Abstract
Objective We deployed a Remote Patient Monitoring (RPM) program to monitor patients with coronavirus disease 2019 (COVID-19) upon hospital discharge. We describe the patient characteristics, program characteristics, and clinical outcomes of patients in our RPM program.
Methods We enrolled COVID-19 patients being discharged home from the hospital. Enrolled patients had an app, and were provided with a pulse oximeter and thermometer. Patients self-reported symptoms, O 2 saturation, and temperature daily. Abnormal symptoms or vital signs were flagged and assessed by a pool of nurses. Descriptive statistics were used to describe patient and program characteristics. A mixed-effects logistic regression model was used to determine the odds of a combined endpoint of emergency department (ED) or hospital readmission.
Results A total of 295 patients were referred for RPM from five participating hospitals, and 225 patients were enrolled. A majority of enrolled patients (66%) completed the monitoring period without triggering an abnormal alert. Enrollment was associated with a decreased odds of ED or hospital readmission (adjusted odds ratio: 0.54; 95% confidence interval: 0.3–0.97; p = 0.039). Referral without enrollment was not associated with a reduced odds of ED or hospital readmission.
Conclusion RPM for COVID-19 provides a mechanism to monitor patients in their home environment and reduce hospital utilization. Our work suggests that RPM reduces readmissions for patients with COVID-19 and provides scalable remote monitoring capabilities upon hospital discharge. RPM for postdischarge patients with COVID-19 was associated with a decreased risk of readmission to the ED or hospital, and provided a scalable mechanism to monitor patients in their home environment.
Keywords: remote patient monitoring, coronavirus disease 2019, patient outcome assessment, remote sensing technology
Background and Significance
The novel coronavirus “severe acute respiratory syndrome coronavirus 2”—which first emerged in Wuhan, China in late 2019 and caused the respiratory illness coronavirus disease 2019 (COVID-19)—has infected millions worldwide, caused unprecedented morbidity and mortality, and overwhelmed many healthcare delivery systems. 1 2 3 4 Hospitals have been forced to fundamentally shift clinical operations, with canceled procedures, repurposed intensive care units (ICUs), and even staff reductions. 5 6 7 8 9 Meanwhile, the COVID-19 pandemic has forced an expansion in the use of virtual care, radically accelerating a shift in care delivery that had already begun, now enabled by U.S. regulatory changes and improved pathways to reimbursement. 10 11 12 13 14
Remote patient monitoring (RPM) is a mechanism for assessing patients outside a typical clinical encounter, for example, in their homes or communities. RPM programs use various telecommunication channels to collect health data—such as a symptom surveys, vital signs, or data from wearable sensors—and transmit these data to a healthcare provider in a different location. 15 16 17 18 19 RPM is an attractive care delivery strategy with promise for lower costs, improved convenience, closer monitoring capabilities, and potentially better outcomes. However, data supporting the use of RPM have so far been mixed, with variable results depending on condition and implementation. 16 20 21 22 23 24 25 26
As COVID-19 cases began to emerge in our region, we identified a need for managing an increasing number of patients admitted to the hospital with COVID-19 in the setting of unparalleled resource constraints. We therefore rapidly designed and deployed an RPM program so that we could monitor and manage patients after discharge in their home environment using pulse oximeters, thermometers, and an app-based symptom assessment tool, monitored by a team of nurses.
We identified several potential benefits from such an RPM program for patients with COVID-19. First, most patients with COVID-19 ultimately recover, even among hospitalized populations, and there is a great need for continuity of care posthospitalization. 27 28 Second, because of the infectious nature of the disease, allowing people to remain in their home could decrease the chance of spread to other patients or staff within the hospital setting. Third, COVID-19 takes a variable clinical course 29 and identifying patients who may clinically worsen after a hospitalization could have important patient safety benefits. Fourth, an RPM program might allow a discharging clinician to feel more comfortable with an earlier discharge, knowing that the patient would be closely monitored after leaving the hospital, thus freeing up additional inpatient capacity. Finally, as COVID-19 rapidly spread in our geographic region, there was significant provider and patient anxiety around its clinical course, epidemiologic characteristics, and outcomes. By providing a visible layer of perceived security, an RPM program could potentially assuage these concerns.
Numerous institutions have implemented RPM programs in response to COVID-19, and initial work has described COVID-19 related RPM programs with a focus on implementation, population characteristics, postdischarge, and cohort isolation settings. 15 30 31 32 33 34 35 36 We describe here the characteristics of our patients and the efficacy of this program in the remote management of patients recently admitted with COVID-19.
Methods
Setting
MassGeneral Brigham (formerly Partners HealthCare) is a large not-for-profit healthcare system based in Boston, Massachusetts founded by Massachusetts General Hospital and Brigham and Women's Hospital. As of this writing, Massachusetts has had over 125,000 cases of COVID-19 and over 9,000 deaths, 37 making Massachusetts one of the hardest-hit states. Our program was launched at our two largest hospitals (Massachusetts General Hospital and Brigham and Women's Hospital) during a pilot period, followed by three additional hospitals several weeks later (Newton-Wellesley Hospital, Brigham and Women's Faulkner Hospital, and North Shore Medical Center).
