Enterohemorrhagic E. coli (EHEC) can cause serious illness and deaths in humans by producing toxins that can severely damage our intestines and kidneys. There is currently no optimal treatment for EHEC infections, as antibiotics can worsen disease development. Consequently, the need for new treatment options is urgent. Environmental factors in our intestines can affect the virulence of EHEC and help our bodies fight EHEC infections. The ruminant intestine, the main reservoir for EHEC, contains high levels of vitamin K, but the levels are variable in humans. This study shows that vitamin K analogs can inhibit the growth of EHEC and/or production of its main virulence factor, the Shiga toxin. They may also inhibit the spreading of the Shiga toxin encoding bacteriophage. Our findings indicate that vitamin K analogs have the potential to suppress the development of serious disease caused by EHEC.
KEYWORDS: EHEC, Escherichia coli, HUS, microbiology, Shiga toxin, vitamin K, enterohemorrhagic E. coli, enteropathogen, foodborne pathogens, virulence
ABSTRACT
Enterohemorrhagic Escherichia coli (EHEC) causes serious foodborne disease worldwide. It produces the very potent Shiga toxin 2 (Stx2). The Stx2-encoding genes are located on a prophage, and production of the toxin is linked to the synthesis of Stx phages. There is, currently, no good treatment for EHEC infections, as antibiotics may trigger lytic cycle activation of the phages and increased Stx production. This study addresses how four analogs of vitamin K, phylloquinone (K1), menaquinone (K2), menadione (K3), and menadione sodium bisulfite (MSB), influence growth, Stx2-converting phage synthesis, and Stx2 production by the EHEC O157:H7 strain EDL933. Menadione and MSB conferred a concentration-dependent negative effect on bacterial growth, while phylloquinone or menaquinone had little and no effect on bacterial growth, respectively. All four vitamin K analogs affected Stx2 phage production negatively in uninduced cultures and in cultures induced with either hydrogen peroxide (H2O2), ciprofloxacin, or mitomycin C. Menadione and MSB reduced Stx2 production in cultures induced with either H2O2 or ciprofloxacin. MSB also had a negative effect on Stx2 production in two other EHEC isolates tested. Phylloquinone and menaquinone had, on the other hand, variable and concentration-dependent effects on Stx2 production. MSB, which conferred the strongest inhibitory effect on both Stx2 phage and Stx2 production, improved the growth of EHEC in the presence of H2O2 and ciprofloxacin, which could be explained by the reduced uptake of ciprofloxacin into the bacterial cell. Together, the data suggest that vitamin K analogs have a growth- and potential virulence-reducing effect on EHEC, which could be of therapeutic interest.
IMPORTANCE Enterohemorrhagic E. coli (EHEC) can cause serious illness and deaths in humans by producing toxins that can severely damage our intestines and kidneys. There is currently no optimal treatment for EHEC infections, as antibiotics can worsen disease development. Consequently, the need for new treatment options is urgent. Environmental factors in our intestines can affect the virulence of EHEC and help our bodies fight EHEC infections. The ruminant intestine, the main reservoir for EHEC, contains high levels of vitamin K, but the levels are variable in humans. This study shows that vitamin K analogs can inhibit the growth of EHEC and/or production of its main virulence factor, the Shiga toxin. They may also inhibit the spreading of the Shiga toxin encoding bacteriophage. Our findings indicate that vitamin K analogs have the potential to suppress the development of serious disease caused by EHEC.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) is a zoonotic pathogen responsible for food- and waterborne outbreaks of bloody diarrhea and hemolytic uremic syndrome (HUS), a disease with severe complications and 2 to 5% fatality (1). The World Health Organization (WHO) estimates that 10% of patients with EHEC infection develop HUS. EHEC infections affect young children most severely and are difficult to treat, as administration of antibiotics may worsen the disease (1). The Shiga toxin (Stx) is considered the main virulence factor of EHEC. Stx binds to the globotriaosylceramide (Gb3) receptor, a glycolipid particularly abundant on kidney cells that is also present on endothelial cells and in the brain (2, 3). It causes cell damage by inhibiting protein synthesis in its target cells, which is the main cause of the development of HUS and neurological symptoms during an EHEC infection (4).
There are two antigenically distinct main types of Stx. Stx1, produced by Shigella dysenteriae and some Stx-producing E. coli (STEC) strains, and Stx2, which is produced mainly by STEC/EHEC (5). Epidemiological data indicate that STEC strains that produce Stx2 are more strongly associated with severe human disease than those that produce only Stx1 (6–9). Stx2 is encoded by a chromosomally integrated (lysogenic) Stx-converting prophage (Stx phage) (10, 11). DNA damage, including that induced by antibiotics or reactive oxygen species (ROS), will trigger the bacterial SOS response. This induces the lysogenic Stx2 phage to enter the lytic (proliferative) cycle, leading to the synthesis and release of phage particles and Stx2 (12, 13). Single STEC cells in a population can start the production of Stx phages even in the absence of an external trigger. This phenomenon is called “spontaneous prophage induction” and occurs at different frequencies in different STEC strains (14).
Ruminants are considered the major reservoir of EHEC (reviewed in reference 15). Adult cattle, with a mature rumen and ruminal microbiota, are usually unaffected by EHEC colonization (16). Neonatal calves (<28 days old) may, however, develop symptoms from exposure to EHEC, such as enterocolitis (17).
It is unknown why EHEC infections manifest differently among infected individuals. It has been reported that various small molecules in the gastrointestinal tract influence the pathogenicity of EHEC. Fucose, cleaved from mucins, inhibits EHEC adhesion to the human epithelial cells (18), succinate enhances EHEC adhesion to human epithelial cells (19), vitamin B12 enhances Stx production (20), vitamin A deficiency exacerbates damage to the intestine and increases EHECs survival in mice (21), vitamin D strengthens tight junctions and, consequently, the intestinal barrier function (22), vitamin B7 (biotin) influences the target site for colonization in the human intestine (23), and manganese blocks intracellular trafficking of Stx and protects against Shiga toxicosis (24).
Vitamin K occurs in various forms and at various levels in the human intestine. The amount of vitamin K present in the intestine is influenced by the diet, the composition of the gut microbiota, and the age of the infected individual (25–27). Vitamin K exists in three main forms, phylloquinone (vitamin K1), menaquinone (vitamin K2), and menadione (vitamin K3) (Fig. 1). Phylloquinone is found in all organisms that perform photosynthesis, as it acts as an electron acceptor in photosystem 1 (28). Therefore, it is available to humans through the diet; fruits and leafy green vegetables are particularly rich in phylloquinone (29–32). Vitamin K is particularly abundant in the intestine of ruminants, mostly since they acquire nutrients from plant-based food, especially grass, which has a high content of phylloquinone, but also because the ruminal microbiota produces large amounts of menaquinone (33). Menaquinone is bacterially produced and, therefore, can be present in fermented foods such as cheese and yogurt (26, 34). Menaquinone is also present in meats and organ meats, as phylloquinone is converted to menaquinone in tissues (35). The human intestinal microbiota also contains bacterial species that produce menaquinones (25, 36). Menadione is the simplest form of vitamin K and an intermediate in the biosynthesis of menaquinone in bacteria (37). It is also an intermediate molecule in the metabolic conversion of phylloquinone to menaquinone in the metabolism of vertebrates (37). Furthermore, menadione is the main vitamin K analog found in enterocytes (38–40). Different forms of menadione, such as menadione and menadione sodium bisulfite (MSB), can also be produced synthetically.
FIG 1.
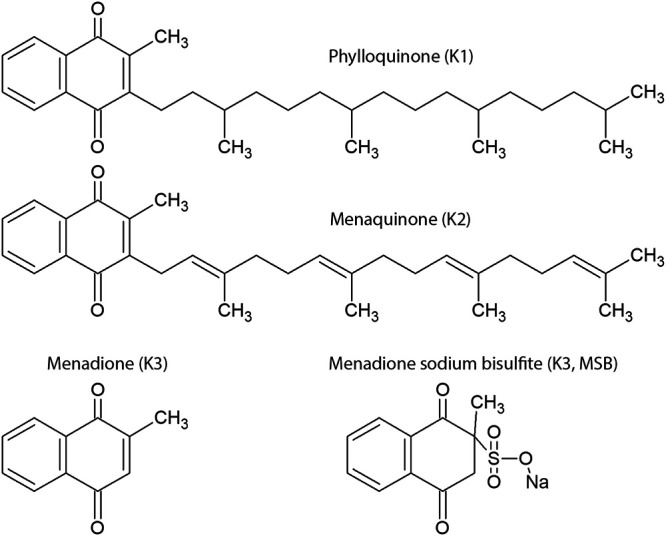
Chemical structure of phylloquinone (vitamin K1), menaquinone (vitamin K2), menadione (vitamin K3), and menadione sodium bisulfite (vitamin K3).
The levels of different types of vitamin K that are present in the intestine, enterocytes, and blood varies between individuals and during different times of life (25). Vitamin K deficiency is quite uncommon in adult humans, but subclinical deficiency can easily be induced by limiting phylloquinone intake and by treatment with antibiotics (41, 42). Although healthy children rarely suffer from vitamin K deficiency, defined with respect to blood clotting, the blood levels of vitamin K in children are much lower than those in adults (43, 44). It has also been reported that menadione inhibits growth and exotoxin production by Staphylococcus aureus, Bacillus anthracis, Streptococcus pyogenes, and Streptococcus agalactiae at a concentration of 10 to 200 μg/ml (45). This was, however, not observed for menaquinone and phylloquinone (45). Furthermore, it was previously shown that menadione exhibits an antibacterial activity against the gastric pathogen Helicobacter pylori (46).
