Abstract
Background
Low immunity, comorbid clinical conditions, and metabolic disorders may be the underlying factors that determine the severity of infection. Diabetes increases the risk of infection and multiple organ damage. In Nepal, the actual burden of fungal infections has not been estimated or is in a limited progress. This study aimed to investigate the status of fungal infection in diabetic and nondiabetic individuals in Bhaktapur, Nepal.
Materials and Methods
A total of 670 samples were collected from 134 participants. From each participant, five samples were collected from different sites like an oral wash, toe swab, midstream urine, hair shaft, and nail scrapings. All samples were cultured on Sabouraud dextrose agar. Gram stain was used to observe yeast cells and lactophenol cotton blue stain was used for hyphae. Chlamydospore production by Candida species was observed in cornmeal agar medium by Dalmau Plate method. Candida species isolated were characterized by germ-tube test and differentiated using CHROM agar Candida medium. Candida species isolates were tested for antibiotic susceptibility.
Results
Overall, 19.4% of the samples showed fungal growth. The prevalence of fungal infection was higher in diabetic (34.0%) than nondiabetic individuals (4.7%). Fungal growth was found to be higher in oral wash followed by toe, urine, hair, and nail samples. Predominant fungi were Candida species (57.5%), Aspergillus species (28.4%), and Trichophyton species (10.7%). Oral wash, toe, and urine samples in diabetics had a significantly higher fungal prevalence when compared between both groups, p value < 0.05. In Candida isolates, higher resistance was seen against fluconazole 36.8% and ketoconazole 28.9%, whereas other drugs showed low resistance.
Conclusion
Diabetic participants are more susceptible to fungal infection than the nondiabetics. Overall, Candida species and Aspergillus species are highly predominant fungi. Candida species are highly resistant to fluconazole and ketoconazole.
1. Introduction
Fungal infections are taken less seriously but these are present as silent killers. Globally, more than 300 million people are at extremely high risk and 25 million people are at high risk of dying due to fungal infections [1]. Diabetes is an endocrine disorder. Patients of uncontrolled diabetes are susceptible to infection due to metabolic disorder, xerostomia, immune-related dysfunctions, and multiple organ disorders. Annually, around 422 million people are affected by diabetes, and the burden is high in low and middle income countries [2]. In Nepal, due to diabetes, the proportional mortality rate has been found to be 3.0% in all ages and its prevalence is much higher in male than female, that is, 10.5% and 7.9%, respectively, as of 2016 [3], which increased up to 3.97% in 2017 [4]. Diabetes leads to the development of opportunistic infections in the skin, foot, urinary tract, surgical sites, and so on through affecting the immune system. In addition, fungal infections have become more severe in the patients with HIV/AIDS, malignancy, and transplant and those who are under steroids therapy and with other immunosuppressive conditions as well [5, 6].
The clinical severity of fungal infections ranges from asymptomatic to mild skin infections to serious invasive infections. According to the Global Action Fund for Fungal Infectious (GAFFI), annually, about 135 million women are affected by vulvovaginal candidiasis (thrush), almost 1 million people are affected by invasive candidiasis, 60,000–100,000 cases of Candida peritonitis occur, above 300,000 patients develop invasive aspergillosis, 400,000 cases of pneumocystis pneumonia are seen, and about 500,000 new infections of histoplasmosis exist globally [7]. Cutaneous fungal infections in the skin, hair, and nails affect around 1 billion people [8]. It was reported that 1.87% of Nepalese population suffer from serious fungal infection and most infections are keratitis (73 per 100,000 annually), invasive mycoses (1119 cases annually), and bronchopulmonary aspergillosis (2673–13 364), pneumocystis pneumonia (990 cases annually), oral infection (10,347 cases), and oesophageal candidiasis (2,950 cases) [5]. Candidiasis is a very common mycotic infection around the world and Candida albicans is the most common etiological agent of candidiasis. Other species such as C. glabrata, C. krusei, and C. tropicalis are opportunistic pathogens [9]. Epidemiological study showed that Aspergillus species including A. flavus, A. niger, and A. fumigatus cause infection in nails, ears, eyes, respiratory tract, and skin [5]. The morbidity and mortality of infection is much higher in patients with other comorbid clinical conditions, like immunological impairment, chemotherapy, cancer, and long-term chronic diseases.