Tool
We deployed MyChart Care Companion, a module embedded in our patient portal software (Epic Systems Inc., Verona, Wisconsin, United States). Care Companion has both mobile and desktop version, and was available in English and Spanish. The mobile version reminds a patient each morning to complete a survey, at which point the patient is able to self-enter their device data (oxygen saturation and temperature), and answer five symptom questions related to shortness of breath, cough, appetite, weakness, and vomiting. The content was developed by Cleveland Clinic and Epic Systems, Inc. 30 There were no major technical issues with care companion during the study period.
Study Population and Program Eligibility
Patients were eligible for the program if they had a diagnosis of COVID-19 (or presumed COVID-19 if nasopharyngeal polymerase chain reaction (PCR) was negative, but clinical suspicion was high, for example, with characteristic symptoms and computed tomography findings), were able to activate a patient portal account, were being discharged home (with or without nursing services), and were able to fill out the survey in English or Spanish (either alone or with the help of a proxy). Exclusion criteria included age <18, comorbid highly symptomatic non-COVID-19 conditions outside the scope of RPM triage providers (e.g., advanced heart failure with dyspnea), cognitive or behavioral health barriers to participation that could not be overcome with caregiver support, conditions limiting the ability to work with the devices, lack of a working phone, or discharge to a facility (e.g., skilled nursing facility, short-term rehabilitation, or field hospital).
Eligible patients were referred to the program at the time of discharge through an order in our electronic health record (EHR) and provided with a pulse oximeter (Masimo MightSat or the Sensogram Sensoscan), a thermometer (Care Line Inc., oral), an instructional packet on the program, and a self-addressed stamped envelope for eventual return of the pulse oximeter. These devices were chosen based on extant knowledge about the clinical course of COVID-19 at the time, as well as the availability of devices within our supply chains. Eligible patients who were not already users of our patient portal were guided through the portal enrollment process. All materials were provided free of charge to the patient.
On the day after discharge, all referred patients were contacted by a nurse from our central call center. Patients who were reachable and were able to confirm that they had the app installed (or could access their desktop computer) were considered enrolled. The contacting nurse would also provide assistance for patients or proxies who were having difficulty with the app as needed. Enrolled patients were subsequently prompted through an automated alert or email to complete the questionnaire on the app every morning. If the patient had no concerning symptoms and their objective data were within defined parameters (O 2 saturation greater than or equal to 92%, temperature less than 100.4F), then no further steps were taken. A message would be sent to a pooled EHR inbox under three conditions: first, if the patient reported worsening symptoms in the questionnaire; second, if their self-entered O 2 saturation was less than 92% or their temperature was greater than 100.3F; and third, if an assigned monitoring task had not been completed in 24 hours. The pooled EHR inbox was staffed by a team of triage nurses 8 A.M. to 8 P.M. in a centralized call-center established specifically for calls related to COVID-19. The nurses would then contact the patient, perform a clinical assessment, and then determine an appropriate plan, for example, refer to the ED, contact the primary care physician, offer empiric treatment, or simply continue monitoring. Given the potential severity of COVID-19 and that our program was started during the early stages of the pandemic, we did not opt for automatic monitoring.
The program was staffed with 24/7 physician backup, and patients were instructed how to reach the on-call physician in the evening hours of 8 P.M. to 8 A.M. when there was no nursing coverage available. After 2 weeks, patients were given the option to complete monitoring or to continue for a third week at patient discretion ( Fig. 1 for detailed enrollment and monitoring workflow).
Fig. 1.
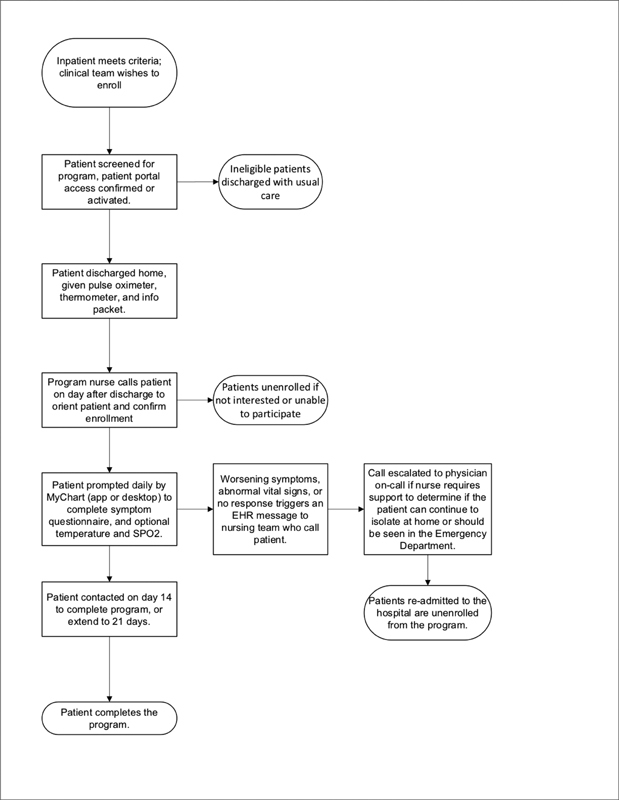
Detailed enrollment workflow for patients with COVID-19 being discharged from one of the five participating hospitals with the Remote Patient Monitoring program.
