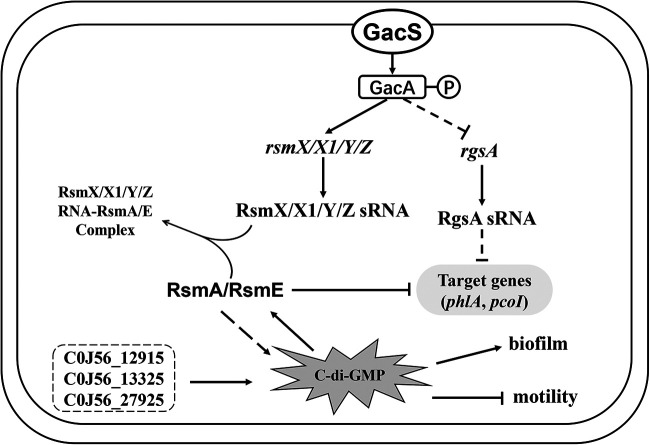
FIG 1.
Model of c-di-GMP regulatory pathway in Pseudomonas fluorescens 2P24. C0J56_12915, C0J56_13325, and C0J56_27925 influence the concentration of c-di-GMP by their c-di-GMP DGC activity. C-di-GMP induces the expression of rsmA and rsmE genes, which regulate phlA and pcoI at the posttranscriptional level. In addition, the concentration of c-di-GMP is induced by RsmA and RsmE. Therefore, a feedback loop mechanism exists between the Gac/Rsm signaling cascade and the c-di-GMP pathway in strain 2P24. Similar to its well-established function, c-di-GMP positively regulates biofilm formation and represses motility in P. fluorescens 2P24.
In the present study, we examined the effects of c-di-GMP on biocontrol traits in P. fluorescens. A total of 23 GGDEF/EAL domain proteins were identified in strain 2P24. Our data revealed that c-di-GMP negatively regulated the QS system and 2,4-DAPG production, and the regulation of biocontrol traits by c-di-GMP was mediated by RsmA and RsmE proteins. In addition, our data demonstrated that c-di-GMP regulated other important biological traits in P. fluorescens, such as motility, biofilm formation, and biocontrol capacity.
RESULTS
Modulation of intracellular c-di-GMP levels by overexpressing yedQ and yhjH in P. fluorescens 2P24.
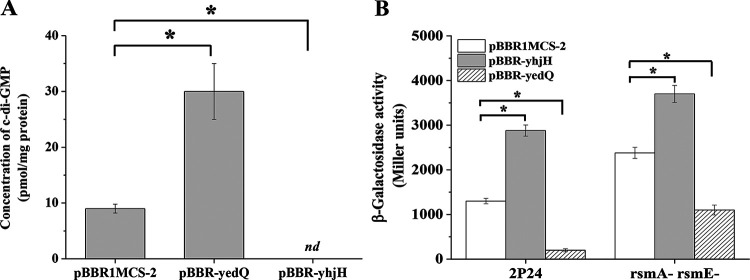
The diguanylate cyclase activity of the YedQ protein for c-di-GMP biosynthesis and phosphodiesterase activity of the YhjH protein for c-di-GMP degradation have been demonstrated in Escherichia coli and used to alter the cellular c-di-GMP concentration in various bacteria (19, 20). To investigate the role of c-di-GMP in the biocontrol strain P. fluorescens 2P24, we constructed two overexpression constructs, pBBR-yedQ and pBBR-yhjH, containing the E. coli genes yedQ and yhjH, respectively, under the control of the E. coli lac promoter, which was constitutively active in P. fluorescens. The two overexpression plasmids were moved into strain 2P24, and the intracellular c-di-GMP concentration of the resulting strains was determined. 2P24(pBBR-yedQ) produced a high intracellular level of c-di-GMP relative to that of 2P24(pBBR1MCS-2), which contained an empty vector, and no c-di-GMP was detected in 2P24(pBBR-yhjH) (Fig. 2A). A reporter plasmid harboring the c-di-GMP-responding Vc2 riboswitch from Vibrio cholerae fused to a promoterless lacZ gene was constructed to measure the relative c-di-GMP concentrations in P. fluorescens (21). Binding of c-di-GMP to the Vc2 riboswitch repressed lacZ expression; thus, a high c-di-GMP concentration will lead to low β-galactosidase activity. Using the generated reporter plasmid, the β-galactosidase activity of 2P24(pBBR-yhjH) or 2P24(pBBR-yedQ) was significantly changed compared with that of 2P24 with the empty vector pBBR1MCS-2, which was consistent with the results of c-di-GMP concentration assays (Fig. 2B). Collectively, these results indicated that the intracellular levels of c-di-GMP of 2P24 can be positively and negatively altered by the heterologous overexpression of E. coli genes yedQ and yhjH, respectively.
FIG 2.
Overexpression of YedQ and YhjH in P. fluorescens 2P24 influenced the intracellular concentration of c-di-GMP. (A) Quantification of the intracellular c-di-GMP levels in strains 2P24 containing pBBR-yedQ, which overexpresses yedQ, pBBR-yhjH, which overexpresses yhjH, or the empty vector pBBR1MCS-2 by mass spectroscopy analysis. (B) Bioreporter assay of c-di-GMP concentration using strain 2P24 and its derivatives harboring plasmid Vc2-p6013 with the c-di-GMP-responding riboswitch. The β-galactosidase assay was performed in LB broth after 16 h of incubation at 28°C. All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
c-di-GMP plays a pleiotropic role in regulation of biocontrol-related phenotypes of P. fluorescens 2P24.
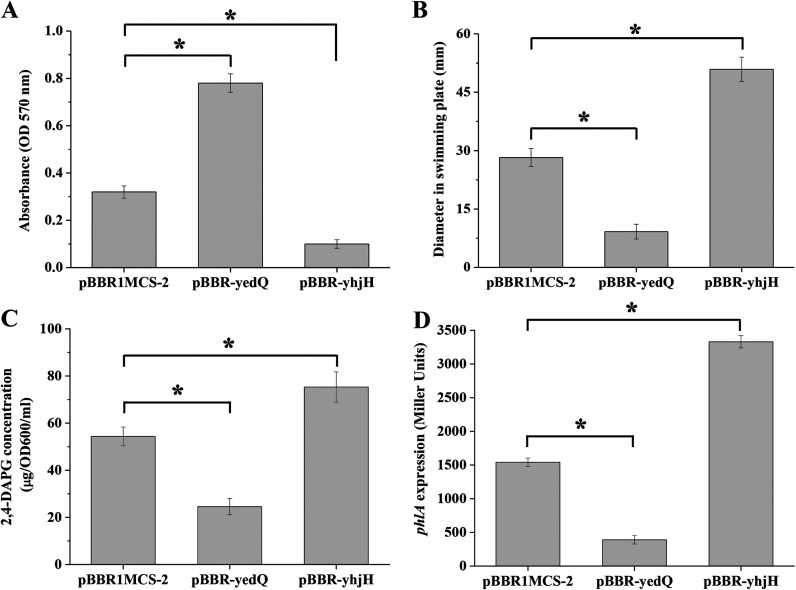
We then tested whether biocontrol traits of 2P24, including biofilm formation, motility, antibiotic 2,4-DAPG production, and the PcoI/PcoR QS system, were affected by the altered intracellular levels of c-di-GMP. The results showed that the biofilm formation of 2P24 was enhanced with the overexpression of yedQ but reduced with the overexpression of yhjH (Fig. 3A). In contrast, the swimming motility of strain 2P24 was reduced with the overexpression of yedQ but enhanced with the overexpression of yhjH (Fig. 3B). These results were consistent with the known role of c-di-GMP in biofilm formation and motility of other strains (3) and suggested that c-di-GMP positively regulated biofilm formation and negatively regulated swimming motility in strain 2P24.
FIG 3.
Role of c-di-GMP on biofilm formation (A), motility (B), and 2,4-DAPG biosynthesis (C and D) of P. fluorescens 2P24. (A) Regulation of biofilm formation by c-di-GMP. Biomasses were analyzed for strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). The biofilms were stained with crystal violet. OD, optical density. (B) Swimming agar plate assay. The swimming abilities of strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH) were determined by the diameter of the zone of motility. (C) HPLC analysis of the concentration of 2,4-DAPG by the strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). (D) The expression of phlA gene was controlled by c-di-GMP. The β-galactosidase activity of the translational fusion phlA′-′lacZ was measured in strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
The production of antibiotic 2,4-DAPG was significantly reduced in 2P24 when overexpressing yedQ but enhanced when overexpressing yhjH (Fig. 3C). Similar regulatory trends were observed in the transcriptional analysis of phlA (2,4-DAPG biosynthase gene)-lacZ (Fig. 3D), suggesting that c-di-GMP negatively regulated the expression of phlA and 2,4-DAPG production in strain 2P24.
