Abstract
Objectives:
Venovenous extracorporeal membrane oxygenation is increasingly being established as a treatment option for severe acute respiratory failure. We sought to evaluate the impact of a dedicated specialist team-based approach on patient outcomes.
Design:
Retrospective cohort study.
Setting:
Single-center medical ICU in an academic tertiary hospital.
Patients:
Adult patients initiated on venovenous extracorporeal membrane oxygenation for severe acute respiratory failure.
Interventions:
Initiation of an extracorporeal membrane oxygenation intensivist-led multidisciplinary team; critical decisions on extracorporeal membrane oxygenation management were jointly made by a dedicated team of extracorporeal membrane oxygenation intensivists, together with the multidisciplinary team.
Measurements and Main Results:
Eighty-one patients (75%) and 27 patients (35%) were initiated on venovenous extracorporeal membrane oxygenation in the preextracorporeal membrane oxygenation intensivist-led multidisciplinary team (before January 2018) and postextracorporeal membrane oxygenation intensivist-led multidisciplinary team period (after January 2018), respectively. Inhospital (14.8% vs 44.4%, p = 0.006) and ICU mortality (11.1% vs 40.7%, p = 0.005) were significantly lower in the postextracorporeal membrane oxygenation intensivist-led multidisciplinary team period. On multivariate analysis correcting for possible confounding factors (ICU severity and extracorporeal membrane oxygenation-specific mortality prediction scores, body mass index, preextracorporeal membrane oxygenation vasopressor support, preextracorporeal membrane oxygenation cardiac arrest, and days on mechanical ventilation before extracorporeal membrane oxygenation initiation), management by an extracorporeal membrane oxygenation intensivist-led multidisciplinary team remained associated with improved hospital survival (odds ratio, 5.06; 95% CI, 1.20–21.28). Patients in the postextracorporeal membrane oxygenation intensivist-led multidisciplinary team period had less nosocomial infections (18.5% vs 46.9%, p = 0.009), a shorter ICU stay (12 days [interquartile range, 6–16 d] vs 15 days [interquartile range, 10–24 d]; p = 0.049), and none suffered an intracranial hemorrhage or nonhemorrhagic stroke.
Conclusions:
An extracorporeal membrane oxygenation intensivist-led multidisciplinary team approach is associated with improved outcomes in patients initiated on venovenous extracorporeal membrane oxygenation for severe acute respiratory failure.
Keywords: acute respiratory distress syndrome, acute respiratory failure, extracorporeal life support, extracorporeal membrane oxygenation, intensive care, multidisciplinary team, pneumonia
There is an increasing global trend of using venovenous extracorporeal membrane oxygenation (VV ECMO) in patients with severe acute respiratory failure. VV ECMO assists in gas exchange, thereby allowing for lung protective ventilatory strategies and “lung rest.” As the mainstay of treatment remains supportive in severe acute respiratory distress syndrome (ARDS), VV ECMO has emerged as an intervention to support lung function while awaiting patient recovery (1–3).
Although advancements in ECMO technology have rendered it safer and more accessible to patients globally, ECMO remains resource-intensive with a potential for severe and life-threatening complications (4). Reported inhospital mortality rates of VV ECMO for severe respiratory failure have remained largely between 40% and 50% in ECMO registries and approximately 35% from research trials performed in experienced centers, over the past decade (5, 6). Considering the complexity of ECMO treatment, an experienced multidisciplinary team, guided by protocols and organized training, should have an impact on clinical outcomes (7, 8). Therefore, Extracorporeal Life Support Organization (ELSO) guidelines and several other consensus opinions recommend ECMO centers to be helmed by interprofessional or multidisciplinary teams, and support a minimum case volume to maintain clinical expertise (9–11). However, the impact of these recommendations on patient outcomes remains unclear. First, evidences supporting VV ECMO have largely been from studies conducted in high-volume and experienced centers (2, 6, 12). Second, although some studies have reported improved outcomes with implementation of an ECMO multidisciplinary team, others have not (13, 14). Finally, the volume-to-outcome relationship remains inconsistent in ECMO (15).
Recent published recommendations from the Joint Society of Critical Care Medicine ELSO task force highlight that intensivists should lead ECMO multidisciplinary teams and play a central role in critical ECMO management decisions, such as patient selection, timing of ECMO initiation, and managing ECMO-related complications (10). Clearly, intensivists should only play this role if they are knowledgeable with ECMO physiology, indications, and complications. We postulate that a multidisciplinary approach to ECMO management, led by a dedicated and consistent team of ECMO intensivists (intensivists with advanced training, experience, and strong subspecialty focus in ECMO), may improve patient outcomes.
MATERIALS AND METHODS
Study Design
We performed a retrospective study of all patients who were initiated on VV ECMO support for acute severe respiratory failure from July 2008 to September 2019 at Singapore General Hospital. Patients who were initiated on venoarterial ECMO were excluded. Our center is the largest tertiary hospital (> 1,700 beds) and one of two national ECMO centers in Singapore. We see a case volume of approximately eight to 10 VV ECMO cases annually—this has increased gradually over time, with an average of seven to nine cases annually from 2008 to 2013 and up to 13–14 cases annually from 2018 to 2019. With implementation of an ECMO intensivist-led multidisciplinary team (ECIT) in January 2018, patients were divided into two subgroups for analysis based on the date of ECMO cannulation: 2008–2017 and 2018–2019.
