Abstract
Two new species of a new genus, Tortomon puer n. gen. n. sp. and T. gejiu n. gen. n. sp. are described from southern Yunnan, southwestern China, based on the morphology and mitochondrial 16S rDNA sequences. The new genus resembles Tenuipotamon Dai, 1990 and Parvuspotamon Dai & Bo, 1994, but can be separated by characters of the male thoracic sternum, male pleon, male first gonopod, and especially the coiled tip of the male second gonopod. The latter character easily separates Tortomon n. gen. from known potamid genera. Notes on the general biology of the two new species are also included.
Keywords: Freshwater crabs, Yunnan, Tortomon n. gen., New species, Potamidae, Systematics, Morphology, Mitochondrial 16S rDNA
BACKGROUND
China has the richest fauna of freshwater crabs in the world (Dai 1999; Liang 2004; Li et al. 2007; Cumberlidge et al. 2011; Shih and Ng 2011; Chu et al. 2018), and the full extent of this biodiversity is yet to be realized (De Graves et al. 2008; Yeo et al. 2008). Situated in southwestern China, the mountainous Yunnan Province is a global freshwater crab hotspot and has the highest freshwater crab genus and species diversity in China (Cumberlidge et al. 2011; Shih and Ng 2011; Chu et al. 2018). The southern areas are relatively low (around 1,500 m a.s.l.) compared to the rest of Yunnan and belong to the Diannan Highlands freshwater zoogeographic province (Huang et al. 2020). In 2013, herpetologist Mian Hou and the second author of this study collected several miniature freshwater crabs from Gejiu City, Yunnan, and gave them to the first author. The male second gonopods of these crabs were distinct as the tips were corkscrew shaped and it was evident that the crabs were not only an unknown species but also from an undescribed genus. Efforts to collect additional material and record the habitat were made by the first and second authors in 2018. On one of these collection trips, ichthyologist Zhuo-Cheng Zhou, who was accompanying the first author, recalls collecting several small purple crabs with Jia-Jun Zhou in a locality in Pu’er City, Yunnan, some years ago. We relocated the locality and successfully collected more specimens. These crabs also had the corkscrew-shaped shaped male second gonopod tip, but differed from the Gejiu new species in the male first gonopod morphology, suggesting the two are different species. A single male specimen of a third species of this genus was found by Jin Chen from Xishuangbanna Prefecture, Yunnan. The morphology of this specimen is very close to that of the Pu’er new species. We therefore refrained from describing this third new species until more specimens can be obtained to assess intraspecific variation. A genetic study of the 16S rDNA supports the uniqueness of these species as they are not clustered with other known Chinese or Indochinese genera in the phylogenetic tree. We herein describe two new species of a new genus, Tortomon puer n. gen. n. sp. and Tortomon gejiu n. gen. n. sp., from southern Yunnan.
MATERIALS AND METHODS
Specimens were collected by hand and preserved in 75% ethanol from 2013 onwards from southern Yunnan, China. They are deposited in the Sun Yat-sen Museum of Biology, Sun Yat-sen University, Guangzhou, China (SYSBM); the Australian Museum, Sydney, Australia (AM); and the Zoology Collection of the National Chung Hsing University, Taichung, Taiwan (NCHUZOOL). Measurements, in millimetres, are of the carapace width and length, respectively. Other abbreviations are as follows: G1, male first gonopod; G2, male second gonopod; CW, carapace width; P2– P5, pereiopods 2–5, respectively. The terminology used primarily follows that of Dai (1999) and Davie et al. (2015).
Specimens used in the molecular study include Tortomon puer (SYSBM 001837, 001839), T. gejiu (SYSBM 001246, 001834), T. sp. (AM P.104576 Xishuangbanna, Yunnan, coll. J. Chen, March, 2015) and Parvuspotamon dixuense Naruse, Chia & Zhou, 2018 (NCHUZOOL 16428, 16429, Zhetu, Guangnan, Yunnan, coll. H.-T. Shih, 2 November 2002). 16S sequences were obtained following Shih et al. (2009), using the primers 16H10 and 16L29 (Schubart 2009), and aligned with the MUSCLE function of MEGA (vers. 10.0.5; Kumar et al. 2018), after verification with the complimentary strand. Sequences of different haplotypes were deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers LC548635 for Parvuspotamon dixuense, LC548634 and LC548635 for Tortomon puer, LC548633 for T. gejiu and LC548636 for T. sp. A preliminary analysis showed that this genus belongs to the “Eastern-Asia Subclade” of the subfamily Potamiscinae and not related directly with the groups of “Socotra”, “SW China” and “Malay Peninsula” (Shih et al. 2009). As a result, to confirm the systematic position of the taxa, 48 additional, related sequences of the 16S sequences of genera from East Asia, Indochina and Southeast Asia in Shih et al. (2009), Huang et al. (2014 2016 2017a b 2018), and Wang et al. (2019) were included for comparison. The variable regions in loop regions of the 16S that could not be aligned adequately for phylogenetic analyses were excluded (Shih et al. 2009).
The best-fitting model for sequence evolution of 16S was determined by jModelTest (vers. 2.1.4; Darriba et al. 2012), selected by the Bayesian information criterion (BIC). The best model obtained was HKY+I+G, which was subsequently applied for Bayesian inference (BI) and minimum evolution (ME) analyses. The BI analysis was performed with MrBayes (vers. 3.2.2; Ronquist et al. 2012) and the search was run with four chains for 10 million generations, with trees sampled every 1000 generations. The convergence of chains was determined by the average standard deviation of split frequency values below the recommended 0.01 (Ronquist et al. 2005) and the first 1500 trees were discarded as the burnin accordingly. The ME tree was constructed on MEGA with the gamma correction obtained from jModeltest, the Kimura (1980) two-parameter model (CNI level = 2, initial tree = NJ, and maximum number of trees to retain = 1) and 2,000 replicates by the interior-branch method (Sitnikova et al. 1995). The pairwise estimates of K2P distances for genetic diversities between haplotypes, and the basepair (bp) differences (by treating the gaps as a fifth character state) were calculated with PAUP* (vers. 4.0b10; Swofford 2003).
RESULTS
SYSTEMATICS
Family Potamidae Ortmann, 1896
Subfamily Potamiscinae Bott, 1970 (sensu Yeo & Ng 2003)
Genus Tortomon n. gen.
(Figs. 1–8)
urn:lsid:zoobank.org:act:06743881-BA7C-4DB9-9AC1-D7E5E55D79FE
Type species: Tortomon puer n. gen. n. sp., by current designation.