The effect of c-di-GMP on the PcoI/PcoR QS system was determined by measuring the production of the QS signal N-acyl homoserine lactone (AHL) in strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). The AHL production in 2P24(pBBR-yedQ) was more than 2-fold lower than that in the wild-type (WT) strain (Fig. 4A). In contrast, 2P24(pBBR-yhjH) produced a significantly larger amount of AHL signal than 2P24(pBBR1MCS-2) (Fig. 4A). Further detection revealed that the regulation occurred at the transcriptional level, i.e., the expression of pcoI (AHL biosynthase gene) in 2P24(pBBR-yedQ) decreased significantly compared with that of 2P24(pBBR1MCS-2) but increased in strain 2P24(pBBR-yhjH) (Fig. 4B). Taken together, these results suggested a negative regulation of c-di-GMP on the transcriptional expression of QS in 2P24.
FIG 4.
Overexpression of YedQ and YhjH influenced the QS system of P. fluorescens 2P24. (A) The production of AHL was regulated by c-di-GMP. The β-galactosidase activity of traG-lacZ fusion in A. tumefaciens NTL4(pZLR4) was determined after incubation with AHL signals that were extracted from 2P24 and its derivatives. (B) The expression of pcoI gene was controlled by c-di-GMP. The β-galactosidase activity of the transcriptional pcoI-lacZ was measured in strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
RsmA and RsmE were required in c-di-GMP-mediated regulation of QS and production of 2,4-DAPG.
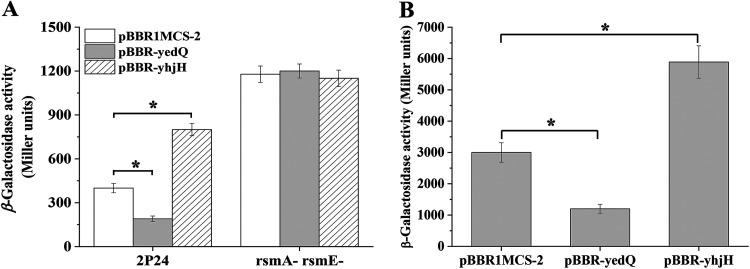
Our prior work demonstrated that the QS system and 2,4-DAPG production are strictly regulated by the Gac/Rsm signaling pathway, which consists of five small regulatory RNAs (sRNA; including RsmZ, RsmY, RsmX, RsmX1, and RgsA) and their binding proteins, RsmA and RsmE (18, 22) (Fig. 1). These Gac/Rsm pathway components are likely required in the regulation of c-di-GMP on QS and 2,4-DAPG production. To test this hypothesis, we assessed the effect of YdeQ and YhjH on the expression of sRNAs and their binding proteins. The expression levels of all five sRNAs were not significantly changed, indicating that these sRNAs were not involved in the c-di-GMP-mediated regulation (Fig. 5A). However, the protein levels of RsmA and RsmE were significantly decreased in strain 2P24(pBBR-yhjH), which had a lower concentration of c-di-GMP (Fig. 2A) than 2P24(pBBR1MCS-2) (Fig. 5B and C). Consistent with this, overexpression of YedQ led to higher RsmA and RsmE levels (Fig. 5B and C). A similar regulation was observed when the lacZ-fused translational reporters of rsmA or rsmE were measured in 2P24 overexpressing YedQ and YhjH (Fig. 5D and E). These data suggested that c-di-GMP positively regulated the expression of RsmA and RsmE.
FIG 5.
Modulating the c-di-GMP levels influenced the levels of RsmA and RsmE proteins. (A) The expression of rsmZ-lacZ, rsmY-lacZ, rsmX-lacZ, rsmX1-lacZ, and rgsA-lacZ transcriptional fusions was determined in strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). Western blot analysis was performed to detect the levels of RsmA-FLAG (B) and RsmE-FLAG (C). The expression of rsmA (D) and the expression of rsmE (E) were controlled by c-di-GMP. The β-galactosidase activity of the translational fusion rsmA′-′lacZ or rsmE′-′lacZ was measured in strains 2P24(pBBR1MCS-2), 2P24(pBBR-yedQ), and 2P24(pBBR-yhjH). All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
To further test whether RsmA and RsmE are required for the c-di-GMP-mediated regulation of the QS system, we determined the AHL production with or without the overexpression of yedQ or yhjH in the rsmA rsmE double mutant. The rsmA rsmE double mutant was used in this experiment due to the functional redundancy of RsmA and RsmE (18). Our results showed that mutation of both rsmA and rsmE enhanced the AHL levels, which was consistent with the known repressive role of RsmA and RsmE on the expression of the QS system (Fig. 4A) (22). Although the intracellular levels of c-di-GMP in the rsmA rsmE double mutant were altered by the overexpression of yedQ or yhjH, their influence on AHL production was abolished (Fig. 2B and 4A). Collectively, these data suggested the positive control of c-di-GMP on the protein levels of RsmA and RsmE in strain 2P24, which in turn negatively regulated the production of QS signals.
Identification of GGDEF/EAL domain proteins that influenced QS in 2P24.
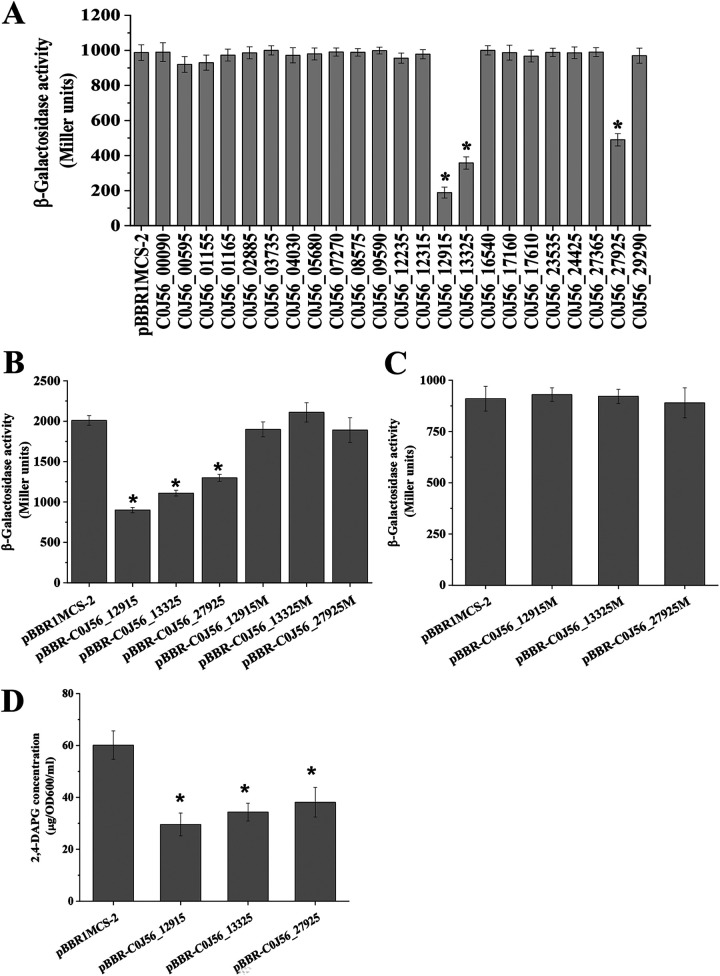
The genome of P. fluorescens 2P24 (GenBank accession number CP025542) encodes 5 EAL, 10 GGDEF, and 8 tandem GGDEF-EAL domain proteins, which may play a role in the c-di-GMP turnover (Table 1). To determine which of these proteins regulate the QS system of 2P24, we cloned each of the 23 genes into pBBR1MCS-2 and moved the generated overexpression plasmids into 2P24 to assess their effects on AHL production. Our data showed that the overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 led to a significant decrease in AHL production in the wild-type 2P24 strain (Fig. 6A).
TABLE 1.
