Abstract
The relative value of and motivation for abused drugs often increases with drug experience and differs based on drug availability. Here, we determined how different intake patterns of fentanyl, a μ-opioid agonist, alter economic demand for fentanyl, and how 5-HT2A receptor stimulation affects economic demand for fentanyl. We used a within-session demand threshold procedure to characterize changes in economic demand for fentanyl before and after intermittent or continuous access schedules. We subsequently tested the acute effects of 5-HT2A receptor stimulation with psychedelic 2,5-dimethoxy-4-iodoamphetamine (DOI) on economic demand for fentanyl. Extended fentanyl experience with both intermittent and continuous schedules increased fentanyl consumption at low cost (Q0), increased total fentanyl consumption, and decreased demand elasticity (α), indicating both schedules elevated motivation to self-administer fentanyl. Overall, the two schedules produced similar alterations in economic demand for fentanyl, although low-cost consumption (Q0) increased more in the continuous access group. Systemic injections of DOI (0.0–0.4mg/kg, i.p.) dose-dependently produced economic demand changes in the opposite direction produced by fentanyl experience. DOI decreased motivation (increased ‘α’), decreased Q0, and decreased total fentanyl consumption. The selective 5-HT2A antagonist, M100907 (0.3mg/kg, i.p.), blocked the effects of DOI, indicating that DOI is acting through 5-HT2A receptors to alter economic demand for fentanyl. In an economic food demand experiment, DOI (0.4mg/kg) also increased demand elasticity and reduced food consumption. These results demonstrate that both intermittent and continuous fentanyl experience raise the economic demand for fentanyl and acute 5-HT2A receptor activation reduces economic demand for fentanyl and food.
Keywords: demand, economic, fentanyl, intermittent, opioid, 5-HT2A
Introduction
Opioid Use Disorder (OUD) is common in the US and its prevalence has brought considerable attention to the opioid overdose epidemic1. The widespread use of μ-opioid agonist, fentanyl, and its derivatives now contributes to a significant fraction of total opioid overdoses2. Treatments for OUD currently consist of other opioids, used either to fully activate (methadone), partially activate (buprenorphine), or fully block (naltrexone) u-opioid receptors. Despite completely different receptor dynamics, all three drugs markedly reduce the economic value of abused opioids3, a primary feature of their efficacy in treating OUD. However, opioid therapeutics suffer from a number of challenges, including dependence4 and compliance5. Researchers are beginning to explore other, non-opioid therapeutics for OUD, including serotonin receptor agonists such as lorcaserin6, a 5-HT2C agonist that reduces opioid self-administration preclinically. Several clinical studies suggest the use of 5-HT2A agonists for alcohol use disorder7,8 and OUD9 may be beneficial. Also, the US Food and Drug Administration (FDA) recently designated the 5-HT receptor agonist, psilocybin, as a breakthrough treatment following clinical trials demonstrating its rapid antidepressant effects10,11, further sparking interest in the potential use of these compounds to treat other mental health disorders. In this study, we examine the effects of 5-HT2A receptor stimulation, using the psychedelic 2,5-dimethoxy-4-iodoamphetamine (DOI), on the economic value of fentanyl in rats with fentanyl self-administration experience.
Researchers posit that experience with drugs of abuse contributes to the addiction process by increasing the economic demand for the drug12. Measures of economic demand for cocaine, which change with extended cocaine experience, predict other addiction-like measures including priming- and cue-induced reinstatement, as well as compulsive (punished) drug-taking13. Prior demand studies demonstrate that unique schedules of reinforcement affect specific economic demand parameters differently14,15. One such economic parameter is demand elasticity, ‘α’, which captures the rate at which consumption falls with increasing price. Relative to continuous long-access schedules, intermittent access reinforcement schedules decrease demand elasticity for cocaine14. Intermittent access schedules are thought to model the repeated rise and fall of brain drug levels that occur in users that do not continuously re-dose15. Continuous access models result in more stable brain drug concentrations, and in the case of cocaine, lead to desensitization, tolerance and higher levels of cocaine consumption at low prices (‘Q0’)14. We aimed to determine whether 1) intermittent access to fentanyl self-administration would similarly decrease demand elasticity, ‘α’, relative to continuous access schedules, and 2) continuous access to fentanyl self-administration would increase consumption at low prices, ‘Q0’, relative to intermittent access.
Previous work has characterized economic demand for fentanyl16,17, but changes in economic demand following different schedules of fentanyl reinforcement have not been examined. Generally, we predicted that additional fentanyl experience in either continuous or intermittent schedules would increase the economic value of fentanyl, expressed as increased fentanyl consumption at low prices (Q0), and decreased demand elasticity, ‘α’. We predicted that intermittent access (INT) to fentanyl self-administration (SA) would decrease demand elasticity to a greater degree than continuous access (CON) to fentanyl SA. After fentanyl experience, we predicted that 5-HT2A/2C agonist, DOI, would decrease the economic value of fentanyl (decrease Q0 and increase α). We also aimed to determine whether DOI exerts its effects through activation of the 5-HT2A receptor, and if these effects were specific to opioid reward.
To test these predictions, we utilized a within-session fentanyl demand threshold procedure18,19 in which we decreased the dose of fentanyl across bins, effectively increasing the price of the drug across the session. This procedure allows for rapid and repeated assessment of economic demand parameters within and between subjects, prior to and following prolonged continuous or intermittent access. This approach is suitable for comparing the increase in economic value of fentanyl in these self-administration models, and subsequently determining the efficacy of 5-HT2A stimulation to decrease fentanyl economic value. This is a preclinical step toward assessing 5-HT2A agonists as potential therapeutics for OUD.
Materials and Methods
Subjects
We received 8-week-old male Sprague Dawley (N=18) rats (Charles River Labs) and maintained them double-housed on a reverse 12-h:12-h light-dark cycle (lights off at 9:00 am) for one week, and then individually housed them following intravenous catheter surgeries. All testing was performed during the dark cycle. We fasted rats overnight (20 hours) prior to the first acquisition session. Subsequently, rats had ad libitum access to standard rat chow (Purina). We performed the experiments in accordance to the “Guide for the care and use of laboratory animals” (8th edition, 2011, US National Research Council) and experimental protocols were approved by the University of Maryland (UMB) Institutional Animal Care and Use Committee. We excluded rats from further testing if they failed to gain weight (N=2, infection) or lost catheter patency (N=10). We checked catheter patency periodically via injection with 100ul sodium methohexital (“Brevital”, 1 mg), and examined rats for loss of muscle tone.
