Abstract
Activating transcription factor 4 (ATF4) is a DNA binding transcription factor belonging to the family of basic Leucine zipper proteins. ATF4 can be activated in response to multiple cellular stress signals including endoplasmic reticulum stress in the event of improper protein folding or oxidative stress because of mitochondrial dysfunction as well as hypoxia. There are multiple downstream targets of ATF4 that can coordinate the regulation between survival and apoptosis of a cell based on time and exposure to stress. ATF4, therefore, has a broad range of control that results in the modulation of immune cells of the innate and adaptive responses leading to regulation of the cellular immunity. Studies provide evidence that ATF4 can regulate immune cells such as macrophages, T cells, B cells, NK cells and dendritic cells contributing to progression of disease. Immune cells can be exposed to stressed environment in the event of a pathogen attack, infection, inflammation, or in the tumor microenvironment leading to increased ATF4 activity to regulate these responses. ATF4 can further control differentiation and maturation of different immune cell types becoming a determinant of effective immune regulation. Additionally, ATF4 has been heavily implicated in rendering effector immune cells dysfunctional that are used to target tumorigenesis. Therefore, there is a need to evaluate where the literature stands in understanding the overall role of ATF4 in regulating cellular immunity to identify therapeutic targets and generalized mechanisms for different disease progressions.
Keywords: ATF4, Unfolded protein response, ER stress, Cellular Immunity
1. Introduction
Activating transcription factor 4 (ATF4) belongs to the basic leucine zipper (bZIP) transcription factor superfamily and is a master regulator that plays a crucial role in the adaptation to stresses.[1] ATF4 participates in the integrated stress response (ISR), involved in amino acid metabolism, differentiation, metastasis, angiogenesis, resistance to oxidative stress and drug resistance. Several ATF4 downstream target genes are themselves transcription factors that regulate the expression of a set of stress-induced target genes and amplify the signal initiated by the original stress. [2]
1.1. ATF4 regulates cellular stress
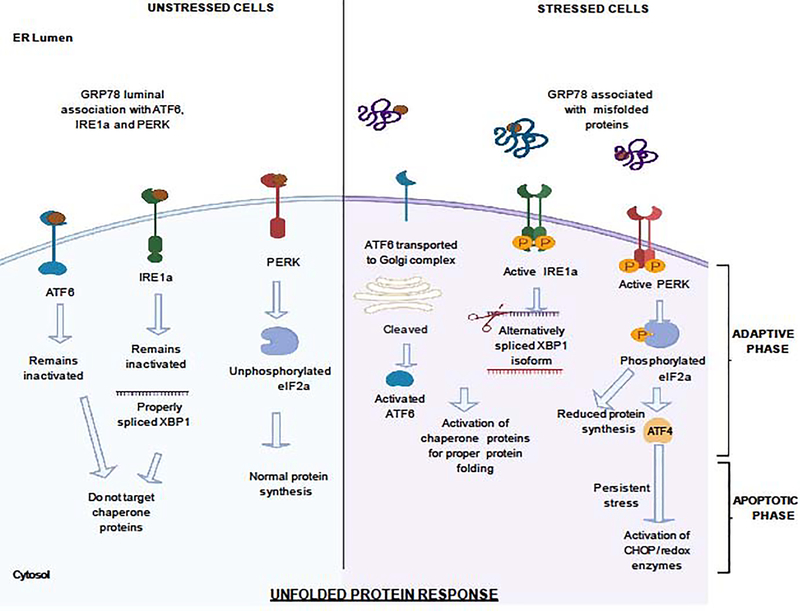
ATF4 mostly responds to immune cells via the endoplasmic reticulum (ER) stress mediated Unfolded Protein Response (UPR) pathway (Figure 1). The endoplasmic reticulum is the organelle responsible for biosynthesis, folding and modification of membrane bound as well as secretory proteins. Different physiological demands might contribute to improper folding of the proteins leading to the accumulation of unfolded or misfolded proteins in the ER lumen, leading to ER stress and is tackled by the UPR. UPR has two distinct phases–the adaptive phase where the response reduces the burden of accumulated proteins via providing chaperones to fold proteins properly or by reducing overall production of the proteins. The translational attenuation is classically attributed to the phosphorylation of the translational initiation factor eIF2α and recently identifies secondary regulation involving a downstream ISR target, 4E-BP, in the inhibition of eIF4E and specifically cap-dependent translation. [3] Misfolded proteins unable to be fold properly via chaperone proteins expressed through ISR result in terminally misfolded proteins which are either targeted for proteasomal degradation or ER associated degradation (ERAD). The major ISR effector molecule that is produced as a result of eIF2α phosphorylation and uORF translation is ATF4. ATF4 can also direct the cell to autophagy. However, if the stress continues to persist, the UPR switches to its apoptotic phase to promote cell death. [4–7] Three ER-resident transmembrane proteins orchestrate the UPR: the protein kinase R (PKR)-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol requiring enzyme-1 alpha (IRE1a). In unstressed cells, these proteins are inactive because their luminal domains are associated with Binding Immunoglobulin protein (BiP) / 78 KDa Glucose -regulated protein (GRP78). During stressed conditions, misfolded/unfolded proteins compete and are preferentially bound to GRP78, disassociating PERK, ATF6 and IRE1, thereby activating them in the process. [8] IRE1 is a transmembrane Ser/Thr kinase with additional endonuclease activity. Mammalian IRE1 occurs as two homologues of the yeast genome, IRE1a and IRE1b, with similar but non-identical cleavage specificities in a temporal as well as tissue specific manner. In presence of unfolded proteins, IRE1a auto-phosphorylates to become active as a ribonuclease and targets a bZIP transcription factor X-Box DNA Binding Protein 1 (XBP-1). XBP-1 mRNA is therefore cleaved by IRE1a with the removal of a 26-nucleotide intron introducing a translational frameshift that forms the XBP-1 isoform (XBP-1s) with a novel carboxy terminus. XBP-1s acts as a potent transcription activator that can produce various ER chaperone proteins restoring ER homeostasis and further upregulating the expression of GRP78. [9–12] Upon ER stress induced disassociation with GRP78, two Golgi localization signals within the ER luminal domain of ATF6a (one of the two homologues of mammalian ATF6) get exposed translocating it to the Golgi apparatus. ATF6a is then cleaved by site1 protease and site2 protease sequentially removing the luminal domain and transmembrane anchor, respectively, resulting in a 50 KDa amino-terminal cytoplasmic fragment that can enter the nucleus and bind to the ER stress response elements (ERSE). [8, 13] ATF6a, like XBP-1s, can upregulate the expression of GRP78 by binding through ERSE in the promoter region of GRP78. ATF6a can further induce the expression of other UPR mediators like XBP-1 providing more substrate for IRE1a along with chaperone proteins. [14, 15] Therefore, a positive feedback loop between GRP78, ATF6 AND XBP-1 is established.
Figure1-. Unfolded protein response (UPR) during cellular stress:
In unstressed cells, the different stress sensors like the protein kinase R (PKR)-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol requiring enzyme-1 alpha (IRE1a) are inactive because they are luminally associated with 78 KDa glucose- regulated protein (GRP78) in the endoplasmic reticulum (ER). This allows unperturbed protein synthesis with no specialized expression of chaperone proteins. During stress conditions, GRP78 dissociates from PERK, ATF6 and IRE1a and preferentially binds to misfolded or unfolded proteins. Golgi localization signals of ATF6 become exposed translocating ATF6 to Golgi complex where it gets cleaved. The 50KDa cleaved ATF6 fragment can enter the nucleus and bind to ER stress response elements encoding chaperones and UPR modulators. IRE1a can be auto-phosphorylated to become active as a ribonuclease to alternatively splice X-Box DNA Binding Protein 1 (XBP-1) including a 26-nucleotide intron. The translational frameshift introduced results in a novel carboxy terminus that acts as a potent transcription factor for encoding chaperone proteins and restore ER homeostasis. Both ATF6 and XBP1-s can also upregulate GRP78 expression establishing a positive feedback loop to deal with the ER stress. PERK auto-phosphorylates to become active and can phosphorylate eIF2a. Phosphorylation of eIF2a reduces global protein translation causing the cell cycle to arrest in the G1 phase. Simultaneously, selective translation of Activator of Transcription Factor-4 (ATF4) is allowed which upregulates expression of genes that can restore ER homeostasis. This is the adaptive phase of the UPR. ATF4 can transition from transcription of pro-survival genes to transcription of pro-apoptotic genes. On exposure to persistent stress, ATF4 can induce the expression of (CHOP). CHOP can mediate apoptosis through various processes like suppressing transcription of BCL2 family of anti-apoptotic proteins and activating pro-apoptotic proteins. Further, ATF4 can work with CHOP to dephosphorylate eIF2a while generating reactive oxygen species (ROS) by controlling gene expression of different redox enzymes. These processes lead to generation of ROS with an increased nascent protein load before restoring protein homeostasis maintaining the signal for apoptosis. This is the apoptotic phase of the UPR.
