Abstract
Aim
To assess associations between statin intensity and adherence, persistence and discontinuation of statin therapy in Scotland.
Method
Retrospective cohort study, using linked electronic health records covering a period from January 2009 to December 2016. The study cohort included adult patients (≥18 years) newly initiating statins within Greater Glasgow and Clyde, Scotland. Study outcomes comprised adherence, discontinuation and persistence to treatment, stratified by three exposure groups (high, moderate and low intensity). Discontinuation and persistence were calculated using the refill‐gap and anniversary methods, respectively. Proportion of days covered (PDC) was used as a proxy for adherence. Kaplan‐Meier survival curves and Cox proportional hazard models were used to evaluate discontinuation, and associations between adherence/persistence and statin intensity were assessed using logistic regression.
Results
A total of 73 716 patients with a mean age of 61.4 ± 12.6 years were included; the majority (88.3%) received moderate intensity statins. Discontinuation rates differed between intensity levels, with high‐intensity patients less likely to discontinue treatment compared to those on moderate intensity (prior cardiovascular disease [CVD]: HR 0.43 [95% CI 0.34‐0.55]; no prior CVD: 0.80 [0.74‐0.86]). Persistence declined over time, and high‐intensity patients had the highest persistence rates. Overall, 52.6% of patients were adherent to treatment (PDC ≥ 80%), but adherence was considerably higher among high‐intensity patients (63.7%).
Conclusion
High‐intensity statins were associated with better persistence and adherence to treatment, but overall long‐term persistence and adherence remain a challenge, particularly among patients without prior CVD. This needs addressing.
Keywords: cardiovascular disease, drug utilisation, high‐intensity therapy, medication adherence, Scotland, statins
1. What is already known about this subject
Long‐term adherence to statin treatment is low and discontinuation rates are high.
Guidelines for cardiovascular disease (CVD) prevention increasingly recommend high‐intensity statin therapy.
As adverse events rates are likely higher with high‐intensity treatment, this could have a negative effect on adherence rates, but evidence so far is scarce.
What this study adds
Adherence to statin treatment was higher among patients treated with high‐intensity statins and discontinuation rates were lower compared to moderate intensity.
Patients with prior CVD (secondary prevention) had better adherence that those without (primary prevention).
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death worldwide. 1 In Scotland, whilst the mortality from coronary heart disease has been decreasing, treating and preventing CDV remains an important priority in health care, particularly as its population is projected to age considerably over the coming years, with an estimated 25% of the population aged 65 years or older by 2041. 2 , 3 In addition, there is a high prevalence of risk factors associated with heart disease in Scotland, including smoking, poor diet and physical inactivity. Accordingly, in 2015, 15% of adults aged 16 years or above had a cardiovascular condition, which represents an estimated 670 000 people living with CVD in Scotland. Both incidence and prevalence of CVD are higher amongst men, the elderly and in deprived areas. 2 , 4
CVD prevention is seen as a cost‐effective strategy in many scenarios. 4 , 5 Evidence from clinical trials and epidemiological studies has shown a strong relationship between high low‐density lipoprotein cholesterol (LCL‐C) levels and the incidence of CVD; lipid‐lowering therapy is associated with significant reductions in CVD morbidity and mortality. 6 , 7 , 8 , 9 Consequently, the use of statins, competitively inhibiting 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG CoA) reductase, is one of the cornerstones of CVD prevention. 4 , 10 , 11
Recent evidence has supported the higher efficacy of high‐intensity statin therapy in comparison to low or moderate intensity, 12 therefore the Scottish guidelines now recommend high‐intensity statin therapy, namely atorvastatin 80 mg, as the most clinically effective and cost‐effective option for secondary prevention in patients with established coronary vascular disease. 4 Nevertheless, there are concerns around the safety of high‐intensity statins as high doses may increase the incidence of statin‐related side effects such as myopathy, hepatotoxicity or diabetes, 13 , 14 , 15 which in turn might impede patients' adherence and persistence to treatment, 16 and subsequently potentially limit the clinical benefit of statins in reducing CVD. 17 , 18 , 19 , 20 This is particularly important given that long‐term adherence to statin therapy has already been shown to be far from optimal, 21 , 22 , 23 , 24 , 25 and a significant proportion of patients stop taking their statins within 2 years of initiation. 22 , 23 A wide range of factors, related to both the patient and the healthcare provider, has been shown to impact adherence to long‐term treatment, for instance insufficient understanding of the disease, concerns about side effects or inconsistent information received from healthcare professionals. 26 There is, however, limited and controversial evidence to date with regard to the effect of statin intensity levels on treatment adherence/persistence. 27 , 28 , 29 A recent systematic review of adherence and persistence with statins showed that only two of the 84 studies included reported information on the statin intensity regime and its correlation with adherence. 30
Prescribing and dispensing data drawn from administrative databases is particularly useful for conducting large observational studies aimed at analysing drug utilisation rates, including adherence/persistence to treatment. 31 , 32 The National Health Service (NHS) Scotland, which provides universal health care with no co‐payments for medicines for all residents in Scotland, offers a wide array of routinely collected data suitable for drug utilisation research, including prescribing and dispensing data. 33
The aim of this study was to assess the association of statin therapy intensity with adherence, persistence and discontinuation to/of treatment, thereby covering two distinct phases of medication‐taking behaviour according to the Ascertaining Barriers for Compliance (ABC) taxonomy: implementation and discontinuation. 34 This information is vital since there has been an increase in prescribing of high‐intensity statins in Scotland in recent years following advice from the Scottish Government and Health Boards. 4 , 35 . The findings from this study will be discussed with key stakeholder groups in Scotland to instigate additional pertinent interventions if needed to further reduce CVD rates.
