Abstract
Background
Congestive heart failure (CHF) is a complex clinical syndrome, with high morbidity and mortality. Serum anion gap (SAG) is associated with the severity of various cardiovascular diseases. However, the role of SAG indicators in CHF is unclear.
Methods and Results
A retrospective analysis of data from Multiparameter Intelligent Monitoring in Intensive Care III version 1.4 was conducted in critically ill patients with CHF. The clinical information of each patient, including demographic data, comorbidities, vital signs, scores, and laboratory indicators, were successfully obtained. Cox proportional hazards models were used to determine the relationship between SAG and mortality in patients with CHF, the consistency of which was further verified by subgroup analysis.
Results
A total of 7426 subjects met the inclusion criteria. Multivariate analysis showed that after adjusting for age, gender, ethnicity, and other potential confounders, increased SAG was significantly related to an increase in 30- and 90-day all-cause mortalities of critically ill patients with CHF compared with decreased SAG (tertile 3 versus tertile 1: adjusted hazard ratio, 95% confidence interval: 1.74, 1.46–2.08; 1.53, 1.32–1.77). Subgroup analysis indicated that the association between SAG and all-cause mortality presented similarities in most strata.
Conclusion
SAG at admission could be a promising predictor of all-cause mortality in critically ill patients with CHF.
1. Introduction
Congestive heart failure (CHF) is the end-stage manifestation of various cardiovascular diseases with structural and functional disruptions in the myocardium, causing restricted ventricular ejection or filling, systemic blood circulation disorder, insufficient perfusion of tissues and organs, and ultimate death [1]. CHF has become a major public health problem with more than 23 million patients affected worldwide, and it still has a high mortality despite great progress in diagnosis and treatment for the last few decades [2]. Thus, how to early identify high-risk patients, determine their prognosis, and formulate effective individualized interventions have attracted growing attention from clinicians in recent years.
Metabolic acidosis is a common complication of CHF; it is closely related to ischemia and hypoxia of tissue caused by hemodynamic disorders and the use of diuretics [3]. In turn, acidosis and accompanying hyperkalemia could further weaken myocardial contractility, creating a vicious circle. Acidosis serves as the independent predictor for the long-term prognosis of CHF, and pH value could help to stratify the risks [4]. However, the predictive value of other laboratory parameters that reflect the acid-base imbalance in CHF requires more evidence, such as serum anion gap (SAG).
SAG refers to the difference between undetermined anions and cations. It indicates the concentration of fixed acids in plasma, and it is a commonly used and easily obtained laboratory parameter of acid-base imbalance [5]. Some recent studies have confirmed that SAG is elevated and closely associated with poor prognosis of various diseases, including acute pesticide poisoning [6], sepsis [7], acute and chronic kidney injury [8, 9], trauma [10], and coronary artery disease [11]. However, whether the anion gap could be used as a prognostic marker for critically ill patients with CHF remains unclear. Therefore, the present study is aimed at investigating the association between SAG and mortality in these patients.
2. Materials and Methods
2.1. Data Source
The Multiparameter Intelligent Monitoring in Intensive Care III version 1.4 (MIMIC-III v1.4) is a freely accessible critical care database developed and operated by the Massachusetts Institute of Technology; it contains detailed clinical data of 53,423 adult patients (age more than 16 years) from June 2001 to October 2012 in the intensive care units (ICUs) of Beth Israel Deaconess Medical Center [12]. Before implementing the present research, author Tang completed and passed the CITI “Data or Specimens Only Research” course (No. 9014457) and obtained authorization for database access. In need of special note here is that this database was approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA), and no additional ethical approval needed to be provided.
2.2. Study Population and Design
In the MIMIC-III database, all adult patients aged over 18 years old, first admitted to the ICU, and diagnosed with CHF were included in the study in accordance with the ninth revision of the International Classification of Diseases (ICD) code (code = 428.0). Patients with any of the following criteria were excluded from the study: (1) ICU stay time of less than 24 hours, (2) no anion gap results within 24 hours of admission to the ICU, and (3) survival time of less than 0 (some organ donors may die earlier than admission). The workflow is shown in Figure 1.
Figure 1.
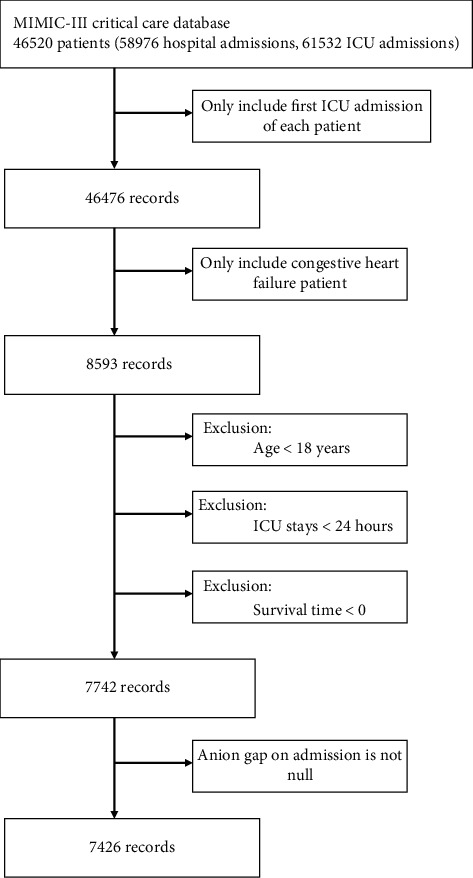
Workflow of the data extraction. The inclusion and exclusion criteria of the study subjects were revealed, and 7426 subjects were finally included. ICU: intensive care unit.
The starting point of the study was defined as the time of admission to the ICU, and the endpoint was the time of death, 30, or 90 days after admission. The patients' 30- and 90-day mortalities were chosen as the primary outcome of interest, and death data were extracted from the Social Security Death Index. The secondary outcomes were the readmission rate and the composite outcome of major adverse cardiac events (MACEs), including all-cause mortality, hospitalization with acute heart failure, heart transplantation, and mechanical circulatory support [13].
