Abstract
Mast cells (MCs) are the initial responders of innate immunity and their degranulation contribute to various etiologies. While the abundance of MCs in the choroid implies their fundamental importance in the eye, little is known about the significance of MCs and their degranulation in choroid. The cause of geographic atrophy (GA), a progressive dry form of age-related macular degeneration (AMD) is elusive and there is currently no therapy for this blinding disorder. Here we demonstrate in both human GA and a rat model for GA, that MC degranulation and MC-derived tryptase are central to disease progression. Retinal pigment epithelium (RPE) degeneration followed by retinal and choroidal thinning, characteristic phenotypes of GA, were driven by continuous choroidal MC stimulation and activation in a slow release fashion in the rat. Genetic manipulation of MCs, pharmacological intervention targeting MC degranulation with ketotifen fumarate or inhibition of MC-derived tryptase with APC 366 prevented all of GA-like phenotypes following MC degranulation in the rat model. Our results demonstrate the fundamental role of choroidal MC involvement in GA disease etiology, and will provide new opportunities for understanding GA pathology and identifying novel therapies targeting MCs.
Keywords: age-related macular degeneration, choroid, degranulation, ketotifen fumarate, retinal pigment epithelium, tryptase, 48/80
INTRODUCTION
Mast cells (MCs) are key effector cells of innate immunity and are resident inflammatory cells in many tissues (1). Mature MCs express a tyrosine kinase receptor c-KIT and high-affinity IgE receptors on their cell membrane through which MC degranulation is triggered in IgE-mediated hypersensitivity reactions (2). Degranulation of MCs is known to release histamine, cytokines, chemokines, and proteases (3). These proteases include MC-specific tryptase and chymase, which initiate an immediate hypersensitivity response to allergens and can activate matrix metalloproteinases (MMPs) that degrade stroma and basement membranes (4). Historically MCs have been implicated in asthma (5), and recent studies have revealed the significance of MC degranulation in the experimental cardiovascular disease models such as deep vein thrombosis, abdominal aortic aneurysms, and atherosclerosis (6–8). In the eye, MCs are abundant in the conjunctiva and uveal tract, which includes the choroid, but are absent in the retina (1, 9). Choroidal MCs are analogous to connective tissue MCs in the skin and mesentery in many ways. They respond to compound 48/80, a snake venom-like compound (1, 9) that induces MC degranulation. Their abundance and location in mammalian choroids suggest that MCs can exert local control of choroidal immunologic and inflammatory reactions as well as basic physiological function (1), however, little is known about the significance of MC degranulation in the choroid.
Age-related macular degeneration (AMD) affects around 200 million people worldwide and is the leading cause of irreversible blindness and visual impairment in developed countries (10). Geographic atrophy (GA) is the advanced dry form of AMD and is characterized by retinal pigment epithelium (RPE) loss and choriocapillaris attenuation, photoreceptor death, and choroidal thinning. While GA is responsible for 20% of the legal blindness in North America (11), to date, there are no proven treatments and experimental animal models for GA are very limited (12). Therefore, new therapies and reliable animal models to evaluate drug efficacy for GA are urgently needed.
Recently, we observed an increase in number and percentage of degranulated choroidal MCs in all types of AMD subjects (13). The possible reason for this is the proinflammatory milieu of AMD choroid, which includes elevated complement factor C3a, C5a, C-reactive protein (CRP), and advanced glycation end products (AGEs), all of which can stimulate MC degranulation (14–16). Human choroidal MCs release tryptase and histamine when they degranulate (17), and tryptase is released specifically from MCs in the first wave of granules during degranulation (18). Tryptase degrades collagens and activates MMPs, which degrade stroma and basement membranes. In GA, MC-derived tryptase was localized to Bruch’s membrane, vascular basement membranes and throughout the choroidal stroma near degranulated MCs (17). These observations imply MC involvement in GA etiology, however, there is still a void in our understanding of how MC degranulation contributes to the progression of GA.
The purpose of the current study was to determine if chronic choroidal MC degranulation results in changes that were phenotypic characteristics of GA. Therefore, RPE atrophy as well as reduced retinal function, and retinal and choroidal thinning were assessed after inducing MC degranulation in a rat model. To evaluate if MCs could be a therapeutic target in GA, ketotifen fumarate, a generic MC stabilizer, was evaluated for prevention of MC degranulation in in vitro and ex vivo assays for MC degranulation, and in our in vivo long-term rat model of GA. In addition, a MC tryptase inhibitor was also evaluated in the rat model. Our results suggest for the first time, choroidal MC involvement in the development of GA and MCs as a potential therapeutic target for GA.
MATERIALS AND METHODS
Human eyes
Human donor eyes were obtained from the National Disease Research Interchange (Philadelphia, PA, USA). All tissues were obtained within 10–35 h of death. All donors were Caucasian. GA was diagnosed when a distinct area of RPE degeneration with sharply defined borders without apparent neovascularization. Utilization of the human tissue was in accordance with the Declaration of Helsinki with approval of the Joint Committee on Clinical Investigation at Johns Hopkins University School of Medicine.
Rats
Sprague/Dawley male rats (200–250 g) were purchased from Envigo (Frederick, MD, USA). MC deficient WsRCWS/WS rats and their littermate wild type (WT) WsRC+/+ rats were purchased from Japan SLC (Hamamatsu, Japan). For all procedures, anesthesia was performed by intramuscular injection of a ketamine (100 mg/ml) and xylazine (100 mg/ml) cocktail. Pupils were dilated with 1% tropicamide and 2.5% phenylephrine eye drops followed by topical anesthesia. All animal experimental procedures were performed according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, following approval from the Animal Care and Use Committee at the Johns Hopkins University.
Images
Images were captured using Zeiss LSM710 confocal microscope with ZEN software (Carl Zeiss, Jena, Germany), Zeiss Photomic II microscope equipped with QCapture Imaging camera and software (Teledyne QImaging, Surrey, BC, Canada), or Leica AF6000 with Leica Application Suite software (Leica Microsystems, Buffallo Grove, IL, USA). Images were analyzed using NIH ImageJ (version 1.50), Adobe Photoshop (CS4 and CS6, Adobe Systems, San Jose, CA), and Imaris (version 8.3.1, Bitplane USA, Concord, MA, USA).
Human flat mount preparation
MCs in human choroidal flat mounts were stained for non-specific esterase (NSE) and alkaline phosphatase (APase) enzyme activities as previously described (13). Briefly, after the retina was carefully removed, eyecup with choroid was soaked in 1% EDTA (Thermo Fisher Scientific, Waltham, MA, USA) in distilled water for 2 h at room temperature (RT) to remove RPE. Any adherent RPE cells were removed by squirting EDTA solution from a syringe with a blunted 25-gauge needle. RPE-denuded choroids were then isolated from the sclera, and fixed for one hour in 2% paraformaldehyde (PFA) in 0.1 M cacodylate buffer at room temperature and incubated for the APase activity as previously described (13). After APase staining, NSE staining was performed using a naphthol AS-D chloroacetate kit (91C-1KT, Millipore Sigma, St Louis, MO, USA) (13). Granulocytes and MC stained red with this method, but the two cell types were easily distinguished due to their size difference. Then, APase and NSE double stained choroids were post-fixed in 2% PFA for 24 h, washed and exposed to 30% hydrogen peroxide (Millipore Sigma, St Louis, MO) to bleach the melanin at 4°C as previously reported (13). Total MC number and degranulated MCs at the border of GA and aged matched eyes were counted in five 1 mm2 areas and averaged per eye for quantification.
For flat mount IHC choroids, eyes were fixed in 2% PFA in TBS overnight after RPE removal (17). After several washes, choroids were incubated with 5% normal goat serum in 0.1% triton X-100 in TBS with 1% BSA overnight at 4°C, followed by incubation with mouse anti-MC tryptase antibody (Ab)(1:500, ab2378, Abcam, Cambridge, MA, USA) for 72 h at 4°C. After washes, the choroid was incubated for 48 h at 4°C with goat anti-mouse Ab conjugated with Cy3 (1:200, Jackson ImmunoResearch, West Grove, PA, USA) and Ulex europaeus agglutinin I (UEA) lectin conjugated with FITC (1:100, GTX01512, GeneTex, Irvine, CA, USA) and then imaged with a Zeiss LSM710.
Rat flat mount preparation
After enucleating the eyes, the anterior segments were removed and the retina was carefully excised. Eyecups were then fixed overnight with 2% PFA at 4°C. After washing, four pie cuts were made to allow flattening of the choroid/sclera eyecup as well as clearly isolate superior and inferior quadrants. Then the choroid was prepared for IHC as previously described (19). The choroid was blocked with 2% normal goat or donkey serum for 4 h at 4°C, washed in 0.1% triton X-100 in TBS, and then incubated with a mixture of the following primary Abs: mouse anti-RPE65 (1:200, NB100–355, Novus Biologicals, Centennial, CO, USA), goat anti-Iba1 (1:200, ab5076, Abcam, Cambridge, MA, USA) and rabbit anti-MC tryptase (1:200, CAU26568, Biomatik, Wilmington, DE, USA) overnight at 4°C. After washing, they were incubated with Alexa-Fluor 488 or Cy3 conjugated goat or donkey secondary Abs (1:300, Jackson ImmunoResearch, West Grove, PA, USA) overnight, followed by mouse anti-ZO-1 Ab conjugated with Alexa-Fluor 594 (1:100, 339194, Thermo Fisher Scientific, Waltham, MA, USA).
Rat cross section preparation
Rat eyecups were cryopreserved as reported previously (17). Eight μm cryosections were permeabilized with absolute methanol at −20°C and blocked with 2% normal donkey serum at RT. After washing, sections were incubated for 2 h at RT with rabbit anti-MC tryptase Ab (1:200, CAU26568, Biomatik, Wilmington, DE, USA). After washing, sections were incubated for 30 min at RT with Isolectin GS-IB4 from Griffonia simplicifolia (GS lectin) conjugated with Alexa-Fluor 488 (1:100, I21411, Thermo Fisher Scientific, Waltham, MA, USA) and donkey anti-rabbit Ab conjugated with Alexa-Fluor 647 (1:500, Jackson ImmunoResearch, West Grove, PA, USA) and then coverslipped with mounting medium (Vector Laboratories, Burlingame, CA, USA).