2. METHODS
2.1. Study design and data sources
This retrospective longitudinal cohort study spanned from January 2009 to December 2016, using linked electronic health records for patients registered within NHS Greater Glasgow & Clyde (NHS GGC), the largest Health Board in Scotland. NHS GGC covers approximately 1.1 million people, or about 20% of the total Scottish population. 36 It comprises both urban and rural areas, as well as some of the most deprived areas within Scotland. Although the share of elderly people (over the age of 65 years) is slightly lower than the Scottish average, NHS GGC is in general representative of Scotland overall, particularly with regards to the prevalence of cardiovascular diseases. 37 , 38
New statin users were identified using the Prescribing Information System (PIS), which covers all prescriptions dispensed within the community (primary care) setting. PIS data contains information with regards to both prescribing and dispensing (prescribed date and dispensed month/year, prescribed and dispensed quantities, and dispensed item name, strength and formulation), but due to the nature of the database, PIS does not contain information about prescriptions that have been issued but were not dispensed in a community pharmacy. 33 Data from PIS was linked to the Scottish Morbidity Records Outpatient attendance (SMR00) and Inpatient and Day Care (SMR01) datasets as well as to the Scottish death records using Community Health Index (CHI) numbers. The CHI number is a unique patient identifier used throughout Scotland and incorporated into all NHS Scotland datasets. 39
2.2. Study cohort
The study population comprised all adult patients (≥18 years old) who newly initiated statin therapy (atorvastatin, fluvastatin, pravastatin, rosuvastatin or simvastatin) between January 2010 and December 2015 (cohort inclusion period); the date of first statin prescription was the index date. New use was defined as having no prescription records for any statin in the year prior to the index date (with the year 2009 used as a run‐in period). Patients were excluded if they used a statin as part of a fixed‐dose combination. In addition, patients were required to have at least 1 year follow‐up time after the index date to ensure sufficient time to evaluate study outcomes. Individual patients were followed from the index date to either death or removal from a Scottish General Practitioner register for other reasons (such as emigration), with the date of emigration/death used as censor date where applicable, or until the study end date (31 December 2016), whichever occurred first. Patients were not censored when being admitted to a care home or hospital, as they are usually provided with medication through primary care or expected to bring their own chronic disease medication, respectively.
2.3. Drug exposure
Detailed information about dispensed statin prescriptions was extracted from PIS and included the nonproprietary name, drug formulation, strength, prescription date and dispensed quantity. While the dispensed quantity was used to calculate patients' days of supply, the prescribing dates were used to estimate time periods between prescriptions as these dates are more accurate than the available dispensing dates (which default to the last day of each month). Statin users were stratified into three exposure groups based on the first statin received according to the current National Institute for Health and Care Excellence (NICE) classifications (high, moderate and low intensity) as detailed in Table 1. 10 Only prescriptions for the first statin intensity level issued to patients were taken into consideration when calculating all outcome measures, with patients being allowed to switch between statins within the same level of intensity (eg, simvastatin 20 mg to atorvastatin 10 mg). Results therefore relate to the level of statin used at the index date.
TABLE 1.
Statin intensity levels according to the National Institute for Health and Care Excellence
| Statin | High intensity | Moderate intensity | Low intensity |
|---|---|---|---|
| Atorvastatin | 20‐80 mg | 10 mg | … |
| Fluvastatin | … | 80 mg | 20‐40 mg |
| Lovastatin | … | 40 mg | 10‐20 mg |
| Pravastatin | … | 40‐80 mg | 10‐20 mg |
| Rosuvastatin | 20‐40 mg | 5‐10 mg | … |
| Simvastatin | 80 mg | 20‐40 mg | 10 mg |
2.4. Outcome measures
Medication‐taking behaviour consists of three elements: initiation, implementation, and discontinuation. While initiation and discontinuation represent the start and end of drug treatment, respectively, the process of implementation describes the extent to which a patient's medication intake corresponds to what has been prescribed. 34 Because of the nature of the data available, the outcome measures used in this study covered the latter two elements and comprised adherence, discontinuation and persistence to statin therapy, stratified by the three exposure groups.
Discontinuation – to stop treatment – was calculated using the refill‐gap method, defined as a gap of more than 60 days without drug supply following the assumed end of a prescription, that is, the last day with assumed medication availability (grace period). Previous oversupply of medicines (eg, due to early refills) was taken into account. 32 The period of 60 days was chosen based on observed prescribing patterns and the recommendation to have a grace period of at least the average prescription supply, 40 which was 56 days in this study. Patients were classified as having discontinued treatment when switching to a different level of statin intensity. In addition, to assess permanence of statin discontinuation, patients reinitiating treatment after an initial discontinuation event were identified, and levels of statin intensity prior and after this discontinuation were compared.
Persistence – being on treatment – was assessed at prespecified points in time (6, 12, 24 and 36 months after treatment initiation) using the anniversary method by assessing whether patients were in possession of drug supply covering specific dates. This method aims to account for both intermediary treatment interruptions and short‐term changes in intensity of therapy (eg, due to temporary shortages of specific drugs). 32 As patients were not censored when switching between different statins or intensity levels, permanent switch of intensity was classified as nonpersistence.