2.3. Data Extraction and Preparation
Structured query language (SQL) was utilized to extract clinical data with PostgreSQL tools (version 9.6), including demographics, comorbidities, vital signs, severity scores, laboratory tests, and interventions. Demographics included age, gender, and ethnicity. For the privacy protection of patients, the database has had the date of birth shifted to exactly 300 years for patients older than 89 years. Before the analysis, the age of these patients was adjusted in accordance with the following formula: realage = age − 300 + 89 [14]. Vital signs consisted of temperature, respiratory rate (RR), heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), and percutaneous oxygen saturation (SpO2). Comorbidities included acute myocardial infarction (AMI), atrial fibrillation, valvular heart diseases, pulmonary circulation diseases, hypertension (HBP), diabetes, pneumonia, respiratory failure, liver disease, renal failure, stroke, and malignancy. The sequential organ failure assessment (SOFA) score and the simplified acute physiology score II (SAPSII) were calculated for each patient when entering the ICU to assess the severity of the disease [15, 16]. Laboratory tests within 24 hours after ICU admission were also extracted, including SAG, white blood cell (WBC), platelet, hemoglobin, blood urea nitrogen (BUN), creatinine, sodium, potassium, chloride, bicarbonate, glucose, prothrombin time (PT), activated partial thromboplastin time (APTT), lactate, and plasma N-terminal probrain natriuretic peptide (NT-proBNP). Interventions included vasopressor, dialysis, and mechanical ventilation.
After extraction was performed, the raw data were merged and disposed with patient identifiers by using STATA version 16 (https://www.stata.com/). The “winsorize” function was used to reduce the effect of outliers, and multivariate multiple imputation with chained equations was utilized to impute the missing values. The details of the missing value are shown in Table 1. More than 20% of the subjects in this study cohort did not have records of lactate and NT-proBNP, which was converted and considered as a dummy variable in the models to avoid possible bias caused by direct filling of missing values [17].
Table 1.
Details of missing values.
| Variables | The number of missing values | The percent of missing values | Variables | The number of missing values | The percent of missing values |
|---|---|---|---|---|---|
| SBP | 39 | 0.5% | Glucose | 48 | 0.6% |
| DBP | 39 | 0.5% | Lactate | 1993 | 26.8% |
| MBP | 32 | 0.4% | NT-proBNP | 6231 | 83.9% |
| RR | 37 | 0.5% | PT | 111 | 1.5% |
| HR | 32 | 0.4% | APTT | 137 | 1.8% |
| SpO2 | 34 | 0.5% | Sodium | 1 | <0.1% |
| Temperature | 255 | 3.4% | Potassium | 1 | <0.1% |
| Weight | 484 | 6.5% | Chloride | 1 | <0.1% |
| WBC | 68 | 0.9% | Bicarbonate | 2 | <0.1% |
| Platelet | 65 | 0.8% | Creatinine | 2 | <0.1% |
| Hemoglobin | 88 | 1.1% | BUN | 4 | <0.1% |
Note: SBP: systolic blood pressure; DBP: diastolic blood pressure; MBP: mean blood pressure; RR: respiratory rate; HR: heart rate; SpO2: percutaneous oxygen saturation; WBC: white blood cell; BUN: blood urea nitrogen; PT: prothrombin time; APTT: activated partial thromboplastin time; NT-proBNP: N-terminal probrain natriuretic peptide.
2.4. Statistical Analysis
The baseline data of all subjects were divided into three groups by SAG tertiles. Continuous variables were presented as mean ± SD or medians and interquartile range. Kruskal-Wallis test was used to conduct a hypothesis test for continuous variables. Categorical variables were expressed by numbers and percentages, which were analyzed using Chi-square (or Fisher's exact) tests. A generalized additive model (GAM) was used to determine the nonlinear association between SAG and 30-day all-cause mortalities in critically ill patients with CHF. In addition, the relationship between SAG and the survival in patients with HF was visually shown through the Kaplan–Meier (K–M) curve and tested using the log-rank test.
Cox proportional hazard model was used to analyze the relationship between SAG and 30-day and 90-day all-cause mortality in critically ill patients with HF and determine the independent prognostic value of SAG in these patients, with the first tertile or quartile as the reference and adjusting for potential confounders. Two multivariate models were conducted, and the results were described as hazard ratio (HR) with 95% confidence intervals (CIs). In model I, the covariates were only adjusted for the confounders' age, sex, and ethnicity. On the basis of model I, model II was further adjusted for other confounders, including temperature, HR, RR, SBP, DBP, SpO2, weight, atrial fibrillation, liver disease, valvular heart diseases, pulmonary circulation diseases, pneumonia, respiratory failure, diabetes, stroke, malignancy, SOFA, SAPSII, lactate, NT-proBNP, PT, WBC, BUN, creatinine, potassium, bicarbonate, glucose, vasopressor, dialysis, and mechanical ventilation. These factors were chosen as confounders on the basis of their association with the outcomes or a change in effect estimate exceeding 10% [18]. Variance inflation factor was used to test the collinearity between variables with 5 as the threshold, and the variable MBP and chloride were deleted. The associations between SAG and readmission rate and MACEs were determined using multivariate logistic regression with results expressed as the odds ratio (OR) with 95% CIs. Besides, a subgroup analysis of the correlation between SAG and 30-day all-cause mortality was performed to examine whether the effect of SAG in various subgroups differed. All statistical analyses were performed on EmpowerStats version 2.20 (http://www.empowerstats.com/cn/, X&Y solutions, Inc., Boston, MA) and R software version 3.4.3; P < 0.05 (two-sided) was considered statistically significant.
3. Results
3.1. Clinical Characteristics of Subjects
A total of 7426 subjects were included in this retrospective study, 3971 of whom were male and 3455 were female. The age of the subjects was generally high, with a median of 75.3 years old. The subjects were mostly white, accounting for 73.3 percent share of the total. And the 30- and 90-day overall mortalities were 17.7% and 24.4%, respectively. The clinical characteristics of these subjects stratified by SAG tertiles are shown in Table 2. A total of 2334 subjects were in the low-SAG group (tertile 1, SAG < 13), 2519 subjects were in the mid-SAG group (tertile 2, SAG ≥ 13, and SAG < 16), and 2573 subjects were in the high-SAG group (tertile 3, SAG ≥ 16). Subjects with higher SAG levels had more comorbidities of HBP, AMI, diabetes, renal failure, and respiratory failure, with higher mortality, higher SOFA and SAPSII scores, and higher rates of use of vasopressor, dialysis, and mechanical ventilation.