Uncontrolled hyperglycemic condition leads to infection due to dysfunction of the immune system by the reduction of T-lymphocyte, neutrophil activity, reduction of secretion of inflammatory cytokines, disorders of antibody mediated immunity along with angiopathy, neuropathy, glycosuria, and increased apoptosis of polymorphonuclear leukocytes [10]. It has been observed that diabetic patients show polymicrobial growth of pathogens. Several studies have shown that the prevalence of fungi ranges from 7.0 to 17.38% in diabetic patients, and fungal species including Candida species, Aspergillus species, Fusarium species, Rhodotorula species, and Trichosporon species are the most frequent [11–13]. However, all fungi are not harmful and most of their infections can be managed if diagnosed timely. In our context, mycological studies are limited because of the lack of sophisticated healthcare facilities and awareness in community. Thus, present study is designed to investigate the prevalence of fungi in diabetic and nondiabetic individuals of Bhaktapur district and also to study the antibiotic pattern of isolated Candida species.
2. Materials and Methods
2.1. Study Site and Sample Collection
A descriptive case control cross-sectional study was conducted in Bhaktapur district from July 2019 to January 2020. A total of 134 volunteers participated in the study, of which 67 were diabetic and 67 were nondiabetic. A total of 670 samples were collected, five each from different sites of each individual. The samples included oral wash, foot swab from interdigital space, midstream urine, hair shaft, and nail scrapings. Exactly 10 ml of sterile normal saline was given to individuals of both the study groups, and they were asked to rinse their oral cavity for 1 min, which was finally collected in sterile wide mouth container. Around 20–40 ml midstream urine sample was collected in a sterile wide-mouthed container. Cotton swab soaked in a sterile normal saline was used for collection of the sample from the interdigital space of toe. Hair shaft sample was collected by hair pluck method using forceps. Nail sample was collected by scraping the nail using a scalpel. All samples were collected aseptically and were transported to laboratory for processing within two hours of collection at the Department of Medical Laboratory Technology, JF Institute of Health Sciences, Hattiban, Lalitpur. Consent and history were taken before sample collection. Volunteer participants having recent history of antibiotic therapy, under immunosuppressive drugs, having cancer and harmful oral habits were excluded from study.
2.2. Processing of Samples
For isolation of fungi, samples were inoculated in Sabouraud dextrose agar (SDA) (HiMedia, Mumbai, India) containing chloramphenicol 50 mg/L concentration, incubated at 370°C and at 250°C for 7–14 days under aerobic conditions. The isolated colonies were further subcultured on Cornmeal Agar (HiMedia, Mumbai, India) containing 1% Tween 80 by applying Dalmau Plate Method for the cultivation of fungi and for the study of Candida species for chlamydospore production after incubation for 24–48 hours at 25–30°C. Fungal colonies morphology were recorded. Each isolates were stained by Gram stain for the detection of yeast cells. Colony growth which showed hyphae spreading on the media was identified by using lactophenol cotton blue mount. Among the isolates, Candida species were identified by subculture on CHROM agar (HiMedia, Mumbai, India) at 37°C for 48 hours.
2.3. Antibiotic Resistance Pattern of Candida Species
Candida species isolated were transferred to broth media and the turbidity of fungal growth was compared with 0.5 McFarland Standard. Samples were evenly streaked onto Mueller–Hinton agar supplemented with 2% glucose and 5 μg/ml methylene blue. For internal control, C. albicans ATCC 90028 and C. tropicalis ATCC 750 were used. The zone of inhibition around the disc was measured after incubating the plates at 37°C for 24 hours in aerobic condition, and antibiotic susceptibility testing was performed as per the Clinical and Laboratory Standards Institute (CLSI) M44-A guidelines.
2.4. Data Analysis
Data was entered and analyzed by using SPSS software version 21. Independent t-test and linear regression analysis were applied and p value <0.05 was taken as significant.