Catheterization surgeries
After a week of acclimatization, we anesthetized nine-week-old rats with isoflurane (5% induction, 2–3% maintenance) and implanted catheters into the right jugular vein. See the supplementary methods for surgical details.
Apparatus
All experiments used self-administration chambers (Med Associates) equipped with two retractable levers (7 cm above the grid floors). A red light was positioned on the top of the back wall, opposite the levers, and its illumination served as discriminative stimulus signaling drug availability, (i.e. off during timeouts and during drug delivery). The active lever was counterbalanced across rats and remained consistent throughout training. Drug was delivered via a syringe pump (Med Associates) and 20 ml syringes containing fentanyl solutions. Drug delivery was accompanied by a compound tone (2900 Hz) plus light (7.5-W white light cue located above the active lever) cue that signaled for the duration of the infusion. We controlled the chambers and collected data using MedPC software (Med Associates).
Drugs
We purchased fentanyl citrate from Sigma, dissolved it in sterile bacteriostatic saline at 12.5mg/ml, and diluted the drug further in sterile saline to a concentration of 44.65 μg/ml/kg. Injection duration controlled the dose, and the rate of infusion was constant at 28 μl/second. Injection duration was 2 seconds during acquisition and intermittent access phases of the experiment (2.5μg/kg/injection, using the weight of the salt - fentanyl citrate). We based the acquisition doses on prior studies20 and our pilot studies in which we observed robust drug lever responding at this dose. We obtained 2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) from Cayman Chemicals, dissolved it in saline, and injected it i.p. at a volume of 1.0 ml/kg. We obtained M100907 from MedChem Express and dissolved it in absolute ethanol (1mg/100μl), followed by the addition of emulphur oils at equal volume. We mixed these vehicles thoroughly with sonication and vortexing before adding saline at 18X the volume of ethanol. M100907 solutions were prepared the day of use and injected at a volume of 0.6 ml/kg at a final concentration of 0.5mg/ml. The M100907 dose of 0.3mg/kg was based on a previous study21 using this dose for effective 5-HT2A blockade. Vehicle for DOI was saline and vehicle for M100907 experiments was 5% EtOH, 5% Emulphur, 90% Saline.
Training phase
We housed all rats in the animal facility and transferred them to the self-administration chambers prior to the five self-administration sessions (sessions 1–5) and returned them to the facility at the end of the 150-minute sessions. Active lever responses resulted in a 2 second infusion and compound light/tone stimuli. Training session intravenous fentanyl infusions were given on an FR1 schedule (2.5μg/kg/injection) and included a 20 second timeout following each infusion. Inactive lever responses were recorded but had no effect. We recorded the timestamp of each lever press and infusion. A total of 17 rats completed this phase.
Demand Phase
Box layouts were identical to all other phases. Demand thresholding sessions were 150 minutes, consisting of ten 15-minute bins. Rats self-administered fentanyl on an FR1 schedule, but with no timeout beyond the length of the injection itself. Successive bins were marked by decreasing doses of fentanyl on a quarter log scale, achieved by reducing the duration of the injection across bins. The red discriminative stimulus light was on, except during the length of an infusion. Infusion duration was also signaled by an equal duration compound light and tone stimulus. The first four demand sessions (sessions 6–9) were completed on the following dose schedule, in units of μg/kg/injection: 4.375, 2.5, 1.425, 0.8125, 0.45, 0.25, 0.1375, 0.075, 0.05, 0.025. For the remainder of the demand sessions (baseline sessions 10–12; and test sessions 18, and 28–38), doses were shifted one quarter log lower: 2.5, 1.425, 0.8125, 0.45, 0.25, 0.1375, 0.075, 0.05, 0.025, 0.0125. We made this shift to better bracket the range of PMax values across the rats and prevent excessive drug loading at the beginning of the demand sessions. We recorded the number of infusions in each bin as the primary measure during this phase.
Intermittent vs. Continuous Access Phase
Following three baseline thresholding sessions at the lower dose range (sessions 10–12), we split rats into 2 groups, continuous access (CON) and intermittent access (INT). Groups were balanced for baseline α, Q0, total fentanyl consumption, and first bin fentanyl consumption measured during these last three baseline sessions. The CON group received fentanyl, 2.5 μg/kg/injection, on an FR1 schedule for 150 minutes under identical conditions as training, with the exception that the 20s timeout was replaced with a timeout identical to the length of the injection (2 s). The CON group received this training schedule for 14 sessions (sessions 13–17, 19–27). The INT group also received fentanyl at the training dose (2.5 μg/kg/injection) on an FR1 schedule (2 s timeout during infusion), but the drug was only available for 5 minute “ON” periods followed by 25 minute “OFF” periods. To facilitate acquisition of the new schedule and limit the potential impact of extinction learning in INT rats, during sessions 13–17, the levers retracted at the beginning of each OFF period and were inserted at the beginning of each ON period, with the 150-minute sessions ending after the 5th OFF period. During sessions 19–27, the levers remained inserted for the length of the 155 minute session, which ended after the 6th ON period. The red light was extinguished during OFF periods and illuminated during ON periods throughout all sessions, except during infusions, when the red light was off. All press and infusion timestamps were recorded for this phase of the experiment. We conducted three demand sessions to examine changes due to drug experience under the two schedules (session 18, & 28–29). A total of 11 rats (N=5–6/group) completed this phase.