Putative diguanylate cyclases and phosphodiesterases investigated in this study
| Locus tag | Name or homologuea | DGC motif | PDE motif | Other domainb |
|---|---|---|---|---|
| C0J56_00090 | GGEEF | |||
| C0J56_00595 | EAL | PAS 3, PAS | ||
| C0J56_01155 | GGEEF | PAS 3 | ||
| C0J56_01165 | GGDEF | EAL | ||
| C0J56_02885 | EAL | GAF, PAS | ||
| C0J56_03735 | GcbA (52) | GGDEF | ||
| C0J56_04030 | YfiN (53) | GGDEF | ||
| C0J56_05680 | WspR (54) | GGEEF | ||
| C0J56_07270 | GGDEF | PAS | ||
| C0J56_08575 | GGEEF | |||
| C0J56_09590 | GGDEF | EAL | PAS | |
| C0J56_12235 | EAL | |||
| C0J56_12315 | MucR (55) | GGDEF | EAL | MHYT |
| C0J56_12915 | GGDEF | GAF | ||
| C0J56_13325 | GGDEF | EAL | CHASE4, PAS | |
| C0J56_16540 | EAL | |||
| C0J56_17160 | GGEEF | PAS | ||
| C0J56_17610 | GGDEF | EAL | GAF, MukB | |
| C0J56_23535 | GGDEF | EAL | REC, PAS 3 | |
| C0J56_24425 | GGDEF | PAS 3 | ||
| C0J56_27365 | GGDEF | EAL | PBP2, PAS | |
| C0J56_27925 | GGDEF | EAL | REC | |
| C0J56_29290 | EAL |
Names for DGCs and PDEs characterized in this study, or homologues (≥65% amino acid identity) that have been characterized in other organisms, are given with references in parentheses.
Other predicted domains are indicated using Pfam designations (http://pfam.xfam.org/).
FIG 6.
Effect of putative DGC and PDE domain proteins on the QS system and 2,4-DAPG production in P. fluorescens 2P24. (A) A quantitative AHL assay analyzing the WT with pBBR1MCS-2 and 23 putative DGC and PDE domain proteins. (B) c-di-GMP concentration measurement in strain 2P24 and its derivatives harboring plasmid Vc2-p6013 with the c-di-GMP-responding riboswitch. The β-galactosidase assay was performed in LB broth after 16 h of incubation at 28°C. (C) Mutations in the GGDEF domain of C0J56_12915, C0J56_13325, and C0J56_27925 abolished the repression of AHL production. (D) HPLC analysis of the concentration of 2,4-DAPG of the strains 2P24(pBBR1MCS-2), 2P24(pBBR-C0J56_12915), 2P24(pBBR-C0J56_13325), and 2P24(pBBR-C0J56_27925). All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
Given that high levels of c-di-GMP inhibited AHL production (Fig. 4), we hypothesized that C0J56_12915, C0J56_13325, and C0J56_27925 acted as the active DGCs to maintain the cellular c-di-GMP pool of 2P24. The c-di-GMP reporter Vc2-p6013 was used to detect the c-di-GMP concentration in 2P24 and its derivatives. Our results showed that the overexpression of C0J56_12915, pBBR-C0J56_13325, and pBBR-C0J56_27925 reduced the β-galactosidase activity in 2P24 compared with that of strain 2P24(pBBR1MCS-2) (Fig. 6B), indicating a high c-di-GMP level of overexpression strains, thereby supporting our hypothesis that these three proteins function as DGCs in strain 2P24.
Sequence analysis revealed that C0J56_12915, C0J56_13325, and C0J56_27925 have a GGDEF domain, which was consistent with the result that the overexpression of these proteins enhanced the c-di-GMP level (Table 1). To confirm the function of the GGDEF domain of these proteins in intracellular c-di-GMP production, we replaced the GGDEF domain of the three proteins with a GGAAF domain in their overexpression plasmids. The results showed that mutation of the GGDEF domain in these three proteins abolished their inhibitory effects on AHL production (Fig. 6C). Furthermore, mutation of the GGDEF motif abolished the effects of C0J56_12915, C0J56_13325, and C0J56_27925 on the expression of the c-di-GMP reporter (Fig. 6B), suggesting that the GGDEF domain was functional in these proteins and contributed to c-di-GMP synthesis in 2P24. The production of 2,4-DAPG is negatively regulated by RsmA and RsmE proteins (18). Given that c-di-GMP influenced the protein levels of RsmA and RsmE (Fig. 5), high-performance liquid chromatography (HPLC) assays further verified that the overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 repressed 2,4-DAPG production in wild-type 2P24 (Fig. 6D). Translation fusion assays showed that rsmA′-′lacZ and rsmE′-′lacZ expression was significantly induced when C0J56_12915, C0J56_13325, and C0J56_27925 were overexpressed (see Fig. S1 in the supplemental material). Taken together, these results indicated that C0J56_12915, C0J56_13325, and C0J56_27925 positively contributed to the c-di-GMP synthesis and negatively regulated the QS system and 2,4-DAPG biosynthesis in 2P24.
Effects of c-di-GMP on rhizosphere colonization and biocontrol capacity of P. fluorescens.
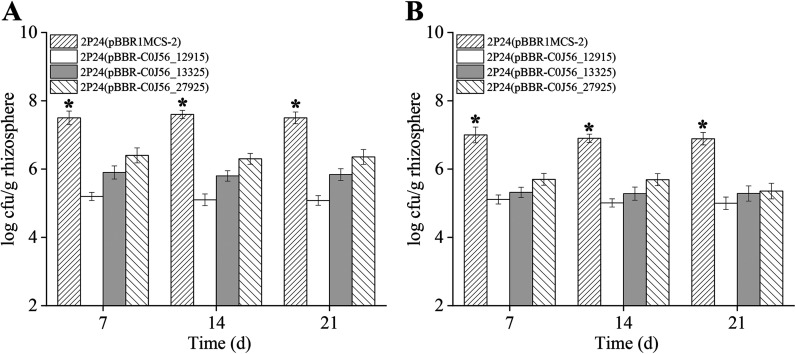
Root colonization is essential for the biocontrol capacity of 2P24 (17), and the effect of c-di-GMP on rhizosphere colonization was assessed under greenhouse conditions. When introduced into sterile soils, the population dynamics of strains 2P24(pBBR-C0J56_12915), 2P24(pBBR-C0J56_13325), and 2P24(pBBR-C0J56_27925) on the wheat rhizosphere were significantly impaired compared with that of 2P24(pBBR1MCS-2) (Fig. 7A). Similar experiments conducted in natural soils revealed a similar tendency (Fig. 7B). These results suggested that c-di-GMP exerted a negative influence on the rhizosphere colonization of P. fluorescens 2P24.
FIG 7.
Colonization of wheat rhizospheres by P. fluorescens 2P24 and its derivatives. The populations of strains 2P24(pBBR1MCS-2), 2P24(pBBR-C0J56_12915), 2P24(pBBR-C0J56_13325), and 2P24(pBBR-C0J56_27925) were determined at 7, 14, and 21 days. (A) Rhizosphere population in sterile soil. (B) Rhizosphere population in natural soil. The experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
Given that the QS system and 2,4-DAPG production are essential for the biocontrol capacity of 2P24 (16, 17), the effect of c-di-GMP on biocontrol capacity was assessed under greenhouse conditions, using tomato as a host plant and Ralstonia solanacearum as a pathogen that causes the tomato bacterial wilt disease. Our data showed that strains 2P24(pBBR-C0J56_12915), 2P24(pBBR-C0J56_13325), and 2P24(pBBR-C0J56_27925) had a reduced disease control effect compared with that of 2P24(pBBR1MCS-2) (Table 2). A control experiment involving the gacA mutant (23) was performed, and the results showed that the level of protection that the gacA mutant conferred to tomato was significantly lower than that of the wild-type strain (Table 2). These results suggested that c-di-GMP had a negative influence on the biocontrol capacity of P. fluorescens 2P24.
TABLE 2.
Control efficacy of 2P24 and its derivatives against tomato bacterial wilt caused by R. solanacearuma
| Strain added | Disease index |
Control efficiency (%) |
||
|---|---|---|---|---|
| 10 days | 14 days | 10 days | 14 days | |
| None | 90a | 100a | ||
| 2P24(pBBR1MCS-2) | 0d | 10d | 100 | 88.5 |
| 2P24(pBBR-C0J56_12915) | 62.4b | 68.5b | 30.5 | 26.7 |
| 2P24(pBBR-C0J56_13325) | 54.6c | 60c | 44.3 | 34.5 |
| 2P24(pBBR-C0J56_27925) | 50.4c | 57.1c | 50.7 | 45.3 |
| PM203 (the gacA mutant) | 60b | 70.5b | 34.5 | 24.5 |
Data are averages from three repeats, and different letters in the same column indicate a significant difference at the 0.05 level.
Gac/Rsm signaling cascade negatively affected c-di-GMP pools.