Proportion of days covered (PDC) was used as a proxy for adherence – to take medication as prescribed. PDC was calculated by dividing the total number of days covered by statin prescriptions by the total number of days in the follow‐up period, capped at 100%. To calculate days covered, dispensed quantities were used with an assumed intake of one tablet/capsule a day. Applying the most commonly used cut‐off point, patients were classified as adherent when their PDC was ≥80% and nonadherent with a PDC < 80%. 31 , 41 In addition, to be able to better distinguish between implementation and persistence, the compliance rate (CR) was calculated: the number of days covered by statin prescriptions (excluding the days' supply obtained at the last dispensation) divided by the number of days in the period up to, but not including, the last recorded prescription. 41
2.5. Covariables
Baseline patient characteristics were captured at index date and included sex, age, first statin prescribed, year of statin initiation, deprivation, comorbidities and overall frailty. Deprivation was measured using the Scottish Index of Multiple Deprivation (SIMD), ranked from 1 (most deprived) to 5 (least deprived). 42 Comorbidities were assessed by calculating a Charlson comorbidity score 43 using International Classification of Diseases, 10th edition (ICD‐10) codes, identified from SMR00 and SMR01 records. The Charlson score is the most widely used indicator of comorbidities and has been adapted by Quan et al for use with ICD‐10 codes. 44 For the general measure of frailty, the number of in‐hospital days – regardless of cause – was counted during the 2‐year period prior to the index date, as recorded in SMR01.
2.6. Statistical analysis
Descriptive statistics were used to assess baseline characteristics. Continuous variables were summarised using means and standard variation, while categorical variables were presented using absolute and relative frequencies.
Kaplan‐Meier survival curves were plotted to calculate the median time to first discontinuation of statin use. Associations between adherence/persistence to treatment and statin intensity were assessed using logistic regression models; Cox proportional hazard models were used to evaluate time to discontinuation. Moderate intensity therapy was chosen as reference based on practical and clinical deliberations. All findings were adjusted for baseline covariables, using complete cases only (ie, excluding patients from the regression analyses when data were missing).
All analyses were performed using the full patient cohort, as well as stratified by whether a statin was prescribed for primary or secondary prevention. Secondary prevention was defined as having a diagnosis of myocardial infarction, angina, stroke or ischaemic heart disease – recorded in SMR00/SMR01 – within the year preceding the index date (ICD‐10 codes I20‐I24, I25.0, I25.1, I25.6, I25.8, I25.9, I51.6, I51.7, I60‐I64, G45.0‐G45.3, G45.8, G45.9, H34.1). Sensitivity analyses were performed for discontinuation and persistence using alternative admissible intervals without drug supply (ie, supply gaps) of 30, 90, 120 and 180 days (grace periods).
All data analyses were conducted using R Studio, version 3.3.0. For the extraction of PIS data, stored on an SQL server, the R package Open Database Connectivity (RODBC) was used. 45 , 46 Data were anonymised, hosted and managed by the NHS GGC Safe Haven, and their use was approved by the appropriate Local Privacy Advisory Committee.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
During the study period, a total of 212 953 patients received at least one prescription for any statin. Of these, 139 237 patients were excluded due to being below the age of 18 years when initiating treatment, having been treated with statins during the run‐in period, having received fixed dose combinations including statins or not having at least one full year of follow‐up after the index date. This resulted in 73 716 patients subsequently being included in the study (Figure 1).
FIGURE 1.
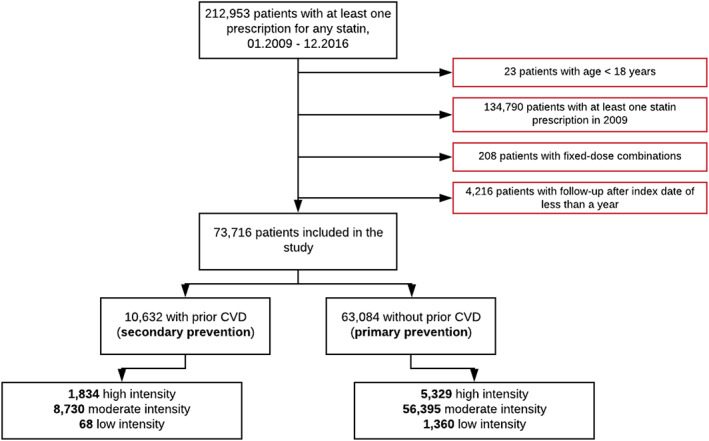
Study cohort identification flow chart
3.1. Baseline characteristics
Of the 73 716 patients included in the study cohort, the majority (88.4%) were initiated on moderate intensity therapy, followed by 9.7% on high intensity and 1.9% on low intensity. Simvastatin was the most commonly prescribed statin, issued to 85.6% of all patients newly initiating treatment and accounting for 95.0% and 79.8% of all moderate and low‐intensity therapy, respectively. In contrast, the vast majority (98.1%) of patients who started high‐intensity treatment received atorvastatin. The mean age of all statin users was 61.4 years (SD 12.6) and 39.5% were elderly (≥ 65 years), with 16.3% being 75 years of age or older. Slightly over half the patients (52.3%) were male. The mean Charlson score was 0.77 (SD 1.32) and 45.2% of patients had hospital admissions in the 2‐year period prior to cohort entry, with an average of 10.4 days (SD 26.1) of in‐hospital stay. There were, however, differences between the three intensity exposure groups. With a mean age of 60.6 years (SD 12.7), patients on high‐intensity therapy were on average younger than those initiating moderate (mean 61.4 years, SD 12.6) or low‐intensity statins (mean 63.9 years, SD 13.3). High‐intensity patients had, nevertheless, more comorbidities, with higher mean Charlson scores, higher proportions of patients with prior CVD (high intensity 25.6%, moderate intensity 13.4%, low intensity 4.8%) and higher proportions of patients with recent admissions to hospital (high intensity 50.3%, moderate intensity 44.7%, low intensity 38.9%). Details of baseline characteristics for the full cohort – overall as well as by statin intensity – can be found in Supporting Information Table S1.