Treatment recommendations for EHEC infections are still mainly supportive in character, despite the serious nature of the disease. As antibiotic treatment remains a controversial issue, there is a dire need for alternative treatment procedures that can restrict the production of Stx and decrease the risk of developing HUS. In this work, we have studied how different vitamin K analogs influence the growth and virulence potential of EHEC. The rationale for studying the role of vitamin K in the complex interplay between EHEC and their Stx phages lies in the fact that vitamin K is particularly abundant in the intestine of ruminants, the main natural habitat for EHEC, and that it is a relevant biomolecule in the human intestine. Additionally, menadione has been shown to have an antibacterial and/or antivirulence effect on several other pathogenic bacteria. Our study addresses how the four vitamin K analogs, phylloquinone, menaquinone, menadione, and menadione sodium bisulfite (MSB), influence growth, Stx2 production, Stx2 phage release, and bacterial survival during the induction of the phage lytic cycle, using the EHEC O157:H7 strain EDL933, which carries the Stx2-converting bacteriophage BP933W, as a model organism. The effect of MSB was also analyzed for the Norwegian EHEC O103:H7 outbreak strain NIPH-11060424 and the O157:H7 NVH-E7 strain.
RESULTS
The effect of vitamin K analogs on growth of EHEC.
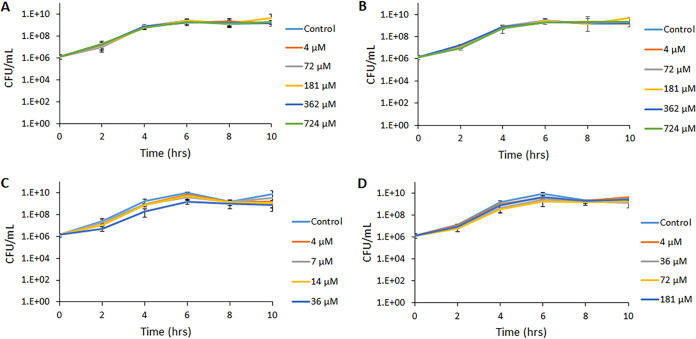
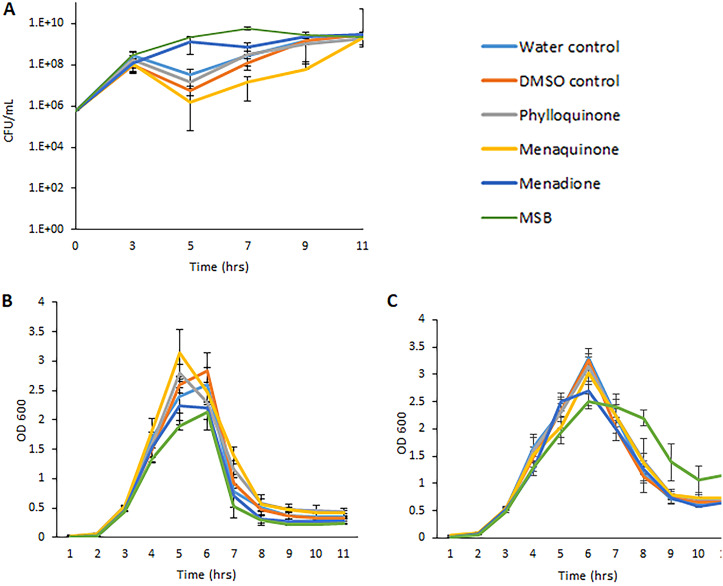
As a first step to explore the effect of different vitamin K analogs on EHEC, we cultured the EHEC strain EDL933 in the presence and absence of different concentrations of phylloquinone, menaquinone, menadione bisulfite (MSB), or menadione. As shown in Fig. 2A and B and Table S1 in the supplemental material, phylloquinone and menaquinone had no discernible effect on bacterial growth, as measured by the number of CFU per milliliter, at all concentrations tested, except for the highest concentration of phylloquinone (724 μM), which reduced the maximum rate of growth (Vmax) by 25% compared to that of the negative control (culture without phylloquinone). The presence of menadione did not influence Vmax significantly at any of the concentrations tested. However, the presence of 36 μM menadione caused a significant reduction of the number of CFU per milliliter at both 2 and 6 h of growth (Fig. 2C and Table S1). MSB reduced the Vmax significantly at all concentrations from 36 μM and above (Fig. 2D and Table S1). Similar results were obtained with strains EDL933, NIPH-11060424, and O157:H7 NVH-E7 when growth was assessed by measuring the optical density at 600 nm (OD600) (Fig. S1A to D and S2 and Table S1).
FIG 2.
Effect of four different vitamin K variants on the growth of EDL933, as measured by the increase in the number of CFU/ml. (A) Phylloquinone; (B) menaquinone; (C) menadione; (D) MSB. The solvents used for solubilization of the different types of vitamin K were used as negative controls. DMSO was used at a concentration of 0.05%. Results are given as means from three independent experiments, with bars showing ± standard deviations (SD).
Effect of vitamin K analogs on Stx production.
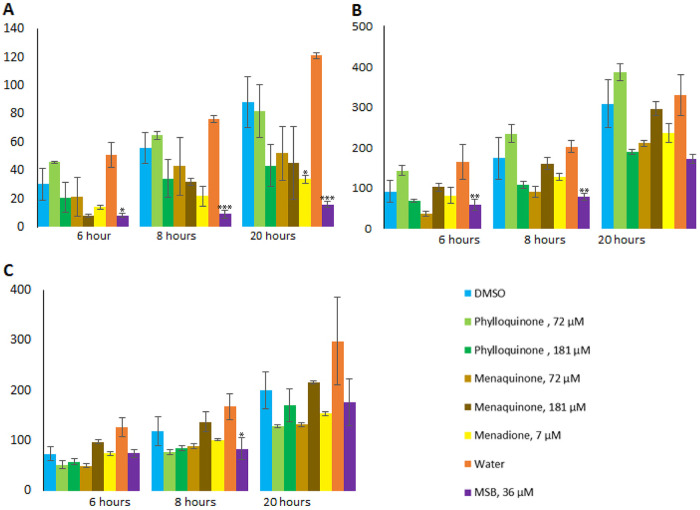
To investigate how different vitamin K analogs influence the production of Stx2, we grew EDL933 in the presence or absence of phylloquinone, menaquinone, MSB, or menadione. After 3 h of growth, BP933W was induced to enter the lytic cycle, with concomitant Stx2 expression, with either H2O2, ciprofloxacin, or MMC. The total level of Stx2 released in bacterial cultures 6, 8, and 20 h postinduction was assessed by liquid chromatography-tandem mass spectrometry (LC-MS/MS), and the effect of vitamin K was determined through comparison with cultures induced with the same agents but without vitamin K. In uninduced cultures (i.e., no added inducing compound), the level of Stx2 was below the detection level (i.e., below 10 ng/ml). Without added vitamin K analogs, ciprofloxacin-induced cultures showed a higher level of released Stx2 than MMC- and H2O2-induced cultures (Fig. 3A to C).
FIG 3.
Stx2 production by EDL933 in the presence or absence of vitamin K analogs. Stx2 production under H2O2-induced (A), ciprofloxacin-induced (B), and MMC-induced (C) conditions, in the presence or absence of menadione, MSB, menaquinone, or phylloquinone, was measured by LC-MS/MS. The error bars represent the SD from three independent experiments. An asterisk indicates statistically significant difference (P < 0.05) in Stx2 levels compared to negative-control cultures with the same inducing agent and same solvent as those used for solubilization of the vitamin K variants, i.e., DMSO or water. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test).
Of the vitamin K analogs tested, MSB demonstrated the strongest reducing effect on Stx2 production. In the presence of MSB, Stx2 production was significantly reduced in samples collected 6, 8, and 20 h postinduction with H2O2 (between 81 and 86% reduction), 6 and 8 h postinduction with ciprofloxacin (56 and 60%, respectively), and 8 h postinduction with MMC (37%) (Fig. 3A to C and Table S3). Menadione-treated samples from 20 h postinduction with H2O2 also showed a 61% reduction in Stx2 production (Fig. 3A and Table S3).
No significant inhibitory effect was seen for the other combinations of inducing agents and vitamin K analogs tested, although cultures containing menaquinone or phylloquinone showed a dose-dependent effect on Stx2 production during induction with H2O2 and ciprofloxacin. The highest concentration (181 μM) of phylloquinone showed a significantly inhibitory effect (47% to 55%) on Stx2 production in H2O2- and ciprofloxacin-induced samples compared to lower concentrations (Fig. 3A and B). Such an effect was not observed in samples induced with MMC (Fig. 3C). In contrast to phylloquinone, the lower concentration of menaquinone showed a trend toward a stronger inhibitory effect on Stx2 production compared to that of the higher concentration in samples induced with ciprofloxacin and MMC (Fig. 3B and C).