3. Results
3.1. Status of Fungi Growth in Diabetic and Nondiabetic Study Groups
Female participants were higher in number as compared with male participants. In diabetic subjects, 38 (56.8%) were female and 29 (43.2%) were male, whereas, in nondiabetic subjects, 40 (59.8%) were female and 27 (40.3%) were male. Out of 670 samples, 130 (19.4%) samples showed fungal growth. The prevalence of fungi amongst diabetics was 114 (34.0%), and in nondiabetics, it was 16 (4.7%). In both diabetic and nondiabetic groups, the most frequently isolated fungus was Candida species 75 (57.6%), followed by Aspergillus species 37 (28.4%), Trichophyton species 14 (10.7%), Mucor species 3 (2.3%), and Rhizopus species 1 (0.8%). The number of fungi isolated was higher in diabetic population than the control group. Among diabetic individuals, the highest number of growths was observed in oral wash sample 45 (67.2%), followed by toe swab 29 (43.3%), midstream urine 22 (32.8%), hair samples 10 (14.9%), and nail samples 8 (11.9%). In nondiabetic individuals, oral wash and toe samples had a prevalence of 5 (7.5%) in each, while no growth was observed from midstream urine. Out of 75 Candida species isolates, 35 (46.6%) were C. albicans, followed by 17 (22.6%) C. krusei, 12 (16.0%) C. glabrata, and 11 (14.6%) C. tropicalis [11]. Similarly, out of 37 Aspergillus species, A. nidulans was found in 13 (35.1%), followed by A. flavus in 11 (29.7%), A. niger in 8 (21.6%), and A. fumigatus in 5 (13.5%). On comparing both groups for fungal growth, samples of oral wash, toe, and urine showed significantly higher prevalence among the diabetic population (p value < 0.05); difference is as shown in Table 1.
Table 1.
Prevalence of fungi in diabetic and nondiabetic individuals from different samples.
| Fungi isolates | Oral wash | Toe sample | Urine sample | Nail sample | Hair sample | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM (n = 67) | NDM (n = 67) | DM (n = 67) | NDM (n = 67) | DM (n = 67) | NDM (n = 67) | DM (n = 67) | NDM (n = 67) | DM (n = 67) | NDM (n = 67) | |
| Candida albicans | 20 (44.4%) | 2 (40.0%) | 7 (24.1%) | 1 (20.0%) | 5 (22.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Candida glabrata | 5 (11.1%) | 0 (0.0%) | 5 (17.2%) | 1 (20.0%) | 1 (4.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Candida tropicalis | 4 (8.9%) | 0 (0.0%) | 2 (6.9%) | 0 (0.0%) | 5 (22.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Candida krusei | 2 (4.4%) | 1 (20.0%) | 3 (10.3%) | 0 (0.0%) | 11 (50%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Aspergillus flavus | 3 (6.7%) | 2 (40.0%) | 3 (10.3%) | 2 (40.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) |
| Aspergillus fumigatus | 3 (6.7%) | 0 (0.0%) | 2 (6.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Aspergillus niger | 2 (4.4%) | 0 (0.0%) | 2 (6.9%) | 1 (20.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) | 0 (0.0%) | 1 (33.3%) |
| Aspergillus nidulans | 2 (4.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (50.0%) | 0 (0.0%) | 6 (60.0%) | 1 (33.3%) |
| Mucor species | 3 (6.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Rhizopus species | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Trichophyton species | 0 (0.0%) | 0 (0.0%) | 5 (17.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (50.0%) | 1 (33.3%) | 4 (40.0%) | 0 (0.0%) |
| Total | 45 | 5 | 29 | 5 | 22 | 0 | 8 | 3 | 10 | 3 |
| P value | 0.00 | 0.00 | 0.00 | 0.526 | 0.372 | |||||
3.2. Relation between Age Group and Distribution of Fungal Isolates in Both Groups
In both study groups, higher numbers of pathogenic fungi were found in individuals in the age group between 46 and 55 years, followed by the age of more than 56 years and the age group between 36 and 46 years. No fungal isolates were observed in the age group of less than 35 years, except from hair samples. P value was significant (<0.05) in samples collected from the oral, toe, and urine, which is shown in Table 2.
Table 2.