Upon dissociation of PERK with GRP78, PERK is activated which can thereafter phosphorylate eIF2a. Phosphorylation of eIF2a reduces ER client protein load by inhibiting global protein synthesis but allowing selective mRNAs such as ATF4 to be preferentially translated. eIF2a phosphorylation resulting in upregulated expression of ATF4 can also be catalysed via general control non-derepressible 2 (GCN2) because of amino acid limitation and UV exposure or via protein kinase RNA-activated (PKR) activated in response to viral infections or via heme-regulated inhibitor (HRI) activated by heme deprivation and oxidative stress. [16] Depending on context, ATF4 can control expression of genes involved in amino acid transport and metabolism, protection from oxidative stress, and protein homeostasis. ATF4 can also push the cell towards autophagy through various processes such as upregulating the expression of a protein called REDD1 that inhibits mTORC1 in a TSC1/TSC2-dependent manner [17, 18] and can induce apoptosis, cell-cycle arrest, and senescence keeping a fine balance between life and death of a cell. [5, 19]
1.2. ATF4 mediates CHOP activity
ATF4, in conjunction with ATF6, transactivates the transcription factor C/EBP homologous protein (CHOP). CHOP was also found to regulate mediators of apoptosis such as B-cell lymphoma 2 (BCL2) and BCL2 interacting mediator of cell death (BIM). [20, 21] Increased expression of CHOP has been reported to lead to upregulation of pro-apoptotic BH3 domain-only proteins genes such as BIM and downregulation of the anti-apoptotic protein Bcl-2 genes, along with disruption of the redox homeostasis. [22] CHOP has further been reported to induce the expression of Tribbles 3 (TRB3), which is a negative regulator of the survival pathway mediated by AKT.[23, 24] Under prolonged stress, CHOP and ATF4 have been reported to dephosphorylate eIF2a by activating Growth arrest and DNA damage-inducible protein (GADD34) increasing the nascent protein load promoting more ER stress and increasing apoptotic cell death. [25] Further, CHOP can induce expression of ER oxidoreductin 1α genes that promote a hyper-oxidising environment through generation of reactive oxygen species (ROS) and lead to apoptosis. Generation of ROS with increased protein synthesis before restoring protein homeostasis marks the apoptotic phase of UPR. [26] However, multiple reports of ATF4 inducing apoptosis in a CHOP independent manner with unexpected increase in cell death after CHOP knockdown has suggested pro-survival functions of CHOP. [27, 28] Therefore, the transcriptional regulation of CHOP via ATF4 is a complex phenomenon.
1.3. Hypoxic stress activates ATF4
Hypoxic stress results in a rapid and sustained inhibition of protein synthesis that is at least partially mediated by eIF2α phosphorylation by the PERK and therefore can also trigger the activation of ATF4. [29, 30] ATF4 is a major transcriptional regulator of the cellular hypoxic response apart from Hypoxia-inducible factor 1α (HIF1α) and is responsible for the activation of genes that provide favourable conditions for normal ER function and promote survival.[6, 31–33] Recently, the 154–181 amino acid region of ATF-4 was identified to interact with Prolyl-4-hydroxylase domain (PHD), which is hydroxylated in an oxygen dependent manner and forms the molecular basis for the hypoxia-induced stability and activity of HIF-1α and HIF-2α. The study found upregulation of ATF-4 protein after treatment with hypoxia or the PHD inhibitor Dimethyloxallyl Glycine (DMOG), demonstrating that mechanisms other than translational control might be additionally involved in the regulation of ATF-4 protein stability. [34]
This has led to a lot of interest in understanding the role of ATF4 in the tumor microenvironment, as it has been seen that cancer cells can survive under hypoxic and metabolic stress. A study reported that translation reprogramming via microenvironmental stress signals through UPR drives phenotypic plasticity and invasion, determining therapeutic outcomes in melanoma using cell-based experiments as well as transcriptomic analysis of data from human melanoma patients from The Cancer Genome Atlas (TCGA) data sets. Translational reprogramming was confirmed by observing tumor colonization in the lungs using B16 melanoma mice model. [35] In addition to the UPR in tumor cells being studied as a cell-intrinsic mechanism for cell survival, the ER stress response has been shown to aid tumor growth in a cell-extrinsic manner by inhibiting antitumor immunity via T cell-independent and -dependent mechanisms. [36] A study investigating ovarian cancer reported that the ER stress response in the tumor microenvironment can result in ROS-dependent activation of PERK in the induction of CHOP in tumor-exposed T cells regulating their antitumor activity. [37] However, the overall role of ATF4 in regulating the activity of immune cells through response towards mitigation of ER stress or as a result of inflammatory response has not been extensively reviewed. In this review, the role of ATF4 in regulating the overall cellular immunity as well as immune cell maturation, polarization state, and responses in progression of diseases will be explored (Table 1).
Table 1-.
Summary of Immune Cell Regulation by ATF4
| Cell type | Phenotype | Major Finding | References |
|---|---|---|---|
| T cells | CFSE labelled CD4+ T Cells | ATF4 deficiency results in reduced Th1 response and increased Th17 response. | Ravindran et al.44, Yang et al.43 |
| CFSE labelled mouse and human CD4+ T cells activated by DMSO expressing CD25, TH1 cells (IFN-ү+ IL-4-), TH2 cells (IL-4+ IFN-ү-), or TH17 cells (IL-17+ IFNү-) | HF selectively inhibits Th17 differentiation by activating Amino acid starvation response which targets ATF4. | Sundrud et al.45 | |
| Human CD4+ T cells activated by antiCD3/CD28 | HOXB9 targets ATF4 to suppress activated T cell function during amino acid insufficiency | Hayashi et al.46 | |
| CD4+ T cells in Intestinal tissue and peripheral blood samples from rhesus macaques following SIV infection after early and chronic stages | Inhibition and enhancing ATF4 suppressed the induction of HIV expression and reactivated latent HIV, respectively. | Jiang G et al.47 | |
| Jurkat Cells following HIV infection | Bystander stimulus of Tat on Jurkat cells resulted in time- dependent overexpression of major UPR markers | Campestrini et al.48 | |
| Jurkat Cells following HTLV infection | All three members of the TORC family of transcriptional regulators but not ATF4 or other bZIP factors act as coactivators of Tax for LTR- driven expression. | Gachon F et al. 49, Siu et al.50 | |
| SKmel28 cells and B16 melanoma mice model with the presence of a 219-geneTNFa response signature | ATF4 activates AXL and suppresses senescence to impose the MITF-low/AXL-high drug-resistant phenotype observed in human tumors. | Faletta et al.35 | |
| CD8+ T cells sorted from tumor bearing mice. | Persistent ER stress activates ATF4 and results in Chop expression which can directly represses Tbet and therefore act as a major negative regulator of the effector function of tumor reactive CD8+ T cells. | Cao et al.37 | |
| Jurkat (clone E6-1) cells, Molt4-Bcl2 and Molt4-hyg cells, DEL, D011.10, Ht1080, and Ht1080mut cells | Specific induction of the PERK-elF2α-ATF3/4 cascade activating apoptosome in Molt4 T lymphoblastic T cells by Farnesol | Joo et al.51 | |
| CD8+ T cells in C57BL/6 recipient mice receiving AMLMLL-PTD FLT3-ITD cells | Sorafenib-related IL-15 production caused metabolic reprogramming of leukemia-reactive T cells in humans via reduced expression of ATF4, thereby blocking negative regulation of interferon regulatory factor-7 (IRF-7) activation | Mathew et al.52cg | |
| Intraepithelial lymphocytes from inflamed Crohn’s disease-like TNFΔARE/+ mice | Inflamed TNFΔARE/+ mice exhibited increased expression of ATF4 in addition to other ER stress proteins in a cytotoxic CD8αβ+ specific manner in the intraepithelial lymphocytes (IEL) | Chang et al. 53 | |
| PBMCs from patients diagnosed with ACLF, chronic hepatitis B (CHB-T) and chronic hepatitis B (CHB-A) differentiated into CD4+ T cells | ATF4 along with other ER stress proteins can be involved in epigenetic regulation of CD4+ T lymphocytes via the modification of H3K9ac in driving acute-on-chronic liver failure (ACLF) | Jin et al.54 | |
| Macrophages | Bone marrow-derived M2 like macrophages (BMDMs) from Lyz2-Cre+-G RP7 8f/f (Lyz- GRP78-/-) mice | GRP78 can regulate macrophage function and insulin resistance via ATF4 in diet induced obesity | Kim et al.57 |
| M2 macrophages | ATF4 promotes the expression of chemokine CCL2 that recruits macrophages contributing to endometrial tumor growth | Liu et al.58 | |
| Raw264.7 macrophage cell line | ATF4 activity in breast cancer cells recruits macrophages via secretion of M-CSF | Liu et al.59 | |
| Bone marrow-derived macrophages | RIG like receptor- based infection of West Nile Virus via the shift to M1 phenotype via suppression of M2 phenotype by down regulating ATF4 | Stone et al.60 | |
| Human monocytes from healthy blood donors, differentiated into MDM. | ATF4 was found to be the hypoxia responsive factor in macrophages early after exposure | Elbarghati et al.61 | |
| M2-polarized macrophages (CD68+/CD163+) | Positive regulation of hypoxia- stimulated ATF4 activation leads to M-CSF based recruitment of M2 polarized macrophages | Xia et al.62 | |
| M2-polarized macrophages (CD68+/CD163+) | Hypoxia elevates ATF4 expression that stimulates RANKL and contributes to OKC development | Zhong et al.63 | |
| PBMCs from healthy donor blood were differentiated into bone marrow-derived macrophages | Cathepsin inhibition can result in mitochondrial stress and ROS production which triggers the ATF4-CHOP pathway to conduct autophagy and form macrophage foam cells in artherosclerosis | Weiss-Sadan et al.64 | |