Table 2.
The clinical characteristics of critically ill patients with CHF according to SAG levels.
| Characteristics | Serum anion gap (mmol/L) | P value | ||
|---|---|---|---|---|
| <13 (n = 2334) | ≥13, <16 (n = 2519) | ≥16 (n = 2573) | ||
| Age (years) | 72.2 ± 13.2 | 73.2 ± 13.2 | 72.5 ± 13.5 | 0.013 |
| Gender, n (%) | 0.278 | |||
| Male | 1280 (54.8) | 1332 (52.9) | 1359 (52.8) | |
| Female | 1054 (45.2) | 1187 (47.1) | 1214 (47.2) | |
| Ethnicity, n (%) | 0.002 | |||
| White | 1744 (74.7) | 1881 (74.7) | 1821 (70.8) | |
| Black | 152 (6.5) | 187 (7.4) | 230 (8.9) | |
| Other | 438 (18.8) | 451 (17.9) | 522 (20.3) | |
| SBP, mmHg | 116.7 ± 15.2 | 117.6 ± 17.0 | 116.2 ± 17.8 | 0.004 |
| DBP, mmHg | 57.3 ± 9.4 | 58.0 ± 10.4 | 58.1 ± 10.9 | 0.013 |
| MBP, mmHg | 75.3 ± 9.7 | 76.1 ± 10.5 | 75.9 ± 11.1 | 0.038 |
| HR, beats/minute | 84.0 ± 14.2 | 84.0 ± 15.6 | 86.8 ± 16.7 | <0.001 |
| RR, beats/minute | 18.7 ± 3.7 | 19.6 ± 3.9 | 20.2 ± 4.3 | <0.001 |
| Temperature, °C | 36.8 ± 0.6 | 36.8 ± 0.6 | 36.7 ± 0.7 | <0.001 |
| SpO2, % | 97.3 ± 1.9 | 96.9 ± 2.0 | 96.8 ± 2.4 | <0.001 |
| Weight, kg | 81.9 ± 24.1 | 81.2 ± 24.3 | 81.3 ± 24.8 | 0.212 |
| Scoring systems | ||||
| SOFA | 4.5 ± 2.7 | 4.4 ± 2.8 | 5.7 ± 3.3 | <0.001 |
| SAPSII | 37.1 ± 11.6 | 38.6 ± 12.6 | 44.2 ± 14.0 | <0.001 |
| Dialysis, n (%) | 60 (2.6) | 115 (4.6) | 440 (17.1) | <0.001 |
| Vasopressor, n (%) | 558 (23.9) | 498 (19.8) | 666 (25.9) | <0.001 |
| Ventilation, n (%) | 624 (26.7) | 784 (31.1) | 1000 (38.9) | <0.001 |
| ICU LOS, hours | 116.7 ± 149.8 | 130.3 ± 164.5 | 147.4 ± 180.9 | <0.001 |
| 30-day mortality, n (%) | 254 (10.9) | 399 (15.8) | 665 (25.8) | <0.001 |
| 90-day mortality, n (%) | 411 (17.6) | 579 (23.0) | 882 (34.3) | <0.001 |
| Comorbidities, n (%) | ||||
| Liver diseases | 97 (4.2) | 89 (3.5) | 119 (4.6) | 0.144 |
| Renal failure | 325 (13.9) | 552 (21.9) | 813 (31.6) | <0.001 |
| Atrial fibrillation | 1053 (45.1) | 1134 (45.0) | 1116 (43.4) | 0.377 |
| Stroke | 105 (4.5) | 152 (6.0) | 134 (5.2) | 0.056 |
| AMI | 121 (5.2) | 207 (8.2) | 270 (10.5) | <0.001 |
| VHD | 178 (7.6) | 248 (9.8) | 264 (10.3) | 0.003 |
| PCD | 130 (5.6) | 140 (5.6) | 139 (5.4) | 0.959 |
| HBP | 278 (11.9) | 467 (18.5) | 673 (26.2) | <0.001 |
| Diabetes | 786 (33.7) | 846 (33.6) | 1021 (39.7) | <0.001 |
| Pneumonia | 440 (18.9) | 581 (23.1) | 640 (24.9) | <0.001 |
| Respiratory failure | 487 (20.9) | 628 (24.9) | 774 (30.1) | <0.001 |
| Malignancy | 94 (4.0) | 145 (5.8) | 118 (4.6) | 0.015 |
| Laboratory tests | ||||
| WBC (K/μl) | 11.4 ± 6.2 | 12.7 ± 27.8 | 13.8 ± 8.3 | <0.001 |
| Platelet (K/μl) | 196.2 ± 95.4 | 218.7 ± 98.2 | 240.1 ± 118.0 | <0.001 |
| Hemoglobin (g/dl) | 10.2 ± 1.9 | 10.8 ± 2.0 | 10.9 ± 2.1 | <0.001 |
| Creatinine (mg/dl) | 1.0 ± 0.6 | 1.4 ± 0.9 | 2.4 ± 2.0 | <0.001 |
| BUN (mg/dl) | 23.2 ± 13.7 | 30.0 ± 19.0 | 45.1 ± 28.8 | <0.001 |
| Glucose (mg/dl) | 129.0 ± 53.7 | 144.7 ± 60.9 | 171.3 ± 94.8 | <0.001 |
| Sodium (mmol/L) | 138.8 ± 4.6 | 138.9 ± 4.6 | 138.2 ± 5.4 | <0.001 |
| Potassium (mmol/L) | 4.1 ± 0.6 | 4.2 ± 0.7 | 4.4 ± 0.8 | <0.001 |
| Chloride (mmol/L) | 106.2 ± 6.2 | 104.6 ± 5.9 | 102.7 ± 6.6 | <0.001 |
| Bicarbonate (mmol/L) | 26.5 ± 4.9 | 24.8 ± 4.2 | 21.5 ± 4.7 | <0.001 |
| PT (second) | 15.9 ± 7.1 | 16.7 ± 9.9 | 17.8 ± 12.0 | <0.001 |
| Laboratory tests | ||||
| APTT (second) | 38.7 ± 23.8 | 38.8 ± 25.2 | 40.4 ± 26.6 | 0.002 |
| Lactate, n (%) | <0.001 | |||
| <1.7 mmol/L | 1076 (46.1) | 865 (34.3) | 694 (27.0) | |
| ≥1.7 mmol/L | 634 (27.2) | 858 (34.1) | 1306 (50.8) | |
| No test | 624 (26.7) | 796 (31.6) | 573 (22.3) | |
| NT-proBNP, n (%) | <0.001 | |||
| <4983 pg/ml | 192 (8.2) | 219 (8.7) | 186 (7.2) | |
| ≥4983 pg/ml | 118 (5.1) | 200 (7.9) | 280 (10.9) | |
| No test | 2024 (86.7) | 2100 (83.4) | 2107 (81.9) | |
Note: CHF: congestive heart failure; SAG: serum anion gap; SBP: systolic blood pressure; DBP: diastolic blood pressure; MBP: mean blood pressure; RR: respiratory rate; HR: heart rate; SpO2: percutaneous oxygen saturation; SOFA: stroke, and malignancy. Calculate the sequential organ failure assessment score; SAPSII: simplified acute physiology score II; ICU: intensive care unit; LOS: length of stay; AMI: acute myocardial infarction; VHD: valvular heart diseases; PCD: pulmonary circulation diseases; HBP: hypertension; WBC: white blood cell; BUN: blood urea nitrogen; PT: prothrombin time; APTT: activated partial thromboplastin time; NT-proBNP: N-terminal probrain natriuretic peptide.
