Abstract
Regulatory science underpins the objective evaluation of medicinal products. It is therefore imperative that regulatory science and expertise remain at the cutting edge so that innovations of ever‐increasing complexity are translated safely and swiftly into effective, high‐quality therapies. We undertook a comprehensive examination of the evolution of science and technology impacting on medicinal product evaluation over the next 5–10 years and this horizon‐scanning activity was complemented by extensive stakeholder interviews, resulting in a number of significant recommendations. Highlighted in particular was the need for expertise and regulatory science research to fill knowledge gaps in both more fundamental, longer‐term research, with respect to technological and product‐specific challenges. A model is proposed to realise these objectives in Europe, comprising a synergistic relationship between the European Medicines Agency, the European Medicines Regulatory Network and academic research centres to establish a novel regulatory science and innovation platform.
What is already known about this subject
The European Medicines Agency and medicinal product regulators around the world, are confronted continuously with advances in science and technology. However, the complexity of innovation is increasing rapidly, requiring regulatory science to evolve in tandem and to develop an effective mechanism to do so in a timely manner.
What this study adds
This study explores regulatory science needs over the next 5–10 years and proposes a mechanism to enable regulatory science to keep pace with innovation.
1. INTRODUCTION
Translating fundamental science into patient‐accessible therapies requires application of diverse scientific disciplines. Regulatory science underpins the objective evaluation of the safety, efficacy and quality of medicinal products and crucially informs the regulatory decision‐making process.
Specifically, therefore, regulatory science must provide medicines' regulators with the knowledge to apply innovative research and novel methodological tools to the objective determination of the benefits and risks associated with the use of a new medicinal product. 1 It is fair to say, however, that rapid progress in the biomedical and related sciences—for example, in areas such as cell‐based therapies, drug‐device combinations, predictive toxicology and artificial intelligence—mean that the most challenging regulatory questions 2 , 3 , 4 are originating from the fastest moving and most competitive scientific disciplines. 5 As a result, it is absolutely imperative that regulatory science remains at the cutting edge so that innovations of ever‐increasing complexity are translated safely into efficacious and affordable therapies in a timely fashion, promoting public health.
The European Medicines Agency (EMA) engages continuously with advances in regulatory science and, in 2017, undertook a comprehensive baseline review examining the evolution of science and technology that will impact its core business of medicinal product evaluation over the next 5–10 years. This horizon‐scanning activity was complemented by an extensive stakeholder outreach exercise across individuals and organisations involved in the entire medicine development lifecycle (and included, inter alia, the pharmaceutical industry, health technology assessors and payers, regulatory science experts, academia, scientific organisations and societies, European Union research infrastructure networks, healthcare professionals, and patient representative groups). The cumulative result of this concerted effort was a document, 6 EMA Regulatory Science to 2025—Strategic Reflection, released for public consultation at the end of 2018 and recently summarised in the literature. 7 A key component of this reflection is a proposed model to strengthen regulatory science and innovation in Europe, the elaboration of which is now described. 6 (pp32–35;pp51–54)
2. METHODS
2.1. Horizon scan (baseline review)
The initial (>60) areas of review (see Supplementary Information, Table S1) across health, science, technology and regulatory science were selected by the EMA's internal scientific leadership, the Scientific Coordination Group. Subsequently, a multidisciplinary research group conducted an initial horizon scanning exercise. This included mining, inter alia, of internal databases and the relevant scientific literature. In each area reviewed, the state‐of‐play and the projected opportunities and challenges over the coming 5–10 years were identified. These results were authenticated within the research group, and then peer‐reviewed by in‐house experts and the Scientific Coordination Group.
2.2. Stakeholder interviews
Interviews were then carried out with external experts and key opinion leaders from the EMA's principal stakeholder groups to validate the internal conclusions. Interviewees were nominated by the European Medicines Regulatory Network (EMRN) and drawn from the Agency's expert database; non‐response error was mitigated through follow‐up reminders. The interviews (n = 70) were either semi‐structured (55) or open (15). The stakeholders were provided with a series of key questions (developed by the research group) and an introduction to the baseline review prior to the interviews. The questions were aligned with the aims of the regulatory science reflection and were trialled with colleagues, and re‐ordered and optimised in terms of timing. The resultant draft script was then tested on an initial panel of interviewees for feedback. This feedback was incorporated into a final master script 7 targeted towards semi‐structured interviews with each stakeholder group. For the open interviews, the script was used after the interviewees had provided their unprompted, initial topics for discussion.