| Mouse macrophage cell line Raw 264.7 and peritoneal and lung macrophages fromOo ATF4+/+ wild-type (WT) and ATF4+/- heterozygous mice in the C57BL/6j background | ATF4 plays an underlying role in pathogenesis of macrophage dysregulation and immune-toxicity of arsenic via down-regulation of CD11 b expression associated with the reduced phagocytic functions of peritoneal and lung macrophages. | Srivastava et al.93 | |
| RAW264.7 macrophage cell line | siRNA mediated knockdown of CHOP attenuated atherogenic lipid-mediated induction of proteins and genes implicated in macrophage mediated inflammation, ER stress (ATF4 and ATF6), and apoptosis (CHOP). | Zahid et al.65 | |
| B cells | IgG for MHV68 lytic antigen in MHV68-transformed SL-1 cells | ATF4 inhibits promoter activity of the MHV68 lytic switch transactivator RTA promoting BCR- mediated lytic replication of gamma herpes virus 68 (MHV68) | Zhou et al.67 |
| B- cell lymphoma cell lines and cells isolated from mice | Artesunate treatment induces anti-tumor activity to treat B cell lymphoma by suppressing ATF4 | Vatsveen et al.68 | |
| SV40-immortalized WT and Atf4-/- MEFs in C57BL/6J Btg1-/- and Btg2-/- mice | Methylation of ATF4 by PRMT1 to allow transcription of a subset of ATF4 target genes leading to increased apoptosis in contrast to survival is regulated by BTG1 | Yuniati et al.69 | |
| NK Cells | NK cells from normal donors and Type II diabetes patients | ER stress contributes to downregulation of NKG2D in-vivo altering NK cell function in Type II diabetic patients. | Berrou et al.76 |
| shXbp1 MODE-K cells with splenic NK cells | Innate immune sensing of ER stress via Chop is important for understanding the mechanism of intestinal inflammation. | Hosomi et al.77 | |
| NK cells from Human liver cell lines (HL-7702) treated with OPN, C57BL/6 male mice under HFD | Obesity can induce Hepatic NK cells to produce ATF4 mediated ER stress response resulting in insulin resistance | Wu et al.78 | |
| Dendritic Cells | Mouse bmDCs activated with pl:C | DCs mount a specific integrated stress response during which ATF4 and GADD34 is expressed with an extensive dephosphorylation of the translation initiation factor elF2α during DC activation. | Clavarino et al.80 |
| Lung, intestinal and mucosal cDCs | ER stress response pathways can mediate tissue specific activation of different mucosal DCs via ATF4. | Tavernier et al.81 | |
| DC2.4 cells, CD11c+ dendritic cells from spleens of sepsis induced C57BL/6J mice | Sestrin2 inhibits PERK-ATF4-CHOP mediated cell death pathway upon high mobility group box-1 protein stimulation rendering a protective effect on DCs. | Wang et al.82 | |
| MDSCs | Splenic and tumor-MDSCs recovered from tumor bearing mice using anti-Gr1 Ab | Chop expression in MDSCs was shown to be driven by ATF4 in the presence of tumor linked reactive oxygen and nitrogen species. | Thevenot et al.87 |
| CD8+ T cells from B6.Gcn2fl/flxLyz2+/Cre mice | GCN2 can promote translation of ATF4 and increase activation of pro-inflammatory MDSCs, macrophages and IFNy expression in intra-tumoral CD8+T cells adversely affecting the tumor microenvironment. | Halaby et al.88 | |
| General/Innate immunity | A. fumigatus strain 3.0772, C57BL/6 female mice, Human corneal epithelial cells (HCECs), THP-1 macrophages; | ATF4 expression was shown to be dependent on TLR4, LOX-1 expression, and MAPKs pathway and therefore involved in host antifungal immune response | Zhang et al.92 |
| Immortalized mouse retinal endothelial cells, and C57BL/6 J mice | ATF4 is critical in regulation of Monocyte chemoattractant protein 1 (MCP1) in retinal and brain microvascular endothelial cells contributing in inflammation- related endothelial injury in diseases such as diabetic retinopathy. | Huang et al.94 | |
| HEK-293FT, RAW264.7, Murine primary macrophages were thioglycolate-elicited and removed from wild-type (WT) or TLR4-knockout (KO) C57BL/6 mice | Knocking down ATF4 in RAW 264.7 macrophage cells decreased NRF2 expression and its nuclear translocation, reduced HO-1 expression and increased nitric oxide production suggesting that ATF4 pathway is involved in parasite survival and progression against Leishmania amazonensis infection. | Dias-Teixeira et al.95 | |
| THP-1, K562 and Jurkat cells | During ER stress, ATF4 directly binds to NLRP1 promoter (core inflammasome component) and reaulate inflammation. | D'Osualdo et al.96 |
2. T cells
T cells are one of the major components of the adaptive immune system that can recognize a specific antigen leading to activation from a naïve T cell to an activated phenotype with effector functions. They can mature to form CD8+ T cells, with the ability to recognize and kill target cells expressing the antigens presented by Major Histocompatibility Complex (MHC) class I, leading to the release of cytotoxic molecules such as perforin and granzymes. CD4+ T cells or helper T cells have a wide range of activity including shaping and regulating other adaptive immune responses. CD4+T cells can differentiate into several different subtypes of T helper (Th) cells such as Th1, Th2, Th9, Th17, and T regulatory cells, maintaining a tighter immune regulation. Memory T cells are a small subset of cells that remain in the body following initial exposure to a specific antigen. Memory T cells are important in quick expansion of effector T cells on re-exposure to the same antigen. [38]
T cell proliferation and function is regulated by a multitude of factors, including the availability of extracellular amino acids and the oxidizing environment. Arginine depletion has been reported to affect T cell proliferation. [39] Additionally, activated T cells have been shown to have higher metabolism of L-arginine, resulting in enhanced CD4+ and CD8+ T cell survival. [40] Another novel finding was reported on the critical role of serine metabolism in activated T cells supporting T cell proliferation. Serine and glycine-limiting conditions can impair anti-CD3/CD28 antibody-driven CD4+ and CD8+ T cell proliferation in vitro, antigen-driven CD8+ effector T cell expansion, and pathogen clearance in mice. [41] ATF4 has been reported to be induced by the extracellular oxidizing environment which can therefore target a network of genes encoding proteins that control the metabolic flux. Enhanced anabolism leads to an overall increase of amino acid and protein synthesis, helping T cells to proliferate under stress. [42] ATF4 has been further shown to contribute to T cell growth in oxygen or amino acid limited environment by participating in catabolism via upregulating genes required in the glycolysis pathway, as well as promoting anaplerotic flux by enhancing glutaminolysis. [43] ATF4 deficiency reportedly results in reduced Th1 and increased Th17 responses in vivo with a reduction of Th1 differentiation and an increase in factors driving Th17 phenotype (characterized by increase in the expression of InterLeukin - 17 (IL-17) in myeloid cells.[43, 44] This is further validated by investigating the small molecule halofuginone (HF) which can inhibit Th17 differentiation in mice and humans. The addition of excess amino acids rescues the inhibition of Th17 differentiation by HF suggesting Th17 differentiation is targeted by activating the amino acid starvation response (AAR), implicating the AAR pathway in regulation of inflammatory T cell differentiation in vivo. [45] As a result of amino acid deprivation or functional inhibition of L-type amino acid transporter 1 (also known as SLC7A5), ATF4 can further induce the expression of Homeobox B9 (HOXB9) in CD4+ T cells activated in the presence of anti-CD3/CD28. HOXB9 can interfere with the activities of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), nuclear factor of activated T-cells (NFAT) and Activator Protein 1 (AP-1), but not the retinoic acid receptor-related orphan receptor. This combination results in attenuation of the production of selective cytokines in activated T cells such as interferon-gamma (IFNγ), IL-2 and IL-4 whereas IL-17 does not significantly change. [46] T cell regulation via ATF4 can therefore be a direct or indirect result of stress and can span different disease progression pathways.
Diseases caused by viral infections have also been shown to involve T cell responses regulated by ATF4. Most viral pathogens need to subvert the innate antiviral response in order to enter the host and replicate. In vitro and in vivo studies using human CD4+ T cell culture models testing a simian immunodeficiency virus (SIV) model of AIDS were used to investigate how human immunodeficiency virus (HIV) can evade early innate defenses. Suppression of protein synthesis and induction of protein kinase GCN2-ATF4 signaling were detected in the gut during acute SIV infection that diminished during chronic viral infection. HIV replication induced in CD4+ T cells reached a similar fate with the induction of ATF4 that was recruited to the HIV long terminal repeat (LTR) to promote viral transcription. Inhibition and enhancing ATF4 suppressed HIV expression and reactivated latent HIV, respectively. [47] Similarly, the link between ER stress mediated apoptosis following a bystander HIV Tat stimulus was studied on Jurkat T cells. The stimulus resulted in time-dependent overexpression of major UPR markers including ER chaperone and stress sensors, as well as an increase in levels of downstream mediators including eIF2α, ATF4, XBP-1. Proapoptotic factors such as CHOP, GADD34, and BIM were also seen to increase.[48] These results provide a mechanism for the continuous depletion of uninfected CD4+ T lymphocytes observed in HIV-related disease. However, a study investigating LTR activation mediated by Tax concerning Human T-cell leukaemia virus type 1 (HTLV-1) showed all three members of the TORC family of transcriptional regulators as coactivators of Tax for LTR-driven expression, but not ATF4 or other bZIP factors. [49, 50] Therefore, the complex process of viruses to evade immune detection may or may not involve T cell regulation by ATF4.