Hydrogel preparation and injection
A hydrogel which slow released compound 48/80 (Millipore Sigma, St Louis, MO, USA), a snake venom-like compound, was formulated using thiolated hyaluronic acid and 4-arm poly (ethylene glycol) acrylate crosslinked using thiol-ene click chemistry (20). The gel consisted of 48/80 (20 mg/ml), 4-arm PEG-acrylate (100 mg/ml, PSB-421, Creative PEGWorks, Chapel Hill, NC, USA), 8-arm PEG SH (150 mg/ml, PSB-851, Creative PEGWorks, Chapel Hill, NC), hyaluronic acid-SH (200 mg/ml, HA-371, Creative PEGWorks, Chapel Hill, NC) added to Irgacure 2959 (Ciba-Geigy, Tarrytown, NY, USA). All components were then mixed together by vortex and placed on ice. The final solution was loaded into an insulin syringe with a 31-gauge needle (BD Biosciences, San Jose, CA, USA) and then exposed to UV light (AnalytikJena, Upland, CA, USA) for 1.5 min. The release and stability of the hydrogel were evaluated in vitro in PBS, pH 7.4 at 37°C (20). The amount of 48/80 released was measured using bicinchoninic acid (BCA) assay. After confirming the gel formation by its consistency, 30 μl of hydrogel with or without 48/80 (blank hydrogel) was implanted into the superior subconjunctival space.
Alcian blue staining
After RPE removal, eyecups were fixed in methanol-formalin-acetic acid cocktail for 30 min and MCs were further stained with 0.05% Alcian blue (pH 5.0) in 0.02 M sodium acetate buffer for 45 min. Choroids were isolated after eyecups were split at the meridian and the number of non-degranulated and degranulated MCs were counted.
RPE degeneration
After the choroid/sclera eyecup was stained with anti-RPE65 Ab, 4–5 fields from the gel and non-gel areas per choroid were captured as optimized z-stacks at 10 or 20x magnification with Zeiss LSM710. Images were exported to ImageJ software, converted into 8-bit grey color and then thresholded. The dark area was considered degenerating RPE. To exclude the area of RPE nucleus (unstained with anti-RPE65 Ab, therefore dark) from the total dark area, particle sizes ranging from 100-infinity (pixels2) were analyzed so nuclei were excluded. Percentage of RPE degeneration was averaged per choroid and then quantified.
Retinal and choroidal thickness
Eight μm cryosections through the optic nerve were stained with Picrosirius Red Stain Kit (Polysciences, Inc., Warrington, PA, USA) for 5 min as recommended by the manufacturer. After rinsing in hydrochloric acid and dehydrating in ethanol, sections were cover-slipped with Permount Mounting Medium (Thermo Fisher Scientific, Waltham, MA, USA). The sections were then imaged with a Zeiss Photomic II microscope at 10x, and adjacent overlapping images of each section were taken from ora serrata to ora serrata. The overlapping images were then stitched using Adobe Photoshop and a panorama image was generated. The entire retina and choroid of each panorama image was precisely hand-traced and areas of the retina and choroid, respectively, were measured using ImageJ.
For morphologic analyses, eyes were cryopreserved and stained with H&E staining as previously described (15) or fixed with 2.5% glutaraldehyde, 2% PFA in 0.1 M cacodylate buffer. After the anterior segments were excised, eyes were cut in half through the optic disk. Then, eyes were washed and dehydrated and embedded in glycol methacrylate and cut with 2 μm thickness as previously published (21).
Electroretinogram (ERG)
After overnight dark adaptation, rats were deeply anesthetized followed by pupil dilation. The rats were placed on a heated platform and full-field scotopic ERGs were elicited by white light flashes with corneal electrodes at intensities ranging from 0.01 to 1 cd.s/m2 using the Celeris ERG system (Diagnosys LLC, Lowell, MA, USA). The amplitude of the a- and b-waves was measured from the a-wave trough to the b-wave peak.
In vitro assays
RBL-2H3 cells (CRL-2256, ATCC, Manassas, VA, USA) were used for the in vitro assay for MC degranulation. Cell degranulation was assessed by the β-hexosaminidase release as previously described (22). A density of 50,000 cells/well was plated overnight in a 96 well plate. Ten μg/ml of 48/80 or 10 μg/ml of ketotifen fumarate (K2628, Millipore Sigma, St Louis, MO, USA) + 10 μg/ml of 48/80 dissolved in 100 μl serum free media (DMEM with 1.0 g/l glucose) (Thermo Fisher Scientific, Waltham, MA) were added to each well and incubated for 45 min. Then, the cells were lysed using 1% triton X-100 to determine MC β-hexosaminidase that was still cell associated. Twenty μl of the supernatant and then the cell lysate was collected and added to 50 μl of cold 4-Nitrophenyl N-acetyl-B-D-glucosaminide (1.3 mg/ml in Citrate Buffer, pH 4.5, Millipore Sigma, St Louis, MO, USA) to detect the amount of β-hexosaminidase released. After the plate was incubated for 90 min, 100 μl of 0.2 M sodium hydroxide with 0.2 M glycine (pH 10) were added to terminate the reaction. The plate was read at 405 nm wavelength using a microplate reader (ELx808, BioTek, Winooski, VT, USA). Degranulation was calculated as the percentage of β-hexosaminidase activity measured in the supernatants relative to the total amount of β-hexosaminidase activity in the cells exposed to 1% triton X-100 (100% degranulation).
ARPE-19 cells (CRL-2302, ATCC, Manassas, VA, USA) incubated in a 24 well plate for 14 d were used for assessing the effect of tryptase on confluent RPE cell monolayer. After washing in PBS, cells were treated with 500 μl serum free media, with or without human lung tryptase (650366-M, Millipore Sigma, St Louis, MO, USA) (1 μg/ml or 5 μg/ml) for 72 h. Then cells were fixed with 4% PFA, permeabilized with 0.1% triton X-100 in PBS and stained with phalloidin-tetramethylrhodamine B isothiocyanate (1:500, Millipore Sigma, St Louis, MO, USA). Images of four fields in the center of the well were captured as optimized z-stacks at 10x magnification with Zeiss LSM710. Images were exported to ImageJ software, converted into 8-bit grey color and thresholded. Percentage of the ARPE-19 cell monolayer degeneration was averaged per well and quantified.
Ex vivo assays
An ex vivo assay was developed to evaluate drugs quickly and efficiently. After enucleation, the anterior segments and retinas were excised and the eyecups were split at the meridian and divided in halves. Then, eyecups were incubated with 300 μg/ml of 48/80 with or without 300 μg/ml of ketotifen fumarate in serum free DMEM in a 48 well plate for 3 h. To evaluate oral ketotifen fumarate potential to prevent MC degranulation, rats were treated orally twice daily with ketotifen fumarate (15 mg/kg) in PBS or with PBS as control for 4 d and 2 h prior to enucleation on the fifth day. Eyes were enucleated and exposed in 48/80 serum free DMEM for 3 h. After washing, RPE was removed and the eyecup fixed for histochemistry. The choroid was isolated from the sclera and MCs were stained for NSE activity and viewed on the Zeiss LSM710 for the autofluorescence of the reaction product. The numbers of non-degranulated and degranulated cells were counted in the whole choroid. MCs were considered as degranulated when they showed irregular shapes or extracellular granules. For WsRC rats, melanin was bleached using the Melanin Bleaching Kits (Polysciences, Inc., Warrington, PA) following the manufacture’s protocol before proceeding to immunohistochemistry.
Volumetric and sphericity measurement
Volumetric and sphericity measurements were performed in the rats to analyze changes in shape and size of choroidal macrophages/monocytes stained with Iba1 as previously reported (23). Briefly, randomly chosen 4–5 fields of optimized 40x magnification z-stacks per choroid captured with Zeiss LSM710 were opened in Surpass View of Imaris software and surfaces (surface detail 1.5 μm) were created, using background subtraction (0.5 μm). Voxel number filtering was applied to remove nonspecific particles in the surfaces created. Cells touching others or ones which had only a portion of the cell body visible were omitted from the volume rendering and then, the cell volume and sphericity were quantified with Imaris software.
Pharmacokinetic study
Ketotifen fumarate quantification in plasma and eye tissue was conducted using high-performance liquid chromatography with tandem mass spectrometry (LC/MS-MS). Briefly, acetonitrile containing 0.5 μM losartan as internal standard was used to extract ketotifen from choroid and retina. Standards were prepared by spiking ketotifen fumarate in naïve tissue from 0.003–100 μmol/g in a half log dilution series. Samples were weighed and placed in low retention microcentrifuge tubes with 5 μl extraction solution/mg tissue and pestle homogenized. Samples were vortexed followed by centrifugation at 16,000x g for 5 min at 4°C. The supernatants were transferred to a 96 well plate and 2 μl were injected for analysis. Samples were analyzed on an UltiMate 3000 UHPLC coupled to Q Exactive Focus orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) using an Agilent EclipsePlus C18 RRHD (1.8 μm; 2.1 × 100 mm) column. The mobile phase consisted of water + 0.1% formic acid, and acetonitrile + 0.1% formic acid. Separation was achieved at a flow rate of 0.4 ml/min using a gradient run. Samples were analyzed in positive ion mode via heated electrospray with capillary temperature set at 350°C and a spray voltage of 3.5 kV. Data acquisition was performed in selected ion monitoring (SIM) mode isolating 310.1260 m/z (ketotifen fumarate) and 423.1695 m/z (losartan) with a 1 m/z isolation window. Data were acquired and quantified with Xcalibur software (Thermo Fisher Scientific, Waltham, MA, USA).
Drug treatments
Rats were pretreated for 1–2 d prior to 48/80 hydrogel injection with either oral treatment of ketotifen fumarate (15 mg/kg) or PBS twice daily. MC tryptase inhibitor APC 366 (5 mg/kg, CAS 258932-85-8, R&D Systems, Minneapolis, MN) (24) or control vehicle was administered subcutaneously once daily for 8 wk.
Statistics
Statistical analyses were performed with Prism 8 (GraphPad Software, San Diego, CA) using a 2-tailed Student’s t test or 1-way ANOVA with a Tukey post-hoc comparison test. Data were expressed as mean ± SD. P values of less than 0.05 were considered statistically significant.