3.2. Primary Outcome: Association between SAG and All-Cause Mortality
As shown in Figure 2, the result of GAM analysis indicated a U-shaped relationship between SAG and 30-day all-cause mortality in critically ill patients with CHF. The K–M survival curve illustrated that subjects with increased SAG levels presented a decreased survival rate and shortened survival time (Log-rank P < 0.0001, Figure 3).
Figure 2.
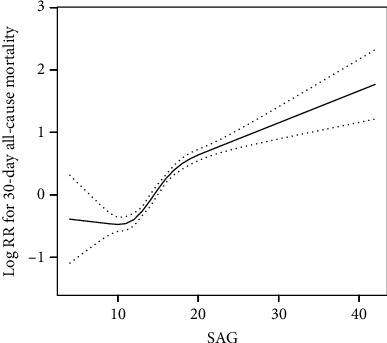
Construction of the smooth curve fitting of the risk of 30-day all-cause mortality and SAG using a generalized additive model. Dashed curves were for the 95% of confidence interval.
Figure 3.
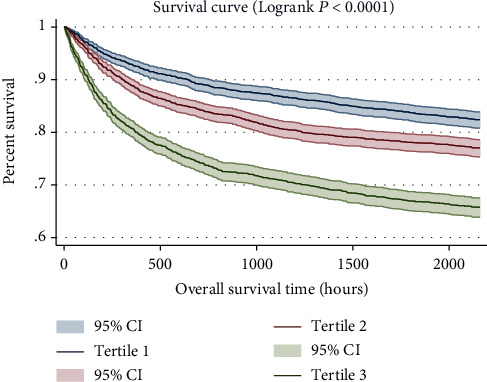
Kaplan–Meier survival curves for critically ill patients with CHF based on tertile of SAG. x-axis: survival time (hours). y-axis: cumulative survival probability. CHF: congestive heart failure. SAG: serum anion gap.
The Cox proportional hazard model was used to assess the association between SAG and all-cause mortality (Table 3). In model I, after adjusting for age, sex, and ethnicity, high levels of SAG were significantly associated with increased risk of 30- and 90-day all-cause mortalities (tertile 3 versus tertile 1: HR, 95% CI: 2.62, 2.27–3.03; 2.23, 1.98–2.51; quartiles 4 versus quartiles 1: HR, 95% CI: 3.20, 2.68–3.82; 2.62, 2.27–3.02). In model II, after adjusting for age, sex, ethnicity, temperature, HR, RR, SBP, DBP, SpO2, weight, atrial fibrillation, liver disease, valvular heart diseases, pulmonary circulation diseases, pneumonia, respiratory failure, diabetes, stroke, malignancy, SOFA, SAPSII, lactate, NT-proBNP, PT, WBC, BUN, creatinine, potassium, bicarbonate, glucose, vasopressor, dialysis, and mechanical ventilation, high levels of SAG were still an independent predictor of 30- and 90-day all-cause mortalities (tertile 3 versus tertile 1: HR, 95% CI: 1.74, 1.46–2.08; 1.53, 1.32–1.77; quartiles 4 versus quartiles 1: HR, 95% CI: 1.97, 1.59–2.45; 1.66, 1.39–1.98).
Table 3.
HRs (95% CIs) for all-cause mortality across groups of serum anion gap.