Evasion of immune system detection by rendering T cells ineffective via ATF4 is also observed during cancer progression and becomes extremely important in the context of effective cancer immunotherapy. The tumor microenvironment can introduce distinct but interconvertible cell types that sustain malignant and therapy resistant phenotype including resistance to anti-PD1 immunotherapy. To understand the mechanisms that help different cancer cells invade and become drug resistant, a study was performed on transcriptionally repressed melanoma identified microphthalmia-associated transcription factor (MITF) by ATF4 in response to translation initiation factor eIF2B. ATF4 further activates AXL Receptor Tyrosine Kinase (AXL) and suppresses senescence to impose the MITF-low/AXL-high drug-resistant phenotype observed in human tumors. However, the ATF4-high/MITF-low state is insufficient to drive invasion without translational reprogramming, suggesting that there are microenvironmental stress signals which drive phenotypic plasticity, invasion and therapeutic outcome by translational reprogramming. [35] Another group investigating the mechanism behind dysfunctionality of CD8+ T cells in cancer immunotherapy reported that CHOP expression is increased in tumor-infiltrating CD8+ T cells and correlates with poor clinical outcome in ovarian cancer patients. [37] CHOP is elevated by ATF4 as a result of persistent ER stress and directly represses Tbet expression. Thus, CHOP acts as a major negative regulator of the effector function of tumor-reactive CD8+ T cells. The authors provide evidence that deletion of CHOP in T cells improves antitumor CD8+ T cell immunity and therefore boosts the efficacy of T cell-based immunotherapy. This study suggests there is a therapeutic potential of blocking CHOP or ER stress to unleash T cell-mediated antitumor immunity.
ATF4 can also be targeted by different cancer therapeutic agents that involves regulation of different T cell responses. While studying the effect of farnesol on T lymphoblastic leukemic Molt4 cells, farnesol was found to activate the apoptosome. Gene expression analysis via microarray revealed the specific induction of the PERK-eIF2α-ATF3/4 cascade in a manner that is independent of the farnesol-induced activation of Mitogen-activated protein kinases (MAPKs). [51] Another study focused on understanding the role of sorafenib in targeting acute myeloid leukemia (AML) with an internal tandem duplication (ITD) in the gene encoding Fms-related tyrosine kinase 3 (FLT3). Sorafenib is a multi-targeted tyrosine kinase inhibitor and was shown to increase IL-15 production in FLT3-ITD+ leukemia cells, which synergized with the allogeneic CD8+ T cell response. Sorafenib-related IL-15 production caused metabolic reprogramming of leukemia-reactive T cells in humans via reduced expression of ATF4, thereby blocking negative regulation of interferon regulatory factor-7 (IRF-7) activation. [52] In other disease conditions, ER stress has been shown to result in the development of Crohn’s disease-like ileitis mediated by cytotoxic CD8αβ+ intraepithelial lymphocytes (IELs). Heterogeneous knockout of Tumor Necrosis Factor (TNFΔARE/+) mice under chronic inflammation exhibited increased expression of ATF4 in addition to GRP78, ATF6, and spliced XBP1 in CD8αβ+ IEL but not in CD8αα+ IEL or in lamina propria lymphocytes. [53] In another study, a group examined the role of epigenetic modification of H3K9ac in regulation of CD4+ T lymphocytes in driving acute-on-chronic liver failure (ACLF) that displays ‘sepsis-like’ immune paralysis. In a study conducted by Jin et al., it was discovered that downstream pathway-related genes of ER stress response such as Heat Shock Protein Family A (Hsp70) Member 5 (HSPA5), BCL2 interacting mediator of cell death (BNIP1), IRF3, Apoliprotein-A4 (APOA4), PolyUbiquitin-C Precursor (UBC) and Period Circadian Regulator 1 (PER1) as well as ATF4, were differentially modified through utilization of ChIP microarray based functional analyses in ACLF. [54] Therefore, ATF4 can interact with T cells to maintain proliferation and differentiation, ensuring proper activity of different kinds of T cells as well as hijacking normal functioning to cause diseases such as viral infections and cancer.
3. Macropahges
Macrophages are derived from blood monocytes and are involved in the detection and phagocytosis of pathogens, antigen presentation to T cells, and initiating inflammation through the release of various cytokines. Macrophages are a heterogeneous set of cells that differentiate and reside in several different tissue locations based on their surrounding microenvironment. [55] Macrophages can respond to environmental cues and are able to change their function by transitioning between different polarization states. Such plasticity renders them the ability to secrete a range of various cytokines that are either pro-inflammatory or anti-inflammatory. [56] Environmental cues received in the form of cellular stress, such as hypoxia or ER stress, generated due to UPR, has been investigated. Mechanistically, ER stress signals have often been found to trigger the ATF4 pathway and interact with multiple factors related to macrophages to alter their plasticity. GRP78 can regulate macrophage function and insulin resistance via ATF4 in diet induced obesity. In GRP78 deficient mice, high levels of ATF4 mRNA lead to the activation of adaptive UPR, resulting in increased IL-6 expression in macrophages that polarizes them to the M2 state. This leads to stimulation of IL-13 signaling via upregulating IL-13 Receptor α1 (IL-13Rα1) and increasing glucose metabolism in skeletal muscle, making macrophage UPR signaling a potential pharmacological therapeutic target for treating skeletal muscle insulin resistance. [57]
Recent reports investigating the tumor microenvironment have implicated the importance of macrophage infiltration for sustenance of tumor cell survival and metastases. Expression of ATF4 in tumors of clinical samples correlated with macrophage infiltration. Studies show that ATF4- knockdown in endometrial cancer cells results in a reduction in M2 macrophage infiltration in xenograft animal models. Upon investigation, the tumor microenvironment related stress was observed to upregulate ATF4 expression, promoting the expression of chemokine CCL2. This resulted in recruitment of macrophages, and therefore contributed to endometrial tumor growth. Thus, ATF4/CCL2 was shown to be a potential therapeutic target for tumor microenvironment. [58] Furthermore, ATF4 activity in breast cancer cells recruits macrophages via secretion of M-CSF. [59] Another intricate regulatory role of ATF4 in macrophages is emphasized by the down-regulation of ATF4 to suppress the M2 polarization phenotype for Retinoic acid-inducible gene (RIG) like receptor- based infection of West Nile Virus via the shift to the M1 phenotype. [60]
Hypoxia leads to up-regulation of certain genes to help respond to the stress caused by a lack of oxygen, out of which HIF-1α is a well-known central mediator. However, recent studies have suggested the induction of ATF4 as a pivotal transcriptional regulator to this stress since hypoxia is a frequent consequence to the UPR following ER stress. While investigating the regulation of macrophages in the ischemic areas of diseased tissue, ATF4 was found to be the hypoxia responsive factor in macrophages early after exposure. [61] Another study investigated Infantile Hemangioma (IH), a condition in which a mark or coloured patch appears within a few weeks after birth as a result of incorrectly formed blood vessels that multiply more than usual. A significant upregulation of ATF4 in proliferating IH was found in comparison to the involuting phase (typically in a year after proliferation and plateau phase). ATF4 was positively correlated with HIF-1α expression in IH specimens using the Spearman correlation and was further corroborated by their synchronous distribution through double labelled immunofluorescence. M2 macrophage polarization closely correlated to ATF4 expression, underlining a positive regulation mechanism of hypoxia-stimulated ATF4, leading to M-CSF based recruitment of M2 polarized macrophages. [62] Following the same route, the role of hypoxia in the induction of M2-polarized macrophage infiltration through ATF4 and its potential relationships with angiogenesis in odontogenic keratocysts (OKC) was also investigated. The pathway resulted in stimulation of the receptor activator of nuclear factor κ-B ligand (RANKL), leading to the development of OKC. This occurred as a result of elevated ATF4 expression in the epithelial lining of OKC in response to hypoxia. [63] Apart from regulating the survival function on exposure to metabolic stress, ATF4 can mediate apoptosis and hence is implicated in autophagy. Arthero-sclerosis is a condition of the cardiovascular tissue suffering from non-resolvable inflammation associating autophagy and formation of macrophage foam cells to contribute towards this dysfunction. While studying the role of cysteine proteases cathepsins in autophagy, cathepsin inhibition led to a mitochondrial stress and ROS production that triggered the ATF4-CHOP pathway. Upon transcriptomic analysis, these cells were genetically similar to inflammatory macrophages. [64] Similar results were reached while studying C/EBPβ regulation in macrophage foam cell formation, driving arthero-sclerosis where they observed (siRNA)-mediated knockdown of C/EBPβ attenuated atherogenic lipid-mediated induction of proteins and genes implicated in macrophage mediated inflammation, ER stress (ATF4 and ATF6), and apoptosis (CHOP). [65] Therefore, ATF4 has an overall ability to affect macrophage polarization as well as the ability to induce various phenotypic changes in the macrophages in order to direct responses to support disease progression.