The effect of MSB on Stx release was also tested for EHEC O103:H25 NIPH-11060424 and EHEC O157:H7 NVH-E7 using a VTEC-RPLA kit. Similar to what was observed for strain EDL933, these strains showed approximately 2- and 6-fold reduced Stx production during treatment with MSB (Fig. S3A).
The effect of vitamin K on production of the Stx2-converting phage BP933W.
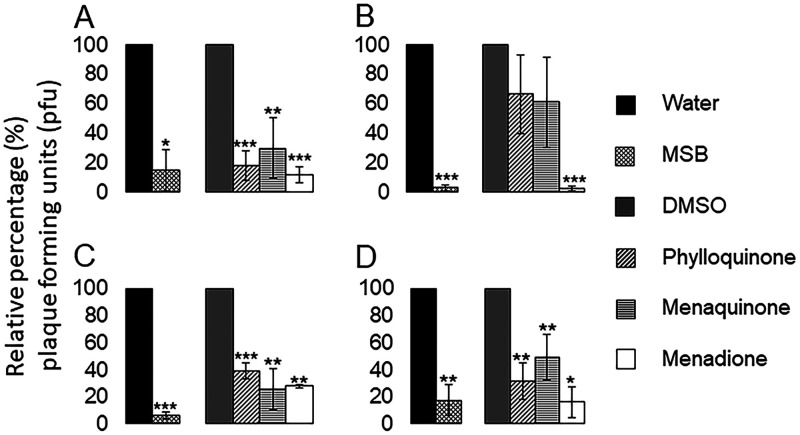
As Stx2 production is linked to the induction of the lytic cycle and release of Stx-converting phages, we wanted to test how different vitamin K analogs influence phage production under uninduced conditions and after induction with either H2O2, ciprofloxacin, or MMC in EDL933. By using a plaque assay for phage enumeration, we found that MMC acted as the most efficient inducer of BP933W production, while H2O2 and ciprofloxacin demonstrated similar, but lower, induction capabilities (Fig. S4). All vitamin K analogs tested reduced the BP933W titer in uninduced cultures, and the strongest reducing effect was observed in cultures containing menadione or MSB (88.4% ± 5.6% and 85.0% ± 13.9%, respectively) (Fig. 4A). In H2O2-induced cultures, the presence of phylloquinone or menaquinone resulted in variable and much weaker reducing effects on the BP933W titer (33.4% ± 26.9% and 39.0% ± 30.8%, respectively) than H2O2-induced cultures containing menadione or MSB (97.6% ± 1.6% and 97.2% ± 1.7%, respectively) (Fig. 4B). MSB also exhibited a strong inhibitory effect on BP933W production in ciprofloxacin-induced cultures (94.2% ± 2.5%), while the other vitamin K analogs showed similar inhibitory effects (from 60% to 74% inhibition) (Fig. 4C). Of the vitamin K analogs tested, menadione and MSB showed the strongest inhibitory effects on BP933W titers in cultures induced with MMC (83.8% ± 11.4% and 82.4% ± 11.1%, respectively) (Fig. 4D).
FIG 4.
Effect of vitamin K analogs on production of BP933W. The level of BP933W produced by EDL933 was investigated under uninduced (A), H2O2-induced (B), ciprofloxacin-induced (C), and MMC-induced (D) conditions, in the presence or absence of different types of vitamin K analogs. The concentrations of vitamin K analogs used were 36 μM for MSB, 72 μM for phylloquinone, 72 μM for menaquinone, and 7 μM for menadione. The error bars represent the SD from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student's t test).
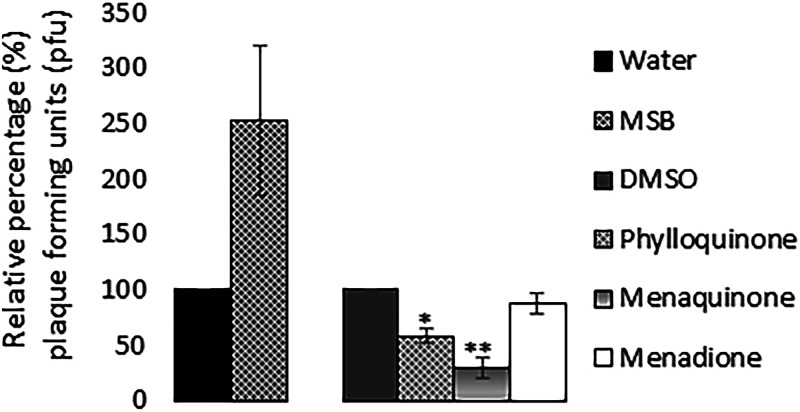
The reduced BP933W titers observed in cultures containing vitamin K analogs could be due to either reduced bacterial growth, reduced synthesis of phages, or a direct effect of these compounds on released phages, affecting their ability to infect the recipient E. coli strain. To determine the effect of vitamin K analogs on the infectivity of BP933W, phage filtrates were incubated with high concentrations of phylloquinone, menaquinone, menadione, or MSB before they were used in the plaque assay. The results showed that both menaquinone and phylloquinone reduced the infectivity of BP933W, i.e., reduced plaque formation. MSB did, on the other hand, cause an increased infectivity of the Stx-converting phage, while no positive or negative effect on phage infectivity was observed for menadione (Fig. 5).
FIG 5.
Effect of the four vitamin K analogs on the infectivity of BP933W on E. coli DH5α. The concentrations of vitamin K analogs used were 362 μM for MSB, 724 μM for phylloquinone, 724 μM for menaquinone, and 72 μM for menadione, and the phage filtrate was treated with the analogs for 2 h. The error bars represent the SD from three independent experiments. *, P < 0.05; **, P < 0.01 (Student's t test).
Effect of vitamin K on stx2 and recA transcription.
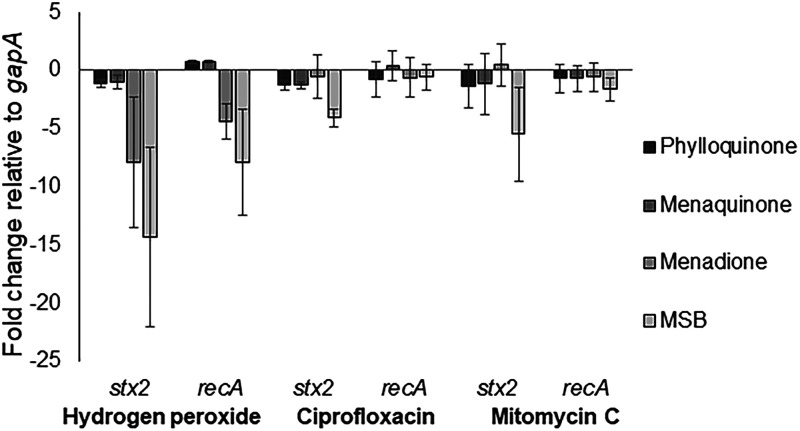
Since vitamin K analogs inhibited the production of BP933W, we wanted to explore if they affected the induction of the SOS response and, thereby, stx2 transcription. EDL933 was grown in the presence or absence of vitamin K analogs and induced with either H2O2, ciprofloxacin, or MMC. Quantitative real-time PCR (qRT-PCR) was used to examine the effect of the different vitamin K analogs on recA (indicative on SOS response activation) and stx2 transcription 2 h postinduction. The results from the qRT-PCR analyses are shown in Fig. 6.
FIG 6.
Relative fold change in transcript levels of stx2 and recA in vitamin K-treated EHEC cultures compared to untreated cultures. The concentrations of vitamin K used were 72 μM for phylloquinone and menaquinone, 36 μM for MSB, and 7 μM for menadione. Data represent means from three individual experiments. The error bars represent the SD from three independent experiments.
Both MSB and menadione had a reducing effect on recA transcription when H2O2 was used as the phage-inducing agent. At the examined time point, the different vitamin K analogs had little or no effect on recA transcription during ciprofloxacin or MMC treatments. MSB had a reducing effect on stx2 transcription, regardless of the inducing agent, while menadione only showed a reducing effect on stx2 transcription during induction with H2O2. Phylloquinone and menaquinone did not confer any noticeable effect on stx2 or recA expression at the time point tested, regardless of phage-inducing agent used (Fig. 6). MSB also conferred an inhibitory effect on both stx2 and recA transcription in EHEC strains NIPH-11060424 and NVH-E7 (Fig. S3B).
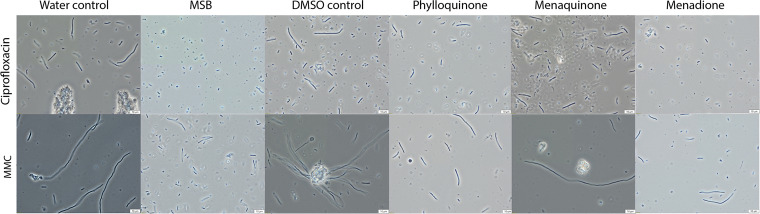
The presence of menadione or MSB prevents antibiotic-induced morphological changes.