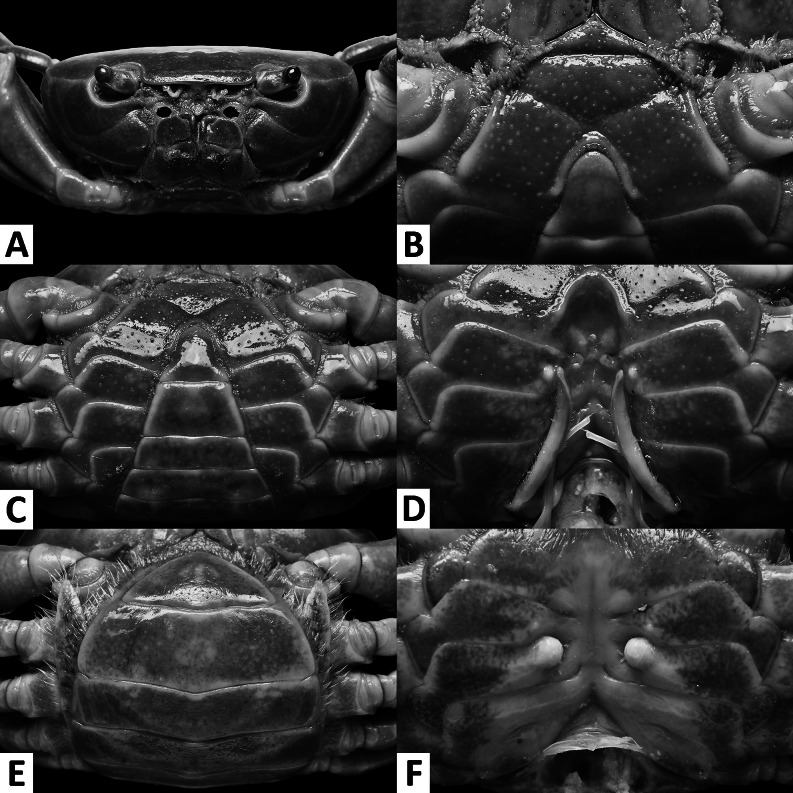
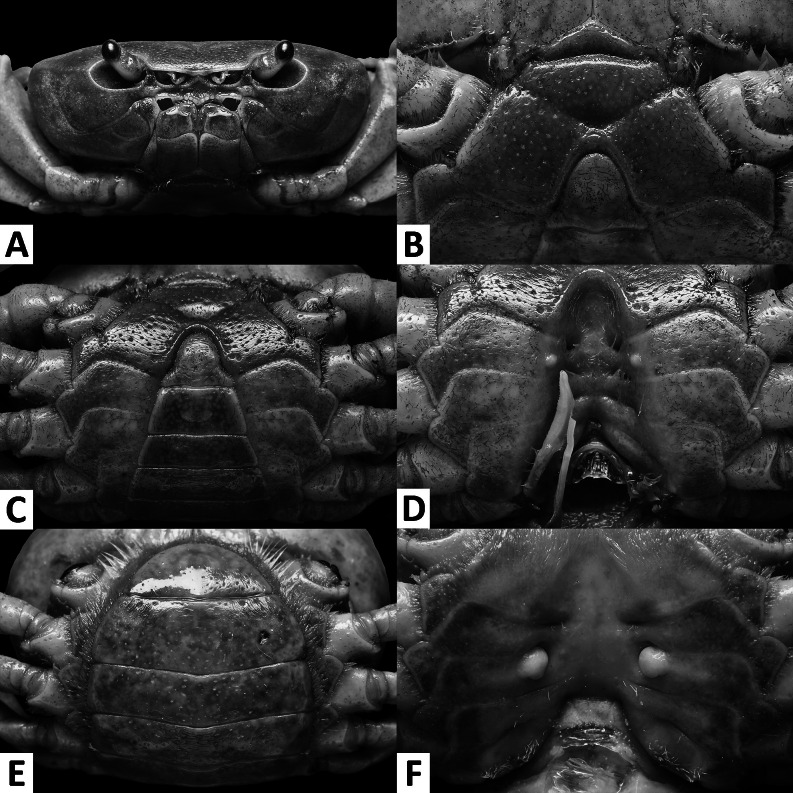
Diagnosis. Small sized (CW < 25 mm). Carapace broader than long, smooth all over, convex; regions indistinct (Figs. 1, 4); epigastric cristae and postfrontal cristae smooth, low, inconspicuous (Figs. 1, 4); external orbital tooth inconspicuous; external orbital angle fused with anterolateral margin (Figs. 1, 2A, 4, 5A). Anterolateral margin smooth to weakly cristate (Figs. 1, 4). Orbits small (Figs. 1, 2A, 4, 5A). Median lobe of epistome broadly triangular (Figs. 2A, 5A). Maxilliped 3 with relatively broad ischium, exopod strongly tapering, reaching beyond anterior edge of ischium, flagellum absent (Figs. 3A, 6A). Cheliped merus margins smooth, palm surface smooth (Figs. 1, 3F–G, 4, 6F–G). Ambulatory legs slender (Figs. 1, 4). Male anterior thoracic sternum relatively wide, width around 1.7 times length (Figs. 2B, 5B). Male pleon narrowly triangular, telson with blunt apex and concave lateral margins (Figs. 2C, 5C). G1 generally straight and slender; terminal segment short, tapering, with blunt tip; pointing upwards to slightly bent outwards (Figs. 3C–E, 6C–E, 7A–D). G2 terminal segment short, with thin coiled tip (Fig. 3B, 6B, 7E). Female vulva with relatively wide space between one another, ovate; medium-sized, mainly situated on sternite 6, reaching to sternite 5 but not sternite 7 (Figs. 2F, 5F). Female pleon ovate (Figs. 2E, 5E).
Fig. 1.
Dorsal habitus of Tortomon puer n. sp. (A) male holotype (21.8 × 16.1 mm), SYSBM 001836; (B) female paratype (19.5 × 14.6 mm), SYSBM 001839.
Fig. 2.
Tortomon puer n. sp. (A–D) male holotype (21.8 × 16.1 mm), SYSBM 001836; (E–F) female paratype (19.5 × 14.6 mm), SYSBM 001839. (A) cephalothorax, anterior view; (B) anterior thoracic sternum; (C) anterior thoracic sternum and pleon, ventral view; (D) sterno-pleonal cavity with G1 in situ, ventral view; (E) pleon, ventral view; (F) vulvae, ventral view.