When stratifying by primary/secondary prevention, patients with prior CVD were older than those without (mean age 63.6 years [SD 14.2] vs 61.0 years [SD 12.3]), predominantly male (58.9% vs 51.1% among patients without CVD) and had a higher mean Charlson score (1.72 [SD 1.47] vs 0.55 [SD 1.17]). In addition, patients with prior CVD had all recently been hospitalised, with an average of 16.5 (SD 30.9) in‐hospital days, in contrast to only 39.5% of patients subject to primary prevention, with an average of 7.6 (SD 23.0) in‐hospital days. Differences between statin intensity levels were also observed in both stratified cohorts (for details, see Table 2).
TABLE 2.
Baseline characteristics of new statin users by intensity level and prior CVD status (type of prevention)
| Prior CVD (secondary prevention) n = 10 632 (14.4%) | No prior CVD (primary prevention) n = 63 084 (85.6%) | |||||
|---|---|---|---|---|---|---|
| Intensity, n (%) | Intensity, n (%) | |||||
| High 1834 (17.2) | Moderate 8730 (82.1) | Low 68 (0.6) | High 5329 (8.4) | Moderate 56 395 (89.4) | Low 1360 (2.2) | |
| Sex, n (%) | ||||||
| Male | 1196 (65.2) | 5041 (57.7) | 30 (44.1) | 2931 (55.0) | 28 728 (50.9) | 608 (44.7) |
| Female | 638 (34.8) | 3689 (42.3) | 38 (55.9) | 2398 (45.0) | 27 667 (49.1) | 725 (53.3) |
| Age categories (years), n (%) | ||||||
| <55 | 685 (37.4) | 2484 (28.5) | 9 (13.2) | 1619 (30.4) | 17 737 (31.5) | 326 (24.0) |
| 55‐64 | 531 (29.0) | 2068 (23.7) | 16 (23.5) | 1587 (29.8) | 17 112 (30.3) | 374 (27.5) |
| 65‐74 | 333 (18.2) | 1740 (19.9) | 18 (26.5) | 1330 (25.0) | 13 340 (23.7) | 368 (27.1) |
| 75+ | 285 (15.5) | 2438 (27.9) | 25 (36.8) | 793 (14.9) | 8206 (14.6) | 292 (24.5) |
| Age (years), mean (SD) | 59.7 (13.1) | 64.4 (14.3) | 68.3 (13.6) | 60.9 (12.6) | 60.9 (12.3) | 63.7 (13.2) |
| Deprivation score (quintile), n (%) a | ||||||
| 1 (most deprived) | 754 (41.1) | 3534 (40.5) | 18 (26.5) | 2034 (38.2) | 22 616 (40.1) | 406 (29.9) |
| 2 | 300 (16.4) | 1548 (17.7) | 13 (19.1) | 928 (17.4) | 10 112 (17.9) | 221 (16.3) |
| 3 | 233 (12.7) | 1192 (13.7) | 13 (19.1) | 740 (13.9) | 7229 (12.8) | 187 (13.8) |
| 4 | 210 (11.5) | 976 (11.2) | 11 (16.2) | 680 (12.8) | 6671 (11.8) | 227 (16.7) |
| 5 (least deprived) | 307 (16.7) | 1366 (15.6) | 11 (16.2) | 869 (16.3) | 9025 (16.0) | 298 (21.9) |
| Calendar year of the first statin prescription, n (%) | ||||||
| 2010 | 384 (20.9) | 1566 (17.9) | 17 (25.0) | 748 (14.0) | 11 513 (20.4) | 315 (23.2) |
| 2011 | 101 (5.5) | 1753 (20.1) | 16 (23.5) | 396 (7.4) | 9594 (17.0) | 251 (18.5) |
| 2012 | 140 (7.6) | 1646 (18.9) | 13 (19.1) | 587 (11.0) | 10 175 (18.0) | 241 (17.7) |
| 2013 | 251 (13.7) | 1434 (16.4) | 12 (17.6) | 954 (17.9) | 9515 (16.9) | 214 (15.7) |
| 2014 | 341 (18.6) | 1332 (15.3) | 5 (7.4) | 1152 (21.6) | 8370 (14.8) | 210 (15.4) |
| 2015 | 617 (33.6) | 999 (11.4) | 5 (7.4) | 1492 (28.0) | 7228 (12.8) | 129 (9.5) |
| Charlson comorbidity score, n (%) b , c | ||||||
| 0 | 90 (4.9) | 677 (7.8) | 9 (13.2) | 2327 (43.7) | 28 482 (50.5) | 616 (45.3) |
| ≥ 1 | 1744 (95.1) | 8053 (92.2) | 59 (86.8) | 1143 (21.4) | 11 571 (20.5) | 353 (26.0) |
| Charlson comorbidity score, mean (SD)2, 3 | 1.61 (1.31) | 1.74 (1.49) | 2.44 (2.15) | 0.63 (1.25) | 0.53 (1.15) | 0.78 (1.44) |
| Hospitalization (yes), n (%) d | 1834 (100) | 8730 (100) | 68 (100) | 1768 (33.2) | 20 402 (36.2) | 487 (35.8) |
| General patient fragility, mean (SD)4 | 12.3 (24.1) | 17.3 (32.1) | 24.6 (34.8) | 6.8 (17.8) | 7.6 (23.5) | 8.5 (21.5) |
Abbreviations: CVD, cardiovascular disease.