VV ECMO Selection Criteria and Workflow
In our center, VV ECMO is considered for patients with severe respiratory failure (Pao2/Fio2 [P/F ratio] < 80) or hypercapnic acidosis (pH < 7.20) despite optimization of conventional lung protective ventilation strategies (tidal volume < 6 mL/kg predicted body weight [PBW] and plateau pressure < 30 cm H2O) and use of neuromuscular blockade or prone positioning. Relative contraindications include greater than 7 days of mechanical ventilation, contraindications to anticoagulation, advanced age (> 70 years old), underlying irreversible organ failure, active malignancy, or significantly immunocompromised states.
Cannulation and decannulation are performed by cardiothoracic surgeons. Cannulation via percutaneous Seldinger technique is generally preferred, but an open cut-down would be performed if this fails. A femoral-femoral VV ECMO configuration is preferred, using Bio-Medicus (Medtronic, Minneapolis, MN) or Maquet HLS (Maquet, Rastatt, Germany) cannulae. A 21-Fr cannula is commonly used for venous drainage and a 19-Fr cannula for venous return. For retrieval cases, a mobile team consisting of cardiothoracic surgeons and perfusionists provides on-site cannulation if required and assists with transport back to our center.
Our VV ECMO circuit uses a Rotaflow centrifugal pump (Maquet), Hilite 7000LT oxygenator (Medos, Stolberg, Germany), and phosphorylcholine-coated ECMO tubings (LivaNova, Arvada, CO). For retrieval cases from 2017 onward, we have been using the Cardiohelp system with an HLS module advanced 7.0 set integrated pump and oxygenator (Maquet), and heparin-coated ECMO tubings (BIOLINE, Maquet). After ECMO initiation, ventilatory strategies include a target tidal volume of approximately 4-mL/kg PBW to keep plateau pressures less than 25 cm H2O. Patients were kept on moderate-to-high positive end-expiratory pressure (PEEP), at least 10 cm H2O with gradual readjustment based on clinical response and pulmonary mechanics. We targeted an Fio2 less than or equal to 0.5, while keeping Sao2 greater than 85%, and respiratory rate set at 10–12 breaths/min. Anticoagulation was preferably achieved with IV heparin infusion, targeting an activated partial thromboplastin (aPTT) of 60–80 seconds.
Before January 2018, the decision of VV ECMO treatment for patients with severe respiratory failure lay with the general intensivist on service. Although most intensivists had some knowledge of ECMO management, each intensivist’s experience was variable, with most covering the ICU for not more than a few months in a year. Similarly, decisions on management of complications, weaning, and decannulation were generally made by the medical intensivist on service, in consultation with a cardiothoracic surgeon on an “as-needed” basis. ICU management was guided by department protocols (including ECMO protocols on patient selection, postcannulation monitoring and nursing care, anticoagulation, sedation and analgesia, and ventilator management) and supported by ICU nurses, perfusionists, and respiratory therapists. ICU nurses and respiratory therapists provided care during the day and night. The medical intensivist on service and perfusionists performed ICU rounds in the day and were available for consultation during the night, including on-site reviews when required.
Implementation of an ECMO Intensivists-Led Multidisciplinary Team
After January 2018, a dedicated specialist team-based approach to ECMO care (ECIT) was initiated. This was led by a group of ECMO intensivists with accreditation in critical care medicine and had received fellowship training in established ECMO centers or completed an ECMO training course with at least 1 year of experience in the ECIT. Assessment of patient suitability for VV ECMO was a joint team decision rather than an individual one. Selection was guided by protocolized-inclusion criteria, with the final decision for ECMO initiation decided by the ECIT based on the clinical assessment of the patient and the presence of relative contraindications. Other members of the team, consisting of medical intensivists, cardiothoracic surgeons, perfusionists, specialized ECMO ICU nurses, respiratory therapists, and pharmacists continue to provide multidisciplinary care. A group of ICU nurses also received training from international ECMO courses to upskill nursing capability to provide 24-hour bedside support and augment the function of the perfusionists. The ECIT performs daily rounds on ECMO patients, together with the ICU team. Assessment and management of ECMO-related complications, ventilator management, weaning, decannulation, and withdrawal are made by ECMO intensivists, with the multidisciplinary team.
ECMO intensivists also provided nighttime call coverage for consultation (with on-site reviews when required) and on-site assistance during ECMO initiation and the immediate postcannulation care. Perfusionists and a cardiothoracic surgeon continued to be available for consultation during the night, including on-site reviews as required. For retrieval cases, the ECMO intensivist may provide on-site reviews, assist with cannulation using real-time ultrasonography, and hold discussions with the patient’s family members, if appropriate. A review of existing ECMO protocols was also initiated in 2018 by the team. In May 2019, our anticoagulation protocol moved toward a more conservative aPTT target of 45–60 seconds and/or an activated clotting time between 180 and 220 seconds. No other major changes in protocolized ICU or ECMO management were made. Finally, along with initiation of the team-based approach to ECMO care, clinical audits, journal clubs, and multidisciplinary case discussions were organized regularly. ECMO survivors are also followed up in the wards and subsequently in the outpatient setting by ECMO intensivists.
