Abstract
Dexterous forelimb movements like reaching, grasping, and manipulating objects are fundamental building blocks of the mammalian motor repertoire. These behaviors are essential to everyday activities, and their elaboration underlies incredible accomplishments by human beings in art and sport. Moreover, the susceptibility of these behaviors to damage and disease of the nervous system can lead to debilitating deficits, highlighting a need for a better understanding of function and dysfunction in sensorimotor control. The cerebellum is central to coordinating limb movements, as defined in large part by Joseph Babinski and Gordon Holmes describing motor impairment in patients with cerebellar lesions over 100 years ago (Babinski, 1902; Holmes, 1917), and supported by many important human and animal studies that have been conducted since. Here, with a focus on output pathways of the cerebellar nuclei across mammalian species, we describe forelimb movement deficits observed when cerebellar circuits are perturbed, the mechanisms through which these circuits influence motor output, and key challenges in defining how the cerebellum refines limb movement.
Keywords: cerebellar nuclei, reach, grasp, internal copy, ataxia, dysmetria
Introduction
The control of forelimb movement is distributed across a broad network of neural circuits dedicated to establishing appropriate spinal motor neuron activity and forelimb muscle contraction. The precise coordination of dozens of muscles required for dexterity depends on descending commands that direct and modulate movement through spinal networks, as well as continuous feedback from sensory pathways that refine motor output (Lemon, 2008; Alstermark and Isa, 2012; Azim and Seki, 2019). Visual, proprioceptive, and tactile pathways provide crucial sensory information about the consequences of moving the limb and any environmental disturbances (Scott et al., 2015; Tuthill and Azim, 2018). Yet, a major challenge for the motor system is that sensory feedback carries inherent temporal delays; thus relying only on peripheral signals for refinement can result in ill-timed and inaccurate movements (Wolpert and Miall, 1996; Shadmehr et al., 2010; Azim and Alstermark, 2015).
One proposed solution to these temporal delays is that the cerebellum uses internal copies of motor commands to predict movement outcome, by implementing a forward model that represents the dynamics of the body (Wolpert and Miall, 1996) (Fig. 1A). In principle, these predictions can be used to compensate for delayed sensory feedback and rapidly update the state estimate of the limb, enabling online adjustments of outgoing motor signals and ensuring precise trajectories (Scott, 2004; Shadmehr and Krakauer, 2008; Adrian and John, 2012; Azim et al., 2014a). On longer timescales, the cerebellum is also thought to reshape this forward model. Mismatch between predictions and actual sensory outcome can be corrected over time, leading to sensorimotor adaptation of subsequent movements (Thach et al., 1992a; Blakemore et al., 2001; Ilg et al., 2008; Ito et al., 2014). Thus, theory predicts that the cerebellum uses a combination of internal copies of motor commands and sensory information about the state of the limb to refine movement across timescales (Wolpert et al., 1998; Kawato, 1999).
Figure 1. Cerebellar circuits for forelimb movement.
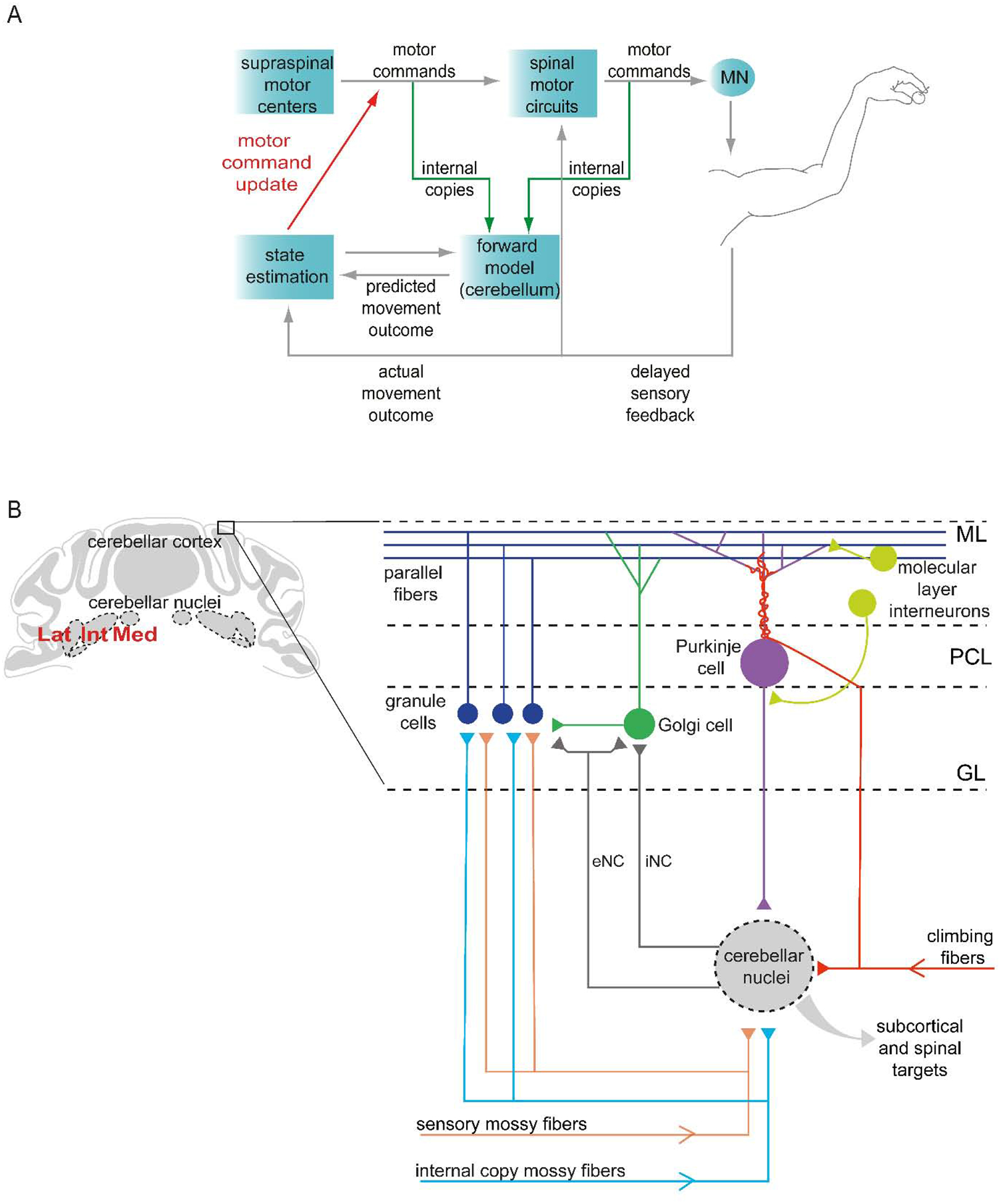
(A) During limb movement, motor commands elicit muscle contraction, generating sensory feedback that is used to update the estimate of limb state and adjust motor output from supraspinal and spinal targets. Yet sensory feedback delays imply a need for a more rapid internal feedback mechanism. Copies of motor commands (internal copies) are thought to be conveyed to the cerebellum, where a forward model generates a prediction of movement outcome, enabling more rapid online refinement. Reducing mismatch between predictions and sensory-reported outcome can also be used to adapt subsequent movements. Cerebellar output is thus tasked with recruiting the necessary motor structures to update motor commands and adjust limb movement (Azim and Alstermark, 2015). (B) The cerebellar cortex receives inputs that deliver sensory and internal copy signals from the spinal cord and brainstem (mossy fibers), and teaching-related signals from the inferior olive (climbing fibers) (Huang et al., 2013; Ishikawa et al., 2015; Miall, 2016; Lang et al., 2017). Neurons in the cerebellar nuclei, the primary output of the cerebellum, also receive input from mossy fiber and climbing fiber collaterals. The activity of neurons in the cerebellar nuclei is shaped by inhibition from Purkinje cells located in the Purkinje cell layer (PCL). Granule cells located in the granular layer (GL) receive mossy fiber signals and provide excitatory input to Purkinje cells via parallel fibers that extend through the molecular layer (ML). Purkinje cells also receive strong excitatory input from climbing fibers, as well as inhibitory input from molecular layer interneurons. Mossy fiber inputs to granule cells are regulated by inhibitory Golgi cells. Neurons in the cerebellar nuclei send inhibitory nucleo-cortical fibers (iNC) to Golgi cells, and excitatory nucleo-cortical fibers (eNC) to granule cells and Golgi cells. Cerebellar nuclear neurons project to many subcortical and spinal targets (Miall, 2016).
How does the cerebellum influence limb movement? Ultimately, the cerebellum affects forelimb muscle activity through output from the cerebellar nuclei, which are linked to many subcortical and spinal targets (Bentivoglio, 1982; Teune et al., 2000; Houck and Person, 2015; Fujita et al., 2020). Projection neurons in the cerebellar nuclei receive a convergence of inputs from Purkinje cells in the cerebellar cortex, as well as collateral input from mossy fibers and climbing fibers conveying motor and sensory information (Fig. 1B). Purkinje cell activity, also driven by mossy fibers (via granule cells) and climbing fibers, shapes the rate and timing of cerebellar nuclear neuron activity through inhibition (Chan-Palay, 1977; Person and Raman, 2011; Uusisaari and De Schutter, 2011; Wu and Raman, 2017) (Fig. 1B). As forward models are thought to be implemented in the cerebellar cortex, projection neurons in the cerebellar nuclei are an ideal candidate to carry out rapid online correction based on predicted movement outcomes, and longer-term adaptation of future movements (Wolpert et al., 1998) (Fig. 1A).
In this review, we focus on the final conduit of cerebellar signaling – the cerebellar nuclei – and describe how delineating their organizational and functional logic in several mammalian species is clarifying how the cerebellum contributes to dexterous motor control. We begin by describing how the execution of skilled forelimb movements is affected in patients with cerebellar disease. We then outline the organization of cerebellar output circuits and summarize insights gained from experimental perturbation and electrophysiological recording in the cerebellar nuclei of animal models. Finally, we review recent studies using molecular-genetic strategies in mice that have begun to define specific cell types and neural circuits that control and refine limb movements. By describing key studies across model systems, we aim to provide a perspective on how cerebellar output controls dexterous behaviors, and highlight critical challenges that remain.
Insights from patients with cerebellar dysfunction
Deficits in reaching movements
Key evidence for the importance of the cerebellum in controlling limb movements has come from analysis of humans with cerebellar pathology (Babinski, 1902; Holmes, 1917). Patients with cerebellar ataxia exhibit uncoordinated movements of the extremities known as dysmetria (Hore et al., 1991; Schmahmann, 2004; Manto, 2009), and often display deficits in endpoint precision that are either hypermetric (overshoot) or hypometric (undershoot) during reaching (Holmes, 1917; Gilman et al., 1976; Schmahmann, 2004). Moreover, limb movements in these patients have irregular trajectories towards a target, suggesting disruptions in online motor adjustments (Gilman et al., 1976; Bastian et al., 1996; Tseng et al., 2007).
Another defining feature of cerebellar disease is limb oscillations, known as intention tremor. Intention tremor is most apparent during goal-directed movements of the extremities, and becomes more pronounced as the effector (forelimb/hindlimb) reaches the end of the movement (Flament and Hore, 1986; Deuschl et al., 2000). A potential explanation for this characteristic tremor is that cerebellar dysfunction disrupts forward model-based prediction, leaving delayed sensory signals as the primary determinant of limb state estimation (Azim and Alstermark, 2015) (Fig. 1A). Thus, delayed sensory information leads to outdated corrections that accumulate and manifest as a tremor as the target is approached (Miall and King, 2008). Recent studies in patients with cerebellar ataxia have shown that providing artificial phase-advanced visuomotor feedback from self-motion in a virtual reality setup is able to improve limb control (Zimmet et al., 2019), supporting the idea that cerebellar predictions are needed to compensate for sensory feedback delays during movement.