Statistical Analysis
Our overall study population included all patients with COVID-19 (or presumed COVID-19) discharged home from one of the five participating hospitals. We split this population into three groups: (1) patients who were not referred to the program, (2) patients who were referred, but not successfully enrolled on post-discharge day 1, and (3) patients ordered for the program and successfully enrolled on postdischarge day 1. Group 1 served as our control group. We performed descriptive statistics for each of these groups. We assessed program engagement by analyzing survey responses, length of time in the program, and symptom patterns across the submitted questionnaires. We examined the frequency of questionnaire responses per patient, segmented by whether the questionnaire triggered an alert to the central nursing pool.
A mixed-effects multivariable logistic regression model was used to compute odds ratios (ORs) with 95% confidence intervals (CIs) for the odds that a patient with COVID-19 who was discharged home from one of our participating hospitals would be readmitted to the emergency department (ED) or the hospital within 30 days of the initial discharge. Covariates included age, ethnicity, language, race (for all groups with at least five readmission events), length of stay of index admission, whether the patient was in an ICU at any point during their admission, income quartile (estimated by zip code), and whether the patient was ordered for the remote monitoring program but not ultimately enrolled, or ordered for the program and enrolled on discharge day 1 (i.e., Group 2 or Group 3 above). Our model included a random-effect term to account for the discharging hospital. COVID-19 status was determined by having an ICD-10 billing code matching COVID.
Data analysis was conducted using R statistical software version 3.5.1 (R Project for Statistical Computing, Vienna, Austria). As a retrospective study on a quality improvement project, the MassGeneral Brigham institutional review board exempted this study from review.
Results
A total of 1,356 patients with COVID-19 were discharged from one of the five hospitals participating in the RPM program during the study period (April 8, 2020 to June 10, 2020 when the program ended due to declining COVID-19 admissions), 295 were referred for RPM by a discharging provider, and 225 patients were enrolled on post-hospital day 1. Our two largest hospitals launched first; the remaining three hospitals launched a few weeks later, and 181 patients completed either 14 or 21 days, while 44 were enrolled, but did not end up completing 14 or 21 days of monitoring ( Fig. 2 ). Table 1 shows the baseline characteristics of COVID-19 patients not referred to the program, referred but not ultimately enrolled, or referred and successfully enrolled in the program.
Fig. 2.
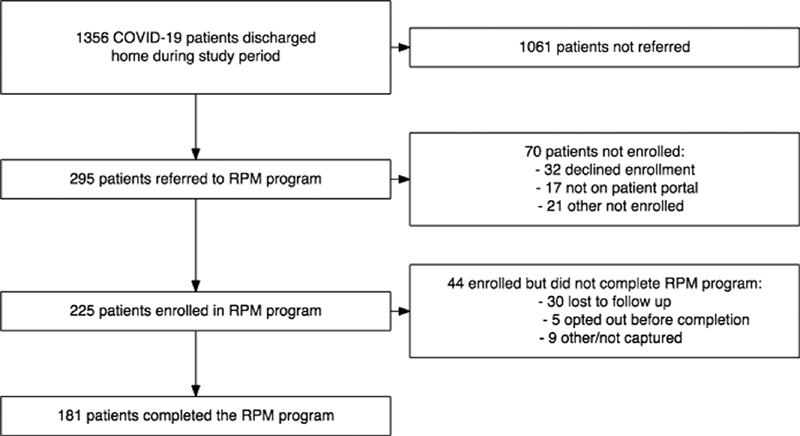
Remote Patient Monitoring enrollment funnel.
Table 1. Demographics of discharged patients with COVID-19 not ordered for Remote Patient Monitoring, ordered for Remote Patient Monitoring (but not successfully enrolled), and patients ordered as well as successfully enrolled.
| Characteristic | RPM not ordered, n = 1,061 a | RPM ordered (not enrolled), n = 70 a | RPM ordered ( enrolled), n = 225 a | p -Value b |
|---|---|---|---|---|
| Gender (%) | 0.7 | |||
| Female | 536 (51) | 32 (46) | 114 (51) | |
| Male | 525 (49) | 38 (54) | 111 (49) | |
| Age | 55 (41–66) | 56 (41–69) | 54 (41–65) | 0.6 |
| Language (%) | 0.028 | |||
| English | 567 (53) | 41 (59) | 142 (63) | |
| Spanish | 386 (36) | 24 (34) | 65 (29) | |
| Not available/other | 108 (10) | 5 (7.1) | 18 (8.0) | |
| Race (%) | 0.14 | |||
| Black or African American | 179 (17) | 12 (17) | 50 (22) | |
| White | 396 (37) | 26 (37) | 85 (38) | |
| Not available/other | 486 (46) | 32 (46) | 90 (40) | |
| Ethnicity (%) | 0.4 | |||
| Hispanic or Latino | 426 (40) | 31 (44) | 81 (36) | |
| Not Hispanic or Latino | 544 (51) | 35 (50) | 126 (56) | |
| Other/not available | 91 (8.6) | 4 (5.7) | 18 (8.0) | |
| Length of stay | 5.0 (3.0–8.0) | 5.0 (2.0–10.0) | 5.0 (3.0–8.0) | 0.4 |
| Required ICU (%) | 255 (24) | 23 (33) | 67 (30) | 0.068 |
| Readmission status (%) | 0.15 | |||
| Not readmitted | 954 (90) | 62 (89) | 211 (94) | |
| Readmitted to ED | 46 (4.3) | 7 (10) | 11 (4.9) | |
| Readmitted to hospital | 61 (5.7) | 1 (1.4) | 3 (1.3) | |
| Median income by zip code (%) | 0.066 | |||
| 4,836–41,406.5 | 515 (49) | 30 (43) | 88 (39) | |
| 41,406.5–51,897 | 272 (26) | 20 (29) | 72 (32) | |
| 51,897–65,903.5 | 169 (16) | 12 (17) | 37 (16) | |
| 65,903.5–244,671 | 99 (9.3) | 8 (11) | 27 (12) | |
| Non-U.S., invalid, or missing | 6 (0.6) | 0 (0) | 1 (0.4) |
Abbreviations: ED, emergency department; ICU, intensive care unit; IQR, interquartile range; RPM, Remote Patient Monitoring; US, United States.