1.3% (n = 987) of missing data: secondary prevention n = 146 (1.4%), primary prevention n = 841 (1.3%).
Based on all ICD codes recorded in hospital records during the 2‐year period preceding the index date.
25.2% (n = 18,592) of missing data: secondary prevention n = 0 (0%), primary prevention n = 18,592 (29.5%).
In‐hospital days, assessed during the 2‐year period preceding the index date.
3.2. Discontinuation of treatment
During the study period, 46 730 patients (62.9%) discontinued statin treatment and the median time to first discontinuation was 522 days (95% confidence interval [CI] 510‐535). Discontinuation rates as well as time to discontinuation differed between intensity levels, however, ranging from 50.9% with a median time to discontinuation of 911 days (95% CI 843‐1007) among patients treated with high‐intensity statins to 75.5% (median time to discontinuation 207 days (95% CI 168‐244) among low‐intensity patients (see also Supporting Information Figures S1 and S2). Discontinuation also differed considerably between patients with and without prior CVD, with overall rates of 48.0% and 65.4%, respectively. Similar to the overall cohort, discontinuation rates in both these subgroups were lowest among patients treated with high‐intensity statins and highest among low‐intensity statin patients. Time to discontinuation varied widely between the different patient groups, but was consistently longer for secondary prevention (see Table 3 and Figure 2 for details).
TABLE 3.
Crude discontinuation rates, time to discontinuation and adjusted HRs by intensity level and prior CVD status (type of prevention)
| Crude discontinuation rate (%) a | Median time to first discontinuation (95% CI) (days) b | Hazard Ratio (95% CI), adjusted c | |
|---|---|---|---|
| All patients | 62.9 | 522 (510‐535) | |
| Low intensity | 75.5 | 207 (168‐244) | 1.56 (1.45‐1.69) |
| Moderate intensity | 63.9 | 504 (493‐517) | 1 |
| High intensity | 50.9 | 911 (843‐1007) | 0.74 (0.69‐0.79) |
| Prior CVD (secondary prevention) | 48.0 | 1232 (1168‐1296) | |
| Low intensity | 58.8 | 209 (87‐NR) | 1.85 (1.30‐2.63) |
| Moderate intensity | 50.4 | 1,126 (1064‐1201) | 1 |
| High intensity | 36.3 | 2,028 (1676‐NR) | 0.43 (0.34‐0.55) |
| No prior CVD (primary prevention) | 65.4 | 440 (429‐450) | |
| Low intensity | 76.3 | 203 (168‐244) | 1.55 (1.43‐1.67) |
| Moderate intensity | 66.0 | 435 (424‐448) | 1 |
| High intensity | 55.9 | 615 (561‐705) | 0.80 (0.74‐0.86) |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; NR, not reached.
Refill‐gap method, admissible gap 60 days.
Kaplan‐Meier survival, unadjusted.
Reference category is moderate intensity, adjusted for baseline variables: year of treatment initiation, drug prescribed, sex, age category, deprivation (SIMD), Charlson score (0 vs ≥1), prior CVD yes/no, hospitalisation yes/no.
FIGURE 2.
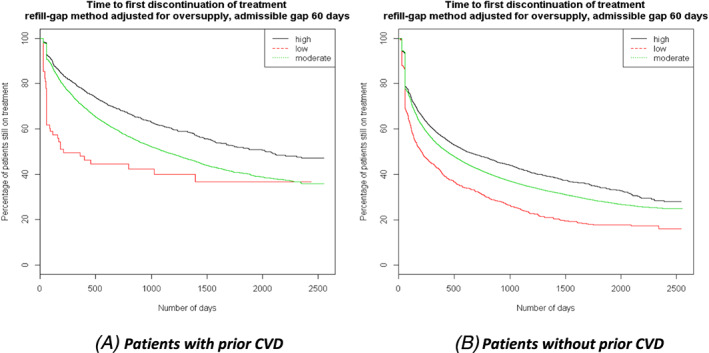
Kaplan‐Meier curves of time to first treatment discontinuation by CVD status (type of prevention)
After adjusting for baseline patient characteristics (year of treatment initiation, drug prescribed, sex, age category, deprivation (SIMD), Charlson score [0 vs ≥1], prior CVD yes/no and hospitalisation yes/no), patients on high‐intensity statin therapy were less likely to prematurely discontinue treatment than those on moderate intensity therapy (Hazard Ratio [HR] 0.74, 95% CI 0.69‐0.79). Although Hazard Ratios differed considerably between patients with and without prior CVD, stratified findings were in general similar to those observed in the complete cohort. Compared to moderate intensity, high‐intensity statin patients were less likely to discontinue treatment regardless of prior CVD status (with prior CVD: HR 0.43, 95% CI 0.34‐0.55; without prior CVD: HR 0.80, 95% CI 0.74‐0.86) (Table 3).
