Abstract
Angelman Syndrome (AS) is characterized by severe developmental delays including marked speech impairment, movement abnormalities(ataxia, tremor), and unique behaviors such as frequent laughter and is caused by dysfunctional maternal UBE3A gene (maternal 15q11–13 deletions, maternal specific UBE3A mutation, uniparental disomy, and imprinting defect). Intractable epileptic seizures since early childhood with characteristic EEG abnormalities are present in 80–90% patients with AS. Underlying pathophysiology may involve neocortical and thalamocortical hyperexcitability secondary to severe reduction of GABAergic input, as well as dysfunctional synaptic plasticity, deficient synaptogenesis, and neuronal morphological immaturity. The onset of epilepsy is most prevalent between 1–3 years of age; however, approximately 25% of patients developed epilepsy before one year of age. Various types of generalized seizures are most prevalent, with most common types are myoclonic and atypical absence. More than 95% of epilepsy patients may have daily seizures at least for a limited time during early childhood, and two-third patients develop disabling seizures. Fever provoked seizures, and frequent occurrence of nonconvulsive status epilepticus are two unique features. Seizures are frequently pharmacoresistant. Considering underlying prominent GABAergic dysfunction, clinicians had used AEDs that target GABAergic signaling such as valproate, phenobarbital, and clonazepam as first-line therapies for AS. However, due to the unfavorable side effect profile of these AEDs, a recent treatment approach involves priority use of levetiracetam, clobazam, topiramate, lamotrigine, ethosuximide, VNS, and carbohydrate-restricted diets. Besides symptomatic management, there has been recent progress to find a curative treatment with the following approaches: 1. Gene/protein replacement therapy (Adeno and lentiviral vector therapy to deliver a gene or secretory protein); 2. Activation of the intact but silent paternal copy of UBE3A (antisense oligonucleotide therapy and artificial transcription factors); and 3. Downstream therapies (OV101/gaboxadol, ketone supplement, novel compounds/peptides, anti-inflammatory/regenerative therapy).
Keywords: Angelman syndrome, UBE3A, epilepsy, seizures, EEG, gene therapy, molecular therapy
Introduction
Angelman Syndrome (AS), a severe neurodevelopmental disorder, is caused by the dysfunctional maternal UBE3A gene. First described in 1965, the prevalence of AS is estimated as 1 in 12,000– 20,000 live births. AS is characterized by severe developmental delays, including marked speech impairment, movement abnormalities (ataxia, tremor), and unique behaviors such as frequent laughter. Intractable epileptic seizures since early childhood with characteristic electroencephalographic(EEG) abnormalities are present in 80–90% patients with AS.[1–3] Although epilepsy phenotype, neurophysiological abnormalities, the relative efficacy of antiepileptic drugs(AEDs), and natural history of epilepsy have been described in detail for a while, a better understanding of underlying pathogenesis and development of molecular therapeutics with hope towards the development of curative therapy for AS is relatively recent.
Pathophysiology
Genetics
Between chromosome 15 pairs, only maternal UBE3A is functionally active in the brain as paternal UBE3A undergoes functional silencing by the process of antisense transcription(ATS).[4] Genomic imprinting occurs in a tissue-specific manner during gametogenesis, and only maternal expression of Ube3a is seen in the brain. Large (5–7 Mb) deletions of 15q11–13- containing critical UBE3A gene- in the maternal allele, which occurs in 70% of AS patients, cause deficient production brain-specific Ube3a proteins.[5] These proteins (enzymes) are essential for the ubiquitin-ligase pathway to facilitate protein degradation(by tagging) in the proteasomes. Appropriate expression of Ube3a is also crucial for synaptic plasticity, synaptogenesis, neuronal morphological maturity, and maintenance of proper levels of neurotransmitter gamma-Aminobutyric acid(GABA). Deficient expression of Ube3a protein also occurs secondary to maternal UBE3A mutation (approximately 11% of AS patients), uniparental disomy (noted in about 7% of AS patients with both copies of 15q 11–13 inherited from the father and none from the mother), and imprinting defect (rarest known genetic cause of AS and noted in approximately 3% of AS patients).
GABAergic neurotransmission
Due to characteristic presence of intractable seizures and EEG changes associated with AS, initial research on pathogenesis was focused on GABA(the principal inhibitory neurotransmitter in the cortex) that maintains inhibitory tone in the brain and counterbalances excitatory glutamatergic outputs.[6,7] GABA mediates its action via GABA receptors, which composed of 5 subunits. Commonly deleted region in AS, 15q11-q13, contains genes that codes for different subunits of GABAA receptors (α 5, β 3, and γ 3) and thus possibly indicate disruption of GABAergic neurotransmission in AS. Knock-out animal models using these GABA receptor genes had been previously utilized in the AS research.[8] Particularly Gabrb3 knock-out mice expressed some similarities to AS patients with seizures susceptibility and EEG changes.[9,10] However, 30% of AS patients (AS patients without 15q11–13 deletion) do not have GABARB3 involvement. Additionally, Gabrb3 knock- out mice do not express all the characteristic features of AS. There is also no evidence of imprinting of GABARB3 in humans. As all patients with AS have a deficient function of UBE3A, the recent focus of research has shifted to the understanding of pathogenesis-related to the ubiquitin pathway. Although maternal UBE3A gene dysfunction is causative for AS, adjacent GABA genes may contribute to the neuronal hyperexcitability and may be responsible for more severe phenotypes associated with the deletion subtype compared to the other three causes of AS. Moreover, recent studies have demonstrated GABAergic dysfunction caused by loss of function of Ube3a in a mouse model(detailed in the next section).[11] The decrement of inhibitory synaptic input to the principal neuron may originate from the defective presynaptic vesicle cycling in multiple interneuron populations. Additionally, Ube3a deficient mice might have a surplus of GABA transporter 1 due to a lack of binding of the transporter with Ube3a.[12] This excess level of transporter might induce decreased tonic inhibition by diminishing GABA concentration in the extrasynaptic space.
Circuit hyperexcitability
Neocortical hyperexcitability in layers 2 and 3 of pyramidal neurons, secondary to severe reduction of GABAergic input, has been established in an AS mouse model specific to UBE3A mutation. The reduction of the GABAergic input might be disproportionately affected than the loss of excitatory glutamatergic input, even with the isolated loss of Ube3a expression. In a Ube3a mouse model, an increase in the seizure susceptibility was noted with the depletion of Ube3a in GABAergic neurons without any obvious neuronal loss or mossy fiber sprouting. Interestingly, this increased seizure susceptibility was reversed with increased Ube3a expression.[13] The authors also reported that the rescue was successful in juvenile mice (postnatal day 21), but not during adulthood, and speculated that there are “critical periods” for such intervention. However, the treatment windows for the prevention of epilepsy may persist for a longer duration compared to other AS phenotypes such as developmental delay, repetitive behaviors, and anxiety. This relatively longer treatment period may be due to the gradual accumulation of cellular and circuit-level pathologies over a prolonged period of time prior to the emergence of epilepsy. Besides the alteration of neocortical circuits, hyperexcitability has also been postulated secondary to the abnormal oscillatory activity of the thalamic reticular nucleus. Further studies showed an accumulation of clathrin-coated vesicles in presynaptic terminals of the pyramidal neurons to suggest a disruption in the presynaptic recycling, which is critical for synaptic physiology. Neuron-specific studies revealed that specific GABAergic Ube3a deletion produced characteristic delta slowing in the electrophysiological studies as well as an increase in seizure susceptibility.[14] On the contrary, glutamatergic Ube3a loss decreased tonic GABAergic inhibition without any change in the EEG or seizure susceptibility pattern. However, glutamatergic Ube3a loss may be relevant for other neurologic functions, such as cerebellar granule cells mediated locomotor activities.