Statistics presented: n (%); median (IQR).
Statistical tests performed: Kruskal–Wallis test.
During the study period, 210 patients completed at least one questionnaire, with a total of 2,291 patient days in the program and 2,161 total questionnaires completed. Among patients who completed at least one questionnaire, engagement was high, with a median program duration of 12 days (interquartile range [IQR]: 10–13 days) and a median of 11 (IQR: 8–13) questionnaires completed per patient. The most common symptom that triggered an alert was temperature greater than 100.3, present in 11% (239/2161) of all questionnaires, and 76% (239/315) of those leading to an alert ( Table 2 ).
Table 2. Remote patient monitoring program patient engagement characteristics.
| Characteristics | Value |
|---|---|
| Number of patient days in program | 2,291 |
| Number of questionnaires submitted | 2,161 |
| Program duration, per patient (day, median [IQR]) | 12 (10–13) |
| Number of questionnaire responses, per patient (median [IQR]) | 11 (8–13) |
| Patients that completed at least one survey | 210 |
| Patients that triggered a symptom/vital sign alert | 72/210 (34%) |
| Number of questionnaires triggering alerts | 315/2,161 (15%) |
| Number of phone calls to patients | 868 |
| Escalated questionnaires that reported: | |
| O 2 saturation < 92% | 11/315 (3%) |
| Temperature > 100.3 | 239/315 (76%) |
| Vomiting | 16/315 (5%) |
| Worse appetite | 17/315 (5%) |
| Worse cough | 28/315 (9%) |
| Worse shortness of breath | 27/315 (9%) |
| Worse weakness | 31/315 (10%) |
Abbreviation: IQR, interquartile range.
A majority of patients were monitored without intervention for their entire postdischarge monitoring period. Of the 210 who completed at least one questionnaire, only 72/210 (34%) triggered a symptom alert to the central nursing pool during their monitoring enrollment period, and only 15% (315/2161) of questionnaires across all patients triggered an alert to the central nursing pool ( Table 2 ). Fig. 3 shows the number of questionnaires completed by program day, split by whether the questionnaire resulted in an alert or escalation to the nursing pool. There was a steady decline in the number of questionnaires per program day over time.
Fig. 3.
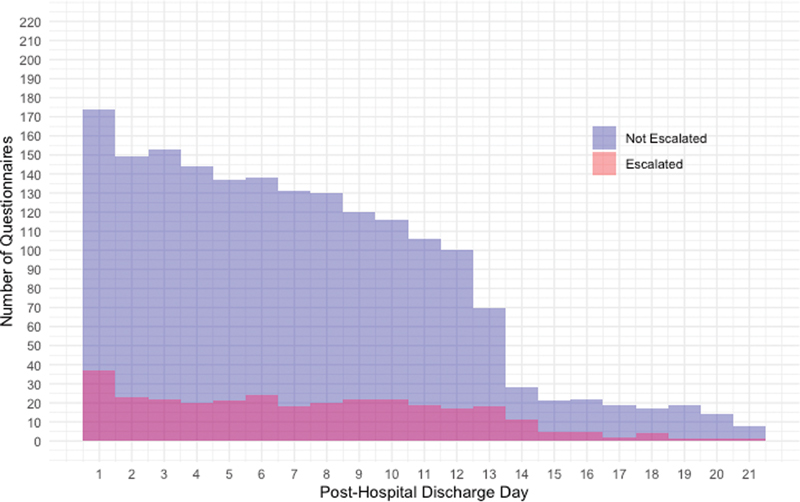
Frequency of completed questionnaires by post-hospital discharge day. Shown are the frequency of surveys triggering escalation based on post-hospital discharge day for the entire population of enrolled patients.
In a multivariable model, we found that being successfully enrolled in the RPM program on postdischarge day 1 was associated with a decreased odds of the combined endpoint of presentation to the ED or readmission (adjusted odds ratio [OR]: 0.54; 95% CI: 0.3–0.97; p = 0.039), but being ordered for the RPM program without a successful enrollment was not associated with a decreased odds of the combined endpoint (adjusted OR: 1.11; 95% CI: 0.51–2.41; p = 0.793). There were no important readmission associations for other demographic variables such as age, sex, race, ethnicity, or language. Full results are listed in Table 3 .
