Abstract
TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis selectively via its interaction with death receptors TRAILR1/death receptor 4 (DR4) and TRAILR2/DR5 in a wide range of cancers while sparing normal cells. Despite its tremendous potential for cancer therapeutics, the translation of TRAIL into the clinic has been confounded by TRAIL-resistant cancer populations. In this review we discuss different molecular mechanisms underlying TRAIL mediated apoptosis and widespread resistance to TRAIL. We also discuss the successes and failures of recent preclinical and clinical studies surrounding TRAIL-induced apoptosis and current attempts to overcome TRAIL resistance and provide a perspective for improving the prospects of clinical implementation in the future.
Keywords: TRAIL, apoptosis, cancer, therapeutic resistance
Introduction:
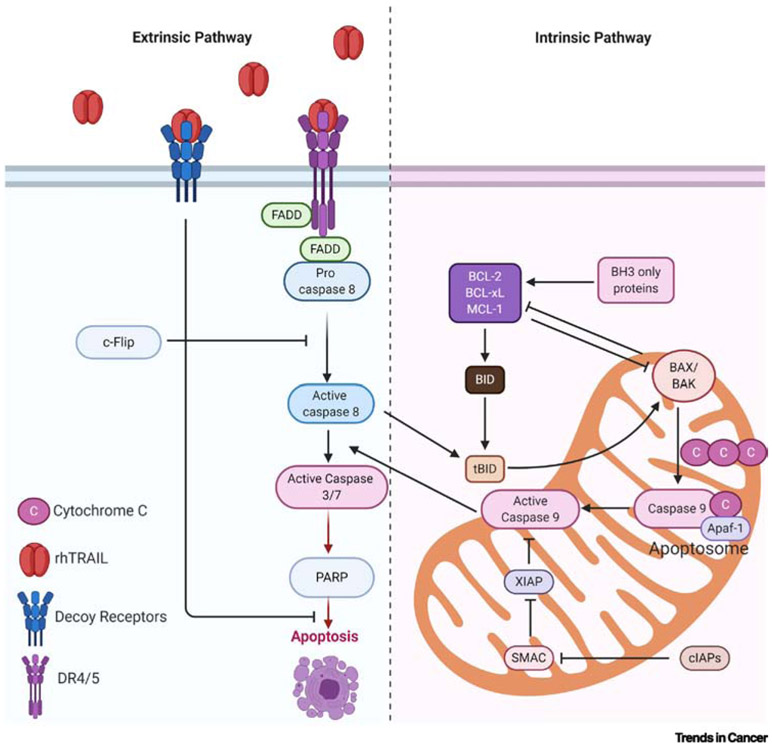
Despite much effort and decades of research and clinical trials, cancer remains one of the most devastating human diseases and it is the leading cause of premature death in humans. One of the hallmarks of cancer that has drawn particular attention is the immortality of cancer.[1] There are two known pathways of apoptosis (see Glossary), extrinsic and intrinsic apoptotic pathways. The major players in the extrinsic pathway belongs to the TNF receptor superfamily called Death Receptor 4/5 (DR4/5 or TRAIL R1/R2 to represent TRAIL receptor 1 and 2) [2]. The intrinsic pathway is initiated in the mitochondria and involves caspase family proteins as well as p53 activation to initiate cell death. Even though TRAIL only physically binds to DR4/5 to initiate the extrinsic pathway, altered protein expression in the intrinsic apoptotic pathway is known to influence TRAIL resistance [2]. Major crosstalk occurs between extrinsic and intrinsic signaling, generating a complicated network of cell-death program [2] (Fig. 1). In order to maintain proper control of apoptosis versus survival, multiple levels of modulators must maintain a careful balance with each other. In the 1980s, a pro-apoptotic cytokine, tumor necrosis factor (TNF)-α was injected systematically with a hope to trigger apoptosis specifically in cancer cells, but the enthusiasm was quickly waned by the unacceptable toxicity in the clinical trials.[3] However, a new member of the same superfamily named TNF-related apoptosis-inducing ligand (TRAIL) was soon discovered to have a wide therapeutic index in healthy tissues and could selectively induce apoptosis in cancer cells. [4,5]
Fig. 1. Intrinsic and Extrinsic Apoptosis Pathways:
Caption: A simplified diagram of the major crosstalk between extrinsic and intrinsic signaling, generating a complicated network of cell-death program. FADD: Fas-associated protein with death domain; c-FLIP: cellular FADD-like IL-1β converting enzyme inhibitory protein; PARP: poly(ADP-ribose) polymerase; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra-large; Mcl-1: induced myeloid leukemia cell differentiation protein; Bid: BH3-interacting domain death agonist; Bax: Bcl-2-associated X protein; Bak: Bcl-2 homologous antagonist killer; Apaf-1: Apoptotic peptidase activating factor 1; XIAP: X-linked inhibitor of apoptosis; c-IAP: cellular inhibitor of apoptosis protein; SMAC: second mitochondria-derived activator of caspases
The initial limitations of using systematic injection of TRAIL to cancer was attributed to the short half-life of TRAIL in the circulatory system.[6] Since then, numerous delivery methods have been developed to prolong TRAIL half-life in tumors. Many traditional materials used to deliver drugs have been used on TRAIL and shown to successfully exhibit endosomal disruption and desired biodistribution [7], those include iron oxide [8], gold [9], PLGA biopolymer [10,11], PEI cationic polymer [12] and liposome-based nanoparticles [13,14]. Much of the mechanism behind specific aggregation of TRAIL in tumor tissue is attributed to the enhanced permeation and retention effect (EPR), in which the nanoparticles can freely traverse through tumor vasculature but accumulate in the solid tumors because of the absence of lymphatic drainage [15]. Aside from the development of TRAIL delivery methods, there have also been attempts to activate TRAIL-induced apoptosis pathway using small molecules. A successful example is the TRAIL transcriptional activator ONC201 that can inactivate pro-survival kinases Akt and ERK, which decreases phosphorylation of the transcription factor FOXO3a to activate TRAIL expression. ONC201 has since entered clinical trials for pediatric gliomas.[16] Other novel approaches include utilizing trimeric receptor aggregates to improve the thermodynamic and kinetic favorability of TRAIL pathway activation and can often produce a much stronger TRAIL response [17]. Despite its selective toxicity towards cancer, TRAIL resistance is evident in a vast proportion of cancer samples. Such resistance has yielded disappointing clinical trials despite pre-clinical success. A majority of the current TRAIL related studies are focused on how to enhance TRAIL efficacy and how to overcome TRAIL resistance in different cancer types. This review attempts to address the mechanisms behind TRAIL resistance and means to circumvent them and also discusses promising pre-clinical and clinical studies and the potential future outlooks to translate TRAIL successfully into clinic use.