Fig. 3.
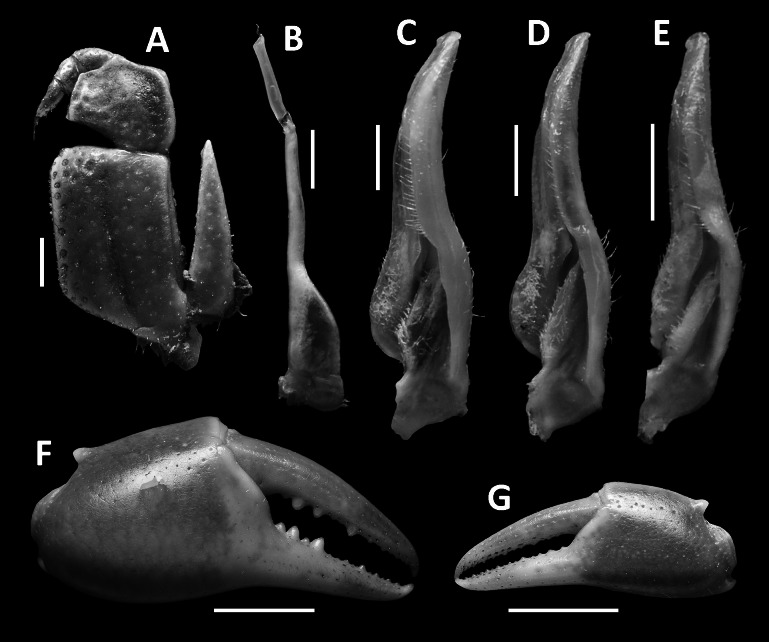
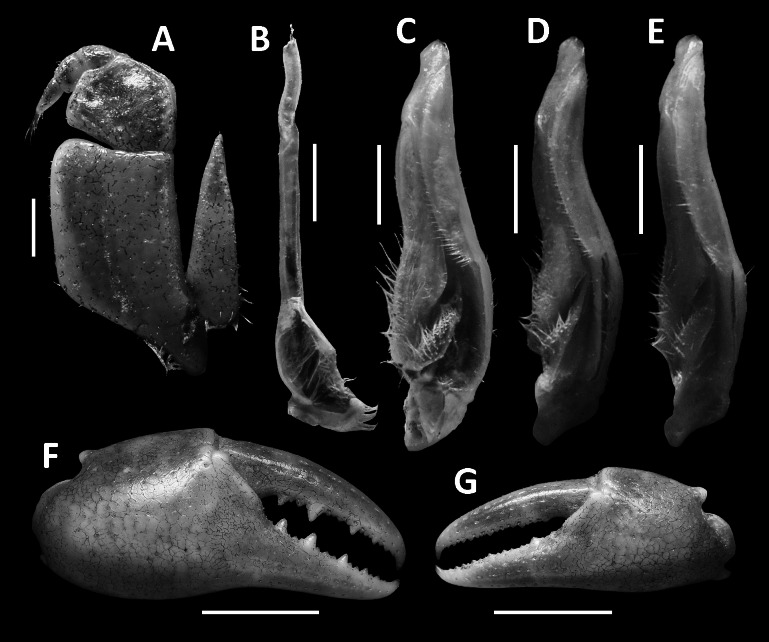
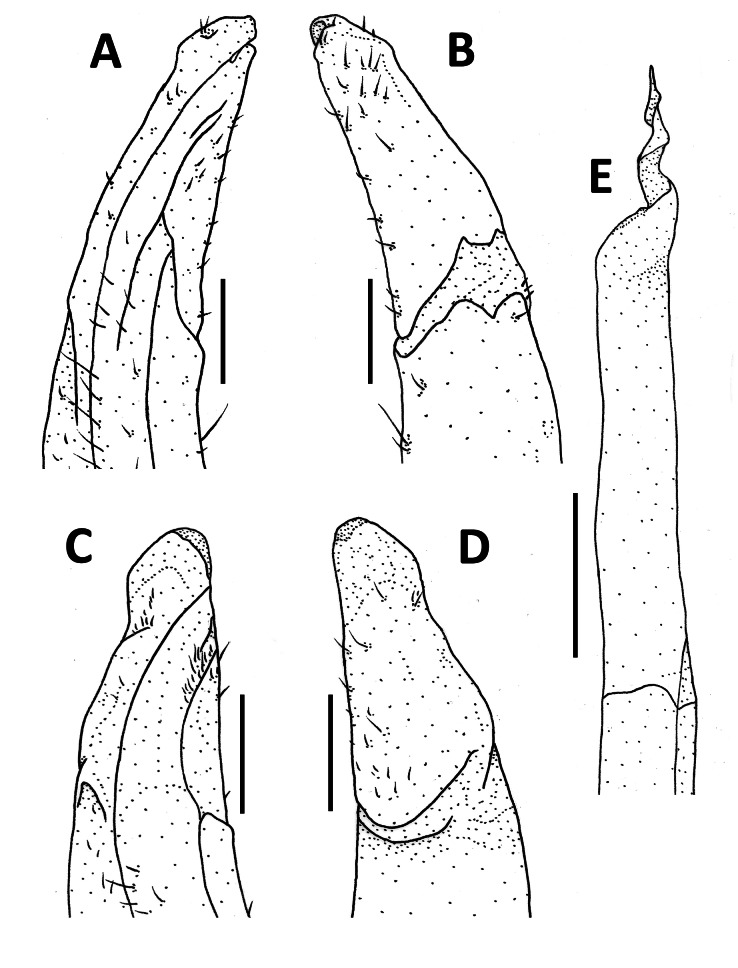
Tortomon puer n. sp. (A–C, F–G) male holotype (21.8 × 16.1 mm), SYSBM 001836; (D) male paratype (19.8 × 15.0 mm), SYSBM 001837; (E) male paratype (15.3 × 11.8 mm), SYSBM 001838. (A) left maxilliped 3; (B) left G2, ventral view; (C–E) left G1, ventral view; (F) major cheliped; (G) minor cheliped. Scale bars: A–E = 1.0 mm; F–G = 5.0 mm.
Fig. 4.
Dorsal habitus of Tortomon gejiu n. sp. (A) male holotype (20.1 × 14.0 mm), SYSBM 001834; (B) female paratype (19.1 × 14.2 mm), SYSBM 001835.
Fig. 5.