RESULTS
Sustained MC activation by subconjunctival 48/80-hydrogel implantation in the rat eye
Immunohistochemical localization of tryptase in human GA choroids demonstrated that many choroidal MCs were degranulated at the border of non-atrophic and atrophic choroid (Fig. 1A) (13). In addition, there was a significant increase in percent degranulated MCs at the border of GA, where RPE loss occurs, compared to the age matched healthy eyes (Fig. 1B). Given the results in human subjects, we next investigated whether MC degranulation could be continuously stimulated in the rat choroid with compound 48/80 (hereafter called 48/80), a snake venom-like compound and a well-established MC stimulator. It was previously reported that direct injection of 48/80 into the subconjunctival space induced anterior segment inflammation within 30 min in parallel with MC degranulation, resulting in severe uveitis (25). This suggested that 48/80 must be delivered in slow release fashion from a hydrogel pellet allowing continuous activation and degranulation of choroidal MCs but avoiding acute uveitis. The release and stability of the hydrogel containing 48/80 was first evaluated in vitro utilizing bicinchoninic acid (BCA) assay. Injectable hydrogels in PBS released 48/80 in a sustained manner for a period of 3 d with an initial burst releasing up to 28% within 5 h (Supplemental Fig. 1A). This rapid release was assessed in an aqueous solution but a much slower sustained release was predicted in vivo in the subconjunctival space.
Figure 1.
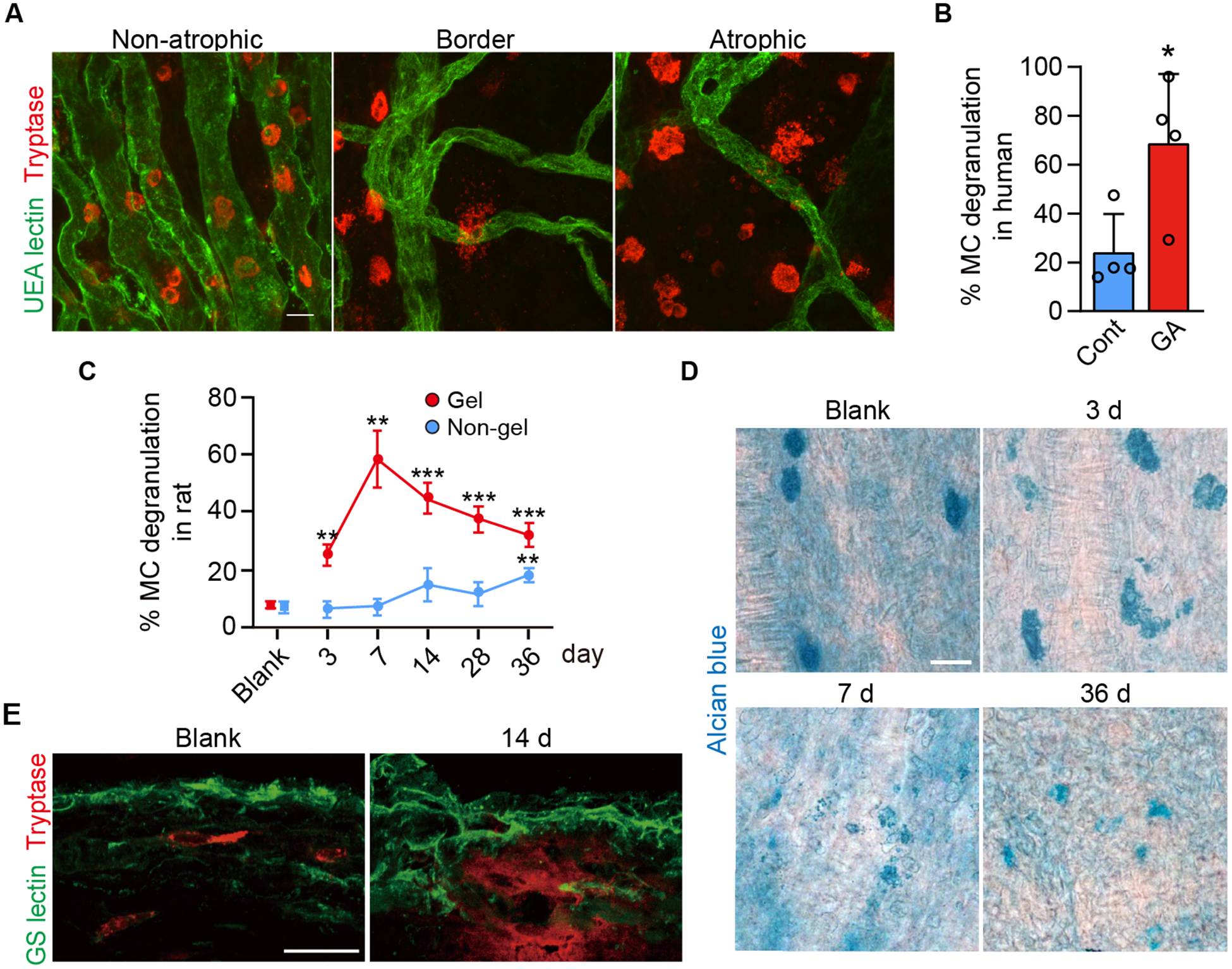
Choroidal MC degranulation in human GA and in the rat model of choroidal MC degranulation. A) Choroidal flat mount immunohistochemistry showing choriocapillaris (UEA lectin; green) and MCs (anti-tryptase; red) in a human GA subject in the non-atrophic region, at the border of atrophy, and in the atrophic lesion. B) Quantification of percentage MC degranulation, as assessed by NSE staining, in the border region of the human GA subjects compared to aged control (n = 4, per group). C) The percentage of degranulated MCs over time after subconjunctival implantation of the 48/80-hydrogel in the rat on the implanted or gel region (superior choroid) and the non-gel region (inferior choroid), as assessed with Alcian blue staining. Quantification was performed on the 48/80 eyes throughout the time course and was compared to blank hydrogel eyes (without 48/80) at day 36 (blank, n = 3; 48/80, n = 4, per time point). D) Time course of MC degranulation in choroidal flat mounts stained with Alcian blue. E) Immunofluorescence of rat choroidal cross sections showing MC (tryptase+; red) and choroidal vessels (GS lectin+; green). Release of tryptase in the choroidal stroma was seen at 14 d post implantation. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, 2-tailed, unpaired Student’s t test. Scale bars, 20 μm.
Given the distribution of choroidal MCs primarily along the long posterior ciliary arteries in the rat choroid (1, 9), the hydrogel with 48/80 was injected subconjunctivally in Sprague/Dawley (S/D) rats in the superior quadrant of the eye, distant from the midline of eye and the greatest concentration of MCs (Supplemental Fig. 1B). A blank hydrogel (without 48/80) was injected in control rats. The hydrogel solidified in the subconjunctival space and is called an implant herein. As predicted, a significant MC degranulation in the gel region of 48/80 implanted eyes was observed using Alcian blue stained choroidal flat mounts; the degranulation peaked at 7 d post implantation, and MCs were still degranulated even at day 36 (Fig. 1C–D). In contrast, no significant degranulation was observed on the non-gel side until day 36, i.e. the inferior quadrant distant from the hydrogel, suggesting that hydrogel interacted focally over the first week with a gradual diffusion to the inferior choroid (Fig. 1C). Consistent with human GA subjects, tryptase was released from degranulating MCs into the choroidal stroma (Fig. 1E) (17).
Time course after MC degranulation
RPE degeneration associated with MC degranulation was assessed next in S/D rats. Intriguingly, RPE cells stained for RPE65 in whole mount choroids showed degeneration over time following MC degranulation, with significant RPE degeneration and loss after 4 wk (Fig. 2A, B). Degenerating RPE cells were seen adjacent to the areas where MC degranulation was confirmed using non-specific esterase (NSE) staining of the choroidal whole mounts (Supplemental Fig. 2A) (13). This indicated that RPE degeneration followed MC degranulation and there were less MCs activated and less RPE degenerated on the non-gel side (Supplemental Fig. 2A). Abnormal autofluorescence of RPE cells was also detected in these lesion (Supplemental Fig. 2B), consistent with human GA subjects (26). In addition, healthy hexagonal RPE cell shape was lost, RPE65 expression was greatly reduced, and there were misaligned cells suggesting tight junction deterioration, which was confirmed with co-labeling for zonula occludins-1 (ZO-1) and RPE65 (Supplemental Fig. 2C). The retinas and choroids were examined histologically through the entire time course using glycol methacrylate-embedded tissue and picrosirius red stained cryosections of eyes. Following the significant RPE loss at 4 and 6 wk post implantation, the retina and choroid demonstrated significant thinning by 8 wk, while no significant changes were seen in the blank hydrogel implanted eyes (Fig. 2C, D). The outer nuclear layer was thinned at 10 wk post implantation with a gradient of severity in loss with transition from gel to the border of gel and non-gel area (Fig. 2C). This appeared to be due to photoreceptor death; therefore, visual function of the retina was assessed using scotopic electroretinogram (ERG) recordings, which revealed significant reduction in both a- and b-wave amplitude after 6 wk (Fig. 2E, F). Reduction of the a-wave amplitude reflects decreased photoreceptor function and the reduction in the b-wave reflects increased photoreceptor dysfunction ensuing an altered post-synaptic signal transduction cascade (27). A similar reduction of scotopic ERG response in the fovea is reported in AMD patients(28). Therefore, functional disturbance of the ERG at 6 wk likely has occurred in advance of the morphological changes seen at 8 and 10 wk post implantation.
Figure 2.

Morphological changes seen after MC degranulation in the 48/80- hydrogel implanted rats. A) Choroidal flat mount labeled for RPE65. Healthy hexagonal RPE cells decreased with time, area of degenerated RPE expanded, and the presence of enlarged cells with multi-nuclei increased with time. Scale bar, 100 μm. B) Percentage of RPE degenerated in the 48/80-hydrogel implanted eyes on the gel and the non-gel side with time after implantation of 48/80-hydrogel. The blank hydrogel implanted eye values were at 6 wk (n = 12–13, per group). C) Representative cross sections of eyes embedded in glycol methacrylate that received a blank hydrogel or 48/80 on the non-gel, at the border of atrophy, and atrophic region on the gel side at 10 wk post implantation. Brackets (U) designate outer nuclear layer or photoreceptor nuclei. Scale bar, 50 μm. D) Area of retina and choroid evaluated in cross sections stained with picrosirius red (n = 6, per time point). E, F) Representative scotopic ERGs at 10 wk. Graphs showing comparison of amplitude of a- and b-waves with the stimuli of 0.01, 0.1 and 1 cd.s/m2 at 6 wk (n = 9), 8 wk (n = 6) and 10 wk (n = 7) compared to the blank gel (n = 8). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, 2-tailed, unpaired Student’s t test.