Data Collection and Analysis
Clinical, laboratory, and outcome data were retrieved from healthcare records. Data prior to ECMO implantation that was retrieved included the duration of mechanical ventilation, ventilator settings (peak inspiratory pressure [PIP], PEEP), arterial blood gas samples, and other therapies, for example, prone-positioning and neuromuscular blockade. ICU severity scores, for example, Sequential Organ Failure Assessment (SOFA), and ECMO-specific mortality prediction scores, for example, Respiratory Extracorporeal membrane oxygenation Survival Prediction (RESP) score and the PRedicting dEath for SEvere ARDS on VV ECMO (PRESERVE) score, were also derived from collected data (16, 17). For the RESP and PRESERVE scores, a score of 0 was assigned to variables where information was not available, for example, ventilator PIP, bicarbonate infusion before ECMO initiation, or central nervous dysfunction. Outcomes of interest were duration of mechanical ventilation and ECMO, ICU and hospital length of stay (LOS), ICU and inhospital mortality, and ECMO-related complications. ECMO-related mechanical complications included cannula malpositioning requiring readjustment, vessel perforation, accidental decannulation, or an ECMO circuit change required. Major bleeding was defined as intracranial hemorrhage or any bleeding requiring blood transfusion/s or medical intervention. Approval from our institutional review board was obtained for this study (CIRB 2016/2929).
Statistical Analysis
Data are presented as n (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. Patients were divided into two groups: a pre-ECIT period (before January 2018) and a post-ECIT period (after January 2018). Patients in the pre- and post-ECIT periods were compared with respect to demographic and pre-ECMO clinical characteristics by using the two-sided t test or Mann-Whitney U test for continuous variables and the Chi-square test or Fisher exact test as appropriate for categorical variables. Univariate and multivariate logistic regression analyses were performed to identify the factors associated with survival, and covariates that were significant (p ≤ 0.10) on univariate analysis were included in the multivariate analysis. Statistical difference was considered significant at p ≤ 0.05. All statistical analyses were performed using the SPSS software (IBM SPSS Statistics Version 22, Chicago, IL).
RESULTS
Patient Demographics and Pre-ECMO Clinical Characteristics
One hundred and eight patients underwent VV ECMO during the study period, of which 27 patients (25%) were managed by a multidisciplinary team led by ECMO intensivists. A summary of baseline clinical characteristics and physiologic data prior to ECMO initiation is presented in Table 1. The mean age was 46.8 ± 15.4 years and 62 patients (57.4%) were male. Almost half of cases (49.1%) were retrieved from other hospitals. The median P/F ratio before ECMO initiation was 63 (IQR, 53–79). Sixty-one (56.5%) and 15 (13.9%) patients received prior neuromuscular blockade and prone-positioning, respectively. Pneumonia was the primary etiology of ARDS in 93 patients (86.1%). Among these patients, 22 (23.6%) were bacterial pneumonia, whereas 44 (47.3%) were viral, with influenza being the most common (Table 2). Eleven patients (10.2%) were immunocompromised: five had underlying hematological or solid organ malignancies, one had AIDS, and five were on long-term immunosuppressive therapy.
TABLE 1.
Demographics and Clinical Characteristics of Patients Before and After Initiation of an Extracorporeal Membrane Oxygenation Intensivist-Led Multidisciplinary Team
| All Patients (n = 108) | Pre-ECIT (n = 81) | Post-ECIT (n = 27) | p | |
|---|---|---|---|---|
| Age, yr | 46.8 ± 15.4 | 45 ± 15 | 52 ± 17 | 0.042 |
| Male gender | 62 (57.4) | 46 (56.8) | 16 (59.2) | 0.822 |
| Body mass index, kg/m2 | 26.11 ± 6.81 | 25.7 ± 7.0 | 27.3 ± 6.2 | 0.289 |
| Charlson comorbidity index | 1 (0–2) | 1 (0–2) | 0 (0–1) | 0.054 |
| Immunocompromised host | 11 (10.2) | 8 (9.9) | 3 (11.1) | 1.000 |
| Sequential Organ Failure Assessment score | 10 (8–14) | 10 (8–14) | 11 (8–15) | 0.435 |
| Respiratory Extracorporeal membrane oxygenation Survival Prediction score | 4 (2–6) | 4 (3–6) | 4 (2–7) | 0.693 |
| PRedicting dEath for SEvere Acute Respiratory Distress Syndrome on Venovenous ECMO score | 3 (1–5) | 3 (1–5) | 4 (2–5) | 0.577 |