Epilepsy phenotype
Approximately 80–90% of patients with AS develop epilepsy.[15] The onset of epilepsy is most prevalent between 1–3 years of age; however, about 25% of patients develop epilepsy before one year of age.[16] Early-onset of seizures may be strongly associated with autistic symptoms, even after controlling the particular genetic abnormality.[17]
The deletion subtype is associated with the most severe epilepsy phenotype, followed by isolated UBE3A mutation.[18] Uniparental disomy subtype is associated with the lowest frequency of epilepsy and exhibits the least severe epilepsy phenotype. Non-deletion patients, in general, may have relatively late-onset seizures. Moncla et al. compared 20 deletion and 20 non-deletion AS patients and noted that all non-deletion patients had rare seizures, and 7/20 patients (age 4–30 years) were seizure-free and several patients successfully stopped seizure medications.[19] In this study, atypical absence and myoclonic seizures were the most prevalent seizure type in the non-deletion cohort, but in contrast to the deletion AS patients, tonic-clonic seizures, infantile spasms, and myoclonic status epilepticus were not reported.
Seizure types
Both generalized and focal seizures have been reported, but isolated focal seizure is rare. Reflex seizures are also have been described.[20,21] Multiple types of seizures are usually present concurrently. Various types of generalized seizures are most prevalent, and myoclonic seizure is the most common type during infancy. The other two common seizure types are atonic and atypical absence seizures. Approximately 50% may have focal seizures.[22] Fever aggravated seizure is present in 50% of cases. Fever can trigger the 1st seizure, and seizure can be triggered with a modest change in temperature.[23] Although febrile seizure can rarely be the only seizure event, it most commonly follows with other seizure types. More than 95% of epilepsy patients may have daily seizures at least for a limited time during early childhood, and 2/3 patients develop disabling seizures.[22,24]
Status epilepticus
Status epilepticus is particularly common and seen in 35–85% of cases.[22,25] Generalized tonic-clonic seizures can be present, but convulsive status epilepticus is rare. Myoclonic status in nonprogressive disorders has been frequently described in AS when the child presents with continuous/ semi-continuous fragmentary myoclonus (rhythmic or arrhythmic symmetric and asymmetric jerks involving limbs, trunk, and face with or without atypical absence) with altered mental status and drooling.[26] Regression in milestones can be prominent such as an inability to walk or worsening of the gait. This particular type of status is more prevalent in 15q11–13 deletion and UBE3A mutation subtypes and less frequently associated with uniparental disomy.[26]Other than typical myoclonic features, nonconvulsive status epilepticus can be manifested as increased seizure activity or cluster of seizure activity at the onset of the episodes in association with some features of the following: Intermittent eye-rolling, eyelid fluttering, head nods, altered mental status, fatigue, sleepiness, developmental regression, and diminished communication. Due to the indistinct nature of many features, heightened suspicion is necessary for prompt diagnosis of these subtle status episodes. Sometimes triggers can be identified, such as infection, recent change or noncompliance with the AED regimen, or worsening sleep dysfunction. EEG shows high voltage, frontally dominant, slow spike-wave discharges comprising 50–95% of the recording. AS patients typically have less frequent episodes of status after puberty.
Infantile spasms
Several reports of infantile spasm exist in association with EEG findings of hypsarrythmia. However, misclassification is possible due to the presence of pseudo-hypsarrythmia and episodes of nonepileptic or epileptic myoclonus or atonic seizures. This is particularly relevant as first-line AED for infantile spasms such as vigabatrin may worsen myoclonic and/or absence seizures with overall clinical deterioration.
There are few nonepileptic events that can be misclassified as seizures in AS, such as continuous tremors like movement that disappears during sleep, nonepileptic myoclonus(no EEG correlates present), and paroxysmal laughter. The prevalence of nonepileptic myoclonus increases with age. Frequently, myoclonus may involve hands first followed by spreading to the face.[27] These episodes are not associated with alteration of consciousness and typically refractory to treatment.
Electroencephalography(EEG)
Characteristic EEG features
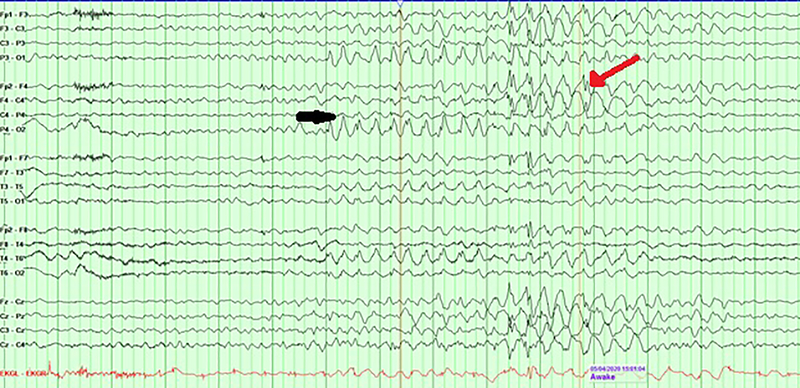
AS patients have characteristic EEG abnormalities, and almost all patients had EEG abnormalities with or without seizures.[25,28–31] Boyd et al. reported EEG in 11 children with clinical features of AS (age range: 11 months-11 years, most individuals were < 5 years of age) and identified three high-voltage features in isolation or combination: 1. Persistent rhythmic 4–6 Hz, ≥ 200 μV activities during wakefulness 2.Prolonged runs of anterior dominant, rhythmic 2–3 Hz (200–500 μV) with or without admixed spike-wave discharges 3. Posterior dominant 3–4 Hz ≥ 200 μV notched delta and theta activities (admixed with ill-defined spike-wave) facilitated with eye closure. (Fig. 1). This last finding has been predominantly seen in children less than 12 years old. Vendrame et al. showed an age-dependent decrease in the central-temporal/occipital delta waves with advancing age.[32] EEG abnormalities have been proposed to be used as a reliable biomarker in research studies. Frohlich et al. reported spectral power of clinical EEG recording in AS children and noted an elevated theta power (peak frequency: 5.3 Hz) and diminished beta power (peak frequency: 23 Hz) in the deletion genotype (37 patients) compared with the nondeletion genotype(21 patients).[33] The authors also noted an excess broadband EEG power (1–32 Hz) peaking in the delta frequency range (peak frequency: 2.8 Hz), shared by both genotypes but stronger for the deletion genotype at younger ages to suggest non-UBE3A neuronal pathophysiology in deletion AS with more severe phenotype. However, EEG features such as delta activity, sleep spindles, etc. need to be evaluated more robustly for clinical relevance and reversibility before they can be used reliably in clinical trials as an objectively quantifiable measure.
Fig 1.
Representative EEG showing posterior dominant 3–4 Hz notched delta and theta activities (black arrow) and anterior dominant, rhythmic 2–3 Hz activities intermixed with spike-wave discharges(red arrow).