GA-like features lacking in MC deficient rats implanted with 48/80
MC deficient WsRCws/ws and their wild type (WT) littermates (WsRC+/+) were treated with subconjunctival 48/80-hydrogel to assess whether 48/80 affected only MCs and MC degranulation alone directly contributed to RPE degeneration. WsRCws/ws rats carry a defective gene for c-KIT, which is required for MC differentiation (29). MCs were identified with NSE staining of the conjunctiva in WsRC+/+ rats but not in WsRCws/ws rats (Supplemental Fig. 3A). In addition, WsRCws/ws rats showed no morphological signs of RPE degeneration at 6 wk post 48/80-hydrogel implantation, indicating 48/80 stimulated only MCs and MC activation was responsible for RPE degeneration (Fig. 3A, B). Choroidal macrophages were also shown to be activated in WsRC+/+ adjacent to MC degranulation as determined by an increase in sphericity and decrease in volume as previously reported in human GA lesion (23), but macrophages were not activated in the MC deficient WsRCws/ws choroids (Fig. 3C, D). The macrophages were likely activated by inflammatory cytokines released during MC degranulation and, in addition, possibly by death of adjacent RPE cells (23, 30). Furthermore, supra-RPE and/or subretinal macrophages were detected in WsRC+/+ animals where RPE cells were lost, which has also been reported in human GA (31). However, this was not observed in WsRCws/ws rats (Supplemental Fig. 3B). ERG amplitude declined in WsRC+/+ rats but not in WsRCws/ws rats further suggesting MC degranulation as the cause of degeneration, whereas no difference was observed between WsRCws/ws and WsRC+/+ rats at baseline (Fig. 3E, F).
Figure 3.
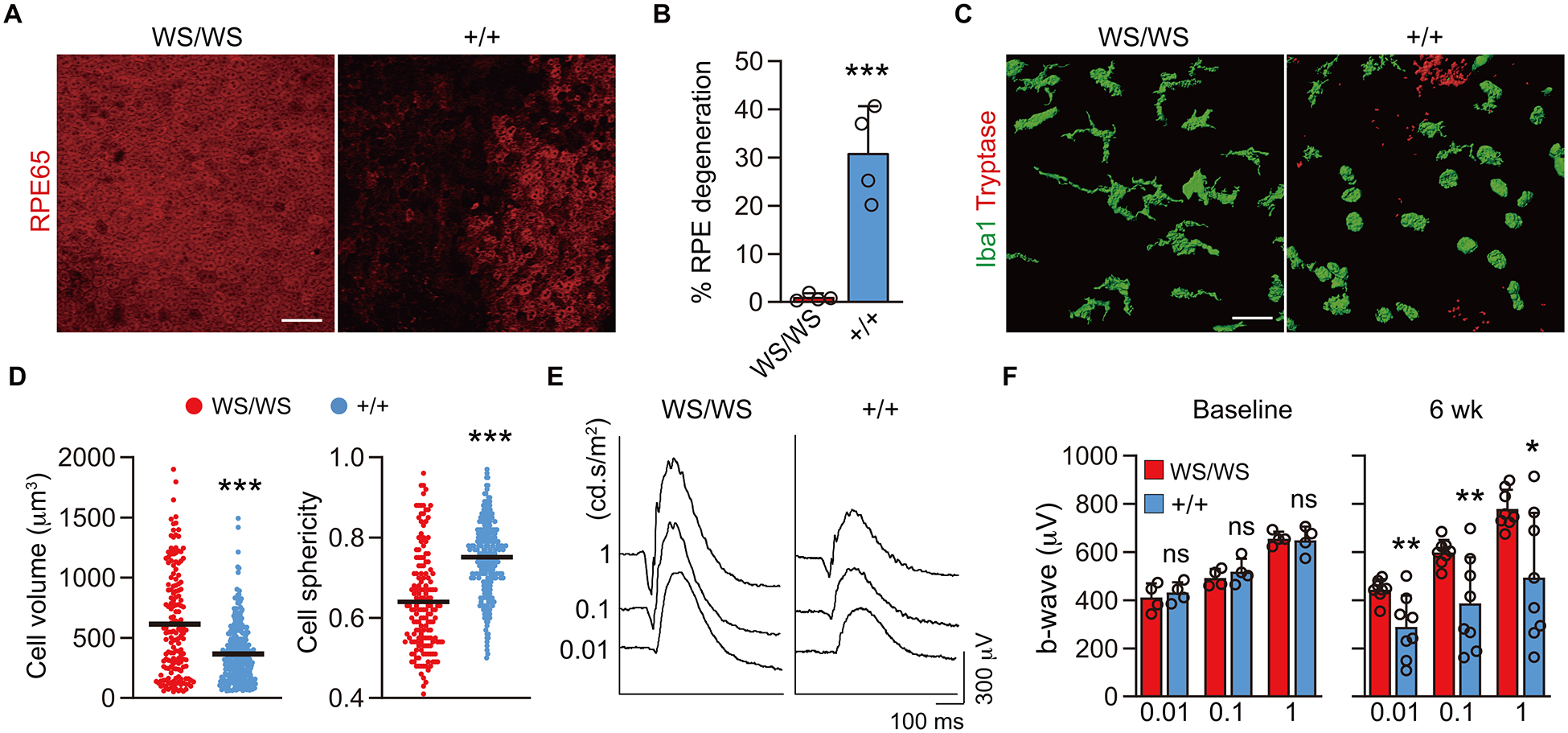
Morphological analyses after 48/80 implantation in MC deficient rats and WT littermates. A, B) Choroidal whole mounts labeled for RPE65 at 6 wk post implantation of 48/80-hydrogel in MC deficient WsRCws/ws (WS/WS) and their WT littermate WsRC+/+ (+/+) rats. The graph shows quantification of percentage RPE degeneration (n = 4, per group). Scale bar, 100 μm. C) Images of three-dimensional volume renderings of macrophage/monocytes (Iba1+; green) and MCs (tryptase+; red) in the choroid. No MCs were detected in WsRCws/ws, while MCs were degranulated in WsRC+/+ WT rats at 6 wk. D) The graph shows comparison of volume and sphericity of Iba+ cells in WsRC+/+ (n = 327) and WsRCws/ws (n = 162). Scale bar, 50 μm. E, F) Representative scotopic electroretinogram (ERG) with the stimuli of 0.01, 0.1 and 1 cd.s/m2 at 6 wk. The graph shows b-wave amplitude with the stimulus of 0.01, 0.1 and 1 cd.s/m2 at baseline (n = 4, per group) and 6 wk post implantation (n = 8, per group). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, 2-tailed, unpaired Student’s t test.
In vitro and ex vivo assays to evaluate MC degranulation and drug efficacy
Given that MCs contributed to the GA-like changes in the rat model, it appeared that MC degranulation could be a therapeutic target and, therefore, quiescing MCs might prevent this phenotype. To this end, the therapeutic potential of a MC stabilizing drug was assessed first by co-incubating the established RBL-2H3 rat MCs with ketotifen fumarate, a MC stabilizer, with or without 48/80 and analyzing the degranulation response using a β-hexosaminidase release assay, as previously reported (22). Ketotifen fumarate is a second-generation non-competitive histamine H-1 receptor antagonist. The mechanism of stabilizing MCs is assumed to be by blocking the intracellular calcium channels needed to form granules (32). It has been used to treat conditions such as asthma, allergic conjunctivitis and mastocytosis (33–35). Ketotifen fumarate treatment in the in vitro assay yielded a 56% inhibition of β-hexosaminidase activity (Fig. 4A). However, this cell line has shortcomings in that the cells share similarities to basophils and are derived from a basophilic leukemia (36). Therefore, an acute ex vivo assay was developed to rapidly evaluate drug’s efficacy and potential to stabilize choroidal MCs specifically and prevent their degranulation. After enucleation and removal of the anterior segments and retina, the eyecup from S/D rats with choroid attached was incubated for 3 h in serum-free medium. Degranulation of MCs as well as choroidal macrophage sphericity and volume, as an indicator of macrophage activation, were evaluated in this assay. To determine that 48/80 only activated MCs ex vivo and not choroidal macrophages, MC deficient WsRCws/ws rats were utilized first in this ex vivo assay. As expected, no significant activation of macrophages was observed with or without 48/80 exposure in eyecups from WsRCws/ws rats (Fig. 4B, C). However, littermate WsRC+/+ rats had significantly increased macrophage sphericity and decreased volume, indicating MC degranulation evoked macrophage activation (Supplemental Fig. 4A, B). Given that 90 min incubation significantly induced MC degranulation and macrophage activation (Supplemental Fig. 4C, D), we concluded that 3 h would be sufficient to evaluate drug efficacy. After 3 h, approximately 70% of the MCs were degranulated with 48/80 (Fig. 4D, E) and co-incubation with ketotifen fumarate prevented MC degranulation, equivalent to the level of untreated eyecups (Fig. 4E). In addition, choroidal macrophage activation was prevented by quiescing MCs with ketotifen fumarate (Fig. 4F, G).
Figure 4.