During multi-joint limb behaviors, movement of one joint often results in dynamic interaction torques at adjacent joints (Hollerbach and Flash, 1982). Kinetic analysis of the torque generated at the elbow and shoulder joints during reaching movements shows that patients with cerebellar ataxia are unable to generate suitable muscle torques during fast reaching movements. Moreover, kinematic analysis of elbow and shoulder joints shows abnormalities in the relative timing of joint movements, curved trajectories, and poor endpoint precision (Bastian et al., 1996) (Fig. 2A). These deficits are thought to result from loss of the ability to produce appropriate muscle activity that predictively compensates for interaction torques, leading to a loss of coordination during movement (Bastian et al., 2000). Together, these studies uncover a critical role for the cerebellum in controlling precision and stability during goal-directed reaching movements.
Figure 2. Reaching and grasping deficits in patients with cerebellar ataxia.
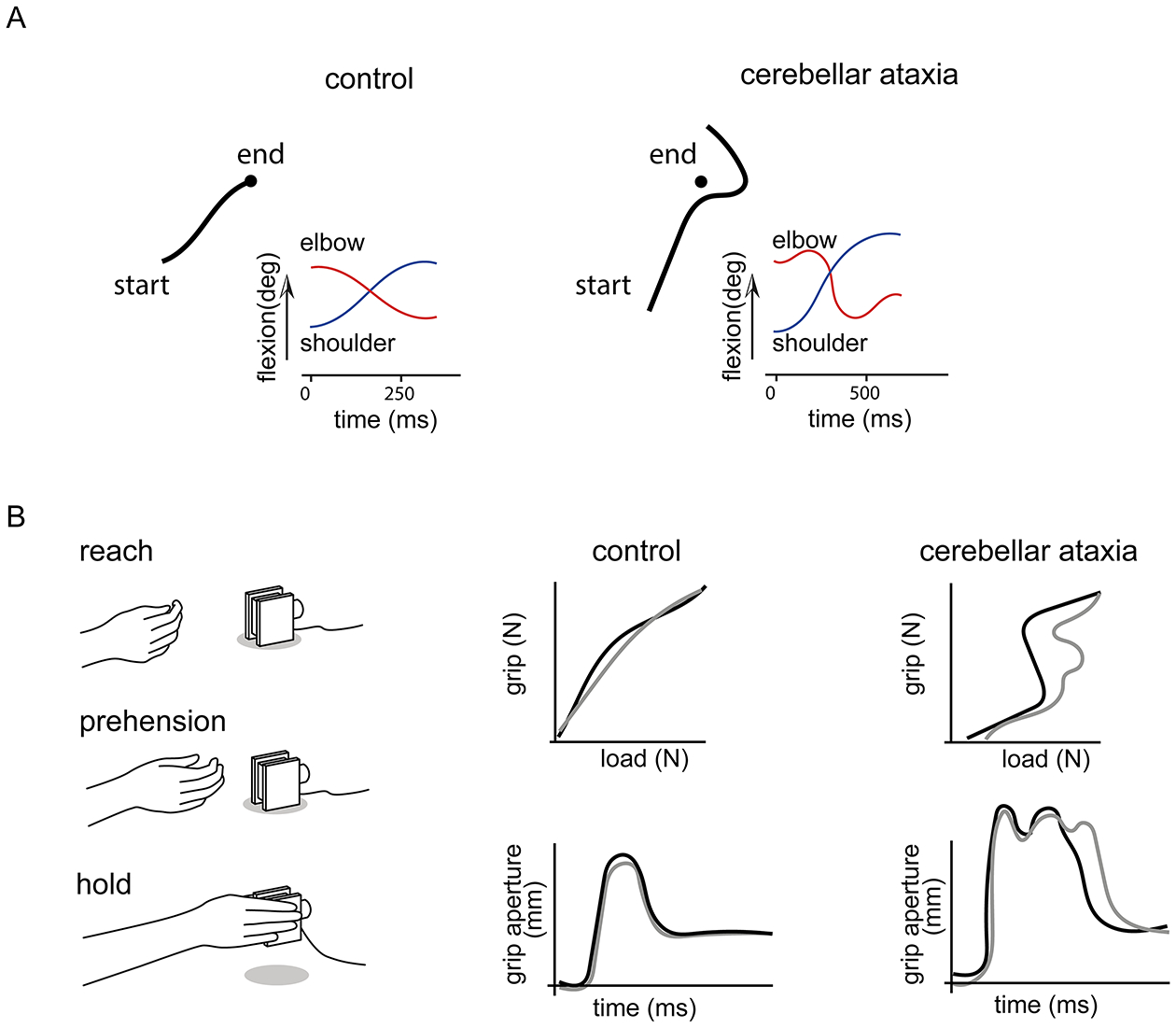
(A) When asked to perform fast, accurate reaches toward a target (filled black circles), control subjects exhibit straight trajectories, while patients with cerebellar ataxia display dysmetria, in this case showing a tendency to overshoot the target (traces show the trajectory of the index finger). Patients also exhibit curved trajectories, increased shoulder flexion (blue traces), and inappropriate flexion and extension of the elbow (red traces), as compared to controls (Bastian et al., 1996). (B) During reach-to-grasp movements (left), patients with cerebellar ataxia show abnormal grip force relative to load force during the grasp (top), and exaggerated grip aperture (bottom) compared to control subjects (traces show two example trials) (Brandauer et al., 2008).
Deficits in grasping movements
The ability to grip, rotate, manipulate, and stabilize an object with appropriate force during movement is a major attribute of dexterity, and critical for the development and use of tools (Gibson, 1991; Nowak et al., 2013). Individuals predictively shape their hands when grasping an object, and modify the grip force appropriate for the load once contact is made (Brandauer et al., 2008). With changes in load, grip force is dynamically adjusted to maintain stability (Nowak et al., 2013). In addition to perturbed reaching, patients with cerebellar ataxia also have characteristic deficits when grasping an object, exhibiting an exaggerated grip aperture (i.e. the distance between the thumb and index finger), and increased grip force relative to load (Brandauer et al., 2008; Nowak et al., 2013) (Fig. 2B).
Adjusting grasp and grip force on an object can be reactive or predictive. Reactive adjustments occur in variable environmental conditions when the load of the object is unknown. Conversely, predictive adjustments occur when the load of an object can be anticipated, such as during voluntary movement of the limb with an object already in hand (Zackowski et al., 2002; Nowak et al., 2013). Patients with cerebellar ataxia have difficulty adjusting grip forces to predictable changes, but their reactive responses appear less affected (Lang and Bastian, 1999). This disruption of predictive grasp control could be due to defective implementation of forward models necessary for anticipatory adjustments that produce appropriate force.
Studies of reaching and grasping in patients with cerebellar ataxia reveal the importance of the cerebellum in fine-tuning proximal and distal limb movements. While this work highlights critical roles for the cerebellum in controlling trajectories of the limb and adapting movements to changes in the environment, pathophysiology in cerebellar patients varies widely and can be difficult to attribute to deficits in specific cerebellar circuit elements. In order to gain mechanistic insight into cerebellar pathways that control limb movement, we now turn to the organization of cerebellar circuits, and animal studies examining the role of substructures within the cerebellum that contribute to motor output.
Cerebellar circuits
The cerebellum is broadly divided into the cerebellar cortex and nuclei (Fig. 1B). The cerebellar cortex consists of three distinct layers: the molecular layer (ML), the Purkinje cell layer (PCL), and the granular layer (GL) (Eccles et al., 1967; Miall, 2016). The PCL contains a single layer of Purkinje cells (PCs) that receive convergent internal copy and sensory input from granule cells that contact PCs through long parallel fibers extending through the ML. PCs also receive strong input from climbing fibers originating in the inferior olive. The final output of PCs in cerebellar cortical regions that influence limb movement is channelled through the cerebellar nuclei, which then target extra-cerebellar structures (Chan-Palay, 1977) (Fig. 1B, Fig. 3A,B). Thus, defining the input-output organization of the cerebellar nuclei is an important step in addressing the contribution of the cerebellum to forelimb behavior.
Figure 3. Major targets of the cerebellar nuclei.
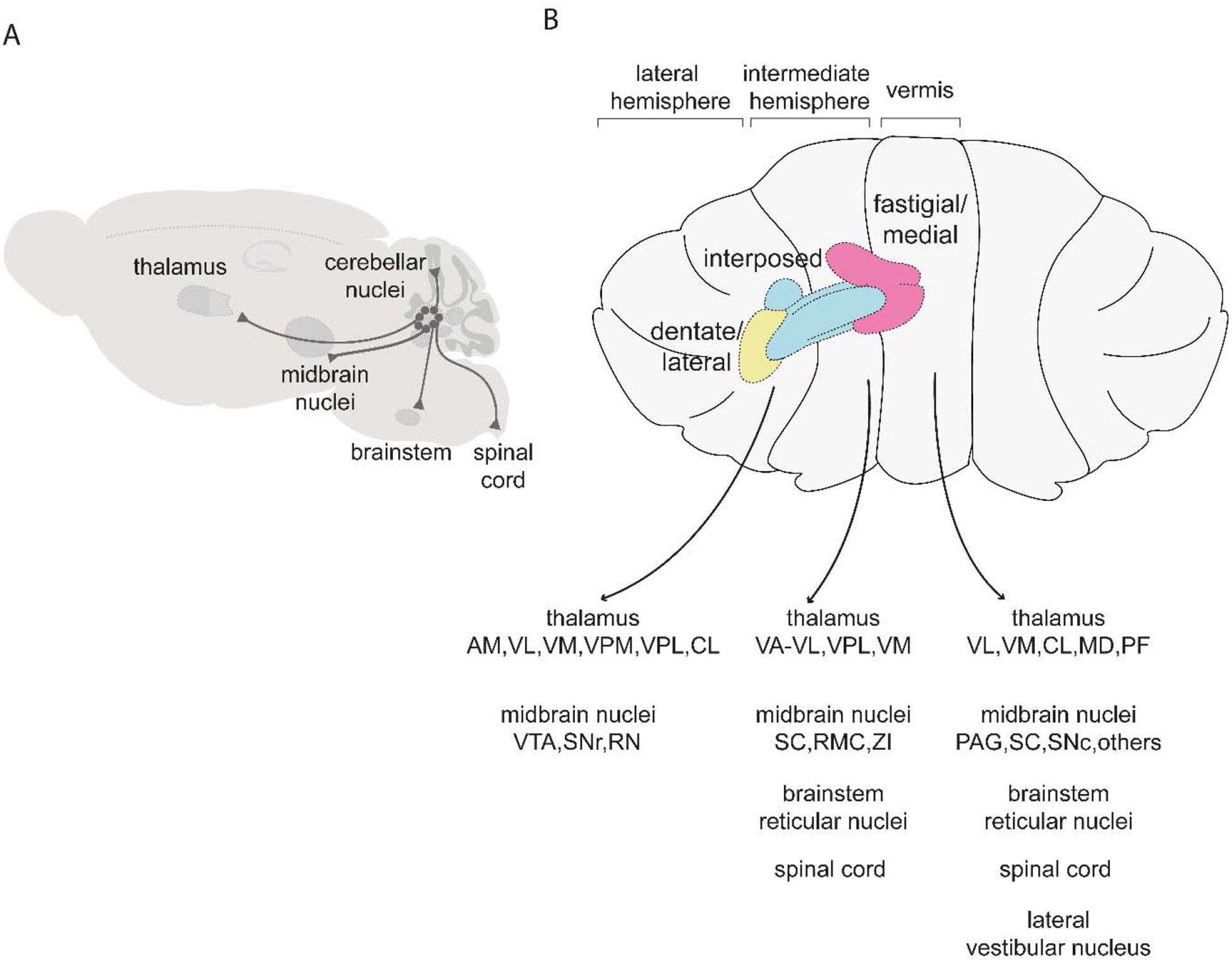
(A) Schematic of pathways originating from the cerebellar nuclei. (B) Targets of the fastigial/medial nucleus include the thalamus (VL: ventrolateral, VM: ventromedial, CL: centrolateral, MD: mediodorsal, PF: parafascicular), midbrain nuclei (PAG: periaqueductal gray, SC: superior colliculus, SNc: substantia nigra pars compacta), brainstem reticular nuclei, spinal cord, lateral vestibular nucleus, and other regions described in (Fujita et al., 2020). Targets of the interposed nuclei include the thalamus (VA-VL: ventral anterior-ventrolateral, VPL: ventral posterolateral, VM: ventromedial), midbrain nuclei (SC: superior colliculus, RMC: magnocellular red nucleus, ZI: zona incerta), brainstem reticular nuclei, and spinal cord (Houck and Person, 2015; Low et al., 2018; Sathyamurthy et al., 2020). Targets of the dentate/lateral nucleus include the thalamus (AM: anteromedial, VL: ventrolateral, VM: ventromedial, VPM: ventral posteromedial: VPL ventral posterolateral, CL: centrolateral) and midbrain nuclei (VTA: ventral tegmental area, SNr: substantia nigra pars reticulata, RN: red nucleus) (Carta et al., 2019; Dacre et al., 2019; Sakayori et al., 2019). For simplicity, schematics are restricted to tracing studies performed in mice.