| Retrieval from other hospitals | 53 (49.1) | 34 (42.0) | 19 (70.4) | 0.011 |
| Primary diagnosis | ||||
| Pneumonia | 93 (86.1) | 72 (88.9) | 21 (77.8) | 0.197 |
| Viral pneumonia | 48 (44.4) | 40 (49.4) | 8 (29.7) | 0.074 |
| Pre-ECMO characteristics | ||||
| Hypotension requiring vasopressors | 59 (54.6) | 43 (53.1) | 16 (59.2) | 0.577 |
| Cardiac arrest before ECMO | 7 (6.5) | 6 (7.4) | 1 (4.0) | 0.677 |
| Days on mechanical ventilation | 1 (0–3) | 1 (0–3) | 1 (0–1) | 0.013 |
| Pre-ECMO ventilator settings | ||||
| Peak inspiratory pressure, cm H2O | 35 (32–34)a | 35 (32–40)a | 35 (30–38)a | 0.605 |
| Positive end-expiratory pressure, cm H2O | 15 (12–18)b | 16 (14–18)b | 15 (12–16)b | 0.079 |
| Tidal volume/predicted body weight, mL/kg | 5.95 ± 1.11c | 6.18 ± 1.44c | 5.82 ± 0.89c | 0.414 |
| Pre-ECMO arterial blood gas | ||||
| pH | 7.23 ± 0.14 | 7.25 ± 0.14 | 7.17 ± 0.15 | 0.020 |
| Paco2, mm Hg | 61.6 ± 23.2 | 60.5 ± 24.0 | 64.8 ± 21.0 | 0.424 |
| Pao2, mm Hg | 67.6 ± 23.8 | 64.0 ± 19.5 | 78.5 ± 31.5 | 0.007 |
| Pao2/Fio2 ratio | 63 (53–79) | 60 (51–77) | 68 (56–84) | 0.059 |
| Pre-ECMO treatment | ||||
| Proning | 15 (13.9) | 8 (9.9) | 7 (25.9) | 0.053 |
| Neuromuscular blockade | 61 (56.5) | 35 (43.2) | 26 (96.3) | < 0.001 |
ECIT = extracorporeal membrane oxygenation intensivist-led multidisciplinary team, ECMO = extracorporeal membrane oxygenation.
aData missing for 48, 39, and nine patients in all patients, pre-ECIT, and post-ECIT groups, respectively.
bData missing for 25, 21, and four patients in all patients, pre-ECIT, and post-ECIT groups, respectively.
cData missing for 78, 70, and eight patients in all patients, pre-ECIT, and post-ECIT groups, respectively.
TABLE 2.
Primary Etiology of Respiratory Failure
| All Patients (n = 108) | Pre-ECIT (n = 81) | Post-ECIT (n = 27) | |
|---|---|---|---|
| Pneumonia (no organism identified) | 20 (18.5) | 15 (18.5) | 5 (18.5) |
| Bacterial pneumonia | 22 (20.4) | 16 (19.8) | 6 (22.2) |
| Streptococcus pnuemoniae | 5 (4.6) | 2 (2.5) | 3 (11.1) |
| Mycobacterium tuberculosis | 5 (4.6) | 5 (6.2) | 0 (0.0) |
| Viral pneumonia | 44 (41.9) | 36 (44.4) | 8 (29.6) |
| Influenza | 28 (25.9) | 21 (25.9) | 7 (25.9) |
| Viral and bacterial pneumonia | 5 (4.6) | 4 (4.9) | 1 (3.7) |
| Pneumocystis pneumonia | 2 (1.9) | 1 (1.2) | 1 (3.7) |
| Near drowning | 3 (2.8) | 2 (2.5) | 1 (3.7) |
| Drug/chemical pneumonitis | 3 (2.8) | 3 (3.7) | 0 (0.0) |
| Nonpulmonary sepsis with acute respiratory distress syndrome | 3 (2.8) | 3 (3.7) | 0 (0.0) |
| Interstitial lung disease | 3 (2.8) | 1 (1.2) | 2 (7.4) |
| Asthma | 3 (2.8) | 0 (0.0) | 3 (11.1) |
ECIT = extracorporeal membrane oxygenation intensivist-led multidisciplinary team.
Data are expressed as n (%).
Comparing Clinical Characteristics and Outcomes Between the Pre- and Post-ECIT Periods
A comparison of clinical characteristics between the patients in the pre-ECIT and post-ECIT groups is summarized in Table 1. Between the two periods, there were no significant differences in Charlson comorbidity index (CCI), SOFA, RESP, and PRESERVE scores, or the proportion of immunocompromised hosts. A higher pre-ECMO Pao2 was seen in the post-ECIT period although this was not associated with a significant difference in P/F ratios. A larger proportion of patients in the post-ECIT period was retrieval cases from other hospitals (70.4% vs 42.0%, p = 0.011) and received neuromuscular blockade (96.3% vs 43.2%, p < 0.001) before ECMO initiation. They were also older in age and had fewer days on mechanical ventilation before ECMO.
VV ECMO clinical outcomes and complications are summarized in Table 3. Inhospital mortality was three-fold lower during the post-ECIT period (14.8% vs 44.4%, p = 0.006). In addition, ICU mortality (11.1% vs 40.7%, p = 0.005), ICU LOS (12 days [IQR, 6–16 d] vs 15 days [IQR, 10–24 d], p = 0.049] and the occurrence rate of nosocomial infections (18.5% vs 46.9%, p = 0.009) were lower in the post-ECIT period. There was also a trend toward a lower incidence of major bleeding (4.0% vs 19.8%, p = 0.065). None of the patients in the post-ECIT period had intracranial hemorrhage or suffered a nonhemorrhagic stroke. There were no significant differences observed with inhospital and ICU mortalities between the patients managed during the time periods 2008–2013 and 2014–2017 (Fig. 1).