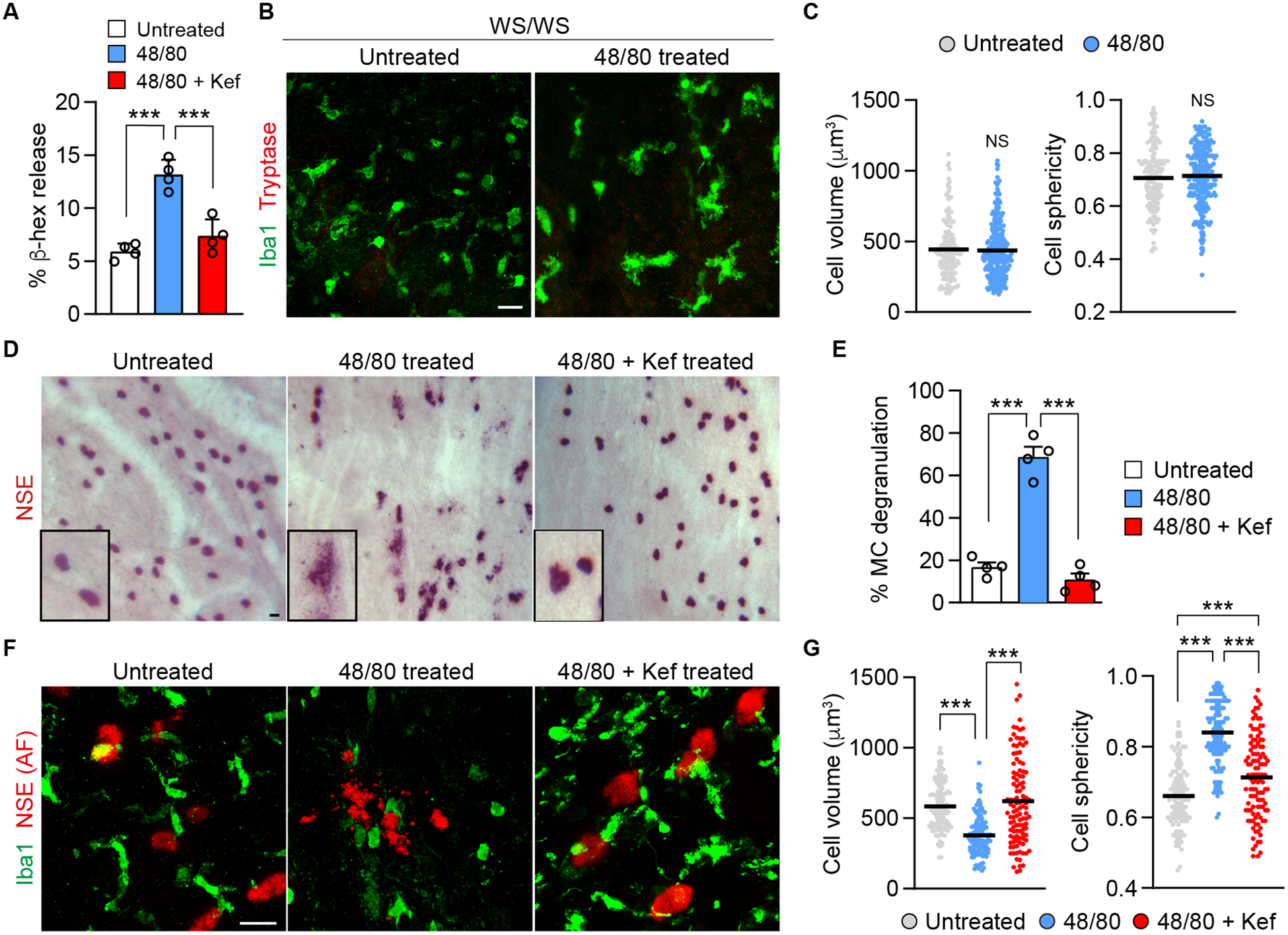
Assays to evaluate MC degranulation and drug efficacy. A) In vitro microplate assay evaluating 48/80-induced (10 μg/ml) release of β-hexosaminidase from RBL-2H3 cells. Cells were co-incubated with 48/80 with or without ketotifen fumarate (Kef) (10 μg/ml) or untreated (no 48/80) medium for 45 min (n = 4). B, C) Choroidal flat mount from ex vivo eyecup assay in MC deficient WsRCws/ws (WS/WS) rats showing macrophages (Iba1+) and MC (tryptase+). Eyecups were incubated for 3 h with 48/80 (300 μg/ml). MCs were not detected in these rats. Graph showing comparison of volume and sphericity of Iba1+ cells in untreated and 48/80 treated eyes of WsRCws/ws rats (untreated, n = 165; 48/80 treated, n = 286). D) Bright field image of the choroid showing MCs (NSE+) after 3 h ex vivo in S/D rats. The boxes are higher magnification images of MCs. E) Quantification of 48/80-induced MC degranulation after 3 h, co-incubated with or without ketotifen fumarate and untreated (n = 4, per group). F) Ex vivo immunohistochemistry of S/D choroid showing MC [NSE, auto-fluorescence (AF)] at 633 nm wavelength and macrophages (Iba1+) after 3 h treatment. G) Comparison of cell volume and sphericity of Iba1+ cells treated with 48/80 with or without ketotifen fumarate and untreated (untreated, n = 114; 48/80, n = 127; 48/80 with ketotifen fumarate, n = 112). Data are mean ± SD. ***P < 0.001, 1-way ANOVA with Tukey post-hoc comparison (A, E, G), 2-tailed, unpaired Student’s t test (C). Scale bars, 20 μm.
Ketotifen fumarate and tryptase inhibitor as therapeutic drugs
To assess whether ketotifen fumarate could prevent GA-like changes in vivo in our rat model, pharmacokinetic (pK) evaluation of oral ketotifen fumarate was performed first. Rats were given 3 mg/kg of ketotifen fumarate in PBS or PBS as control once daily by gavage until sacrifice. The animals for pK were sacrificed on the fifth day and the retina and choroid were dissected from the globes. The pK evaluation indicated that there was 13–29 nM ketotifen fumarate in choroid and 12–52 nM in retina. Knowing that the drug reached choroid, 15 mg/kg of ketotifen fumarate in PBS was administered twice daily by gavage, while controls received PBS orally. Pre-treatment of rats with ketotifen fumarate for 4 d prior to enucleation prevented 37% of MC degranulation and significantly prevented macrophage activation in the ex vivo assay (Supplemental Fig. 5) compared to PBS treatment, indicating the potential of oral ketotifen fumarate administration in vivo. In the rat in vivo model, twice daily of oral treatment with ketotifen fumarate (15 mg/kg) significantly prevented RPE degeneration (Fig. 5A, B) and MC degranulation (Fig. 5C,D) at 4 wk post 48/80-hydrogel implantation. Choroidal macrophage/monocyte activation (increased sphericity and decreased volume) was also prevented (Fig. 5C and E). In addition, treatment with ketotifen fumarate prevented decline of the ERG amplitude in both a- and b-waves (Fig. 5F, G). Histologic evaluation of the retina and choroidal thickness at 8 wk post implantation demonstrated that retinochoroidal thinning was inhibited by quiescing MCs with ketotifen fumarate (Fig. 5H, I). Treating animals with ketotifen fumarate did not affect RPE morphology or viability (Supplemental Fig. 6A) nor did it affect growth of the animals as assessed by body weight measurements (Supplemental Fig. 6B). ERG amplitudes were normal after 6 wk of drug treatment alone (Supplemental Fig. 6C). This indicated that administration of ketotifen fumarate orally did not manifest any adverse effects on the rats in our study.
Figure 5.
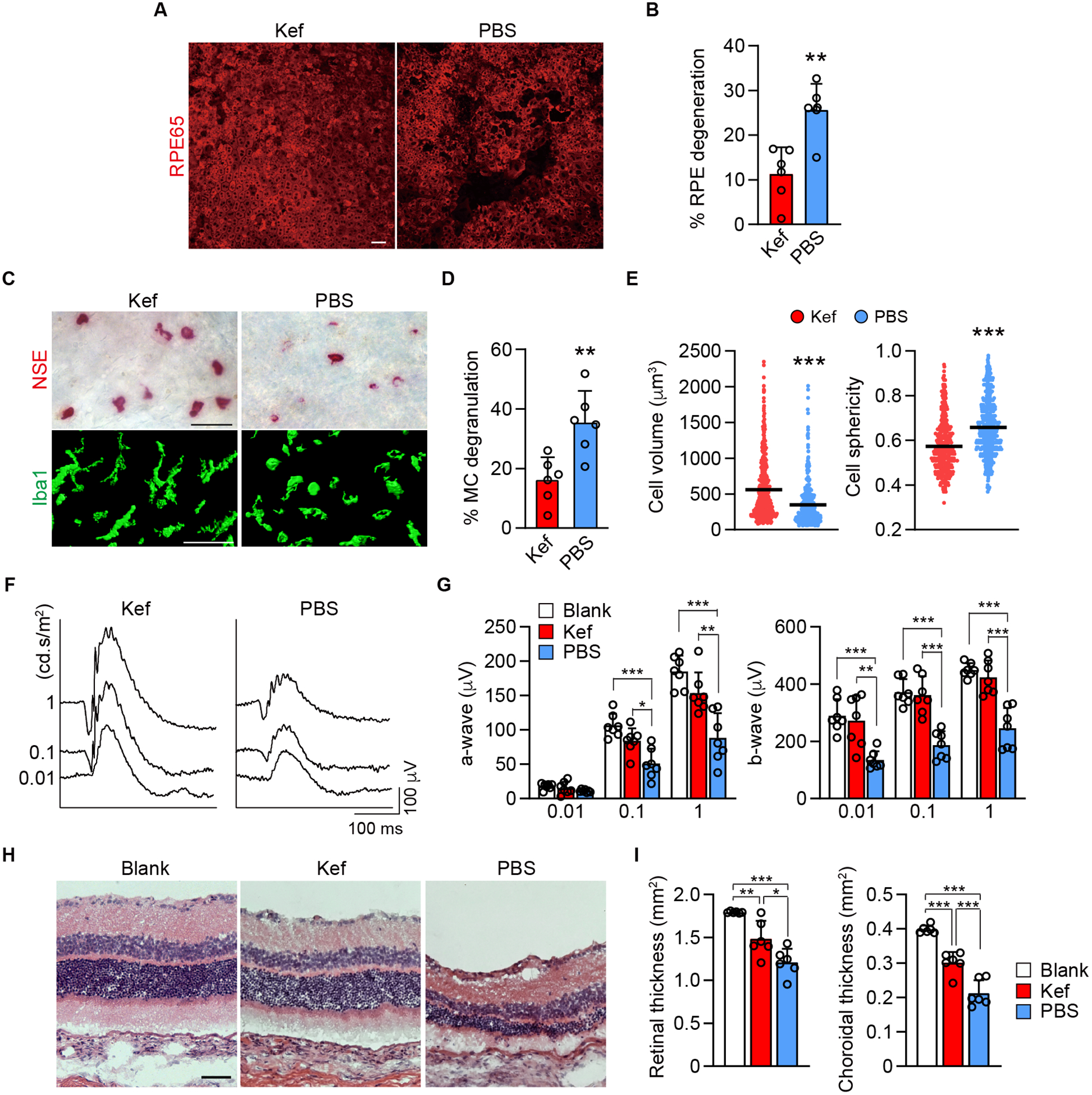
Results of quiescing MCs with the generic MC stabilizer, ketotifen fumarate, in the rat model. A, B) Choroidal whole mount labeled for RPE65 at 4 wk post implantation of 48/80-hydrogel in rats treated orally twice daily with ketotifen fumarate (Kef) or PBS. The graph shows quantification of percentage RPE degeneration in S/D rats (n = 6, per group). C-E) Bright field images of the choroid which show MCs (NSE+) (top) and the images of three-dimensional volume renderings shows choroidal macrophage (Iba1+) (bottom) in ketotifen fumarate and PBS treated rats 4 wk after implanting 48/80-hydrogel. The graph shows percentage of MC degranulation in the two groups (n = 6, per group) and comparison of the volume and sphericity of Iba1+ cells in the choroid after treating with ketotifen fumarate and PBS for 4 wk (ketotifen fumarate, n = 570; PBS, n = 601). F, G) Representative ERG with the stimuli of 0.01, 0.1 and 1 cd.s/m2 at 8 wk after treating with ketotifen fumarate or PBS in the 48/80 implanted eyes. The graph shows a- and b-wave amplitude blank-hydrogel implanted eyes and ketotifen fumarate or PBS treated eyes with the stimulus of 0.01, 0.1 and 1 cd.s/m2 at 8 wk post implantation (n = 7, per group). H, I) Representative H&E sections of blank-hydrogel implanted eye and treatments with ketotifen fumarate and PBS for 8 wk in the rat model. The graphs show area of retina and choroid evaluated in cross sections stained with picrosirius red (n = 6, per group). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, 2-tailed, unpaired Student’s t test. Scale bars, 50 μm.