5-HT2A Agonist and Antagonist Testing Phase
For 5-HT2A agonist and antagonist test sessions, we transferred rats to the behavioral room one hour prior to experiments to administer DOI and/or M100907 while in the testing room but still in their home cages. Following the INT/CON phase, we gave all rats alternating injections of saline (control) and ascending doses of 5-HT2A agonist, DOI (0.1, 0.2, and 0.4 mg/kg), 30 minutes prior to demand testing. To test the role of 5HT2A receptors in DOI action, we co-administered M100907 (0.3mg/kg, i.p., 50 min prior to testing) with DOI or saline (0.4mg/kg, i.p., 30 min prior to testing) in two demand sessions and conducted a final demand session with co-administration of both vehicles. We gave DOI doses in ascending order with at least two days off between successive doses to minimize tolerance. We counterbalanced the order of vehicle and DOI injections, such that half the rats were receiving DOI or vehicle before any particular demand session during this phase of the experiment (sessions 30–38). A total of N=10 rats completed the 0.1mg/kg phase, N=10 rats completed the 0.2 mg/kg phase, N=7 rats completed the 0.4 mg/kg phase, and N=5 rats completed the DOI/M100907 phase.
Demand Data Analysis
Primary data from demand sessions were the number of infusions earned in each bin. These data were transformed by the dose to give the consumption of fentanyl in each bin for each rat. The price of the drug was defined as the number of responses required to reach 12.5μg/kg (price units = responses/(12.5μg/kg)). Consumption for each bin is expressed in units of μg/kg/15min. For each session, we calculated the price bin with the maximum active presses (PMax) and the number of active presses in the PMax bin (OMax).
Before fitting the data to the demand equation, the lowest price bin (the first bin) was removed from the consumption data because of drug loading19,22. See the supplemental methods for details regarding binning of data.
We fitted the data to the exponentiated demand equation23:
Where:
k = logarithmic range of the positive consumption data = log10(ConsumptionMax)−log10(ConsumptionMin). This value was taken directly from the log10 range of the actual non-zero consumption data for each rat session (referred to as kfitted in supplemental methods).
Q0 = fitted parameter representing consumption at very low prices
α = fitted parameter representing demand elasticity
Q = consumption at a given price (consumption during a particular bin)
We fit the data in Matlab using the “fitnlm” function, which minimizes mean squared error between the actual values and fitted curve. See the supplement for further details on curve fitting and parameter extraction.
We calculated essential value (EV) for each session using the following formula:
Food Demand
A separate group of rats (N=6) was trained in a food demand procedure before being tested with DOI (See Supplemental Methods for details).
Statistical Analyses
Data were fitted in Matlab (Mathworks, Natick, MA), organized and transformed in Excel (Microsoft, Redmond, WA), and statistical analyses were performed in Prism (GraphPad, La Jolla, CA). To examine the effect of reinforcement schedule on fentanyl demand, for each demand measurement we baseline-normalized each rat’s individual data to their average measurement from baseline demand sessions 10–12. Similarly, to examine the effect of 5-HT2A drugs on fentanyl demand, for each demand measurement, we baseline-normalized each rat’s individual data to their average of all completed vehicle injection sessions. We plot all demand measures on a log2(MeasureSession/MeasureBaseline) scale to display data as fold change from baseline. We performed standard two-way (CON/INT experiments) or one-way (5-HT2A experiments) ANOVAS on the displayed measurements. In 5-HT2A drug experiments, significant main effects (ME) in one-way ANOVAs were followed by a post-hoc Dunnet’s multiple comparison correction procedure, comparing all doses to the vehicle average. In repeated measures tests, violations of sphericity were corrected for by using the Geisser-Greenhouse correction. We performed t-tests on planned comparisons between groups (CON vs. INT) for demand parameters between the baseline average (sessions 10–12) values and the average values of the final two demand tests (session 28–29), as well as on total fentanyl consumption across the CON vs. INT phases of the experiment. We excluded three demand sessions for low R2 values (R2<.25), and we excluded one rat from the final three demand sessions (36–38) due to highly erratic behavior during the final vehicle session (‘α’ exhibited >10 fold-change deviation from the average of all vehicle sessions). We note that performance during control demand sessions following vehicle was very stable otherwise (see Fig. S3A). Displayed data is mean +/− SEM.
Results
Intermittent vs. Continuous Access Training
Self-administration data for all rats is shown in Fig. 1 and Fig. S1, plotted by INT and CON groups. All rats rapidly acquired fentanyl self-administration as indicated by fentanyl infusions earned (Fig. 1A) and differential responding for active vs. inactive levers for each acquisition session (Fig. S1A). We found no effects of subsequent group assignment and no group X session interaction for fentanyl infusions, active presses, and inactive presses during acquisition. After 5 days of self-administration acquisition, rats began 7 days of baseline demand testing. We used performance during the last three days of baseline demand testing (sessions 10–12) to counterbalance subject assignment between INT and CON groups. An analysis including all CON and INT sessions (sessions 13–17; 19–27) indicated a trend for all rats to earn more fentanyl infusions across sessions (effect of session, F2.73,24.60=2.502, p=.088, Fig. 1), with CON rats earning more infusions than INT rats (main effect of schedule, F1,9=6.723, p<.05, Fig. 1). CON rats consumed more fentanyl than INT rats, summing across the first 14 CON/INT sessions (CON: 1,951 +/− 474 μg/kg vs. INT: 586 +/−53 μg/kg, p<.05, Fig. S1C inset), and across all 29 CON/INT sessions of the experiment (CON: 2778 +/− 613 μg/kg vs. INT: 1154 +/− 208 μg/kg, p<.05, Fig. S1C inset).
Figure 1:
A) Fentanyl infusions earned across all experimental phases (29 sessions). Each phase of the experiment is indicated above the corresponding session data. Data are shown for rats that completed all phases displayed (N=5–6/group). Phases (Acquisition, Demand, CON vs. INT) were analyzed separately. B-D) Comparison CON vs. INT phase: B) Average bout size across session for CON vs. INT groups. C) Average pause length across session for CON vs. INT groups. D) Latency to earn 1st infusion of session for CON vs. INT groups. E) Average number of presses during INT sessions across bins (sessions 19–27). Note scalloped responding between each pair of ON periods. A-D) “*” indicates main effect of group, 2-way RM ANOVA.