Tortomon gejiu n. sp. (A–D) male holotype (20.1 × 14.0 mm), SYSBM 001834; (E–F) female paratype (19.1 × 14.2 mm), SYSBM 001835. (A) Cephalothorax, anterior view; (B) anterior thoracic sternum; (C) anterior thoracic sternum and pleon, ventral view; (D) sterno-pleonal cavity with right G1 in situ, ventral view; (E) pleon, ventral view; (F) vulvae, ventral view.
Fig. 6.
Tortomon gejiu n. sp. (A–C, F–G) male holotype (20.1 × 14.0 mm), SYSBM 001834; (D) male paratype (17.0 × 12.1 mm), SYSBM 001247; (E) male paratype (16.7 × 12.1 mm), SYSBM 001248. (A) left maxilliped 3; (B) left G2, ventral view; (C–E) left G1, ventral view; (F) major cheliped; (G) minor cheliped. Scale bars: A–E = 1.0 mm; F–G = 5.0 mm.
Fig. 7.
(A–B) Tortomon puer n. sp., male holotype (21.8 × 16.1 mm), SYSBM 001836; (C–D) Tortomon gejiu n. sp., male holotype (20.1 × 14.0 mm), SYSBM 001834; (E) Tortomon gejiu n. sp., male paratype (23.7 × 16.4 mm), SYSBM 001246. G1 terminal segment, ventral view (A, C); G1 terminal segment, dorsal view (B, D); G2 terminal segment, ventral view (E).
Etymology: The genus name is an arbitrary combination of Latin tortus and the type genus of Potamidae, Potamon. It alludes to the coiled tip of the G2 of the new genus, which is its most distinctive character. Gender: neuter. See the Supplementary Material for its proposed Chinese name.
Distribution: Yunnan, China.
Remarks: Tortomon n. gen. is included in the Potamiscinae due to the absence of a transverse ridge on the eighth thoracic sternite (Yeo and Ng 2003). Its distribution in southern Yunnan also fits that of Potamiscinae, which occurs in East Asia and Southeast Asia (Yeo and Ng 2004). The new genus is quite unique, with only Tenuipotamon Dai, 1990 and Parvuspotamon Dai & Bo, 1994 being comparable to it within the Potamidae. Most notably, the coiled tip of the G2 terminal segment is a rare character in brachyurans (see DISCUSSION) and is not seen in any other known potamid. It is closest to Tenuipotamon in morphology, but differs in its narrowly triangular male pleon (Figs. 2C, 5C) (versus widely triangular in Tenuipotamon; Dai, 1999: fig. 205 (2)), G1 terminal segment that points upwards to slightly bent outwards (Figs. 3C–E, 6C–E, 7A–D) (versus bent inwards in Tenuipotamon; Dai, 1999: fig. 205 (5)), and G2 terminal with a thin coiled tip (Fig. 3B, 6B, 7E) (versus tip without this structure in Tenuipotamon; Dai, 1999: fig. 205 (6)). Tortomon n. gen. is also similar to Parvuspotamon, but differs in that its external orbital angle is fused with anterolateral margin (Figs. 1, 4) (versus separated by narrow gap in Parvuspotamon; Naruse et al. 2018: fig. 24B), small orbits (Figs. 2A, 5A) (versus larger orbits in Parvuspotamon; Naruse et al. 2018: fig. 24), anterior thoracic sternum relatively wide, width around 1.7 times the length, sternite 2 relatively small (Figs. 2B, 5B) (versus relatively narrow, width around 1.5 times the length, sternite 2 relatively large in Parvuspotamon; Naruse et al. 2018: fig. 25), male telson lateral margins concave (Figs. 2C, 5C) (versus slightly convex in Parvuspotamon; Naruse et al. 2018: fig. 25A), G1 terminal segment short (Figs. 3C–E, 6C–E, 7A–D) (versus slender and long in Parvuspotamon; Dai, 1999: fig. 216(4)), and the G2 terminal segment with a thin coiled tip (Figs. 3B, 6B, 7E) (versus tip without this structure in Parvuspotamon; Dai, 1999: fig. 205 (6)).
Tortomon puer n. gen. n. sp.
(Figs. 1–3, 7A, B, 8A)
urn:lsid:zoobank.org:act:A640B2B4-40C2-4EB8-8F92-41311FF8B9BE
Material examined: Holotype: SYSBM 001836, male (21.8 × 16.1 mm), Simao District (22.66°N, 101.08°E), Pu’er City, Yunnan, China, mud burrow next to small hillstream, 1,500 m a.s.l., coll. C. Huang, July, 2018. Paratypes: SYSBM 001837-001838, 2 males (19.8 × 15.0 mm, 15.3 × 11.8 mm), same data as holotype. SYSBM 001839-001840, female (19.5 × 14.6 mm, 18.3 × 14.3 mm), same data as holotype. AM P.104575, male (19.5 × 14.8 mm), same data as holotype. NCHUZOOL 16431, 2 males (22.2 × 17.0 mm, 12.6 × 9.6 mm), same data as holotype.
Description: Male: Small sized (CW < 23 mm). Carapace broader than long, width 1.3 times length (n = 7), regions indistinct; dorsal surface smooth, finely pitted, convex (Fig. 1). Front deflexed, margin almost straight in dorsal view (Fig. 1). Epigastric cristae and postfrontal cristae smooth, low, inconspicuous (Fig. 1). Branchial regions swollen; cervical groove indiscernible; mesogastric region convex (Fig. 1). External orbital tooth inconspicuous; external orbital angle fused with anterolateral margin (Figs. 1, 2A). Epibranchial tooth indiscernible (Figs. 1, 2A). Anterolateral margin slightly cristate, lined with numerous fused granules (Figs. 1, 2A). Posterolateral surface smooth (Fig. 1). Orbits small, supraorbital and infraorbital margins ridged, smooth (Figs. 1, 2A). Sub-orbital, sub-hepatic and pterygostomial regions divided by sutures; surfaces smooth (Fig. 2A). Epistome median lobe broadly triangular, posterior margin slightly sinuous (Fig. 2A).
Maxilliped 3 merus width about 1.1 times length; ischium width about 0.7 times length; merus subtrapezoidal with median depression; ischium subtrapezoidal, with distinct median sulcus, mesial margin rounded; exopod strongly tapering, reaching to proximal quarter of merus height, flagellum absent (Fig. 3A).