Relation between age group and distribution of fungal isolates in study subjects.
| Sites | Age group (in year) | DM | NDM | P value |
|---|---|---|---|---|
| Oral samples | Less than 35 | 0 (0.0%) | 0 (0.0%) | 0.00 |
| 36 to 45 | 6 (13.3%) | 1 (20.0%) | ||
| 46 to 55 | 21 (46.7%) | 1 (20.0%) | ||
| More than 56 | 18 (40.0%) | 3 (60.0%) | ||
|
| ||||
| Toe samples | Less than 35 | 0 (0.0%) | 0 (0.0%) | 0.00 |
| 36 to 45 | 5 (17.2%) | 1 (20.0%) | ||
| 46 to 55 | 10 (34.5%) | 2 (40.0%) | ||
| More than 56 | 14 (48.3%) | 2 (40.0%) | ||
|
| ||||
| Urine samples | Less than 35 | 0 (0.0%) | 0 (0.0%) | 0.00 |
| 36 to 45 | 2 (9.1%) | 0 (0.0%) | ||
| 46 to 55 | 12 (54.5%) | 0 (0.0%) | ||
| More than 56 | 8 (36.4%) | 0 (0.0%) | ||
|
| ||||
| Nail samples | Less than 35 | 0 (0.0%) | 0 (0.0%) | 0.469 |
| 36 to 45 | 1 (12.5%) | 1 (33.3%) | ||
| 46 to 55 | 4 (50.0%) | 0 (0.0%) | ||
| More than 56 | 3 (37.5%) | 2 (66.7%) | ||
|
| ||||
| Hair samples | Less than 35 | 0 (0.0%) | 1 (33.3%) | 0.439 |
| 36 to 45 | 2 (20.0%) | 0 (0.0%) | ||
| 46 to 55 | 4 (40.0%) | 1 (33.3%) | ||
| More than 56 | 4 (40.0%) | 1 (33.3%) | ||
3.3. Relation between Education Levels and Prevalence of Fungi
In this study, uneducated study subjects were higher in number (74), followed by lower secondary level [14], bachelor level [12], and primary level [10]. Uneducated subjects showed the highest fungal prevalence, which was 44.6%, followed by lower secondary level 15.3% and primary level 12.3%. Surprisingly, no fungi were isolated from the participants having a master degree and more qualifications. P value was found significant (<0.05) in the sample collection from oral, toe, and urine, as shown in Table 3.
Table 3.
Relation between education levels and the prevalence of fungi in diabetic and nondiabetic groups.
| Oral sample | Toe sample | Urine sample | Nail sample | Hair sample | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM | NDM | DM | NDM | DM | NDM | DM | NDM | DM | NDM | |
| Uneducated | 21 (46.7%) | 5 (100.0%) | 13 (43.3%) | 4 (80.0%) | 6 (27.3%) | 0 (0.0%) | 3 (37.5%) | 2 (66.7%) | 3 (30.0%) | 2 (66.7%) |
| Preprimary level | 1 (2.2%) | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) | 2 (9.1%) | 0 (0.0%) | 2 (25.0%) | 0 (0.0%) | 2 (20.0%) | 0 (0.0%) |
| Primary level | 6 (13.3%) | 0 (0.0%) | 4 (13.3%) | 0 (0.0%) | 3 (13.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (20.0%) | 1 (33.3%) |
| Lower secondary level | 9 (20.0%) | 0 (0.0%) | 5 (16.7%) | 1 (20.0%) | 3 (13.6%) | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) |
| Secondary level | 3 (6.7%) | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) | 2 (9.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Higher secondary level | 3 (6.7%) | 0 (0.0%) | 4 (13.3%) | 0 (0.0%) | 4 (18.2%) | 0 (0.0%) | 1 (12.5%) | 1 (33.3%) | 1 (10.0%) | 0 (0.0%) |
| Bachelor level | 2 (4.4%) | 0 (0.0%) | 2 (6.7%) | 0 (0.0%) | 2 (9.1%) | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) |
| Master level | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total | 45 | 5 | 30 | 5 | 22 | 0 | 8 | 3 | 10 | 3 |
| p value | 0.00 | 0.00 | 0.00 | 0.802 | 0.603 | |||||
3.4. Antibiotic Susceptibility Pattern of Candida Species
Of the total isolates, Candida species were isolated in 75 samples and were subjected to antibiotic sensitivity test (Figure 1(b)). Under standard in vitro conditions, 36.8% [15] isolates were resistant to fluconazole and 28.9% [16] were resistant to ketoconazole, while 10.5% [8] were resistant to miconazole. All isolates were sensitive to nystatin. Higher resistance was observed in C. albicans, followed by C tropicalis, C. glabrata, and C. krusei as shown in Table 4.