Table 3. Mixed-effects adjusted logistic regression model for odds that a patient with COVID-19, initially discharged home, would be readmitted to the hospital or emergency department.
| Characteristic | Unadjusted by readmission status | Readmission to ED/hospital | |||
|---|---|---|---|---|---|
| Not readmitted, n = 1,227 a | Readmitted, n = 129 a | OR | 95% CI | p -Value | |
| Gender (%) | |||||
| Female | 616 (50) | 66 (51) | |||
| Male | 611 (50) | 63 (49) | 1.04 | 0.72–1.51 | 0.84 |
| Age | 54 (17) | 55 (17) | 0.99 | 0.98–1.01 | 0.29 |
| Language (%) | |||||
| English | 663 (54) | 87 (67) | |||
| Spanish | 443 (36) | 32 (25) | 0.83 | 0.42–1.66 | 0.60 |
| Not available/other (%) | 121 (9.9) | 10 (7.8) | 0.89 | 0.43–1.84 | 0.76 |
| Race (%) | |||||
| Black or African American | 215 (18) | 26 (20) | |||
| White | 441 (36) | 66 (51) | 1.17 | 0.71–1.95 | 0.54 |
| Not available /other | 571 (47) | 37 (29) | 0.65 | 0.35–1.18 | 0.16 |
| Ethnicity (%) | |||||
| Hispanic or Latino | 499 (41) | 39 (30) | |||
| Not Hispanic or Latino | 619 (50) | 86 (67) | 1.12 | 0.57–2.23 | 0.74 |
| Other/not available | 109 (8.9) | 4 (3.1) | 0.34 | 0.11–1.10 | 0.073 |
| Length of stay | 5.0 (3.0–8.0) | 4.0 (2.0–8.0) | 1.02 | 0.99–1.04 | 0.16 |
| Required ICU | 311 (25) | 34 (26) | 0.92 | 0.58–1.47 | 0.73 |
| Median income by zip code | |||||
| 4,836–41,406.5 | 589 (48) | 44 (34) | |||
| 41,406.5–51,897 | 325 (26) | 39 (30) | 1.45 | 0.90–2.31 | 0.13 |
| 51,897–65,903.5 | 191 (16) | 27 (21) | 1.60 | 0.93–2.74 | 0.089 |
| 65,903.5–244,671 | 116 (9.5) | 18 (14) | 1.65 | 0.87–3.13 | 0.12 |
| Non-U.S., invalid, or missing | 6 (0.5) | 1 (0.8) | 2.05 | 0.23–18.2 | 0.52 |
| Program status | |||||
| RPM not ordered | 954 (78) | 107 (83) | |||
| RPM ordered not enrolled | 62 (5.1) | 8 (6.2) | 1.11 | 0.51–2.41 | 0.79 |
| RPM ordered enrolled | 211 (17) | 14 (11) | 0.54 | 0.30–0.97 | 0.039 |
Abbreviations: ED, emergency department; ICU, intensive care unit; OR, odds ratio; IQR, interquartile range; RPM, Remote Patient Monitoring; SD, standard deviation; US, United States.
Statistics presented: n (%); mean (SD); median (IQR).
Discussion
We describe the implementation and impact of a posthospitalization portal-based RPM program for patients with COVID-19. We found that the RPM program was associated with a significantly decreased risk of our combined endpoint of ED or hospital readmission for patients who were referred to the program and enrolled on postdischarge day 1. Furthermore, among patients who completed at least one questionnaire while enrolled in the program (210 patients out of 225 enrolled), 66% did not product a symptom alert that needed manual follow-up, suggesting that RPM for discharged patients with COVID-19 can provide broad monitoring without the need to directly contact patients after hospital discharge beyond the initial enrollment call. This is an important finding because it demonstrates a scalable mechanism for monitoring patients with high risk for clinical worsening without requiring one-to-one engagement.
COVID-19 has forced healthcare systems around the world to innovate and adapt to unprecedented operational and clinical strain. In many ways, the particular challenges presented by the COVID-19 pandemic create a variety of needs for which RPM is uniquely well suited. First, RPM offers the potential to partially ameliorate capacity constraints. By reducing the risk of undetected decompensation after discharge, RPM has the potential to reduce length of stay for medically stable patients who might otherwise get another day of observation. With many hospitals operating well above capacity, the value of even a small reduction in occupancy would be meaningful. Second, RPM may allow health systems to make better use of human resources. Many health systems have redeployed providers to assist with COVID-19 follow-up calls to ensure adequate surveillance for decompensations. In our experience, these calls are often done by prescriber-level providers, and typically take between 8 and 15 minutes. By automating a significant share of time-intensive phone calls, RPM may decrease the cost of COVID-19 follow-up and increase the capacity of providers to provide other higher-value forms of care.