Several studies have shown that mutation of the Gac/Rsm signaling cascade resulted in high intracellular c-di-GMP levels (24, 25). To evaluate the contribution of the Gac/Rsm signaling cascade to c-di-GMP levels in P. fluorescens 2P24, we evaluated the expression of the c-di-GMP reporter in the rsmA rsmE double mutant and the retS mutant (the retS gene encodes a hybrid sensor kinase that affects the phosphorylation state of GacS) (26) and found that the β-galactosidase activities were significantly increased in the rsmA rsmE double mutant and the retS mutant compared to that of the wild type, suggesting that RsmA/RsmE and RetS positively affected c-di-GMP pools (Fig. 8). Thus, these results indicated a feedback loop mechanism between the Gac/Rsm signaling cascade and c-di-GMP signaling pathway.
FIG 8.
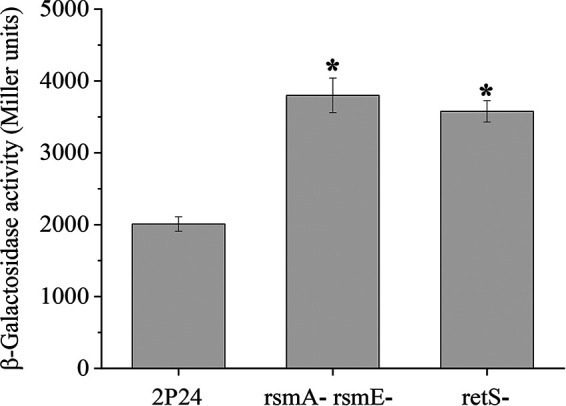
RsmA/RsmE and RetS influenced the c-di-GMP levels in P. fluorescens. Shown is the c-di-GMP concentration measurement in the WT, the rsmA rsmE double mutant, and the retS mutant harboring plasmid Vc2-p6013 with the c-di-GMP-responding riboswitch. The β-galactosidase activities then were determined in LB broth. All experiments were performed in triplicate, and the mean values ± standard deviations are indicated. *, P < 0.05.
DISCUSSION
In this study, the secondary messenger c-di-GMP was involved in the regulation of biocontrol traits, such as the antibiotic 2,4-DAPG, the QS system, and biocontrol capacity, in P. fluorescens 2P24 (Fig. 3, 4, and 7). The P. fluorescens 2P24 genome encodes 23 GGDEF/EAL domain proteins, and our data suggested that the overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 decreased the production of AHL signals and 2,4-DAPG (Fig. 6). In addition to C0J56_12915, C0J56_13325, and C0J56_27925, overexpression of the genes encoding other GGDEF/EAL domain proteins had no significant effect on AHL production (Fig. 6A). These results suggested that these 20 GGDEF/EAL domain proteins are not involved in the production of the AHL signals under the conditions used in this study.
Many genetic elements that regulate the production of 2,4-DAPG and AHL have been characterized in P. fluorescens, and the Gac/Rsm signaling cascade plays a vital role in activating the biocontrol traits (27). The Gac/Rsm signaling cascade includes the GacS/GacA two-component system and the Csr/Rsm regulatory pathway. In the presence of an unknown environmental cue, the GacS histidine kinase promotes the phosphorylation of the cognate response regulator GacA, which activates the transcription of RsmZ-like sRNAs. Ultimately, these sRNAs sequester RsmA and RsmE proteins, which inhibit the translation of the target mRNAs by binding to single-stranded GGA motifs formed near the ribosome binding site (28). Previous studies have shown that RsmZ-like sRNA genes are controlled by several regulatory proteins (29–32). However, the impact of the function of RsmA and RsmE proteins was rarely investigated. Here, Western blot assays confirmed that RsmA and RsmE protein levels were controlled by c-di-GMP in P. fluorescens cells (Fig. 5). Genetic evidence further showed that the overexpression of yedQ and yhjH influenced the production of AHL and 2,4-DAPG (Fig. 3 and 4). These results suggested that the RsmA and RsmE proteins were the downstream targets of the c-di-GMP signaling. Previous work showed that the transcriptional regulator AlgR directly activates rsmA expression in P. aeruginosa (33). The function of AlgR is highly conserved in pseudomonads (34), and two AlgR binding sites are found in the promoter regions of the rsmA and rsmE genes (unpublished data). These results suggested that c-di-GMP could regulate RsmA and RsmE through AlgR in strain 2P24. The specific link between the Gac/Rsm cascade and c-di-GMP signaling also has been illustrated (25). In P. aeruginosa, the retS mutant showed high levels of c-di-GMP (24). Further analysis indicated that the sadC gene, which encodes the diguanylate cyclase, is tightly repressed by RsmA (35). The results of the present study showed the effect of c-di-GMP on the protein levels of RsmA and RsmE (Fig. 5). Several genes that are involved in c-di-GMP turnovers are regulated by RsmA and RsmE (unpublished data). Considering that the Gac/Rsm signaling pathway is conserved in many Gram-negative bacteria, our data revealed additional complexity in the regulatory network between the Gac/Rsm cascade and the c-di-GMP regulatory pathway.
In P. fluorescens 2P24, C0J56_12915 is predicted to be a cytoplasmic protein that contains a GAF domain and a GGDEF domain, C0J56_13325 contains a PAS sensory domain, a CHASE4 domain, a GGDEF domain, and an EAL domain, and C0J56_27925 encodes a protein with three predicted domains, namely, REC, GGDEF, and EAL domains (Pfam designations). Our data suggested that these proteins are active DGCs, because the overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 in 2P24 significantly decreased AHL production, which was consistent with the increased cellular levels of c-di-GMP of these overexpressing strains (Fig. 4). The β-galactosidase activity assay further showed that mutation in the GGDEF domain failed to decrease the production of AHL (Fig. 6), indicating that C0J56_12915, C0J56_13325, and C0J56_27925 were involved in c-di-GMP turnover. Phylogenetic analysis indicated that no homologs of C0J56_12915, C0J56_13325, and C0J56_27925 were found in other biocontrol strains, such as P. protegens Pf-5, P. protegens CHA0, and P. fluorescens Pf0-1 (Table 1). This suggests that different strains have evolved diverse pathways to control biocontrol traits via the secondary messenger c-di-GMP pathway.
A previous study indicated five proteins containing the GGDEF domain are highly conserved in pseudomonads, whereas the functions of these proteins differ (36). For example, in P. fluorescens F113, inactivation of the conserved wspR gene increased the swimming motility (37), whereas overexpression of C0J56_05680 (homolog of WspR) showed no effect on motility and biofilm formation in 2P24 (unpublished data). These results suggested that bacteria adapted to their immediate environments using their own unique c-di-GMP signaling pathways.
Rhizosphere colonization is important for PGPR to suppress plant diseases, and many factors are involved in determining the efficient colonization of bacteria (17). In this work, overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 increased the cellular concentrations of c-di-GMP (Fig. 6B) and decreased the root colonization of strain 2P24 (Fig. 7), suggesting that c-di-GMP negatively regulates rhizosphere colonization of 2P24. The PcoI/PcoR QS system positively regulates root colonization on the wheat rhizosphere in strain 2P24 (17). In this study, our results demonstrated that c-di-GMP negatively regulates the PcoI/PcoR QS system (Fig. 4 and 6A). Based on these data, it is possible that c-di-GMP levels regulate root colonization through the QS system. In fact, the PhzI-PhzR QS system of P. chlororaphis PA23 negatively regulates a PDE (EY04_RS05905), MotD, and MotY, which favors induced motility (38). The mechanism by which the PcoI/PcoR QS system affects root colonization is unknown, and further investigations are needed to elucidate the relationship between the QS system and c-di-GMP signal cascade and their regulation of root colonization in P. fluorescens 2P24.
2,4-DAPG plays a critical role in protecting tomato against the phytopathogen R. solanacearum (16). Here, the overexpression of C0J56_12915, C0J56_13325, and C0J56_27925, which are involved in c-di-GMP synthesis, negatively regulated 2,4-DAPG production (Fig. 3 and 6). The overexpression of C0J56_12915, C0J56_13325, and C0J56_27925 in P. fluorescens 2P24 resulted in a partial loss of biocontrol efficacy. These results suggested that c-di-GMP plays an important role in regulating the biocontrol ability of P. fluorescens. It should be noted that a higher concentration of 2,4-DAPG in some Pseudomonas strains can lead to notable phytotoxicity to plants (16). However, high levels of c-di-GMP inhibit the production of 2,4-DAPG, indicating the increased severity of wilting symptoms on tomato in the biocontrol assay was not caused by the phototoxicity of 2,4-DAPG but by the impaired biocontrol activity due to the negative influence of c-di-GMP on the biocontrol traits of strain 2P24.