Figure 1.
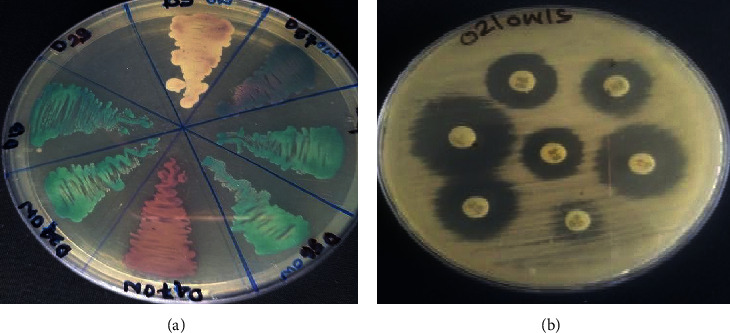
(a) Growth of Candida species in CHROMagar medium; (b) antibiotic susceptibility testing.
Table 4.
Antibiotic resistance pattern of Candida species isolated from both groups.
| Antifungal agents | Candida species | Total C. species (N = 75) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans (N = 35) | C. krusei(N = 17) | C. tropicalis (N = 11) | C. glabrata (N = 12) | ||||||||||
| S | I | R | S | I | R | S | I | R | S | I | R | R | |
| Ketoconazole | 14 | 2 | 19 | 13 | 3 | 1 | 10 | 1 | 0 | 8 | 2 | 2 | 22 (28.9%) |
| Voriconazole | 33 | 1 | 1 | 13 | 4 | 0 | 7 | 2 | 2 | 8 | 1 | 3 | 6 (8.0%) |
| Amphotericin | 7 | 28 | 0 | 7 | 10 | 0 | 3 | 7 | 1 | 5 | 7 | 0 | 1 (1.3%) |
| Itraconazole | 24 | 8 | 3 | 14 | 2 | 1 | 11 | 0 | 0 | 10 | 1 | 1 | 5 (6.5%) |
| Miconazole | 30 | 2 | 3 | 14 | 1 | 2 | 6 | 2 | 3 | 11 | 1 | 0 | 8 (10.5%) |
| Fluconazole | 18 | 1 | 16 | 13 | 1 | 3 | 5 | 0 | 6 | 8 | 1 | 3 | 28 (36.8%) |
N = number, S = sensitive, I = intermediate, and R = resistant.
4. Discussion
Diabetes increases the risk of infection and damages multiple organs, which may affect the ability of protection against variety of pathogens. Poor glycemic control and chronic diabetes mellitus cause several complications like micro- and macrovascular complications, diabetic foot ulcers, eye infection, nephritis, and nerve infections, which are responsible for high morbidity and mortality [14, 17]. According to GAFFI, fungal infection kills more people than tuberculosis or malaria. Annually, approximately 11.5 million people are severely affected and more than 1.5 million die due to fungal infection. Fungal infections are not taken seriously in our community as a public health concern [1, 18]. Globally, 422 million are affected by diabetes and 1.6 million people lose their lives annually [2]. Hyperglycemic state causes immune dysfunction which leads to local and systemic infection due to overgrowth of microflora and causes an opportunistic infection. Uncontrolled diabetes allows fungal colonization in epithelial cells by sequentially increasing the number of receptors for colonization. Meanwhile, glucose, maltose, and sucrose boost the adhesion and suppression of the killing capacity of neutrophils [19]. Previous studies have shown that the incidence of fungal infection is higher in diabetic patients in comparison with nondiabetics. High blood sugar promotes the binding of fungus to the host cell surface, and high glucose in salivary secretions with low pH, poor oral hygiene, and very low salivary secretions also allow the growth of more than 50 Candida species colonies in the oral cavity [20].