TRAIL Resistance
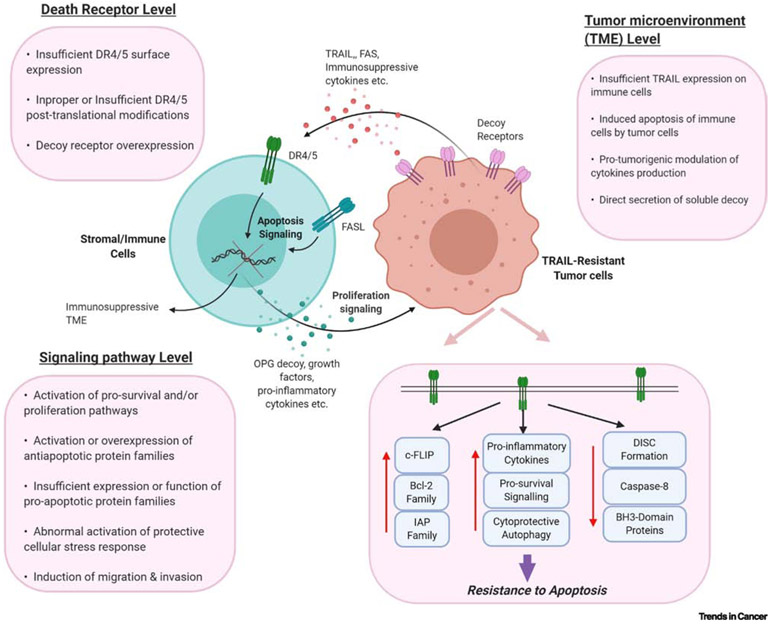
Although TRAIL has demonstrated tremendous promises in pre-clinical studies, both in vitro studies and in vivo studies have shown that while most cancer cell lines display to some degree sensitivity to TRAIL killing, a proportion of them harbors innate resistance. Furthermore, as tumor genome evolves under the selective pressure, many tumors can also later acquire TRAIL resistance and relapse. Multiple studies have demonstrated that resistance to TRAIL mediated apoptosis is diverse and can occur in virtually every step of the cell signaling cascade [2]. Here, we summarize some of the most prominent resistance mechanisms studied in recent years (Figure 2).
Fig. 2. Molecular and Cellular Mechanisms of TRAIL resistance:
Caption: Four main categories of TRAIL resistance. DR4/5: Death receptor 4/5; FasL: Tumor necrosis factor superfamily Fas Ligand
Death-Receptor
Death Receptor 4/5 (DR4/5) are two essential components of the extrinsic apoptosis pathway. Transcription factors for DR4 and DR5 are mostly stress-induced genes such as p53, NF-kB and FOXO3a, and upregulation of DR4/5 mediated by increased expression of such transcription factors should sensitize cancer cells to apoptosis [18]. However, studies have shown that the localization of DR4/5 in the surface membrane contributes significantly to differential sensitivities among cancer cells [19]. Specifically, upregulation of clathrin-mediated endocytosis (CME) results in loss of surface expression and abolishes apoptotic signaling in TRAIL-resistant breast cancer cell lines [20]. Such receptor-trafficking appears to be modulated by an autoregulating feedback loop, in which activation of caspase-8 by the DISC complex can activate Dyn1 to trigger internalization of DR4/5 and decrease apoptotic signaling. siRNA knockdown of Dyn1 has been shown to confirm sensitivity to TRAIL-mediated apoptosis, thus validating its potential as a target for combination therapy [20]. Recent microRNA (miRNA) studies have also revealed several regulatory mechanisms and potential therapeutic targets for TRAIL-resistance. siRNA screening studies have shown that miR-145 and miR-216 are capable of decreasing DR4/5 expression in breast cancer cell lines [21]. Another study showed that miR-25 overexpression can dramatically decrease DR4 expression via direct targeting of the 3’ UTR region of the transcript in liver cancer cell lines [22]. TRAIL is also known to bind to three other decoy receptors TRAIL-R3, TRAIL-R4 and OPG but is unable to induce apoptosis. Overexpression of some decoy receptors have been shown to correlate with profound TRAIL resistance [23].
Caspase-8 and c-FLIP
Upon TRAIL binding to the endogenous death receptors, their intracellular carboxyl terminal death domains (DD) oligomerize and recruit an adaptor protein called Fas-associated death domain (FADD). Through their death effector domains (DEDs), pro-caspase-8 and pro-caspase-10 can bind to FADD, forming the DISC to cleave pro-caspases into active caspase forms and transduce pro-apoptotic signals [2].This process can be antagonized and regulated by the cellular FLICE inhibitory protein (c-FLIP), which contains a DED that can bind to FADD but unable to act as a protease to activate caspases [24]. In cancer cells, multiple resistance mechanisms can occur on the (pro)-caspase-8 and c-FLIP levels to evade TRAIL killing. For example, histone hypoacetylation has shown to result in downregulation of CASP8 expression in medulloblastoma [25]. Interestingly, two contrasting studies have shown that ubiquitination of caspase-8 can either enhance or inhibit TRAIL killing depending on the cancer cell line tested [26,27]. Additionally, a wide range of kinases have also been shown to phosphorylate multiple tyrosine residues of caspase 8 resulting in suppressing its activity and subsequently increasing TRAIL resistance and invasiveness in colon cancer [28,29].
While the exact molecular mechanism by c-FLIP in apoptosis continues to be studied, it is known that high c-FLIP expression can sequester FADD and prevent effective DISC formation [2]. Being a master negative regulator of apoptosis, c-FLIP is reportedly overexpressed in a wide variety of cancers and implicated with poor prognosis due to lack of response to chemotherapy [30-32]. Additionally, c-FLIP can also activate proliferation and pro-survival signaling such as ERK and NF-kB while preventing apoptotic cell death signaling such as Fas-induced JNK activation [33,34]. An important regulator of c-FLIP is the calcium/calmodulin-dependent protein kinase II (CaMK II), which phosphorylates and upregulates c-FLIP and its overexpression is seen in melanoma [35]. It is important to note that, phosphorylation of c-FLIP does not always correlate with TRAIL resistance. The phosphorylation of c-FLIP by protein kinase C or bile acid glycochenodeoxycholate results in decrease association between c-FLIP and FADD in hepatocellular carcinoma cell lines and it is correlated with TRAIL sensitivity [36]. Thus, it appears different residues of phosphorylation have different effects on c-FLIP, and more research is needed to elucidate the functional outcome.