| Variable | Crude | Model I | Model II | |||
|---|---|---|---|---|---|---|
| HR (95%CIs) | P value | HR (95%CIs) | P value | HR (95%CIs) | P value | |
| 30-day all-cause mortality | ||||||
| Anion gap | 1.10 (1.09, 1.11) | <0.0001 | 1.11 (1.10, 1.12) | <0.0001 | 1.05 (1.03, 1.07) | <0.0001 |
| Anion gap (tertile) | ||||||
| <13 | 1 (ref) | 1 (ref) | 1 (ref) | |||
| ≥13, <16 | 1.50 (1.28, 1.75) | <0.0001 | 1.47 (1.25, 1.72) | <0.0001 | 1.28 (1.09, 1.50) | 0.0031 |
| ≥16 | 2.63 (2.27, 3.03) | <0.0001 | 2.62 (2.27, 3.03) | <0.0001 | 1.74 (1.46, 2.08) | <0.0001 |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
| Anion gap (quartile) | ||||||
| <12 | 1 (ref) | 1 (ref) | 1 (ref) | |||
| ≥12, <14 | 1.41 (1.15, 1.73) | 0.0009 | 1.39 (1.13, 1.70) | 0.0016 | 1.22 (0.99, 1.49) | 0.0621 |
| ≥14, <17 | 1.80 (1.50, 2.17) | <0.0001 | 1.75 (1.46, 2.11) | <0.0001 | 1.53 (1.26, 1.86) | <0.0001 |
| ≥17 | 3.16 (2.65, 3.78) | <0.0001 | 3.20 (2.68, 3.82) | <0.0001 | 1.97 (1.59, 2.45) | <0.0001 |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
| 90-day all-cause mortality | ||||||
| Anion gap | 1.09 (1.07, 1.10) | <0.0001 | 1.09 (1.08, 1.10) | <0.0001 | 1.04 (1.02, 1.06) | <0.0001 |
| Anion gap (tertile) | ||||||
| <13 | 1 (ref) | 1 (ref) | 1 (ref) | |||
| ≥13, <16 | 1.36 (1.20, 1.54) | <0.0001 | 1.33 (1.17, 1.51) | <0.0001 | 1.17 (1.03, 1.34) | 0.0170 |
| ≥16 | 2.22 (1.98, 2.50) | <0.0001 | 2.23 (1.98, 2.51) | <0.0001 | 1.53 (1.32, 1.77) | <0.0001 |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
| Anion gap (quartile) | ||||||
| <12 | 1 (ref) | 1 (ref) | 1 (ref) | |||
| ≥12, <14 | 1.33 (1.14, 1.56) | 0.0004 | 1.31 (1.12, 1.54) | 0.0008 | 1.17 (0.99, 1.37) | 0.0637 |
| ≥14, <17 | 1.57 (1.35, 1.82) | <0.0001 | 1.53 (1.32, 1.77) | <0.0001 | 1.35 (1.15, 1.57) | <0.0001 |
| ≥17 | 2.57 (2.23, 2.97) | <0.0001 | 2.62 (2.27, 3.02) | <0.0001 | 1.66 (1.39, 1.98) | <0.0001 |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
Models were derived from Cox proportional hazards regression models. Crude model adjusted for none. Model I adjusted for age, gender, and ethnicity. Model II adjusted for age, gender, ethnicity, temperature, systolic blood pressure, diastolic blood pressure, respiratory rate, heart rate, percutaneous oxygen saturation, weight, atrial fibrillation, liver disease, valvular heart diseases, pulmonary circulation diseases, pneumonia, respiratory failure, diabetes, stroke, malignancy, lactate, prothrombin time, white blood cell, blood urea nitrogen, creatinine, potassium, bicarbonate, glucose, vasopressor, dialysis, mechanical ventilation, SOFA, SAPSII, and NT-proBNP. Note: HR: hazard ratio; CI: confidence interval; SOFA: stroke, and malignancy. Calculate the sequential organ failure assessment score; SAPSII: simplified acute physiology score II; NT-proBNP: N-terminal probrain natriuretic peptide.
3.3. Secondary Outcome: Association between SAG and Readmission and MACEs
In this study, readmission rate and MACEs were regarded as the secondary outcome. Multivariate logistic regression indicated that increased SAG was associated with increased risk of MACEs in critically ill patients with CHF (tertile 3 versus tertile 1: OR, 95% CI: 1.76, 1.50–2.07). No obvious correlation between SAG and readmission rate was observed in critically ill patients with CHF (tertile 3 versus tertile 1: OR, 95% CI: 1.05, 0.88–1.24). The detailed data are shown in Supplementary Table 1.
3.4. Subgroup Analyses
Subgroup analyses were employed to assess the association between SAG and 30-day all-cause mortality. No significant interactions were found in most strata, as shown in Table 4, except for pneumonia, malignancy, respiratory failure, and mechanical ventilation. Among patients with CHF and high SAG, those with a comorbidity of pneumonia had a significantly lower 30-day mortality risk (HR, 95% CI: 1.44, 1.13–1.85 versus 3.27, 2.73–3.91). Similar trends also appeared in patients with a history of malignancy, respiratory failure, and mechanical ventilation (HR, 95% CI: 1.84, 1.10–3.07 versus 2.71, 2.33–3.15; 1.49, 1.22–1.83 versus 3.51, 2.85–4.32; 1.89, 1.55–2.30 versus 2.95, 2.38–3.65).
Table 4.
Subgroup analysis of the correlation between serum anion gap and 30-day all-cause mortality in critically ill patients with congestive heart failure.
| N | Serum anion gap (mmol/L) | P for interaction | |||
|---|---|---|---|---|---|
| <13 | ≥13, <16 | ≥16 | |||
| Liver diseases | 0.4454 | ||||
| No | 7121 | 1.0 (ref) | 1.45 (1.23, 1.70) | 2.56 (2.20, 2.97) | |
| Yes | 305 | 1.0 (ref) | 1.98 (0.97, 4.06) | 3.85 (2.04, 7.30) | |
| Renal failure | 0.0908 | ||||
| No | 5736 | 1.0 (ref) | 1.56 (1.31, 1.85) | 2.60 (2.21, 3.05) | |
| Yes | 1690 | 1.0 (ref) | 1.17 (0.79, 1.73) | 2.74 (1.94, 3.88) | |
| Atrial fibrillation | 0.1283 | ||||
| No | 4123 | 1.0 (ref) | 1.33 (1.07, 1.66) | 2.25 (1.84, 2.76) | |
| Yes | 3303 | 1.0 (ref) | 1.62 (1.30, 2.03) | 3.07 (2.50, 3.78) | |
| Stroke | 0.6586 | ||||
| No | 7035 | 1.0 (ref) | 1.43 (1.21, 1.68) | 2.63 (2.26, 3.06) | |
| Yes | 391 | 1.0 (ref) | 1.68 (1.01, 2.80) | 2.61 (1.59, 4.27) | |
| AMI | 0.4511 | ||||