Third, by offering an extra layer of monitoring, relative to usual care, RPM may increase safety and enhance the patient experience. The significant heterogeneity observed in COVID-19 patient trajectories represents a focus of clinical risk and significant worry for patients, their families, and their medical providers. The variable and frequently prolonged clinical course of COVID-19 in particular make RPM appealing, as median symptom duration is heterogeneous and many patients take weeks to recover. 29 38 Fortunately, most patients have mild disease and do not require hospitalization or escalated care. However, some patients develop moderate or severe disease, and RPM enables a healthcare system to monitor a large set of patients to ensure that the patients that do require escalated care are rapidly identified. Fourth, by reducing direct patient contact, RPM may help reduce the spread of a highly contagious illness, keeping infectious patients in their homes instead of clinical settings (although this could also cause spread to family members). Fifth, as we demonstrate, RPM may have the potential to reduce readmissions to the hospital or ED. We hypothesize that this may be due to the patients having a reliable point of contact, as well as vital sign monitoring for reassurance. Because of significant anxiety around COVID-19, particularly early in the pandemic, in our experience patients had a low threshold for presenting to the ED or hospital, and it is possible that our program led to fewer unnecessary presentations. Finally, by embedding real-time knowledge generation processes into routine care, RPM facilitates the functions of a so-called “learning health system.” The real-time adaptation that typifies learning health systems is invaluable when dealing with a previously unknown illness, such as COVID-19.
Using technology to remotely monitor patients in their home environment has been of interest for decades across many different diseases, but unfortunately has mixed results from hundreds of randomized trials. 39 Remote monitoring for congestive heart failure, for example, a common condition leading to hospitalization (and subsequent re-admissions)—has had variable results 21 40 41 42 —as have numerous other conditions. 16 43 44 There is significant heterogeneity in this space; clinical condition, usage of devices, or wearables and program implementation are just some of the variables that might impact program success. Early evidence, as well as numerous press releases, have indicated a significant interest in using RPM for COVID-19. Our work suggests that RPM can be an effective method of monitoring patients with COVID-19 being discharged from the hospital, that it may lead to a decreased likelihood of presenting to the ED or hospital, and that most patients were monitored without active intervention by our monitoring nurses.
Unfortunately, COVID-19 has disproportionately impacted racial and ethnic minorities, especially African American, Native American, Latinx, and other underserved groups. 46 47 48 This was true in our population as well; more than 30% of our total population with COVID-19 spoke Spanish, for example, which is substantially higher than baseline (in Boston, ∼14% of residents speak Spanish at home). 49 Our program implementation team worked diligently to ensure access to the RPM program to all patients, and our results demonstrated reasonably equitable enrollment, with no statistically significant difference in enrollment between racial or ethnic groups, though there were statistically fewer patients who spoke Spanish that ended up fully enrolled. More broadly, it is important to recognize that requiring a smart phone or desktop computer, having only English and Spanish translations, and relying on provider discretion for referral created the potential for systemic structural inequities in access to the RPM program. We attempted to mitigate these systemic exclusions by enabling proxy access for data entry and 24-hour phone-interpreter support, by using inpatient staff to help with portal access and by explicitly acknowledging concerns around disparities with our team members, but we recognize this may have not been sufficient. Future work should build on this program with an increased focus on health equity concerns.
Our study has several limitations. First, as a five-hospital study, it may not generalize to other hospitals or geographies and we only had access to readmission within our healthcare system. Second, referral into the program was left up to the discretion of the discharging physician, and there is likely uncaptured bias related to which patients were referred to the program; our results do not imply causality. Third, our program required a smart phone or a desktop computer (and internet or a data plan); we did not put other models in place (like telephone only) for this program. Fourth, we did not look at specific associations between responses and readmissions risk (for example, associations between fever and readmission). Finally, we did not look at outcomes beyond re-admission, like overall morbidity and mortality associated with RPM. While we would not expect RPM to worsen morbidity and mortality, it is possible that the tendency toward less readmission may have also delayed necessary presentations, leading to worse outcomes.
Conclusion
We describe the implementation of a COVID-19 RPM program and show that RPM for patients with COVID-19 discharged from one of five hospitals in a single healthcare system was associated with decreased risk of readmission to a combined endpoint of ED or hospital. We also show that a majority of patients enrolled could be passively monitored, which might enable scalable delivery models for COVID-19 in the post-acute setting. Further work is needed to understand the financial implications of these types of programs, as well as impact on overall clinical outcomes.
Clinical Relevance Statement
RPM for patients with COVID-19 being discharged home from the hospital was associated with a decreased risk of readmission to the ED or hospital. Additionally, RPM provided a scalable mechanism to monitor patients in their home environment, with most patients not requiring active intervention from nursing staff.
Multiple Choice Questions
-
Among patients recovering from COVID-19 at home who reported symptoms or abnormal vital signs through an RPM platform, which abnormality was most common?
Temperature
Oxygen saturation
Worsening shortness of breath
Vomiting
Correct Answer: The correct answer is option a. According to our data, for all abnormal surveys completed by patients with COVID-19 discharged from the hospital, the presence of a temperature >100.3 was the most common abnormal vital sign or symptom reported. This is an important finding, as it suggests that fever can remain an important symptom of patients with COVID-19 even after they have been hospitalized.