Chelipeds unequal (Figs. 1, 3F–G). Merus trigonal in cross section, surfaces and margins smooth (Figs. 1, 2A). Carpus with blunt spine at inner-distal angle, spinule at base barely discernible, surfaces smooth (Fig. 1). Major cheliped palm length about 1.2–1.3 times height (n = 4); dactylus 1.1 times palm length (n = 4) (Fig. 3F, G). Palm surface smooth, pitted. Occlusal margin of fingers lined with triangular teeth of different size; small gape when closed, large gape in large males (Fig. 3F, G).
Ambulatory legs (P2–5) slender, with sparse short setae. P3 merus 0.6 times carapace length (n = 5). P5 propodus 2.2–2.4 times as long as broad (n = 5), shorter than dactylus (Fig. 1A).
Thoracic sternum generally smooth, pitted; sternites 1–4 wide, width 1.7 times length; sternites 1, 2 fused, forming a subtriangular structure; sternites 2, 3 fused, separated by a deep transverse sulcus; sternites 3, 4 fused, with deep “v” shaped sulcus (Fig. 2B). Sterno-pleonal cavity reaching anteriorly to level of midlength of chelipeds coxae base (Fig. 2B); median longitudinal groove separating sternites 7, 8 deep (Fig. 2D). Pleonal locking tubercle positioned at mid-length of sternites 5 (Fig. 2D).
Pleon narrowly triangular (Fig. 2C). Pleonites 3–6 progressively narrower, lateral margins almost straight; pleonite 6 1.8 times as broad as long; telson 1.3 times as broad as long, with blunt apex and concave lateral margins (Fig. 2C).
G1 generally straight and slender, reaching beyond pleonal locking tubercle in situ (Fig. 2D). Subterminal segment 2.8–2.9 times as long as terminal segment (n = 3), inner mesial margin slightly concave, outer mesial margin slightly convex. Terminal segment short, tapering, with blunt tip, pointed slightly outwards (Figs. 3C–E, 7A, B). G2 subterminal segment 2.3 times as long as terminal segment; terminal segment with thin coiled tip (Fig. 3B).
Female: Nonsexual characters similar to males. Major cheliped palm length about 1.3–1.4 times height (n = 2); dactylus 1.1 times palm length (n = 2) (Fig. 1B). P3 merus 0.6 times carapace length (n = 2). P5 propodus 2.2–2.3 times as long as broad (n = 2), shorter than dactylus (Fig. 1B). Vulva ovate, with relatively wide space between one another; medium-sized, mainly situated on sternite 6, reaching to sternite 5 but not sternite 7 (Fig. 2F). Pleon broadly ovate (Fig. 2E).
Etymology: The new species is named as a noun after the type locality, which is in Pu’er City, Yunnan.
Colour in life: Generally dark purple on dorsal surfaces with orbital margins, cheliped tips, joints of chelipeds and joints of ambulatory legs brightly orange (Fig. 8A).
Habitat: This species was collected from a small hillstream in a tea tree plantation at around 1,500 m a.s.l. In the initial sightings of this species by Z.-C. Zhou and J.-J. Zhou, they were found in the hillstream, underwater. All the specimens collected by the first author, however, were found in burrows in high density from a patch of wet ground at the hillstream bank. Therefore, it is likely that the new species is a semi-terrestrial burrower that prefers living in soft wet mud but also sometimes visits the stream.
Remarks: Tortomon puer n. sp. is very similar to Tortomon gejiu n. sp. in overall external morphology, but can be separated by its relatively narrower carapace (width of carapace 1.3 times the length versus width 1.4 times the length in Tortomon gejiu), the G1 terminal segment more is proportionately slender and points slightly outwards (Figs. 3C–E, 7A, B) (versus G1 terminal segment stouter, points upwards in Tortomon gejiu; Figs. 6C–E, 7C, D), and the G2 terminal segment is more slender, with the subterminal segment 2.3 times as long as the terminal segment (Fig. 3B) (versus G2 terminal segment stouter, subterminal segment 2.1 times as long as terminal segment in Tortomon gejiu; Fig. 6B, 7E). The two species can also be separated by their life colours in the field, with Tortomon puer sp. nov. being dark purple (Fig. 8A) and Tortomon gejiu being generally light brown to a light turquoise (Fig. 8B).
Fig. 8.
Colour in life. (A) Tortomon puer n. sp. from type locality, Simao, Pu’er, Yunnan; (B) Tortomon gejiu n. sp. from type locality, Gejiu, Honghe, Yunnan.
Tortomon gejiu n. gen. n. sp.
(Figs. 4–6, 7C, D, 8B)
urn:lsid:zoobank.org:act:D7161B21-695D-4094-B1E7-4A168460C585
Material examined: Holotype: SYSBM 001834, male (20.1 × 14.0 mm), Yangjiatian Village (23.33°N, 103.15°E), Gejiu City, Honghe Hani and Yi Autonomous Prefecture, Yunnan, China, small hillstream, 1,800 m a.s.l., coll. J. Wang, May, 2018. Paratypes: SYSBM 001246-001248, 3 males (23.7 × 16.4 mm, 17.0 × 12.1 mm, 16.7 × 12.1 mm), Yangjiatian Village, Honghe Hani and Yi Autonomous Prefecture, Yunnan, China, small hillstream, 1800 m a.s.l., coll. M. Hou, October, 2013. SYSBM 001835, 1 female (19.1 × 14.2 mm), same data as holotype. AM P.104574, 1 male (17.6 × 13.3 mm), same data as holotype. NCHUZOOL 16430, 2 males (19.3 × 14.1 mm, 18.5 × 13.3 mm), same data as holotype.
Description: Male: Small sized (CW < 24 mm). Carapace broader than long, width 1.4 times length (n = 8), regions indistinct; dorsal surface smooth, finely pitted, convex (Fig. 4). Front deflexed, margin slightly ridged in dorsal view (Fig. 4). Epigastric cristae and postfrontal cristae smooth, low, inconspicuous (Fig. 4). Branchial regions swollen; cervical groove indiscernible; mesogastric region convex (Fig. 4). External orbital tooth inconspicuous; external orbital angle fused with anterolateral margin (Figs. 4, 5A). Epibranchial tooth indiscernible (Figs. 4, 5A). Anterolateral margin smooth, lined with smoothly fused granules (Figs. 4, 5A). Posterolateral surface smooth (Fig. 4). Orbits small, supraorbital and infraorbital margins ridged, smooth (Figs. 4, 5A). Sub-orbital, sub-hepatic and pterygostomial regions divided by sutures; surfaces smooth (Fig. 5A). Epistome median lobe broadly triangular, posterior margin almost straight (Fig. 5A).