Over the past few decades, principles of the organization of PC convergence in cortico-nuclear circuits and how nuclear neurons respond to PC inputs have emerged (Heck et al., 2013). The inhibitory nature of PCs suggests that increases in their activity should correlate with a decrease in cerebellar nucleus output. Yet while nuclear neurons have been shown to reduce their firing in response to sensory stimuli (Cody et al., 1981), increases in firing in both PCs and nuclear neurons have also been observed during movement (Thach, 1970a, b; Armstrong and Edgley, 1984). A key study examining the convergence of cortico-nuclear projections determined that mimicking asynchronous PC activity suppresses nuclear neurons, while synchronous PC activity drives phase-locked nuclear neuron activity, providing a mechanism through which PCs in the cortex can control precise firing in cerebellar output pathways (Person and Raman, 2011, 2012). Another recent study found that PCs increase or decrease their activity during saccadic eye movements, and their simple spike population response can predict movements of the eye. Importantly, the PC population code could reflect anatomical organization – PCs that project to a nuclear neuron group together based on similar complex spike activity driven by input from the inferior olive (Herzfeld et al., 2015). Thus, cortico-nuclear convergence is functionally organized based on olivocerebellar projections, and this grouping of PCs can be used to communicate specific information to target neurons in the cerebellar nuclei (Shadmehr, 2020). The organizational logic of these circuit will be further clarified as approaches for simultaneous large-scale recording from PCs and nuclear neurons continue to evolve.
While PCs are important regulators of nuclear neuron activity, the cerebellar nuclei also receive input from mossy fibers delivering motor and sensory information from the brain and periphery (Glickstein, 1997). Mossy fibers have been shown to control nuclear activity in a PC dependent manner. For instance, excitatory input from mossy fibers is more effective at driving nuclear neuron activity when coupled with convergent synchronous PC activity (Wu and Raman, 2017). Additionally, similar to climbing fiber mediated plasticity at parallel fiber-PC synapses, LTP and LTD have also been observed at mossy fiber-cerebellar nuclear neuron synapses, highlighting the potential role of these nuclear inputs in motor learning (Raymond et al., 1996; Ohyama et al., 2006; Pugh and Raman, 2006; Zhang and Linden, 2006). Taken together, convergent PC circuit organization combined with dynamic sensory and motor inputs make the cerebellar nuclei a central hub of sensorimotor processing equipped to exert precise control over downstream circuit activity.
The cerebellum is implicated in a wide range of motor and cognitive behaviors (Schmahmann and Sherman, 1998; Ito, 2008; Popa and Ebner, 2018; Schmahmann et al., 2019). This functional diversity is attributed, in part, to the cerebellum’s widespread connections to many regions of the brain and spinal cord (Bentivoglio, 1982; Schmahmann and Pandya, 1997; Teune et al., 2000; Kelly and Strick, 2003; Schmahmann et al., 2004; Liang et al., 2011; Fujita et al., 2020; Sathyamurthy et al., 2020). Moreover, the extensive motor and sensory convergence and the diversity of neuronal subtypes within the cerebellar nuclei suggest that nuclear neurons perform computational roles, rather than simply serving as a relay station for processed signals from the cerebellar cortex (Pugh and Raman, 2008; Uusisaari and De Schutter, 2011; Herzfeld et al., 2020).
The cerebellar nuclei can be divided into the dentate (lateral in rodents), interposed, and fastigial (medial in rodents) nuclei (Chan-Palay, 1977; Paxinos and Franklin, 2007) (Fig. 1B,3B). In rodents, these three major, anatomically-distinct nuclei are further subdivided into nine subnuclei: interposed anterior (IntA), interposed posterior (IntP), interposed posterior parvicellular (IntPPC), interposed dorsolateral (IntDL), medial (Med), medial dorsolateral (MedDL), medial lateral part (MedL), lateral (Lat), and lateral parvicellular (LatPPC) (Paxinos and Franklin, 2007; Sugihara, 2011). The cerebellar cortex, which provides input to the nuclei, can be subdivided into sagittal zones (Sugihara and Shinoda, 2004; Apps and Hawkes, 2009). Each zone is a longitudinal area of the cerebellar cortex defined by climbing fiber innervation from distinct areas of the inferior olive (Voogd and Ruigrok, 2004; Apps and Hawkes, 2009; Sotelo, 2020). Sagittal zones of the cerebellum have been proposed to represent operational units, with paramedian and vermal zones controlling sensorimotor behaviors and the laterally expanded zones also influencing non-motor behaviors (Oscarsson, 1979; Bostan and Strick, 2013; Hawkes, 2014; Cerminara et al., 2015). Climbing fiber input also divides the cerebellar nuclei into subregions that receive a convergence of input from zones with similar climbing fiber receptive fields, together forming modules of cerebellar processing (Garwicz and Ekerot, 1994; Sugihara, 2011; Voogd, 2014). Thus, distinct cerebellar nuclear regions may implement a specific set of output functions.
Neurons in the cerebellar nuclei are not homogenous. Through electrophysiological and anatomical studies, cerebellar nuclear neurons have been classified into diverse subtypes (Raman et al., 2000; Czubayko et al., 2001; Aizenman et al., 2003; Sultan et al., 2003; Uusisaari and Knopfel, 2011; Canto et al., 2016). Excitatory and inhibitory projection neuron classes link the cerebellar nuclei with extra-cerebellar targets (Chan-Palay, 1977; Uusisaari and Knopfel, 2011). Glutamatergic projection neurons in the cerebellar nuclei innervate subcortical motor regions and collateralize to form nucleo-cortical mossy fibers in the granular layer (Houck and Person, 2015; Gao et al., 2016). Inhibitory neurons in the cerebellar nuclei form a feedback loop that modulates activity within the glomeruli of the inferior olive, as well as project back to the cerebellar cortex to regulate Golgi cells (Llinas, 2013; Ankri et al., 2015; Lang et al., 2017) (Fig. 1B). Additionally, glycinergic cerebellar nuclear neurons also make functional connections with brainstem targets (Bagnall et al., 2009).
Tracing experiments have shown that axons originating from neurons within each cerebellar nucleus target multiple subcortical motor nuclei (Teune et al., 2000; Kelly and Strick, 2003; Houck and Person, 2015; Low et al., 2018; Fujita et al., 2020) (Fig. 3A,B). Based on this target selectivity, each nucleus may have distinct functions. For example, within the Med nucleus is a subclass of neurons that project to the lateral vestibular nucleus, known to be involved in balance and control of head/trunk movements (Ilg et al., 2008; Bagnall et al., 2009; Murray et al., 2018). Neurons in both the Lat and Int nuclei project to the motor thalamus and red nucleus, and are implicated in voluntary, goal-directed movements (Keifer and Houk, 1994; Tlamsa and Brumberg, 2010; Houck and Person, 2015; Low et al., 2018). Additional brain and spinal cord regions are also targeted by the cerebellar nuclei, such as the brainstem reticular nuclei, superior colliculus, zona incerta, and ipsilateral and contralateral spinal cord (Teune et al., 2000; Liang et al., 2011; Chen et al., 2014; Sathyamurthy et al., 2020) (Fig. 3A,B). Details of the connectivity and functional relevance of many of these output projections are beginning to be explored. Overall, diversity in cerebellar output pathways suggests that subtypes of neurons in each nucleus engage specific pathways to regulate distinct features of motor and non-motor control. In the following sections, we provide an overview of key studies in several mammalian species that have begun to uncover roles of specific cerebellar outputs for dexterous limb movement.
Cerebellar nuclei and the control of forelimb movements
Inactivation of the cerebellar nuclei disrupts limb movements
Supporting observations from patients with cerebellar pathology, reversible inactivation studies in animals have revealed important roles for the cerebellar nuclei in forelimb movement. Focal muscimol inactivation of the cerebellar nuclei in cats and non-human primates have shown that each nucleus contributes to different features of movement (Thomas Thach and Bastian, 2004). Inactivation of the fastigial nucleus in cats results in deficits in balance, head and trunk control, and locomotion (Martin et al., 2000). In monkeys, inactivation of the fastigial nucleus causes animals to fall to the ipsilateral side. In both instances, reaching and grasping movements are not obviously affected when the animal’s body is supported (Thach et al., 1992b; Martin et al., 2000), suggesting that the fastigial nucleus does not have a primary role in controlling forelimb behavior. In cats, muscimol inactivation of the dentate nucleus results in slowed movement and a modest increase in reach path curvature, though success in a reach-to-grasp task is not affected (Martin et al., 2000). Inactivation of the dentate nucleus in monkeys results in hypermetria during reaching, perturbs wrist movements, and impairs grasping and coordination of the digits during food retrieval (Thach et al., 1992b; Ishikawa et al., 2014). Thus, the dentate nucleus influences the control of forelimb movement, with potential distinctions in functional roles across different species.
Perhaps the most extensively studied nucleus in the context of forelimb movement is the Int nucleus. Inactivation of the IntA and IntP subnuclei in cats produces pronounced effects on limb movement, resulting in dysmetria and changes in the speed of wrist movement during reaching (Bracha et al., 1999; Martin et al., 2000) (Fig. 4A). In monkeys, IntA and dentate nuclei inactivation results in an inability to extend the hand during a grasping movement, and IntP inactivation results in instability of the arm during reach and poor accuracy (Mason et al., 1998). Inactivation of the Int nucleus in monkeys also triggers dynamic tremor during reaching, causing the animal to miss the target (Thach et al., 1992b). When predictable force pulses were applied during the hold phase of a reach-to-grasp task, monkeys also had difficulty anticipating and maintaining appropriate grip force (Monzee et al., 2004; Monzee and Smith, 2004) (Fig. 4B). Additionally, IntA inactivation in cats causes a loss of anticipatory limb adjustments to an obstruction, while sensory reactive adjustments are left intact (Milak et al., 1997; Martin et al., 2000). The importance of the Int nucleus in coordinating limb behavior is not restricted to reaching and grasping movements. Inactivation of the Int nucleus in cats also affects conditioning of the withdrawal reflex as well as paw placement and movement precision during locomotion, indicating a critical role in controlling limb movement across behavioral contexts (Bracha et al., 1999).
Figure 4. Reversible inactivation of the Int nucleus results in abnormal forelimb movement.