Previous reports have shown that exposure to ciprofloxacin and mitomycin induces morphological and biochemical changes in E. coli cells (47, 48). Similar to these reports, we observed that EHEC cells demonstrated elongated (filamentous) morphology when grown in the presence of ciprofloxacin or mitomycin. Ciprofloxacin-induced samples containing menadione or MSB did, on the other hand, contain fewer filamentous cells or much shorter filaments (Fig. 7). MMC-treated cells showed an even more elongated appearance than ciprofloxacin-treated cells; also, the presence of menadione or MSB reduced the level of cell elongation. Cells induced with H2O2 did not show a markedly different morphology, and there was no obvious difference between H2O2-induced samples with or without vitamin K (data not shown).
FIG 7.
Microscopy analysis of EHEC cultured with four vitamin K variants and their respective controls induced with ciprofloxacin and mitomycin C. Slightly different shades were observed between quadrants in all images. The reason for this is a mismatch in the graphic board requirements between the camera and the laboratory computer. This artifact does not influence the aim of this figure, which is to show differences in cell morphology.
Menadione and MSB influence the survival of EHEC in the presence of phage-inducing agents.
As some vitamin K analogs had a negative effect on the production of BP933W, it seemed likely that these compounds also prevent phage-mediated lysis of the bacterial cells (i.e., bacterial death). A decline in bacterial growth, as measured by the number of CFU per milliliter, was observed in all cultures immediately after addition of H2O2. The decline in bacterial growth was, however, much less pronounced in cultures containing menadione or MSB (Fig. 8A). As the exposure of EHEC to ciprofloxacin or MMC made EHEC grow into unseptated filaments, it was not relevant to measure bacterial growth in cultures containing these antibiotics by counting the number of CFU per milliliter, since one filament containing multiple bacterial genomes will count as one colony. As the measurement of optical density provided results similar to those of measurement of the number of CFU per milliliter (Fig. 2 and Fig. S1B and C), the effect of vitamin K analogs on bacterial survival was determined by the measurement of OD600 in cultures containing ciprofloxacin or MMC. The addition of 1× MIC of ciprofloxacin or 0.5 μg/ml of MMC did not confer an immediate negative effect on bacterial growth (Fig. 8B and C). The presence of MSB slowed down growth after induction with ciprofloxacin (Table S5). After 5 to 6 h of growth, there was a strong decline in OD600 in both ciprofloxacin- and MMC-induced cultures, indicating cell lysis (Fig. 8B and C). In MMC-induced cultures, MSB seemed to have a slight positive effect on bacterial survival (Fig. 8B). This was not seen for the other types of vitamin K tested. MSB also conferred a positive effect on bacterial survival when strains NIPH-11060424 and NVH-E7 were treated with 0.5 μg/ml MMC in the presence or absence of 72 μM MSB (Fig. S5).
FIG 8.
Effect of vitamin K analogs on the growth and survival of strain EDL933 in the presence of H2O2, ciprofloxacin, and MMC. (A) H2O2 (measured by the increase in the number of CFU/ml); (B) ciprofloxacin (measured by the increase in OD600); (C) MMC (measured by the increase in OD600). Phylloquinone and menaquinone were used at a concentration of 72 μM, menadione at a concentration of 7 μM, and MSB at a concentration of 36 μM. The results are given as means from three independent experiments, with bars showing ±SD.
MSB reduces uptake of ciprofloxacin into the bacterial cells.
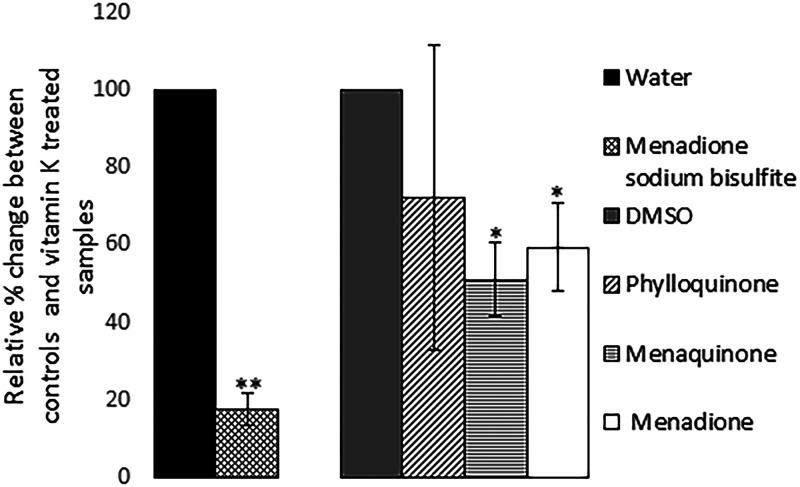
To test whether the vitamin K analogs decreased phage and/or Stx2 production by reducing the uptake of ciprofloxacin into the bacterial cell, we cultured strain EDL933 in the presence or absence of different types of vitamin K and induced the cultures with 1× MIC (0.06 μg/ml) of ciprofloxacin. The presence of MSB in the growth medium caused an approximately 6× reduction in the intracellular level of ciprofloxacin. Both menaquinone and menadione reduced uptake of ciprofloxacin (48.9% ± 9.6% and 40.5% ± 11.4%, respectively). No significant effect on ciprofloxacin uptake was observed for phylloquinone (P = 0.42) (Fig. 9).
FIG 9.
Influence of vitamin K on the intracellular concentration of ciprofloxacin. The concentrations of vitamin K used were 72 μM for phylloquinone and menaquinone, 36 μM for MSB, and 7 μM for menadione. The error bars represent the SD from three independent experiments. *, P < 0.05; **, P < 0.01 (Student's t test).
DISCUSSION
In this work, we have studied the effect of four vitamin K analogs on the growth, Stx2 production, infectivity of the Stx2 phage, and survival of the EHEC O157:H7 strain EDL933 during the induction of the phage lytic cycle. The study was done as part of the search for novel treatment regimens for EHEC infections. Our strategy was to study the vitamin K analogs, as previous data have shown that these biomolecules could limit the growth and virulence of other pathogenic bacteria. First, we showed that two of the vitamin K analogs tested, menadione and MSB, inhibited growth of strain EDL933, while the presence of phylloquinone or menaquinone did not seem to have a pronounced effect on growth under the tested conditions. Menadione generates reactive oxygen species, such as superoxide anions (O2−), which are toxic for the bacteria, and this could explain the negative effect of these compounds on bacterial growth (49).
The effects of the various vitamin K analogs on the production of the Stx2-converting phage and Stx2 were tested under uninduced conditions and by using H2O2, ciprofloxacin, or MMC as phage-inducing agents. H2O2 represents a natural inducing agent in the host, as it is produced by neutrophils and other cells in infected humans and by protists that prey on bacterial cells (50, 51). Ciprofloxacin is clinically relevant, as it is used to treat different types of E. coli infections, but for fear that antibiotic treatment will exacerbate the symptoms, it is not used to cure EHEC infections (52, 53). MMC is an efficient inducer of the SOS response in E. coli and is frequently used in research to induce phages to enter the lytic cycle, but normally it is not used for treating human infections due to its toxicity and mutagenicity (54–58). The presence of MSB reduced the levels of released Stx2 in H2O2-induced cultures, and the same outcome was observed for MSB in ciprofloxacin-induced cultures. A similar reducing effect of MSB on Stx2 production was also observed in two other EHEC strains tested, which suggests that this effect is a general response among EHEC strains. Results from the plaque assay showed that the presence of all four types of vitamin K reduced plaque formation independently of which inducing agent was used to trigger the activation of the lytic cycle. MSB exhibited the strongest reducing effect on plaque production. The reduction in plaque formation was not due to MSB treatment reducing the ability of BP933W to infect the recipient strain, as the plaque count increased when phage filtrates were treated with MSB. The mechanism for the positive effect of MSB on the infection rate of BP933W is unknown. However, as phage production results in lysis and death of the bacterial cell, the reduced production of phages in cultures containing menadione or MSB could explain why menadione and MSB had a positive effect on bacterial growth/survival in the presence of H2O2 and that MSB had a slight positive effect on bacterial survival in cultures containing MMC.
The reduced Stx2 phage synthesis and Stx2 production led us to the hypothesis that vitamin K analogs could inhibit the uptake of molecules into the bacterial cell. Indeed, by LC-MS/MS measurements, we could show that the intracellular level of ciprofloxacin taken up into the bacterial cell was reduced when strain EDL933 was grown in the presence of MSB, menadione, and menaquinone. In E. coli, exposure to redox cycling drugs, such as menadione, leads to the activation of the OxyR protein, a global transcriptional regulator important in oxidative stress resistance (reviewed in reference 59). This leads to increased expression of the homologous MarA, SoxS, and Rob proteins that are involved in the regulation of the adaptive response of E. coli to chemical stresses, oxidative stressors, and antibiotic compounds (60–62). Their upregulation is associated with the altered expression of genes involved in the efflux of antibiotics (acrAB and tolC), decrease in outer membrane permeability (ompF), and superoxide resistance (fpr and sodA) (63–68). The reduced uptake of ciprofloxacin suggests that the mar-sox-rob regulon is involved in the increased tolerance against H2O2 and the reduced uptake of ciprofloxacin in MSB-treated EHEC strains observed in the present study. Furthermore, the increased tolerance against H2O2 and reduced uptake of antibiotics could inhibit the activation of the SOS response and induction of the lytic cycle of the Stx2 phage, followed by reduced production of Stx2.