Maxilliped 3 merus width about 1.3 times length; ischium width about 0.7 times length; merus subtrapezoidal with median depression; ischium subtrapezoidal, with distinct median sulcus, mesial margin rounded; exopod strongly tapering, reaching to proximal quarter of merus height, flagellum absent (Fig. 6A).
Chelipeds unequal (Figs. 4, 6F–G). Merus trigonal in cross section, surfaces and margins smooth (Figs. 4, 5A). Carpus with blunt spine at inner-distal angle, spinule at base barely discernible, surfaces smooth (Fig. 4). Major cheliped palm length about 1.2–1.3 times height (n = 5); dactylus 1.0–1.2 times palm length (n = 5) (Figs. 6F–G). Palm surface smooth, pitted. Occlusal margin of fingers lined with triangular teeth of different size; small gape when closed (Figs. 6F–G).
Ambulatory legs (P2–5) slender, with sparse short setae. P3 merus 0.6–0.7 times carapace length (n = 6). P5 propodus 2.2–2.6 times as long as broad (n = 5), shorter than dactylus (Fig. 4A).
Thoracic sternum generally smooth, pitted; sternites 1–4 wide, width 1.7 times length; sternites 1, 2 fused, forming a subtriangular structure; sternites 2, 3 fused, separated by a deep transverse sulcus; sternites 3, 4 fused, with deep “v” shaped sulcus (Fig. 5B). Sterno-pleonal cavity reaching anteriorly to level of midlength of chelipeds coxae base (Fig. 2B); median longitudinal groove separating sternites 7, 8 deep (Fig. 5D). Pleonal locking tubercle positioned at mid-length of sternites 5 (Fig. 5D).
Pleon narrowly triangular (Fig. 5C). Pleonites 3–6 progressively narrower, lateral margins almost straight; pleonite 6 2.0 times as broad as long; telson 1.3 times as broad as long, with blunt apex and concave lateral margins (Fig. 5C).
G1 generally straight, slender, not reaching pleonal locking tubercle in situ (Fig. 5D). Subterminal segment 2.9–3.0 times as long as terminal segment (n = 3), inner mesial margin slightly concave, outer mesial margin slightly convex. Terminal segment short, tapering, with large rounded tip, pointed upwards (Figs. 6C–E, 7C, D). G2 relatively stout, subterminal segment 2.1 times as long as terminal segment; terminal segment with thin coiled tip (Figs. 6B, 7E).
Female: Nonsexual characters similar to males. P3 merus 0.6 times carapace length (n = 1). P5 propodus 2.4 times as long as broad (n = 1), shorter than dactylus (Fig. 4B). Vulva ovate, with relatively wide space between one another; medium-sized, mainly situated on sternite 6, reaching to sternite 5 but not sternite 7 (Fig. 5F). Pleon broadly ovate (Fig. 5E).
Etymology: The new species is named as a noun after the type locality, which is in Gejiu City, Yunnan. See the Supplementary Material for its proposed Chinese name.
Colour in life: Dorsal surface generally light brown to light turquoise (Fig. 8B).
Habitat: This species was collected from a small hillstream at around 1,800 m a.s.l. Not much is known about its habits other than that it tends to be more active at night and can be found amongst aquatic plants. They were found in low densities at the type locality, which may be the result of the agricultural land use at the banks of the hillstream. It is possible that this species is also a semi-terrestrial burrower like its congener (see above) and the few specimens we found originated from a source population in the undisturbed environment further upstream.
Remarks: See remarks for Tortomon puer n. sp.
Phylogenetic Analysis
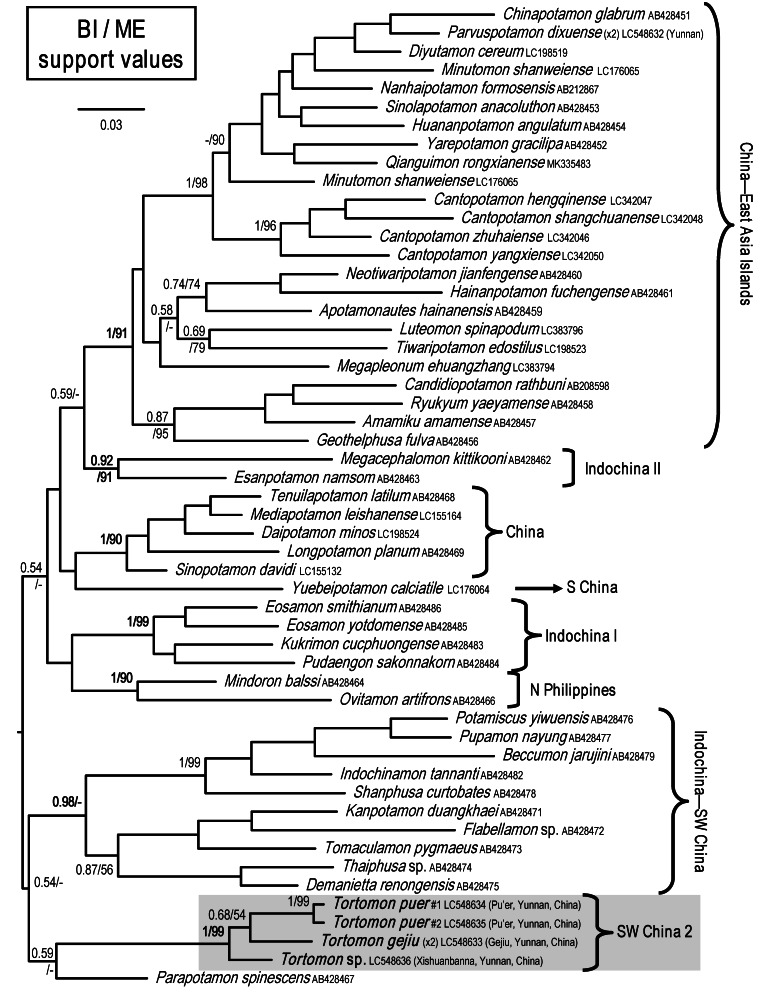
The molecular analysis of the 16S gene (505 bps after removing the variable regions) yielded 51 species in the Potamiscinae (Fig. 9). The five specimens of Tortomon formed three subclades within a larger clade corresponding to the new genus. The three subclades correspond well with T. gejiu, T. puer and T. sp., with the former two being sister species with weak support from both BI and ME methods. Although the new genus is closest to Parapotamon De Man, 1907 in the tree, the support values are too low to suggest this. The new clade formed by Tortomon is named “SW China 2” to discriminate it from the “SW China” clade in Shih et al. (2009).