4. B Cells
B cells are also a part of the adaptive immune response and mediate immunity by producing antigen-specific immunoglobulins directed against invasive pathogens. [66] Dysregulation in proper development and maturation of B cells is implicated in different kinds of abnormalities, spanning from immunodeficiency and autoimmunity to haematological malignancies, some of which arise as a result of interaction with the ATF4 pathway. B cell receptor (BCR) expression is necessary for survival and development of B cells. BCR signaling has been found to trigger CHOP to promote BCR-mediated lytic replication of gamma herpes virus 68 (MHV68) by suppressing upstream Bip and ATF4 expression. BCR-mediated MHV68 lytic gene expression in CHOP knockout cells was rescued by knocking out Bip and this rescue was blocked by ectopic ATF4 expression. Further, ATF4 inhibited promoter activity of the MHV68 lytic switch transactivator replication and transcriptional activator (RTA) implicating a complex interconnectedness between BCR signaling and ER stress mediated UPR to regulate the gammaherpes virus infection cycle. [67] Similar interplay was also indicated in a study investigating anti-tumor activity of artesunate for the treatment of B cell lymphoma. Gene expression analysis identified ER stress and UPR as the most affected pathways with a distinct upregulation of markers ATF4 and CHOP in malignant cells vs. normal cells and artesunate treatment significantly suppressed overall cell metabolism as a treatment strategy. [68]
B cells go through a complicated maturation process and many of its developmental stages have malignant counterparts where the dominance of a particular sub-clone leads to development of leukemia or lymphoma. Mutations in the tumor suppressor B-cell Translocation Gene 1 (BTG1) have been known to cause Acute Lymphoblastic Leukemia and diffused large B cell lymphoma along with its lower expression levels correlating with poor clinical outcomes for many solid malignancies. In a study investigating the loss of Btg1, they found Btg1 renders a survival advantage to primary mouse embryonic fibroblasts (MEFs) under stress conditions. Under stress, BTG interacts with ATF4 to recruit protein arginine methyl transferase (PRMT1) which methylates ATF4 on arginine residue 239 modulating cellular adaptation positively. Loss of BTG1 shifts the balance and allows for cells to survive instead of targeting genes downstream of ATF4 that are pro apoptotic. [69] Further implications of the ATF4 pathway in haematological malignancies were discovered while investigating the single agent and synergistic combinatorial efficacy of first in class small molecule impridone ONC201. ONC201 induced caspase-dependent apoptosis that involved activation of the integrated stress response (ATF4/CHOP) pathway along with the inhibition of Akt phosphorylation, Foxo3a activation, downregulation of cyclin D1, Inhibitor of Apoptosis Protein (IAP), and B cell lymphoma (Bcl-2) family members in multiple different haematological malignancies. [70] Thus, specific targeting of ATF4 as a part of the UPR could lead to new therapeutic approaches for treating haematological malignancies.
5. NK Cells
Natural Killer (NK) cells are a part of the innate immune response known for defense against viral infections and tumors without any priming. NK cells can secrete cytokines such as IFN-γ and TNF-α that can enhance the immune response by interacting with other immune cells. The Natural Killer Group 2D (NKG2D) receptor plays an important role in protecting the host from infections and cancer via recognition of cells expressing induced self-proteins acting as a primary activation signal for NK cells. [71] It can override inhibitory signals received by other NK cell receptors. [72] Since the ER stress elicits inflammatory response, UPR related proteins were hypothesized to induce surface expression of NKG2D ligands. [73] As a result, one of the ligands for NKG2D receptor, UL16 Binding Protein 1 (ULBP1), which is a cell surface glycoprotein related to MHC Class I molecules and functions as a stress induced ligand [74] was studied. A forward genetic screen study found that ATF4 is a critical protein involved in ULBP1 transcription and surface expression, and was therefore important for the induction of ULBP1, but not other NKG2DLs, showing a specificity for ER stress induced NKG2DL. The result was further confirmed by demonstrating that knockdown of ATF4 strongly decreased ULBP1 transcription. ATF4 was shown to have direct ULBP1 promotor binding sites that directly transactivates the ULBP1 promoter. [75] However, the study did not report on the interaction of the NKG2DL with NK cells. The functional response of NK cells as a result of upregulated expression of NKG2DL would provide more understanding of NK cell activity under ER stress. Another study investigated NK cell function in Type II diabetes patients and ER stress was found to be an important mediator. ER stress was induced in vitro in normal NK cells through tunicamycin treatment which resulted in a significant decrease in NKG2D expression. This was coupled with an increase in the markers of the UPR including XBP-1s, ATF4 and CHOP in the patient NK cells indicating that ER stress is activated in vivo through both PERK and IRE1 sensors implicating the UPR pathway as a potential mechanism. [76] Similarly, in a study investigating enteritis or inflammation in intestinal epithelial cells, Xbp1 (downstream target of UPR) deletion in the epithelium (Xbp1ΔIEC ) is shown to cause increased expression of (ULBP)-like transcript 1 and its human orthologue cytomegalovirus ULBP via CHOP, downstream target of ATF4. Increased numbers of intraepithelial NKG2D-expressing group 1 innate lymphoid cells (ILCs; NK cells or ILC1) were observed in Xbp1ΔIEC cells, which when blocked, suppressed cytolysis against ER-stressed epithelial cells in vitro and spontaneous enteritis in vivo. Depletion of NK1.1+ NK cells also significantly improved enteritis revealing innate immune sensing of ER stress in IECs as an important mechanism of intestinal inflammation. [77] Further, a study to investigate the role of NK cells in promoting insulin resistance in normal human liver cell line (HL-7702 cells) pre-treated with osteopontin (OPN) discovered that hyperactivation of JNK and subsequent decrease of tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1) is responsible for impaired insulin signaling. This was reversed by inhibiting ER stress, since hepatic NK cells were able to induce obesity-induced hepatic ATF4 mediated ER stress. [78] Additional research into how ATF4 affects NK cell ligands as well as tissue specific responses to drive different conditions such as insulin resistance could open up new avenues of immune cell based therapeutics.
6. Dendritic cells
Dendritic cells (DCs) develop in different tissue types for antigen presentation and have been found to respond in a variety of manners to cellular stress. [79] It was discovered that DCs mount a specific integrated stress response during which ATF4 and the GADD34/Ppp1r15a, a phosphatase 1 (PP1) cofactor, was expressed with an extensive dephosphorylation of the translation initiation factor eIF2α during DC activation. GADD34 was shown to be required for normal cytokine production both in vitro and in vivo, displaying the importance of pathogen detection with the integrated stress response pathways. [80] Similarly, in another study investigating responses to ER stress, different mucosal DCs were reported to respond in a tissue specific manner either via the ATF4- dependent cellular stress adaptation or via IRE1-dependent ER stress adaptive mechanism, a signaling pathway that also controls development and survival of immune cells. Lung circulating dendritic cells (cDC1s) die, whereas intestinal cDC1s survive via their ability to shut down protein synthesis through a protective integrated stress response by marked increase in regulated IRE1-dependent messenger RNA decay. [81] Furthermore, Sestrin2, a highly evolutionarily conserved protein, was reported to be expressed in dendritic cells after high mobility group box-1 protein stimulation to inhibit the apoptotic ER stress signaling based PERK-ATF4-CHOP mediated cell death pathway exerting a protective effect on DCs in the event of sepsis. [82] Further work in the field in understanding interactions of ATF4 with DCs will present a better understanding of the crossroads of antigen presentation, immune activation and cellular stress.
7. Myeloid-derived Suppressor Cells
On exposure to pathogenic stimuli, the innate immunity fights infection/inflammation via non-specific defenses. Pathogen associated molecular patterns (PAMPs) or danger associated molecular patterns (DAMPs) send strong signals to activate and expand neutrophils and monocytes followed by phagocytosis, respiratory burst and release of inflammatory cytokines. This innate response is short-lived and stops with the signal. However, persistent stimulation associated with chronic infection, inflammation, or cancer involves relatively low-strength signals that result in a different cell type with distinct genomic and biochemical properties through myelopoiesis. The main functional characteristic of these cells is their ability to suppress various types of immune responses and are therefore called myeloid derived suppressor cells (MDSCs). [83] They are of two types: granulocytic/polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs). MDSCs mostly suppress immune activity by targeting T cells. M-MDSC suppress T cell responses both in antigen-specific and non-specific manners whereas PMN-MDSC primarily suppress T cell response in an antigen-specific manner. ATF4 can also interact with MDSCs to regulate their activity. [84–86] Chop has been reported in the accumulation and immune inhibitory activity of tumor-infiltrating MDSCs. Chop expression in MDSCs was shown to be driven by ATF4 in the presence of tumor-mediated reactive oxygen and nitrogen species. [87] In contrast, Chop-deficient MDSCs display reduced signaling through CCAAT/enhancer-binding protein-β which leads to a reported decrease in the production of interleukin-6 (IL-6) and low expression of phospho-STAT3. This study further suggests Chop as a therapeutic target for cancer immunotherapy. [87] Another putative therapeutic target for immuno-oncology is GCN2, which is an environmental sensor in response to nutrient availability. Using mass cytometry as well as transcriptomics and transcription factor binding analyses, myeloid lineage deletion of GCN2 was shown to drive a shift in MDSCs and tumor associated macrophages promoting anti-tumor immunity. [88] Further assessment on the mechanisms showed that GCN2 promotes translation of ATF4 and increases activation of pro-inflammatory MDSCs, macrophages and IFN-γ expression in intratumoral CD8+T cells adversely affecting the microenvironment. Thus, ATF4 can regulate overall MDSC activity in the tumor microenvironment.