BCL-2 Family and IAP Family
After caspase-8/10 activation, the extrinsic apoptosis pathway involves a direct cleavage of caspase-3 and subsequent PARP-induced cell death. However, in type-II cells, in order to activate intrinsic apoptosis, the BH3-interacting domain pro-apoptotic protein, Bid is truncated by caspase-8 and translocated to further transduce apoptotic signals in the mitochondria [2]. The oligomerization of Bcl-2-associated X protein (Bax) and the mitochondria-residing Bcl-2 homologous antagonist killer protein (Bak) induces a change in conformation and converts the dimer into pore-forming proteins to puncture the mitochondrial outer membrane (OM) in a fashion possibly similar to pore-forming toxins in bacteria. Subsequently, mitochondria release the mitochondria-derived activator of caspase (Smac) and cytochrome c. The latter can then bind apoptotic peptidase activating factor 1 (Apaf-1) and pro-caspase-9 to form apoptosome, which can cleave and activates pro-caspase-9 to relays the signals to activate caspases 3, 6 and 7 and leads to cell death [37]. Additionally, anti-apoptotic proteins in the Bcl-2 family are important for regulating intrinsic apoptosis. Specifically, Bcl-xL and Mcl-1 can both inhibit apoptosis activation by competing with Bax for binding with tBid, while Bcl-2 can inhibit tBid from translocating into mitochondrial OM [37]. Therefore, overexpression of these proteins is also common in cancer, and its expression level is negatively correlated with TRAIL response and survival rates [38]. Therefore, BH3-mimetics and SMAC-mimetics have been designed to antagonize the anti-apoptotic programs in cancer cells [2]. Various small molecules have been shown to help overcome chemotherapy resistance in both solid and hematological cancer and some of them have already entered clinical trials [39,40]. In a properly functional apoptosis program, IAP family proteins serve as a checkpoint to regulate both extrinsic and intrinsic pathways, preventing unwanted activation of cell death. XIAP has been shown to bind caspases 3, 7 and 9 and potently inhibit their activities while cIAP-1 and cIAP-2 contain E3 ligase domain to polyubiquitinate caspase-3 and 7 to target them for degradation [2]. In addition, IAP family proteins can create complexes with NF-kB and activate pro-survival pathway. As a result, IAP family proteins are also widely overexpressed and associated with poor clinical outcomes in solid cancer [41] and many blood cancers such as leukemia [42].
PI3K-Akt
PI3K-Akt pathway is a major signaling transduction pathway involved in diverse cellular functions, including proliferation, survival under stress and growth. Since its discovery, its importance in tumorigenesis has been gradually uncovered, and one of which is its crucial role in driving apoptosis resistance in cancer cells [2]. The negative regulator of this pathway, PTEN, is one of the most often inactivated tumor suppressor genes in cancer such as glioblastoma. [43] Studies have extensively shown that overactivation of PI3K/Akt/mTOR pathway increases TRAIL resistance in a range of cancer cells [44]. Notably, some cancers can actively take advantage of the TRAIL resistance and become more aggressive and invasive. For example, KRAS-driven NSCLC and pancreatic ductal adenocarcinoma activate PI3K/Akt axis in response to TRAIL and phosphorylate GTPases involved in cellular migration such as Rho [45]. These results suggest that large kinome landscape changes occur after the activation of PI3K/Akt pathway, and together result in cell survival, proliferation, migration and invasion in TRAIL resistant cell. [46] Additionally, PI3K/Akt pathway also drives activation of cytoprotective autophagy through downstream activation of mTOR signaling [18]. As previous studies have shown, autophagy could contribute to TRAIL resistance via sequestering DR4/5 in the autophagosomes, thus preventing them from their cell surface expression [47].
Tumor Microenvironment (TME)
Increasing understanding of TRAIL signaling has revealed a far more complex network that could have either anti-tumor effects or pro-tumor effects depending on the cell types and specific mutations that hijack apoptosis and pro-survival pathways [2]. Both cellular and non-cellular components of the TME can transmit multiple signals and modulate TRAIL-induced apoptosis. The resistance could come from simple physical barriers that prevent delivery of TRAIL-expressing leukocytes, or from active evasion of cancer. [48] As TRAIL sensitive cell lines can become resistant by the interactions with their stroma, it is critical to understand mechanisms behind which TME can contribute to TRAIL resistance when one thinks about clinical relevance [48].
One mechanism seen in murine lymphoma is the downregulation of TRAIL on dendritic cells (DCs). One study showed that tumor cells or whole tumor lysate is capable of completely impairing TRAIL expression on DCs to achieve immune evasion and such impairment seems to be irreversible even with exogenous IL-15 stimulation of DCs [49]. Additionally, both neutrophils and macrophages can express TRAIL and migrate to DR4/5 expressing tumor cells. They can release soluble TRAIL but protect themselves from TRAIL killing by overexpressing their own decoy receptors and downregulate their own DR4/5 expressions [50]. However, previous studies have shown that neutrophils and macrophages can also exhibit pro-tumorigenic effects by actions of various cytokines from the tumor secretome or immunosuppressive TME [48, 51]. One such cytokine is IL-35 and it can enhance tumor infiltration and invasion via the activation of MMP9 pathway and downregulate neutrophil TRAIL expression via activation of the STAT3 pathway [51].
Many cancer-associated stromal cells can significantly contribute to tumor resistance to TRAIL as well. Notably, cancer-associated fibroblasts (CAFs) have been observed to highly express the two TRAIL decoy receptors TRAIL R3 and TRAIL R4 to create a pro-tumorigenic environment, particularly around TRAIL-resistant cancer cells [23]. Moreover, myofibroblasts can secrete platelet-derived growth factor BB (PDGF-BB) and can activate the hedgehog (Hh) survival pathway in cholangiocarcinoma to counter TRAIL-induced apoptosis [52]. Finally, resistant cancer cells can also actively direct antitumor immune cells to become tumor-associated stromal cells. For example, one such study showed that TRAIL resistant NSCLC, pancreatic and colorectal cells can use DR4/5 signaling for tumor-supportive role, by secreting CCL2 and induce polarization of myeloid cells into M2-like cells and potently attract immunosuppressive MDSCs [53].
Overcoming TRAIL Resistance
With increasing number of resistance mechanisms being discovered, different strategies have been implemented to overcome various TRAIL resistance. One of the most common strategies is combining different modes of therapeutics. Not only does targeting multiple pathways decrease the likelihood of two simultaneous mutations arising in the same clone, functional redundancy and compensation between pathways also rationalize combination therapy. Here we summarize some previous promising pre-clinical studies related to overcoming TRAIL resistance or sensitizing TRAIL apoptotic signaling (see Table 1).
Table 1:
Promising Pre-clinical Studies of TRAIL-induced Apoptosis in Cancer Therapeutics.