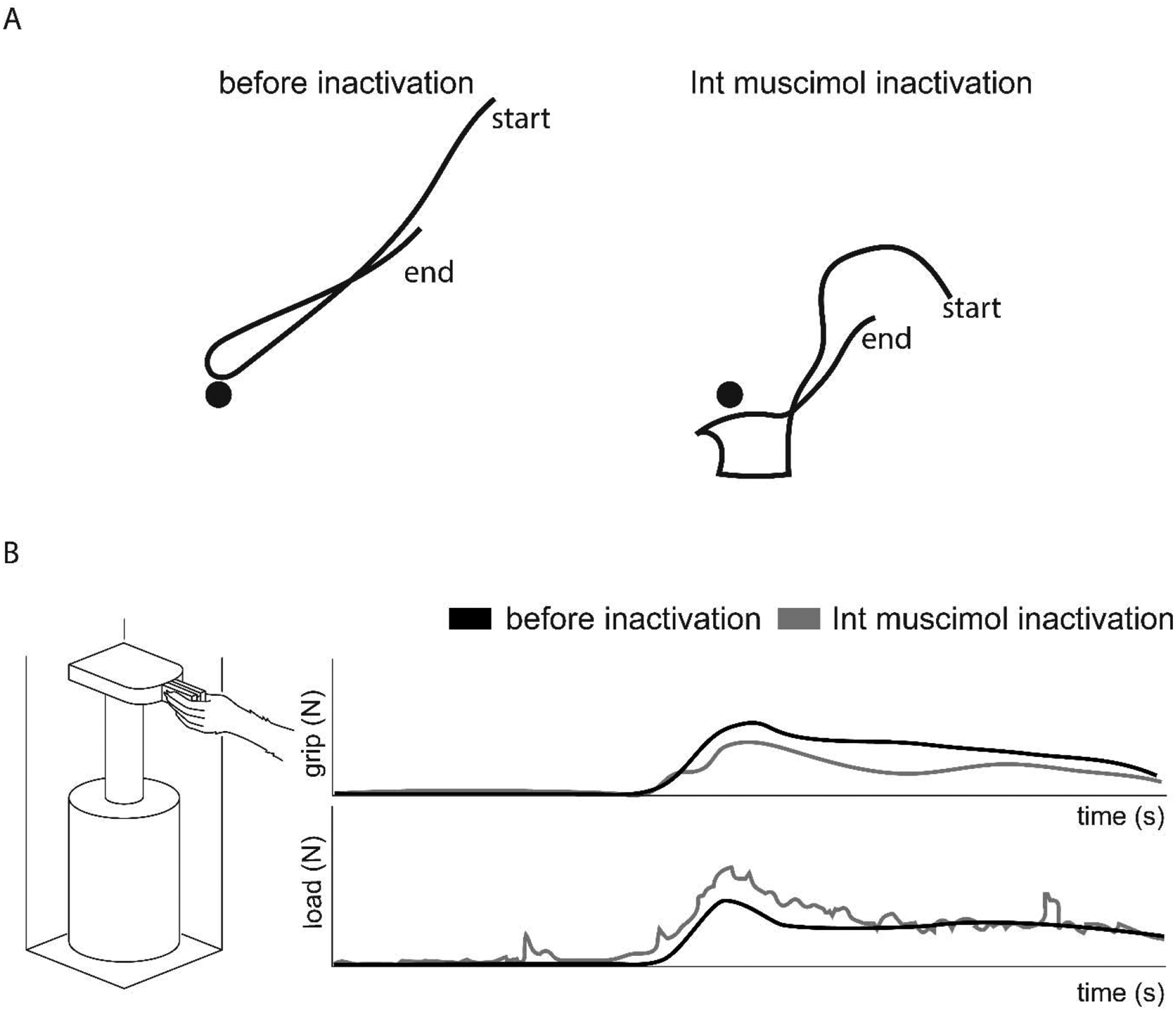
(A) Cats exhibit dysmetric trajectories when reaching toward a target (filled black circles) and during limb retraction after muscimol inactivation of the Int nucleus (Bracha et al., 1999). (B) During a grasp, lift, and hold task (left), muscimol inactivation of the Int nucleus in monkeys results in reduced grip force during the hold phase (top) and ataxic movement with dynamic tremor visible in the load force (bottom) (Monzee et al., 2004).
Supporting distinct contributions of cerebellar substructures to forelimb movement, patients with focal cerebellar lesions in the intermediate and lateral areas of cerebellum including the interposed and dentate nuclei show characteristic deficits in reaching and grasping behavior. Lesions of the Int nucleus result in increased lift off times during movement while dentate lesions result in slowed reaching and prehensile deficits (Bastian and Thach, 1995; Küper et al., 2011). Together, inactivation and lesion studies across species reveal the importance of the cerebellar nuclei in controlling dexterous movements through adjustment of limb kinematics and grip force.
Electrophysiological mapping of forelimb circuits of the interposed nucleus
Electrophysiological recording and stimulation experiments have provided insight into the organization of the cerebellar nuclei defined by sensory input and movements evoked. A series of studies investigated the functional organization of cerebellar nuclear neurons for forelimb control in anesthetized cats (Ekerot et al., 1997). Using cerebellar zones as an anatomical reference point, somatosensory input receptive fields in the cerebellar cortex and corresponding output regions of the cerebellar nuclei were mapped by peripheral stimulation (Ekerot et al., 1991). Forelimb cutaneous and nociceptive climbing fiber receptive fields in motor zones of the cerebellar cortex were mapped via stimulation of forelimb skin, and climbing fibers with similar receptive fields were found to terminate in a single longitudinal zone (Ekerot et al., 1991; Jorntell et al., 1996). In addition, analysis of tactile receptive fields through extracellular field recording within the IntA nucleus revealed a topographical organization of nuclear neurons. Nuclear regions that respond to climbing fiber input from specific forelimb areas receive a convergence of Purkinje cell input from cortical microzones corresponding to the same forelimb regions (Garwicz and Ekerot, 1994) (Fig. 5A). Thus, climbing fiber inputs relaying somatosensory information to the cerebellum appear to be organized into specific cortico-nuclear circuits.
Figure 5. Organization of forelimb areas in the IntA nucleus.
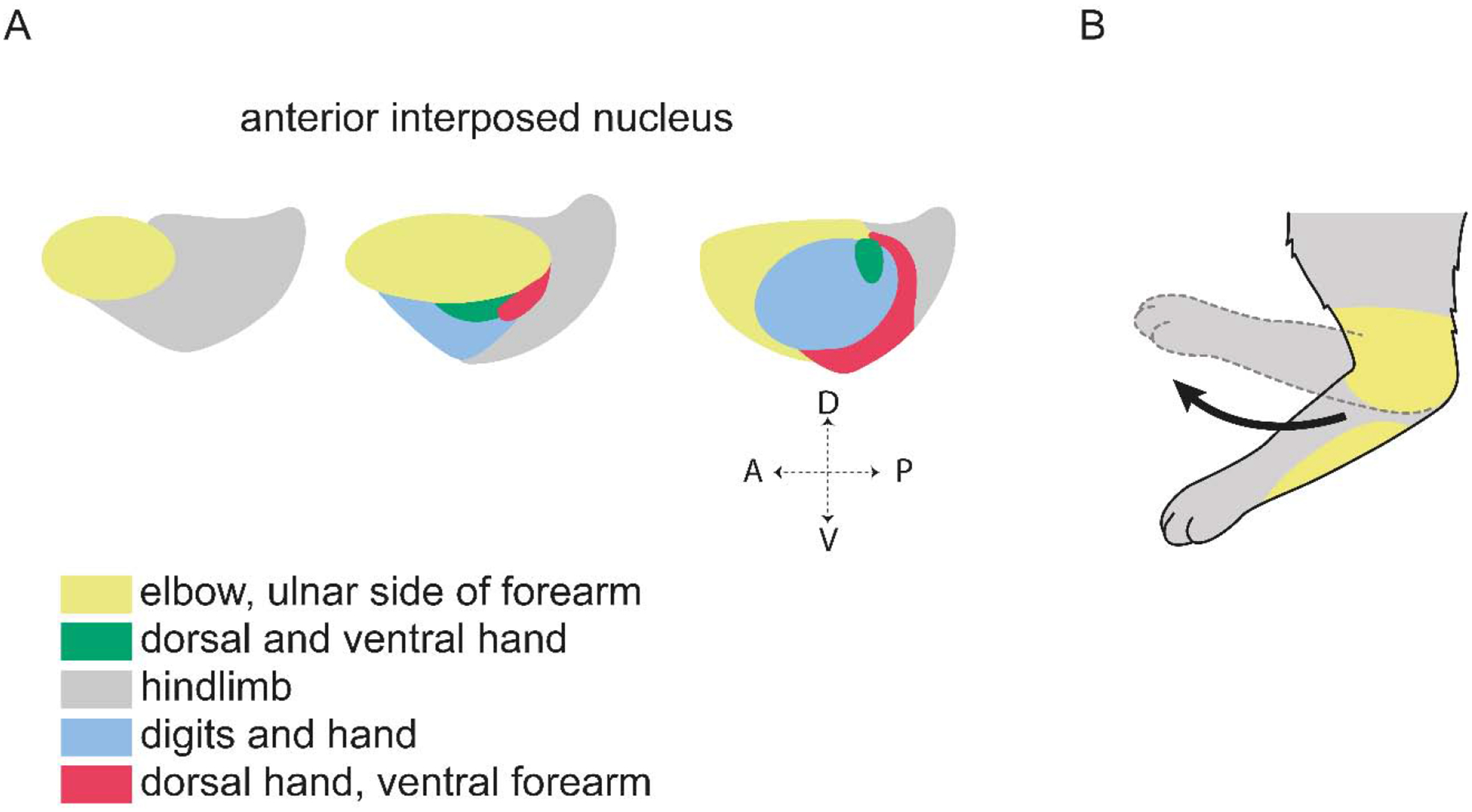
(A) The IntA nucleus is topographically organized based on Purkinje cell termination from microzones with specific climbing fiber receptive fields. Shown here are forelimb and hindlimb cutaneous and nociceptive receptive fields (Garwicz and Ekerot, 1994; Ekerot et al., 1997). (B) Microstimulation of the nuclear zone receiving indirect cutaneous input from the ulnar side of the forelimb results in flexion of the elbow, moving the limb region corresponding to the receptive field away from the putative stimulus (Ekerot et al., 1995).
Microstimulation of cerebellar nuclear neurons was also used to explore how output pathways of the cerebellum influence limb movement (Schultz et al., 1979; Ekerot et al., 1995). IntA stimulation in cats evokes multi-joint movements consisting predominantly of flexion of the elbow and shoulder joints, and both flexion and extension of the wrist (Ekerot et al., 1995). Importantly, stimulation of a specific nuclear region elicits withdrawal of the limb segment with corresponding cutaneous receptive fields; for example, stimulation of sites with receptive fields on the ulnar surface of the forelimb produces flexion at the elbow and withdrawal of the ulnar surface of the limb (Ekerot et al., 1997) (Fig. 5B). Thus, cutaneous input appears to be an important determinant of which limb regions are recruited by specific IntA neurons. The topographic organization of sensory inputs to cerebellar cortico-nuclear circuits provides a potentially important link between feedback pathways that report unexpected environmental perturbations, and feedforward pathways that move the corresponding parts of the body.
The role of cerebellar nuclear neurons during forelimb movements has also been investigated in monkeys (van Kan et al., 1993; Monzee and Smith, 2004). Neural activity correlated to forelimb movements has been identified in the IntA, IntP, and adjacent dentate regions. Recording single neuron discharge within these regions during reach to grasp and joint movements revealed that nuclear neurons are modulated strongly by multi-joint forelimb movements, but show limited specificity to single joint actions (van Kan et al., 1993). In another primate study, after training animals to lift and hold an object for a fixed period of time, a downward force pulse perturbation was applied to assess the ability to respond and adapt to the perturbation (Monzee and Smith, 2004). Single unit recording of neurons with proprioceptive receptive fields that correspond to the trained forelimb movement were mapped to the IntA nucleus. A majority of these neurons encode reflex-like forelimb movements that respond to the force pulse, and a smaller subset of responsive neurons exhibit preparatory activity prior to the perturbation (Monzee and Smith, 2004). This preparatory IntA activity could reflect the anticipatory increase in grip force that occurs during the perturbation to maintain a stable grasp. More generally, these studies suggest that neurons in the Int nucleus respond to somatosensory information about environmental perturbations, and may coordinate anticipatory forelimb responses to make corrective adjustments.