-
Which of the following percentages most accurately describes the percent population of patients with COVID-19 discharged from the hospital that can be monitored passively, that is, without nursing intervention?
36%
46%
56%
66%
Correct Answer: The correct answer is option d. Enrollment in a postdischarge COVID-19 RPM program resulted in many patients completing surveys each day; however, only 34% of those patients reported abnormal symptoms requiring direct nursing follow-up during their enrollment in the program. This suggests that the majority of patients can be monitored passively, and that an RPM program is a scalable mechanism of providing clinical monitoring for patients with COVID-19 discharged from the hospital.
-
Enrollment in a postdischarge COVID-19 RPM program was associated with which of the following outcomes?
Improved 30-day all cause-mortality
Decreased combined ED or inpatient readmission
Less utilization of primary care resources
Improvement in time to being COVID-19 PCR negative
Correct Answer: The correct answer is option b. Enrollment in a postdischarge COVID-19 RPM program was associated with a statistically significant decreased odds of a combined endpoint of re-admission to the hospital or ED. We did not look at mortality of primary care resource utilization as an outcome, and (D) is incorrect as we did not routinely reassess test-status (e.g., PCR positivity) once the patient was discharged from the hospital.
Conflict of Interest W.J.G. reports research funding from IBM, outside the scope of this study. W.J.G. reports consulting income from the Office of the National Coordinator, U.S. Department of Health and Human Services, outside the scope of this study. D.M.L. reports grants from Biofourmis, outside the submitted work.
Authors' Contributions
W.J.G. was involved in article conception. W.J.G., A.D., J.J., M.Y.S., L.M., and D.S. were involved in data collection. W.J.G., D.H., A.D., D.M.L., L.M., D.S., and R.B. were involved in data interpretation and visualization. W.J.G., D.M.L., D.H., and R.B. were involved in statistical analysis. W.J.G. wrote the manuscript draft. All authors were involved in critical revisions, editing, and manuscript approval.
Protection of Human and Animal Subjects
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, and as a quality improvement project, the MassGeneral Brigham IRB exempted this study from review.
References
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboardAvailable at: https://COVID19.who.int/. Accessed July 20, 2020
- 2.Helmy Y A, Fawzy M, Elaswad A, Sobieh A, Kenney S P, Shehata A A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9(04):E1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X.Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study Lancet 2020395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White D B, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323(18):1773–1774. doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 5.Kliff S.Hospitals knew how to make money. Then coronavirus happenedThe New York Times. Available at: https://www.nytimes.com/2020/05/15/us/hospitals-revenue-coronavirus.html. Published May 15, 2020. Accessed July 20, 2020
- 6.Reuters California hospitals struggle financially after preparing for COVID-19 surge that never cameThe New York Times. Available at: https://www.nytimes.com/reuters/2020/05/28/us/28reuters-health-coronavirus-california-hospitals.html. Published May 28, 2020 Accessed July 20, 2020
- 7.Khullar D, Bond A M, Schpero W L. COVID-19 and the financial health of US hospitals. JAMA. 2020;323(21):2127–2128. doi: 10.1001/jama.2020.6269. [DOI] [PubMed] [Google Scholar]
- 8.Peters A W, Chawla K S, Turnbull Z A. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi: 10.1056/NEJMc2010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 10.Webster P.Virtual health care in the era of COVID-19 Lancet 2020395(10231):1180–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsey E R, Topol E J. State of telehealth. N Engl J Med. 2016;375(02):154–161. doi: 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- 12.Schwamm L H, Erskine A, Licurse A. A digital embrace to blunt the curve of COVID19 pandemic. NPJ Digit Med. 2020;3(01):64. doi: 10.1038/s41746-020-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollander J E, Carr B G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 14.Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323(23):2375–2376. doi: 10.1001/jama.2020.7943. [DOI] [PubMed] [Google Scholar]
- 15.Annis T, Pleasants S, Hultman G.Rapid Implementation of a COVID-19 Remote Patient Monitoring Program J Am Med Inform Assoc JAMIAPublished online May 11, 2020 10.1093/jamia/ocaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noah B, Keller M S, Mosadeghi S. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med. 2018;1:20172. doi: 10.1038/s41746-017-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vegesna A, Tran M, Angelaccio M, Arcona S. Remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. 2017;23(01):3–17. doi: 10.1089/tmj.2016.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daley C N, Chen E M, Roebuck A E. Providing patients with implantable cardiac device data through a personal health record: a qualitative study. Appl Clin Inform. 2017;8(04):1106–1116. doi: 10.4338/ACI-2017-06-RA-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed R, Toscos T, Rohani Ghahari R. Visualization of cardiac implantable electronic device data for older adults using participatory design. Appl Clin Inform. 2019;10(04):707–718. doi: 10.1055/s-0039-1695794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher N DL, Fera L E, Dunning J R. Development of an entirely remote, non-physician led hypertension management program. Clin Cardiol. 2019;42(02):285–291. doi: 10.1002/clc.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telemedical Interventional Monitoring in Heart Failure Investigators . Koehler F, Winkler S, Schieber M. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 22.Pevnick J M, Fuller G, Duncan R, Spiegel B MR. A Large-scale initiative inviting patients to share personal fitness tracker data with their providers: initial results. PLoS One. 2016;11(11):e0165908. doi: 10.1371/journal.pone.0165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group . Ong M K, Romano P S, Edgington S. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition – heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176(03):310–318. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace E L, Rosner M H, Alscher M D. Remote patient management for home dialysis patients. Kidney Int Rep. 2017;2(06):1009–1017. doi: 10.1016/j.ekir.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee P A, Greenfield G, Pappas Y. The impact of telehealth remote patient monitoring on glycemic control in type 2 diabetes: a systematic review and meta-analysis of systematic reviews of randomised controlled trials. BMC Health Serv Res. 2018;18(01):495. doi: 10.1186/s12913-018-3274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasic I, Tomasic N, Trobec R, Krpan M, Kelava T. Continuous remote monitoring of COPD patients-justification and explanation of the requirements and a survey of the available technologies. Med Biol Eng Comput. 2018;56(04):547–569. doi: 10.1007/s11517-018-1798-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ISARIC4C investigators . Docherty A B, Harrison E M, Green C A. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m19. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.the Northwell COVID-19 Research Consortium . Richardson S, Hirsch J S, Narasimhan M. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal P, Choi J J, Pinheiro L C. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina M, Babiuch C, Card M. Home monitoring for COVID-19. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc028. [DOI] [PubMed] [Google Scholar]
- 31.Health System Sinai M.Mount Sinai uses remote patient monitoring to rapidly respond to COVID-19Published April 6, 2020. Available at: https://www.newswise.com/coronavirus/mount-sinai-uses-remote-patient-monitoring-to-rapidly-respond-to-COVID-19/?article_id=729377. Accessed July 20, 2020
- 32.Landi H.Providence St. Joseph using Twistle remote monitoring technology for 700 COVID patientsPublished April 1, 2020. Available at: https://www.fiercehealthcare.com/tech/providence-using-twistle-remote-monitoring-technology-for-700-COVID-patients. Accessed July 20, 2020
- 33.Park P G, Kim C H, Heo Y, Kim T S, Park C W, Kim C H. Out-of-hospital cohort treatment of coronavirus disease 2019 patients with mild symptoms in Korea: an experience from a single community treatment center. J Korean Med Sci. 2020;35(13):e140. doi: 10.3346/jkms.2020.35.e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grutters L A, Majoor K I, Mattern E SK, Hardeman J A, van Swol C FP, Vorselaars A DM.Home telemonitoring makes early hospital discharge of COVID-19 patients possible J Am Med Inform Assoc JAMIAPublished online July 15, 2020 10.1093/jamia/ocaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hron J D, Parsons C R, Williams L A, Harper M B, Bourgeois F C. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;11(03):452–459. doi: 10.1055/s-0040-1713635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grange E S, Neil E J, Stoffel M. Responding to COVID-19: The UW medicine information technology services experience. Appl Clin Inform. 2020;11(02):265–275. doi: 10.1055/s-0040-1709715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massachusetts COVID-19 Response reportingPublished June 8, 2020. Available at: https://www.mass.gov/info-details/COVID-19-response-reporting. Accessed July 20, 2020
- 38.Chen J, Qi T, Liu L. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(05):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wootton R. Twenty years of telemedicine in chronic disease management--an evidence synthesis. J Telemed Telecare. 2012;18(04):211–220. doi: 10.1258/jtt.2012.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhry S I, Mattera J A, Curtis J P. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bashi N, Karunanithi M, Fatehi F, Ding H, Walters D. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res. 2017;19(01):e18. doi: 10.2196/jmir.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inglis S C, Clark R A, McAlister F A, Stewart S, Cleland J GF. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail. 2011;13(09):1028–1040. doi: 10.1093/eurjhf/hfr039. [DOI] [PubMed] [Google Scholar]
- 43.Hameed A S, Sauermann S, Schreier G. The impact of adherence on costs and effectiveness of telemedical patient management in heart failure: a systematic review. Appl Clin Inform. 2014;5(03):612–620. doi: 10.4338/ACI-2014-04-RA-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ralston J D, Cook A J, Anderson M L. Home blood pressure monitoring, secure electronic messaging and medication intensification for improving hypertension control: a mediation analysis. Appl Clin Inform. 2014;5(01):232–248. doi: 10.4338/ACI-2013-10-RA-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kricke G, Roemer P E, Barnard C.Rapid implementation of an outpatient COVID-19 monitoring programNEJM Catal Innov Care Deliv. Published online June 16, 2020. Available at: https://catalyst.nejm.org/doi/abs/10.1056/cat.20.0214. Accessed June 28, 2020
- 46.Price-Haywood E G, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tai D BG, Shah A, Doubeni C A, Sia I G, Wieland M L.The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States Clin Infect Dis Off Publ Infect Dis Soc Am. Published online June 20, 2020 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenaway C, Hargreaves S, Barkati S. COVID-19: exposing and addressing health disparities among ethnic minorities and migrants. J Travel Med. 2020:taaa113. doi: 10.1093/jtm/taaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jimenez C R.New Bostonians demographic reportAvailable at: https://www.cityofboston.gov/newbostonians/pdfs/dem_report.pdf. Accessed September 11, 2020