| Category | Sensitizing Agent | Cancer Type | Mechanism |
|---|---|---|---|
| DNA Damaging Agent | Radiation therapy | Breast cancer, Prostate cancer, Lung cancer, Colorectal cancer, Head and Neck cancer, T-lymphoblastic leukemia [86] | Irradiation-induced activation of p53 and subsequent p53-dependent up-regulation of pro-apoptotic proteins such as DR4/5. |
| DNA Damaging Agent | Cisplatin, gemcitabine and many more (e.g. 5-FU or topoisomerase inhibitor Irinotecan and Etoposide) | NSCLC, Esophageal cancer, mesothelioma, Leukemia [54][87] | DNA Damage-induced activation of p53 and upregulated caspase-8 |
| Manipulation or Recovery of p53 | Ad-p53, p53R3 Nutlin-3 | Glioma, myeloma, Lung cancer, Leukemia, lymphoma, sarcoma [87] | Stabilization and activation of p53 and subsequent upregulation of DR5 |
| Natural Compound | Polyphenols (e.g. wogonin, resveratrol) | Colorectal cancer hepatoblastoma, medulloblastoma, glioblastoma, pancreatic cancer etc. [87] | Pro-oxidant effect that generates ROS-mediated p53 activation and pro-apoptotic protein PUMA and DR5 upregulation |
| Natural Compound | Terpenoids (e.g. nimbolide, andrographolide) | Colon cancer, hepatoma, cervical cancer [87] | ROS production & ERK activation (causing DR4/5 upregulation), downregulation of several anti-apoptotic proteins and stabilization of p53 through phosphorylation |
| Natural Compound | Vitamin-related products (e.g. Vitamin E and related compounds, vitamin A analogs, CD437 retinoid) | Colon cancer, mesothelioma, lung cancer [87] | ROS production & ERK activation and DR4/5 upregulation, enhanced Bid cleavage and mitochondrial apoptosis pathway |
| Epigenetics Modification | HDAC inhibitors (e.g. SAHA, CBHA, TSA and VPA) | Breast cancer, lung cancer, colon cancer, prostate cancer, renal cell carcinoma, melanoma [88] | Upregulation of DR4/5, upregulation of pro-apoptotic Bcl-2 family proteins, reduce cytoprotective autophagy, distribute DR4/5 into lipid raft, downregulation of anti-apoptotic proteins and suppression of c-myc |
| Epigenetics Modification | DNMT inhibitors (e.g. Zeb, decitabine | Breast cancer, lung cancer [88] | Enhancing DR5 fucosylation, caspase-8 upregulation, downregulation of XIAP |
| Molecule mimetics of BH3-only proteins | ABT-737, ABT-199, ABT-263 and Smac-mimetics | Chronic Lymphocytic leukemia, follicular lymphoma, ovarian cancer [54][88] | Mimics the activity of pro-apoptotic protein Bad and suppression of anti-apoptotic Bcl-2 and IAP family proteins |
| Small molecules | Multi-kinase inhibitor (e.g. Sorafenib) | Malignant pleural mesothelioma, colon cancer [89,90] | Shut down MAPK signaling, PI3K/Akt signaling and NF-kB signaling, downregulation of mcl-1, c-FLIP and IAP family proteins |
| Small molecules | PI3K/Akt/mTORC1 inhibitors | Neuroblastoma, breast cancer, leukemia [91] | Suppression of autophagy and proliferation |
| Antibodies | Single-chain variable fragment (scFv) nanobodies | NSCLC, colon cancer, glioma, B-lymphoblastic leukemia, chronic-B-lymphocytic leukemia [66,67] | Simultaneous targeting of proliferation and apoptosis pathway, selective induction of apoptosis, target angiogenesis |
| Immunotherapy | Tumor antigen-specific, MHC-restricted T cells with PD-L1 nanobodies | Melanoma, chronic lymphocytic leukemia [71] | Downregulation of c-FLIP, converting suppressive macrophages/DCs into pro-apoptotic effector cells and IFN-gamma production |
| Immunotherapy | CD3/CD7 fusion with stimulatory TRAIL antibody on T-cells | Pan-carcinoma [72] | Cytolytic granzyme/perforin pathway activation induced by even low DR5 expression |
| Immunotherapy | scFv:CD70-TNC-TRAIL fusion protein | Lymphoma, melanoma, bladder cancer, colon cancer [70] | Cytotoxic T-cells and activate granulocytes, monocytes and DCs |
Chemo- and radiation therapy
It is extensively demonstrated that there is a favorable effect of combining TRAIL and radiotherapy and recent study has shown the impact of p53 status on TRAIL sensitivity [54]. Specifically, p53 can act as a transcription factor to a variety of proapoptotic Bcl-2 family members such as Bax as well as the expression of DR4/5 [55]. Therefore, DNA damages caused by radiation stabilizes the level of p53 and can enhance TRAIL killing synergistically. Using a similar rationale, DNA damaging chemo-agents, such as cisplatin and gemcitabine have been employed in combination with TRAIL to sensitize cancer cell death [54]. Similarly, the use of novel inhibitor of p53 negative regulator MDM2 can lead to stabilization and accumulation of p53 and sensitizes lung, breast and colon cell lines to TRAIL killing [56]. Lastly, Activator of AMP-activated protein kinase (AMPK) has also been shown sensitize TRAIL resistant colon cancer cells [57].
Epigenetic modulation
Epigenetic drugs, in particular, histone deacetylases (HDAC) inhibitors have shown great cooperation with TRAIL therapy by sensitizing cells to TRAIL mediated killing in a panel of cancers including breast, lung, colon, prostate, renal and skin cancer [58]. HDAC inhibitors (such as SBHA, VPA and TSA etc.) can upregulate the expression of DR4/5 and pro-apoptotic Bcl-2 family proteins such as Bax and Bim, simultaneously priming the extrinsic and intrinsic pathways [59, 60]. HDAC inhibitors have also been shown to reduce the expression of various anti-apoptotic protein such as Bcl-2, Bcl-XL, Mcl-1, survivin, XIAP and c-FLIP [59, 61]. It is, however, unclear how much of each function contributes to its sensitization in different cancer cell types. Given that HDAC inhibitors cause global histone acetylation and therefore activate global gene expressions somewhat non-discriminately, it is important to study and conclude their therapeutic efficacy only within the context of specific cancer cell types. Besides HDAC inhibitors, demethylating agents can also promote apoptosis by upregulating XAF1 expression, a negative regulator of anti-apoptotic protein XIAP, as well as DR4/5 and caspase-8 expression [62].
Innovative approaches targeting multiple pathways
Besides targeting anti-apoptosis program, cancer often activates survival pathways to bypass TRAIL killing as well [2]. A number of antibody-based inhibitors exhibit a better synergistic anti-tumor effects with chemotherapy than small-molecule inhibitors [65]. Thus, combination of antibodies against common driver genes and TRAIL has promise to overcome resistance in either single agent treatment. Previously, an innovative approach used a single-chain variable fragment (scFv) or nanobodies (VHH domain fragment) of an antibody targeting EGFR fused to TRAIL. The resulting (ENb2)-TRAIL fusion is much smaller in size compared to a full antibody molecule with TRAIL and targeted both the cell death and proliferation pathways in tumor cells [6]. ENb2-TRAIL, either direct addition or with stem-cell delivery has been shown to have elicit a more potent apoptosis response and overcome single agent TRAIL or ENb resistance [66]. Similarly, fusing only the three type-1 repeat (3TSR) domain of the anti-angiogenic protein thrombospondin-1 (TSP) with TRAIL could overcome apoptosis resistance in glioblastoma by systemic delivery or stem-cell delivery. Importantly, since 3TSR can target angiogenesis in both tumor and tumor-associated endothelial cells, its combination with TRAIL can significantly extends the lifespan of tumorbearing mice [67].