Pseudo-hypsarrythmia
Misclassification of infantile spasms is possible due to the presence of a unique pseudo-hypsarrythmia pattern in EEG, which is seen particularly in 3 months-2 years old AS patients and can be differentiated from hypsarrythmia pattern by the following: predominance of slow waves compared to epileptiform discharges, no change in pattern between wakefulness and sleep, and lack of fragmentation during sleep.[34]
EEG abnormalities associated with AS can also be seen rarely in some other epileptic encephalopathies such as Rett syndrome and 4p deletion syndrome.[35,36]
Magnetic resonance imaging (MRI)
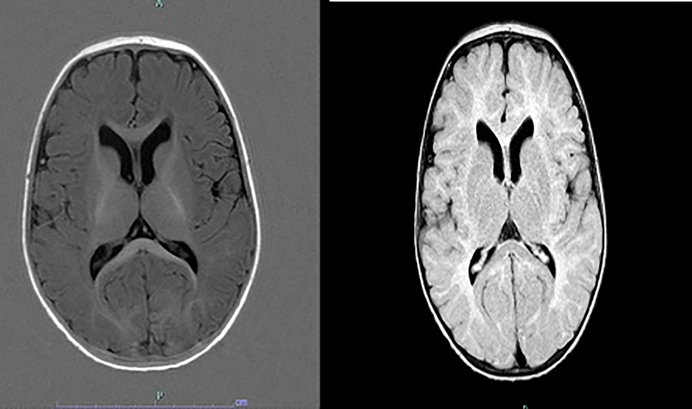
Neuroimaging abnormalities have been described in AS, such as corpus callosum hypoplasia, lateral ventricle enlargement, and cerebral atrophy(Fig. 2).[37] Harting et al. reported five infants with delayed myelination and white matter deficit in a cohort of 9 AS patients (7.5 months - 5 years).[38] Peters et al. reported diffuse white matter microstructure changes in AS by diffusion tensor imaging (DTI), and clinical severity was most correlated with the disruption in the temporal white matter pathway.[39] These microstructure changes were likely due to myelination defect as well as changes in the axonal density, diameter, and organization.
Fig. 2.
MRI of a child with Angelman syndrome at the age of 8 months. Myelination on T1w(left) shows still deficient frontal and temporal white matter and a hypoplastic corpus callosum corresponding to a maturation stage of 5–6 months. T2w image(right) indicates mildly widened ventricles, dorsally thin corpus callosum, a slightly patchy T2 signal of the white matter with particular prominence over the parieto-occipital region.
Treatment
The effectiveness of individual AEDs in the treatment of seizures associated with AS is based on low-quality studies as prospective evaluations are scarce. Most retrospective chart-review and questionnaire-based studies have been beneficial to provide an understanding of relative efficacy and tolerability of one AED compared to others.[40] Considering underlying prominent GABAergic dysfunction, clinicians had used treatment that targets GABAergic signaling such as valproate, phenobarbital, and clonazepam as first-line therapies for AS.[40] However, due to unfavorable side effect profile of these former AEDs, other medicines such as topiramate, lamotrigine, ethosuximide, levetiracetam, clobazam (slightly different chemical structure than other benzodiazepines) have emerged as more favorable options.
Valproate
Previously valproate was used as one of the 1st line AEDs in the management of AS due to its effectiveness against various generalized seizures.[2] However, its use as first-line therapy is declining due to concern about adverse effects as well as the availability of other better tolerated AEDs for generalized epilepsy.[41] Valente et al. reported 18 patients(in a cohort of 19 patients) with improved seizure control from valproate either as a monotherapy or in combination with either clonazepam or phenobarbital. A clear trend of declining valproate use has been noted between 2 studies done in 2006–2007 and 2008–2015.[42,43] Valproate use was decreased from 60% to 40% in just one decade. In the 1st study, valproate was the most commonly used AED.[43] In the latter study, Shaaya et al. reported a cohort of 85 AS patients, among which 63 had epilepsy. [42] Twenty-five subjects (40%) were treated with VPA, and 2/3 of the patients had more than 90% seizure reduction, and the rest of them had more than 50% seizure reduction. However, unfortunately, greater than 70% had adverse events such as worsening behavior, ataxia, and declining gross/fine motor skills. Majority patients (60%) discontinued valproate due to adverse effects.
Topiramate
Thibert et al. reported that topiramate was the 2nd most commonly used AED (similar to clonazepam) following valproate in a questionnaire-based study.[43] Topiramate was used in 1/3 of patients. Topiramate has a GABA mimetic effect and can act as a positive allosteric modulator of GABAA receptors. Franz et al. reported a positive experience of treating 5 AS patients with topiramate.[44] All these patients had 15q11–13 deletions. 4 out of 5 patients were treated with topiramate monotherapy (6–23 mg /kg/day) over 3–12 months. One patient discontinued topiramate due to akathisia and insomnia. Besides the patient who discontinued topiramate, the rest of the patients had a remarkable improvement in seizures, and two patients remained seizure-free for 8–12 months. However, topiramate’s cognitive side effects and appetite suppression remain a big concern in the AS patient population.
Lamotrigine
Lamotrigine use was noted in approximately ¼ AS patients in a questionnaire-based study.[43] Lamotrigine (a sodium channel blocker) has been effective for both focal and generalized epilepsy with broad activity against multiple seizure types associated with severe epileptic encephalopathies such as Lennox -Gastaut syndrome. Dion et al. retrospectively evaluated the effectiveness of adjunctive lamotrigine in 5 AS patients with intractable seizures.[45] Three patients became seizure-free, and one patient was seizure-free for one year with subsequent recurrence, and 1 had more than 50% reduction of seizure frequency in a low-dose but had worsening of seizures with a higher dose. Lamotrigine was relatively well-tolerated, and no patient had consistent worsening of seizures in spite of lamotrigine’s potential pro myoclonic adverse effect. Although other sodium channel blockers such as phenytoin and carbamazepine are relatively contraindicated in AS patients due to potential worsening of absence and/or myoclonic seizure, it is unclear how lamotrigine shows positive response in AS consistently. Mechanisms other than sodium channel blockage have been speculated. In an MRS study, Kuzinecky et al. showed increased cerebral GABA concentration after four weeks of lamotrigine exposure in healthy volunteers.[46] Wang et al. showed that lamotrigine might increase the expression of GABAB3 subunit expression in the hippocampus.[47]
Ethosuximide
Ethosuximide, a T-type Ca2+ channel blocker, is the most preferred AED for typical absence seizures associated with childhood absence epilepsy. However, it has also been widely used for atypical absence seizures. Sugiura et al. demonstrated the use of high dose adjunctive ethosuximide (serum concentration greater than 110 mcg per mL) in combination with valproate for atypical absence seizures in 2 patients with AS. [48] Remarkable clinical improvement was noted in these patients in association with suppression of spike-wave complexes. Valproate may increase the level of ethosuximide, and the combination of valproate and ethosuximide may be preferable in patients with refractory absence seizures. Interestingly, ethosuximide has shown effectiveness in seizure control in mice with deficient GABRB3.[9]
Levetiracetam
Levetiracetam is the most commonly used AED for any indication due to its broad activity against both focal and generalized seizures and favorable side effect profile with no or minimal occurrence of significant life-threatening adverse effects. Previously, levetiracetam has been used in approximately 20% of AS patients, but recently, this has emerged as the most commonly used AED in AS patients (used in 2/3 of the patients).[42,43] In a retrospective study, levetiracetam was associated with a 90% reduction of seizures in 86% of patients.[42]. However, 1/5 had behavioral adverse effects. Finally, 80% remained on levetiracetam therapy but only 1/3 on monotherapy with a dose range of 6.15 −210 mg/kg/day to primarily support its role as a well-tolerated adjunctive AED.
Clonazepam
Clonazepam, an old and inexpensive 1,4-benzodiazepine, had been used extensively as a chronic AED in AS patients. In one study, clonazepam was the 2nd most commonly used AED in AS patients.[43] However, clonazepam’s side effects, such as sedation, decreased muscle tone, and increased drooling, are not favorable for AS patients. Specifically, due to concern about tolerance, its use as a daily AEDs is declining in the developed world.