In conclusion, our data indicated that c-di-GMP negatively influenced the production of 2,4-DAPG and the QS system through the RsmA and RsmE proteins. Furthermore, among 23 GGDEF/EAL domain proteins, DGCs C0J56_12915, C0J56_13325, and C0J56_27925 in strain 2P24 played a specific role in regulating its biocontrol traits (Fig. 1). Our work also provided new insights that the connection between the Gac/Rsm cascade and c-di-GMP signaling pathway could be more complicated than expected (Fig. 1). Further study is necessary to illustrate the molecular mechanism of how the levels of RsmA and RsmE are regulated by c-di-GMP in P. fluorescens 2P24.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and culture conditions.
The bacterial strains, plasmids, and oligonucleotides used in this study are listed in Tables 3 and 4. Escherichia coli DH5α (39) was used as the host for DNA cloning and routinely grown in lysogeny broth (LB) medium at 37°C. Unless otherwise indicated, P. fluorescens strains were grown in LB medium, King’s B (KB) medium (40), or ABM medium (41) at 28°C. The concentrations of antibiotics were the following: ampicillin (50 μg/ml), gentamicin (5 μg/ml), kanamycin (50 μg/ml), and tetracycline (20 μg/ml).
TABLE 3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Strains | ||
| Pseudomonas fluorescens | ||
| 2P24 | Wild type, Apr | 16 |
| PM203 | In-frame deletion of gacA, Apr | 23 |
| PM206 | In-frame deletion of retS, Apr | 26 |
| WPM13 | Double deletion of rsmA and rsmE, Apr | 18 |
| WPM26 | Strain 2P24 with a FLAG epitope sequence tagged to the C terminus of RsmA, Apr | This work |
| WPM27 | Strain 2P24 with a FLAG epitope sequence tagged to the C terminus of RsmE, Apr | This work |
| Agrobacterium tumefaciens NTL4(pZLR4) | A. tumefaciens NT1 derivative carrying a traG-lacZ reporter fusion, QS biosensor | 45 |
| Ralstonia solanacearum | Bacterial pathogen causing tomato bacterial wilt | Laboratory stock |
| E. coli DH5α | supE44 lacU169 (ϕ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 39 |
| Plasmids | ||
| pBBR1MCS-2 | Broad-host-range cloning vector, Kmr | 42 |
| pBBR-yedQ | pBBR1MCS-2 containing the yedQ gene, Kmr | This work |
| pBBR-yhjH | pBBR1MCS-2 containing the yhjH gene, Kmr | This work |
| pBBR-C0J56_00090 | pBBR1MCS-2 containing the C0J56_00090-encoding gene, Kmr | This work |
| pBBR-C0J56_00595 | pBBR1MCS-2 containing the C0J56_00595-encoding gene, Kmr | This work |
| pBBR-C0J56_01155 | pBBR1MCS-2 containing the C0J56_01155-encoding gene, Kmr | This work |
| pBBR-C0J56_01165 | pBBR1MCS-2 containing the C0J56_01165-encoding gene, Kmr | This work |
| pBBR-C0J56_02885 | pBBR1MCS-2 containing the C0J56_02885-encoding gene, Kmr | This work |
| pBBR-C0J56_03735 | pBBR1MCS-2 containing the C0J56_03735-encoding gene, Kmr | This work |
| pBBR-C0J56_04030 | pBBR1MCS-2 containing the C0J56_04030-encoding gene, Kmr | This work |
| pBBR-C0J56_05680 | pBBR1MCS-2 containing the C0J56_05680-encoding gene, Kmr | This work |
| pBBR-C0J56_07270 | pBBR1MCS-2 containing the C0J56_07270-encoding gene, Kmr | This work |
| pBBR-C0J56_08575 | pBBR1MCS-2 containing the C0J56_08575-encoding gene, Kmr | This work |
| pBBR-C0J56_09590 | pBBR1MCS-2 containing the C0J56_09590-encoding gene, Kmr | This work |
| pBBR-C0J56_12235 | pBBR1MCS-2 containing the C0J56_12235-encoding gene, Kmr | This work |
| pBBR-C0J56_12315 | pBBR1MCS-2 containing the C0J56_12315-encoding gene, Kmr | This work |
| pBBR-C0J56_12915 | pBBR1MCS-2 containing the C0J56_12915-encoding gene, Kmr | This work |
| pBBR-C0J56_13325 | pBBR1MCS-2 containing the C0J56_13325-encoding gene, Kmr | This work |
| pBBR-C0J56_16540 | pBBR1MCS-2 containing the C0J56_16540-encoding gene, Kmr | This work |
| pBBR-C0J56_17160 | pBBR1MCS-2 containing the C0J56_17160-encoding gene, Kmr | This work |
| pBBR-C0J56_17610 | pBBR1MCS-2 containing the C0J56_17610-encoding gene, Kmr | This work |
| pBBR-C0J56_23535 | pBBR1MCS-2 containing the C0J56_23535-encoding gene, Kmr | This work |
| pBBR-C0J56_24425 | pBBR1MCS-2 containing the C0J56_24425-encoding gene, Kmr | This work |
| pBBR-C0J56_27365 | pBBR1MCS-2 containing the C0J56_27365-encoding gene, Kmr | This work |
| pBBR-C0J56_27925 | pBBR1MCS-2 containing the C0J56_27925-encoding gene, Kmr | This work |
| pBBR-C0J56_29290 | pBBR1MCS-2 containing the C0J56_29290-encoding gene, Kmr | This work |
| pBBR-C0J56_12915M | pBBR-C0J56_12915 derivative with GGDEF to GGAAF point mutation, Kmr | This work |
| pBBR-C0J56_13325M | pBBR-C0J56_13325 derivative with GGDEF to GGAAF point mutation, Kmr | This work |
| pBBR-C0J56_27925M | pBBR-C0J56_27925 derivative with GGDEF to GGAAF point mutation, Kmr | This work |
| Vc2-pRS414 | c-di-GMP biosensor plasmid, Apr | 21 |
| Vc2-pME6013 | c-di-GMP biosensor plasmid, Tetr | This work |
| p970Gm-rsmXp | rsmX-lacZ transcriptional fusion, Gmr | 18 |
| p970Gm-rsmX1p | rsmX1-lacZ transcriptional fusion, Gmr | 18 |
| p970Gm-rsmYp | rsmY-lacZ transcriptional fusion, Gmr | 18 |
| p970Gm-rsmZp | rsmZ-lacZ transcriptional fusion, Gmr | 18 |
| p970Gm-rgsAp | rgsA-lacZ transcriptional fusion, Gmr | 18 |
| p970Gm-phlAp | phlA-lacZ transcriptional fusion, Gmr | 18 |
| p6013-rsmAp | rsmA′-′lacZ translational fusion, Tetr | 18 |
| p6013-rsmEp | rsmE′-′lacZ translational fusion, Tetr | 18 |
| p2P24Km | Sucrose-based counterselectable plasmid, Kmr | 47 |
| p2P24-RsmA-FLAG | p2P24Km with a FLAG epitope sequence tagged to the C terminus of RsmA, Kmr | This work |
| p2P24-RsmE-FLAG | p2P24Km with a FLAG epitope sequence tagged to the C terminus of RsmE, Kmr | This work |
Ap, ampicillin; Gm, gentamicin; Km, kanamycin; Tet, tetracycline.