Together, inactivation and electrophysiological studies in cats and monkeys have provided compelling evidence that the cerebellar nuclei, and the Int nucleus in particular, are essential for dexterous forelimb control. These findings also raise many questions about how distinct cerebellar output circuits influence motor output. Even within a single cerebellar nucleus, neurons possess diverse electrophysiological properties, neurotransmitter identities, and input-output connectivity patterns. The employment of molecular-genetic tools in mice to access discrete circuits has begun to extend findings from work in other species by clarifying the connectivity and functional logic of neuronal subtypes within the cerebellar nuclei.
Molecular-genetic dissection of cerebellar circuits and their contributions to dexterous movement
The advent of modern genetic and viral approaches in mice is enabling a more fine-grained investigation of pathways that send input into the cerebellum, specific classes of neurons in the cerebellar nuclei, and cerebellar output pathways involved in forelimb control.
Input to the cerebellum is essential for the execution and adaptation of forelimb movement
The cerebellum is thought to integrate internal copies of motor commands with sensory input to support adjustments over several timescales for smooth and precise execution of movement (Thach et al., 1992a; Adrian and John, 2012; Azim and Alstermark, 2015). Internal copies are hypothesized to arise from multiple levels in the descending flow of motor commands, including the motor cortex, red nucleus, brainstem, and spinal cord, providing a variety of motor-related information to the cerebellum (Huang et al., 2013; Azim et al., 2014b; Ishikawa et al., 2015; Beitzel et al., 2017). Similarly, peripheral input is conveyed to the cerebellum from diverse sensory modalities and is used to provide feedback that reports the consequences of movement and facilitate feedforward control over future movement (Shadmehr et al., 2010; Azim and Alstermark, 2015) (Fig. 1A).
The most prominent mossy fiber input to the cerebellum arises from the pontine nucleus, which receives descending input from corticofugal neurons across the neocortex, including the motor cortex (Leergaard et al., 2004; Huang et al., 2013). Optogenetic activation of pontine neurons during a skilled pellet reaching task in mice results in hypermetria and impaired grasping. Additionally, reversible inactivation of ponto-cerebellar neurons reduces task success and impairs the ability of mice to correct movements after a failed first attempt (Guo et al., 2019). Thus, disrupting circuits that convey descending cortical signals to the cerebellum affects the endpoint accuracy of ongoing reaching movements as well as the correction of future movements. The cortico–ponto–cerebellar pathway is also instrumental in coordinating network dynamics across the cerebral and cerebellar cortices. A recent study found that the activity of cerebellar granule cells is coupled to the activity of layer 5 pyramidal cells in the neocortex during a learned forelimb movement planning task. This correlated activity emerges during the learning process and depends on cortical input to the cerebellum via the pontine nucleus (Wagner et al., 2019). Although the specific role of this shared activity for motor control is unclear, results from this study suggest that the cerebellum works in conjunction with the cerebral cortex to improve forelimb motor performance during learning.
In addition to descending input from the motor cortex, the pontine nucleus also receives signals from sensory cortical areas. Cortico-pontine input from both sensory and motor whisker related cortical areas has been shown to converge in lateral regions of the cerebellar cortex, which then form a closed loop with the whisker motor cortex (Proville et al., 2014). Moreover, anatomical convergence of cortico-pontine input with proprioceptive sensory streams from the external cuneate nucleus has also been described at individual granule cells (Huang et al., 2013). Integration of sensory signals with motor internal copy information is an important element of feedforward control over motor output (Fig. 1A), and these studies provide compelling evidence for this convergence in the cerebellar cortex. The sensory cortex is also implicated in forelimb sensorimotor adaptation. Mice subjected to a forcefield perturbation in a joystick task show impaired sensorimotor adaptation when S1 neurons are optogenetically inhibited (Mathis et al., 2017), suggesting that the somatosensory cortex is involved in providing sensory input that updates cerebellar internal models in response to environmental disturbances. Thus, cerebellar input from the sensory and motor cortices through the pons is likely to contribute to learning, execution, and adaptation of limb movements.
Another important source of internal copies to the cerebellum originates from dual-projecting propriospinal neurons (PNs) in the cervical spinal cord (Azim and Alstermark, 2015). These cervical PNs receive direct input from descending motor pathways, and send axons that bifurcate, innervating forelimb motor neurons as well as neurons in the lateral reticular nucleus (LRN), a major source of mossy fiber input to the cerebellum (Alstermark and Isa, 2012; Azim et al., 2014b). The extensive connectivity of the LRN with the cerebellar cortex and cerebellar nuclei suggests that this structure plays an important role in transmitting copies of the penultimate motor command signals received by forelimb motor neurons (Matsushita and Ikeda, 1976; Alstermark and Ekerot, 2013; Mukherjee et al., 2018). Selective ablation of a genetically-defined subpopulation of neurons (V2a interneurons) that include cervical PNs results in dysmetric reaching movements and impaired success in a pellet retrieval task. Moreover, selective optogenetic activation of the ascending internal copy input to the LRN results in severe perturbation of forelimb trajectories during reach, caused by short latency modulation of motor neuron activity that requires intact cerebellar input from the LRN (Azim et al., 2014b). Several classes of functionally distinct spinal interneurons have been shown to send bifurcating projections to forelimb motor neurons and the LRN (Pivetta et al., 2014). Thus, spinal interneuron pathways provide a diversity of internal copy inputs to cerebellar circuits, establishing spinal-cerebellar-spinal loops capable of rapid adjustment of forelimb movements.
Spinal pathways also convey sensory information directly and indirectly to the cerebellum. A major source of proprioceptive input to the cerebellum comes from spinocerebellar tract neurons that transmit proprioceptive information from primary sensory neurons (Chen et al., 2003), some of which are subject to descending modulation by corticospinal circuits (Hantman and Jessell, 2010). Single-cell RNA sequencing recently revealed genetic diversity amongst spinocerebellar neurons, identifying segment-specific transcriptional codes required for appropriate connectivity between proprioceptive, spinal, and cerebellar circuits (Baek et al., 2019). In addition, the external cuneate nucleus in the brainstem, which sends mossy fiber projections to the cerebellum, directly receives proprioceptive input (Quy et al., 2011), and movement related modulation of activity in this nucleus is important for gating self-generated sensory signals (Tiriac and Blumberg, 2016). Thus, the cerebellum receives feedback from multiple ascending sensory streams that convey information about the state of the body.
The characterization of discrete pre-cerebellar pathways in rodents has begun to reveal the importance of copy and sensory signals for the precision of ongoing limb movements and the adaptation of subsequent movements. Resolving how the cerebellum uses these inputs to ultimately refine motor output requires a closer dissection of cerebellar nuclear circuits that influence forelimb movement.
Cerebellar nuclei are required for the initiation and execution of movement
Cerebellar nuclear neurons are linked to many subcortical motor nuclei, providing several potential pathways through which the cerebellum might implement online correction and sensorimotor adaptation (Tlamsa and Brumberg, 2010; Houck and Person, 2015; Gao et al., 2016; Low et al., 2018). Downstream targets of cerebellar nuclei neurons include the motor thalamus, red nucleus, reticular nuclei, and spinal cord (Fig. 3A,B). However, due to the challenges in selectively accessing and manipulating subpopulations of neurons in the cerebellar nuclei, the role of each of these pathways in forelimb control is not well defined. Several studies employing genetic and viral strategies in mice have begun to uncover functional distinctions within the cerebellar nuclei for the control of dexterous movement.
Two recent studies provide direct evidence that neurons in the Int nucleus are required for the control of forelimb kinematics to ensure precision. Selective ablation of a genetically-defined subpopulation of glutamatergic projection neurons in the IntA nucleus (Urocortin3/Ucn3+ neurons) that preferentially target the motor thalamus and magnocellular red nucleus results in reduced success in a skilled forelimb reaching task, disrupted endpoint accuracy, and hypermetria (Fig. 6A, B). Ablation of Ucn3+ IntA neurons also disrupts limb trajectories during locomotion, and optogenetic activation of these neurons leads to increased vertical displacement during the swing phase of limb movement (Low et al., 2018). This study provides evidence that a discrete subpopulation of IntA neurons plays an essential role in controlling the accuracy of forelimb movements, raising the possibility that additional, molecularly-distinct subpopulations of Int neurons influence other aspects of limb movement.
Figure 6. The IntA nucleus ensures endpoint precision during skilled reaching.
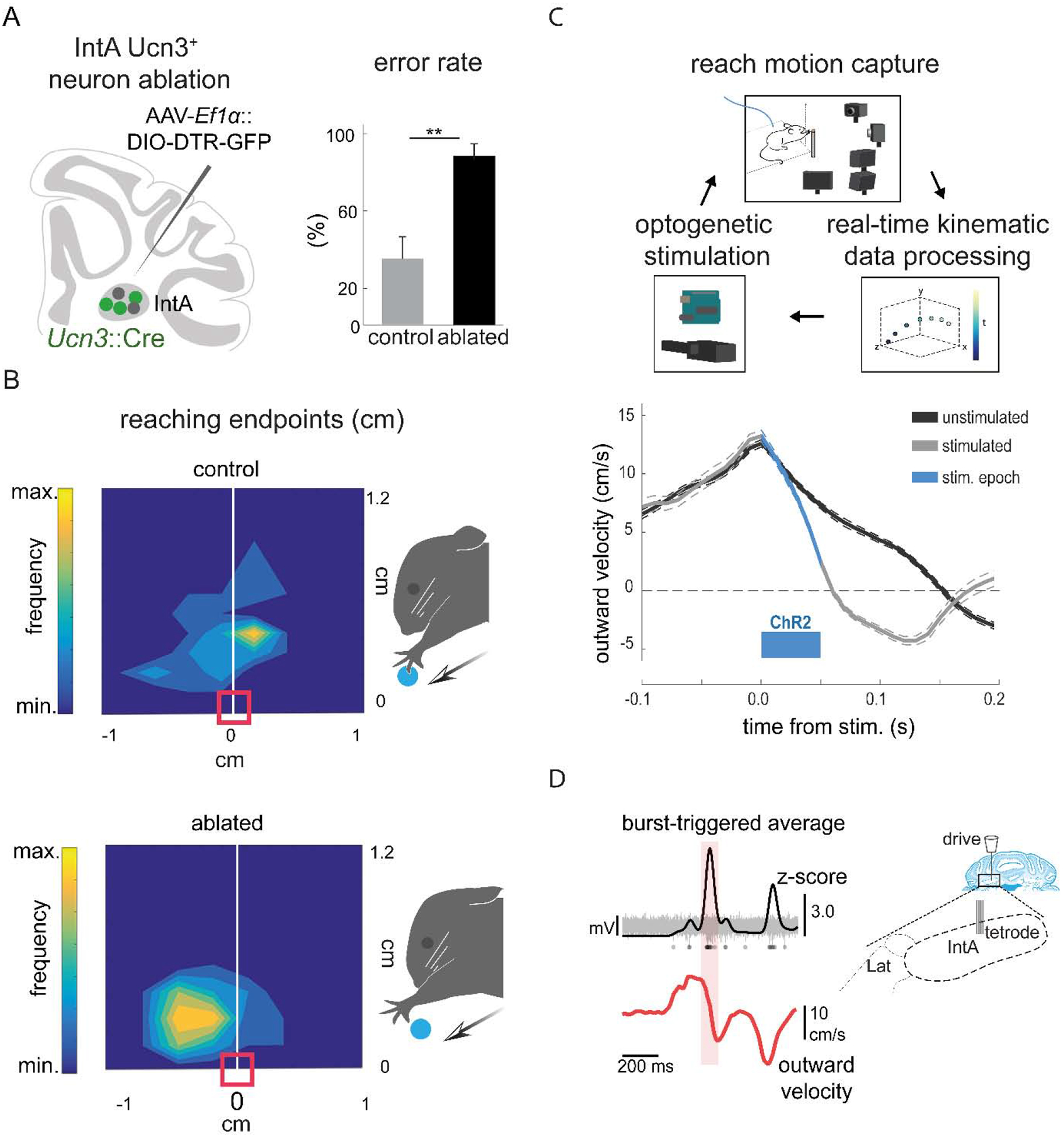
(A) Schematic summarizing strategy for selective ablation of Ucn3+ IntA neurons using a viral diphtheria toxin receptor-mediated strategy (left). Ablation of Ucn3+ IntA neurons results in increased pellet reach-to-grasp errors and reduces pellet retrieval success (right) (Low et al., 2018). (B) Contour density plots depict the endpoint position of the wrist with respect to the pellet. Arrows depict the direction of reach and the red box indicates the position of the pellet. Ablation of Ucn3+ IntA neurons results in hypermetric reaching and overshooting the target (Low et al., 2018). (C) Schematic of the behavioral setup for real-time kinematic analysis and closed-loop optogenetic manipulation of the IntA nucleus (top). Averaged velocity traces from a single animal illustrate that optogenetic activation of IntA neurons during reaching reduces outward velocity of the limb (bottom) (Becker and Person, 2019). (D) Tetrode recordings in IntA during reaching reveal that the amplitude of IntA neuronal activity (top) peaks during abrupt reductions in limb outward velocity (Becker and Person, 2019).