Clobazam
Clobazam, a 1,5-benzodiazepine, has been reported to exhibit less adverse effects and better tolerance profile than the classic 1,4-benzodiazepines such as diazepam and clonazepam. Clobazam had been very rarely utilized due to its unavailability in the United States previously. However, a recent study in the AS population showed this as the 2nd most commonly used AED (used in 50% of patients) after levetiracetam. [42]With exposure to clobazam, 93% had greater than 90% reduction in seizure frequency. Long term effect was evident, with 75% remained on the therapy and 1/3 on monotherapy. In this evaluation, tachyphylaxis was not found to be a significant factor.
Cannabidiol(CBD)
Acute CBD treatment (100 mg/kg (intraperitoneally) was noted to suppress hyperthermia and acoustically induced seizures in the AS mouse model but did not change myoclonic or tonic-clonic seizure susceptibility after kindling immediately and after two weeks of therapy.[49] A mild sedative effect was seen in the mice, and no definite improvement was noted in motor skill or memory secondary to CBD treatment. Interestingly, CBD treatment reversed some electrophysiological features, such as theta and delta slowing in the AS mice.
A highly purified form of pharmaceutical CBD has been approved for drug-resistant seizures associated with Dravet syndrome and Lennox-Gastaut syndrome.[50,51] Open-label trials of a pharmaceutical-grade CBD and other artisanal CBD products have shown long-term efficacy and safety of CBD in various other treatment-resistant epilepsy such as tuberous sclerosis, CDKL5 deficiency disorders, Aicardi syndrome, 15q duplication, and Doose syndrome. Anecdotal reports exist about CBD’s effectiveness in AS patients, but a rigorous randomized clinical trial is needed in the AS population. Some other new antiepileptic drugs such as rufinamide and perampanel have been utilized in small case series for management of nonepileptic myoclonus, but limited evidence currently exists for their efficacy against epileptic seizures.[52]
Dietary therapies
The ketogenic diet can be very useful for the management of refractory seizures associated with AS.[53] Almost 1/3 of AS patients endorsed the ketogenic diet as the most successful treatment. However, tolerance may be a significant issue. Due to the restrictive nature of the ketogenic diet, Grocott and colleagues from the center for dietary therapy of epilepsy at the Massachusetts General Hospital evaluated 23 AS patients with low glycemic index dietary therapy.[54] Among these patients, three became seizure-free, and an additional 10 had only seizures in the presence of intercurrent infection. All these patients were treated with additional AEDs. Among the other eight patients, all had decreased seizure frequency after initiation of the diet except in one patient where detailed information was not available. Despite low ketone (beta-hydroxybutyrate) level, these patients had better seizure control to suggest an alternative mechanism of action, such as stabilization of blood glucose. Many patients tolerated higher than the required carbohydrate intake without any deleterious effect. Side effects (acidosis requiring supplementation with potassium citrate, carnitine supplementation, constipation) were benign, and compliance and tolerance were excellent with minimal rate of loss to follow-up. The robust effect of the low glycemic index diet was also noted in a prospective pediatric cohort of 6 AS patients.[55]
Vagus nerve stimulation(VNS)
VNS has been utilized in medically intractable seizures. Tomei et al. reported a small case series of 3 AS patients treated with VNS.[56] All three patients had marked reduction of seizure frequency with improved alertness. No major side effect was reported. One patient even became clinically seizure-free. However, none of these patients could decrease their AEDs. Vagal hypertonicity has been speculated in AS secondary to a central mechanism or due to intrathoracic pressure changes associated with frequent laughter. Preoperative use of anticholinergics has been recommended as a unique management consideration for AS patients.[57] Although the vagal hypertonicity may indicate an increased risk of bradyarrhythmia, this has not been noted in clinical experience. On the contrary, augmented vagal responsiveness with better efficacy of VNS has been hypothesized in a study. Based on a survey-based study, Thibert et al. described 16 patients with VNS use, out of which 3 reported VNS as the most effective therapy. The exact mechanism of action of VNS in AS (as with any other forms of epilepsy) is unknown, but decreased excitability may be associated with improvement in GABAergic signal and inhibition of brainstem mediated fast synchronous oscillation (nucleus tractus solitarious mediated brainstem reticular formation activity). Chronic VNS administration has noted to be associated with increased cerebrospinal fluid(CSF) GABA levels and may be particularly relevant for the management of AS.
Corpus callosotomy
Palliative surgical procedures such as corpus callosotomy have been utilized for the management of intractable drop seizures and disabling falls associated with various types of generalized seizures. Although corpus callosotomy can cause an improvement in generalized tonic-clonic and atonic seizures in several epileptic encephalopathies, effectiveness and the role of corpus callosotomy in the management of AS is currently unknown due to paucity of good quality research.
Corticosteroid
Forrest et al. described four children with AS who showed excellent response to prednisone therapy.[58] Improvement in both clinical (decrease seizure frequency of spasms, atypical absence, and atonic seizures) and EEG appearance were seen. Moreover, improvement in sleep, myoclonus, development, and social interaction was noted. Activation of the GABA receptor by steroid has been hypothesized as a potential mechanism.
Management of nonconvulsive status epilepticus
Management of nonconvulsive status epilepticus is particularly important in AS patients. Worden et al. reported that approximately 50% AS patients had nonconvulsive status epilepticus with features of myoclonic or absence seizures.[59] This study showed the success of oral diazepam in the treatment of nonconvulsive status in ambulatory patients. In 13 pediatric patients with AS, a course of oral tapering diazepam (mean dose of 0.32 mg/kg /day in 2–3 divided doses over 4–12 days) successfully terminated 80% (20/25) episodes. No major side effects, such as respiratory depression was observed. Occasional patients required the 2nd course of therapy. If ambulatory treatment with diazepam fails, prednisone therapy has been tried and/or inpatient hospitalization with more aggressive management, including therapeutic coma. Other than diazepam, alternative benzodiazepines such as clonazepam, clobazam, lorazepam, a higher dose of valproate, levetiracetam, and diet with carbohydrate restriction have been utilized for ambulatory and inpatient management of status.
AED induced worsening of seizures
Carbamazepine and oxcarbazepine can cause a worsening of seizures in AS patients. Interestingly vigabatrin, a GABAergic drug, does not effectively manage seizures in AS patients and may induce worsening of seizures. [60] Possibly increasing the GABA level without enhancing the GABA receptor function may be responsible for its ineffectiveness.
Outcomes
Several studies in AS reported severe seizures in early childhood with a distinct improvement during later childhood.[61–63] Seizure remission in late teens and early twenties followed by recurrence of seizures in third or fourth decades has been reported.[64] However, studies also described the persistence of subtle absence and myoclonic seizures throughout adulthood.[65] Larson et al. reported increase seizure frequency and severity in individuals over 25 years age compared to those 16–20 years old.