TABLE 4.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′–3′)a | Comment |
|---|---|---|
| yedQ-F | GCGAATTCAGCTTTTCATGAAACCGAGCTTGTTC | For cloning the yedQ gene |
| yedQ-R | ATGAGCTCTGCGCCCTTACTGGAGGTAATGG | |
| yhjH-F | ATGAATTCGGACATAGTCGTGAACCTGATCTG | For cloning the yhjH gene |
| yhjH-R | AAGGATCCAGTCAAAAGTTGCAATCAAAATGATC | |
| C0J56_00090-F | ATGGATCCGTAGGTTGAACAAAAGAAGTCGCG | For cloning the C0J56_00090-encoding gene |
| C0J56_00090-R | AATGAGCTCATCGAAGGAACAGTGGAAACGTC | |
| C0J56_00595-F | ATGTCGACTCGTTGAGGGAGTTCAGCAGAAG | For cloning the C0J56_00595-encoding gene |
| C0J56_00595-R | ATGGATCCAATGTCACTGGCGTCAGTATTTTC | |
| C0J56_01155-F | ATTAAGCTTACCCTCAAGCGCTGTG | For cloning the C0J56_01155-encoding gene |
| C0J56_01155-R | TAGAGCTCAAAGAGCACTTCGCCAT | |
| C0J56_01165-F | ATGAAGCTTGCGGTGGATGGCGGTCCACTCG | For cloning the C0J56_01165-encoding gene |
| C0J56_01165-R | ATGAATTCCTTACGTTCGATCTGGACCACC | |
| C0J56_02885-F | AGTCTAGATCGCGAAATCCACGTCCATG | For cloning the C0J56_02885-encoding gene |
| C0J56_02885-R | ATGGATCCACGACCGGGATGGCAACCCTTTG | |
| C0J56_03735-F | ATGAAGCTTCAATCACTTGAGTTCACGGTTC | For cloning the C0J56_03735-encoding gene |
| C0J56_03735-R | ATGGATCCAGGGCCTGGGTCGCTGCGCTC | |
| C0J56_04030-F | ATGGTACCCAATACACCGACGATCT | For cloning the C0J56_04030-encoding gene |
| C0J56_04030-R | ATTCTAGACCTGAGCAAGCATTCATT | |
| C0J56_05680-F | ATGGATCCATTGGCTGGCTGATTGGTGAGC | For cloning the C0J56_05680-encoding gene |
| C0J56_05680-R | ATGAGCTCTTGCGCACCAGGCGGTGCAC | |
| C0J56_07270-F | ATGAATTCGCTCCTTATGCCGGAATGGAG | For cloning the C0J56_07270-encoding gene |
| C0J56_07270-R | AAGGATCCCGCTTCAAGCCTCTTCGTTCGATC | |
| C0J56_08575-F | ATGGTACCTGGAGCGTTGCATGCAA | For cloning the C0J56_08575-encoding gene |
| C0J56_08575-R | TGCAAGCTTCAACGCGCAATAACTGC | |
| C0J56_09590-F | GCGAATTCAACGCGACATTATGTCTTGCGTC | For cloning the C0J56_09590-encoding gene |
| C0J56_09590-R | ATGAAGCTTCACAGTTCAGGCACTTGGCGATG | |
| C0J56_12235-F | ATGAGCTCACACAAAACGGGCGACATGTATTG | For cloning the C0J56_12235-encoding gene |
| C0J56_12235-R | ATGGATCCTATCGAGACCTGCACCCAGGGATC | |
| C0J56_12315-F | ATGAAGCTTCGATAGTAGCTGAACGACCTTG | For cloning the C0J56_12315-encoding gene |
| C0J56_12315-R | GTGAGCTCGGCCAAAGTCTTCAAGCTCAAG | |
| C0J56_12915-F | ATGAAGCTTCATCGGTCGGCGTCCTCGTCTTG | For cloning the C0J56_12915-encoding gene |
| C0J56_12915-R | ATGAATTCAATGTCCATACGAGCACCAGTC | |
| C0J56_13325-F | ACTCTAGAGCTTGTAAGGGGATTGAGCTTGC | For cloning the C0J56_13325-encoding gene |
| C0J56_13325-R | ATGAATTCGGCGGCGACACCGCCAAGGC | |
| C0J56_16540-F | ATGGATCCTGGACCTGCACTACATCGTGC | For cloning the C0J56_16540-encoding gene |
| C0J56_16540-R | AGGAAGCTTTCCCATGAACCAGAGTCCCTC | |
| C0J56_17160-F | AGGAATTCACGACTCGGAAATCGCC | For cloning the C0J56_17160-encoding gene |
| C0J56_17160-R | ATTCTAGACGCCTAGCACCTCATGG | |
| C0J56_17610-F | ATGGTACCTCAGTCTGTTGTCTGCCGGTG | For cloning the C0J56_17610-encoding gene |
| C0J56_17610-R | AGTCTAGAAGCTCGCCAGCACCCTGAAAC | |
| C0J56_23535-F | ATGAAGCTTCGTCATCGACCAGCAGCACCGTC | For cloning the C0J56_23535-encoding gene |
| C0J56_23535-R | AAGAGCTCGGTATCGTCCAGTGAGTC | |
| C0J56_24425-F | ATGAAGCTTCACATGGCAGGTGAGTCGGGTTTG | For cloning the C0J56_24425-encoding gene |
| C0J56_24425-R | TAGGATCCGTGCGCTATAGCGCTGCAGATG | |
| C0J56_27365-F | ATGAATTCGTCAGATCGAAGCCAAGGCG | For cloning the C0J56_27365-encoding gene |
| C0J56_27365-R | GATCTAGAACAGTTCAAGAAACAGTCGCGGGAG | |
| C0J56_27925-F | AAGGTACCAACGAGAGGCTCTAGCCCGCGCTTG | For cloning the C0J56_27925-encoding gene |
| C0J56_27925-R | ATGGATCCTTGTCGATGGCTGGCGCGTTAC | |
| C0J56_29290-F | ATGAAGCTTGCTGCCGGGGATTGAGTC | For cloning the C0J56_29290-encoding gene |
| C0J56_29290-R | AGTCTAGATGAAGCGGATGATTCGCAGTG | |
| C0J56_12915-GGAAFM-F | GATCGGTCGCCTGGGTGGTGCTGCGTTCGTGGCGTTG | Site-directed mutagenesis of the GGDEF domain of C0J56_12915 |
| C0J56_12915-GGAAFM-R | GCAGCACCACCCAGGCGACCGATCACATCACTTTC | |
| C0J56_13325-GGAAFM-F | GTGGCACGCCTGGGCGGTGCTGCATTTGTCATTATC | Site-directed mutagenesis of the GGDEF domain of C0J56_13325 |
| C0J56_13325-GGAAFM-R | GCAGCACCGCCCAGGCGTGCCACCAGATCAGTGT | |
| C0J56_27925-GGAAFM-F | CCTGGCGCGGCTGGGCGGGGCCGCGTTCACGGCGC | Site-directed mutagenesis of the GGDEF domain of C0J56_27925 |
| C0J56_27925-GGAAFM-R | GCGGCCCCGCCCAGCCGCGCCAGGATATCGAATG | |
| RsmA-FLAG-F1 | ATGAATTCGAGCACACGGTATACCTACAAG | For constructing the RsmA-FLAG fusion |
| RsmA-FLAG-R1 | GATTACAAGGACGACGATGACAAGTAATTTTTATCGATTTTTATGTTTGC | |
| RsmA-FLAG-F2 | CTTGTCATCGTCGTCCTTGTAATCATGGCTTGGTTCGTCGTCCTTCTCTT | |
| RsmA-FLAG-R2 | AGTCTAGATCGCCAAGGTCTCGATCGTCGGTG | |
| RsmE-FLAG-F1 | ATGGATCCGTCGGGTAATCCACCAC | For constructing the RsmE-FLAG fusion |
| RsmE-FLAG-R1 | CTTGTCATCGTCGTCCTTGTAATCGGGGGTTTGCGGCTTGTCGGGGGCGG | |
| RsmE-FLAG-F2 | GATTACAAGGACGACGATGACAAGTGAACATTGCAGCAGTCCGCAGCCAG | |
| RsmE-FLAG-R2 | GCGAATTCGGCGGACTGAAGTCTTA |
Restriction sites inserted in the primer for the cloning strategy are underlined.
Construction of the plasmids for overexpression of GGDEF/EAL domain proteins.
Overexpression studies utilized the broad-host-range plasmid pBBR1MCS-2 (42). Target genes were amplified by PCR using primers listed in Table S1 in the supplemental material. The successful construction of each plasmid was confirmed by sequencing and electroporation into P. fluorescens cells.
Site-directed mutagenesis.
Site-directed mutagenesis was performed in the plasmids pBBR-C0J56_12915, pBBR-C0J56_13325, and pBBR-C0J56_27925 using a site-directed mutagenesis system (Sangon Biotech, Shanghai, China) to substitute critical residues in the GGDEF domain (from GGDEF to GGAAF) of C0J56_12915, C0J56_13325, and C0J56_27925. Substitutions were confirmed by DNA sequencing.
Construction of c-di-GMP reporter plasmid.
To report the c-di-GMP level in P. fluorescens, plasmid Vc2-pMS414 (21) was digested with EcoRI and BamHI, and the resulting 369-bp restriction fragment was inserted into pME6013 at the corresponding sites, resulting in Vc2-p6013; in this construct, the 7th codon of the COG3070 open reading frame was fused in frame to the 5th codon of the lacZ reporter gene. Plasmid Vc2-p6013 was then electrotransformed into P. fluorescens 2P24 and its derivatives, and the β-galactosidase activity was determined using the Miller method (43).
Determination of the intracellular c-di-GMP concentration.