| No | 6828 | 1.0 (ref) | 1.44 (1.23, 1.70) | 2.58 (2.22, 2.99) | |
| Yes | 598 | 1.0 (ref) | 2.14 (1.02, 4.51) | 3.90 (1.95, 7.83) | |
| VHD | 0.0653 | ||||
| No | 6736 | 1.0 (ref) | 1.52 (1.28, 1.80) | 2.78 (2.38, 3.24) | |
| Yes | 690 | 1.0 (ref) | 1.08 (0.69, 1.67) | 1.63 (1.08, 2.46) | |
| PCD | 0.0510 | ||||
| No | 7017 | 1.0 (ref) | 1.53 (1.30, 1.81) | 2.77 (2.38, 3.22) | |
| Yes | 409 | 1.0 (ref) | 0.88 (0.51, 1.51) | 1.43 (0.87, 2.34) | |
| HBP | 0.2921 | ||||
| No | 6008 | 1.0 (ref) | 1.49 (1.26, 1.76) | 2.59 (2.21, 3.03) | |
| Yes | 1418 | 1.0 (ref) | 1.61 (1.00, 2.58) | 3.52 (2.29, 5.42) | |
| Diabetes | 0.6109 | ||||
| No | 4773 | 1.0 (ref) | 1.41 (1.17, 1.69) | 2.56 (2.20, 2.97) | |
| Yes | 2653 | 1.0 (ref) | 1.61 (1.19, 2.17) | 2.76 (2.10, 3.62) | |
| Pneumonia | <0.0001 | ||||
| No | 5765 | 1.0 (ref) | 1.57 (1.29, 1.92) | 3.27 (2.73, 3.91) | |
| Yes | 1661 | 1.0 (ref) | 1.16 (0.89, 1.50) | 1.44 (1.13, 1.85) | |
| Respiratory failure | <0.0001 | ||||
| No | 5537 | 1.0 (ref) | 1.72 (1.37, 2.16) | 3.51 (2.85, 4.32) | |
| Yes | 1889 | 1.0 (ref) | 1.06 (0.85, 1.32) | 1.49 (1.22, 1.83) | |
| Malignancy | 0.0320 | ||||
| No | 7069 | 1.0 (ref) | 1.42 (1.20, 1.68) | 2.71 (2.33, 3.15) | |
| Yes | 357 | 1.0 (ref) | 1.69 (1.03, 2.77) | 1.84 (1.10, 3.07) | |
| Dialysis | 0.8370 | ||||
| No | 6811 | 1.0 (ref) | 1.45 (1.23, 1.70) | 2.51 (2.16, 2.92) | |
| Yes | 615 | 1.0 (ref) | 1.48 (0.69, 3.19) | 2.34 (1.18, 4.60) | |
| Vasopressor | 0.0736 | ||||
| No | 5704 | 1.0 (ref) | 1.46 (1.21, 1.77) | 2.33 (1.94, 2.80) | |
| Yes | 1722 | 1.0 (ref) | 1.62 (1.23, 2.13) | 3.15 (2.48, 4.01) | |
| Ventilation | 0.0088 | ||||
| No | 5018 | 1.0 (ref) | 1.57 (1.25, 1.98) | 2.95 (2.38, 3.65) | |
| Yes | 2408 | 1.0 (ref) | 1.22 (0.99, 1.52) | 1.89 (1.55, 2.30) | |
| SOFA | 0.3416 | ||||
| <4 | 2785 | 1.0 (ref) | 1.33 (1.00, 1.78) | 2.01 (1.50, 2.69) | |
| ≥4 | 4641 | 1.0 (ref) | 1.56 (1.30, 1.89) | 2.59 (2.19, 3.07) | |
| SAPSII | 0.8125 | ||||
| <39 | 3701 | 1.0 (ref) | 1.50 (1.13, 2.00) | 2.17 (1.62, 2.91) | |
| ≥39 | 3725 | 1.0 (ref) | 1.36 (1.12, 1.64) | 2.09 (1.77, 2.48) | |
Note: AMI: acute myocardial infarction; VHD: valvular heart diseases; PCD: pulmonary circulation diseases; HBP: hypertension; SOFA: stroke, and malignancy. Calculate the sequential organ failure assessment score; SAPSII: simplified acute physiology score II.
4. Discussion
CHF is a common critical illness in the field of cardiovascular disease, with characteristics of high prevalence, hospital admissions, readmissions, and even mortality [19]. According to the Framingham study, the 5-year survival rate of patients with CHF for men is only 25%, whereas that for women is 38%, which heavily threatens human health [20, 21]. Unfortunately, a lack of clinical objective indicators to predict the prognosis of patients with CHF exists at present. Various laboratory tests, such as blood gas analysis and biochemical testing, are often clinically used to determine the status of the acid-base balance of patients, but the relationship between these indicators and the prognosis of patients with CHF is rarely studied. Therefore, finding a simple and effective laboratory parameter to predict the prognosis of patients with CHF is particularly important and valuable for clinical treatment.
In this study, the possible prognostic value of SAG in patients with CHF was explored. Increased SAG was associated with increased risk of 30- and 90-day all-cause mortalities, and it remained as an independent predictor for all-cause mortality in critically ill patients with CHF after adjusting for age, gender, ethnicity, and another confounder. Besides, no significant interactions were found between SAG and most covariables for 30-day mortality, thus enhancing the stability and consistency of the results. To the best of the author's knowledge, this research was the first to clarify that high levels of SAG were associated with poor prognosis of critically ill patients with CHF.
As a routine examination that almost all admitted patients complete, SAG has the characteristics of simple calculation and easy acquisition, even without arterial puncture [14]. By retrospectively analyzing the electrolyte test results of 6868 hospitalized patients, Lolekha et al. [22] showed that there are SAG abnormalities in 40.5% of patients, including an increase in 37.6% of patients, and a decrease in 2.9%. Moreover, a series of studies in recent years further indicated that SAG could be used as a new prognosis biomarker to stratify patients at risk of deterioration, and patients could benefit from further treatment [5]. Sahu and his colleagues [23] reported that SAG acidosis was independently associated with in-hospital all-cause mortality in patients with AMI (OR, 95% CI: 4.2, 2.3–7.5), with longer average length of stay (5.1 versus 3.3 days). In critical ill patients with aortic aneurysm, Chen et al. [14] also demonstrated that SAG could effectively predict ICU mortality, and the risk of ICU death could increase by 38% for every increase of 1 mEq/L in SAG. Regrettably, the specific mechanism of SAG as a prognostic biomarker remains unclear.