A previous study showed that nitric oxide (NO) exhibits an inhibitory effect on the production of Stx-converting phages, stx expression, and MMC-induced killing of strain EDL933 (69). This effect resembles the menadione- and MSB-mediated resistance to the growth-inhibitory/killing effect of H2O2 and MMC observed in our study. Like menadione, NO also activates the SoxRS response system in E. coli, which confers protection against subsequent exposure to harmful compounds, such as H2O2 and antibiotics, which induce the lytic cycle of Stx phages (69). However, further studies are required to elucidate the mechanisms behind the antibacterial/antivirulence effects of menadione, MSB, and vitamin K on EHEC. Nontargeted proteomic or transcriptomic methods could be employed to get a global view on the biological processes behind their effects on EHEC. Further studies should also include strains of different serotypes and Stx profiles.
Earlier studies suggested that Stx-converting phages exhibit a diverse host range and are able to infect commensal E. coli strains (14, 70). Gamage et al. showed that commensal non-O157 E. coli strains were susceptible to both lytic and lysogenic infections by Stx2-converting phages from an EHEC O157:H7 strain (70). Based on their findings, they suggested that commensal E. coli strains can amplify Stx production if they are susceptible to infection by Stx phages. Similarly, the Stx2 phage (Φ734) from the Norwegian outbreak strain NIPH-11060424 was shown to lysogenize commensal E. coli strains from healthy children below 5 years of age (14). When commensal Φ734 lysogens were induced to enter the lytic cycle by H2O2, most of the commensal strains produced more Φ734 phages than the donor NIPH-11060424 strain. Notably, five of the commensal strains spontaneously (not induced) produced more Φ734 phages than the NIPH-11060424 strain did under either H2O2- or MMC-induced conditions (14). Altogether, the reports by Gamage and Iversen suggest that phages that are released by EHEC and subsequently infect commensal strains can increase the pathogenic potential of EHEC during infection. With this in mind, it is tempting to speculate that if vitamin K analogs, from the diet and from the metabolism of the host intestinal microbiota, inhibit the production and dissemination of infective Stx phages, they may also restrict the development of severe disease. A similar effect could be achieved by using MSB or menadione therapeutically. It is also tempting to speculate that the high concentrations of vitamin K in the ruminant intestine contribute to the persistence and long-term carriage of EHEC by ruminants by preventing phage induction with concomitant lysis of the EHEC cells.
Menadione has been shown to produce carbon monoxide (CO) endogenously in vivo in rat brain microsomes and also in vitro (71). CO has a reputation primarily as a toxic gas when inhaled in large quantities. It does, however, have important anti-inflammatory, cytoprotective, and vasodilatory properties in vivo that are beneficial to health and have many therapeutic applications. CO also has antimicrobial properties, and CO-releasing molecules (CORMs) are pointed out as potential antimicrobial agents in a postantibiotic era (72–75). It could be that the inhibitory effect of menadione and MSB we see on the growth of EHEC could, at least partly, be due to CO production.
Together, our results suggest that MSB, menadione, phylloquinone, and menaquinone function as supportive agents to prevent severe outcomes from EHEC infections by reducing the virulence of the infecting EHEC strain. In theory, by targeting virulence factors, the resilience of EHEC within the human host could be impaired, allowing the host immune system to combat the infection. To evaluate the potential of vitamin K analogs as therapeutic agents, we need an increased understanding of the effects of these compounds on the interaction between EHEC and the human host. By studying the effect of vitamin K analogs on the interaction between EHEC and cultured cells, we could gain increased knowledge on how these compounds affect the initial infection process, colonization, and pathogenesis. However, the intestinal environment is complex and cannot be adequately simulated in vitro. For example, the host immune response and the normal microbiota are factors that are not considered in in vitro models and are probably of utmost importance for the outcome of EHEC infections. The use of in vivo models is, therefore, necessary to further evaluate if vitamin K analogs could be used to treat EHEC infections.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
EHEC O157:H7 strain EDL933 (76) was used to study the effect of vitamin K on the growth and virulence potential of EHEC. EDL933 carries both stx1 and stx2 genes, but it has previously been shown that stx1 is poorly expressed in this strain and not upregulated under MMC treatment (69). The E. coli laboratory strain DH5α (77) was used as a recipient strain in the plaque assay. The Norwegian outbreak strain NIPH-11060424 of serotype O103:H25 (78), and NVH-E7, a non-sorbitol-fermenting O157:H7 strain belonging to MLST type 11, were also used to test the effect of MSB on growth and Stx2 production. NIPH-11060424 is Stx1 negative and Stx2 positive, while NVH-E7 is both Stx1a and Stx2 positive. The stx2-negative EHEC O157:H7 strain NVH-E961 was used as an Stx2 negative matrix in LC-MS/MS samples. EDL933 and DH5α were grown under agitation (200 rpm) in Luria-Bertani (LB) broth at 37°C. Strains NIPH-11060424 and NVH-E7 were grown in brain heart infusion (BHI) broth (Oxford Limited, Basingstoke, UK) under agitation (225 rpm) at 37°C. Ciprofloxacin (AppliChem, Darmstadt, Germany) at 0.03 μg/ml (1/2 MIC for EHEC EDL933 [79]), MMC (Sigma-Aldrich) at 0.5 μg/ml (80), or H2O2 (NAF, Oslo, Norway) at 3 mM was used to induce the phage lytic cycle when the cultures had reached an optical density at 600 nm (OD600) of 0.5, i.e., when the cultures had reached the exponential growth phase. After the addition of inducing agents, the cultures were incubated in the dark.
To determine the dose-response effect of menadione sodium bisulfite (MSB) (Sigma-Aldrich, St. Louis, MO), menaquinone (Supelco, Bellefonte, PA), phylloquinone (Supelco, Bellefonte, PA), and menadione (Sigma-Aldrich, St. Louis, MO), the EHEC strains were grown overnight at 37°C under agitation. Twenty microliters of the overnight culture was inoculated into an Erlenmeyer flask containing 20 ml of LB broth, when appropriate, a defined concentration of menadione, MSB, or either of the two types of vitamin K tested. MSB was solved in water, while phylloquinone, menaquinone, and crystalline menadione are lipid soluble and, therefore, were solved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the cultures was 0.05%. The OD600 was monitored every 30 min for 10 h, and samples were taken every 2 h for 10 h for the enumeration of the number of CFU per milliliter.
Sampling for Stx measurements.
EDL933 was grown in the presence or absence of the different forms of vitamin K and induced as described above. The induced cultures were incubated at 37°C in the dark with shaking at 200 rpm, and samples were collected at 6, 8, and 20 h postinduction. The samples collected after 6 and 8 h were kept at 0°C on ice overnight for minimal loss of Stx during storage. The choice of storing the samples on ice was done after examining what temperatures were ideal for storing Stx overnight with minimal loss of toxin. The temperatures tested were −80°C, −20°C, 0°C, and 4°C. Storage at 0°C (in the dark) on ice showed the best yield of Stx2 toxin.
Protein reduction, alkylation, and digestion.
Protein reduction, alkylation, and digestion were done using a modified version of the method previously described by Silva et al. in 2014. (81). After harvesting EDL933 cells at 20 h postinduction, 100 μl of the bacterial cultures was added to a 1.5-ml centrifuge tube and diluted with 20 μl of MilliQ water. A volume of 100 μl of the Stx-negative matrix strain was spiked with 20 μl of MilliQ water with 0, 10, 28, 52.5, 70, and 140 ng/ml the peptide standard YNEDDTFTVK (Biomatik, Cambridge, Canada) to create a calibration curve. Thereafter, the EDL933 samples and the calibration standards were treated identically throughout the preparation for LC-MS analysis. Disulfide bond reduction was performed by adding 2 μl of 100 mM dithiothreitol (DTT; Sigma-Aldrich), solved in 25 mM ammonium bicarbonate (buffer A), to the samples and incubating them for 1 h at 37°C. To ensure alkylation of the free sulfhydryl groups on cysteine residues in the toxin, the samples were cooled to room temperature, and 8 μl of 100 mM iodoacetamide (IAA; Sigma-Aldrich) solved in buffer A was added. The samples were then incubated in darkness at room temperature for 1 h. Subsequently, 4 μl of 100 mM DTT, solved in buffer A, was added to quench excess iodoacetamide, followed by the addition of 10 μl of sequencing-grade modified trypsin (100 μg/ml; Promega). The samples were incubated at 37°C for 2 h (82). All samples then were transferred to 0.3-ml PP short-thread microvials (VWR) and capped. Samples that were expected to have Stx2 levels above the highest point in the calibration curve were diluted 1/5 in an Stx2-free matrix. An MS standard and a spiked matrix blind were also created to ensure the accuracy of the analyses.
LC-MS analysis.
The quantification of Stx2 was done by LC-MS/MS. The analysis was performed using an Agilent 1290 Infinity high-performance LC (HPLC) system (Agilent Technologies, Waldbronn, Germany) coupled with an Agilent G6490 MS/MS (Agilent Technologies, Santa Clara, CA, USA) containing an Agilent jet stream electrospray ion source. Separation was done using a 2.1- by 50-mm Agilent Zorbax SB-C18 column (1.8 μm). The chromatographic method was 5.5 min. The gradient started at 98% mobile phase A, and that within 2 min was decreased to 60% A. Mobile phase B was increased to 100% in 0.2 min, held for 1.8 min, and then returned to 98% mobile phase A, which was held for 1.5 min. The flow was held constant at 0.6 ml/min. Mobile phase A consisted of 0.5% acetic acid in water, and mobile phase B consisted of 0.5% acetic acid in 90% acetonitrile. The column compartment and autosampler were held at 25°C and 4°C, respectively. Stx2 was detected using multiple reaction monitoring (MRM), with mass transitions set at 616.3 m/z→135.9 m/z for quantification and 616.3 m/z→277.9 m/z as the qualifier transition.