Future therapy
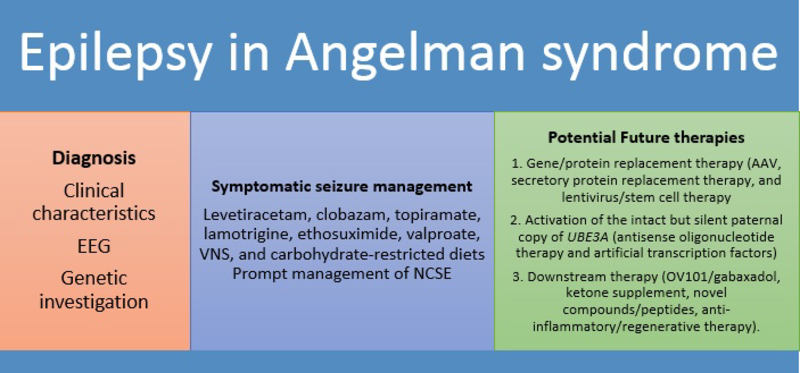
There is no approved curative treatment of AS, and available therapies for epilepsy are focused on the symptomatic management of seizures to minimize seizure frequency and severity. However, this field is rapidly changing, with advancements in various molecular therapies.[66,67] Discovery of the abnormal phosphorylation state of calcium/ calmodulin-dependent protein kinase II in AS demonstrated postnatal biochemical and signal abnormalities as a causative pathway for many phenotypes (rather than irreversible global developmental defects) and raised interest in AS therapeutics to rescue clinical phenotypes with postnatal intervention.[68,69] The characteristic of ideal therapy will be a minimally invasive intervention without significant toxicity that can cross the blood-brain barrier to produce a long-term, gene-specific regulation with a maximum and durable phenotypic reversal.[70] The treatment development can be categorized as follows: 1. Gene/protein replacement therapy (Adeno and lentiviral vector therapy to deliver a gene or secretory protein); 2. Activation of the intact but silent paternal copy of UBE3A (antisense oligonucleotide therapy and artificial transcription factors); and 3. Downstream therapies (OV101/gaboxadol, ketone supplement, novel compounds/peptides, anti-inflammatory/regenerative therapy). (Fig. 3)
Fig 3.
Current and potential future therapies in Angelman syndrome
AAV and other viral vector therapy(gene/protein replacement therapy)
The most common genetic abnormality of AS is de novo 5–7 Mb maternal loss of 15q11–13. However, it would be challenging to replace this large deleted region with viral vectors due to size limitation, and a pragmatic approach involves only the replacement of the causative UBE3A gene. Adenovirus associated virus serotype 9 (AAV-9), with the highest transgene expression in the brain compared to other serotypes, has been used with a TR-2 flank UBE3A to directly injected into the hippocampus of AS mice with an improvement of associative learning. AAV-9 therapy produced a limited distribution of viral vector in the brain and only achieved partial rescue of memory and learning but no improvement in motor coordination(possibly due to the AAV9 not reaching the cerebellum).[71] Lentiviral vectors have also been proposed to deliver non-integrating episomes in the neurons. Rather than cDNA delivery, alternative approaches to providing the protein to rescue phenotype have been considered.
Besides the restriction of viral distribution around the injection site, another potential limitation is the immune reaction against AAV. Although the AAV study was encouraging to show partial phenotype reversibility, other less traditional approaches had been explored in search of full phenotypic rescue.
Reactivation of the intact but silent paternal copy of UBE3A
Paternal UBE3A is epigenetically silenced by a long antisense RNA transcript overlapping the open reading frame of paternal UBE3A. By using a screening method in primary cortical neurons of mice, Huang et al. identified several topoisomerase 1 and 2 inhibitors that can unsilence the paternal Ube3a by downregulating antisense transcript overlapping the paternal copy.[72] Among these topoisomerase inhibitors, an FDA approved cancer chemotherapy agent topotecan can powerfully turn on the inactivated paternal allele of UBE3A with improved Ube3a expression in several regions of the nervous systems, such as the hippocampus, neocortex, striatum, cerebellum, and spinal cord in a mouse model. The effect was evident chronically with an enduring impact of increased expression of Ube3a in spinal neurons for up to 12 weeks after intrathecal administration. As a chemotherapeutic agent, neutropenia and systemic toxicity are concerning side effects of Topotecan. Additionally, research has not been expanded to assess its potential off-target effects, and no recent advancement toward human trials has been reported.
However, the concept of reducing Ube3a-ATS expression to improve paternal Ube3a expression has further augmented by other studies using a transcriptional termination signal.[4,73] Meng et al. reported the use of antisense oligonucleotide therapy by reduction of UBE3A- ATS (antisense transcript is an atypical transcript made by the nuclear-localized long non-coding RNA polymerase II) and sustained unsilencing of paternal antisense transcript in a murine model. The decrease in neuronal UBE3A-ATS was up to 90% within 48 hours of treatment -both in vitro and in vivo examinations- as a dose-dependent manner after single intracerebral ventricular injection in adult mice. After four weeks of injection, partial restoration of Ube3a protein was observed. Following a single injection of antisense oligonucleotides(ASOs), decreased ATS expression persisted for four months in cortical neurons.[74]
However, widespread and high-level cortical expression and Ube3a restoration remain a challenging task. Behavioral phenotypes were compared between ASO treated mice with controls. Although ASO treated mice showed some improvement in memory, they did not show significant improvement in many behavioral tests such as in the open field assay during testing for locomotor and anxiety-like behavior, in the marble-burying test during the examination of anxiety and obsessive-compulsive behaviors as well as during testing on an accelerating rotarod to evaluate motor coordination. A complete phenotypic reversal may need treatment before the closure of the critical window or may need a higher Ube3a induction level. Additionally, improvement may appear later than the testing period due to a gradual recovery of neuronal circuits, much delayed after the correction of the Ube3a protein.
Engineered DNA-binding protein (Artificial transcription factor/ATF)
AS is caused by mutation of a single gene and thus an ideal candidate for gene expression therapy, especially with advanced technology to engineer smaller DNA-binding particle such as artificial transcription factor (based on zinc finger, TALENS- Transcription activator-like effector nucleases, CRISPR/CAS9- clustered, regularly interspaced, short, palindromic repeats) that can cross the blood-brain barrier and inhibit UBE3A-ATS with subsequent expression of Ube3a.[75] Research studies involving mice using transcription factors are applicable to the future human research as the imprinting control centers are highly conserved among species. Bailus reported the use of an engineered Zinc finger-based artificial transcription factor in an adult mouse model.[76] After intraperitoneal or subcutaneous injection, these ATFs cross the blood-brain barrier with an improved expression of Ube3a in the hippocampus and cerebellum. CRISPR technology has been used to produce a large deletion Ube3a rats and AS pig models. Additionally, CRISPR /CAS13 b technology is gathering attention in the treatment sphere as it can cut RNA directly compared to CRISPR /CAS9 that can only cut DNA. However, several shortcomings exist, such as difficulty in the distribution of UBE3A throughout the neurons of the brain, potential off-target effect, and potential immune response against the agent. Additionally, more research is needed regarding the critical window of restoration.[77] Deng et al. reported the use of a mouse mesenchymal (bone marrow) stem-cell that can secrete an artificial transcription factor as a delivery platform to reactivate silent UBE3A with an enhanced expression of the protein in hippocampus, cerebellum, and cortex in a mouse model.
Downstream therapeutics
In conjunction with molecular therapy to increase UBE3A expression, several research groups have attempted to investigate therapeutics to attack synaptic abnormality associated with AS. This is particularly relevant as an alteration of synaptic physiology in AS may be similar to other neurodevelopmental disorders, and a broader application might be possible of the effective intervention.