3.3. Reinitiation of treatment
Overall, 72.3% of patients reinitiated statin therapy after a first discontinuation event, the majority of which (72.7%) with the same statin intensity that was used prior to treatment interruption, and 4.7% of patients reduced and 23.2% increased statin intensity. The proportion of patients changing intensity levels differed, however, between patients with and without prior CVD (Figure 3).
FIGURE 3.
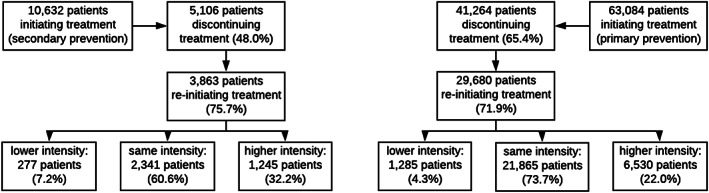
Discontinuation and reinitiation of treatment by CVD status (type of prevention)
3.4. Persistence to treatment
In line with discontinuation rates, persistence to treatment also varied significantly between statin intensity levels, with considerable differences between patients with and without prior CVD. Persistence declined over time; nevertheless, patients subject to high‐intensity therapy had the highest persistence rates at all analysed time points. Details are presented in Table 4.
TABLE 4.
Crude persistence rates at specific points in time since treatment initiation by intensity level and prior CVD status (type of prevention)
| Persistence rate (%) a , b | 6 months | 12 months | 24 months | 36 months |
|---|---|---|---|---|
| All patients | 76.8 | 69.9 | 63.7 | 59.2 |
| Low intensity | 62.7 | 53.1 | 43.4 | 36.2 |
| Moderate intensity | 76.7 | 69.8 | 63.8 | 59.4 |
| High intensity | 80.1 | 74.7 | 68.0 | 63.3 |
| Prior CVD (secondary prevention) | 88.6 | 81.6 | 74.0 | 68.2 |
| Low intensity | 59.1 | 50.8 | 42.6 | 39.6 |
| Moderate intensity | 88.7 | 81.0 | 73.5 | 67.6 |
| High intensity | 89.7 | 85.5 | 78.0 | 73.5 |
| No prior CVD (primary prevention) | 74.8 | 68.0 | 62.1 | 57.8 |
| Low intensity | 62.9 | 53.2 | 43.4 | 36.1 |
| Moderate intensity | 74.9 | 68.1 | 62.3 | 58.2 |
| High intensity | 76.8 | 71.0 | 64.8 | 60.0 |
Abbreviations: CVD, cardiovascular disease.
Anniversary method, admissible gap 60 days.
Denominators are patients with sufficient follow‐up time within a specific category.
In general, after adjusting for baseline characteristics, patients initiating high‐intensity statins were more likely to still be on treatment at any point in time compared to patients initiating moderate intensity therapy (6 months: Odds Ratio [OR] 1.48, 95% CI 1.30‐1.68; 12 months: OR 1.54, 95% CI 1.37‐1.74; 24 months: OR 1.47, 95% CI 1.29‐1.68). Among patients with prior CVD, the ORs for being persistent with high‐intensity statins as compared to moderate intensity were 3.41 (95% CI 2.24‐5.12) after 6 months, 2.94 (95% CI 1.97‐4.34) after 12 months and 2.82 (95% CI 1.82‐4.34) after 24 months. Although differing in magnitude, the findings paint a similar picture for patients without prior CVD. Patients were more likely to be persistent with high‐intensity statins when compared to moderate intensity (6 months: OR 1.38, 95% CI 1.20‐1.57; 12 months: OR 1.41, 95% CI 1.24‐1.60; 24 months: OR 1.36, 95% CI 1.18‐1.56) (see Table 5 for details).
TABLE 5.
Adjusted ORs for persistence at specific points in time since treatment initiation by intensity level and prior CVD status (type of prevention)
| OR (95% CI), adjusted a , b | 6 months | 12 months | 24 months |
|---|---|---|---|
| All patients | |||
| Low intensity | 0.45 (0.39‐0.52) | 0.45 (0.40‐0.52) | 0.40 (0.34‐0.46) |
| Moderate intensity | 1 | 1 | 1 |
| High intensity | 1.48 (1.30‐1.68) | 1.54 (1.37‐1.74) | 1.47 (1.29‐1.68) |
| Prior CVD (secondary prevention) | |||
| Low intensity | 0.17 (0.10‐0.32) | 0.22 (0.12‐0.39) | 0.23 (0.12‐0.43) |
| Moderate intensity | 1 | 1 | 1 |
| High intensity | 3.41 (2.24‐5.12) | 2.94 (1.97‐4.34) | 2.82 (1.82‐4.34) |
| No prior CVD (primary prevention) | |||
| Low intensity | 0.47 (0.41‐0.54) | 0.47 (0.41‐0.54) | 0.41 (0.35‐0.48) |
| Moderate intensity | 1 | 1 | 1 |
| High intensity | 1.38 (1.20‐1.57) | 1.41 (1.24‐1.60) | 1.36 (1.18‐1.56) |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; OR, Odds Ratio.
Anniversary method, admissible gap 60 days.
Reference category is moderate intensity, adjusted for baseline variables: year of treatment initiation, drug prescribed, sex, age category, deprivation (SIMD), Charlson score (0 vs ≥1), prior CVD yes/no, hospitalisation yes/no.