Semiquantification of Stx2 levels using VTEC RPLA kit.
The VTEC RPLA toxin detection kit (Oxford Limited, Basingstoke, United Kingdom) was used to determine Stx2 production in culture supernatants of strains NIPH-11060424 and NVH-E7. The assay was performed according to the manufacturer's instructions. The cultures were induced by MMC as described above, and the samples were harvested 4 h after induction. The amount of toxin in each test well was reduced 2-fold at each dilution. The reciprocal of the highest dilution causing latex agglutination was considered the Stx titer.
Plaque assay.
The plaque assay used for quantification of infectious phage particles was modified from a method previously described by O’Brien et al. (10). Briefly, cultures of EDL933, grown to an OD600 of 0.3 to 0.6, were induced with either ciprofloxacin, H2O2, or mitomycin and incubated overnight under dark conditions. The cultures were centrifuged (3,900 × g for 10 min) and filtered using 0.22-μm pore filters (Minisart syringe filters; Sartorius, Göttingen, Germany). To eliminate the bias of cell lysis by colicins (83), tryptic digestion of the phage filtrates were performed using 0.1 mg/ml trypsin-EDTA (Gibco, Fischer Scientific, Loughborough, England) for 1 h at 37°C with shaking (200 rpm). A volume of 100 μl of the phage filtrate was mixed with 900 μl of a culture of the E. coli strain DH5α (OD600, 0.3 to 0.6), and the mixture was added to 3 ml of liquid soft agar (0.7% agar, 55°C) supplemented with 10 mM CaCl2 and overlaid on LB agar plates. The plates were incubated at 37°C overnight, and the phage titers were determined by visual plaque recognition and counting the following day.
Vitamin K’s effect on the infectivity of BP933W.
A phage stock was made by inoculating 150 ml of LB broth in an Erlenmeyer flask with 1.5 ml of overnight culture of EDL933. After growth to an OD600 of 0.3 to 0.6, the cultures were induced with 0.5 μg/ml mitomycin C, covered with aluminum foil to deprive the cultures of light, and incubated at 37°C with shaking at 200 rpm for 24 h. The cultures then were centrifuged (10 min, 4,000 × g, 4°C) and sterile filtered with 0.22-μm Minisart syringe filters. The different vitamin K variants and solvents were added to 20 ml of the phage filtrates. DMSO was added to a final concentration of 0.05%, the same concentration as that in the cultures with vitamin added. The vitamin K concentrations were 724 μM for phylloquinone and menaquinone, 36 μM for MSB, and 72 μM for menadione. The samples were incubated for 2 h at 37°C, in the dark, under shaking at 200 rpm. The vitamin K-treated phage stocks were treated with trypsin as described above and tested with a plaque assay.
qPCR.
Quantitative real-time PCR (qRT-PCR) was used to measure the expression level of stx2 and recA. EDL933 was cultured in the presence or absence of different forms of vitamin K and induced as described above. Two hours after induction, the cultures were mixed with ice-cold methanol and kept at −80°C before isolation of RNA. Total RNA was extracted using the PureLink RNA minikit (Life Technologies, Carlsbad, CA), and the DNA was removed using the Turbo DNA-free kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The quantity (A260) and purity (A260/280) of the RNA were measured in a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and an Agilent 2100 bioanalyzer was used to assess the quality of the RNA. Only mRNA samples with a purity (A260/280) of 1.90 to 2.10 and with integrity over RIN 9 were used for cDNA synthesis. cDNA then was synthesized from 500 ng of RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. Five-microliter samples of a 1:100 dilution of the cDNA preparations were used as the templates for qPCR amplification in a total volume of 25 μl containing 12.5 μl of PowerUp SYBR green master mix (Applied Biosystems, Carlsbad, CA) and primers at a concentration of 400 nM. The primers used for qPCR are listed in the supplemental material (Table 1). The qPCR amplification was performed using a StepOne system (Applied Biosystems, Carlsbad, CA). The thermal cycling conditions were 2 min at 50°C, 2 min at 95°C, and then 40 cycles of 15 s at 95°C and 30 s at 60°C. The fluorescence was recorded during each extension phase, and a melting curve analysis was carried out after each run to verify the amplification of specific transcripts. Each assay was performed in three biological replicates and three technical replicates. Samples containing no cDNA template functioned as negative controls. The slope of the standard curve and PCR efficiency for each primer pair were obtained by amplifying serial dilutions of genomic DNA of EDL933 containing the target sequence. The mRNA level for each gene was determined relative to the reference gene gapA (glyceraldehyde-3-phosphate dehydrogenase), and the results were analyzed using the Pfaffl method (84).
TABLE 1.
Primers used in this study
| Gene | Primer sequence |
Slopea | % Effb | |
|---|---|---|---|---|
| Forward (5′ to 3′) | Reverse (5′ to 3′) | |||
| stx2 | GAACGTTCCGGAATGCAAA | CCATTAACGCCAGATATGATGA | −3.40 | 98.00 |
| recA | TTGACCTGGGCGTAAAAGAG | CGGTTTCCGGGTTATCTTTC | −3.10 | 90.00 |
| gapA | AGGTCTGATGACCACCGTTC | AACGGTCAGGTCAACTACGG | −3.30 | 99.70 |
The slope was calculated from the regression line of the standard curve.
The efficiency (Eff) was calculated using the slope of the regression line of the standard curve.
Microscopy.
The EDL933 strain was grown in the presence or absence of different forms of vitamin K and induced as described above. The samples were incubated for 20 h postinduction, and the samples were immediately prepared for microscopy analysis. The microscopy was done with an Olympus BX51 microscope, and the pictures were taken with an Olympus UC-90 color camera (Olympus, Tokyo, Japan) and examined with cellSens software (Olympus, Tokyo, Japan). Slightly different shades were observed between quadrants in all images. The reason for the different shades is due to a mismatch in the graphic board requirements between the camera and the laboratory computer connected to the camera. The computer connected to the camera has too low a capacity (Windows VGA 1,920 × 1,080 × 32 bit compared to what the camera requires (3,840 × 2,160 pixels at 30 Hz). However, this should not influence the results presented.
Survival assay.
EHEC was grown overnight in LB broth, and the next day 20 μl was inoculated into 20 ml LB in Erlenmeyer flasks. Vitamin K variants were used in the following concentrations: phylloquinone and menaquinone (72 μM), MSB (36 μM), and menadione (7 μM). The OD600 was determined every hour, and H2O2 (3 mM) or ciprofloxacin (0.06 μg/ml, i.e., 1× the MIC) and 0.5 μg/ml MMC were added when the cultures had reached an OD600 of 0.5 ± 0.05. The OD600 was measured every hour after induction for eight hours, and samples were taken for enumeration of CFU per milliliter every second hour.
LC-MS/MS quantification of intracellular ciprofloxacin.
Cytoplasmic extracts were prepared from EDL933 grown in LB with or without vitamin K analogs (phylloquinone and menaquinone, 72 μM; MSB, 36 μM; menadione, 7 μM) at 37°C. The cultures were induced with 0.06 μg/ml ciprofloxacin when they reached an OD600 of 0.5. After 20 min of growth, 6-ml aliquots of the cultures were harvested and pelleted by centrifugation at 18,000 × g for 30 s and washed three times in PBS (pH 7.4). Prior to the last wash, a 10-μl portion of the sample was harvested, diluted, and plated on LB agar for enumeration. The samples were pelleted and vacuum dried (Savant Spd 121P speed vac concentrator; Thermo Scientific, Waltham, MA, USA) for 5 to 10 min at 35°C. The pellets were solubilized in a solution of 200 μl water and 10 μl chloroform and centrifuged for 8 min at 18,000 × g. The supernatants were transferred to 0.3-ml PP short-thread microvials (VWR) and capped. Aliquots of 5 μl were analyzed for the concentration of ciprofloxacin with LC-MS/MS as described previously (85). The instrumentation used was an Agilent 1200 SL HPLC system equipped with an Agilent G6490 triple-quadrupole mass spectrometer with an electrospray ion source. An Agilent Zorbax Rx C18 column, 150 mm by 3.0 mm (inner diameter) with 3.5-μm particles, was used for separation. Calibration standards were prepared in a filtered cell extract matrix of EDL933 without ciprofloxacin added and ciprofloxacin at concentrations of 0, 0.5, 1, 5, 7.5, and 10 ng/ml. The calibration curve was forced through zero and was linear, with a correlation coefficient above 0.99. The values from the MS analysis were normalized according to the number of CFU per milliliter counted, and relative percentages of the uptake of ciprofloxacin were calculated.
Supplementary Material
ACKNOWLEDGMENTS
We thank senior researcher and laboratory manager John Aasen, Marine Algal Toxin Laboratory, Department of Paraclinical Science, NMBU, for providing the equipment and guidance for performing the Shiga toxin quantification.