TABLE 3.
Outcomes and Complications of Venovenous Extracorporeal Membrane Oxygenation
| All Patients (n = 108) | Pre-ECIT (n = 81) | Post-ECIT (n = 27) | p | |
|---|---|---|---|---|
| Outcomes | ||||
| Inhospital mortality | 40 (37.0) | 36 (44.4) | 4 (14.8) | 0.006 |
| ICU mortality | 36 (33.3) | 33 (40.7) | 3 (11.1) | 0.005 |
| Hospital LOS, d | 26 (15–44) | 25 (14–41) | 25 (17–47) | 0.723 |
| ICU LOS, d | 15 (10–22) | 15 (10–24) | 12 (6–16) | 0.049 |
| Duration of ECMO, d | 6 (4–11) | 7 (5–12) | 6 (4–11) | 0.295 |
| Days on mechanical ventilation | 13 (9–20) | 14 (9–22) | 12 (6–15) | 0.065 |
| Renal replacement therapy required | 50 (46.3) | 37 (45.7) | 13 (48.1) | 0.824 |
| Conversion to venoarterial extracorporeal membrane oxygenation | 7 (6.5) | 7 (8.6) | 0 (0.0) | 0.189 |
| Complications | ||||
| ECMO-related mechanical complications | 16 (14.8) | 13 (16.0) | 3 (11.1) | 0.756 |
| Thrombosis (deep vein thrombosis and/or pulmonary embolism) | 24 (22.2) | 17 (21.0) | 7 (25.9) | 0.600 |
| Major bleeding | 17 (15.7) | 16 (19.8) | 1 (4.0) | 0.065 |
| Intracranial hemorrhage | 8 (7.4) | 8 (9.9) | 0 (0) | 0.197 |
| Pneumothorax | 18 (16.7) | 16 (19.8) | 2 (7.4) | 0.231 |
| Nosocomial infections | 43 (39.8) | 38 (46.9) | 5 (18.5) | 0.009 |
| Nonhemorrhagic stroke | 3 (2.8) | 3 (3.7) | 0 (0.0) | 0.572 |
ECIT = extracorporeal membrane oxygenation intensivist-led multidisciplinary team, ECMO = extracorporeal membrane oxygenation, LOS = length of stay.
Figure 1.
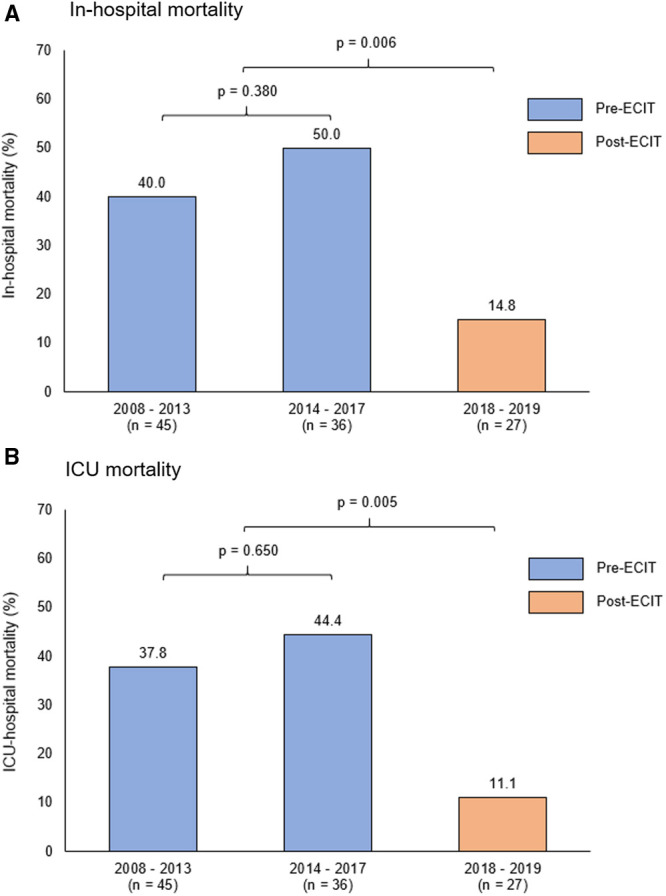
Comparison of outcomes during time periods: 2008–2013, 2014–2017, and 2018–2019 (postextracorporeal membrane oxygenation intensivist-led multidisciplinary team [ECIT]). (A) Inhospital mortality and (B) ICU mortality rates are illustrated showing significantly lower mortality rates in the post-ECIT period, with no significant difference seen between the time periods 2008–2013 and 2014–2017.