For both CON and INT schedules, cumulative response records indicate that all rats exhibited characteristic infusion bouts, defined as two or more consecutive infusions with inter-infusion intervals less than one minute, followed by pauses in fentanyl seeking (Fig. S1B). We found no difference in bout sizes between CON vs. INT groups, across groups or sessions (Fig. 1B). However, the structure of the INT schedule consisted of 5 min “ON” periods followed by 25 min “OFF” periods, forcing average pause lengths to be much longer in INT relative to CON conditions across sessions (ME of schedule, F1,9=59.13, p<0.0001, Fig. 1C). We also observed a group difference in latency to first injection, with INT rats earning their first fentanyl infusion significantly faster than CON rats (ME of schedule, F1,9=8.658, p<0.05, Fig. 1D). In the second phase of intermittent access when the levers remained extended for the length of the session (sessions 19–27), INT rats developed a scalloped pattern of active lever responding whereby pressing fell to very low levels in the first few minutes of the OFF period, and then rose to a maximum near the beginning of the next ON period (Fig. 1E). In summary, intermittent access resulted in significantly less fentanyl consumption than continuous access, shorter latencies to respond for fentanyl, and >3.5-fold longer pause times between fentanyl infusion bouts, without affecting average bout size.
Continuous vs. Intermittent Access: Effects on Economic Demand for Fentanyl
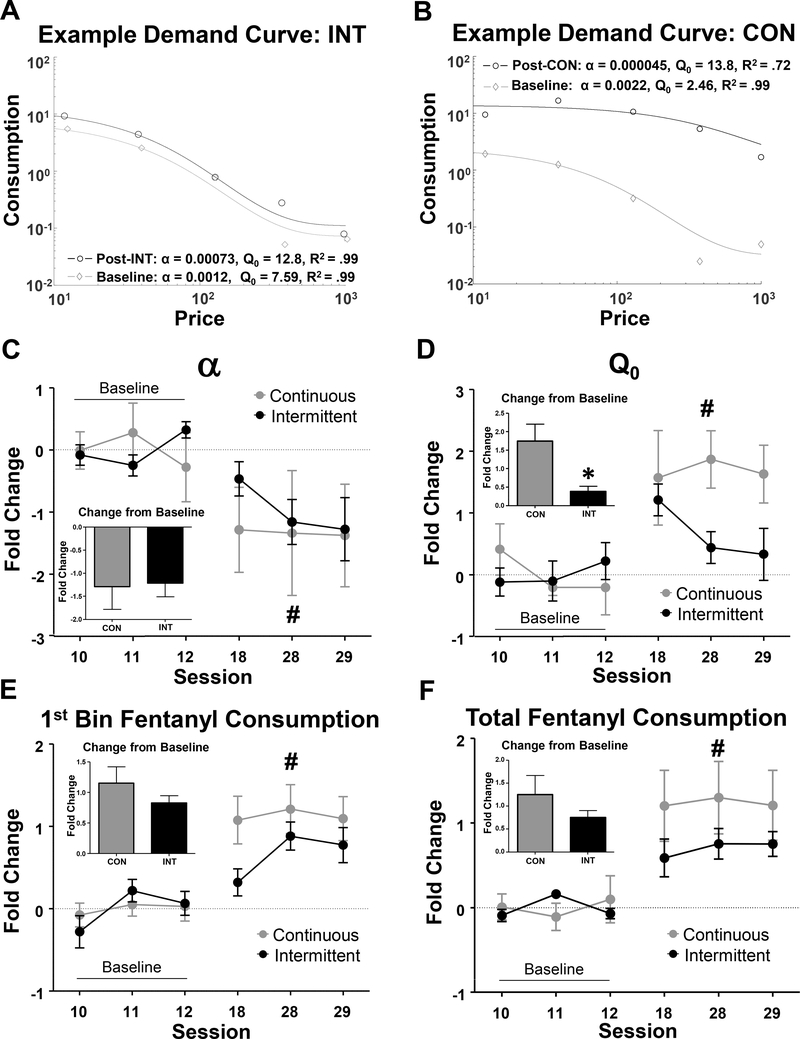
Example demand curves from CON and INT groups are shown in Fig. 2A,B. Fentanyl economic demand parameters during baseline (prior to introduction of CON and INT schedules) and post-schedule sessions are shown in Fig. 2C–F,S3A–E. Prior to initiating CON and INT schedules, rats showed stable fentanyl demand as indicated by two demand parameters, consumption at low price (Q0) and demand elasticity (α), which exhibit stability between baseline sessions and no differences between groups (Fig. 2C–D, Fig. S3). Following CON or INT training, we observed shifts in fentanyl economic demand parameters, with decreasing α and increasing Q0 across sessions, but no main effects of group or interactions (ME of session for α: F2.429,21.86 = 3.275, p<0.05; and ME of session for Q0: F2.71,24.38 = 5.393, p<0.01, Fig. 2C,D). We also observed main effects of session on 1st bin fentanyl consumption (F4.16,37.45 = 13.02, p<0.0001, Fig. 2E) and total fentanyl consumption (F2.45,22.07 = 9.905, p<0.0001, Fig. 2F) during demand testing sessions, but no group effects or interactions, indicating fentanyl consumption increased with self-administration experience, independent of schedule. We performed planned comparisons between CON and INT groups to examine group differences in the total change in economic demand parameters from baseline. For the last two days of demand testing, the only group difference was in consumption at low price (Q0), for which CON rats showed a greater increase from baseline Q0 than INT rats (ΔQ0 (fold-change): CON: 1.75 +/− 0.46; INT: 0.39 +/− 0.14, p<0.05. Fig. 2C–F, insets).
Figure 2:
Economic demand parameters from fentanyl demand sessions before and after CON vs INT access phase. A) Example pre- and post-INT access demand curve from a single rat. B) Example pre- and post CON access demand curve from a single rat. C-F) Log2 fold-change in indicated economic demand parameter across baseline (sessions 10–12) through post-schedule (sessions 18, 28–29) testing. Insets for each parameter show the average fold change from baseline (average of sessions 28–29 relative to average of three baseline sessions). ‘#’ indicates main effect of session, 2-way ANOVA. “*” indicates p<0.05 between CON/INT groups, 2-tailed t-test.