The Department of Paraclinical Sciences at the Faculty of Veterinary Medicine, the Norwegian University of Life Sciences, contributed financially to this project.
The work was done under the supervision of M.A. as the principal investigator. She significantly contributed to the study design, drafting, revisions, and interpretation of data. A.K. was the major player in the conception, design, conduct, revision, analysis, and interpretation. T.L., I.L.W., H.T.R., T.L.-L., and Y.W. contributed to the design and conducting of different sections of the work as well as to editing the manuscript. All authors have approved the final version of the manuscript before submission.
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.WHO. 2016. E. coli fact sheet. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs125/en/. [Google Scholar]
- 2.Obata F, Tohyama K, Bonev AD, Kolling GL, Keepers TR, Gross LK, Nelson MT, Sato S, Obrig TG. 2008. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J Infect Dis 198:1398–1406. doi: 10.1086/591911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes AK, Ergonul Z, Stricklett PK, Kohan DE, Ergonal Z. 2002. Molecular basis for high renal cell sensitivity to the cytotoxic effects of shigatoxin-1: upregulation of globotriaosylceramide expression. J Am Soc Nephrol 13:2239–2245. doi: 10.1097/01.asn.0000027873.85792.52. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Tesh VL. 2019. Roles of Shiga toxins in immunopathology. Toxins 11:212. doi: 10.3390/toxins11040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melton-Celsa AR. 2014. Shiga toxin (Stx) classification, structure, and function. Microbiol Spectr 2:EHEC. doi: 10.1128/microbiolspec.EHEC-0024-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. 1999. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 37:497–503. doi: 10.1128/JCM.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostroff SM, Tarr PI, Neill MA, Lewis JH, Hargrett-Bean N, Kobayashi JM. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J Infect Dis 160:994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 8.De Rauw K, Buyl R, Jacquinet S, Pierard D. 2018. Risk determinants for the development of typical haemolytic uremic syndrome in Belgium and proposition of a new virulence typing algorithm for Shiga toxin-producing Escherichia coli. Epidemiol Infect 2018:1–5. doi: 10.1017/S0950268818002546. [DOI] [PubMed] [Google Scholar]
- 9.Brandal LT, Wester AL, Lange H, Lobersli I, Lindstedt BA, Vold L, Kapperud G. 2015. Shiga toxin-producing Escherichia coli infections in Norway, 1992–2012: characterization of isolates and identification of risk factors for haemolytic uremic syndrome. BMC Infect Dis 15:324. doi: 10.1186/s12879-015-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien A, Newland J, Miller S, Holmes R, Smith H, Formal S. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 11.Smith HW, Green P, Parsell Z. 1983. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens and pigs. J Gen Microbiol 129:3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- 12.Muhldorfer I, Hacker J, Keusch GT, Acheson DW, Tschape H, Kane AV, Ritter A, Olschlager T, Donohue-Rolfe A. 1996. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect Immun 64:495–502. doi: 10.1128/IAI.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis 181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 14.Iversen H, L' Abée-Lund TM, Aspholm M, Arnesen LPS, Lindbäck T. 2015. Commensal E. coli Stx2 lysogens produce high levels of phages after spontaneous prophage induction. Front Cell Infect Microbiol 5:5. doi: 10.3389/fcimb.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferens WA, Hovde CJ. 2011. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog Dis 8:465–487. doi: 10.1089/fpd.2010.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cray WC Jr, Moon HW. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl Environ Microbiol 61:1586–1590. doi: 10.1128/AEM.61.4.1586-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean-Nystrom EA, Bosworth BT, Cray WC, Moon HW. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect Immun 65:1842–1848. doi: 10.1128/IAI.65.5.1842-1848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature 492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis Meredith M, Hu Z, Klimko C, Narayanan S, Deberardinis R, Sperandio V. 2014. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16:759–769. doi: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordonnier C, Le Bihan G, Emond-Rheault J-G, Garrivier A, Harel J, Jubelin G. 2016. Vitamin B(12) uptake by the gut commensal bacteria Bacteroides thetaiotaomicron limits the production of shiga toxin by enterohemorrhagic Escherichia coli. Toxins 8:14. doi: 10.3390/toxins8010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera G, Fernández-Brando RJ, Abrey-Recalde MJ, Baschkier A, Pinto A, Goldstein J, Zotta E, Meiss R, Rivas M, Palermo MS. 2014. Retinoid levels influence enterohemorrhagic Escherichia coli infection and shiga toxin 2 susceptibility in mice. Infect Immun 82:3948–3957. doi: 10.1128/IAI.02191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. 2014. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis 210:1296–1305. doi: 10.1093/infdis/jiu235. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Feng L, Wang F, Wang L. 2015. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6:6592. doi: 10.1038/ncomms7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay S, Linstedt AD. 2012. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science 335:332–335. doi: 10.1126/science.1215930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karl JP, Meydani M, Barnett JB, Vanegas SM, Barger K, Fu X, Goldin B, Kane A, Rasmussen H, Vangay P, Knights D, Jonnalagadda SS, Saltzman E, Roberts SB, Meydani SN, Booth SL. 2017. Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am J Clin Nutr 106:1052–1061. doi: 10.3945/ajcn.117.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Booth SL. 2012. Vitamin K: food composition and dietary intakes. Food Nutrition Res 2012:56. doi: 10.3402/fnr.v56i0.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G, Franchini M. 2011. Vitamin K in neonates: facts and myths. Blood Transfusion 9:4–9. doi: 10.2450/2010.0034-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palace GP, Franke JE, Warden JT. 1987. Is phylloquinone an obligate electron carrier in photosystem I? FEBS Lett 215:58–62. doi: 10.1016/0014-5793(87)80113-8. [DOI] [PubMed] [Google Scholar]
- 29.Damon M, Zhang NZ, Haytowitz DB, Booth SL. 2005. Phylloquinone (vitamin K-1) content of vegetables. J Food Composition Anal 18:751–758. doi: 10.1016/j.jfca.2004.07.004. [DOI] [Google Scholar]
- 30.Harshman SG, Finnan EG, Barger KJ, Bailey RL, Haytowitz DB, Gilhooly CH, Booth SL. 2017. Vegetables and mixed dishes are top contributors to phylloquinone intake in US adults: data from the 2011–2012 NHANES. J Nutr 147:1308–1313. doi: 10.3945/jn.117.248179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duggan P, Cashman KD, Flynn A, Bolton-Smith C, Kiely M. 2004. Phylloquinone (vitamin K-1) intakes and food sources in 18-year-old to 64-year-old Irish adults. Br J Nutr 92:151–158. doi: 10.1079/BJN20041157. [DOI] [PubMed] [Google Scholar]
- 32.Dismore ML, Haytowitz DB, Gebhardt SE, Peterson JW, Booth SL. 2003. Vitamin K content of nuts and fruits in the US diet. J Am Diet Assoc 103:1650–1652. doi: 10.1016/j.jada.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Nestor KE Jr, Conrad HR. 1990. Metabolism of vitamin K and influence on prothrombin time in milk-fed preruminant calves. J Dairy Sci 73:3291–3296. doi: 10.3168/jds.S0022-0302(90)79022-4. [DOI] [PubMed] [Google Scholar]
- 34.Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L. 2019. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci 20:896. doi: 10.3390/ijms20040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson RT, Foley AL, Engelke JA, Suttie JW. 1998. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr 128:220–223. doi: 10.1093/jn/128.2.220. [DOI] [PubMed] [Google Scholar]
- 36.Karl JP, Fu X, Wang X, Zhao Y, Shen J, Zhang C, Wolfe BE, Saltzman E, Zhao L, Booth SL. 2015. Fecal menaquinone profiles of overweight adults are associated with gut microbiota composition during a gut microbiota-targeted dietary intervention. Am J Clin Nutr 102:84–93. doi: 10.3945/ajcn.115.109496. [DOI] [PubMed] [Google Scholar]
- 37.Card DJ, Gorska R, Cutler J, Harrington DJ. 2014. Vitamin K metabolism: current knowledge and future research. Mol Nutr Food Res 58:1590–1600. doi: 10.1002/mnfr.201300683. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. 2006. Menadione is a metabolite of oral vitamin K. Br J Nutr 95:260–266. doi: 10.1079/bjn20051630. [DOI] [PubMed] [Google Scholar]
- 39.Hirota Y, Tsugawa N, Nakagawa K, Suhara Y, Tanaka K, Uchino Y, Takeuchi A, Sawada N, Kamao M, Wada A, Okitsu T, Okano T. 2013. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem 288:33071–33080. doi: 10.1074/jbc.M113.477356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goncalves A, Margier M, Roi S, Collet X, Niot I, Goupy P, Caris-Veyrat C, Reboul E. 2014. Intestinal scavenger receptors are involved in vitamin K(1) absorption. J Biol Chem 289:30743–30752. doi: 10.1074/jbc.M114.587659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferland G, Sadowski JA, O'Brien ME. 1993. Dietary induced subclinical vitamin K deficiency in normal human subjects. J Clin Investig 91:1761–1768. doi: 10.1172/JCI116386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirakawa H, Komai M, Kimura S. 1990. Antibiotic-induced vitamin K deficiency and the role of the presence of intestinal flora. Int J Vitam Nutr Res 60:245–251. [PubMed] [Google Scholar]