8. Other inflammatory diseases
ATF4 can regulate survival, apoptosis and differentiation in immune cells and can therefore use them to drive tumorigenesis, autophagy and viral entry. However, the immune cell regulation via ATF4 is not limited to these kinds of disease progression and has been reported in broader ranges. Fungal keratitis driven by Aspergillus fumigatus (A. fumigatus) can damage visual acuity and cause blindness. [89–91] In a study investigating the response to the fungal infection, ATF4 was increased in corneas from two kinds of A. fumigatus keratitis models after 3 days as well as in the conidia in both the human corneal epithelial cells (HCECs) and the THP-1 macrophages 16 hours after stimulation. The ATF4 expression was shown to be dependent on Toll-like receptor 4 (TLR4), lectin-type oxidized LDL receptor 1 (LOX-1) expression, and MAPKs pathway and is therefore involved in the host antifungal immune response. [92] Arsenic has been shown to induce immunosuppression on chronic exposure. While investigating ATF4 in regulating arsenic trioxide (ATO)-mediated dysregulation of macrophage functions, ATO-treated ATF4(+/+) wild-type mice were compared to ATO-treated ATF4(+/−) heterozygous mice where the wild type mice showed a significant down-regulation of CD11b expression associated with the reduced phagocytic functions of peritoneal and lung macrophages. Further, ATF4 knockdown rescued ATO-mediated impairment of macrophage functions including cytokine production, bacterial engulfment, and clearance of engulfed bacteria in RAW 264.7 cells, suggesting that ATF4 plays an underlying role in pathogenesis of macrophage dysregulation and immune-toxicity of arsenic. [93] Another report showed that ATF4 is critical in the regulation of Monocyte chemoattractant protein 1 (MCP1) in retinal and brain microvascular endothelial cells. MCP1 is a chemokine that recruits monocytes to site of tissue injury and plays a role in microvascular complications of diabetes. In cases where Lipopolysaccharide (LPS) treatment was used to induce MCP-1, it was shown that overexpression of ATF4 enhanced retinal levels of MCP-1 and promoted inflammatory cell infiltration into the vitreous and retina, whereas LPS-induced MCP-1 upregulation was markedly attenuated in ATF4-deficient endothelial cells and in the retinas of ATF4 knockout mice. Furthermore, pharmacological inhibition of NF-κB, P38, or c-Jun N-terminal kinase JNK, significantly reduced the ATF4-stimulated MCP-1 secretion from endothelial cells. This suggests that regulation of MCP1 via ATF4 may contribute to inflammation-related endothelial injury in diseases such as diabetic retinopathy. [94] In another report investigating overcoming host defenses by the parasite Leishmania to cause Leishmania amazonensis infection, the PERK/eIF2α/ATF4 signaling branch of the integrated ER stress response was shown to be activated like many viral entry systems. Infected patient lesions showed increased expression of ATF4 and Heme oxygenase-1 (HO-1) mRNAs, whereas knocking down ATF4 in RAW 264.7 macrophage cells decreased nuclear factor erythroid 2-related factor 2 (NRF2) expression and its nuclear translocation, reducing HO-1 expression and increasing nitric oxide production. This suggests the importance of the ATF4 pathway in parasite survival and progression, especially because human leishmaniasis infection is also associated with HIV infection. [95] A more general regulatory role of ATF4 as a part of the innate immune response was recently explored where expression of NACHT, LRR, FIIND, CARD domain and PYD domains-containing protein 1 (NLRP1), a core inflammasome component, is specifically up-regulated during severe ER stress conditions in human cell lines. Using mutagenesis, chromatin immunoprecipitation and Clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9-mediated genome editing technology, the ATF4 transcription factor was shown to directly bind to the NLRP1 promoter during ER stress and regulate inflammation. [96] Therefore, the ability of ATF4 to regulate cell survival or lead to apoptosis in response to stress makes interactions with multiple stages of innate immune system homeostasis essential.
8. Conclusion
Activating Transcription Factor 4 is a stress induced transcription factor that can regulate a multitude of different immune cell responses. The regulation of immunity via ATF4 can be indirect by initiating inflammation that will secrete a cascade of cytokine directed immune responses. In contrast, ATF4 can also directly interfere with maturation, development, and polarization states of different immune cells, rendering tissue specific responses and therefore contributing to an overall modulation in immune cell regulation (Figure 2). ATF4 can be triggered by different stressors leading to its involvement in a range of different immune cell regulation processes. As tumor cells survive under extreme metabolic stresses, ATF4 is suggested to have an extensive role in dysfunctionality of different effector immune cells. Therefore, there is a huge potential in targeting ATF4 as an immunotherapeutic approach to cancer. However, more in-depth analyses of its involvement in tissue specific regulation of microenvironment is required in the field. ATF4 has also been reported to aid viral entry and evade the immune system by interacting with multiple immune cell types. A holistic approach in utilising ATF4 as a target for defense against viral entry can help explore different therapeutic potentials. The exposure to stress is time sensitive and the transition between the adaptive and apoptotic phase of the unfolded protein response is mediated by ATF4. Henceforth, many autophagy mediated disease progressions involve immune cell regulation via ATF4 and requires further research for a better understanding. This review has highlighted many investigations involving immune cell regulation by ATF4 and further mechanistic findings involving downstream signaling will provide new insights for the basic understanding of different immunological processes as well as to find previously unexplored therapeutic targets for different diseases.
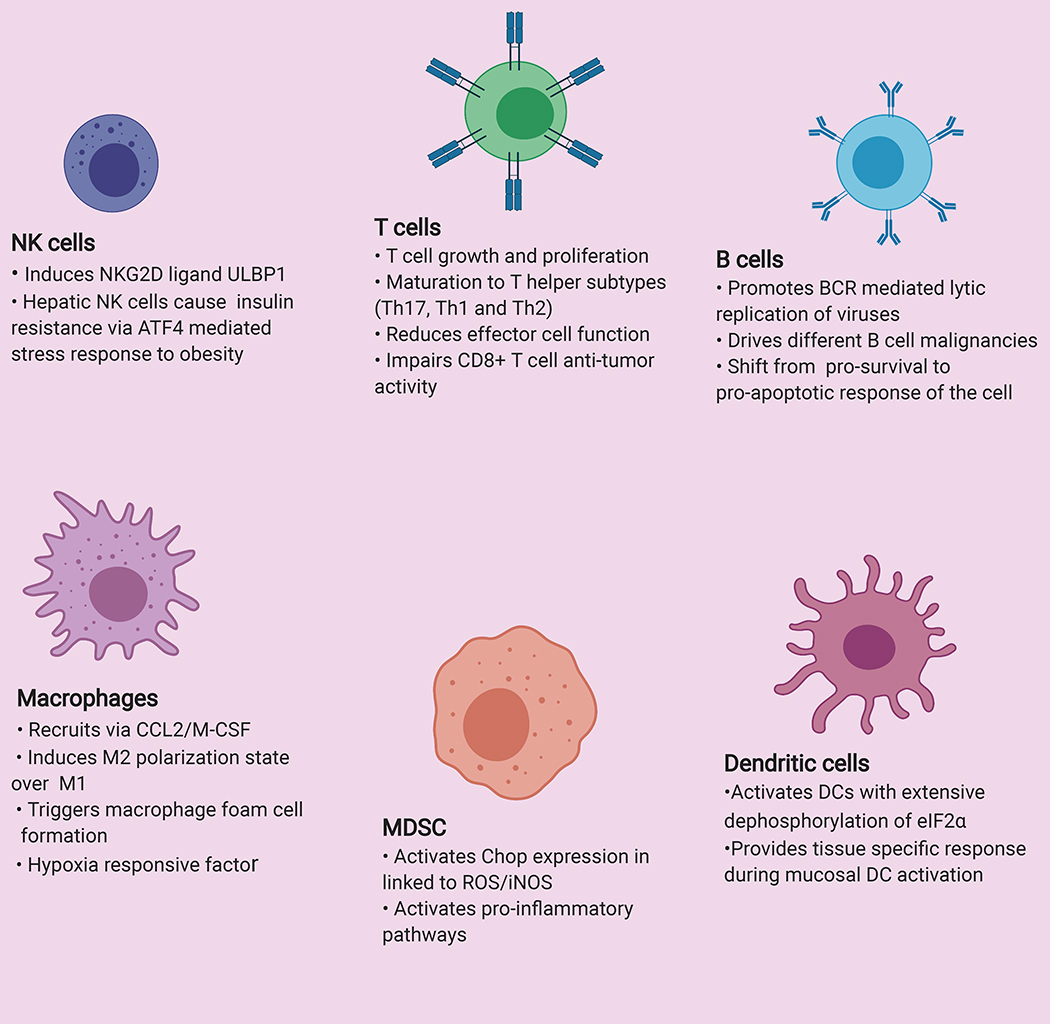
Figure 2-. Overall regulation of immune cells by ATF4:
Interaction of ATF4 in regulating different immune cell types such as T cell, B cell, Natural Killer cell (NK cell), Macrophages, Dendritic cells and Myeloid Derived Suppressor Cell (MDSC).
Highlights.
ATF4 is activated as a result of cellular or oxidative stress and is a component of the unfolded protein response.
Activation of ATF4 can affect immune cell growth and differentiation
Viral and pathogen entry and evasion of immune detection can be regulated by ATF4.
ATF4 can be targeted for therapeutic strategies against cancer, viral pathogenesis and inflammatory disorders
Acknowledgments
Funding Support: This project was supported by the OSU Comprehensive Cancer Center (OSUCCC) Biostatistics, Genomics, Target Validation, Analytical Cytometry, and Comparative Pathology & Mouse Phenotyping Shared Resources supported in part by NCI grant P30 CA016058. Project was also supported by an IDEA award from the intramural research program at OSUCCC. Project was supported by the Biliary System and Liver Cancer Research Fund at the OSUCCC.