Comparing Survivors and Nonsurvivors
A comparison in clinical characteristics of survivors versus nonsurvivors is summarized in Table 4. Patients who survived to hospital discharge had lower CCI and SOFA scores, and higher BMI and RESP scores. A lower proportion of these patients had vasopressor support before ECMO initiation. On multivariate analysis, treatment in the post-ECIT period remained associated with improved survival (odds ratio, 5.06; 95% CI, 1.20–21.28), after correction for possible confounders (Table 5). A higher RESP score also remained significantly associated with improved survival on multivariate analysis. Patients who survived to hospital discharge had a lower incidence of major bleeding (10.3% vs 25.0%, p = 0.043), nonhemorrhagic stroke (0.0% vs 7.5%, p = 0.048), and a lower incidence of intracranial hemorrhage (2.9% vs 15.2%, p = 0.050).
TABLE 4.
Comparing Clinical Characteristics and Extracorporeal Membrane Oxygenation Complications of Survivors and Nonsurvivors
| Nonsurvivors (n = 40) | Survivors (n = 68) | p | |
|---|---|---|---|
| Age, yr | 48 ± 14 | 46 ± 16 | 0.582 |
| Male gender | 22 (55.0) | 40 (58.8) | 0.698 |
| Body mass index, kg/m2 | 23.9 ± 6.01 | 27.4 ± 6.9 | 0.009 |
| Charlson comorbidity index | 1 (0–2) | 0 (0–1) | 0.018 |
| Immunocompromised host | 6 (15.0) | 5 (7.4) | 0.323 |
| Sequential Organ Failure Assessment score | 13 (8–16) | 10 (8–13) | 0.003 |
| Respiratory Extracorporeal membrane oxygenation Survival Prediction score | 3 (0–4) | 4 (3–6) | < 0.001 |
| PRedicting dEath for SEvere ARDS on Venovenous ECMO score | 4 (2–5) | 3 (1–4) | 0.121 |
| Retrieval from other hospitals | 22 (55.0) | 31 (45.6) | 0.345 |
| Primary diagnosis | |||
| Pneumonia | 34 (85.0) | 59 (86.8) | 0.798 |
| Viral pneumonia | 16 (40.0) | 32 (47.1) | 0.479 |
| Pre-ECMO characteristics | |||
| Hypotension requiring vasopressors | 29 (72.5) | 30 (44.1) | 0.004 |
| Cardiac arrest before ECMO | 5 (12.5) | 2 (2.9) | 0.099 |
| Days on mechanical ventilation | 2 (0–4) | 1 (0–2) | 0.084 |
| Pre-ECMO ventilator settings | |||
| Peak inspiratory pressure, cm H2O | 34 (32–42)a | 35 (30–39)a | 0.565 |
| Positive end-expiratory pressure, cm H2O | 15 (12–18)b | 15 (12–18)b | 0.846 |
| Tidal volume/predicted body weight, mL/kg | 5.94 ± 1.49c | 5.96 ± 0.87c | 0.880 |
| Pre-ECMO arterial blood gas | |||
| Ph | 7.21 ± 0.13 | 7.24 ± 0.15 | 0.306 |
| Paco2, mm Hg | 65.4 ± 24.0 | 59.4 ± 22.7 | 0.209 |
| Pao2, mm Hg | 65.0 ± 21.9 | 69.1 ± 24.8 | 0.398 |
| Pao2/Fio2 ratio | 60 (51–77) | 68 (56–84) | 0.455 |
| Pre-ECMO treatment | |||
| Proning | 4 (10.0) | 11 (16.2) | 0.370 |
| Neuromuscular blockade | 19 (47.5) | 42 (61.8) | 0.149 |
| Treatment during post-ECMO intensivist-led multidisciplinary team period | 4 (10.0) | 23 (33.8) | 0.006 |
| Complications | |||
| ECMO-related mechanical complications | 7 (17.5) | 9 (13.2) | 0.547 |
| Thrombosis (deep vein thrombosis and/or pulmonary embolism) | 6 (15.0) | 18 (26.5) | 0.166 |
| Major bleeding | 10 (25.0) | 7 (10.3) | 0.043 |
| Intracranial hemorrhage | 6 (15.2) | 2 (2.9) | 0.050 |
| Pneumothorax | 10 (25.0) | 8 (11.8) | 0.075 |
| Nosocomial infections | 20 (50.0) | 23 (33.8) | 0.097 |
| Nonhemorrhagic stroke | 3 (7.5) | 0 (0) | 0.048 |
ECMO = extracorporeal membrane oxygenation.
aData missing for 19 and 29 patients in nonsurvivors and survivors, respectively.
bData missing for 12 and 13 patients in nonsurvivors and survivors, respectively.
cData missing for 29 and 49 patients in nonsurvivors and survivors, respectively.
TABLE 5.