In this study, the prevalence of fungus was observed to be 19.4%, which is similar to a recent study from India, in which the prevalence was 17.4% [13]. We found that the prevalence of fungal colonization in diabetics is eight times higher than that in nondiabetic participants, that is, 34.0% in diabetics and 4.7% in nondiabetics. Colonization in male and female was found to be in an equal proportion for both the study groups. Several studies have shown that the colonization rate of fungi in patients with diabetes is higher than in the nondiabetic individuals, that is, 84.0% and 27.0%, respectively [21]. Similarly, Candida species were isolated more frequently in diabetic population as compared with nondiabetic population. C. albicans among diabetics was 68.9% and 40.0% among healthy individuals [22]. However, another study found that there are no significant differences in Candida species isolated from diabetic individuals in comparison with healthy individuals [23].
In this study, Aspergillus species accounted for 28.4% prevalence, among which A. nidulans was 10%, followed by 8.46% of A. flavus and 3.84% A. niger of the total. A study from India also found A. flavus and A. niger in diabetic patients [11]. Prevalence of Trichophyton was 10.7%, which is similar to a study conducted in Portugal (14.3%) [16]. In the present study, we used CHROM agar for differentiation of isolated Candida species in standard in vitro conditions. CHROM agar had been used as selective and differential media for isolation of Candida species according to its colony color (Figure 1(a)). Various authors noted that CHROM agar has very good sensitivity and specificity for C. albicans ranging from 96.55% to 100% and from 96.42% to 100%, respectively [9, 24–26]. However, sensitivity and specificity are less when identifying C. tropicalis, where sensitivity ranged from 66.7% to 100%, and specificity, from 78.8% to 100%. C. krusei has sensitivity and specificity of 100% [24, 26]. Another study also showed that CHROM agar has a similar positivity as PCR-RFLP test method for Candida species differentiation.
In a study carried out on nondiabetic patients, it was found that there is no significant relationship between fungal infection in nails and level of education. It depended upon manual handwork practice, occupation and quality of life, socioeconomic status, gender, and age [27]. However, our study shows that there are no fungal isolates from participants with a master level of education in both study subjects.
Notwithstanding, the use of antibiotics for the treatment of bacterial infection increases the risk of fungal infection in hosts. For treatment of severe Candida infection, three classes of drugs (azoles, echinocandins, and amphotericin B) have been mainly used. According to the Center for Disease Control and Prevention, USA, nearly 7.0% of fungal bloodstream infection cases developed drug resistance [15]. In this study, out of 75 Candida species, 37.3% isolates were resistant to fluconazole, followed by 29.3% to ketoconazole and 10.6% to itraconazole. Other studies have also reported resistance of Candida spp. to flucytosine, ketoconazole, miconazole, and econazole, and the rate of resistance is higher in diabetic than nondiabetic individuals [22, 23]. In our study, fluconazole drug resistance in Candida species was 37.3%, which is higher than that in the study done in India and Singapore, that is, 9.3% and 3.2%, respectively [28]. Out of 75 isolates, 29.3% were resistant to ketoconazole, followed by 10.6% to miconazole and 8.0% to voriconazole. The cause for high resistance is unclear; perhaps, it could be due to the easy availability and increased use of drugs, irrational use, consumption without clinician's consultation, selling drugs without prescription, and limited diagnostic centers for fungus culture and identification in Nepal. In addition, it is also believed that fluconazole is one of the safest and effective drugs for topical and oral use in local and systemic fungal infections.
Fungal infection in diabetic patients causes serious morbidity and mortality. To minimize the burden of diseases, there is a need of proper use of prophylaxis and precise diagnosis of infectious disease, selection of proper drug and antibiotics, health hygiene awareness, and regular communication with patients. Most of the hospitals and laboratories in Nepal do not have the facility and required capacity to identify and test for antifungal drugs resistance. Developing countries have a lack of reliable, cost-effective diagnostic tools and limited therapeutic options. Increase in the morbidity of diabetes and the development of drug resistance among pathogenic fungi is a major threat to health and economy. The need for proper diagnosis of fungal diseases and availability of effective therapeutic agents at a low cost is a must for decreasing the morbidity. Moreover, diabetic patients need to be counseled about the need for proper hygiene, sanitation, and healthy living.