Quantitative analysis of intracellular c-di-GMP concentration was performed using ultrahigh-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), as described previously (44). Briefly, P. fluorescens 2P24 and its derivatives were inoculated with shaking in 40 ml of LB medium at 28°C until the optical density at 600 nm (OD600) of the bacterial culture was 1.0. One milliliter of bacterial culture was used for protein quantification by bicinchoninic acid (BCA) assay (TaKaRa). Twenty milliliters of cells was collected from each sample after centrifugation at 4°C for 15 min at 4,000 × g. The supernatant was removed and the pellet resuspended in 1 ml of extraction buffer (40% acetonitrile and 40% methanol in 0.1 N formic acid) and incubated at −20°C for 2 h. Cell debris was removed by centrifugation at 4°C for 5 min at 15,000 × g, and supernatants containing c-di-GMP were dried to remove lysis buffer. Dried samples were reconstituted in 100 μl of LC-MS-grade water and analyzed by UPLC-MS/MS. The intracellular concentration of c-di-GMP was calculated and normalized against protein levels.
Quantification of AHL.
The concentration of AHL was measured as previously described (22). In brief, strain 2P24 and its derivatives were grown in 20 ml LB medium at 28°C for 24 h. One milliliter of the culture was extracted with 0.8 ml ethyl acetate. The ethyl acetate extracts were then dried under vacuum and suspended in 100 μl methanol. For quantitative analysis of AHL, a portion (5 μl) was incubated with 0.2 ml Agrobacterium tumefaciens NTL4(pZLR4) (OD600 = 1.0) (45), and the mixture was incubated at 28°C for 2 h. The β-galactosidase activity was then measured as described previously (43).
Quantification of 2,4-DAPG production.
For quantitative analysis of 2,4-DAPG production, strain 2P24 and its derivatives were cultured in 20 ml KB liquid medium at 28°C for 24 h. 2,4-DAPG was extracted from the culture supernatant and assayed by HPLC according to the method described previously (46).
Swimming motility assay.
Swimming motility assays were determined on soft LB plates (0.2% agar). The centers of the plates were inoculated with 5-μl samples of overnight bacterial cultures. The plates were then incubated at 28°C. Every assay was performed at least three times with three replicates in each experiment.
Biofilm formation assay.
The quantification of biofilm formation was performed by a modified version of a previously described method (17). In brief, overnight bacterial cultures were inoculated 1:100 in LB in a 2-ml EP tube and cultured statically at 28°C for 2 days. Crystal violet (0.1%) was used to stain biofilm adhered to the tubes for 15 min. The tubes were washed gently three times with double-distilled water, the remaining crystal violet was fully dissolved in 200 μl of 95% ethanol, and the absorbance was detected at 570 nm. Experiments were repeated three times with nine replicates in each assay.
Construction of the FLAG-tagged strains and Western blot analysis.
To construct a C-terminal FLAG-RsmA/RsmE fusion, a PCR-generated fragment with the sequence 5′-GATTACAAGGACGACGATGACAAG-3′ inserted in frame to the 3′ end of the rsmA or rsmE gene was cloned into p2P24Km (47). The resulting plasmids were mobilized into strain 2P24 by electroporation, and chromosome FLAG-tagged mutants were selected after a two-step homologous recombination.
P. fluorescens cells were grown in LB at 28°C for 12 h, and 1-ml samples were taken. Cell lysates were then prepared by sonication in phosphate-buffered saline (PBS) buffer. The protein levels in cell lysates were quantified using the Bradford protein assay (Bio-Rad). The soluble fractions separated by 12% SDS-PAGE were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore). Blots were washed with PBS containing 0.05% Tween 20 and probed with an anti-Flag antibody (1:2,000). Anti-RNA polymerase monoclonal antibody (1:2,000) (Neoclone) was used as a control. Signals were then developed using the chemiluminescence detection kit (Thermo Fisher).
Rhizosphere colonization assays.
Root colonization assays were conducted as described previously (48). Briefly, strain 2P24 and its derivatives were labeled by streptomycin sulfate resistance (49). Surface-sterilized wheat seeds were soaked inside the cultures (108 CFU/ml) as described previously (50). The wheat seeds were then planted into a pot containing sterile soil or natural soil (not autoclaved). After 7, 14, and 21 days, soil was gently removed from roots, and population densities of the introduced strains were determined as described previously (51). The experiment was repeated three times, and population data, collected as CFU counts, were log10 transformed before statistical analysis.
Plant disease suppression assay.
The biocontrol assays were conducted as described previously (18). Tomato seedlings were uprooted carefully at the four-leaf stage, and the tips of the roots were cut off to create an injury. The roots were then dipped in a culture suspension of R. solanacearum GX02 (1 × 107 CFU/ml) for 1 h. The seedlings were placed in separate flasks containing sterile soil. P. fluorescens strains were added to soil in distilled water to give 1 × 107 CFU per gram of soil. Control flasks received the same amount of sterile water. Seedling survival was measured at regular intervals. The disease index was recorded as described previously (18): 0, no wilt symptoms; 1, wilt symptoms on 1 to 25% of the leaves; 2, wilt symptoms on 26 to 50% of the leaves; 3, wilt symptoms on 51 to 75% of the leaves; 4, wilt symptoms on more than 76% of the leaves.
Statistical analysis.
Data were tested for normality and analyzed using an unpaired Student's t test. Asterisks indicate P values (*, P < 0.05), and results are presented as the means ± standard deviations. Each experiment was performed three times with similar results.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by grants from the National Natural Science Foundation of China (31760533), the National Key Research and Development Program (2017YFD02011083), the Natural Science Foundation of Guangxi (2017GXNSFAA198341), and the Science and Technology Major Project of Guangxi (AA17204041). The funders had no role in study design.
We thank Ching-Hong Yang for thoughtful suggestions about this research.
We have no competing financial interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 3.Schirmer T, Jenal U. 2009. Structural and mechanistic determinants of c-di-GMP signalling. Nat Rev Microbiol 7:724–735. doi: 10.1038/nrmicro2203. [DOI] [PubMed] [Google Scholar]
- 4.Alm RA, Bodero AJ, Free PD, Mattick JS. 1996. Identification of a novel gene, pilZ, essential for type 4 fimbrial biogenesis in Pseudomonas aeruginosa. J Bacteriol 178:46–53. doi: 10.1128/jb.178.1.46-53.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sondermann H, Shikuma NJ, Yildiz FH. 2012. You’ve come a long way: c-di-GMP signaling. Curr Opin Microbiol 15:140–146. doi: 10.1016/j.mib.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breaker RR. 2011. Prospects for riboswitch discovery and analysis. Mol Cell 43:867–879. doi: 10.1016/j.molcel.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan RP, Tolker-Nielsem T, Dow JM. 2012. When the PilZ don’t work: effectors for cyclic di-GMP action in bacteria. Trends Microbiol 86:557–567. doi: 10.1016/j.tim.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Fang X, Ahmad I, Blanka A, Schottkowski M, Cimdins A, Galperin MY, Römling U, Gomelsky M. 2014. GIL, a new c-di-GMP-binding proteins domain involved in regulation of cellulose synthesis in enterobacteria. Mol Microbiol 93:439–452. doi: 10.1111/mmi.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roelofs KG, Jones CJ, Helman SR, Shang X, Orr MW, Goodson JR, Galperin MY, Yildiz FH, Lee VT. 2015. Systematic identification of cyclic-di-GMP binding proteins in Vibrio cholerae reveals a novel class of cyclic-di-GMP-binding ATPases associated with type II secretion systems. PLoS Pathog 11:e1005232. doi: 10.1371/journal.ppat.1005232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulesekara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. 2006. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3'-5')-cyclic-GMP in virulence. Proc Natl Acad Sci U S A 103:2839–2844. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solano C, García B, Latasa C, Toledo-Arana A, Zorraquino V, Valle J, Casals J, Pedroso E, Lasa I. 2009. Genetic reductionist approach for dissecting individual roles of GGDEF proteins within the c-di-GMP signaling network in Salmonella. Proc Natl Acad Sci U S A 106:7997–8002. doi: 10.1073/pnas.0812573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tischler AD, Camilli A. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 73:5873–5882. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobrov AG, Kirillina O, Ryjenkov DA, Waters CM, Price PA, Fetherston JD, Mack D, Goldman WE, Gomelsky M, Perry RD. 2011. Systematic analysis of cyclic-di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol Microbiol 79:533–551. doi: 10.1111/j.1365-2958.2010.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan RP, Fouhy Y, Lucey JF, Jiang B, He Y, Feng J, Tang J, Dow JM. 2007. Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris. Mol Microbiol 63:429–442. doi: 10.1111/j.1365-2958.2006.05531.x. [DOI] [PubMed] [Google Scholar]