3.5. Adherence to treatment
Only patients who had at least two dispensing records for the same statin intensity were included in the adherence analysis, totalling 66 248 patients (89.9% of all new statin users during the study period). Based on PDC, the overall adherence rate for the full cohort was 52.6%, but adherence to treatment was significantly higher among high‐intensity statin users, with 63.7% classified as adherent in contrast to 51.6% and 40.5% among moderate‐ and low‐intensity users, respectively. There were also differences in adherence between patients with and without prior CVD, with higher adherence rates among patients subject to secondary prevention at all levels of statin intensity (Table 6).
TABLE 6.
Crude adherence rates, median PDC and adjusted ORs by intensity level and prior CVD status (type of prevention)
| PDC ≥ 80% | Median PDC (IQR) | OR (95% CI), adjusted a | |
|---|---|---|---|
| All patients | 52.6 | 83.3 (36.7‐99.9) | |
| Low intensity | 40.5 | 58.2 (16.8‐96.8) | 0.58 (0.50‐0.68) |
| Moderate intensity | 51.6 | 82.0 (35.6‐99.5) | 1 |
| High intensity | 63.7 | 93.4 (59.0‐100.0) | 1.54 (1.37‐1.74) |
| Prior CVD (secondary prevention) | 64.7 | 94.3 (58.4‐100.0) | |
| Low intensity | 58.3 | 97.4 (24.4‐100.0) | 0.78 (0.41‐1.50) |
| Moderate intensity | 62.7 | 93.1 (54.1‐100.0) | 1 |
| High intensity | 74.1 | 98.7 (78.7‐100.0) | 3.06 (2.10‐4.46) |
| No prior CVD (primary prevention) | 50.4 | 80.5 (34.0‐99.1) | |
| Low intensity | 39.6 | 56.4 (16.4‐96.3) | 0.57 (0.48‐0.67) |
| Moderate intensity | 49.7 | 79.7 (33.3‐98.9) | 1 |
| High intensity | 59.8 | 90.1 (51.7‐100.0) | 1.39 (1.22‐1.58) |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; IQR, inter‐quartile range; OR, Odds Ratio; PDC, proportion of days covered.
Reference category is moderate intensity, adjusted for baseline variables: year of treatment initiation, drug prescribed, sex, age category, deprivation (SIMD), Charlson score (0 vs ≥1), prior CVD yes/no, hospitalisation yes/no.
When using the CR instead of the PDC, the overall adherence rate was considerably higher at 69.3% and differences between intensity levels were less pronounced, with between 68.5% and 76.1% of patients considered adherent to treatment. Again, patients with prior CVD had higher adherence rates at all levels of statin intensity (for details see Table 7).
TABLE 7.
Crude adherence rates, median CR and adjusted OR by intensity level and prior CVD status (type of prevention)
| CR ≥ 80% | Median CR (IQR) | OR (95% CI), adjusted a | |
|---|---|---|---|
| All patients | 69.3 | 93.2 (72.9‐101.1) | |
| Low intensity | 72.5 | 94.9 (76.9‐103.2) | 1.19 (1.00‐1.41) |
| Moderate intensity | 68.5 | 92.8 (71.9‐101.0) | 1 |
| High intensity | 76.1 | 96.0 (81.3‐102.1) | 1.27 (1.11‐1.45) |
| Prior CVD (secondary prevention) | 82.5 | 97.6 (87.6‐103.1) | |
| Low intensity | 85.4 | 99.7 (94.4‐108.3) | 1.19 (0.53‐3.08) |
| Moderate intensity | 81.9 | 97.4 (87.0‐103.0) | 1 |
| High intensity | 85.1 | 98.3 (89.8‐103.2) | 1.98 (1.25‐3.08) |
| No prior CVD (primary prevention) | 66.9 | 92.0 (70.1‐100.7) | |
| Low intensity | 71.9 | 94.5 (75.9‐103.0) | 1.19 (1.00‐1.42) |
| Moderate intensity | 66.3 | 91.7 (69.4‐100.6) | 1 |
| High intensity | 72.7 | 94.6 (77.4‐101.7) | 1.23 (1.06‐1.41) |
Abbreviations: CI, confidence interval; CR, compliance rate; CVD, cardiovascular disease; IQR, inter‐quartile range; OR, Odds Ratio.
Reference category is moderate intensity, adjusted for baseline variables: year of treatment initiation, drug prescribed, sex, age category, deprivation (SIMD), Charlson score (0 vs ≥1), prior CVD yes/no, hospitalisation yes/no.
After adjusting for baseline characteristics, compared to moderate intensity, patients on high‐intensity statin therapy in general were more likely to be adherent to treatment, regardless of outcome measurement used (PDC: OR 1.54, 95% CI 1.37‐1.74; CR: OR 1.27, 95% CI 1.11‐1.45). Adherence was similar to the overall results when looking only at patients without prior CVD/primary prevention (PDC: OR 1.39, 95% CI 1.22‐1.58; CR: 1.22, 95% CI 1.06‐1.41), but was considerably higher among patients with prior CVD/secondary prevention (PDC: OR 3.06, 95% CI 2.10‐4.46; CR: 1.98, 95% CI 1.25‐3.08) (Tables 6 and 7).