5. Conclusion
Diabetic patients are more prone to fungal colonization in comparison with nondiabetic individuals. The spread of fluconazole and ketoconazole-resistant Candida species is rising at an alarming rate in the community. Thus, there is a need to increase awareness regarding fungal infection and its impact on health in the community to reduce the burden of disease.
Abbreviations
- C:
Candida
- GAFFI:
Global Action Fund for Fungal Infections.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request. The authors agree that anyone interested can have access to the raw data from the research upon request to the authors. The data will be provided to anyone based on two conditions: upon use of the data, the authors should be acknowledged and also the paper needs to be cited. An agreement in the aforementioned situation will be reached.
Ethical Approval
Ethical approval was obtained from the Nepal Health Research Council (NHRC) (assigned number: 277/2019).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Bhuvan Saud and Prajuna Bajgain designed the study. Bhuvan Saud, Prajuna Bajgain, and Govinda Paudel carried out data collection, laboratory work, and data analysis. Bhuvan Saud, Govinda Paudel, Vikram Shrestha, and Saroj Adhikari prepared the manuscript. Bhuvan Saud, Prajuna Bajgain, and Dipendra Bajracharya carried out the statistical analysis. Gunaraj Dhungana and Mamata Sherpa Awasthi provided critical comments on the manuscript. All of the authors finalized and approved the manuscript.
References
- 1.Global Action Fund for Fungal Infectious (GAFFI) Our Vision Is to Reduce Illness and Death Associated with Fungal Disease Worldwide. Hale, UK: Global Action Fund for Fungal Infectious (GAFFI); 2013. https://www.gaffi.org/why/fungal-disease-frequency/ [Google Scholar]
- 2.World Health Organisation (WHO) World Health Organisation (WHO), Geneva, Switzerland: World Health Organisation (WHO); Diabetes. https://www.who.int/health-topics/diabetes. [Google Scholar]
- 3.World Health Organisation (WHO) Diabetes. Nepal Country Profile. Geneva, Switzerland: World Health Organisation (WHO); 2000. https://www.who.int/diabetes/country-profiles/npl_en.pdf?ua=1. [Google Scholar]
- 4.World Health Ranking. Nepal: Diabetes Mellitus. Geneva, Switzerland: World Health Organisation (WHO); 2000. https://www.worldlifeexpectancy.com/nepal-diabetes-mellitus. [Google Scholar]
- 5.Khwakhali U. S., Denning D. W. Burden of serious fungal infections in Nepal. Mycoses. 2015;58:45–50. doi: 10.1111/myc.12393. [DOI] [PubMed] [Google Scholar]
- 6.Low C.-Y., Rotstein C. Emerging fungal infections in immunocompromised patients. F1000 Medicine Reports. 2011;3 doi: 10.3410/m3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Fungal Infection Trust. How Common Are Fungal Diseases? Fungal Research Trust 20th Anniversary Meeting. London, UK: The Fungal Infection Trust; 2017. https://www.gaffi.org/wp-content/uploads/How-Common-are-Fungal-Diseases-v12.2.pdf. [Google Scholar]
- 8.Vos T., Flaxman A. D., Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baradkar V., Mathur M., Kumar S. Hichrom candida agar for identification of Candida species. Indian Journal of Pathology and Microbiology. 2010;53(1):p. 93. doi: 10.4103/0377-4929.59192. [DOI] [PubMed] [Google Scholar]
- 10.Casqueiro J., Casqueiro J., Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian Journal of Endocrinology and Metabolism. 2012;16(1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal E., Garg A., Bhatia S., Attri A. K., Chander J. Spectrum of microbial flora in diabetic foot ulcers. Indian Journal of Pathology and Microbiology. 2008;51(2):p. 204. doi: 10.4103/0377-4929.41685. [DOI] [PubMed] [Google Scholar]