Multivariate Logistic Regression Analysis for Survival to Hospital Discharge
| OR (95% CI) | p | |
|---|---|---|
| Body mass index, kg/m2 | 1.087 (0.997–1.185) | 0.058 |
| Charlson comorbidity index | 1.034 (0.755–1.415) | 0.836 |
| Sequential Organ Failure Assessment score | 0.858 (0.718–1.025) | 0.092 |
| Respiratory Extracorporeal membrane oxygenation Survival Prediction score | 1.412 (1.092–1.826) | 0.008 |
| Hypotension requiring vasopressor support | 0.741 (0.181–3.029) | 0.677 |
| Cardiac arrest before ECMO initiation | 0.617 (0.075–5.046) | 0.652 |
| Days on Mechanical ventilation | 0.964 (0.802–1.158) | 0.693 |
| Management by an ECMO intensivist-led team | 5.056 (1.201–21.280) | 0.027 |
ECMO = extracorporeal membrane oxygenation, OR = odds ratio.
DISCUSSION
Our results suggest that management by an ECIT is associated with improved survival and a shorter ICU LOS in patients supported with VV ECMO for severe respiratory failure. The magnitude of our findings (14.8% mortality rate in the post-ECIT period) is remarkable, considering that reported mortality rates for VV ECMO in respiratory failure range between 35% and 40% (6, 18).
It is unlikely that differences in pre-ECMO clinical characteristics alone can account for this significant improvement in survival. In the post-ECIT period, patients were in fact older in age. In our cohort, pre-ECMO hypotension, lower BMI, and worse clinical severity scores (e.g., CCI, RESP, and SOFA) were associated with increased mortality, but no significant difference in these factors between the pre- and post-ECIT periods was found. On multivariate analyses correcting for above factors, management during the post-ECIT period remained associated with improved survival. A higher proportion of patients in the post-ECIT period received neuromuscular blockade, with a trend toward an increased use of prone-positioning. Although the evidence for neuromuscular blockade remains uncertain (19), prone-positioning improves survival in moderate-to-severe ARDS (20), including patients initiated on ECMO for respiratory failure (16). Although it is plausible that improved management of ARDS may have led to improved outcomes, it is unlikely that these measures per se would result in mortality reduction of this magnitude. Retrieval cases were more common in the post-ECIT period, but unlikely to improve survival in any significant way. Finally, although general ICU care may have improved over the long pre-ECIT period of 10 years (2008–2017), there were no differences in outcomes between the patients managed before 2014, compared with the 2014–2017 time period to support this.
We report significant mortality reduction with a multidisciplinary team approach, noting the slight difference that our team is helmed by a group of ECMO intensivists that remained unchanged during the study period. Current literature on the impact of a multidisciplinary approach to ECMO management remains unclear with conflicting results (13, 14). Therefore, it is our opinion that overall benefit is likely to be a synergistic effect of these two administrative criteria in team setup. Second, complex decision-making is often required at multiple time points of each ECMO run. For instance, patient selection requires a comprehensive assessment of the anticipated clinical trajectory, underlying etiology, patient’s overall health and physiologic reserve, risk of bleeding complications, optimal timing of ECMO initiation, and location of cannulation in interhospital referrals, among many other factors. “Multiconsultant decision-making,” where decisions on ECMO initiation and withdrawal are not made by a single ECMO intensivist but a second (or third) opinion sought, has been proposed by several experts (10, 11, 21). We find the joint decision-making invaluable, especially when challenging clinical scenarios related to patient selection, complications, and troubleshooting are encountered.
A noteworthy finding is that survival rates in our cohort are not inferior to international standards despite our modest volume of 108 VV ECMO cases over a 12-year period. Barbaro et al analyzed over 10,000 adult ECMO cases from registry data and reported lower mortality rates with higher annual volume of cases (12). Conversely, an analysis of ECMO outcomes based on a large publicly available database reported lower mortality in low-volume centers (15). However, there exist inherent limitations to such databases as they often lack important details (e.g., ECMO mode and disease severity scores) and rely on diagnosis coding for case identification, making interpretation difficult (15). Nevertheless, it is our opinion that the benefits of a dedicated and consistent team are likely to have a greater impact in centers with low or modest volumes. Such a team allows for accumulation of experience (despite lower volumes) and consistency in management, which would not be feasible in a “decentralized” model of rotating ECMO or general intensivists. One potential downside of a specialized team is deskilling of other members of the ICU team. In our center, multidisciplinary case discussions and audits are regularly held. In addition, during day-to-day management of the patient, the ECMO intensivist generally plays a supportive and educational role and the general intensivist and ICU team continue to provide direct ICU care.
The lower nosocomial infections rates in the post-ECIT period and trend toward reduced major bleeding events suggest that minimizing ECMO-related complications may contribute to mortality reduction. In addition, none of the patients in the post-ECIT period suffered from an intracranial hemorrhage or nonhemorrhagic stroke, factors that were significantly associated with higher mortality in our cohort. Management of anticoagulation is central to ECMO care as patients are both at risk of venous thrombosis and circuit clotting, and devastating consequences of bleeding such as an intracranial hemorrhage (22). Furthermore, because the optimal anticoagulation strategy for ECMO is still poorly understood, there is a need to examine the hemostatic system holistically with contextual interpretation of laboratory tests according to the clinical status of the patient and ECMO circuit—specialized areas that will benefit from management by a multidisciplinary team led by ECMO intensivists (23–25). Reviewing and improving our anticoagulation protocol shortly after inception of the ECIT, with a more conservative aPTT target, is likely to have contributed to this as well.