We did not observe any effects of session or group differences for PMax (the bin of maximum responding; Fig. S3A) or essential value (EV), which is related to PMax and often used as a normalized metric to compare reinforcing efficacy across reinforcers (see equation in methods) (Fig. S3B). We did observe a trend for an increase in OMax (the number of infusions obtained at PMax) across sessions (effect of session, F2.26,20.4=2.619, p=.09, Fig. S3C), with no group main effect or group X session interaction (Fig. S3C). In comparing responses/bin before and after experience, we found no significant differences between INT vs. CON groups, and collapsing across groups, we found a main effect of experience and bin, with no interaction (ME of experience, F1,20=12.03, p<.01; ME of bin, F3.91=2.97, p<.05, Fig S3F). In summary, experience with fentanyl self-administration decreased demand elasticity (α), increased consumption at low cost (Q0), and fentanyl consumption (1st bin and total), but did not affect EV or PMax. Consistent with our prediction, the increase in consumption at low cost, Q0, was greatest following CON access. Contrary to our prediction, decreased demand elasticity with fentanyl experience was independent of schedule.
5-HT2A Activation: Effects on Economic Demand for Fentanyl
After the final post-schedule demand session, we examined the effects of acute 5-HT2A receptor stimulation on economic demand for fentanyl in drug experienced rats. We conducted a within-subject dose-response with the 5-HT2A/2C agonist, DOI, and the selective 5-HT2A antagonist, M100907 (see Fig. 3A for timeline). In this phase, the data from both CON and INT rats were combined.
Figure 3:
Economic demand parameters from fentanyl demand sessions with DOI and/or M100907 administration. A) Timeline of 5HT2A agonist/antagonist testing phase. B) Example saline and 0.4mg/kg DOI curves from a single rat. C-F) Log2 fold-change in indicated economic demand parameter for each drug condition relative to the log-average of all vehicle sessions. DOI 0.1mg/kg (N=10), 0.2mg/kg (N=10), 0.4 mg/kg (N=7), M100907 + DOI (N=5), M100907 alone (N=5). “*” = p<.05, “**” = p<.01, “***” = p<.001, Results of Dunnet’s post-hoc test, compared to vehicle, following significant ANOVA overall effect (p<.05).
Example demand curves for one rat during a vehicle and 0.4 mg/kg DOI session are overlaid in Fig. 3B. Baseline measurements of ‘α’ were consistent between all vehicle sessions (Fig. S4A). We found main effects of dose for ‘α’ (F5,64=3.77, p<.01, Fig. 3C), Q0 (F5,64=3.36, p<.01, Fig. 3D), fentanyl consumption (F5,64=22.0, p<.0001, Fig. 3E), and 1st bin fentanyl consumption (F5,64=9.13, p<.0001, Fig. 3F). Specifically, multiple-comparison adjusted post-hoc tests of all doses vs. vehicle reveal that the dose of 0.4mg/kg DOI increased demand elasticity (α) (q=2.76, p<.05), decreased Q0 (q=3.802, p<0.01), decreased 1st bin fentanyl consumption (q=5.239, p<.001), and decreased total fentanyl consumption (q=9.383, p<.001), relative to vehicle (Fig. 3C–F). The 0.2mg/kg dose of DOI also reduced 1st bin fentanyl consumption (q=5.239, p<.001) and total fentanyl consumption (q=3.038, p<.05) relative to vehicle (Fig. 3E,F). No other drug treatments, including M100907 in combination with DOI or M100907 alone, had any significant effects on these 4 parameters (‘α’, Q0, 1st bin intake, & total fentanyl intake) relative to vehicle, indicating that 0.3 mg/kg M100907 was able to completely block all observed effects of 0.4mg/kg DOI. We also note that M100907 alone tended to decrease ‘α’ (non-significant), opposite of the effects of DOI (Fig. 3C). We also observed a decrease in OMax following 0.4 mg/kg DOI (F5,64=4.354, p<.01; post-hoc test: q=3.587, p<.01, Fig. S4B), which was also completely reversed by M100907 (Fig. S4B). Together, these data indicate that the effects of DOI on fentanyl demand are through the 5-HT2A receptor.
Similar to our results of the CON/INT phase, there were no effects of DOI or M100907 on PMax or EV (Fig. S4C,D). The effects of DOI (0.4 mg/kg) relative to vehicle were apparent throughout the session, with a main effect of drug (ME of drug, F1,8=18.49, p<.001, Fig S4F) to reduce responses, and no effect of bin nor any interaction between bin and drug. We also note that all rats that completed the DOI 0.4mg/kg session exhibited an increase in demand elasticity (‘α’) relative to the associated vehicle session and subsequent return to vehicle levels during the M100907+DOI session (see Fig. S4E for individual data points for the last five sessions). In summary, we found that the effects of fentanyl self-administration experience on economic demand parameters (‘α’, Q0) and fentanyl consumption (1st bin and total), were changed in the opposite direction by 0.4 mg/kg DOI, and that 0.3mg/kg M100907 blocked DOI’s effects. Additionally, the parameters that were not altered by fentanyl experience (EV, PMax), were also unmodified by 5-HT2A stimulation and blockade.
5-HT2A Activation: Effects on Economic Demand for Food
To determine whether the effects of DOI on economic demand parameters were specific to opioid demand, we performed a control experiment to determine the effects of DOI on demand for food pellets in hungry rats. Example demand curves for one rat during a vehicle and 0.4 mg/kg DOI session are overlaid in Fig. S5B. We found main effects of dose for ‘α’ (F3,32=5.218, p<.01, Fig. S5C), total food consumption (F3,32=7.674, p<.001, Fig. S5E), and 1st bin food consumption (F3,32=3.699, p<.05, Fig. S5F). Specifically, multiple-comparison adjusted post-hoc tests of all doses vs. vehicle reveal that the dose of 0.4mg/kg DOI increased demand elasticity (α) (q=3.92, p<.01), decreased 1st bin food consumption (q=3.038, p<.05), and decreased total food consumption (q=4.443, p<.001), relative to vehicle (Fig. S5C–F). The 0.2mg/kg dose of DOI also reduced total food consumption (q=2.858, p<.05) relative to vehicle (Fig. S5E).