A second study employed closed-loop neural manipulation and recording strategies to clarify specific roles for IntA neurons during reaching. Selective optogenetic activation of IntA neurons results in a reduction of outward velocity during reach, premature termination of the movement, and hypometria (Becker and Person, 2019) (Fig. 6C). Consistent with the effects of ablating Ucn3+ IntA neurons, optogenetic inhibition of IntA neurons results in an increase in outward velocity and hypermetria (Low et al., 2018; Becker and Person, 2019). This ability to manipulate cerebellar output over millisecond timescales has thus provided new insight into the role of the Int nucleus in controlling forelimb kinematics. Moreover, single unit recordings determined that the magnitude of activity of IntA neurons is proportional to the outward velocity of the limb, suggesting that IntA adaptively decelerates the limb to ensure endpoint precision (Becker and Person, 2019) (Fig. 6D). These studies demonstrate the importance of IntA neurons in establishing appropriate limb kinematics, at least in part by modulating outward velocity of the limb. Thus, the online control of forelimb kinematics by IntA output neurons could provide a means to accommodate the speed accuracy tradeoff observed during movement, permitting rapid movements until deceleration is needed for precision (Harris and Wolpert, 1998).
In addition to online execution, the Int nucleus has also been implicated in sensorimotor adaptation of limb movements during locomotion. Selective chemogenetic inhibition of Purkinje cells that target the Int nucleus disrupts the ability of mice to adapt to differing speeds on a split-belt treadmill, leading to perturbed spatial and temporal adaptation of the limbs (Darmohray et al., 2019). Although the role of the Int nucleus in the adaptation of forelimb reaching has not been examined, the deficits in complex locomotor behaviors described in this study suggest a general role for the Int nucleus in sensorimotor adaptation of limb movements across behavioral contexts.
The cerebellar nuclei influence movement through many downstream targets. In a recent study, direct projections to the spinal cord from the Int and Med nuclei were found to be important for success in a forelimb reaching task and for adaptation to increasing speed on a rotarod. Moreover, distinct cerebellospinal circuits differentially contribute to reaching and rotarod adaptation, revealing separate roles for discrete pathways from the cerebellar nuclei in limb movement (Sathyamurthy et al., 2020). The contributions of cerebellar–thalamo–cortical pathways to goal-directed movement have been the focus of several recent studies. Intersectional labeling by retrograde tracing from M1 and anterograde tracing from the Int and Lat nuclei was used to map specific cerebellar-recipient regions of the motor thalamus (Low et al., 2018; Dacre et al., 2019). Muscimol inactivation of this thalamic region reduces the success of goal-directed forelimb movements, and produces a specific deficit in the timing of movement initiation (Dacre et al., 2019). The Med nucleus is also involved in a reciprocal loop between the cerebellum and the anterior lateral motor cortex (ALM). A recent study showed that activity in the Med nucleus is linked to preparatory activity in ALM in a cued discriminative lick task, and manipulation of neurons in the Med nucleus disrupts behavioral choice, biasing the licking behavior in one direction, but leaving execution of licking movements intact. Moreover, motor preparatory activity can be observed in ALM and Med, and is maintained by bidirectional communication between these two structures (Gao et al., 2018). Similar to the Med nucleus, neurons in the Lat nucleus also exhibit preparatory activity, and silencing the Lat nucleus suppresses preparatory activity in ALM (Chabrol et al., 2019). These findings demonstrate that thalamus-projecting cerebellar nuclear neurons shape motor cortical preparatory activity, raising the possibility that the modulation of anticipatory motor signals in the cerebral cortex is a fundamental role of the cerebellar nuclei during voluntary limb movements.
Molecular-genetic dissection of input and output pathways of the cerebellar nuclei are beginning to reveal some of the circuit-level details of cerebellar function. In many cases, the anatomical, electrophysiological, and behavioral findings generated by these mouse studies provide support for long-standing theories of cerebellar computation, while also raising new questions.
Future perspectives
Studies across mammalian species are clarifying how specific cerebellar output circuits contribute to forelimb movements. Through the recent work in mice described here, at least two notable properties of the cerebellar nuclei emerge: 1) Neurons in the Int nucleus that project to subcortical motor pathways control the kinematics of dexterous forelimb movements, modulating online control of the limb to ensure accuracy; 2) Cerebellar-thalamo-cortical loops play a key role in the generation of preparatory signals that are likely involved in the planning and initiation of learned movements, and may be essential for sensorimotor adaptation in response to changing conditions.
Much remains to be explored. The cerebellar nuclei send a diversity of ascending and descending projections to a variety of targets (Teune et al., 2000; Liang et al., 2011; Houck and Person, 2015; Low et al., 2018; Fujita et al., 2020; Sathyamurthy et al., 2020). Yet while populations of neurons in the cerebellar nuclei can be distinguished by their target selectivity, the wide array of functions attributed to the cerebellum have yet to be assigned to individual output circuits. The identification of molecularly-defined neuronal subpopulations in the cerebellar nuclei suggests that, in addition to target selectivity, distinctions in the transcriptional identities of cerebellar nuclear neurons might correlate with functional specialization (Chung et al., 2009; Locke et al., 2018; Low et al., 2018; Sarpong et al., 2018; Fujita et al., 2020). With the rapidly growing arsenal of molecular-genetic and quantitative behavioral tools, cerebellar nuclear neurons can now be molecularly profiled, recorded, and manipulated during behavior with unprecedented resolution. This work in genetically-tractable animals must continue to occur alongside work in animal models in which established experimental approaches and sophisticated behavioral assays can provide more direct relation to human motor function. Collectively, these ongoing efforts to understand how cerebellar output influences limb movement promise to provide insight into more general mechanisms of sensorimotor control, and clarify how motor, and non-motor, functions are disrupted by cerebellar injury and disease.
Highlights.
Cerebellar dysfunction impacts the execution and adaptation of dexterous limb movements
The cerebellar nuclei receive diverse sensorimotor inputs and project to many brain and spinal targets to influence movement
Perturbation of distinct cerebellar nuclei produces specific limb movement deficits across species
Electrophysiological approaches identify somatotopic organization and movement-related activity in the cerebellar nuclei
Molecular-genetic dissection is revealing the functional organization of the cerebellar nuclei and their inputs and outputs
Acknowledgments
We thank Sho Aoki, Matthew Becker, Kee Wui Huang, Masakazu Igarashi, Aloysius Low, and Abigail Person for helpful discussions and feedback. We thank Amy Cao and Salk Institute Communications for help with preparation of figure schematics. A.I.C. was supported by Singapore Ministry of Education Tier 2 (MOE2018-T2-1-065) and Tier 3 (MOE2017-T3-1-002). E.A. was supported by the National Institutes of Health (DP2NS105555, R01NS111479, and U19NS112959), the Searle Scholars Program, The Pew Charitable Trusts, and the McKnight Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian MH, and John WK (2012). Theoretical Models of Motor Control and Motor Learning In Routledge Handbook of Motor Control and Motor Learning (Routledge; ). [Google Scholar]
- Aizenman CD, Huang EJ, and Linden DJ (2003). Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. J Neurophysiol 89, 1738–1747 doi: 10.1152/jn.01043.2002. [DOI] [PubMed] [Google Scholar]
- Alstermark B, and Ekerot CF (2013). The lateral reticular nucleus: a precerebellar centre providing the cerebellum with overview and integration of motor functions at systems level. A new hypothesis. J Physiol 591, 5453–5458 doi: 10.1113/jphysiol.2013.256669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, and Isa T (2012). Circuits for skilled reaching and grasping. Annual review of neuroscience 35, 559–578 doi: 10.1146/annurev-neuro-062111-150527. [DOI] [PubMed] [Google Scholar]
- Ankri L, Husson Z, Pietrajtis K, Proville R, Lena C, Yarom Y, Dieudonne S, and Uusisaari MY (2015). A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. eLife 4, e06262 doi: 10.7554/eLife.06262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, and Hawkes R (2009). Cerebellar cortical organization: a one-map hypothesis. Nature reviews Neuroscience 10, 670–681 doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, and Edgley SA (1984). Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol 351, 411–432 doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, and Alstermark B (2015). Skilled forelimb movements and internal copy motor circuits. Curr Opin Neurobiol 33, 16–24 doi: 10.1016/j.conb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Fink AJ, and Jessell TM (2014a). Internal and External Feedback Circuits for Skilled Forelimb Movement. Cold Spring Harb Symp Quant Biol 79, 81–92 doi: 10.1101/sqb.2014.79.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, and Jessell TM (2014b). Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508, 357–363 doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, and Seki K (2019). Gain control in the sensorimotor system. Curr Opin Physiol 8, 177–187 doi: 10.1016/j.cophys.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski J (1902). Sur le role du cervelet dans les actes volitionnels necessitant une succession rapide de mouvements (1)(Diadocoeinesie) (Masson).
