Before the coronavirus disease 2019 (COVID-19) pandemic, antimicrobial resistance was the world's most pressing health crisis. The United Nations, the World Health Organization (WHO) and the European Center for Disease Control and Prevention have made antimicrobial resistance a top priority, establishing a task force in the G-20 and organizing a joint program initiative on antimicrobial resistance including countries from al over the world [1]. However, with the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus pandemic in January 2020, all efforts are now focused on COVID-19, including controlling infections and developing vaccines [2].
Viral infections not only produce disease but also pave the way for secondary bacterial infections that can be more invasive and life-threatening than the initial viral infection. With SARS-CoV-2, the macrolide antibiotic azithromycin has been one of the recommended therapies against COVID-19, along with hydroxychloroquine or remdesivir [3,4], apart from other drugs like tocilizumab [5]. Although there is no robust evidence of the efficacy of azithromycin against SARS-CoV-2, a significant increase in the use of the antibiotic is likely to occur. This could trigger a second post–COVID-19 scenario of bacterial coinfections caused by pathogens that are more resistant to antibiotics than the ones we know today, putting our current healthcare system at risk.
The first COVID-19 patient in Spain was diagnosed on 31 January 2020 in the Canary Islands; by 31 March, Spain had 62,095 hospitalized patients, 6730 patients in intensive care units and 8674 deaths (https://cnecovid.isciii.es/covid19/#niveles-de-gravedad).
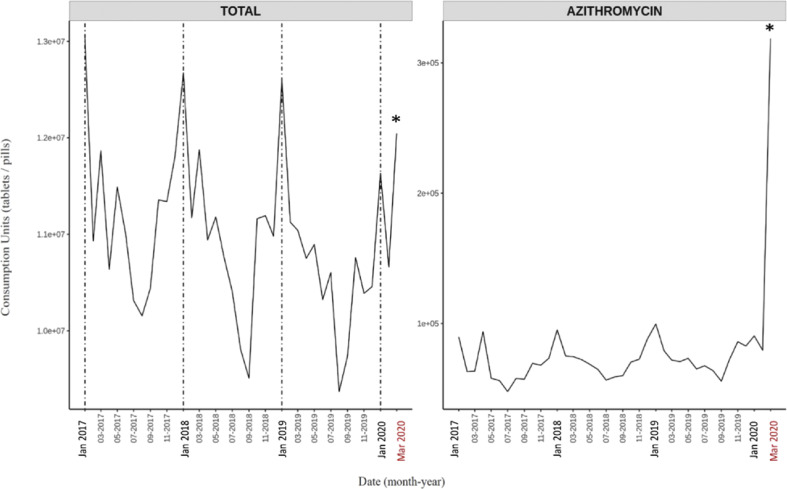
In order to assess the use of antibiotics during the COVID-19 crisis, I have recorded the use of the antibacterials for systemic use in Spain in March 2020 and compared the data with the antibiotic consumption from January 2017 to February 2020 (IQVIA, Spain, Fig. 1 ).
Fig. 1.
Use of antibiotics in Spanish hospitals from January 2017 to 31 March 2020. Total intrahospital antibiotic (left) and azithromycin (right) use show consumption peaks in March 2020 during the coronavirus disease 2019 (COVID-19) pandemic. Data used for analysis are based on hospital consumption information of molecules included in ATC (EphMRA Anatomical Classification System) class J01 provided by IQVIA's EMH database from January 2017 to March 2020 (IQVIA Information SA, all rights reserved). EMH database contains drug consumption data from a representative sample of hospitals (n = 97) projected to the national level. Analysis was performed in units indicating each intravenous bottle, pill, syringe and so on consumed. Asterisks denote statistical significance (α = 0.05).
The timeline since January 2017 shows a typical seasonal curve, with maximum antibiotic use in the winter months, peaking in January and an overall decrease over the years, likely as a result of the Spanish National Action Plan [6,7]. In March 2020, with a surge of COVID-19 cases in Spain, antibiotic consumption increased significantly 11.5% compared to February 2020, surpassing the level of the January 2020 peak.