- 43.Theuwissen E, Magdeleyns EJ, Braam LA, Teunissen KJ, Knapen MH, Binnekamp IA, van Summeren MJ, Vermeer C. 2014. Vitamin K status in healthy volunteers. Food Funct 5:229–234. doi: 10.1039/c3fo60464k. [DOI] [PubMed] [Google Scholar]
- 44.Andrew M. 1995. Developmental hemostasis: relevance to hemostatic problems during childhood. Semin Thromb Hemost 21:341–356. doi: 10.1055/s-2007-1000655. [DOI] [PubMed] [Google Scholar]
- 45.Schlievert PM, Merriman JA, Salgado-Pabon W, Mueller EA, Spaulding AR, Vu BG, Chuang-Smith ON, Kohler PL, Kirby JR. 2013. Menaquinone analogs inhibit growth of bacterial pathogens. Antimicrob Agents Chemother 57:5432–5437. doi: 10.1128/AAC.01279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee MH, Yang JY, Cho Y, Woo HJ, Kwon HJ, Kim DH, Park M, Moon C, Yeon MJ, Kim HW, Seo WD, Kim SH, Kim JB. 2019. Inhibitory effects of menadione on Helicobacter pylori growth and Helicobacter pylori-induced inflammation via NF-kappaB inhibition. Int J Mol Sci 20:1169. doi: 10.3390/ijms20051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki H, Pangborn J, Kilgore WW. 1967. Filamentous cells of Escherichia coli formed in the presence of mitomycin. J Bacteriol 93:683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wickens HJ, Pinney RJ, Mason DJ, Gant VA. 2000. Flow cytometric investigation of filamentation, membrane patency, and membrane potential in Escherichia coli following ciprofloxacin exposure. Antimicrob Agents Chemother 44:682–687. doi: 10.1128/aac.44.3.682-687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Hong Y, Drlica K. 2015. Moving forward with reactive oxygen species involvement in antimicrobial lethality. J Antimicrob Chemother 70:639–642. doi: 10.1093/jac/dku463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner PL, Acheson DW, Waldor MK. 2001. Human neutrophils and their products induce Shiga toxin production by enterohemorrhagic Escherichia coli. Infect Immun 69:1934–1937. doi: 10.1128/IAI.69.3.1934-1937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lainhart W, Stolfa G, Koudelka GB. 2009. Shiga toxin as a bacterial defense against a eukaryotic predator, Tetrahymena thermophila. J Bacteriol 191:5116–5122. doi: 10.1128/JB.00508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimmitt PT, Harwood CR, Barer MR. 2000. Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis 6:458–465. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowicki D, Maciąg-Dorszyńska M, Kobiela W, Herman-Antosiewicz A, Węgrzyn A, Szalewska-Pałasz A, Węgrzyn G. 2014. Phenethyl isothiocyanate inhibits shiga toxin production in enterohemorrhagic Escherichia coli by stringent response induction. Antimicrob Agents Chemother 58:2304–2315. doi: 10.1128/AAC.02515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu T, Ohta Y, Noda M. 2009. Shiga toxin 2 is specifically released from bacterial cells by two different mechanisms. Infect Immun 77:2813–2823. doi: 10.1128/IAI.00060-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buzdar AU, Legha SS, Luna MA, Tashima CK, Hortobagyi GN, Blumenschein GR. 1980. Pulmonary toxicity of mitomycin. Cancer 45:236–244. doi:. [DOI] [PubMed] [Google Scholar]
- 57.Hama-Inaba H, Sato K, Moustacchi E. 1988. Survival and mutagenic responses of mitomycin C-sensitive mouse lymphoma cell mutants to other DNA cross-linking agents. Mutat Res 194:121–129. doi: 10.1016/0167-8817(88)90014-4. [DOI] [PubMed] [Google Scholar]
- 58.de Oliveira JT, Barbosa M, de Camargos LF, da Silva IVG, Varotti FP, da Silva LM, Moreira LM, Lyon JP, Dos Santos V, Dos Santos FV. 2017. Digoxin reduces the mutagenic effects of mitomycin C in human and rodent cell lines. Cytotechnology 69:699–710. doi: 10.1007/s10616-017-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu M, Imlay JA. 2011. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol Microbiol 79:1136–1150. doi: 10.1111/j.1365-2958.2010.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller PF, Sulavik MC. 1996. Overlaps and parallels in the regulation of intrinsic multiple-antibiotic resistance in Escherichia coli. Mol Microbiol 21:441–448. doi: 10.1111/j.1365-2958.1996.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Demple B. 1994. SoxS, an activator of superoxide stress genes in Escherichia coli. Purification and interaction with DNA. J Biol Chem 269:18371–18377. [PubMed] [Google Scholar]
- 63.Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol 16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 64.Okusu H, Ma D, Nikaido H. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol 178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen SP, McMurry LM, Levy SB. 1988. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol 170:5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liochev SI, Hausladen A, Beyer WF Jr, Fridovich I. 1994. NADPH: ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc Natl Acad Sci U S A 91:1328–1331. doi: 10.1073/pnas.91.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krapp AR, Tognetti VB, Carrillo N, Acevedo A. 1997. The role of ferredoxin-NADP+ reductase in the concerted cell defense against oxidative damage–studies using Escherichia coli mutants and cloned plant genes. Eur J Biochem 249:556–563. doi: 10.1111/j.1432-1033.1997.00556.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, Lee KL, Yeo WS, Park SJ, Roe JH. 2009. SoxRS-mediated lipopolysaccharide modification enhances resistance against multiple drugs in Escherichia coli. J Bacteriol 191:4441–4450. doi: 10.1128/JB.01474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vareille M, de Sablet T, Hindre T, Martin C, Gobert AP. 2007. Nitric oxide inhibits Shiga-toxin synthesis by enterohemorrhagic Escherichia coli. Proc Natl Acad Sci U S A 104:10199–10204. doi: 10.1073/pnas.0702589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gamage SD, Strasser JE, Chalk CL, Weiss AA. 2003. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infect Immun 71:3107–3115. doi: 10.1128/iai.71.6.3107-3115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odozor CU, Peterson N, Pudwell J, Smith GN. 2018. Endogenous carbon monoxide production by menadione. Placenta 71:6–12. doi: 10.1016/j.placenta.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Motterlini R. 2007. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans 35:1142–1146. doi: 10.1042/BST0351142. [DOI] [PubMed] [Google Scholar]
- 73.Knauert M, Vangala S, Haslip M, Lee PJ. 2013. Therapeutic applications of carbon monoxide. Oxid Med Cell Longev 2013:360815. doi: 10.1155/2013/360815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilson JL, Jesse HE, Poole RK, Davidge KS. 2012. Antibacterial effects of carbon monoxide. Curr Pharm Biotechnol 13:760–768. doi: 10.2174/138920112800399329. [DOI] [PubMed] [Google Scholar]
- 75.Wareham LK, Poole RK, Tinajero-Trejo M. 2015. CO-releasing metal carbonyl compounds as antimicrobial agents in the post-antibiotic era. J Biol Chem 290:18999–19007. doi: 10.1074/jbc.R115.642926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perna NT, Plunkett G III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Pósfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 77.Hanahan D. 1985. Techniques for transformation of Escherichia coli, p 109–129. In Glover DM (ed), DNA cloning: a practical approach, vol I. IRL Press, McLean, VA. [Google Scholar]
- 78.Schimmer B, Nygard K, Eriksen HM, Lassen J, Lindstedt BA, Brandal LT, Kapperud G, Aavitsland P. 2008. Outbreak of haemolytic uraemic syndrome in Norway caused by stx2-positive Escherichia coli O103:H25 traced to cured mutton sausages. BMC Infect Dis 8:41. doi: 10.1186/1471-2334-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. 2012. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob Agents Chemother 56:3277–3282. doi: 10.1128/aac.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshimura K, Fujii J, Taniguchi H, Yoshida S. 1999. Chemotherapy for enterohemorrhagic Escherichia coli O157:H infection in a mouse model. FEMS Immunol Med Microbiol 26:101–108. doi: 10.1111/j.1574-695X.1999.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 81.Silva CJ, Erickson-Beltran ML, Skinner CB, Dynin I, Hui C, Patfield SA, Carter JM, He X. 2014. Safe and effective means of detecting and quantitating Shiga-like toxins in attomole amounts. Anal Chem 86:4698–4706. doi: 10.1021/ac402930r. [DOI] [PubMed] [Google Scholar]
- 82.Silva C, Erickson-Beltran M, Skinner C, Patfield S, He X. 2015. Mass spectrometry-based method of detecting and distinguishing type 1 and type 2 Shiga-like toxins in human serum. Toxins 7:5236–5253. doi: 10.3390/toxins7124875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gordon DM, O’Brien CL. 2006. Bacteriocin diversity and the frequency of multiple bacteriocin production in Escherichia coli. Microbiology 152:3239–3244. doi: 10.1099/mic.0.28690-0. [DOI] [PubMed] [Google Scholar]
- 84.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.B’Hymer C, Connor T, Stinson D, Pretty J. 2015. Validation of an HPLCMS/MS and wipe procedure for mitomycin C contamination. J Chromatogr Sci 53:619–624. doi: 10.1093/chromsci/bmu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.