Fig. 9.
A Bayesian inference (BI) tree for species of Potamiscinae from East Asia, Indochina and Southeast Asia, based on mitochondrial 16S rRNA gene. Probability values at nodes represent support values for BI and minimum evolution (ME).
The pairwise nucleotide divergences and differences in the total bp numbers (gaps considered), for the 520-bp 16S segment are shown in table 1. The genetic distances (and number of differences) among T. gejiu, T. puer and T. sp. are from 3.78% (19 bp) to 5.63% (30 bp).
Table 1.
Matrix of percentage pairwise nucleotide divergences with K2P distance and number of bp differences based on 16S rDNA between specimens of three species of Tortomon. Lower-left values are K2P and upper-right ones are bp differences
| T. gejiu (SYSBM 001246) | T. gejiu (SYSBM 001834) | T. puer (SYSBM 001837) | T. puer (SYSBM 001839) | T. sp. | |
| T. gejiu (SYSBM 001246) | 0 | 30 | 30 | 28 | |
| T. gejiu (SYSBM 001834) | 0 | 30 | 30 | 28 | |
| T. puer (SYSBM 001837) | 5.63% | 5.63% | 2 | 19 | |
| T. puer (SYSBM 001839) | 5.63% | 5.63% | 0.39% | 19 | |
| T. sp. | 5.21% | 5.21% | 3.78% | 3.78% |
DISCUSSION
The reconstructed 16S tree (Fig. 9) is largely similar to those in Shih et al. (2009), Huang et al. (2016 2017a b 2018) and Wang et al. (2019). While the new genus is superficially similar to Tenuipotamon and Parvuspotamon in morphology (see Remarks under the genus Tortomon), they do not appear to be genetically close. Tenuipotamon belongs to the “SW China group” in Shih et al. (2009), which was excluded from our phylogenetic analysis during a preliminary run (see MATERIALS AND METHODS), whereas Parvuspotamon is situated in the “China-East Asia Islands “group”,” which is also not closely related (Fig. 9). It is apparent that Tortomon is genetically distinct from other known genera, but the position of the new genus within the Potamiscinae remains unresolved. As mentioned in the Remarks for Tortomon, the coiled-tip of the G2 is a remarkable character that is only seen in a few crab species, namely some bythograeids such as Segonzacia mesatlantica (Williams, 1988) (Guinot 1989: figs. 6, 7A, C) and to a lesser extent some species in the genera Hypothalassia Gistel, 1848 (Koh and Ng 2000: fig. 11), Mursia Desmarest, 1823 (Galil 1993: figs. 3, 6, 10) and Notonyx A. Milne-Edwards, 1873 (Clark and Ng 2006: figs. 3G, 5I). The function of such a character, if indeed there is one, remains unknown for now.
With regard to the genetic distances of the 16S rDNA, the interspecific divergences among the species of Tortomon are at least 3.78% (Table 1), larger than other interspecific distances of potamid crabs (e.g., ≥ 0.93% for most Geothelphusa species from southern Taiwan in Shih et al. 2004; ≥ 2.05% for most Geothelphusa species from southwestern Taiwan in Shih et al. 2007; ≥ 0.93% for most Nanhaipotamon species in Shih et al. 2011). As a result, T. puer, T. gejiu and T. sp. are supported genetically. The single specimen that we currently have—Tortomon sp. from Xishuangbanna, Yunnan—is very similar to T. puer morphologically. More specimens of T. sp. will be needed to properly assess intraspecific variation before we attempt to describe it.
Supplementary materials
The proposed Chinese names of the new taxa published this study. (download)
Acknowledgments
This work, the new genus name, and new species names have been registered with ZooBank under urn:lsid:zoobank.org:pub:F1CFE119-FC48-46E9-8786-07A2BC05A763. This study was supported by a grant from the Ministry of Science and Technology (MOST 108-2313-B-005-007-MY3), Executive Yuan, Taiwan, to HTS. We would like to thank Mian Hou (Sichuan Normal University), Zhuo-Cheng Zhou (Professional committee of native aquatic organisms and water ecosystems of China Fisheries Association) and Jia-Jun Zhou (Zhejiang Forest Resource Monitoring Center) for discovering the two new species and providing critical information for this study. We would also like to acknowledge reviewer Peter K. L. Ng (National University of Singapore) for greatly improving our manuscript.
Footnotes
Authors’ contributions: CH did field collections and drafted the manuscript; JW collected and processed the samples, performed the ecological observation, and drafted the manuscript; HTS performed the DNA analyses and drafted the manuscript. All authors read and approved the final manuscript.
Competing interests: The authors declare that they have no conflict of interests.
Availability of data and materials: Sequences generated in the study have been deposited in the DNA Data Bank of Japan (DDBJ) database (accession numbers in Fig. 9).
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Chu KL, Ma XP, Zhang ZW, Wang PF, Lu LN, Zhao Q, Sun HY. 2018. A checklist for the classification and distribution of China’s freshwater crabs. Biodivers Sci 26:274–282. doi:10.17520/biods.2018062. (in Chinese)
- Clark PF, Ng PKL. 2006. A new species of Notonyx A. Milne-Edwards, 1873 (Crustacea, Brachyura, Goneplacidae) from the intertidal zone of Phuket, Thailand. Zoosystema 28:539–551.
- Cumberlidge N, Ng PKL, Yeo DC, Naruse T, Meyer KS, Esser LJ. 2011. Diversity, endemism and conservation of the freshwater crabs of China (Brachyura: Potamidae and Gecarcinucidae). Integr Zool 6:45–55. doi:10.1111/j.1749-4877.2010.00228.x. [DOI] [PubMed]
- Dai AY. 1999. Fauna Sinica. Arthropoda: Crustacea: Malacostraca: Decapoda: Parathelphusidae, Potamidae. Science Press, Beijing, 501 pp, 30 pls. (in Chinese)
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Meth 9:772. doi:10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed]
- Davie PJF, Guinot D, Ng PKL. 2015. Anatomy and functional morphology of Brachyura. In: Castro P, Davie PJF, Guinot D, Schram FR, von Vaupel Klein JC (eds) Treatise on zoology – anatomy, taxonomy, biology. The Crustacea. Volume 9C-I. Decapoda: Brachyura (Part 1), pp. 11–163. Brill, Leiden, The Netherlands. doi:10.1163/9789004190832_004.