Previous high-throughput screening efforts have also provided clues for synergistic interactions between TRAIL and natural or synthetic product compounds. Out of a screening of 16,480 compounds on renal adenocarcinoma, 18 compounds were identified to sensitize cells to TRAIL cytotoxicity, 12 of which activate caspase-8 in the presence, but not the absence of TRAIL [68]. Another small screen was tested on TRAIL resistant pancreatic and prostate cancer cells with 55 FDA or foreign-approved antineoplastic drugs. The identified sensitizers likely represent many of the previously described mechanisms, including inducing DNA-damage, inhibiting XIAP or Mcl-1 and promoting DR4/5 lipid raft distribution [69].
Recently, a few studies have explored direct regulation of the apoptotic pathways for overcoming resistance by simultaneously affecting multiple players in the apoptotic program. For example, a recent study has shown that selective CDK9 inhibitor can prime the highly TRAIL-resistant cancer cells to apoptosis through a concomitant suppression of c-Flip and Mcl-1].[63] Similarly, proteasome inhibitors have also shown to overcome resistance via a variety of common TRAIL-resistance mechanisms including reducing the levels of XIAP, decoy receptors DcR1 and DcR2 and dominant negative DR5 mutants. [64]
Immunotherapy
Most recent breakthrough in cancer therapeutics is the development of immunotherapy. Cancer immunotherapy usually attempts to stimulate the immune system by direct activation or inhibition of negative immune regulation. A number of lymphocyte-mediated tumor cell apoptosis programs including T-cell or NK cell response are dependent on TRAIL signaling pathway and thus combination therapy could potentiate a synergistic effect [70]. Among cell-based therapeutic approaches, activating tumor antigen-specific, MHC-restricted T cells by using immune checkpoint blockers has been tested. Specifically, scFv fragment of anti-PD-L1 fused with TRAIL, was shown to exhibit a multi-fold therapeutic effect by converting suppressive macrophages/DCs into pro-apoptotic effector cells and IFNγ production, thus leading to sensitization of TRAIL killing through downregulation of c-FLIP [71]. Additionally, the scFv targeting domain has been utilized to selectively deliver activated T-cells to cancer. The CD3 or CD7 stimulatory antibody fragment and TRAIL fusion protein was shown to bind to DR5 on the surface of the cancer cells to simultaneously initiate pro-apoptotic pathway and stimulate the tumoricidal activity of T-cells including intrinsic cytolytic granzyme/perforin pathway [72].The resulting treatment has been characterized against a wide range of cancers and is believed to have the potential as a pan-carcinoma reagent. Remarkably, even a low level of DR5 surface expression can be sufficient to induce immunological synapse with CD3 and activate T-cell functions [72]. Similar strategy has also been used with scFv antibody fragment specific to C-type lectin-like molecule-1 (CLL1) to activate granulocytes, monocytes and dendritic cells [73]. Aside from cytotoxic T-cells, another combinatorial approach with TRAIL was to engineer NK cells ex-vivo to secrete glycosylated TRAIL and the engineered NK cells were shown to infiltrate peritoneal tumors and inhibit peritoneal colorectal carcinomatosis [74]. Lastly, outside of the realm of cell-based immunotherapy, there has been a recent development of multi-valent TRAIL construct. Among many different variants, one study generates TRAIL trimer dimerization by fusing Fc portion of human IgG1 as a dimerization domain. This recombinant protein provides in total of 6 binding domain to induce a superior clustering and has since entered phase 1 clinical trial [75].
Clinical Trials
Despite rapid development in creative and novel approaches to overcome TRAIL resistance, not many pre-clinical studies have since entered the stage of clinical trials yet. Previous clinical trials have largely failed to synergize with other existing chemotherapies and to extend cancer patient survival significantly. We here summarized some of the most current or ongoing oncology clinical trials utilizing the principles of TRAIL-induced apoptosis (see Table 2).
Table 2:
Clinical trials and Applications of TRAIL-induced Apoptosis in Cancer Treatments.
| Cancer Type | Intervention | Outcome | Status | Clinical Trial Identifier |
|---|---|---|---|---|
| Peritoneal carcinomatosis | SCB-313 (recombinant human TRAIL-Trimer Fusion Protein) | Ongoing, recruiting | Phase 1 | NCT03443674 |
| Non-small cell lung Cancer | AMG 951 (rhApo2L/TRAIL) with or without chemo | Terminated | Phase 2 | NCT00508625 |
| Non-small cell lung Cancer | Recombinant human Apo-2 ligand (Injection) | Ongoing, not recruiting | Phase 3 | NCT03083743 |
| Follicular and Low-grade CD20+ B-cell Non-Hodgkin’s Lymphoma | Combination of dulanermin (TRAIL ligand) with rituximab | Terminated | Phase 2 | NCT00400764 |
| Metastatic or recurrent colorectal cancer | Dulanermin administered in combination with the FOLFOX regimen and bevacizumab | Completed | Phase 1 | NCT00873756 |
| Metastatic, triple negative breast cancer | Combination of tigatuzumab with abraxane | Completed | Phase 2 | NCT01307891 |
| Advanced colorectal Cancer | Combination of DS-8273a with nivolumab (anti-PDl) | Terminated | Phase 1 | NCT02991196 |
| Unresectable stage III or IV melanoma | Combination of DS-8273a with nivolumab | Ongoing, recruiting | Phase 1 | NCT02983006 |
| Metastatic renal Cancer | 2G-1 TCR retroviral vector-transduced lymphocytes IV transfusion | Terminated | Phase 1 | NCT00923390 |
| Advanced cervical Cancer | Mapatumumab, cisplatin and radiotherapy | Completed | Phase 2 | NCT01088347 |
| Lung adenocarcinoma | TRAIL-expressing Mesenchymal stem cell (MSC) | Ongoing, recruiting | Phase 1/2 | NCT03298763 |
| Relapsed or refractory NSCLC | TRAIL-R1 mAb | Completed | Phase 2 | NCT00092924 |
| Advanced solid tumor or lymphoma | DS-8273a | Completed | Phase 1 | NCT02076451 |
| Various types of cancer | Tigatuzumab (TRA-8) (anti-DR5) | VARIED | Phase 1/2 | Breast cancer-(NCT01307891) Lymphomas-(NCT00320827) Ovarian cancer-(NCT: 00945191) Liver cancer-(NCT01033240) Pancreatic cancer-(NCT00521404) |
| Pediatric solid tumor | Lexatumumab (HGS-ETR2) | Terminated | Phase 1 | NCT00428272 |
| Various types of cancer | Conatumumab (AMG 655) | VARIED | Phase 2 | Ovarian cancer-(NCT01940172) Colorectal cancer-(NCT00630786) Sarcoma-(NCT01327612) |
| Multiple Myeloma | Mapatumumab and Bortezomib | Completed | Phase 2 | NCT00315757 |
| Advanced Hepatocellular Carcinoma | CS-1008 (DR5 antibody agonist) and sorafenib | Completed | Phase 2 | NCT01033240 |
| Metastatic Pancreatic cancer | AMG655 or AMG 479 and Gemcitabine | Completed | Phase 1b/2 | NCT00630552 |
| Unresectable Soft tissue Sarcoma | AMG 655 and Doxorubicin | Completed | Phase 1b/2 | NCT00626704 |
| Ovarian cancer | CS-1008 and Carboplatin/paclitaxel | Completed | Phase 2 | NCT00945191 |
| Pre-treated advanced solid and hematological tumors | Hexavalent TRAIL receptor agonist ABBV-621 | Ongoing | Phase 1 | NCT03082209 |
Main challenges that exist currently for TRAIL therapy in clinics include the weak apoptosis-inducing potency and unfavorable pharmacokinetics of TRAIL in vivo. Recombinant TRAIL that simultaneously targets both DR4 and DR5 can result in a stronger apoptotic signaling than either DR4/5 specific agonist antibody [76]. Such rhTRAIL has been tested in phase 1b/2 randomized clinical trials in combination with proposed sensitizing compounds such as Rituximab. The study showed good tolerance but lack of improved responses with the addition of rhTRAIL Dulanermin in Non-Hodgkin’s Lymphoma [77]. Moreover, it appears that DR4 and DR5 have varying functions in different cancer cell types. For example, colon and breast cancer cells have been shown to rely more on DR5 for apoptosis induction whereas lymphoma and pancreatic cancer cells use DR4 [77-79]. For DR4 agonistic antibodies, the only widely tested one in clinical trials is mapatumumab whereas for DR5 agonistic antibodies, there are several such as conatumumab, lexatumumab and tigatuzumab [80-84]. For second-generation TRAIL receptor agonist, there is currently one ongoing clinical trial that utilizes a multi-valent TRAIL receptor agonist ABBV-621 which is a fusion protein consisting of six receptor binding domains of TRAIL fused to the Fc-domain of a human immunoglobulin G1 (IgG1) antibody [75, 85]. Recent trial report has indicated evidence of antitumor activity. [85] For most currently concluded clinical trials, the addition of any of these agonists to various existing chemotherapies has not shown statistically significant anticancer efficacy although almost all of them are well-tolerated [76]. While it is disappointing to see the discrepancy between pre-clinical effectiveness and poor clinical potency, it is certainly possible to overcome the resistance through new pre-clinical developments that both enhance TRAIL potency and identify and screen for genetic locus associated for TRAIL resistance in patients before initiating future clinical trials.