To assess if this sudden increase had an impact in the type of antibiotics administered, 60 classes of antibiotics belonging to all antimicrobial families were analysed. The use of azithromycin in March 2020 was 400% the use of the same molecule in February 2020, and more than 320% the consumption of azithromycin in January 2019, the month with the highest rate recorded since January 2017 (Fig. 1). Thus, COVID-19 has contributed to a substantial increase in the use of azithromycin in Spanish hospitals. This is of great concern, as this could have an effect on the resistance levels to this drug and can rapidly lessen the usefulness of this macrolide in the future. However, when other antibiotics were analysed, another seven antibiotics reached the highest rates of use in March 2020, up to levels not seen since January 2017 (Supplementary Fig. S1). These antimicrobials and their increase with respect to February 2020 were ceftaroline fosamil (183%), ceftolozane/tazobactam (103%), cefditoren (261%), ceftriaxone (204%), colistin (145%), doxycycline (517%) and linezolid (189%). A further four antibiotics – amoxicillin, cefixime, erythromycin and levofloxacin – underwent a significant increase with respect to February 2020. Most of these antibiotics are not specifically targeted against respiratory bacteria and are broad-spectrum antibiotics acting against a wide variety of diseases and bacterial families, including Gram-positive and Gram-negative pathogens.
The data presented here show antibiotic use until 31 March 2020, as the SARS-CoV-2 pandemic was just beginning in most countries, including Spain. The increase of antibiotics includes last-resort antibiotics that the WHO in 2019 classified as critical antimicrobials due to their resistance levels [8]. The data indicate that we will have a post–COVID-19 scenario where antimicrobial resistance and antimicrobial resistant nosocomial infections could come back with greater strength. However, if the COVID-19 crisis has shown us anything, it is that an increase in intrahospital infection control measures is effective [9]. These same measures could be equally effective in controlling the spread of multidrug-resistant bacteria within hospitals, which could lead to a better management of nosocomial infections. We do not yet know if these measures will be able to counterbalance the potential increase in resistance to last-resort antibiotics in hospitals. We do know, however, that the use of antibiotics will be higher as the number of clinical COVID-19 cases rises and that our therapeutic options to treat bacterial infections will be even more limited in the future. Following antibiotic stewardship programmes worldwide in these months of the COVID-19 pandemic, especially in this second wave, is and will be critical to the welfare and preservation of society.
Acknowledgements
I would like to thank Jose Luis Fernández, Ramón Pérez and Patricia Frías from IQVIA for their kind support and help with the data; Carlos Serna from ARU at UCM for help with the figures; Marina Cuesta from the DSLAB at the URJC for assistance with statistical analysis; and Heather Benit for suggestions on the text.
Editor: J. Rodriguez-Baño
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.09.055.
Transparency declaration
All authors report no conflicts of interest relevant to this article.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.van Hengel A.J., Marin L. Research, innovation, and policy: an alliance combating antimicrobial resistance. Trends Microbiol. 2019;27:287–289. doi: 10.1016/j.tim.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Nature. COVID research updates. 2020. https://www.nature.com/articles/d41586-020-00502-w Available at: [Google Scholar]
- 3.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez-Baño J., Pachón J., Carratalà J., Ryan P., Jarrín I., Yllescas M. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol Infect. 2021;27:244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control (ECDC) 2020. Antimicrobial consumption database (ESAC-Net)https://www.ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database Available at: [Google Scholar]
- 7.Spanish Medical Agency . 2020. Strategic action plan to reduce the risk of selection and dissemination of antibiotic resistance.http://www.resistenciaantibioticos.es/en/node/388 Available at: [Google Scholar]
- 8.World Health Organization (WHO) 6th rev. ed. WHO; Geneva: 2019. Critically important antimicrobials for human medicine.https://www.who.int/foodsafety/publications/antimicrobials-sixth/en/ Available at: [Google Scholar]
- 9.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. Erratum in: Nat Med 2020;26:1491–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.