There are limitations to our study. Due to its retrospective nature, we were unable to obtain complete data pertaining to mechanical ventilator settings before ECMO initiation, particularly in retrieval cases. Hence, the RESP and PRESERVE scores that require information on ventilatory support are incomplete for some of our patients. This is likely to explain the higher RESP scores in our cohort compared with other studies. Second, it is probable that improvements in overall ICU care or better compliance with ECMO or ICU protocols may have contributed to the improved outcomes seen in the post-ECIT period. Arguably, the presence of an ECIT that encourages increased compliance with ECMO protocols may serve as a mechanism for improved outcomes. There were, however, no major changes in ICU management protocols going into the post-ECIT period. Third, we were unable to analyze data on rejected ECMO referrals, which may shed more light on the influence of patient selection on outcomes. Finally, patients were only followed up to hospital discharge or death, and it remains unclear if these benefits translated to improved long-term outcomes.
CONCLUSIONS
An ECIT approach was associated with improved patient survival in VV ECMO for severe respiratory failure. This deliberate team organization allows for consistency in ECMO care, guided by joint decisions made by dedicated ECMO intensivists. We believe that this administrative change in team makeup may yield positive clinical outcomes, especially in ECMO centers with low or modest case volumes.
ACKNOWLEDGMENTS
We thank all the nurses, respiratory therapists, pharmacists, occupational and physiotherapists of the Medical ICU, Singapore General Hospital, and the perfusion unit and staff from the Department of Cardiothoracic Surgery, National Heart Centre Singapore, without whom our extracorporeal membrane oxygenation service would not have been possible.
Footnotes
Drs. Goh and Tan contributed equally.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR Trial Collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 2.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018; 378:1965–1975 [DOI] [PubMed] [Google Scholar]
- 3.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: A review. JAMA. 2019; 322:557–568 [DOI] [PubMed] [Google Scholar]
- 4.Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012; 18:99–104 [DOI] [PubMed] [Google Scholar]
- 5.Extracorporeal Life Support Organization. ECLS Registry Report. 2020Available at: https://www.elso.org/Registry/Statistics.aspx. Accessed May 5, 2020
- 6.Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir Med. 2019; 7:163–172 [DOI] [PubMed] [Google Scholar]
- 7.Moll V, Teo EY, Grenda DS, et al. Rapid development and implementation of an ECMO program. ASAIO J. 2016; 62:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotza M, Carboni G, Ballotta A, et al. Modern ECMO: Why an ECMO programme in a tertiary care hospital. Eur Heart J Suppl. 2016; 18:E79–E85 [DOI] [PubMed] [Google Scholar]
- 9.Combes A, Brodie D, Bartlett R, et al. ; International ECMO Network (ECMONet). Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014; 190:488–496 [DOI] [PubMed] [Google Scholar]
- 10.DellaVolpe J, Barbaro RP, Cannon JW, et al. Joint Society of Critical Care Medicine-Extracorporeal Life Support Organization Task Force Position Paper on the role of the intensivist in the initiation and management of extracorporeal membrane oxygenation. Crit Care Med. 2020; 48:838–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullen EC, Teijeiro-Paradis R, Fan E. How I select which patients with ARDS should be treated with venovenous extracorporeal membrane oxygenation. Chest. 2020; 158:1036–1045 [DOI] [PubMed] [Google Scholar]
- 12.Barbaro RP, Odetola FO, Kidwell KM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015; 191:894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komindr A, Abe R, Tateishi Y, et al. Establishing extracorporeal membrane oxygenation team increased number of patients and improved data recording. J Intensive Care. 2019; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Na SJ, Chung CR, Choi HJ, et al. The effect of multidisciplinary extracorporeal membrane oxygenation team on clinical outcomes in patients with severe acute respiratory failure. Ann Intensive Care. 2018; 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy FH, McDermott KM, Spragan D, et al. Unconventional volume-outcome associations in adult extracorporeal membrane oxygenation in the United States. Ann Thorac Surg. 2016; 102:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013; 39:1704–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014; 189:1374–1382 [DOI] [PubMed] [Google Scholar]
- 18.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization registry international report 2016. ASAIO J. 2017; 63:60–67 [DOI] [PubMed] [Google Scholar]
- 19.Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 21.Bohman JK, Ratzlaff RA, DeMartino ES, et al. Approach to adult extracorporeal membrane oxygenation patient selection. Crit Care Med. 2020; 48:618–622 [DOI] [PubMed] [Google Scholar]
- 22.Lorusso R, Gelsomino S, Parise O, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: Findings from the extracorporeal life support organization database. Crit Care Med. 2017; 45:1389–1397 [DOI] [PubMed] [Google Scholar]
- 23.Kurihara C, Walter JM, Karim A, et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 2020; 110:1209–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chlebowski MM, Baltagi S, Carlson M, et al. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020; 24:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aubron C, McQuilten Z, Bailey M, et al. ; Endorsed by the International ECMO Network (ECMONet). Low-dose versus therapeutic anticoagulation in patients on extracorporeal membrane oxygenation: A pilot randomized trial. Crit Care Med. 2019; 47:e563–e571 [DOI] [PubMed] [Google Scholar]