The relationship between SAG and 30-day all-cause mortality in critically ill patients with CHF presented a U-shaped curve, which showed an elevated mortality risk at decreased and increased SAGs. In clinical practice, a decrease in SAG is usually rare, which may be related to the decrease in untested anions (such as hypoalbuminemia) or even laboratory error [24]. Albumin plays a remarkable role in various physiological processes, including maintaining colloidal osmotic pressure and microvascular integrity, ligand binding and material transport, antioxidant and antithrombotic effects, and enzyme activities [25]. Low level of blood albumin could promote and aggravate circulatory congestion and strengthen oxidative stress, inflammatory response, and susceptibility to infection, which could worsen the prognosis of patients with CHF [26]. In patients with acute heart failure, hypoalbuminemia has been proven to be associated with increased hospital mortality, and it served as an independent predictor of long-term mortality [27]. The increase in SAG is relatively more common, and previous studies have illustrated that the accumulation of serum lactate and ketone body accounted for 62% of the cause of the increase in SAG [28]. In CHF, the heart loses its ability to efficiently pump blood, which leads to decreased perfusion of tissues and hypoxia of cells [29]. Under anaerobic conditions, glucose undergoes glycolysis and eventually generates lactate, which may be primarily responsible for the increased SAG in patients with CHF [30]. In addition, sympathetic excitation in CHF contributes to the overproduction of lactate [31]. Under normal condition, the production and clearance of lactate are in balance, and this clearance is mainly responsible for the liver and kidney. Patients with CHF are often complicated by liver and kidney dysfunction, which further aggravates hyperlactataemia and the increase in SAG [32]. In the present study, the relationship between lactate and 30-day all-cause mortality in critically ill patients with CHF was also determined. Consistent with the results of previous studies [33], the increase in lactate was significantly correlated with poor prognosis (HR, 95% CI: 1.73, 1.53–1.95), which may be related to the damage and dysfunction of organs caused by tissue hypoperfusion and neurohormonal abnormalities. After adjusting for covariates including lactate, the anion gap was still associated with poor prognosis, thus showing the unique value of SAG as an independent predictor of the prognosis of critically ill patients with CHF.
This research has some limitations. First, it was a single-center study, whose subjects were relatively seriously ill. The results may not be applicable to all patients with CHF. Second, some unknown or even vital risk factors were not considered, although two multivariate models were used to control the influence of confounder on the outcome variables. Third, the SAG concentration of patients was only used when they entered the ICU to assess the relationship between them and all-cause mortality. It may be more valuable for the prognosis prediction if SAG can be dynamically monitored. Fourth, due to the lack of records of albumin, we did not perform albumin correction on the SAG, although some studies have shown that hypoalbuminemia could affect the SAG concentration [34]. Finally, the accuracy and efficiency of NT-proBNP and SAG in predicting the poor prognosis of critically ill patients with CHF were not compared, hence the possible exclusion bias.
5. Conclusion
SAG can effectively predict the 30- and 90-day all-cause mortalities of critically ill patients with CHF. It is expected to become a simple and effective marker for prognostic evaluation in these patients. Monitoring of SAG concentration could clinically help identify high-risk patients early and choose a more scientific treatment plan for patients.
Acknowledgments
Our study was supported by the National Natural Science Foundation of China (81873416) and the National Science and Technology Major Project (2017ZX0930401405).
Data Availability
Publicly available datasets were analyzed in this study. This data can be extracted from Monitoring in Intensive Care Database III version 1.4 (MIMIC-III v.1.4) after passing on the required courses and obtaining the authorization.
Conflicts of Interest
All authors declare that there is no conflict of interest.
Authors' Contributions
Author Z. X. Y designed the research. Y.Y.T performed the data analysis and drafted the manuscript. W.C.L, M.Q, G.J.L, L.H.Z, X.F.Z, and Z.H.L analyzed the data and revised the manuscript.
Supplementary Materials
Supplementary Table 1: ORs (95% CIs) for MACEs and readmission across groups of serum anion gap.
References
- 1.Sun D., Zhang F., Ma T., Zhang Y., Liang Z. Atorvastatin alleviates left ventricular remodeling in isoproterenol-induced chronic heart failure in rats by regulating the RhoA/Rho kinase signaling pathway. Pharmacological Reports. 2020;72(4):903–911. doi: 10.1007/s43440-020-00085-3. [DOI] [PubMed] [Google Scholar]
- 2.Orso F., Fabbri G., Maggioni A. P. Epidemiology of heart failure. Handbook of Experimental Pharmacology. 2017;243:15–33. doi: 10.1007/164_2016_74. [DOI] [PubMed] [Google Scholar]
- 3.Peixoto A. J., Alpern R. J. Treatment of severe metabolic alkalosis in a patient with congestive heart failure. American Journal of Kidney Diseases. 2013;61(5):822–827. doi: 10.1053/j.ajkd.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Park J. J., Choi D. J., Yoon C. H., et al. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. European Journal of Heart Failure. 2015;17(6):601–611. doi: 10.1002/ejhf.276. [DOI] [PubMed] [Google Scholar]
- 5.Glasmacher S. A., Stones W. Anion gap as a prognostic tool for risk stratification in critically ill patients - a systematic review and meta-analysis. BMC Anesthesiology. 2016;16(1):p. 68. doi: 10.1186/s12871-016-0241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S. B., Kim D. H., Kim T., et al. Anion gap and base deficit are predictors of mortality in acute pesticide poisoning. Human & Experimental Toxicology. 2019;38(2):185–192. doi: 10.1177/0960327118788146. [DOI] [PubMed] [Google Scholar]
- 7.Mitra B., Roman C., Charters K. E., O'Reilly G., Gantner D., Cameron P. A. Lactate, bicarbonate and anion gap for evaluation of patients presenting with sepsis to the emergency department: a prospective cohort study. Emergency Medicine Australasia. 2019;32(1):20–24. doi: 10.1111/1742-6723.13324. [DOI] [PubMed] [Google Scholar]