Tryptase is the most abundant protease released by MCs (37) and tryptase release was increased in choroid/Bruch’s membrane of GA subjects (17). A similar increase in tryptase release was observed in the rat model (Fig. 1D). To test the hypothesis that tryptase directly affects RPE cell viability, in vitro assays were performed first. ARPE-19 cells were cultured with serum-free medium with or without tryptase (either 1 μg/ml or 5 μg/ml). After 72 h incubation, tryptase dose dependently induced ARPE-19 cells loss from a monolayer (Fig. 6A, B), confirming that tryptase can affect RPE adherence and cell viability.
Figure 6.
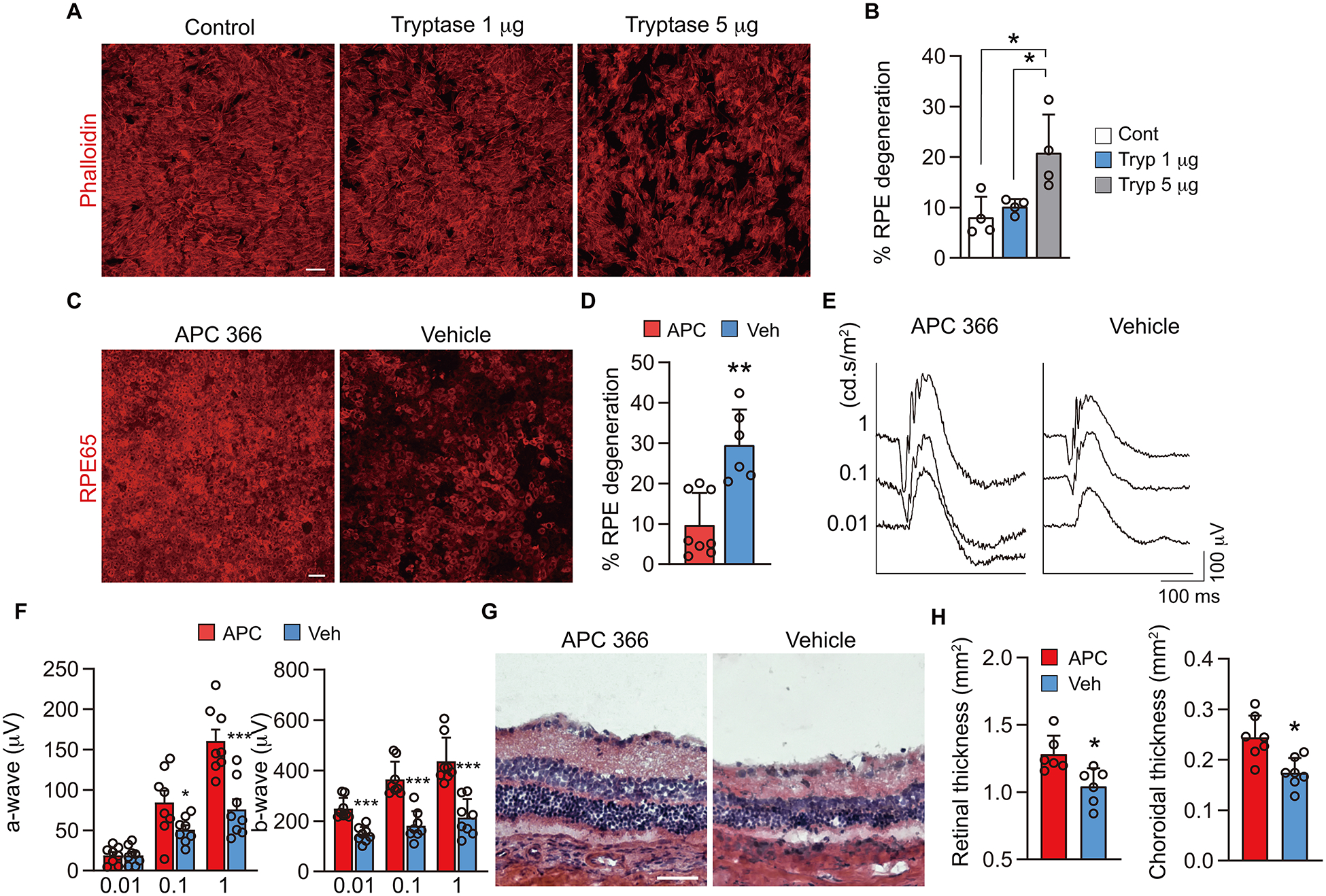
Effects of inhibiting MC-derived tryptase in the rat model. A, B) ARPE-19 cells labeled with phalloidin after 72 h treatment with serum free media (control), tryptase 1 μg/ml and 5 μg/ml in vitro. The graph shows quantification of the percentage of ARPE-19 cells that have degenerated (n = 4, per group). C, D) Choroidal whole mounts labeled for RPE65 at 4 wk post implantation of 48/80-hydrogel in rats treated with daily subcutaneous injections of the tryptase inhibitor APC 366 or vehicle. The graph shows quantification of the percentage of RPE that have degenerated (APC 366; n = 8, vehicle; n = 6). E, F) 8 wk ERG with the stimulus of 0.01, 0.1 and 1 cd.s/m2 at 8 wk after treating with APC 366 or vehicle in the 48/80 implanted eyes. The graph shows a- and b-wave amplitude with the stimuli of 0.01, 0.1 and 1 cd.s/m2 at 8 wk post implantation treated with APC 366 or vehicle (n = 8, per group). G, H) Representative H&E sections after treating with APC 366 and vehicle for 8 wk after administrating 48/80 in the rat. The graphs show area of retina and choroid evaluated in cross sections stained with picrosirius red (n = 6, per group). Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001; 1-way ANOVA with Tukey post-hoc comparison (B), 2-tailed, unpaired Student’s t test (D, F, H). Scale bars, 50 μm.
Therefore, the tryptase inhibitor APC 366, which is reported to prevent allergic, inflammatory or fibrotic changes in human and animal models (24, 38, 39) was evaluated in our 48/80-hydrogel implant rat model. Rats were given a subcutaneous injection of APC 366 (5 mg/kg) or vehicle daily after 48/80 implantation until sacrifice. After 4 wk treatment, RPE degeneration was significantly inhibited (Fig. 6C, D) and choroidal macrophage/monocyte activation was prevented (Supplemental Fig. 7). Furthermore, reduction in ERG amplitude of both a- and b-waves was prevented at 8 wk (Fig. 6E, F). When retinal and choroidal thickness were evaluated, APC 366 treated animals had significantly less retinochoroidal thinning (Fig. 6G, H); therefore all the GA-like changes were suppressed. Collectively, these findings suggest that MC-derived tryptase is a key molecule in development of GA-like changes following chronic MC degranulation.
DISCUSSION
GA is a multifactorial disease characterized by RPE loss and choriocapillaris attenuation and retinal and choroidal atrophy that is clinically manifested over many years. There is increasing evidence that hypoxia-initiated oxidative stress and inflammation contribute to GA pathogenesis (40). Nevertheless, there is insufficient understanding of the cellular or molecular mechanism underlying the development of GA, as well as, few animal models reproducing a GA-like phenotype (12). This paucity of knowledge and animal models has hampered drug development and targeting. In the current study, we present evidence that chronic degranulation of choroidal MCs can induce many phenotypic changes in retina and choroid that occur in GA: RPE degeneration, visual function decline, and retinal and choroidal thinning. However, another hallmark of GA, attenuation of choriocapillaris (21), did not occur in our model, perhaps due to the regenerative capability of the rodent choriocapillaris (41). Furthermore, we developed both ex vivo and in vivo models, which demonstrate that choroidal MC degranulation alone can stimulate choroidal macrophage activation as observed in human GA (23). Using these models, we demonstrated that MC stabilization and tryptase inhibition can prevent these MC-induced GA-like phenotypes.
MCs are the initial responders of innate immunity and their abundance in the uveal tract and choroid suggest their fundamental importance in the eye (1). Indeed, they respond to toxins and microbes as well as substances found in GA choroid such as AGEs, C3a, C5a, and CRP, all of which are implicated in AMD (14, 15, 42, 43). Recently, we reported that choroidal MCs were increased in number and their degranulation was increased in GA subjects (13). We do not know exactly when abnormal MC degranulation occurs in GA choroid, but in that study we found a significant increase in MC numbers and number of degranulated in early AMD subjects, suggesting it is occurring early in the disease process. In addition, MC-derived tryptase was localized in Bruch’s membrane (13, 17). Nevertheless, the precise role of MCs in the development of GA had yet to be elucidated. Here, we demonstrate that hydrogel containing 48/80 implanted subconjunctivally resulted in a gradual and protracted activation and degranulation of choroidal MCs in the rat. Continuous MC degranulation mediated chronic macrophage activation and induced RPE degeneration with subsequent retina and choroidal thinning, four characteristics of human GA (21, 23). We further show evidence that MC-derived tryptase, at least in part, through degradation of the choroid stroma and Bruch’s membrane, contributed to RPE degeneration. The pathological phenotype was prevented by genetically eliminating MCs and by pharmacological intervention targeting MCs with a generic MC stabilizer ketotifen fumarate and a MC specific tryptase inhibitor APC 366. Therefore, MCs with aberrant degranulation and specifically MC-derived tryptase appear to play a central role in developing a GA-like pathology. Therefore, we propose the hypothesis that choroidal MC degranulation and release of MC-derived tryptase (Fig. 7), results in degradation of Bruch’s membrane and choroidal stroma, which ultimately progresses to RPE degeneration as well as retinal and choroidal thinning, which are hallmarks of GA.
Figure 7.