- De Grave S, Cai Y, Anker A. 2008. Global diversity of shrimps (Crustacea: Decapoda: Caridea) in freshwater. Hydrobiologia 595:287–293. doi:10.1007/s10750-007-9024-2.
- Galil BS. 1993. Crustacea Decapoda: A revision of the genus Mursia Desmarest, 1823 (Calappidae). In: Crosnier A (ed), Résultats des Campagnes MUSORSTOM, Vol. 10. Mémoires du Muséum national d’Histoire naturelle, Paris 156:347–379.
- Guinot D. 1989. Description de Segonzacia gen. nov. et remarques sur Segonzacia mesatlantica (Williams): campagne HYDROSNAKE 1988 sur la dorsale médio-Atlantique (Crustacea Decapoda Brachyura). Bull Mus Nat Hist Nat Paris (4e)11:203–231.
- Huang C, Ahyong ST, Shih HT. 2017a. Cantopotamon: a new genus of freshwater crabs from Guangdong, China, with descriptions of four new species (Crustacea: Decapoda: Brachyura: Potamidae). Zool Stud 56:41. doi:10.6620/ZS.2017.56-41. [DOI] [PMC free article] [PubMed]
- Huang C, Ebach M, Ahyong ST. 2020. Bioregionalisation of the freshwater zoogeographical areas of mainland China. Zootaxa 4742:271–298. doi:10.11646/zootaxa.4742.2.3. [DOI] [PubMed]
- Huang C, Mao SY, Huang JR. 2014. Two new potamid crabs, Yuexipotamon arcophallus new genus, new species and Minutomon shanweiense new genus, new species, (Crustacea: Decapoda: Brachyura: Potamidae) from southern China. Zootaxa 3764:455–466. doi:10.11646/zootaxa.3764.4.5. [DOI] [PubMed]
- Huang C, Shih HT, Ahyong ST. 2018. Two new genera and two new species of narrow-range freshwater crabs from Guangdong, China (Decapoda: Brachyura: Potamidae). J Crustacean Biol 38:614–624. doi:10.1093/jcbiol/ruy050.
- Huang C, Shih HT, Mao SY. 2016. Yuebeipotamon calciatile, a new genus and new species of freshwater crab from southern China (Crustacea, Decapoda, Brachyura, Potamidae). ZooKeys 615:61–72. doi:10.3897/zookeys.615.9964. [DOI] [PMC free article] [PubMed]
- Huang C, Shih HT, Ng PKL. 2017b. A new genus and new species of Potamidae (Crustacea: Decapoda: Brachyura: Potamoidea), the first stygomorphic cave crab known from China and East Asia. Zootaxa 4232:71–84, 4250:600. doi:10.11646/zootaxa.4232.1.5. [DOI] [PubMed]
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. [DOI] [PubMed]
- Koh SK, Ng PKL. 2000. A revision of the spiny crabs of the genus Hypothalassia Gistel, 1848 (Crustacea: Decapoda: Brachyura: Eriphiidae). Raffles Bull Zool 48:123–141.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi:10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Li XZ, Liu RY, Liang XQ, Chen GX. 2007. Fauna Sinica. Invertebrata: Crustacea: Decapoda: Palaomonoidea. Science Press, Beijing, 381 pp. (in Chinese)
- Liang X. 2004. Fauna Sinica. Invertebrata: Crustacea: Decapoda: Atyidae. Science Press, Beijing, 375 pp. (in Chinese)
- Naruse T, Chia JE, Zhou XM. 2018. Biodiversity surveys reveal eight new species of freshwater crabs (Decapoda: Brachyura: Potamidae) from Yunnan Province, China. PeerJ 6:e5497. doi:10.7717/peerj.5497. [DOI] [PMC free article] [PubMed]
- Ronquist F, Huelsenbeck JP, Teslenko M, Nylander JAA. 2005. MrBayes 3.2 Manual. http://mrbayes.csit.fsu.edu/manual.php (Accessed 22 June 2020).
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi:10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed]
- Schubart CD. 2009. Mitochondrial DNA and decapod phylogenies: the importance of pseudogenes and primer optimization. Decapod Crustacean Phylogenetics. Crustacean Issue 18:47–65.
- Shih HT, Ng PKL. 2011. Diversity and biogeography of freshwater crabs (Crustacea: Brachyura: Potamidae, Gecarcinucidae) from East Asia. Syst Biodivers 9:1–16. doi:10.1080/14772000.2011.5 54457.
- Shih HT, Ng PKL, Chang HW. 2004. Systematics of the genus Geothelphusa (Crustacea, Decapoda, Brachyura, Potamidae) from southern Taiwan: a molecular appraisal. Zool Stud 43:561– 570.
- Shih HT, Yeo DCJ, Ng PKL. 2009. The collision of the Indian Plate with Asia: molecular evidence for its impact on the phylogeny of freshwater crabs (Brachyura: Potamidae). J Biogeogr 36:703– 719. doi:10.1111/j.1365-2699.2008.02024.x.
- Shih HT, Zhou XM, Chen GX, Chien IC, Ng PKL. 2011. Recent vicariant and dispersal events affecting the phylogeny and biogeography of East Asian freshwater crab genus Nanhaipotamon (Decapoda: Potamidae). Mol Phylogen Evol 58:427–438. doi:10.1016/j.ympev.2010.11.013. [DOI] [PubMed]
- Sitnikova T, Rzhetsky A, Nei M. 1995. Interior-branch and bootstrap tests of phylogenetic trees. Mol Biol Evol 12:319–333. [DOI] [PubMed]
- Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, Massachusetts, USA.
- Wang SB, Huang C, Zou JX. 2019. Description of a new species of freshwater crab of the genus Qianguimon Huang, 2018 (Crustacea: Decapoda: Brachyura: Potamidae) from Yulin, Guangxi, southern China. Zool Stud 58:31. doi:10.6620/ZS.2019.58-31. [DOI] [PMC free article] [PubMed]
- Yeo DCJ, Ng PKL. 2003. Recognition of two subfamilies in the Potamidae Ortmann, 1896 (Brachyura, Potamidae) with a note on the genus Potamon Savigny, 1816. Crustaceana 76:1219– 1235. doi:10.1163/156854003773123456.
- Yeo DCJ, Ng PKL, Cumberlidge N, Magalhães C, Daniels SR, Campos MR. 2008. Global diversity of crabs (Crustacea: Decapoda: Brachyura) in freshwater. Hydrobiologia 595:275– 286. doi:10.1007/s10750-007-9023-3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The proposed Chinese names of the new taxa published this study. (download)