2.3. Data acquisition and analysis
The semi‐structured interviews lasted around 1 hour, the open interviews up to 2 hours. A written record of the interviews was made by 2 or more of the research team and then cross‐checked for accuracy and consistency. Analysis of the information obtained involved open and axial coding 8 , 9 whereby the research team attributed codes to meaningful sections of text (words, statements and sentences). These codes were compared and a subset agreed before undertaking additional rounds of axial coding. The findings were eventually reported using Consolidated Criteria for Reporting Qualitative Research. 10 Finally, the codes were grouped into themes, which were compared to and merged with the results of the horizon scan and baseline review. From this exercise, a set of overarching strategic goals for regulatory science emerged along with several core recommendations and associated underlying actions necessary to achieve these aims.
3. RESULTS
The baseline review, horizon scan and stakeholder outreach resulted in over 600 comments and recommendations. Many of these identified the need for expertise and regulatory science research to fill knowledge gaps in 2 broad areas as discussed in detail in the published EMA document, EMA Regulatory Science to 2025—Strategic reflection 6 and summarised elsewhere 7 : (i) those requiring more fundamental, longer‐term research; and (ii) where technology or product‐specific challenges were evident. Relatedly, the limited funds available for regulatory science research, and the clear need for more resource in this area, represented very strong signals.
Regarding expertise, a deficit in the area of regulatory science know‐how was identified, particularly in rapidly evolving domains of research and innovation such as drug–device combinations, predictive toxicology and artificial intelligence. 6 (pp32–35;pp51–54)combinations, predictive toxicology and artificial A more proportionate approach to access international expertise was a recurring suggestion in this regard. Enhanced training in the relevant science for stakeholders and regulators alike was also highlighted.
4. DISCUSSION
The primary role of medicines regulatory agencies may be summarised as protecting and promoting public health and, increasingly, of catalysing and enabling science to be translated into patient‐centred healthcare. 1 To meet these objectives, the regulatory agency must understand the fundamentals of the relevant science, and their application in the medicinal product review and approval process, and be critically informed of key areas of scientific innovation that have the potential to impact on its core business. 5 , 6
4.1. A model to underpin regulatory science and innovation in Europe
A mechanism with which these goals can be achieved in Europe is a synergistic relationship between the EMA, the EMRN and distributed academic research centres to establish a novel science and innovation platform—provisionally termed the Regulatory Science and Innovation Programme for Europe (ReScIPE)—that undertakes both long‐term, fundamental research in strategic areas of regulatory science (Figure 1, upper panel), and shorter‐term investigations to address emerging regulatory science questions (Figure 1, lower panel).
FIGURE 1.
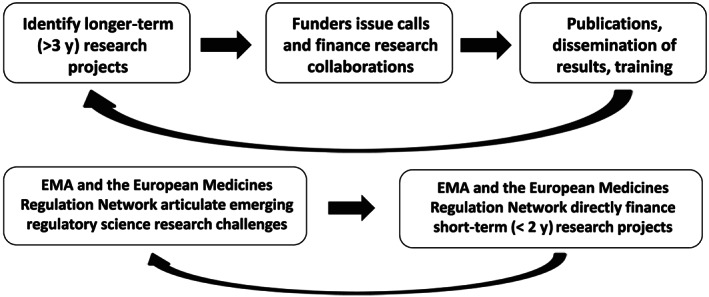
Upper panel: an iterative partnership between regulators, European public funding agencies and academic scientists to strategically focus basic research in regulatory science. The potential funding agencies include those at the European level, such as the Directorate General for Research and Innovation (DG RTD) and Innovative Medicines Initiative (IMI), and national funders. Lower panel: research collaboration between network scientists and academia to tackle rapidly evolving regulatory science questions and to translate innovation efficiently into regulatory tools and processes
4.2. ReScIPE: goals and deliverables
It is anticipated that ReScIPE will identify research priorities that promote the field of regulatory science—including innovative research, development of regulatory tools, education and scientific exchange—together with not‐for‐profit and commercial entities striving to produce safe, effective, affordable and high‐quality medical products. Self‐evidently, collaboration involving ReScIPE and the European pharmaceutical, biotechnology, and high‐tech industries is particularly important to the long‐term aims articulated above. With the governance of these collaborations being carefully decided by funders at the call stage. It is also envisaged that partnerships between EMA, the EMRN and academia will also develop regulatory training modules and undertake horizon scanning in emerging areas of innovation, and that ReScIPE will drive a data‐sharing culture to foster open science that is mutually beneficial for all stakeholders.