Concluding Remarks
TRAIL represents one of the most promising approaches to treat cancer owing to its specificity, safety and encouraging results in vitro. However, its complex interactions with other pathways in a diverse, cell-dependent manner and its widespread resistance pose as significant hurdles for its development of clinical use. Nonetheless, attempts to understand and circumvent the resistance mechanism remain enthusiastic in both clinical and pre-clinical studies (see outstanding questions). The dual roles of anti- and pro-tumor effect of TRAIL in different cell types suggest that more research is needed to understand how TRAIL pathway cross-talks in the context of large signaling network. Moreover, despite the lack of efficacy in previous clinical trials, genetic epidemiological studies and meta-analyses exploring the genetic signatures of TRAIL-responders v.s. TRAIL-non-responders could aid in the stratification of candidates to unmask potential therapeutic effects. CRISPRi or ORF transduction aided with computational modelling has also been recently developed to look for the best combinatorial small molecule targeted therapies with minimized likelihood of relapse and such technique could potentially be deployed in TRAIL resistance study as well. In addition, tumor microenvironment and rapid evolutionary plasticity by tumor heterogeneity are two other frontiers that could potentially hold the explanations for the disappointing clinical results since they are usually poorly modelled in tumor cell lines. Advances in bioengineering such as cancer-on-a-chip will allow studying multiple physical and biological parameters not easily accessible in traditional 2D or 3D cultures. Finally, immune system represents another important way of modulating TRAIL efficacy and combinations with immunotherapy remain a large unexploited area of research.
Outstanding Questions:
What are the molecular determinants for both pro- and anti- tumor effects of TRAIL?
What are some additional molecular mechanisms that can lead to TRAIL resistance?
What are the difference in genetic signatures between TRAIL responders and TRAIL non-responders in clinical trials? Why do some patients respond to TRAIL therapy while others do not?
Why is there a huge disparity between TRAIL efficacy in pre-clinical studies v.s. clinical studies. Do parameters not available in traditional 2D or 3D cultures play a role in TRAIL sensitivity?
How can effective immunotherapies be designed based on synergistic efficacy between TRAIL and immune cell response?
Highlights:
TRAIL is a promising non-toxic highly tumor-specific apoptosis-inducing agent that has tremendous potential for cancer treatment.
New delivery systems have been developed to enhance TRAIL half-life and its potency against tumor growth in vivo.
Widespread resistance to TRAIL-induced apoptosis can happen on almost every level of extrinsic and intrinsic pathways and prevents TRAIL therapeutic efficacy against a variety of cancer types
TRAIL resistance can be circumvented through multiple means, including the use of genotoxic agents, epigenetic modulators, synthetic small molecules or antibodies, and can take advantage of combination therapy as well as immunotherapy.
Recent Investigation of TRAIL has generated a great number of promising pre-clinical that have the potential to be translated into clinics.
Acknowledgments
This work was supported by NIH grants R01-CA201148 and by DoD grant LC180495 (K.S.).
Glossary:
- Apoptosis
A type of programmed cell death that could result from two pathways: the extrinsic apoptosis pathway and the intrinsic apoptosis pathway. The former is mediated by TNF receptor superfamily that includes the canonical death receptors and the latter is involves mitochondrial proteins and activation of p53. Although acting independently of each other, research has shown major cross-talks between the pathways in mediating apoptosis and TRAIL resistance.
- Caspases
Cysteine-aspartic proteases are a family of enzymes central to apoptosis. They are usually sub-divided into initiator caspases and effector caspases. The former can cleave the pro-caspase (inactive) form of the latter and trigger apoptosis cascade.
- Death Receptors and Decoys
Endogenous receptors for the TRAIL ligand. Death receptors have the intracellular domain and can initiate extrinsic cellular apoptosis upon activation by TRAIL whereas decoys do not have intracellular domains and can sequester TRAIL and inhibit apoptosis.
- Immunotherapy
a class of treatment that activates or suppresses immune system. It has recently become the interest of cancer therapeutics. It often involves antibodies, immunomodulating factors and immune cells.
- MicroRNA
A type of small non-coding RNA that has implications in transcriptional regulation of genes.
- Nanobody
a fragment of antibody consisting of a single monomeric variable domain. It retains the specificity of the antibody binding ability but has a much smaller molecular weight.
- TRAIL
A cytokine ligand in the TNF superfamily that could be recognized by death receptor 4/5 (DR4/5) and initiate extrinsic apoptosis pathway.
- Tumor Microenvironment (TME)
Broadly defined as the environment around a tumor, usually referred to immune cells, tumor-associated fibroblasts, cytokines, extracellular matrix and blood vessels etc.