Discussion
Summary
The first phase of this study compared the changes in economic demand for fentanyl following extended experience with continuous (CON) vs. intermittent (INT) access to fentanyl. We found that, irrespective of access conditions, extended experience with fentanyl led to an increase in fentanyl intake during post-schedule demand sessions, along with an increase in Q0 (consumption at low prices), and a decrease in ‘α’ (demand elasticity). We observed no change in PMax (the price at which most responses occur) or essential value (EV) in either group, although OMax (the number of responses at PMax) showed a non-significant tendency to increase following both schedules. Expectedly, Q0 increased to a greater extent in the CON group, but contrary to our predictions, the decrease in ‘α’ was similar following both schedules.
The next phase of this study was designed to test the acute effects of 5-HT2A receptor stimulation on the economic demand for fentanyl after fentanyl experience. In rats with extensive fentanyl experience (28 sessions), the 5-HT2A/2C agonist DOI dose-dependently reduced the economic value of fentanyl in the opposite direction of the changes induced by fentanyl experience. Specifically, DOI increased ‘α’, while decreasing Q0, OMax, and fentanyl consumption. PMax and EV were again unchanged. Pre-administration of M100907 completely blocked all effects of DOI, and M100907 alone had no significant effects on any parameters. These experiments provide evidence that the 5-HT2A/2C agonist, DOI, reduces fentanyl demand after extensive drug experience through activation of the 5-HT2A receptor. Importantly, DOI also produced a reduction in food economic demand elasticity (‘α’) at the 0.4 mg/kg dose, indicating the effects of DOI are not specific to opioid demand.
Methodological Considerations
Both phases of the experiment used within-session economic demand procedures to assess the economic value of fentanyl. The overall design of these sessions was similar to several previous reports using fentanyl17, remifentanil24, and cocaine13,22,25 as the reinforcer. However, we extended the bin length to fifteen minutes (from ten minutes), which helped limit the number of zero consumption bins in the data. Furthermore, we made several adjustments to the analysis to improve the quality of model fitting and parameter estimation (see Methods and supplemental methods). Importantly, we found that within-subject parameter estimation using this approach was stable during baseline demand thresholding, as well as during vehicle sessions.
Intermittent versus Continuous Access
Based on studies comparing intermittent and continuous schedules with respect to cocaine demand14,15, we expected that ‘α’ (demand elasticity) would decrease most in the INT group, and that Q0 (free consumption) would increase most in the CON group. In fact, we did observe a larger increase in Q0 in the CON group, but the decreases in ‘α’ were unexpectedly similar between groups. We adopted the same lengths of ON/OFF periods (5 min/25 min) that many have used for intermittent cocaine access14,15,25. However, the plasma half-life of cocaine (~11 min26) is shorter than that of fentanyl (~50 min27), likely producing a less pronounced spike in fentanyl blood levels in our rats compared to what is observed with intermittent cocaine access15. Theoretically, it is conceivable that longer OFF periods might further increase spiking of plasma fentanyl levels and decrease demand elasticity in INT rats. Nevertheless, the effective half-life of fentanyl with respect to its reinforcing effects is much shorter than its plasma half-life, as fentanyl produced average pause lengths of 8–9 minutes between bouts of self-administration. Indeed, the 25 min OFF period in the INT schedule forced rats to wait 3–4 times longer to administer a subsequent bout of fentanyl than in the CON group. This waiting period caused the INT group to develop increased anticipation of drug availability, indicated by short latencies to earn injections at the beginning of a session (relative to the CON group), and a pronounced scalloped pattern of non-reinforced responding during OFF periods. A scalloped response pattern is typical of fixed-interval schedules, which result in different neurochemical changes than fixed ratio schedules for food28. With cocaine, long access conditions lead to tolerance to cocaine’s effects on the dopamine transporter, while intermittent access leads to sensitization29. We speculate that decreases in demand elasticity (‘α’) in CON/INT groups may also reflect different underlying processes: an increased sensitivity to fentanyl availability in INT rats compared to increased tolerance in CON rats.
Likewise, the greater increase in Q0 we observed in the CON group may be related to an overall increase in tolerance to fentanyl due to the higher cumulative dose taken over the course of all sessions prior to post-schedule demand testing. Behavioral tolerance to the rate-decreasing effects of opioids30 may also underlie the increases in Q0 and decreases in ‘α’ observed with fentanyl experience, particularly in the CON group.
Despite extended experience with fentanyl, we did not observe changes in PMax or the EV of fentanyl. EV is a measure that corresponds to relative changes in α after taking differences in Q0 into account. This measure is often used as a way to compare different reinforcers, as it is insensitive to the magnitude of the reinforcer used during testing12. Here, the decreases we measured in ‘α’ were largely offset by increases in Q0. Following both schedules, the shapes of the price/response curves were similar before and after extended fentanyl experience (Fig S3F). Notably, the curves were shifted higher after experience because on average the rats consumed more fentanyl at all prices. Indeed, PMax was not modified by continuous or intermittent fentanyl experience, though we observed a trend for OMax to increase in both groups.
Typically, as PMax increases, EV also increases, indicating that a subject is willing to exert maximum effort at higher prices to defend the preferred level of reinforcer intake. Others have suggested that EV may be the best parameter for tracking the changes in drug value that occur in addiction12, and supporting this idea, cocaine experience tends to increase the EV of cocaine in rats31. Furthermore, a study in monkeys showed that in 3 out of 4 monkeys, dependence induced by chronic morphine injections lowered ‘α’ both during withdrawal and several weeks post withdrawal32. Additional evidence suggests that motivation for opioids, using progressive ratio measures, differs depending on access schedule33. Also, choice for opioids relative to cocaine32, and relative to non-drug reinforcers increases during opioid withdrawal34. However, our data suggests that considerable experience with fentanyl may not raise EV. It remains a distinct possibility that long-access conditions would lead to greater tolerance and dependency development that might be reflected by increases in EV.
With respect to human drug taking, the increases in economic demand for fentanyl we observe following experience in both preclinical models predicts that, regardless of the pattern intake, extended exposure to fentanyl is likely to raise aspects of its economic value.