- 12.Saseedharan S., Sahu M., Chaddha R., et al. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Brazilian Journal of Microbiology. 2018;49(2):401–406. doi: 10.1016/j.bjm.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arun C., Raju P., Lakshmanan V., Kumar A., Bal A., Kumar H. Indian Journal of Community Medicine. 5. Vol. 44. Official Publication of Indian Association of Preventive & Social Medicine; 2019. Emergence of fluconazole-resistant candida infections in diabetic foot ulcers: implications for public health; pp. 74–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce I., Simó R., Lövestam‐Adrian M., Wong D. T., Evans M. Association between diabetic eye disease and other complications of diabetes: implications for care. A systematic review. Diabetes, Obesity and Metabolism. 2019;21(3):467–478. doi: 10.1111/dom.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centre for Disease Control and Prevention (CDC) Antibiotic Resistance Threats in the United States. Atlanta, Georgia, USA: Centre for Disease Control and Prevention (CDC); 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/candida-508.pdf. [Google Scholar]
- 16.Parada H., Veríssimo C., Brandão J., et al. Dermatomycosis in lower limbs of diabetic patients followed by podiatry consultation. Revista Iberoamericana de Micología. 2013;30(2):103–108. doi: 10.1016/j.riam.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Unnikrishnan R., Anjana R. M., Mohan V. Diabetes mellitus and its complications in India. Nature Reviews Endocrinology. 2016;12(6):357–370. doi: 10.1038/nrendo.2016.53. [DOI] [PubMed] [Google Scholar]
- 18.Tudela J. L. R., Denning D. W. Recovery from serious fungal infections should be realisable for everyone. The Lancet Infectious Diseases. 2017;17(11):1111–1113. doi: 10.1016/s1473-3099(17)30319-5. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues C., Rodrigues M., Henriques M. Candida sp. infections in patients with diabetes mellitus. Journal of Clinical Medicine. 2019;8(1):p. 76. doi: 10.3390/jcm8010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammadi F., Javaheri M. R., Nekoeian S., Dehghan P. Identification of Candida species in the oral cavity of diabetic patients. Current Medical Mycology. 2016;2(2):p. 1. doi: 10.18869/acadpub.cmm.2.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar B. V., Padshetty N. S., Bai K. Y., Rao M. S. Prevalence of Candida in the oral cavity of diabetic subjects. The Journal of the Association of Physicians of India. 2005;53:599–602. [PubMed] [Google Scholar]
- 22.Al-Attas S. A., Amro S. O. Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Annals of Saudi Medicine. 2010;30(2):101–108. doi: 10.4103/0256-4947.60514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremenkamp R. M., Caris A. R., Jorge A. O. C., et al. Prevalence and antifungal resistance profile of Candida spp. oral isolates from patients with type 1 and 2 diabetes mellitus. Archives of Oral Biology. 2011;56(6):549–555. doi: 10.1016/j.archoralbio.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Shettar S. K., Patil A. B., Nadagir S. D., Shepur T. A., Mythri B. A., Gadadavar S. Evaluation of HiCrome differential agar for speciation of candida. Journal of Academy of Medical Sciences. 2012;2(3):101–104. [Google Scholar]
- 25.Peng C. F., Lee K. M., Lee S. H. Characterisation of two chromogenic media of Candida ID2 and CHROMagar Candida for preliminary identification of yeasts. Journal of Biomedical and Laboratory Sciences. 2007;19:63–68. [Google Scholar]
- 26.Yücesoy M., Esen N., Yuluğ N. Use of chromogenic tube and methyl blue-sabouraud agar for the identification of Candida albicans strains. The Kobe Journal of Medical Sciences. 2001;47(4):161–167. [PubMed] [Google Scholar]
- 27.Oomar J., Rajesh J. A survey of nail infection and awareness among non-diabetic patients in mauritius. Our Dermatology Online. 2013;4(3):265–271. doi: 10.7241/ourd.20133.65. [DOI] [Google Scholar]
- 28.Tan T. Y., Tan A. L., Tee N. W., Ng L. S. A retrospective analysis of antifungal susceptibilities of Candida bloodstream isolates from Singapore hospitals. Annals of the Academy of Medicine, Singapore. 2008;37(10):835–840. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request. The authors agree that anyone interested can have access to the raw data from the research upon request to the authors. The data will be provided to anyone based on two conditions: upon use of the data, the authors should be acknowledged and also the paper needs to be cited. An agreement in the aforementioned situation will be reached.