Egawa et al. showed that selective extrasynaptic GABAA receptor agonist gaboxadol improved the abnormal firing properties of a population of Purkinje cells in cerebellar brain slices in Ube3a-deficient mice in vivo.[12] OV101/gaboxadol, a small molecule derived from muscimol (naturally found in mushroom), has been evaluated in the STARS study (a phase II RCT) over 12 weeks in adolescents and adults (13–49 years) AS patients and showed global improvement in a subjective improvement scale with a once-daily dose. [78] However, no specific data was available in terms of seizure control. Ganaxolone, another modulator of extrasynaptic GABAA receptors, also noted to decrease seizure susceptibility in AS mice in association with improvement in motor deficits.[79]
A double-blind, placebo-controlled, trial investigated beneficial effects of two nutritional products(by potentially increasing methylation), betaine and folic acid, in 48 children with AS over one year but did not find any statistically significant changes between the two groups.[80] Patented synthetic analogs of cyclic glycine proline is a breakdown product of Insulin-like growth factor-1(IGF-1), which is a growth factor that’s naturally present in the brain and can exert a widespread effect in biological process in the brain, such as reduction of neuroinflammation, action against overactivated microglia, and improvement of synaptic signaling. Although ketogenic and low glycemic index diets have been noted to be safe and effective in the management of AS patients, noncompliance and micronutrient deficiencies are challenging for chronic use. Although ketone ester supplementation reduced seizure activity in an AS mouse model, the effectiveness and safety of consumption of exogenous ketones in humans are unknown.[81] A 16 week double-blind, randomized control trial using beta hydroxybutyrate has been planned in AS patients. Similar to the fragile X mouse model, lovastatin suppressed the epileptiform activity and audiogenic seizures in an AS mouse model.[82] The supplementation of taurine has been studied in an AS mouse model.[83,84]
Abnormal dendritic spine morphology and density in hippocampal pyramidal neurons leading to abnormal synaptic physiology have been described in AS. Minocycline, a second-generation tetracycline antibiotic medication, has been shown to recover aberrant synaptic function by reducing matrix metalloproteinases and positively altering dendritic spine structure.[85] In a prospective open-label study of minocycline, Grieco et al. showed improvement in communication and fine motor activities in 25 AS children (4–12 years), but the Phase II, randomized trial for minocycline therapy up to 16 weeks failed to show significant improvements in the developmental status of the patients or in the EEG.[85,86] Patients with uncontrolled seizures were excluded from the study.
Conclusion
Current therapy of intractable epilepsy associated with AS is primarily symptomatic. However, in the last 20 years, there has been remarkable progress in the understanding of causative gene, elucidation of subsequent signal abnormalities, and the delineation of paternal UBE3A gene silencing: essential discoveries not only for the management of epilepsy but likely improvement in the health of AS patient as a whole. Several biotechnology companies have received orphan drug designation of various products from the FDA and European Commission for faster development of commercially viable therapeutics. There has been significant progress in the establishment of a global patient registry to identify suitable patients for recruitments in the clinical trials.[87,88] However, proper cost-effective analysis is needed to balance the economic burden of the disease with these potential ultra-costly therapeutics.
Acknowledgments
Funding:
The author is supported by the Translational Research Institute (TRI), grant UL1 TR003107 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures:
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pelc K, Boyd SG, Cheron G, Dan B. Epilepsy in Angelman syndrome.Seizure. 2008;17:211–7. [DOI] [PubMed] [Google Scholar]
- [2].Park SH, Yoon JR, Kim HD, Lee JS, Lee YM, Kang HC. Epilepsy in Korean patients with Angelman syndrome.Korean J Pediatr. 2012;55:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thibert RL, Larson AM, Hsieh DT, Raby AR, Thiele EA. Neurologic manifestations of Angelman syndrome.Pediatr Neurol. 2013;48:271–9. [DOI] [PubMed] [Google Scholar]
- [4].Meng L, Person RE, Beaudet AL. Ube3a-ATS is an atypical RNA polymerase II transcript that represses the paternal expression of Ube3a.Hum Mol Genet. 2012;21:3001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khatri N, Man HY. The Autism and Angelman Syndrome Protein Ube3A/E6AP: The Gene, E3 Ligase Ubiquitination Targets and Neurobiological Functions.Front Mol Neurosci. 2019;12:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borgatti R, Piccinelli P, Passoni D, Romeo A, Viri M, Musumeci SA, et al. Peripheral markers of the gamma-aminobutyric acid (GABA)ergic system in Angelman’s syndrome.J Child Neurol. 2003;18:21–5. [DOI] [PubMed] [Google Scholar]
- [7].Liljelund P, Handforth A, Homanics GE, Olsen RW. GABAA receptor beta3 subunit gene-deficient heterozygous mice show parent-of-origin and gender-related differences in beta3 subunit levels, EEG, and behavior.Brain Res Dev Brain Res. 2005;157:150–61. [DOI] [PubMed] [Google Scholar]
- [8].DeLorey TM, Olsen RW. GABA and epileptogenesis: comparing gabrb3 gene-deficient mice with Angelman syndrome in man.Epilepsy Res. 1999;36:123–32. [DOI] [PubMed] [Google Scholar]
- [9].DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, et al. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome.J Neurosci. 1998;18:8505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liljelund P, Ferguson C, Homanics G, Olsen RW. Long-term effects of diazepam treatment of epileptic GABAA receptor beta3 subunit knockout mouse in early life.Epilepsy Res. 2005;66:99–115. [DOI] [PubMed] [Google Scholar]
- [11].Wallace ML, Burette AC, Weinberg RJ, Philpot BD. Maternal loss of Ube3a produces an excitatory/inhibitory imbalance through neuron type-specific synaptic defects.Neuron. 2012;74:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Egawa K, Kitagawa K, Inoue K, Takayama M, Takayama C, Saitoh S, et al. Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of Angelman syndrome.Sci Transl Med. 2012;4:163ra157. [DOI] [PubMed] [Google Scholar]
- [13].Gu B, Carstens KE, Judson MC, Dalton KA, Rougié M, Clark EP, et al. Ube3a reinstatement mitigates epileptogenesis in Angelman syndrome model mice.J Clin Invest. 2019;129:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Judson MC, Wallace ML, Sidorov MS, Burette AC, Gu B, van Woerden GM, et al. GABAergic Neuron-Specific Loss of Ube3a Causes Angelman Syndrome-Like EEG Abnormalities and Enhances Seizure Susceptibility.Neuron. 2016;90:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fiumara A, Pittalà A, Cocuzza M, Sorge G. Epilepsy in patients with Angelman syndrome.Ital J Pediatr. 2010;36:31,7288–36-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Van Lierde A, Atza MG, Giardino D, Viani F. Angelman’s syndrome in the first year of life.Dev Med Child Neurol. 1990;32:1011–6. [DOI] [PubMed] [Google Scholar]