- 15.Yi X, Yamazaki A, Biddle E, Zeng Q, Yang CH. 2010. Genetic analysis of two phosphodiesterases reveals cyclic diguanylate regulation of virulence factors in Dickeya dadantii. Mol Microbiol 77:787–800. doi: 10.1111/j.1365-2958.2010.07246.x. [DOI] [PubMed] [Google Scholar]
- 16.Wei HL, Wang Y, Zhang LQ, Tang WH. 2004. Identification and characterization of biocontrol bacterial strain 2P24 and CPF-10. Acta Phytopathol Sin 34:80–85. [Google Scholar]
- 17.Wei HL, Zhang LQ. 2006. Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 89:267–280. doi: 10.1007/s10482-005-9028-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang B, Wu XG, Zhang LQ. 2020. Characterization the role of GacA-dependent small RNAs and RsmA family proteins on 2,4-diacetylphloroglucinol production in Pseudomonas fluorescens 2P24. Microbiol Res 233:126391. doi: 10.1016/j.micres.2019.126391. [DOI] [PubMed] [Google Scholar]
- 19.Gjermansen M, Ragas P, Tolker-Nielsen T. 2006. Proteins with GGDEF and EAL domains regulate Pseudomonas putida biofilm formation and dispersal. Microbiol Res 265:215–224. doi: 10.1111/j.1574-6968.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang T, Cai Z, Shao X, Zhang W, Xie Y, Zhang Y, Hua C, Schuster SC, Yang L, Deng X. 2019. Pleiotropic effects of c-di-GMP content in Pseudomonas syringae. Appl Environ Microbiol 85:e00152-19. doi: 10.1128/AEM.00152-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudarsan N, Lee ER, Weinberg Z, Moy RH, Kim JN, Link KH, Breaker RR. 2008. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science 321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Q, Wu XG, Wei HL, Wang HM, Zhang LQ. 2009. Differential control of the PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24 by sigma factor RpoS and the GacS/GacA two-component regulatory system. Microbiol Res 164:18–26. doi: 10.1016/j.micres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Yan XX, Zhang LQ, Yang WZ, Tang WH. 2004. The role of regulatory gene gacA in the suppression of soil-borne disease by Pseudomonas fluorescens 2P24. Acta Phytopathol Sin 34:272–279. [Google Scholar]
- 24.Moscoso JA, Mikkelsen H, Heeb S, Williams P, Filloux A. 2011. The Pseudomonas aeruginosa sensor RetS switches type III and type VI secretion via c-di-GMP signalling. Mol Microbiol 13:3128–3138. [DOI] [PubMed] [Google Scholar]
- 25.Allsopp LP, Wood TE, Howard SA, Maggiorelli F, Nolan LM, Wettstadt S, Filloux A. 2017. RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 114:7707–7712. doi: 10.1073/pnas.1700286114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhang W, Wu X, Zhang LQ. 2013. Effect of retS gene on biosynthesis of 2,4-diacetylphloroglucinol in Pseudomonas fluorescens 2P24. Acta Microbiol Sin 53:118–126. [PubMed] [Google Scholar]
- 27.Haas D, Défago D. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 28.Vakulskas CA, Potts AH, Babitzke P, Ahmer BM, Romeo T. 2015. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev 79:193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeb S, Haas D. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact 14:1351–1363. doi: 10.1094/MPMI.2001.14.12.1351. [DOI] [PubMed] [Google Scholar]
- 30.Petrova OE, Sauer K. 2010. The novel two-component regulatory system BfiSR regulates biofilm, development by controlling the small RNA rsmZ through CafA. J Bacteriol 192:5275–5288. doi: 10.1128/JB.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi K, Yamada K, Haas D. 2018. ppGpp controlled by the Gac/Rsm regulatory pathway sustains biocontrol activity in Pseudomonas fluorescens CHA0. Mol Plant Microbe Interact 31:274–282. doi: 10.1094/MPMI-05-17-0120-R. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarty S, Melton CN, Bailin A, Yahr TL, Anderson GG. 2017. Pseudomonas aeruginosa magnesium transporter MgtE inhibits type III secretion system gene expression by stimulating rsmYZ transcription. J Bacteriol 199:e00268-17. doi: 10.1128/JB.00268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stacey SD, Williams DA, Pritchett CL. 2017. The Pseudomonas aeruginosa two-component regulator AlgR directly activates rsmA expression in a phosphorylation-independent manner. J Bacteriol 199:e00048-17. doi: 10.1128/JB.00048-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peñaloza-Vázquez A, Fakhr MK, Bailey AM, Bender CL. 2004. AlgR functions in algC expression and virulence in Pseudomonas syringae pv. syringae. Microbiology 150:2727–2737. doi: 10.1099/mic.0.27199-0. [DOI] [PubMed] [Google Scholar]
- 35.Moscoso JA, Jaeger T, Valentini M, Hui K, Jenal U, Filloux A. 2014. The diguanylate cyclase SadC is a central player in Gac/Rsm-mediated biofilm formation in Pseudomonas aeruginosa. J Bacteriol 196:4081–4088. doi: 10.1128/JB.01850-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Q, Leclercq S, Bhasme P, Xu A, Zhu B, Zhang Y, Zhang M, Wang S, Ma LZ. 2019. Diguanylate cyclases and phosphodiesterases required for basal-level c-di-GMP in Pseudomonas aeruginosa as revealed by systematic phylogenetic and transcriptomic analyses. Appl Environ Microbiol 85:e01194-19. doi: 10.1128/AEM.01194-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navazo A, Barahona E, Redondo-Nieto M, Martínez-Granero F, Rivilla R, Martín M. 2009. Three independent signaling pathways repress motility in Pseudomonas fluorescens F113. Microb Biotechnol 2:489–498. doi: 10.1111/j.1751-7915.2009.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah N, Gislason AS, Becker M, Belmonte MF, Fernando WGD, de Kievit TR. 2020. Investigation of the quorum-sensing regulon of the biocontrol bacterium Pseudomonas chlororaphis strain PA23. PLoS One 15:e0226232. doi: 10.1371/journal.pone.0226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 40.King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307. [PubMed] [Google Scholar]
- 41.Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nester EW. 1974. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A 71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 43.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 44.Wu X, Zeng Q, Koestler BJ, Waters CM, Sundin GW, Hutchins W, Yang CH. 2014. Deciphering the components that coordinately regulate virulence factors of the soft rot pathogen Dickya dadantii. Mol Plant Microbe Interact 27:1119–1131. doi: 10.1094/MPMI-01-14-0026-R. [DOI] [PubMed] [Google Scholar]
- 45.Cha C, Gao P, Chen YC, Shaw PD, Farrand SK. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant Microbe Interact 11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 46.Shanahan P, O'Sullivan DJ, Simpson P, Glennon JD, O'Gara F. 1992. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol 58:353–358. doi: 10.1128/AEM.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Zhang Y, Zhang B, Wu X, Zhang LQ. 2018. Effect of carbon sources on production of 2,4-diacetylphloroglucinol in Pseudomonas fluorescens 2P24. Acta Microbiol Sin 58:1202–1212. [Google Scholar]
- 48.Wu X, Duan H, Tian T, Yao N, Zhou H, Zhang L. 2010. Effect of the hfq gene on 2,4-diacetylphloroglucinol production and the PcoI/PcoR quorum-sensing system in Pseudomonas fluorescens 2P24. FEMS Microbiol Lett 309:16–24. doi: 10.1111/j.1574-6968.2010.02009.x. [DOI] [PubMed] [Google Scholar]
- 49.Weller DM. 1983. Colonization of wheat roots by a fluorescent pseudomonad suppressive to take-all. Phytopathology 73:1548–1553. doi: 10.1094/Phyto-73-1548. [DOI] [Google Scholar]
- 50.Pierson EA, Wood DW, Cannon JA, Blachere FM, Pierson LS III. 1998. Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol Plant Microbe Interact 11:1078–1084. doi: 10.1094/MPMI.1998.11.11.1078. [DOI] [Google Scholar]
- 51.Hoben HJ, Somasegaran P. 1982. Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl Environ Microbiol 44:1246–1247. doi: 10.1128/AEM.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newell PD, Yoshioka S, Hvorecny KL, Monds RD, O'Toole GA. 2011. Systematic analysis of diguanylate cyclases that promoter biofilm formation by Pseudomonas fluorescens Pf0-1. J Bacteriol 193:4685–4698. doi: 10.1128/JB.05483-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malone JG, Jaeger T, Spangler C, Ritz D, Spang A, Arrieumerlou C, Kaever V, Landmann R, Jenal U. 2010. YfiBNR mediates cyclic di-GMP dependent small colony variant formation and persistence in Pseudomonas aeruginosa. PLoS Pathog 6:e1000804. doi: 10.1371/journal.ppat.1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hickman JW, Tifrea DF, Harwood CS. 2005. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci U S A 102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hay ID, Remminghorst U, Rehm BHA. 2009. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl Environ Microbiol 75:1110–1120. doi: 10.1128/AEM.02416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.