4.3. Precedence for success in Europe
Given the strength of the pharmaceutical and biotechnology industries in Europe, the established importance of leading scientific professional societies (such as the European Federation for Pharmaceutical Scientists), the considerable regulatory expertise at EMA and across the EMRN, and the world‐leading quality of biomedical research related to medical product innovation and development in European universities and research centres, the present situation also affords a real opportunity to accomplish a paradigm‐shift in regulatory science and innovation through the establishment of ReScIPE. 6 (pp32–35;pp51–54) This concept must build upon precedents at the national level, including the Dutch Medicines Evaluation Board (MEB) Regulatory Science Program, which has led to the creation of a broad network of partnerships between academic and other external parties. 11 In this way, MEB has committed a budget to catalyse and facilitate both short‐term projects and longer‐term PhD theses to enhance its ability to deliver high quality benefit/risk assessment. Three specific areas of the medicinal product lifecycle have been targeted: development and innovation, regulation and decision‐making, and consumer use and safety. At the same time, MEB is actively participating in regulatory education and learning, for example, via internships to bachelor‐ and masters‐level students. Other similar research models include Germany's Federal Institute for Drugs and Medical Devices, 12 which conducts research in collaboration with national, EU and international research centres and academia, and the Paul‐Ehrlich‐Institut, 13 which interacts with leading research institutes, academia and international organisations to set new standards in the field of vaccines/biomedicines. Another example is the European Center of Pharmaceutical Medicine, 14 based at the University of Basel, that provides training which covers the entire medicinal product development process from molecule identification to commercialisation, including an understanding of essential aspects of regulatory science.
Most recently, a new EU‐funded project entitled Strengthening Training of Academia in Regulatory Science, 15 was initiated. The consortium involved includes the EMA and 20 regulatory bodies. The 3‐year project aims to analyse and improve the training of academia in regulatory science and to enhance regulatory protocol assistance in academic‐driven health research. These measures are designed to facilitate translational clinical research in academia, and to accelerate the availability of innovative, cutting‐edge therapies to patients across Europe.
4.4. Centers of Excellence in Regulatory Science and Innovation: an American model
Furthermore, evidence, from the USA in particular, suggests that this model of synergistic partnership between a regulatory agency, academic researchers and key stakeholders, such as established pharmaceutical companies and small and/or medium‐sized enterprises, is a fruitful approach to ensure that research ideas are effectively translated into new and effective medical products and that technological advances resulting in novel tools are applied to catalysing and facilitating the regulatory review and approval process, thereby accelerating patient access to innovative therapies. 16 The US Food and Drug Administration currently funds 5 Centers of Excellence in Regulatory Science and Innovation, each with a particular focus associated with the Agency's priority areas. 17 The UCSF‐Stanford Center, for example, is addressing the over‐arching strategic aim to develop new models and methods for moving drugs and other medical products, such as devices and cell‐based therapies, from the laboratory to clinical trials. 18 In parallel, the Center provides training and educational programs (including internships and laboratory rotations) for PhD students, postdoctoral fellows, faculty and scientists in the industry and at the Food and Drug Administration.
5. CONCLUSIONS
Scientific challenges in regulatory science and innovation span the entire spectrum of the medicinal product lifecycle—for both human and veterinary drug product development 19 —from, for example, the conception and development of new cell‐based treatments, through new thinking in predictive toxicology, and the rapidly increasing variety of imaginative drug‐device combination products, to new ideas concerning the personalisation and precision of medical therapy (including the manufacturing challenges). 5 , 6 As such, there is a strong rationale for ReScIPE to use a distributed model, and to benefit from the collaboration of expertise across different academic centres that each concentrate on specific target areas of investigation.
The scale of investment required is logically a function of the number and complexity of the transformational research questions to be addressed, the requirements for associated infrastructure, and the perspective taken on the specific role of ReScIPE in training early‐career scientists in this important field. In developing existing interactions between the EMA, the EMRN and academia (as well as integrating with ongoing key European activities as mentioned above) to ensure that regulatory science keeps up‐to‐date, these resources must also be proportional to the public health aim of ensuring that regulation of medicines not only guarantees safe and effective therapies that meet the highest standards of quality, but that it also facilitates patient access to these innovative and important medicines. 6 (p.1) While regulators are wrestling with this latter challenge to an ever‐increasing extent, further discussion of how to achieve better and more uniform access to novel (and almost always expensive) therapies, and to a high standard of healthcare in general, is beyond the scope of this article.
COMPETING INTEREST
There are no competing interests to declare. The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the agencies or organisations with which the authors are affiliated.
CONTRIBUTORS
All the authors have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. All the authors have been involved in drafting the manuscript or revising it critically for important intellectual content. All the authors have given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. All the authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Supporting info item
TABLE S1 Areas of regulatory science and innovation subjected to horizon‐scanning
Hines PA, Guy RH, Brand A, Humphreys AJ, Papaluca‐Amati M. Regulatory Science and Innovation Programme for Europe (ReScIPE): A proposed model. Br J Clin Pharmacol. 2020;86:2530–2534. 10.1111/bcp.14099
DATA AVAILABILITY STATEMENT
Due to the unforeseen publication of this article, and lack of consent to share interview data, the authors elect to not share data.