Footnotes
Conflict of interests
K.S. owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell-based therapies for cancer. K.S.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners Healthcare in accordance with their conflict of interest policies. The other authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D & Weinberg RA Hallmarks of Cancer: The Next Generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Ralff MD & El-Deiry WS TRAIL pathway targeting therapeutics. Expert Review of Precision Medicine and Drug Development 3, 197–204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs SF et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med 16, 242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walczak H et al. Tumoricidal Activity of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Vivo. Nat Med. 5, 157–163 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A et al. Safety and Antitumor Activity of Recombinant Soluble Apo2 Ligand. J Clin. Invest 104, 155–162 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuckey DW & Shah K TRAIL on trial: preclinical advances in cancer therapy. Trends in Molecular Medicine 19, 685–694 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guimrães PPG et al. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 12, 912–931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K et al. Nanoparticle-Mediated Target Delivery of TRAIL as Gene Therapy for Glioblastoma. Adv. Healthcare Mater 4, 2719–2726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke S et al. Gold nanoparticles enhance TRAIL sensitivity through Drp1-mediated apoptotic and autophagic mitochondrial fission in NSCLC cells. IJN. 12, 2531–2551 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Hu C-MJ et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 526, 118–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell MJ et al. Polymeric mechanical amplifiers of immune cytokine-mediated apoptosis. Nat Commun. 8, 14179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren H, Zhou L, Liu M, Lu W & Gao C Peptide GE11–Polyethylene Glycol–Polyethylenimine for targeted gene delivery in laryngeal cancer. Med Oncol 32, 185 (2015). [DOI] [PubMed] [Google Scholar]
- 13.De Miguel D et al. High-order TRAIL oligomer formation in TRAIL-coated lipid nanoparticles enhances DR5 cross-linking and increases antitumour effect against colon cancer. Cancer Letters 383, 250–260 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Nair PM et al. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc Natl Acad Sci USA 112, 5679–5684 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura Y & Maeda H A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 7 6387–6392 (1986). [PubMed] [Google Scholar]
- 16.Stein MN et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin Cancer Res 23, 4163–4169 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen JE et al. Targeting TRAIL Death Receptor4 with Trivalent DR4 Atrimer Complexes. Molecular Cancer Therapeutics 11, 2087–2095 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Twomey JD, Kim S-R, Zhao L, Bozza WP & Zhang B Spatial dynamics of TRAIL death receptors in cancer cells. Drug Resistance Updates 19, 13–21 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Haselmann V et al. Nuclear Death Receptor TRAIL-R2 Inhibits Maturation of let-7 and Promotes Proliferation of Pancreatic and Other Tumor Cells. Gastroenterology 1, 278–290 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Reis CR, Chen P-H, Bendris N & Schmid SL TRAIL-death receptor endocytosis and apoptosis are selectively regulated by dynamin-1 activation. Proc Natl Acad Sci USA 114, 504–509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21..Zhu J, Zhou Q & Tan S Targeting miRNAs associated with surface expression of death receptors to modulate TRAIL resistance in breast cancer. Cancer Letters 383, 154–160 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Lu T, Shao N & Ji C Targeting microRNAs to modulate TRAIL-induced apoptosis of cancer cells. Cancer Gene Ther 20, 33–37 (2013). [DOI] [PubMed] [Google Scholar]
- 23.O’Leary L et at Decoy receptors block TRAIL sensitivity at a supracellular level: the role of stromal cells in controlling tumour TRAIL sensitivity. Oncogene 35, 1261–1270 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Irmler M et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Crowder RN & El-Deiry WS CASPASE-8 REGULATION OF TRAIL-MEDIATED CELL DEATH. Experimental Oncology 5 (2012). [PubMed] [Google Scholar]
- 26.Jin Z et al. Cullin3-Based Polyubiquitination and p62-Dependent Aggregation of Caspase-8 Mediate Extrinsic Apoptosis Signaling. Cell 137, 721–735 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Bellail AC, Olson JJ, Yang X, Chen ZJ & Hao C A20 Ubiquitin Ligase–Mediated Polyubiquitination of RIP1 Inhibits Caspase-8 Cleavage and TRAIL-induced Apoptosis in Glioblastoma. Cancer Discovery 2, 140–155 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powley IR, Hughes MA, Cain K & MacFarlane M Caspase-8 tyrosine-380 phosphorylation inhibits CD95 DISC function by preventing procaspase-8 maturation and cycling within the complex. Oncogene 35, 5629–5640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P et al. Targeting CDK1 and MEK/ERK Overcomes Apoptotic Resistance in BRAF-Mutant Human Colorectal Cancer. Mol Cancer Res 16, 378–389 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Haag C et al. Identification of c-FLIPL and c-FLIPS as critical regulators of death receptor-induced apoptosis in pancreatic cancer cells. Gut 60, 225–237 (2011). [DOI] [PubMed] [Google Scholar]
- 31.McLornan DP et at Prognostic Significance of TRAIL Signaling Molecules in Stage II and III Colorectal Cancer. Clinical Cancer Research 16, 3442–3451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCourt C et al. Elevation of c-FLIP in Castrate-Resistant Prostate Cancer Antagonizes Therapeutic Response to Androgen Receptor-Targeted Therapy. Clinical Cancer Research 18, 3822–3833 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golks A, et al. The C-FLIP-NH2 terminus (p22-FLIP) induces NFKB activation. J Exp Med. 203:1295–1305 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima A, et al. An antiapoptotic protein, C-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. EMBO J. 25:5549–59 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao C et al. Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Experimental Cell Research 304, 244–255 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Higuchi H et al. Bile Acids Stimulate cFLIP Phosphorylation Enhancing TRAIL-mediated Apoptosis. J. Biot Chem. 278, 454–461 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Trivedi R & Mishra DP Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front. Oncol. 5, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hari Y, Harashima N, Tajima Y & Harada M Bcl-xL inhibition by molecular-targeting drugs sensitizes human pancreatic cancer cells to TRAIL. Oncotarget 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stilgenbauer S, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 17, 768–778 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Tolcher AW, et al. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother. Pharmacol 76, 1025–1032 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Park SH et al. Down-Regulation of Survivin by Nemadipine-A Sensitizes Cancer Cells to TRAIL-induced Apoptosis. Biomolecules and Therapeutics 21, 29–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraei R et al. The role of XIAP in resistance to TNF-related apoptosis-inducing ligand (TRAIL) in Leukemia. Biomedicine & Pharmacotherapy 107, 1010–1019 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Benitez JA, et al. PTEN regulates glioblastoma oncogenesis through chromatin-associated complexes of DAXX and histone H3.3. Nat. Commun 8, 15223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, et al. Activation of the Akt Survival Pathway Contributes to TRAIL Resistance in Cancer Cells. Plos One 5, e10226 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Karstedt S et al. Cancer Cell-Autonomous TRAIL-R Signaling Promotes KRAS-Driven Cancer Progression, Invasion, and Metastasis. Cancer Cell 27, 561–573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azijli K et al. Kinome profiling of non-canonical TRAIL signaling reveals RIP1-Src-STAT3-dependent invasion in resistant non-small cell lung cancer cells. Journal of Cell Science 125, 4651–4661 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Di X, et al. Accumulation of autophagosomes in breast cancer cells induces TRAIL resistance through downregulation of surface expression of death receptors 4 and 5. Oncotarget 4, 1349–1364. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Looff M, de Jong S & Kruyt FAE Multiple Interactions Between Cancer Cells and the Tumor Microenvironment Modulate TRAIL Signaling: Implications for TRAIL Receptor Targeted Therapy. Front. Immunol 10, 1530 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar Hira S, Mondal I, Bhattacharya D & Manna PP Downregulation of endogenous STAT3 augments tumoricidal activity of interleukin 15 activated dendritic cell against lymphoma and leukemia via TRAIL. Experimental Cell Research 327, 192–208 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Liguori M et al. Functional TRAIL receptors in monocytes and tumor-associated macrophages: A possible targeting pathway in the tumor microenvironment. Oncotarget 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou J-M et al. IL-35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fingas CD et al. Myofibroblast-derived PDGF-BB promotes hedgehog survival signaling in cholangiocarcinoma cells. Hepatology 54, 2076–2088 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartwig T et al. The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Molecular Cell 65, 730–742.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kretz AL et al. TRAILblazing Strategies for Cancer Treatment. Cancers 11, 456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willms A et al. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS ONE 14, e0214847 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh AK et al. Dual targeting of MDM2 with a novel small-molecule inhibitor overcomes TRAIL resistance in cancer. Carcinogenesis. 37 1027–1040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su RY, et al. 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and TNF alpha-induced cytotoxicity in colon cancer cells through AMP-activated protein kinase signaling, Mol. Cancer Ther 6 1562–1571. (2007). [DOI] [PubMed] [Google Scholar]
- 58.Elmallah MIY & Micheau O Epigenetic Regulation of TRAIL Signaling: Implication for Cancer Therapy. Cancers 11, 850 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillespie S, et al. Bim plays a crucial role in synergistic induction of apoptosis by the histone deacetylase inhibitor SBHA and TRAIL in melanoma cells. Apoptosis 11, 2251–2265 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Facchetti F, et al. Modulation of pro- and anti-apoptotic factors in human melanoma cells exposed to histone deacetylase inhibitors. Apoptosis 9, 573–582 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Chueh AC, et al. ATF3 repression of BCL-XL determines apoptotic sensitivity to HDAC inhibitors across tumor types. Clin. Cancer Res 23, 5573–5584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straszewski-Chavez SL, et al. XAF1 mediates tumor necrosis factor-alpha-induced apoptosis and X-linked inhibitor of apoptosis cleavage by acting through the mitochondrial pathway. J. Biol. Chem 282, 13059–13072 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Lemke J et al. Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death & Differentiation 21, 491–502 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menke C, et al. Distinct TRAIL Resistance Mechanisms Can Be Overcome by Proteasome Inhibition but not Generally by Synergizing Agents. Cancer Res. 71: 1883–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imai K & Takaoka A Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6, 714–727 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Zhu Y et al. Bi-specific molecule against EGFR and death receptors simultaneously targets proliferation and death pathways in tumors. Sci Rep 7, 2602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi SH et al. Antiangiogenic Variant of TSP-1 Targets Tumor Cells in Glioblastomas. Molecular Therapy 23, 235–243 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Booth NL et al. A cell-based high-throughput screen to identify synergistic TRAIL sensitizers. Cancer Immunol Immunother 58, 1229–1244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor DJ, Parsons CE, Han H, Jayaraman A & Rege K Parallel screening of FDA-approved antineoplastic drugs for identifying sensitizers of TRAIL-induced apoptosis in cancer cells. BMC Cancer 11, 470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beyer et al. Interactions of Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL) with the Immune System: Implications for Inflammation and Cancer. Cancers 11, 1161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hendriks D et al. Programmed Death Ligand 1 (PD-LI)-targeted TRAIL combines PD-L1-mediated checkpoint inhibition with TRAIL-mediated apoptosis induction. OncoImmunology 5, e1202390 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Bruyn M et al. Cell Surface Delivery of TRAIL Strongly Augments the Tumoricidal Activity of T Cells. Clinical Cancer Research 17, 5626–5637 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Wiersma VR et al. C-type lectin-like molecule-1 (CLLI)-targeted TRAIL augments the tumoricidal activity of granulocytes and potentiates therapeutic antibody-dependent cell-mediated cytotoxicity. mAbs 7, 321–330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song X et al. Secretory TRAIL-Armed Natural Killer Cell–Based Therapy: In Vitro and In Vivo Colorectal Peritoneal Carcinomatosis Xenograft. Mol Cancer Ther 15, 1591–1601 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gieffers C et al. APG350 Induces Superior Clustering of TRAIL Receptors and Shows Therapeutic Antitumor Efficacy Independent of Cross-Linking via Fc Receptors. Molecular Cancer Therapeutics 12, 2735–2747 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Lemke J, von Karstedt S, Zinngrebe J & Walczak H Getting TRAIL back on track for cancer therapy. Cell Death Differ 21, 1350–1364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheah CY et al. Dulanermin with rituximab in patients with relapsed indolent B-cell lymphoma: an open-label phase 1b/2 randomised study. The Lancet Haematology 2, e166–e174 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Kelley RF, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem 280: 2205–2212 (2005). [DOI] [PubMed] [Google Scholar]
- 79.MacFarlane M, et al. TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer Res. 65: 11265–11270 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Lemke J, et al. TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5. J Mol Med (Berl) 88: 729–740 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Belch A, et al. A multicenter randomized phase ii trial of mapatumumab, a TRAIL-R1 agonist monoclonal antibody, in combination with bortezomib in patients with relapsed/refractory multiple myeloma (MM). Blood 116: abstracts 5031 (2010). [Google Scholar]
- 82.Fuchs CS, et al. TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: A randomized phase 1b/2 trial. Cancer 119: 4290–4298 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Merchant MS, et al. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol 30: 4141–4147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reck M, et al. A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naive metastatic/unresectable non-small cell lung cancer. Lung Cancer 82: 441–448 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Ratain MJ, et al. Phase 1, first-in-human study of TRAIL receptor agonist fusion protein ABBV-621. Journal of Clinical Oncology 37, no. 15_suppl (2019). [Google Scholar]
- 86.Niemoeller OM, & Belka C Radiotherapy and TRAIL for cancer therapy. Cancer Lett. 332, 184–193 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Zhao J, Lu Y & Shen H-M Targeting p53 as a therapeutic strategy in sensitizing TRAIL-induced apoptosis in cancer cells. Cancer Letters 314, 8–23 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Wong SHM et al. The TRAIL to cancer therapy: Hindrances and potential solutions. Critical Reviews in Oncology/Hematology 143, 81–94 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Ricci MS et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or Sorafenib Sensitizes Resistant Human Cancer Cells to TRAIL-Induced Death. Cancer Cell 12, 66–80 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Katz SI et al. Sorafenib inhibits ERK1/2 and MCL-1L phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma. Cancer Biology & Therapy 8, 2406–2416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puduvalli VK et al. TRAIL-induced apoptosis in gliomas is enhanced by Akt-inhibition and is independent of JNK activation. Apoptosis 10, 233–243 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]