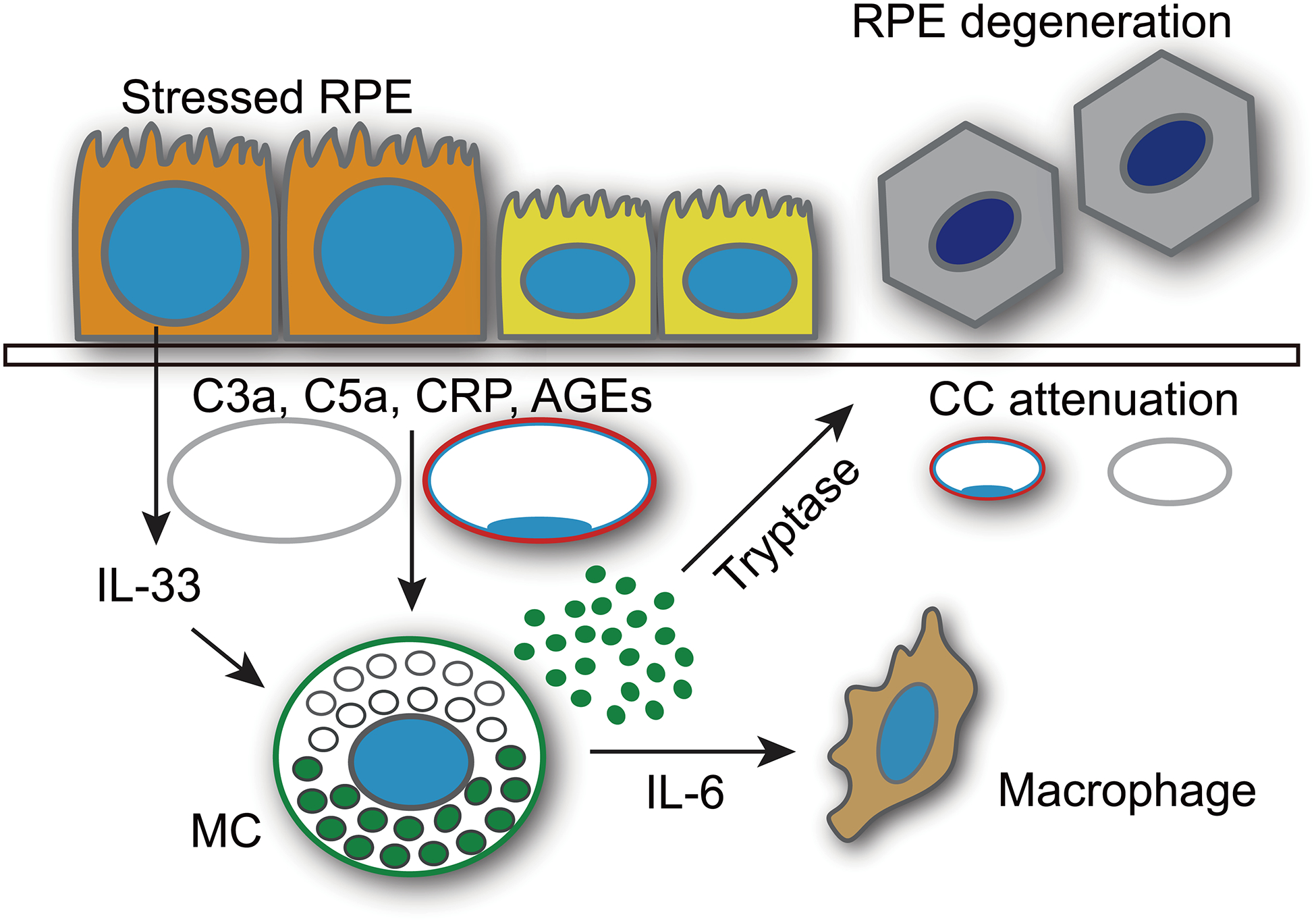
Schematic of hypothesis that MCs contribute to GA. The working hypothesis is that choroidal MCs are activated by hypoxia-induced IL-33 production by RPE or CRP, AGEs, C3a or C5a, which are known to be elevated in AMD choroid. Upon activation and degranulation, tryptase is released into choroidal stroma and Bruch’s membrane. Tryptase can degrade choroidal stroma and Bruch’s membrane resulting in thinning of choroid and degeneration of RPE, which are hallmarks of GA.
To evaluate the efficacy of targeting MCs in GA, development of an assay to screen potential therapeutic compounds was deemed indispensable. To this end, we utilized the well-established rat MC line, RBL-2H3 cells, and their production of β-hexosaminidase, an MC granule enzyme. Although RBL-2H3 cells have been broadly used to assess MC degranulation and drug screening, various contradictions and limitations have also been reported (36). There remains controversy in the lineage of RBL-2H3 cells in that they resemble basophils rather than MCs, which is highly likely because these cells originate from a basophilic leukemia (36). The ex vivo assay developed for these studies overcame those limitations and provided a rapid assay in a tissue milieu involved in AMD, the choroid and its inflammatory cells. This assay enabled sufficient choroidal MC degranulation and effective drug screening in 3 h and yielded data on 3 important parameters: percentage of MCs degranulated in choroid, and volume and sphericity of choroidal macrophages, indicating macrophage activation. The lack of blood flow enabled direct evaluation of only choroidal MCs and resident macrophages and not interaction with circulating cells. In order to exclude the possibility of 48/80 influencing macrophages directly and not activating MCs specifically, MC deficient WsRCWS/WS and their WT control rats were first evaluated in this ex vivo assay. Choroidal macrophages were only activated in WT, not in MC deficient rat choroids, suggesting cytokines released after MC activation and degranulation induced macrophage activation. Similar findings were confirmed in vivo, collectively indicating that cytokines released from activated MCs induced focal inflammation and contributed at least in part to the disease progression. However, we realize that our model only represents MC interaction with macrophages and macrophages in AMD choroid are exposed to an activated complement cascade and CRP as well as MC granule contents.
In the current study, the pharmaceutical benefit of ketotifen fumarate, an FDA-approved generic MC stabilizer, was evaluated in vitro, ex vivo and then in vivo, where it prevented MC degranulation efficiently in all experiments. One of the major complications reported with oral daily treatment was drowsiness but this is said to decrease with time (33). Patients with mastocytosis and chronic asthma are managed chronically with oral MC stabilizers like ketotifen fumarate without severe complications, suggesting that the drug is rather innocuous (44, 45). However, a recent report demonstrated that ketotifen fumarate dose dependently decreased non-rapid eye movement (REM) sleep and increased REM sleep in rats; therefore, it might function as an aid for desirable sleep (46). Nevertheless, no adverse effects with oral ketotifen fumarate treatments were observed in our study.
Inhibiting MC-derived tryptase activity with APC 366 successfully inhibited all GA-like changes in our rat model from RPE degeneration to macrophage activation after MC degranulation. Degeneration of RPE and retinal and choroidal thinning was expected because tryptase degrades collagens and activates MMPs, which further degrade stroma and basement membranes. Previous reports have shown that tryptase released via MC degranulation amplified the production of intercellular adhesion molecule-1, CC-motif ligand 2 and IL-8 in endothelial and epithelial cells (47, 48). Most recently, treating mice with APC 366 suppressed inflammation as assessed by the transcriptional expression of multiple mediators including IL-1β, IL-6, IL-8, CC-motif ligand 2 and MMP-3 in an osteoarthritis model (38). Thus, the effect of tryptase released in degranulation resulting in macrophage activation might well be expected in our model. Also, MCs release IL-6 and tumor necrosis factor when activated, further stimulating macrophage activation (49). Taken together, monocyte/macrophage activation in the choroid and activation of resident macrophages was presumably prevented by inhibiting tryptase with APC 366, hence inhibiting focal inflammation. We have clarified a critical role of MCs in RPE degeneration and the presence of MC-derived pro-inflammatory cytokines and proteases can be presumed to contribute to disease development based on other studies (38, 47). One scenario might be that MCs are activated by stressed RPE pro-inflammatory molecules in the GA choroid and then catalyze tissue destruction via tryptase and also trigger inflammation and macrophage activation, which facilitates the vicious cycle of degeneration in the GA photoreceptor/RPE/Bruch’s membrane/choriocapillaris complex (Fig. 7). Our model (Figure 7) is obviously a simplified scenario in which the effects of mast cell degranulation are highlighted. Our model does not address the complexities of human GA where the complement cascade is activated (50), perhaps in the presence of a defective complement factor H, complement can then activate choroidal macrophages (23), and oxidative stress is known to play a key role (40). Even with those challenges lacking tissue destruction and a GA phenotype resulted in the model, suggesting that MCs could initiate or at least contrbute to GA. Alternatively, MC degranulation like complement activation could be a result of AMD and not the cause. In AMD choroid, complement is activated (elevated C3a and C5a)(14), Complement factor H declines and CRP is greatly elevated (15), providing three potential stimuli for MC migration and degranulation.
In conclusion, this is the first study to demonstrate the involvement of MC degranulation and release of tryptase in initiating GA-like pathologic changes in the eye. It would be interesting in the future to investigate if there is any relationship between incidence of GA and MC activating diseases like mastocytosis, MC activating syndrome, Ehlers-Danos syndrome, and fibromyalgia. The etiology of RPE degeneration and choroidal thinning in GA was not known prior to this study. Our model suggests a potential role for MCs in GA without addressing the pro-inflammatory milieu of GA RPE/choroid complex where complement is activated and oxidative stress is present. A MC stabilizer or inhibition of MC-derived tryptase prevented the disease onset and progression in the rat model. These results support the novel concept that MCs contribute to the development of GA and choroidal MCs could be a viable therapeutic target for the currently untreatable GA.
Supplementary Material
Acknowledgments
The authors are grateful to the eye donors and their relatives for their generosity. This work was supported by National Eye Institute (NIH) Grants EY-01765 (Wilmer), R01-EY016151 (G.A.L), Bayer AG, Arnold and Mabel Beckman Foundation and unrestricted funds from Research to Prevent Blindness (Wilmer).
Nonstandard Abbreviations
- Ab
antibody
- AGEs
advanced glycation end products
- AMD
age-related macular degeneration
- APase
alkaline phosphatase
- BCA
bicinchoninic acid
- CRP
C-reactive protein
- ERG
electroretinogram
- GA
geographic atrophy
- H&E
hematoxylin and eosin
- MC
mast cell
- MMP
matrix metalloproteinase
- NSE
non-specific esterase
- pK
pharmacokinetic
- RPE
retinal pigment epithelium
- RT
room temperature
- S/D rats
Sprague/Dawley rats
- UEA
Ulex europaeus agglutinin
- WsRCWS/WS rats
rats with defective c-KIT gene and have no MCs
- WT
wild type
Footnotes
Conflict of Interest: W. Schubert is an employee of the Bayer AG Pharmaceuticals. The other authors declare no conflict of interest.