5-HT2A Activation Effects on Fentanyl Demand
This study examining the effects of 5-HT2A stimulation on fentanyl demand was motivated by renewed clinical interest in 5-HT2A agonists, such as psilocybin, for treating psychiatric illnesses including depression10,11 and addiction35,36. Previous studies in rats compared the effects of morphine alone with morphine in combination with 5-HT2A stimulation using 2,5-dimethoxy-4-methylamphetamine DOM37, an analogue of DOI similarly biased to activate 5-HT2A receptors38. This work demonstrated DOM had very little effect on morphine-induced antinociception or the discriminative stimulus effects of morphine in rats. However, DOM reduced the acute hyperlocomotion produced by morphine (10 mg/kg)37. In another study in non-human primates, acute DOM administration reduced heroin self-administration and the self-administration of a DOM/heroin mixture was less than self-administration of heroin alone39. Our finding that the economic demand for fentanyl is reduced by 5-HT2A activation in rats is consistent with these prior studies37,39.
Animal studies suggest that 5-HT2A agonists do not support robust self-administration and can even produce negative reinforcement (eg.40,41), though non-human primates occasionally self-administer certain 5-HT2A agonists41,42.Other studies have reported decreases in intracranial self-stimulation (ICSS) following acute 5-HT2A agonist administration43. Similarly, DOI reduces the conditioned place preference (CPP) induced by ethanol, as well as ethanol consumption in mice44. Notably, food self-administration is also dose-dependently reduced by 5-HT2A agonists45,46. The results of the economic food demand studies reported here indicate a similar dose response function for the reduction of food and opioid demand. We note that at the 0.4 mg/kg dose of DOI, response rates in the food demand study were much higher than responding for drug under vehicle conditions, indicating that motoric effects of this dose of DOI are unlikely contributing to the rate decreasing effects of DOI on lever pressing for drug, though it remains possible the combination of fentanyl and DOI may effect motor competence.
With respect to clinical implications, our data indicating that DOI reduces economic demand for fentanyl suggests that 5-HT2A agonists are unlikely to increase opioid seeking, and may in fact be useful for reducing opioid motivation, though additional testing is needed. An important outstanding question is whether acute, post-acute, or chronic 5-HT2A stimulation preferentially reduces economic demand for drug vs. non-drug reinforcers. Choice studies may be the best way to address this question, as manipulations that alter the demand for two commodities in the same direction may differ with respect to concurrent choice between those commodities32. Also, a high concordance between positive preclinical and clinical results is found when assessing choice of drug relative to non-drug reinforcers47. For example, several drugs that increase choice for non-drug vs. drug rewards are also efficacious in humans with OUD3,34. Additionally, the long-term effects on behavior following acute and repeated 5-HT2A agonist administration is of paramount clinical relevance. The rapid tolerance development and profound psychological effects of 5-HT2A agonists48 likely preclude chronic therapeutic use. However, clinical studies demonstrate the potential of one or a few acute treatments to lead to long-lasting therapeutic benefits10,11. Additional preclinical work will aid in elucidating mechanisms for these long-term changes. Interestingly, a recent study found that a single systemic or intra-VTA dose of a 5-HT2A agonist, 4-acetoxy-dimethyltryptamine, reverses some changes in opioid reward after chronic opioid administration49.
Limitations and Conclusions
The intermittent and continuous access schedules used here were designed to produce changes in economic demand measures, but not physical dependence, which is a critical factor to consider for any translational model of OUD. While measuring the acute effects of 5-HT2A receptor activation on opioid demand is an important first step to understanding their interaction, modelling the impact of chronic and/or post-acute 5-HT2A activation in drug-free contexts may be more clinically relevant10. Measuring opioid responding under extinction conditions will also determine if experiencing the unconditioned stimulus during 5-HT2A agonism is essential for the reduction in responding, disambiguating between mechanisms whereby reductions in motivation are independent of, or are preceded by, a change in the reinforcing properties of the reward itself. Also, testing 5-HT2A agonists other than DOI, which is not commonly taken by humans, would expand the generality of the findings to drugs more likely to be utilized in the clinic. A final limitation is that only male rats were included and sex differences were not considered here. For a clear picture of the interaction between opioid reward and 5-HT2A activation future studies will be needed to determine potential differences between the sexes.
In conclusion, we find that the same economic demand parameters that increase following fentanyl experience are decreased by acute activation of the 5-HT2A receptor. This work provides additional preliminary evidence that this receptor warrants further study as a potentially useful target in addiction pharmacotherapy.
Supplementary Material
Acknowledgments
This work was supported by a McKnight Memory and Cognitive Disorders Award (DJC), a NARSAD Young Investigator Grant #24950 (DJC), NIDA grants R01DA043533 (DJC), and the Department of Anatomy and Neurobiology at the University of Maryland, School of Medicine. The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript.