- [17].Bakke KA, Howlin P, Retterstøl L, Kanavin Ø, Heiberg A, Nærland T. Effect of epilepsy on autism symptoms in Angelman syndrome.Mol Autism. 2018;9:2,017–0185-1. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luk HM, Lo IF. Angelman syndrome in Hong Kong Chinese: A 20 years’ experience.Eur J Med Genet. 2016;59:315–9. [DOI] [PubMed] [Google Scholar]
- [19].Moncla A, Malzac P, Voelckel M, Auquier P, Girardot L, Mattei M, et al. Phenotype–genotype correlation in 20 deletion and 20 non-deletion Angelman syndrome patients. Eur J Hum Genet. 1999;7:131–9. [DOI] [PubMed] [Google Scholar]
- [20].Ferlazzo E, Sueri C, Elia M, D’Agostino T, Aguglia U. Reflex seizures in a patient with Angelman syndrome and trisomy 21.Neurol Sci. 2016;37:1373–4. [DOI] [PubMed] [Google Scholar]
- [21].Pellinen J, Hasan H, Ortiz N, Bluvstein J, Miles D. Reflex micturition defecation epilepsy in Angelman syndrome.Neurol Clin Pract. 2019;9:510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Valente KD, Koiffmann CP, Fridman C, Varella M, Kok F, Andrade JQ, et al. Epilepsy in patients with angelman syndrome caused by deletion of the chromosome 15q11–13.Arch Neurol. 2006;63:122–8. [DOI] [PubMed] [Google Scholar]
- [23].Viani F, Romeo A, Viri M, Mastrangelo M, Lalatta F, Selicorni A, et al. Seizure and EEG patterns in Angelman’s syndrome.J Child Neurol. 1995;10:467–71. [DOI] [PubMed] [Google Scholar]
- [24].Valente KD, Fridman C, Varela MC, Koiffmann CP, Andrade JQ, Grossmann RM, et al. Angelman syndrome: uniparental paternal disomy 15 determines mild epilepsy, but has no influence on EEG patterns.Epilepsy Res. 2005;67:163–8. [DOI] [PubMed] [Google Scholar]
- [25].Laan LA, Renier WO, Arts WF, Buntinx IM, vd Burgt IJ, Stroink H, et al. Evolution of epilepsy and EEG findings in Angelman syndrome.Epilepsia. 1997;38:195–9. [DOI] [PubMed] [Google Scholar]
- [26].Elia M Myoclonic status in nonprogressive encephalopathies: an update.Epilepsia. 2009;50 Suppl 5:41–4. [DOI] [PubMed] [Google Scholar]
- [27].Pollack SF, Grocott OR, Parkin KA, Larson AM, Thibert RL. Myoclonus in Angelman syndrome.Epilepsy Behav. 2018;82:170–4. [DOI] [PubMed] [Google Scholar]
- [28].Buoni S, Grosso S, Pucci L, Fois A. Diagnosis of Angelman syndrome: clinical and EEG criteria.Brain Dev. 1999;21:296–302. [DOI] [PubMed] [Google Scholar]
- [29].Laan LA, Brouwer OF, Begeer CH, Zwinderman AH, van Dijk JG. The diagnostic value of the EEG in Angelman and Rett syndrome at a young age.Electroencephalogr Clin Neurophysiol. 1998;106:404–8. [DOI] [PubMed] [Google Scholar]
- [30].Leyser M, Penna PS, de Almeida AC, Vasconcelos MM, Nascimento OJ. Revisiting epilepsy and the electroencephalogram patterns in Angelman syndrome.Neurol Sci. 2014;35:701–5. [DOI] [PubMed] [Google Scholar]
- [31].Robinson AA, Goldman S, Barnes G, Goodpaster L, Malow BA. Electroencephalogram (EEG) duration needed to detect abnormalities in angelman syndrome: is 1 hour of overnight recording sufficient? J Child Neurol. 2015;30:58–62. [DOI] [PubMed] [Google Scholar]
- [32].Vendrame M, Loddenkemper T, Zarowski M, Gregas M, Shuhaiber H, Sarco DP, et al. Analysis of EEG patterns and genotypes in patients with Angelman syndrome.Epilepsy Behav. 2012;23:261–5. [DOI] [PubMed] [Google Scholar]
- [33].Frohlich J, Miller MT, Bird LM, Garces P, Purtell H, Hoener MC, et al. Electrophysiological phenotype in Angelman syndrome differs between genotypes.Biol Psychiatry. 2019;85:752–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Darteyre S, Mazzola L, Convers P, Lebrun M, Ville D. Angelman syndrome and pseudo-hypsarrhythmia: a diagnostic pitfall.Epileptic Disord. 2011;13:331–5. [DOI] [PubMed] [Google Scholar]
- [35].Laan LA, Vein AA. A Rett patient with a typical Angelman EEG.Epilepsia. 2002;43:1590–2. [DOI] [PubMed] [Google Scholar]
- [36].Sgrò V, Riva E, Canevini MP, Colamaria V, Rottoli A, Minotti L, et al. 4p(−) syndrome: a chromosomal disorder associated with a particular EEG pattern.Epilepsia. 1995;36:1206–14. [DOI] [PubMed] [Google Scholar]
- [37].Leyser M, Gonsalvez Mde C, Vianna PE, Fernandes PA, Carvalho RS, Vasconcelos MM, et al. Scrutinizing brain magnetic resonance imaging patterns in Angelman syndrome.Neurol India. 2016;64:228–32. [DOI] [PubMed] [Google Scholar]
- [38].Harting I, Seitz A, Rating D, Sartor K, Zschocke J, Janssen B, et al. Abnormal myelination in Angelman syndrome.Eur J Paediatr Neurol. 2009;13:271–6. [DOI] [PubMed] [Google Scholar]
- [39].Peters SU, Kaufmann WE, Bacino CA, Anderson AW, Adapa P, Chu Z, et al. Alterations in white matter pathways in Angelman syndrome.Dev Med Child Neurol. 2011;53:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ruggieri M, McShane MA. Parental view of epilepsy in Angelman syndrome: a questionnaire study.Arch Dis Child. 1998;79:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Deda G, Caksen H, Kansu A, Girgin N, Suskan E, Uysal S, et al. Toxic hepatitis in a case of Angelman syndrome associated with Lennox-Gastaut syndrome.Genet Couns. 2004;15:357–61. [PubMed] [Google Scholar]
- [42].Shaaya EA, Grocott OR, Laing O, Thibert RL. Seizure treatment in Angelman syndrome: A case series from the Angelman Syndrome Clinic at Massachusetts General Hospital.Epilepsy Behav. 2016;60:138–41. [DOI] [PubMed] [Google Scholar]
- [43].Thibert RL, Conant KD, Braun EK, Bruno P, Said RR, Nespeca MP, et al. Epilepsy in Angelman syndrome: a questionnaire-based assessment of the natural history and current treatment options.Epilepsia. 2009;50:2369–76. [DOI] [PubMed] [Google Scholar]
- [44].Franz DN, Glauser TA, Tudor C, Williams S. Topiramate therapy of epilepsy associated with Angelman’s syndrome.Neurology. 2000;54:1185–8. [DOI] [PubMed] [Google Scholar]
- [45].Dion MH, Novotny EJ Jr, Carmant L, Cossette P, Nguyen DK. Lamotrigine therapy of epilepsy with Angelman’s syndrome.Epilepsia. 2007;48:593–6. [DOI] [PubMed] [Google Scholar]
- [46].Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults.Neurology. 2002;58:368–72. [DOI] [PubMed] [Google Scholar]
- [47].Wang J, Sun X, Chen B, Young LT. Lamotrigine increases gene expression of GABA-A receptor Î23 subunit in primary cultured rat hippocampus cells.Neuropsychopharmacology. 2002;26:415–21. [DOI] [PubMed] [Google Scholar]
- [48].Sugiura C, Ogura K, Ueno M, Toyoshima M, Oka A. High-dose ethosuximide for epilepsy in Angelman syndrome: implication of GABA(A) receptor subunit.Neurology. 2001;57:1518–9. [DOI] [PubMed] [Google Scholar]
- [49].Gu B, Zhu M, Glass MR, Rougié M, Nikolova VD, Moy SS, et al. Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice.J Clin Invest. 2019;129:5462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Samanta D Cannabidiol: A Review of Clinical Efficacy and Safety in Epilepsy.Pediatr Neurol. 2019;96:24–9. [DOI] [PubMed] [Google Scholar]
- [51].Samanta D Changing Landscape of Dravet Syndrome Management: An Overview.Neuropediatrics. 2020;51:135–45. [DOI] [PubMed] [Google Scholar]
- [52].Kawano O, Egawa K, Shiraishi H. Perampanel for nonepileptic myoclonus in Angelman syndrome. Brain Dev. 2020;42:389–92. [DOI] [PubMed] [Google Scholar]
- [53].Stein D, Chetty M, Rho JM. A “happy” toddler presenting with sudden, life-threatening seizures.Semin Pediatr Neurol. 2010;17:35–8. [DOI] [PubMed] [Google Scholar]