- 8.Cheng B., Li D., Gong Y., Ying B., Wang B. Serum anion gap predicts all-cause mortality in critically ill patients with acute kidney injury: analysis of the MIMIC-III database. Disease Markers. 2020;2020:10. doi: 10.1155/2020/6501272.6501272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee T., Crews D. C., Wesson D. E., et al. Elevated serum anion gap in adults with moderate chronic kidney disease increases risk for progression to end-stage renal disease. American Journal of Physiology. Renal Physiology. 2019;316(6):F1244–F1253. doi: 10.1152/ajprenal.00496.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leskovan J. J., Justiniano C. F., Bach J. A., et al. Anion gap as a predictor of trauma outcomes in the older trauma population: correlations with injury severity and mortality. The American Surgeon. 2013;79(11):1203–1206. doi: 10.1177/000313481307901126. [DOI] [PubMed] [Google Scholar]
- 11.Yang S. W., Zhou Y. J., Zhao Y. X., et al. The serum anion gap is associated with disease severity and all-cause mortality in coronary artery disease. Journal of Geriatric Cardiology. 2017;14(6):392–400. doi: 10.11909/j.issn.1671-5411.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson A. E., Pollard T. J., Shen L., et al. MIMIC-III, a freely accessible critical care database. Scientific Data. 2016;3(1):p. 160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W., Huang A., Zhu H., et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. The Medical Journal of Australia. 2020;213(8):374–379. doi: 10.5694/mja2.50781. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q., Chen Q., Li L., et al. Serum anion gap on admission predicts intensive care unit mortality in patients with aortic aneurysm. Experimental and Therapeutic Medicine. 2018;16(3):1766–1777. doi: 10.3892/etm.2018.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allard J., Cotin S., Faure F., et al. SOFA--an open source framework for medical simulation. Studies in Health Technology and Informatics. 2007;125:13–18. [PubMed] [Google Scholar]
- 16.Le Gall J. R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 17.Feng M., McSparron J. I., Kien D. T., et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Medicine. 2018;44(6):884–892. doi: 10.1007/s00134-018-5208-7. [DOI] [PubMed] [Google Scholar]
- 18.Jaddoe V. W., de Jonge L. L., Hofman A., Franco O. H., Steegers E. A., Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348(jan23 1):p. g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Q., Luo Y., Guo P., et al. Clinical effects of Xinmailong therapy in patients with chronic heart failure. International Journal of Medical Sciences. 2013;10(5):624–633. doi: 10.7150/ijms.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart S., MacIntyre K., Hole D. J., Capewell S., McMurray J. J. More 'malignant' than cancer? Five-year survival following a first admission for heart failure. European Journal of Heart Failure. 2001;3(3):315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q., Dong L., Jian Z., Tang X. Effectiveness of a PRECEDE-based education intervention on quality of life in elderly patients with chronic heart failure. BMC Cardiovascular Disorders. 2017;17(1):p. 262. doi: 10.1186/s12872-017-0698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lolekha P. H., Vanavanan S., Lolekha S. Update on value of the anion gap in clinical diagnosis and laboratory evaluation. Clinica Chimica Acta. 2001;307(1-2):33–36. doi: 10.1016/S0009-8981(01)00459-4. [DOI] [PubMed] [Google Scholar]
- 23.Sahu A., Cooper H. A., Panza J. A. The initial anion gap is a predictor of mortality in acute myocardial infarction. Coronary Artery Disease. 2006;17(5):409–412. doi: 10.1097/00019501-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez-Cherit G., Namendys-Silva S. A. Changes in the anion gap. Critical Care Medicine. 2013;41(1):336–337. doi: 10.1097/CCM.0b013e318270e799. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan G. J., Martin G. S., Evans T. W. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41(6):1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 26.Gotsman I., Shauer A., Zwas D. R., Tahiroglu I., Lotan C., Keren A. Low serum albumin: a significant predictor of reduced survival in patients with chronic heart failure. Clinical Cardiology. 2019;42(3):365–372. doi: 10.1002/clc.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonilla-Palomas J. L., Gamez-Lopez A. L., Moreno-Conde M., et al. Hypoalbuminemia in acute heart failure patients: causes and its impact on hospital and long-term mortality. Journal of Cardiac Failure. 2014;20(5):350–358. doi: 10.1016/j.cardfail.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Gabow P. A., Kaehny W. D., Fennessey P. V., Goodman S. I., Gross P. A., Schrier R. W. Diagnostic importance of an increased serum anion gap. The New England Journal of Medicine. 1980;303(15):854–858. doi: 10.1056/NEJM198010093031505. [DOI] [PubMed] [Google Scholar]
- 29.Ali Sheikh M. S., Salma U., Zhang B., Chen J., Zhuang J., Ping Z. Diagnostic, prognostic, and therapeutic value of circulating miRNAs in heart failure patients associated with oxidative stress. Oxidative Medicine and Cellular Longevity. 2016;2016:13. doi: 10.1155/2016/5893064.5893064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulop M., Horowitz M., Aberman A., Jaffe E. R. Lactic acidosis in pulmonary edema due to left ventricular failure. Annals of Internal Medicine. 1973;79(2):180–186. doi: 10.7326/0003-4819-79-2-180. [DOI] [PubMed] [Google Scholar]
- 31.Zymlinski R., Biegus J., Sokolski M., et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. European Journal of Heart Failure. 2018;20(6):1011–1018. doi: 10.1002/ejhf.1156. [DOI] [PubMed] [Google Scholar]
- 32.Orn S., van Hall G. Does a normal peripheral lactate value always indicate an aerobic tissue metabolism? European Journal of Heart Failure. 2017;19(8):1034–1035. doi: 10.1002/ejhf.863. [DOI] [PubMed] [Google Scholar]
- 33.Kawase T., Toyofuku M., Higashihara T., et al. Validation of lactate level as a predictor of early mortality in acute decompensated heart failure patients who entered intensive care unit. Journal of Cardiology. 2015;65(2):164–170. doi: 10.1016/j.jjcc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Kotake Y. Unmeasured anions and mortality in critically ill patients in 2016. Journal of Intensive Care. 2016;4(1):p. 45. doi: 10.1186/s40560-016-0171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: ORs (95% CIs) for MACEs and readmission across groups of serum anion gap.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be extracted from Monitoring in Intensive Care Database III version 1.4 (MIMIC-III v.1.4) after passing on the required courses and obtaining the authorization.