- Baek M, Menon V, Jessell TM, Hantman AW, and Dasen JS (2019). Molecular Logic of Spinocerebellar Tract Neuron Diversity and Connectivity. Cell Reports 27, 2620–2635.e2624 doi: 10.1016/j.celrep.2019.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, and du Lac S (2009). Glycinergic projection neurons of the cerebellum. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 10104–10110 doi: 10.1523/JNEUROSCI.2087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, and Thach WT (1996). Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76, 492–509 doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, and Thach W (1995). Cerebellar outflow lesions: A comparison of movement deficits resulting from lesions at the levels of the cerebellum and thalamus. Annals of neurology 38, 881–892 doi: 10.1002/ana.410380608. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Zackowski KM, and Thach WT (2000). Cerebellar Ataxia: Torque Deficiency or Torque Mismatch Between Joints? Journal of neurophysiology 83, 3019–3030 doi: 10.1152/jn.2000.83.5.3019. [DOI] [PubMed] [Google Scholar]
- Becker MI, and Person AL (2019). Cerebellar Control of Reach Kinematics for Endpoint Precision. Neuron 103, 335–348 e335 doi: 10.1016/j.neuron.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitzel CS, Houck BD, Lewis SM, and Person AL (2017). Rubrocerebellar Feedback Loop Isolates the Interposed Nucleus as an Independent Processor of Corollary Discharge Information in Mice. The Journal of Neuroscience 37, 10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentivoglio M (1982). The organization of the direct cerebellospinal projections. Progress in brain research 57, 279–291 doi: 10.1016/s0079-6123(08)64134-5. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, and Wolpert DM (2001). The cerebellum is involved in predicting the sensory consequences of action. Neuroreport 12, 1879–1884. [DOI] [PubMed] [Google Scholar]
- Bostan AC, and Strick PL (2013). Cerebellar Outputs in Non-human Primates: An Anatomical Perspective Using Transsynaptic Tracers. In Handbook of the Cerebellum and Cerebellar Disorders, pp. 549–569. [Google Scholar]
- Bracha V, Kolb FP, Irwin KB, and Bloedel JR (1999). Inactivation of interposed nuclei in the cat: classically conditioned withdrawal reflexes, voluntary limb movements and the action primitive hypothesis. Exp Brain Res 126, 77–92 doi: 10.1007/s002210050718. [DOI] [PubMed] [Google Scholar]
- Brandauer B, Hermsdorfer J, Beck A, Aurich V, Gizewski ER, Marquardt C, and Timmann D (2008). Impairments of prehension kinematics and grasping forces in patients with cerebellar degeneration and the relationship to cerebellar atrophy. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 119, 2528–2537 doi: 10.1016/j.clinph.2008.07.280. [DOI] [PubMed] [Google Scholar]
- Canto CB, Witter L, and De Zeeuw CI (2016). Whole-Cell Properties of Cerebellar Nuclei Neurons In Vivo. PLOS ONE 11, e0165887 doi: 10.1371/journal.pone.0165887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott AL, Dorizan S, and Khodakhah K (2019). Cerebellar modulation of the reward circuitry and social behavior. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerminara NL, Lang EJ, Sillitoe RV, and Apps R (2015). Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nature reviews Neuroscience 16, 79–93 doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol FP, Blot A, and Mrsic-Flogel TD (2019). Cerebellar contribution to preparatory activity in motor neocortex. Neuron 103, 506–519. e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V (1977). Cerebellar dentate nucleus : organization, cytology and transmitters (Berlin; New York: Springer-Verlag; ). [Google Scholar]
- Chen CH, Fremont R, Arteaga-Bracho EE, and Khodakhah K (2014). Short latency cerebellar modulation of the basal ganglia. Nature Neuroscience 17, 1767–1775 doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-H, Hippenmeyer S, Arber S, and Frank E (2003). Development of the monosynaptic stretch reflex circuit. Current Opinion in Neurobiology 13, 96–102 doi: 10.1016/S0959-4388(03)00006-0. [DOI] [PubMed] [Google Scholar]
- Chung SH, Marzban H, and Hawkes R (2009). Compartmentation of the cerebellar nuclei of the mouse. Neuroscience 161, 123–138 doi: 10.1016/j.neuroscience.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Cody FW, Moore RB, and Richardson HC (1981). Patterns of activity evoked in cerebellar interpositus nuclear neurones by natural somatosensory stimuli in awake cats. J Physiol 317, 1–20 doi: 10.1113/jphysiol.1981.sp013810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubayko U, Sultan F, Thier P, and Schwarz C (2001). Two types of neurons in the rat cerebellar nuclei as distinguished by membrane potentials and intracellular fillings. J Neurophysiol 85, 2017–2029 doi: 10.1152/jn.2001.85.5.2017. [DOI] [PubMed] [Google Scholar]
- Dacre J, Colligan M, Ammer J, Schiemann J, Clarke T, Chamosa-Pino V, Claudi F, Harston JA, Eleftheriou C, Pakan JMP, et al. (2019). Cerebellar-recipient motor thalamus drives behavioral context-specific movement initiation. bioRxiv, 802124 doi: 10.1101/802124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmohray DM, Jacobs JR, Marques HG, and Carey MR (2019). Spatial and Temporal Locomotor Learning in Mouse Cerebellum. Neuron 102, 217–231 e214 doi: 10.1016/j.neuron.2019.01.038. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Wenzelburger R, Loffler K, Raethjen J, and Stolze H (2000). Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain 123 (Pt 8), 1568–1580 doi: 10.1093/brain/123.8.1568. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, and Szentágothai J (1967). The cerebellum as a neuronal machine (Berlin, New York: etc.: Springer-Verlag; ). [Google Scholar]
- Ekerot CF, Garwicz M, and Jorntell H (1997). The control of forelimb movements by intermediate cerebellum. Prog Brain Res 114, 423–429. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Garwicz M, and Schouenborg J (1991). Topography and nociceptive receptive fields of climbing fibres projecting to the cerebellar anterior lobe in the cat. J Physiol 441, 257–274 doi: 10.1113/jphysiol.1991.sp018750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H, and Garwicz M (1995). Functional relation between corticonuclear input and movements evoked on microstimulation in cerebellar nucleus interpositus anterior in the cat. Exp Brain Res 106, 365–376 doi: 10.1007/bf00231060. [DOI] [PubMed] [Google Scholar]
- Flament D, and Hore J (1986). Movement and electromyographic disorders associated with cerebellar dysmetria. Journal of neurophysiology 55, 1221–1233 doi: 10.1152/jn.1986.55.6.1221. [DOI] [PubMed] [Google Scholar]
- Fujita H, Kodama T, and du Lac S (2020). Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. eLife 9, e58613, doi: 10.7554/eLife.58613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, and Li N (2018). A cortico-cerebellar loop for motor planning. Nature 563, 113–116 doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Proietti-Onori M, Lin Z, Ten Brinke MM, Boele HJ, Potters JW, Ruigrok TJ, Hoebeek FE, and De Zeeuw CI (2016). Excitatory Cerebellar Nucleocortical Circuit Provides Internal Amplification during Associative Conditioning. Neuron 89, 645–657 doi: 10.1016/j.neuron.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwicz M, and Ekerot CF (1994). Topographical organization of the cerebellar cortical projection to nucleus interpositus anterior in the cat. J Physiol 474, 245–260 doi: 10.1113/jphysiol.1994.sp020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K (1991). Tools, language and intelligence: Evolutionary implications. Man, 255–264. [Google Scholar]
- Gilman S, Carr D, and Hollenberg J (1976). Kinematic effects of deafferentation and cerebellar ablation. Brain 99, 311–330 doi: 10.1093/brain/99.2.311. [DOI] [PubMed] [Google Scholar]
- Glickstein M (1997). Chapter 14 Mossy-fibre sensory input to the cerebellum In Progress in brain research, De Zeeuw CI, Strata P, and Voogd J, eds. (Elsevier), pp. 251–259. [DOI] [PubMed] [Google Scholar]
- Guo J-Z, Sauerbrei B, Cohen JD, Mischiati M, Graves A, Pisanello F, Branson K, and Hantman AW (2019). The Pontine Nuclei are an Integrative Cortico-Cerebellar Link Critical for Dexterity. bioRxiv, 637447 doi: 10.1101/637447. [DOI] [Google Scholar]
- Hantman AW, and Jessell TM (2010). Clarke’s column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13, 1233–1239 doi: 10.1038/nn.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, and Wolpert DM (1998). Signal-dependent noise determines motor planning. Nature 394, 780–784 doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hawkes R (2014). Purkinje cell stripes and long-term depression at the parallel fiber-Purkinje cell synapse. Front Syst Neurosci 8, 41 doi: 10.3389/fnsys.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, De Zeeuw CI, Jaeger D, Khodakhah K, and Person AL (2013). The neuronal code(s) of the cerebellum. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 17603–17609 doi: 10.1523/jneurosci.2759-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Hall NJ, Tringides M, and Lisberger SG (2020). Principles of operation of a cerebellar learning circuit. eLife 9, e55217 doi: 10.7554/eLife.55217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Kojima Y, Soetedjo R, and Shadmehr R (2015). Encoding of action by the Purkinje cells of the cerebellum. Nature 526, 439–442 doi: 10.1038/nature15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerbach MJ, and Flash T (1982). Dynamic interactions between limb segments during planar arm movement. Biol Cybern 44, 67–77 doi: 10.1007/bf00353957. [DOI] [PubMed] [Google Scholar]
- Holmes G (1917). The Symptoms of Acute Cerebellar Injuries Due to Gunshot Injuries. Brain 40, 461–535 doi: 10.1093/brain/40.4.461. [DOI] [Google Scholar]
- Hore J, Wild B, and Diener HC (1991). Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol 65, 563–571 doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- Houck BD, and Person AL (2015). Cerebellar Premotor Output Neurons Collateralize to Innervate the Cerebellar Cortex. The Journal of comparative neurology 523, 2254–2271 doi: 10.1002/cne.23787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, and Hantman AW (2013). Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. eLife 2, e00400 doi: 10.7554/eLife.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg W, Giese MA, Gizewski ER, Schoch B, and Timmann D (2008). The influence of focal cerebellar lesions on the control and adaptation of gait. Brain 131, 2913–2927 doi: 10.1093/brain/awn246. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Shimuta M, and Häusser M (2015). Multimodal sensory integration in single cerebellar granule cells in vivo. eLife 4, e12916 doi: 10.7554/eLife.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Tomatsu S, Tsunoda Y, Lee J, Hoffman DS, and Kakei S (2014). Releasing dentate nucleus cells from Purkinje cell inhibition generates output from the cerebrocerebellum. PloS one 9, e108774–e108774 doi: 10.1371/journal.pone.0108774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M (2008). Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9, 304–313 doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Ito M, Yamaguchi K, Nagao S, and Yamazaki T (2014). Long-term depression as a model of cerebellar plasticity. Prog Brain Res 210, 1–30 doi: 10.1016/B978-0-444-63356-9.00001-7. [DOI] [PubMed] [Google Scholar]
- Jorntell H, Garwicz M, and Ekerot CF (1996). Relation between cutaneous receptive fields and muscle afferent input to climbing fibres projecting to the cerebellar C3 zone in the cat. The European journal of neuroscience 8, 1769–1779 doi: 10.1111/j.1460-9568.1996.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Kawato M (1999). Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9, 718–727 doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Keifer J, and Houk JC (1994). Motor function of the cerebellorubrospinal system. Physiol Rev 74, 509–542 doi: 10.1152/physrev.1994.74.3.509. [DOI] [PubMed] [Google Scholar]
- Kelly RM, and Strick PL (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper M, Hermsdörfer J, Brandauer B, Thürling M, Schoch B, Theysohn N, and Timmann D (2011). Lesions of the dentate and interposed nuclei are associated with impaired prehension in cerebellar patients. Neuroscience letters 499, 132–136 doi: 10.1016/j.neulet.2011.05.055. [DOI] [PubMed] [Google Scholar]
- Lang CE, and Bastian AJ (1999). Cerebellar Subjects Show Impaired Adaptation of Anticipatory EMG During Catching. J Neurophysiol 82, 2108–2119 doi: 10.1152/jn.1999.82.5.2108. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Apps R, Bengtsson F, Cerminara NL, De Zeeuw CI, Ebner TJ, Heck DH, Jaeger D, Jorntell H, Kawato M, et al. (2017). The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum 16, 230–252 doi: 10.1007/s12311-016-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leergaard TB, Alloway KD, Pham TA, Bolstad I, Hoffer ZS, Pettersen C, and Bjaalie JG (2004). Three-dimensional topography of corticopontine projections from rat sensorimotor cortex: comparisons with corticostriatal projections reveal diverse integrative organization. The Journal of comparative neurology 478, 306–322 doi: 10.1002/cne.20289. [DOI] [PubMed] [Google Scholar]
- Lemon RN (2008). Descending pathways in motor control. Annual review of neuroscience 31, 195–218 doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Liang H, Paxinos G, and Watson C (2011). Projections from the brain to the spinal cord in the mouse. Brain structure & function 215, 159–186 doi: 10.1007/s00429-010-0281-x. [DOI] [PubMed] [Google Scholar]