REFERENCES
- 1.McMenamin PG (1997) The distribution of immune cells in the uveal tract of the normal eye. Eye 11 183–193 [DOI] [PubMed] [Google Scholar]
- 2.Wernersson S, and Pejler G (2014) Mast cell secretory granules: armed for battle. Nature reviews. Immunology 14, 478–494 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz LB (1987) Mediators of human mast cells and human mast cell subsets. Annals of allergy 58, 226–235 [PubMed] [Google Scholar]
- 4.Iddamalgoda A, Le QT, Ito K, Tanaka K, Kojima H, and Kido H (2008) Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Archives of dermatological research 300 Suppl 1, S69–76 [DOI] [PubMed] [Google Scholar]
- 5.Bradding P, Walls AF, and Holgate ST (2006) The role of the mast cell in the pathophysiology of asthma. The Journal of allergy and clinical immunology 117, 1277–1284 [DOI] [PubMed] [Google Scholar]
- 6.Ponomaryov T, Payne H, Fabritz L, Wagner DD, and Brill A (2017) Mast Cells Granular Contents Are Crucial for Deep Vein Thrombosis in Mice. Circulation research 121, 941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, and Shi GP (2007) Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. The Journal of clinical investigation 117, 3359–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krystel-Whittemore M, Dileepan KN, and Wood JG (2015) Mast Cell: A Multi-Functional Master Cell. Frontiers in immunology 6, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfrey WA (1987) Characterization of the choroidal mast cell. Transactions of the American Ophthalmological Society 85, 557–599 [PMC free article] [PubMed] [Google Scholar]
- 10.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, and Wong TY (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2, e106–116 [DOI] [PubMed] [Google Scholar]
- 11.Holz FG, Strauss EC, Schmitz-Valckenberg S, and van Lookeren Campagne M (2014) Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 121, 1079–1091 [DOI] [PubMed] [Google Scholar]
- 12.Pennesi ME, Neuringer M, and Courtney RJ (2012) Animal models of age related macular degeneration. Mol Aspects Med 33, 487–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhutto IA, McLeod DS, Jing T, Sunness JS, Seddon JM, and Lutty GA (2016) Increased choroidal mast cells and their degranulation in age-related macular degeneration. The British journal of ophthalmology 100, 720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, and Ambati J (2006) Drusen complement components C3a and C5a promote choroidal neovascularization. Proceedings of the National Academy of Sciences of the United States of America 103, 2328–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, and Lutty GA (2011) C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. The British journal of ophthalmology 95, 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada Y, Ishibashi K, Bhutto IA, Tian J, Lutty GA, and Handa JT (2006) The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Experimental eye research 82, 840–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod DS, Bhutto I, Edwards MM, Gedam M, Baldeosingh R, and Lutty GA (2017) Mast Cell-Derived Tryptase in Geographic Atrophy. Investigative ophthalmology & visual science 58, 5887–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne V, and Kam PC (2004) Mast cell tryptase: a review of its physiology and clinical significance. Anaesthesia 59, 695–703 [DOI] [PubMed] [Google Scholar]
- 19.Bhutto IA, Ogura S, Baldeosingh R, McLeod DS, Lutty GA, and Edwards MM (2018) An Acute Injury Model for the Phenotypic Characteristics of Geographic Atrophy. Investigative ophthalmology & visual science 59, AMD143–AMD151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soiberman U, Kambhampati SP, Wu T, Mishra MK, Oh Y, Sharma R, Wang J, Al Towerki AE, Yiu S, Stark WJ, and Kannan RM (2017) Subconjunctival injectable dendrimer-dexamethasone gel for the treatment of corneal inflammation. Biomaterials 125, 38–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, and Lutty GA (2009) Relationship between RPE and choriocapillaris in age-related macular degeneration. Investigative ophthalmology & visual science 50, 4982–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weatherly LM, Kennedy RH, Shim J, and Gosse JA (2013) A microplate assay to assess chemical effects on RBL-2H3 mast cell degranulation: effects of triclosan without use of an organic solvent. J Vis Exp, e50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLeod DS, Bhutto I, Edwards MM, Silver RE, Seddon JM, and Lutty GA (2016) Distribution and Quantification of Choroidal Macrophages in Human Eyes With Age-Related Macular Degeneration. Investigative ophthalmology & visual science 57, 5843–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Chen B, Li S, and Sun Q (2014) Tryptase inhibitor APC 366 prevents hepatic fibrosis by inhibiting collagen synthesis induced by tryptase/protease-activated receptor 2 interactions in hepatic stellate cells. Int Immunopharmacol 20, 352–357 [DOI] [PubMed] [Google Scholar]
- 25.Bousquet E, Zhao M, Thillaye-Goldenberg B, Lorena V, Castaneda B, Naud MC, Bergin C, Besson-Lescure B, Behar-Cohen F, and de Kozak Y (2015) Choroidal mast cells in retinal pathology: a potential target for intervention. The American journal of pathology 185, 2083–2095 [DOI] [PubMed] [Google Scholar]
- 26.Delori FC, Fleckner MR, Goger DG, Weiter JJ, and Dorey CK (2000) Autofluorescence distribution associated with drusen in age-related macular degeneration. Investigative ophthalmology & visual science 41, 496–504 [PubMed] [Google Scholar]
- 27.Pardue MT, and Peachey NS (2014) Mouse b-wave mutants. Doc Ophthalmol 128, 77–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter P, Widder RA, Luke C, Konigsfeld P, and Brunner R (1999) Electrophysiological abnormalities in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 237, 962–968 [DOI] [PubMed] [Google Scholar]
- 29.Niwa Y, Kasugai T, Ohno K, Morimoto M, Yamazaki M, Dohmae K, Nishimune Y, Kondo K, and Kitamura Y (1991) Anemia and mast cell depletion in mutant rats that are homozygous at “white spotting (Ws)” locus. Blood 78, 1936–1941 [PubMed] [Google Scholar]
- 30.Omri S, Behar-Cohen F, de Kozak Y, Sennlaub F, Verissimo LM, Jonet L, Savoldelli M, Omri B, and Crisanti P (2011) Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCzeta in the Goto Kakizaki rat model. The American journal of pathology 179, 942–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillonneau X, Eandi CM, Paques M, Sahel JA, Sapieha P, and Sennlaub F (2017) On phagocytes and macular degeneration. Progress in retinal and eye research 61, 98–128 [DOI] [PubMed] [Google Scholar]
- 32.Craps LP, and Ney UM (1984) Ketotifen: current views on its mechanism of action and their therapeutic implications. Respiration 45, 411–421 [DOI] [PubMed] [Google Scholar]
- 33.Grant SM, Goa KL, Fitton A, and Sorkin EM (1990) Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs 40, 412–448 [DOI] [PubMed] [Google Scholar]
- 34.Kidd M, McKenzie SH, Steven I, Cooper C, Lanz R, and Australian Ketotifen Study G (2003) Efficacy and safety of ketotifen eye drops in the treatment of seasonal allergic conjunctivitis. The British journal of ophthalmology 87, 1206–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valent P, Sperr WR, Schwartz LB, and Horny HP (2004) Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. The Journal of allergy and clinical immunology 114, 3–11; quiz 12 [DOI] [PubMed] [Google Scholar]
- 36.Passante E, and Frankish N (2009) The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflamm Res 58, 737–745 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz LB, Irani AM, Roller K, Castells MC, and Schechter NM (1987) Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. Journal of immunology 138, 2611–2615 [PubMed] [Google Scholar]
- 38.Wang Q, Lepus CM, Raghu H, Reber LL, Tsai MM, Wong HH, von Kaeppler E, Lingampalli N, Bloom MS, Hu N, Elliott EE, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove J, Fukuoka Y, Schwartz LB, Galli SJ, and Robinson WH (2019) IgE-mediated mast cell activation promotes inflammation and cartilage destruction in osteoarthritis. Elife 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishna MT, Chauhan A, Little L, Sampson K, Hawksworth R, Mant T, Djukanovic R, Lee T, and Holgate S (2001) Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. The Journal of allergy and clinical immunology 107, 1039–1045 [DOI] [PubMed] [Google Scholar]
- 40.Datta S, Cano M, Ebrahimi K, Wang L, and Handa JT (2017) The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Progress in retinal and eye research 60, 201–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi A, Majji AB, Fujioka S, Kim HC, Fukushima I, and de Juan E Jr. (1999) Surgically induced degeneration and regeneration of the choriocapillaris in rabbit. Graefes Arch Clin Exp Ophthalmol 237, 668–677 [DOI] [PubMed] [Google Scholar]
- 42.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, and Zuberbier T (1997) C3a and C5a stimulate chemotaxis of human mast cells. Blood 89, 2863–2870 [PubMed] [Google Scholar]
- 43.Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, and Yamamotos H (1998) Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia 41, 1435–1441 [DOI] [PubMed] [Google Scholar]
- 44.Kabra SK, Pandey RM, Singh R, and Seth V (2000) Ketotifen for asthma in children aged 5 to 15 years: a randomized placebo-controlled trial. Ann Allergy Asthma Immunol 85, 46–52 [DOI] [PubMed] [Google Scholar]
- 45.Marone G, Spadaro G, Granata F, and Triggiani M (2001) Treatment of mastocytosis: pharmacologic basis and current concepts. Leuk Res 25, 583–594 [DOI] [PubMed] [Google Scholar]
- 46.Unno K, Ozaki T, Mohammad S, Tsuno S, Ikeda-Sagara M, Honda K, and Ikeda M (2012) First and second generation H(1) histamine receptor antagonists produce different sleep-inducing profiles in rats. European journal of pharmacology 683, 179–185 [DOI] [PubMed] [Google Scholar]
- 47.Kinoshita M, Okada M, Hara M, Furukawa Y, and Matsumori A (2005) Mast cell tryptase in mast cell granules enhances MCP-1 and interleukin-8 production in human endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 25, 1858–1863 [DOI] [PubMed] [Google Scholar]
- 48.Cairns JA, and Walls AF (1996) Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. Journal of immunology 156, 275–283 [PubMed] [Google Scholar]
- 49.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, and Kalogeromitros D (2012) Mast cells and inflammation. Biochim Biophys Acta 1822, 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, and Kaarniranta K (2016) Inflammation and its role in age-related macular degeneration. Cellular and molecular life sciences : CMLS 73, 1765–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