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- AKT
Protein Kinase B
- AML
Acute Myeloid Leukemia
- APOA4
Apoliprotein A4
- AP-1
Activator Protein 1
- ATO
Arsenic Trioxide
- ATF
Activating Transcription Factor
- AXL
AXL Receptor Tyrosine Kinase
- BCL2
B-cell lymphoma 2
- BCR
B-Cell Receptor
- BIP
Binding Immunoglobulin Protein
- BIM
BCL2 interacting mediator of cell death
- BNIP1
BCL2 interacting protein 1
- BTG1
B-cell Translocation Gene 1
- bZIP
basic leucine zipper
- CCL2
Chemokine (C-C motif) ligand 2
- cDC
Conventional Dendritic cell
- CHOP
C/EBP homologous protein
- CRISPR
Clustered regularly interspaced short palindromic repeats
- DAMPs
Damage associated molecular patterns
- DC
Dendritic cells
- DDIT3
DNA damage-inducible transcript 3
- DMOG
Dimethyloxallyl Glycine
- eIF2a
Eukaryotic translation initiation factor 2A
- ER
Endoplasmic Reticulums
- ERAD
ER associated degradation
- ERSE
ER Stress Response Element
- FLT3
Fms-related tyrosine kinase 3
- GADD34
Growth arrest and DNA damage-inducible protein
- GCN2
General control nonderepressible 2
- GRP78
Glucose-regulated protein 78
- HOXB9
Homeobox B9
- HIF1a
Hypoxia-inducible factor 1-alpha
- HIV
Human Immunodeficiency Virus
- HO-1
Heme oxygenase-1
- HSPA5
Heat Shock Protein Family A (Hsp70) Member 5
- HTLV-1
Human T-cell lymphotropic virus type 1
- IAP
Inhibitor of Apoptosis Protein
- IEL
intraepithelial lymphocytes
- IH
Infantile Hemangioma
- IFNᵞ
Interferon ᵞ
- ILC
Innate Lymphoid cells
- IL
Interleukin
- IRE1a
Inositol requiring enzyme-1 alpha
- IRF
Interferon regulatory factor
- IRS-1
Insulin receptor substrate 1
- ISR
Integrated Stress Response
- ITD
Internal tandem duplication
- JNK
c-Jun N-terminal kinase
- Lox1
lectin-type oxidized LDL receptor 1
- LPS
Lipopolysaccharide
- LTR
Long Terminal Repeat
- MAPK
Mitogen-activated protein kinase
- MCP1
Monocyte chemoattractant protein 1
- M-CSF
Macrophage colony-stimulating factor
- MDSC
Myeloid Derived Suppressor Cells
- MEF
Mouse Embryonic Fibroblasts
- MHV68
Gamma herpes virus 68
- MITF
Microphthalmia-associated transcription factor
- mTORC1
Mammalian target of rapamycin complex 1
- M1/2
Activated/Alternately activated Macrophages
- NFAT
Nuclear factor of activated T-cells
- NF Kb
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural Killer cells
- NKG2D
The Natural Killer Group 2D
- NLRP1
NACHT, LRR, FIIND, CARD domain and PYD domains-containing protein 1
- NRF2
Nuclear factor erythroid 2-related factor 2
- PAMPs
Pathogen associated molecular patterns
- PER1
Period Circadian Regulator 1
- PERK
Protein kinase R (PKR)-like ER kinase
- PpIr15a
Protein phosphatase 1 regulatory subunit 15A
- PRMT1
Protein arginine N-methyltransferase 1
- PHD
Prolyl-4-hydroxylase domain
- OKC
Odontogenic keratocysts
- RANKL
Receptor activator of nuclear factor kappa-Β ligand
- REDD1
regulated in development and DNA damage response 1
- RIG
Retinoic acid-inducible gene
- RTA
Replication and transcriptional activator
- SIV
Simian Immunodeficiency Virus
- SLC7A5
Solute carrier family 7 member 5
- STAT3
Signal transducer and activator of transcription 3
- Tbet
T-box expressed in T cells
- TLR4
Toll-like receptor 4
- TNFa
Tumor Necrosis Factor alpha
- TRB3
Tribbles 3
- TSC1/2
Tuberous sclerosis 1 /2
- UBC
PolyUbiquitin-C precursor
- ULBP1
UL16 Binding Protein 1
Footnotes
Declaration of Interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ameri K and Harris AL, Activating transcription factor 4. Int J Biochem Cell Biol, 2008. 40(1): p. 14–21. [DOI] [PubMed] [Google Scholar]
- 2.Zinszner H, et al. , CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev, 1998. 12(7): p. 982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryoo HD and Vasudevan D, Two distinct nodes of translational inhibition in the Integrated Stress Response. BMB Rep, 2017. 50(11): p. 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo R, et al. , Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol, 2013. 301: p. 215–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harding HP, et al. , An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell, 2003. 11(3): p. 619–33. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Villanueva JF, Diaz-Molina R, and Garcia-Gonzalez V, Protein Folding and Mechanisms of Proteostasis. Int J Mol Sci, 2015. 16(8): p. 17193–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding HP, Zhang Y, and Ron D, Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature, 1999. 397(6716): p. 271–4. [DOI] [PubMed] [Google Scholar]
- 8.Schroder M and Kaufman RJ, ER stress and the unfolded protein response. Mutat Res, 2005. 569(1–2): p. 29–63. [DOI] [PubMed] [Google Scholar]
- 9.Tirasophon W, et al. , The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev, 2000. 14(21): p. 2725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niwa M, et al. , A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell, 1999. 99(7): p. 691–702. [DOI] [PubMed] [Google Scholar]
- 11.Wang XZ, et al. , Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J, 1998. 17(19): p. 5708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfon M, et al. , IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature, 2002. 415(6867): p. 92–6. [DOI] [PubMed] [Google Scholar]
- 13.Hillary RF and FitzGerald U, A lifetime of stress: ATF6 in development and homeostasis. J Biomed Sci, 2018. 25(1): p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida H, et al. , XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 2001. 107(7): p. 881–91. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, et al. , Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem, 1998. 273(50): p. 33741–9. [DOI] [PubMed] [Google Scholar]
- 16.Wek RC, Jiang HY, and Anthony TG, Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans, 2006. 34(Pt 1): p. 7–11. [DOI] [PubMed] [Google Scholar]
- 17.Xu D, et al. , ATF4-Mediated Upregulation of REDD1 and Sestrin2 Suppresses mTORC1 Activity during Prolonged Leucine Deprivation. J Nutr, 2020. 150(5): p. 1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff NC, et al. , Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol Cell Biol, 2011. 31(9): p. 1870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiramatsu N, et al. , Translational and posttranslational regulation of XIAP by eIF2alpha and ATF4 promotes ER stress-induced cell death during the unfolded protein response. Mol Biol Cell, 2014. 25(9): p. 1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozpedek W, et al. , The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr Mol Med, 2016. 16(6): p. 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilberg MS, et al. , The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr, 2012. 3(3): p. 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCullough KD, et al. , Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol, 2001. 21(4): p. 1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohoka N, et al. , TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J, 2005. 24(6): p. 1243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, et al. , Transcription Factor C/EBP Homologous Protein in Health and Diseases. Front Immunol, 2017. 8: p. 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brush MH, Weiser DC, and Shenolikar S, Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol, 2003. 23(4): p. 1292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, et al. , ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol, 2013. 15(5): p. 481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, et al. , Anacardic acid induces cell apoptosis associated with induction of ATF4-dependent endoplasmic reticulum stress. Toxicol Lett, 2014. 228(3): p. 170–8. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong JL, et al. , Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem, 2010. 285(9): p. 6091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouschop KM, et al. , The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest, 2010. 120(1): p. 127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blais JD, et al. , Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol, 2004. 24(17): p. 7469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siwecka N, et al. , Dual role of Endoplasmic Reticulum Stress-Mediated Unfolded Protein Response Signaling Pathway in Carcinogenesis. Int J Mol Sci, 2019. 20(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke Q and Costa M, Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol, 2006. 70(5): p. 1469–80. [DOI] [PubMed] [Google Scholar]
- 33.Vandewynckel YP, et al. , The paradox of the unfolded protein response in cancer. Anticancer Res, 2013. 33(11): p. 4683–94. [PubMed] [Google Scholar]
- 34.Koditz J, et al. , Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood, 2007. 110(10): p. 3610–7. [DOI] [PubMed] [Google Scholar]
- 35.Falletta P, et al. , Translation reprogramming is an evolutionarily conserved driver of phenotypic plasticity and therapeutic resistance in melanoma. Genes Dev, 2017. 31(1): p. 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadevan NR, et al. , Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8(+) T cell priming. PLoS One, 2012. 7(12): p. e51845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Y, et al. , Publisher Correction: ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat Commun, 2019. 10(1): p. 3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennock ND, et al. , T cell responses: naive to memory and everything in between. Adv Physiol Educ, 2013. 37(4): p. 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez PC, Quiceno DG, and Ochoa AC, L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood, 2007. 109(4): p. 1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geiger R, et al. , L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell, 2016. 167(3): p. 829–842 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma EH, et al. , Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab, 2017. 25(2): p. 345–357. [DOI] [PubMed] [Google Scholar]