- [54].Grocott OR, Herrington KS, Pfeifer HH, Thiele EA, Thibert RL. Low glycemic index treatment for seizure control in Angelman syndrome: A case series from the Center for Dietary Therapy of Epilepsy at the Massachusetts General Hospital.Epilepsy Behav. 2017;68:45–50. [DOI] [PubMed] [Google Scholar]
- [55].Thibert RL, Pfeifer HH, Larson AM, Raby AR, Reynolds AA, Morgan AK, et al. Low glycemic index treatment for seizures in Angelman syndrome.Epilepsia. 2012;53:1498–502. [DOI] [PubMed] [Google Scholar]
- [56].Tomei KL, Mau CY, Ghali M, Pak J, Goldstein IM. Vagal nerve stimulation for medically refractory epilepsy in Angelman syndrome: a series of three cases.Childs Nerv Syst. 2018;34:395–400. [DOI] [PubMed] [Google Scholar]
- [57].Warner ME, Martin DP, Warner MA, Gavrilova RH, Sprung J, Weingarten TN. Anesthetic Considerations for Angelman Syndrome: Case Series and Review of the Literature.Anesth Pain Med. 2017;7:e57826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Forrest KM, Young H, Dale RC, Gill DS. Benefit of corticosteroid therapy in Angelman syndrome.J Child Neurol. 2009;24:952–8. [DOI] [PubMed] [Google Scholar]
- [59].Worden L, Grocott O, Tourjee A, Chan F, Thibert R. Diazepam for outpatient treatment of nonconvulsive status epilepticus in pediatric patients with Angelman syndrome.Epilepsy Behav. 2018;82:74–80. [DOI] [PubMed] [Google Scholar]
- [60].Kuenzle C, Steinlin M, Wohlrab G, Boltshauser E, Schmitt B. Adverse effects of vigabatrin in Angelman syndrome.Epilepsia. 1998;39:1213–5. [DOI] [PubMed] [Google Scholar]
- [61].Giroud M, Daubail B, Khayat N, Chouchane M, Berger E, Muzard E, et al. Angelman syndrome: a case series assessing neurological issues in adulthood.Eur Neurol. 2015;73:119–25. [DOI] [PubMed] [Google Scholar]
- [62].Laan LA, den Boer AT, Hennekam RC, Renier WO, Brouwer OF. Angelman syndrome in adulthood.Am J Med Genet. 1996;66:356–60. [DOI] [PubMed] [Google Scholar]
- [63].Sueri C, Ferlazzo E, Elia M, Bonanni P, Randazzo G, Gasparini S, et al. Epilepsy and sleep disorders improve in adolescents and adults with Angelman syndrome: A multicenter study on 46 patients.Epilepsy Behav. 2017;75:225–9. [DOI] [PubMed] [Google Scholar]
- [64].Larson AM, Shinnick JE, Shaaya EA, Thiele EA, Thibert RL. Angelman syndrome in adulthood.Am J Med Genet A. 2015;167A:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Espay AJ, Andrade DM, Wennberg RA, Lang AE. Atypical absences and recurrent absence status in an adult with Angelman syndrome due to the UBE3A mutation.Epileptic Disord. 2005;7:227–30. [PubMed] [Google Scholar]
- [66].Bi X, Sun J, Ji AX, Baudry M. Potential therapeutic approaches for Angelman syndrome.Expert Opin Ther Targets. 2016;20:601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rotaru DC, Mientjes EJ, Elgersma Y. Angelman Syndrome: From Mouse Models to Therapy. Neuroscience. 2020, in press. doi: 10.1016/j.neuroscience.2020.02.017. [DOI] [PubMed] [Google Scholar]
- [68].van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation.Nat Neurosci. 2007;10:280–2. [DOI] [PubMed] [Google Scholar]
- [69].Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome.J Neurosci. 2003;23:2634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bailus BJ, Pyles B, McAlister MM, O’Geen H, Lockwood SH, Adams AN, et al. Protein Delivery of an Artificial Transcription Factor Restores Widespread Ube3a Expression in an Angelman Syndrome Mouse Brain. Mol Ther. 2016;24:548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Daily JL, Nash K, Jinwal U, Golde T, Rogers J, Peters MM, et al. Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome.PLoS One. 2011;6:e27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Huang HS, Allen JA, Mabb AM, King IF, Miriyala J, Taylor-Blake B, et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons.Nature. 2011;481:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Meng L, Person RE, Huang W, Zhu PJ, Costa-Mattioli M, Beaudet AL. Truncation of Ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model.PLoS Genet. 2013;9:e1004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Meng L, Ward AJ, Chun S, Bennett CF, Beaudet AL, Rigo F. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA.Nature. 2015;518:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Syding LA, Nickl P, Kasparek P, Sedlacek R. CRISPR/Cas9 Epigenome Editing Potential for Rare Imprinting Diseases: A Review.Cells. 2020;9:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bailus BJ, Segal DJ. The prospect of molecular therapy for Angelman syndrome and other monogenic neurologic disorders.BMC Neurosci. 2014;15:76,2202–15-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Silva-Santos S, van Woerden GM, Bruinsma CF, Mientjes E, Jolfaei MA, Distel B, et al. Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model.J Clin Invest. 2015;125:2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].STARS: Results from a safety and efficacy study of OV101 (gaboxadol) in adults and adolescents with Angelman syndrome. American Academy of Neurology 2019 Annual Meeting, Philadelphia, PA; 2019. [Google Scholar]
- [79].Ciarlone SL, Wang X, Rogawski MA, Weeber EJ. Effects of the synthetic neurosteroid ganaxolone on seizure activity and behavioral deficits in an Angelman syndrome mouse model.Neuropharmacology. 2017;116:142–50. [DOI] [PubMed] [Google Scholar]
- [80].Peters SU, Bird LM, Kimonis V, Glaze DG, Shinawi LM, Bichell TJ, et al. Double-blind therapeutic trial in Angelman syndrome using betaine and folic acid.Am J Med Genet A. 2010;152A:1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ciarlone SL, Grieco JC, D’Agostino DP, Weeber EJ. Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model.Neurobiol Dis. 2016;96:38–46. [DOI] [PubMed] [Google Scholar]
- [82].Chung L, Bey AL, Towers AJ, Cao X, Kim IH, Jiang YH. Lovastatin suppresses hyperexcitability and seizure in Angelman syndrome model.Neurobiol Dis. 2018;110:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Guzzetti S, Calzari L, Buccarello L, Cesari V, Toschi I, Cattaldo S, et al. Taurine Administration Recovers Motor and Learning Deficits in an Angelman Syndrome Mouse Model.Int J Mol Sci. 2018;19:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jakaria M, Azam S, Haque ME, Jo SH, Uddin MS, Kim IS, et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms.Redox Biol. 2019;24:101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Grieco JC, Ciarlone SL, Gieron-Korthals M, Schoenberg MR, Smith AG, Philpot RM, et al. An open-label pilot trial of minocycline in children as a treatment for Angelman syndrome.BMC Neurol. 2014;14:232,014–0232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ruiz-Antoran B, Sancho-López A, Cazorla-Calleja R, López-Pájaro LF, Leiva Á, Iglesias-Escalera G, et al. A randomized placebo controlled clinical trial to evaluate the efficacy and safety of minocycline in patients with Angelman syndrome (A-MANECE study). Orphanet J Rare Dis. 2018;13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Napier KR, Tones M, Simons C, Heussler H, Hunter AA, Cross M, et al. A web-based, patient driven registry for Angelman syndrome: the global Angelman syndrome registry. Orphanet J Rare Dis. 2017;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tones M, Cross M, Simons C, Napier KR, Hunter A, Bellgard MI, et al. Research protocol: The initiation, design and establishment of the Global Angelman Syndrome Registry.J Intellect Disabil Res. 2018;62:431–43. [DOI] [PubMed] [Google Scholar]