References
- 1.Scholl L, Seth P, Kariisa M, Wilson N & Baldwin G Drug and Opioid-Involved Overdose Deaths - United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2018; 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer MR, Warner M, Bastian BA, Trinidad JP & Hedegaard H Drug Overdose Deaths Involving Fentanyl, 2011–2016. Natl Vital Stat Rep 2019; 68, 1–19. [PubMed] [Google Scholar]
- 3.Negus SS & Banks ML Medications development for opioid abuse. Cold Spring Harb Perspect Med 2013; 3, a012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung KS, Wu HC, Hsu CY, Lu YS & Li DJ Factors impeding switching from methadone to buprenorphine in heroin users receiving methadone maintenance therapy - A naturalistic cohort study. J Subst Abuse Treat 2019; 105, 51–56. [DOI] [PubMed] [Google Scholar]
- 5.Bell J & Strang J Medication Treatment of Opioid Use Disorder. Biol Psychiatry 2019. [DOI] [PubMed] [Google Scholar]
- 6.Neelakantan H et al. Lorcaserin Suppresses Oxycodone Self-Administration and Relapse Vulnerability in Rats. ACS Chem Neurosci 2017; 8, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogenschutz MP et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of concept study. J Psychopharmacol 2015; 29, 289–299. [DOI] [PubMed] [Google Scholar]
- 8.Krebs TS & Johansen PO Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol 2012; 26, 994–1002. [DOI] [PubMed] [Google Scholar]
- 9.Savage C & McCabe OL Residential psychedelic (LSD) therapy for the narcotic addict. A controlled study. Arch Gen Psychiatry 1973; 28, 808–814. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths RR et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol 2016; 30, 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross S et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 2016; 30, 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hursh SR Behavioral Economics and the Analysis of Consumption and Choice. The Wiley Blackwell handbook of operant and classical conditioning. 1 edn, (Wiley-Blackwell, 2014). [Google Scholar]
- 13.Bentzley BS, Jhou TC & Aston-Jones G Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 2014; 111, 11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MH et al. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry 2019; 85, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmer BA, Oleson EB & Roberts DC The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 2012; 37, 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend EA, Negus SS, Caine SB, Thomsen M & Banks ML Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragale JE, Pantazis CB, James MH & Aston-Jones G The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology 2019; 44, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Espana RA et al. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 2010; 31, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentzley BS, Fender KM & Aston-Jones G The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013; 226, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ree JM, Slangen JL & de Wied D Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther 1978; 204, 547–557. [PubMed] [Google Scholar]
- 21.Fletcher PJ, Zeeb FD, Browne CJ, Higgins GA & Soko AD Effects of 5-HT1A, 5-HT2A and 5-HT2C receptor agonists and antagonists on responding for a conditioned reinforcer and its enhancement by methylphenidate. Psychopharmacology (Berl) 2017; 234, 889–902. [DOI] [PubMed] [Google Scholar]
- 22.Oleson EB, Richardson JM & Roberts DC A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 2011; 214, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koffarnus MN, Franck CT, Stein JS & Bickel WK A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol 2015; 23, 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacy RT, Austin BP & Strickland JC The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addict Biol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawa AB, Bentzley BS & Robinson TE Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 2016; 233, 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman AB, Buesing WR, Norman MK, Tabet MR & Tsibulsky VL The self-administration of WIN 35,428 and cocaine: comparisons of satiety threshold and elimination half-life in rats. Eur J Pharmacol 2004; 483, 281–287. [DOI] [PubMed] [Google Scholar]
- 27.Ohtsuka H, Fujita K & Kobayashi H Pharmacokinetics of fentanyl in male and female rats after intravenous administration. Arzneimittelforschung 2007; 57, 260–263. [DOI] [PubMed] [Google Scholar]
- 28.Barrett JE & Hoffmann SM Neurochemical changes correlated with behavior maintained under fixed-interval and fixed-ratio schedules of reinforcement. J Exp Anal Behav 1991; 56, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC & Jones SR Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 2013; 38, 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altarifi AA & Negus SS Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 2011; 22, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen CJ, Silberberg A, Hursh SR, Roma PG & Riley AL Demand for cocaine and food over time. Pharmacol Biochem Behav 2008; 91, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade-Galuska T, Galuska CM & Winger G Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J Exp Anal Behav 2011; 95, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenoir M & Ahmed SH Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology 2008; 33, 2272–2282. [DOI] [PubMed] [Google Scholar]
- 34.Negus SS Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther 2006; 317, 711–723. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Romeu A et al. Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacol 2019, 269881119845793. [DOI] [PubMed] [Google Scholar]
- 36.Johnson MW, Griffiths RR, Hendricks PS & Henningfield JE The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 2018; 142, 143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li JX, Shah AP, Patel SK, Rice KC & France CP Modification of the behavioral effects of morphine in rats by serotonin 5-HT(1)A and 5-HT(2)A receptor agonists: antinociception, drug discrimination, and locomotor activity. Psychopharmacology (Berl) 2013; 225, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titeler M, Lyon RA & Glennon RA Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 1988; 94, 213–216. [DOI] [PubMed] [Google Scholar]
- 39.Maguire DR, Li JX, Koek W & France CP Effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) and quipazine on heroin self-administration in rhesus monkeys. Psychopharmacology (Berl) 2013; 225, 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmeister F Negative reinforcing properties of some psychotropic drugs in drug-naive rhesus monkeys. J Pharmacol Exp Ther 1975; 192, 468–477. [PubMed] [Google Scholar]
- 41.Fantegrossi WE, Woods JH & Winger G Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav Pharmacol 2004; 15, 149–157. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin AK An intravenous self-administration procedure for assessing the reinforcing effects of hallucinogens in nonhuman primates. J Pharmacol Toxicol Methods 2016; 82, 31–36. [DOI] [PubMed] [Google Scholar]
- 43.Sakloth F et al. Effects of acute and repeated treatment with serotonin 5-HT2A receptor agonist hallucinogens on intracranial self-stimulation in rats. Exp Clin Psychopharmacol 2019; 27, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oppong-Damoah A, Curry KE, Blough BE, Rice KC & Murnane KS Effects of the synthetic psychedelic 2,5-dimethoxy-4-iodoamphetamine (DOI) on ethanol consumption and place conditioning in male mice. Psychopharmacology (Berl) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aulakh CS, Hill JL, Yoney HT & Murphy DL Evidence for involvement of 5-HT1C and 5-HT2 receptors in the food intake suppressant effects of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). Psychopharmacology (Berl) 1992; 109, 444–448. [DOI] [PubMed] [Google Scholar]
- 46.Schechter LE & Simansky KJ 1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI) exerts an anorexic action that is blocked by 5-HT2 antagonists in rats. Psychopharmacology (Berl) 1988; 94, 342–346. [DOI] [PubMed] [Google Scholar]
- 47.Banks ML & Negus SS Insights from Preclinical Choice Models on Treating Drug Addiction. Trends Pharmacol Sci 2017; 38, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollister LE, Macnicol MF & Gillespie HK An hallucinogenic amphetamine analog (DOM) in man. Psychopharmacologia 1969; 14, 62–73. [DOI] [PubMed] [Google Scholar]
- 49.Vargas-Perez H et al. A single administration of the hallucinogen, 4-acetoxy-dimethyltryptamine, prevents the shift to a drug-dependent state and the expression of withdrawal aversions in rodents. Eur J Neurosci 2017; 45, 1410–1417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.