REFERENCES
- 1. Rasi G. EU's innovative medical technology and EMA's measures. Paper presented at Summit of Heads of Medicines Regulatory Agencies Symposium and International Coalition of Medicines Regulatory Authorities (ICMRA); October 27, 2017; Kyoto, Japan; https://www.pmda.go.jp/files/000220949.pdf (2018). Accessed August 30, 2019.
- 2. European Medicines Agency . Draft guideline on quality, non‐clinical and clinical requirements for investigational advanced therapy medicinal products in clinical trials. https://www.ema.europa.eu/en/guideline‐quality‐non‐clinical‐clinical‐requirements‐investigational‐advanced‐therapy‐medicinal. Accessed August 30, 2019.
- 3. European Medicines Agency . Questions & answers on implementation of the medical devices and in vitro diagnostic medical devices regulations ((EU) 2017/745 and (EU) 2017/746). https://www.ema.europa.eu/en/human‐regulatory/overview/medical‐devices. Accessed August 30, 2019.
- 4. Heads of medicines agencies/European medicines agency joint big data taskforce – summary report. https://www.ema.europa.eu/en/about‐us/how‐we‐work/big‐data. Accessed August 30, 2019.
- 5. Altman RB, Khuri N, Salit M, Giacomini KM. Unmet needs: research helps regulators do their jobs. Sci Transl Med. 2015;7(315):315ps22. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . EMA regulatory science to 2025 – strategic reflection. https://www.ema.europa.eu/en/about‐us/how‐we‐work/regulatory‐science‐2025. Accessed August 30, 2019.
- 7. Hines PA, Guy RH, Humphreys AJ, Papaluca‐Amati M. The European medicines agency: regulatory science to 2025. Nat Rev Drug Discov. 2019;18(6):403‐404. [DOI] [PubMed] [Google Scholar]
- 8. Allen M. The Sage Encyclopedia of Communication Research Methods. Thousand Oaks, CA: SAGE Publications, Inc; 2017. [Google Scholar]
- 9. Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3‐21. [Google Scholar]
- 10. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. International J Qual Health Care. 2007;19(6):349‐357. [DOI] [PubMed] [Google Scholar]
- 11. Medicines Evaluation Board . The MEB Regulatory Science Program 2016 and beyond. https://english.cbg‐meb.nl/binaries/medicines‐evaluation‐board/documents/publications/2016/01/01/the‐meb‐regulatory‐science‐program‐2016‐and‐beyond/160205+Regulatory+Science+brochure.pdf. Accessed August 30, 2019.
- 12. Federal Institute for Drugs and Medical Devices . Annual Report 2017|18. https://www.bfarm.de/research/research/BfArM/Publikationen/AnnualReport2017‐18.pdf?__blob=publicationFile&v=4. Accessed August 30, 2019.
- 13. Paul‐Ehrlich‐Institut (PEI) . Research Programme of the Paul‐Ehrlich‐Institut 2016–2020. https://www.pei.de/SharedDocs/Downloads/EN/research/research‐programme.pdf?__blob=publicationFile&v=8. Accessed August 30, 2019.
- 14. European Center of Pharmaceutical Medicine . http://web.ecpm.ch/about. Accessed August 30, 2019.
- 15. Hoffmann S. Training of academia in regulatory science: 18 countries join forces in new consortium called STARS. https://idw‐online.de/en/news709726. Accessed August 30, 2019.
- 16. Bahinski A, Friere MC, McLellan MR, Psaty BM, Roden DM, Steele SJ. Scientific engagement at FDA: a report to the FDA science board from the scientific engagement subcommittee. https://pdxscholar.library.pdx.edu/cgi/viewcontent.cgi?article=1221&context=bio_fac. Accessed August 30, 2019.
- 17. U.S. Food & Drug Administration . Centers of excellence in regulatory science and innovation (CERSIs). https://www.fda.gov/scienceresearch/specialtopics/regulatoryscience/ucm301667.htm. Accessed August 30, 2019.
- 18. UCSF‐Stanford Center of Excellence in Regulatory Science and Innovation (CERSI). https://pharm.ucsf.edu/cersi. Accessed August 30, 2019. [DOI] [PubMed]
- 19. Mochel JP, Tyden E, Hellmann K, et al. Network on veterinary medicines initiated by the European Federation for Pharmaceutical Sciences. J Vet Pharmacol Ther. 2018;41(3):378‐383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
TABLE S1 Areas of regulatory science and innovation subjected to horizon‐scanning
Data Availability Statement
Due to the unforeseen publication of this article, and lack of consent to share interview data, the authors elect to not share data.