- Llinas RR (2013). The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits 7, 96 doi: 10.3389/fncir.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke TM, Soden ME, Miller SM, Hunker A, Knakal C, Licholai JA, Dhillon KS, Keene CD, Zweifel LS, and Carlson ES (2018). Dopamine D1 Receptor-Positive Neurons in the Lateral Nucleus of the Cerebellum Contribute to Cognitive Behavior. Biological psychiatry 84, 401–412 doi: 10.1016/j.biopsych.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low AYT, Thanawalla AR, Yip AKK, Kim J, Wong KLL, Tantra M, Augustine GJ, and Chen AI (2018). Precision of Discrete and Rhythmic Forelimb Movements Requires a Distinct Neuronal Subpopulation in the Interposed Anterior Nucleus. Cell Rep 22, 2322–2333 doi: 10.1016/j.celrep.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Manto M (2009). Mechanisms of human cerebellar dysmetria: experimental evidence and current conceptual bases. J Neuroeng Rehabil 6, 10 doi: 10.1186/1743-0003-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Cooper SE, Hacking A, and Ghez C (2000). Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. J Neurophysiol 83, 1886–1899 doi: 10.1152/jn.2000.83.4.1886. [DOI] [PubMed] [Google Scholar]
- Mason CR, Miller LE, Baker JF, and Houk JC (1998). Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. J Neurophysiol 79, 537–554 doi: 10.1152/jn.1998.79.2.537. [DOI] [PubMed] [Google Scholar]
- Mathis MW, Mathis A, and Uchida N (2017). Somatosensory Cortex Plays an Essential Role in Forelimb Motor Adaptation in Mice. Neuron 93, 1493–1503 e1496 doi: 10.1016/j.neuron.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, and Ikeda M (1976). Projections from the lateral reticular nucleus to the cerebellar cortex and nuclei in the cat. Exp Brain Res 24, 403–421 doi: 10.1007/bf00235006. [DOI] [PubMed] [Google Scholar]
- Miall RC (2016). Cerebellum: Anatomy and Function. In Neuroscience in the 21st Century, pp. 1277–1295. [Google Scholar]
- Miall RC, and King D (2008). State estimation in the cerebellum. Cerebellum 7, 572–576 doi: 10.1007/s12311-008-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Shimansky Y, Bracha V, and Bloedel JR (1997). Effects of Inactivating Individual Cerebellar Nuclei on the Performance and Retention of an Operantly Conditioned Forelimb Movement. Journal of neurophysiology 78, 939–959 doi: 10.1152/jn.1997.78.2.939. [DOI] [PubMed] [Google Scholar]
- Monzee J, Drew T, and Smith AM (2004). Effects of muscimol inactivation of the cerebellar nuclei on precision grip. J Neurophysiol 91, 1240–1249 doi: 10.1152/jn.01124.2002. [DOI] [PubMed] [Google Scholar]
- Monzee J, and Smith AM (2004). Responses of cerebellar interpositus neurons to predictable perturbations applied to an object held in a precision grip. Journal of neurophysiology 91, 1230–1239 doi: 10.1152/jn.01120.2002. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Sokoloff G, and Blumberg MS (2018). Corollary discharge in precerebellar nuclei of sleeping infant rats. eLife 7, e38213 doi: 10.7554/eLife.38213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Croce K, Belton T, Akay T, and Jessell TM (2018). Balance Control Mediated by Vestibular Circuits Directing Limb Extension or Antagonist Muscle Co-activation. Cell Rep 22, 1325–1338 doi: 10.1016/j.celrep.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Timmann D, and Hermsdörfer J (2013). Deficits of Grasping in Cerebellar Disorders In Handbook of the Cerebellum and Cerebellar Disorders, Manto M, Schmahmann JD, Rossi F, Gruol DL, and Koibuchi N, eds. (Dordrecht: Springer Netherlands; ), pp. 1657–1667. [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, and Mauk MD (2006). Learning-induced plasticity in deep cerebellar nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 12656–12663 doi: 10.1523/jneurosci.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O (1979). Functional units of the cerebellum - sagittal zones and microzones. Trends in Neurosciences 2, 143–145 doi: 10.1016/0166-2236(79)90057-2. [DOI] [Google Scholar]
- Paxinos G, and Franklin KBJ (2007). The mouse brain in stereotaxic coordinates, Vol 3rd Edition (Amsterdam; Boston: Elsevier Academic Press; ). [Google Scholar]
- Person AL, and Raman IM (2011). Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481, 502–505 doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, and Raman IM (2012). Synchrony and neural coding in cerebellar circuits. Front Neural Circuits 6, 97 doi: 10.3389/fncir.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivetta C, Esposito MS, Sigrist M, and Arber S (2014). Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell 156, 537–548 doi: 10.1016/j.cell.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Popa LS, and Ebner TJ (2018). Cerebellum, Predictions and Errors. Front Cell Neurosci 12, 524 doi: 10.3389/fncel.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proville RD, Spolidoro M, Guyon N, Dugue GP, Selimi F, Isope P, Popa D, and Lena C (2014). Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat Neurosci 17, 1233–1239 doi: 10.1038/nn.3773. [DOI] [PubMed] [Google Scholar]
- Pugh JR, and Raman IM (2006). Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51, 113–123 doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Pugh JR, and Raman IM (2008). Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 10549–10560 doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quy PN, Fujita H, Sakamoto Y, Na J, and Sugihara I (2011). Projection patterns of single mossy fiber axons originating from the dorsal column nuclei mapped on the aldolase C compartments in the rat cerebellar cortex. The Journal of comparative neurology 519, 874–899 doi: 10.1002/cne.22555. [DOI] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, and Padgett D (2000). Ionic Currents and Spontaneous Firing in Neurons Isolated from the Cerebellar Nuclei. The Journal of Neuroscience 20, 9004 doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, and Mauk MD (1996). The Cerebellum: A Neuronal Learning Machine? Science 272, 1126 doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Sakayori N, Kato S, Sugawara M, Setogawa S, Fukushima H, Ishikawa R, Kida S, and Kobayashi K (2019). Motor skills mediated through cerebellothalamic tracts projecting to the central lateral nucleus. Mol Brain 12, 13 doi: 10.1186/s13041-019-0431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpong GA, Vibulyaseck S, Luo Y, Biswas MS, Fujita H, Hirano S, and Sugihara I (2018). Cerebellar modules in the olivo-cortico-nuclear loop demarcated by pcdh10 expression in the adult mouse. The Journal of comparative neurology 526, 2406–2427 doi: 10.1002/cne.24499. [DOI] [PubMed] [Google Scholar]
- Sathyamurthy A, Barik A, Dobrott CI, Matson KJE, Stoica S, Pursley R, Chesler AT, and Levine AJ (2020). Cerebellospinal Neurons Regulate Motor Performance and Motor Learning. Cell Reports 31, 107595 doi: 10.1016/j.celrep.2020.107595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (2004). Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of neuropsychiatry and clinical neurosciences 16, 367–378 doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Guell X, Stoodley CJ, and Halko MA (2019). The Theory and Neuroscience of Cerebellar Cognition. Annual review of neuroscience 42, 337–364 doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, and Pandya DN (1997). The cerebrocerebellar system. International review of neurobiology 41, 31–60 doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Rosene DL, and Pandya DN (2004). Motor projections to the basis pontis in rhesus monkey. The Journal of comparative neurology 478, 248–268 doi: 10.1002/cne.20286. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, and Sherman JC (1998). The cerebellar cognitive affective syndrome. Brain 121 (Pt 4), 561–579 doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schultz W, Montgomery EB Jr., and Marini R (1979). Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain 102, 127–146 doi: 10.1093/brain/102.1.127. [DOI] [PubMed] [Google Scholar]
- Scott SH (2004). Optimal feedback control and the neural basis of volitional motor control. Nature reviews Neuroscience 5, 532–546 doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- Scott SH, Cluff T, Lowrey CR, and Takei T (2015). Feedback control during voluntary motor actions. Curr Opin Neurobiol 33, 85–94 doi: 10.1016/j.conb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Shadmehr R (2020). Population coding in the cerebellum and its implications for learning from error. bioRxiv, 2020.2005.2018.102376 doi: 10.1101/2020.05.18.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, and Krakauer JW (2008). A computational neuroanatomy for motor control. Exp Brain Res 185, 359–381 doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, and Krakauer JW (2010). Error correction, sensory prediction, and adaptation in motor control. Annual review of neuroscience 33, 89–108 doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Sotelo C (2020). Cellular Mechanisms Involved in Cerebellar Microzonation. Neuroscience doi: 10.1016/j.neuroscience.2020.01.019. [DOI] [PubMed] [Google Scholar]
- Sugihara I (2011). Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum 10, 449–463 doi: 10.1007/s12311-010-0226-1. [DOI] [PubMed] [Google Scholar]
- Sugihara I, and Shinoda Y (2004). Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 8771–8785 doi: 10.1523/JNEUROSCI.1961-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan F, Czubayko U, and Thier P (2003). Morphological classification of the rat lateral cerebellar nuclear neurons by principal component analysis. The Journal of comparative neurology 455, 139–155 doi: 10.1002/cne.10443. [DOI] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, and Ruigrok TJ (2000). Topography of cerebellar nuclear projections to the brain stem in the rat. Progress in brain research 124, 141–172 doi:10.1016/S0079–6123(00)24014–4. [DOI] [PubMed] [Google Scholar]
- Thach WT (1970a). Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol 33, 527–536 doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Thach WT (1970b). Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol 33, 537–547 doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, and Keating JG (1992a). The cerebellum and the adaptive coordination of movement. Annual review of neuroscience 15, 403–442 doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Thach WT, Kane SA, Mink JW, and Goodkin HP (1992b). Cerebellar Output: Multiple Maps and Modes of Control in Movement Coordination In The Cerebellum Revisited, Llinás R, and Sotelo C, eds. (New York, NY: Springer US; ), pp. 283–300. [Google Scholar]
- Thomas Thach W, and Bastian AJ (2004). Role of the cerebellum in the control and adaptation of gait in health and disease. In Progress in brain research (Elsevier), pp. 353–366. [DOI] [PubMed] [Google Scholar]
- Tiriac A, and Blumberg MS (2016). Gating of reafference in the external cuneate nucleus during self-generated movements in wake but not sleep. eLife 5, e18749 doi: 10.7554/eLife.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlamsa AP, and Brumberg JC (2010). Organization and morphology of thalamocortical neurons of mouse ventral lateral thalamus. Somatosens Mot Res 27, 34–43 doi: 10.3109/08990221003646736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, and Bastian AJ (2007). Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98, 54–62 doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, and Azim E (2018). Proprioception. Current biology : CB 28, R194–R203 doi: 10.1016/j.cub.2018.01.064. [DOI] [PubMed] [Google Scholar]
- Uusisaari M, and De Schutter E (2011). The mysterious microcircuitry of the cerebellar nuclei. J Physiol 589, 3441–3457 doi: 10.1113/jphysiol.2010.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari M, and Knopfel T (2011). Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum 10, 637–646 doi: 10.1007/s12311-010-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan PL, Houk JC, and Gibson AR (1993). Output organization of intermediate cerebellum of the monkey. J Neurophysiol 69, 57–73 doi: 10.1152/jn.1993.69.1.57. [DOI] [PubMed] [Google Scholar]
- Voogd J (2014). What we do not know about cerebellar systems neuroscience. Frontiers in systems neuroscience 8, 227–227 doi: 10.3389/fnsys.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, and Ruigrok TJ (2004). The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol 33, 5–21 doi: 10.1023/B:NEUR.0000029645.72074.2b. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Kadmon J, Nguyen ND, Ganguli S, Schnitzer MJ, and Luo L (2019). Shared Cortex-Cerebellum Dynamics in the Execution and Learning of a Motor Task. Cell 177, 669–682 e624 doi: 10.1016/j.cell.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, and Miall RC (1996). Forward Models for Physiological Motor Control. Neural networks : the official journal of the International Neural Network Society 9, 1265–1279 doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, and Kawato M (1998). Internal models in the cerebellum. Trends in cognitive sciences 2, 338–347 doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Wu Y, and Raman IM (2017). Facilitation of mossy fibre-driven spiking in the cerebellar nuclei by the synchrony of inhibition. J Physiol 595, 5245–5264 doi: 10.1113/JP274321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackowski KM, Thach WT Jr., and Bastian AJ (2002). Cerebellar subjects show impaired coupling of reach and grasp movements. Exp Brain Res 146, 511–522 doi: 10.1007/s00221-002-1191-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, and Linden DJ (2006). Long-Term Depression at the Mossy Fiber–Deep Cerebellar Nucleus Synapse. The Journal of Neuroscience 26, 6935 doi: 10.1523/JNEUROSCI.0784-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet AM, Bastian AJ, and Cowan NJ (2019). Cerebellar patients have intact feedback control that can be leveraged to improve reaching. bioRxiv, 827113 doi: 10.1101/827113. [DOI] [PMC free article] [PubMed] [Google Scholar]