- 42.Krokowski D, et al. , A self-defeating anabolic program leads to beta-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J Biol Chem, 2013. 288(24): p. 17202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, et al. , ATF4 Regulates CD4(+) T Cell Immune Responses through Metabolic Reprogramming. Cell Rep, 2018. 23(6): p. 1754–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravindran R, et al. , The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature, 2016. 531(7595): p. 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundrud MS, et al. , Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science, 2009. 324(5932): p. 1334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashi K, et al. , HOXB9 acts as a negative regulator of activated human T cells in response to amino acid deficiency. Immunol Cell Biol, 2016. 94(6): p. 612–7. [DOI] [PubMed] [Google Scholar]
- 47.Jiang G, et al. , HIV Exploits Antiviral Host Innate GCN2-ATF4 Signaling for Establishing Viral Replication Early in Infection. mBio, 2017. 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campestrini J, Silveira DB, and Pinto AR, HIV-1 Tat-induced bystander apoptosis in Jurkat cells involves unfolded protein responses. Cell Biochem Funct, 2018. 36(7): p. 377–386. [DOI] [PubMed] [Google Scholar]
- 49.Gachon F, et al. , Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4). Mol Cell Biol, 2000. 20(10): p. 3470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siu YT, et al. , TORC1 and TORC2 coactivators are required for tax activation of the human T-cell leukemia virus type 1 long terminal repeats. J Virol, 2006. 80(14): p. 7052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joo JH, et al. , Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells. Biochem Pharmacol, 2015. 97(3): p. 256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew NR, et al. , Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med, 2018. 24(3): p. 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang JS, et al. , Endoplasmic reticulum stress response promotes cytotoxic phenotype of CD8alphabeta+ intraepithelial lymphocytes in a mouse model for Crohn’s disease-like ileitis. J Immunol, 2012. 189(3): p. 1510–20. [DOI] [PubMed] [Google Scholar]
- 54.Jin L, et al. , Genomewide Histone H3 Lysine 9 Acetylation Profiling in CD4+ T Cells Revealed Endoplasmic Reticulum Stress Deficiency in Patients with Acute-on-chronic Liver Failure. Scand J Immunol, 2015. 82(5): p. 45–29. [DOI] [PubMed] [Google Scholar]
- 55.Hirayama D, Iida T, and Nakase H, The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int J Mol Sci, 2017. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills CD, et al. , M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol, 2000. 164(12): p. 6166–73. [DOI] [PubMed] [Google Scholar]
- 57.Kim JH, et al. , Endoplasmic reticulum chaperone GRP78 regulates macrophage function and insulin resistance in diet-induced obesity. FASEB J, 2018. 32(4): p. 2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu B, et al. , ATF4 regulates CCL2 expression to promote endometrial cancer growth by controlling macrophage infiltration. Exp Cell Res, 2017. 360(2): p. 105–112. [DOI] [PubMed] [Google Scholar]
- 59.Liu C, et al. , Activating transcription factor 4 promotes angiogenesis of breast cancer through enhanced macrophage recruitment. Biomed Res Int, 2015. 2015: p. 974615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stone AEL, et al. , RIG-I-like receptors direct inflammatory macrophage polarization against West Nile virus infection. Nat Commun, 2019. 10(1): p. 3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elbarghati L, Murdoch C, and Lewis CE, Effects of hypoxia on transcription factor expression in human monocytes and macrophages. Immunobiology, 2008. 213(9–10): p. 899–908. [DOI] [PubMed] [Google Scholar]
- 62.Xia HF, et al. , Association of ATF4 Expression With Tissue Hypoxia and M2 Macrophage Infiltration in Infantile Hemangioma. J Histochem Cytochem, 2017. 65(5): p. 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong WQ, et al. , Elevated ATF4 Expression in Odontogenic Keratocysts Epithelia: Potential Involvement in Tissue Hypoxia and Stromal M2 Macrophage Infiltration. J Histochem Cytochem, 2019. 67(11): p. 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss-Sadan T, et al. , Cathepsins Drive Anti-Inflammatory Activity by Regulating Autophagy and Mitochondrial Dynamics in Macrophage Foam Cells. Cell Physiol Biochem, 2019. 53(3): p. 550–572. [DOI] [PubMed] [Google Scholar]
- 65.Zahid MDK, et al. , CCAAT/enhancer-binding protein beta (C/EBPbeta) knockdown reduces inflammation, ER stress, and apoptosis, and promotes autophagy in oxLDL-treated RAW264.7 macrophage cells. Mol Cell Biochem, 2020. 463(1–2): p. 211–223. [DOI] [PubMed] [Google Scholar]
- 66.Chaplin DD, Overview of the immune response. J Allergy Clin Immunol, 2010. 125(2 Suppl 2): p. S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou XC, et al. , Regulation of gammaherpesvirus lytic replication by endoplasmic reticulum stress-induced transcription factors ATF4 and CHOP. J Biol Chem, 2018. 293(8): p. 2801–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vatsveen TK, et al. , Artesunate shows potent anti-tumor activity in B-cell lymphoma. J Hematol Oncol, 2018. 11(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuniati L, et al. , Tumor suppressor BTG1 promotes PRMT1-mediated ATF4 function in response to cellular stress. Oncotarget, 2016. 7(3): p. 3128–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prabhu VV, et al. , Single agent and synergistic combinatorial efficacy of first-in-class small molecule imipridone ONC201 in hematological malignancies. Cell Cycle, 2018. 17(4): p. 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paul S and Lal G, The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front Immunol, 2017. 8: p. 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilfillan S, et al. , NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol, 2002. 3(12): p. 1150–5. [DOI] [PubMed] [Google Scholar]
- 73.Hosomi S, et al. , New Insights Into the Regulation of Natural-Killer Group 2 Member D (NKG2D) and NKG2D-Ligands: Endoplasmic Reticulum Stress and CEA-Related Cell Adhesion Molecule 1. Front Immunol, 2018. 9: p. 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cosman D, et al. , ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity, 2001. 14(2): p. 123–33. [DOI] [PubMed] [Google Scholar]
- 75.Gowen BG, et al. , A forward genetic screen reveals novel independent regulators of ULBP1, an activating ligand for natural killer cells. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berrou J, et al. , Natural killer cell function, an important target for infection and tumor protection, is impaired in type 2 diabetes. PLoS One, 2013. 8(4): p. e62418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hosomi S, et al. , Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J Exp Med, 2017. 214(10): p. 2985–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu J, et al. , NK cells induce hepatic ER stress to promote insulin resistance in obesity through osteopontin production. J Leukoc Biol, 2020. 107(4): p. 589–596. [DOI] [PubMed] [Google Scholar]
- 79.Steinman RM and Banchereau J, Taking dendritic cells into medicine. Nature, 2007. 449(7161): p. 419–26. [DOI] [PubMed] [Google Scholar]
- 80.Clavarino G, et al. , Protein phosphatase 1 subunit Ppp1r15a/GADD34 regulates cytokine production in polyinosinic:polycytidylic acid-stimulated dendritic cells. Proc Natl Acad Sci U S A, 2012. 109(8): p. 3006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tavernier SJ, et al. , Regulated IRE1-dependent mRNA decay sets the threshold for dendritic cell survival. Nat Cell Biol, 2017. 19(6): p. 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang LX, et al. , Sestrin2 protects dendritic cells against endoplasmic reticulum stress-related apoptosis induced by high mobility group box-1 protein. Cell Death Dis, 2020. 11(2): p. 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabrilovich DI, Myeloid-Derived Suppressor Cells. Cancer Immunol Res, 2017. 5(1): p. 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabrilovich DI, Ostrand-Rosenberg S, and Bronte V, Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol, 2012. 12(4): p. 253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koehn BH, et al. , GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood, 2015. 126(13): p. 1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marigo I, et al. , Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity, 2010. 32(6): p. 790–802. [DOI] [PubMed] [Google Scholar]
- 87.Thevenot PT, et al. , The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity, 2014. 41(3): p. 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halaby MJ, et al. , GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci Immunol, 2019. 4(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal S, et al. , Clinical profile of pythium keratitis: perioperative measures to reduce risk of recurrence. Br J Ophthalmol, 2018. 102(2): p. 153–157. [DOI] [PubMed] [Google Scholar]
- 90.Karthikeyan RS, et al. , Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis, 2011. 204(6): p. 942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong J, et al. , Tacrolimus interacts with voriconazole to reduce the severity of fungal keratitis by suppressing IFN-related inflammatory responses and concomitant FK506 and voriconazole treatment suppresses fungal keratitis. Mol Vis, 2018. 24: p. 187–200. [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang S, et al. , ATF4 Involvement in TLR4 and LOX-1-Induced Host Inflammatory Response to Aspergillus fumigatus Keratitis. J Ophthalmol, 2018. 2018: p. 5830202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srivastava RK, et al. , Activating transcription factor 4 underlies the pathogenesis of arsenic trioxide-mediated impairment of macrophage innate immune functions. Toxicol Appl Pharmacol, 2016. 308: p. 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang H, et al. , ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J Inflamm (Lond), 2015. 12: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dias-Teixeira KL, et al. , Emerging Role for the PERK/eIF2alpha/ATF4 in Human Cutaneous Leishmaniasis. Sci Rep, 2017. 7(1): p. 17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Osualdo A, et al. , Transcription Factor ATF4 Induces NLRP1 Inflammasome Expression during Endoplasmic Reticulum Stress. PLoS One, 2015. 10(6): p. e0130635. [DOI] [PMC free article] [PubMed] [Google Scholar]