3.6. Sensitivity analyses
Results from the sensitivity analyses were consistent with the main analysis, although changing the length of permissible gaps had an impact on absolute numbers of patients discontinuing treatment, as well as on the median time to treatment discontinuation. When allowing for a short gap of 30 days, the overall proportion of patients discontinuing treatment increased to 75.0%, with a median time to discontinuation of 256 days (95% CI 251‐261). In contrast, extending the admissible gap to 90, 120 and 180 days resulted in decreased discontinuation rates of 56.0% (median time to discontinuation 798 days, 95% CI 778‐818), 52.1% (median time to discontinuation 1029 days, 95% CI 1002‐1052) and 47.5% (median time to discontinuation 1409 days, 95% CI 1372‐1445), respectively. In line with these findings, overall persistence rates decreased when shortening the admissible gap and increased when expanding it. Overall trends and differences between patients with or without prior CVD, or between different levels of statin intensity, were not affected (detailed results not shown).
4. DISCUSSION
This longitudinal cohort study assessed the impact of statin intensity on all medication‐taking behaviours (adherence, persistence and discontinuation) over a 7‐year period. Unexpectedly, compared to moderate statin intensity, high‐intensity statin therapy was associated with better adherence and persistence to treatment, and lower discontinuation rates. Nevertheless, in line with other published studies which evaluated discontinuation of and persistence to statins, 21 , 22 , 23 , 24 , 25 , 30 discontinuation rates were high overall, persistence to treatment decreased over time, and adherence was in general suboptimal. However, none of these studies assessed the impact of statin intensity.
Although differences between intensity levels have previously been reported, albeit to varying degrees depending on the specific population studied (eg, a study conducted in Israel found adherence to be lowest among high‐intensity statin users, 27 while a Japanese study observed both higher and lower adherence with high‐intensity statin therapy in comparison to moderate intensity depending on the database used), 28 this is, to the best of our knowledge, the first study to comprehensively analyse all aspects of medication‐taking behaviour (ie, discontinuation, persistence and adherence) with regard to statin therapy, taking into consideration both the level of statin intensity and whether patients have been subject to primary or secondary prevention.
Discontinuation was consistently lowest among high‐intensity patients in the full cohort as well as in the subgroups when stratifying by primary/secondary prevention. This is encouraging given the initial concerns that discontinuation rates may be higher among high‐intensity patients due to potentially increased side effects with increased dosage. Interestingly, atorvastatin, which accounted for the vast majority of high‐intensity therapy in our study, has recently been associated with lower odds of discontinuing statin treatment than simvastatin in a study conducted in Australia. 25 This was despite atorvastatin having been linked with higher levels of adverse events in comparison to simvastatin. 47 Differences in discontinuation rates were still observed when adjusting for patients' baseline characteristics, potentially indicating that higher intensity statins are either not associated with a significantly higher occurrence of side effects; or that patients are more tolerant towards minor side effects than anticipated if there is sufficient motivation to continue treatment. Unfortunately, adverse events data were not available to confirm these hypotheses. Bearing in mind that a considerable proportion of patients (72.3%) reinitiated treatment after a first discontinuation event, the latter might at least partially explain the results, especially when considering that patients being treated with high‐intensity statins will have a higher risk of serious cardiovascular events and thus might be more receptive to being prescribed a statin, while simultaneously prescribers might be more vigilant with continuing treatment in a high‐risk patient group.
Persistence in all treatment groups decreased over time. While 76.8% of all patients were still on treatment after 6 months, this decreased to 69.9% after 1 year, albeit with significant differences between intensity levels, with persistence consistently highest among high‐intensity patients. Similarly, adherence to statin therapy was poor overall, with only just over half of all patients (52.6%) categorised as adherent during the study period; again, high intensity patients generally displayed better adherence. When assessing adherence using CRs instead of the frequently applied PDC, a noticeably higher percentage of patients (overall 69.3%) was considered adherent to treatment. Differences between these two outcome measures were particularly marked within the low‐intensity treatment groups, highlighting the potentially substantial impact of early treatment discontinuation on measurements of adherence. Remarkably, adherence remained higher among patients treated with high‐intensity statins even when using CRs. As in previous studies, both persistence and adherence to treatment (regardless of measurement used) were higher among patients with a recent previous cardiovascular event such as a myocardial infarction or stroke. 21 , 22 , 25 , 28 , 48
Low adherence to long‐term treatment may be related to a range of parameters involving prescribers, patients, or both, and it is conceivable that factors such as insufficient explanation about the treatment and the impact of noncompliance, an underestimation of disease risks or the probability of drug‐related adverse events (which have all been shown to influence adherence to medication 20 , 26 , 49 ) are distributed differently between distinct patient groups, with subsequent effects on outcomes. These factors, however, could not be analysed in this study. Consequently, the reasons for the observed substantial differences in treatment adherence among the study participants remain unclear and need to be investigated further to enhance future adherence rates in Scotland.
Keeping the findings of not only this study but also results from other studies in mind, it seems pertinent to consider how best to increase adherence to statin therapy, as statins are a proven and cost‐effective means of preventing cardiovascular diseases, which are, despite recent improvements, still a major cause of death and disability in Scotland. 2 Interventions to improve adherence that have been suggested include interactive voice response reminders, regular medication reviews by community pharmacists, and repeat disease counselling through nurses or physicians. 50 Several studies 26 , 49 , 51 have highlighted that the patients who were most satisfied with their physician's explanations were more likely to remain current medicines users and to have better adherence. With pharmacists increasingly being introduced into GP practices in Scotland, 52 there is scope to explore options for improving the dialogue between patients and healthcare professionals, and to potentially implement other interventions tailored to patients that could improve adherence to statins ‐ such as providing verbal and written information about the disease and/or the treatment, adapting treatment regimens or using dispensing support tools such as dosette boxes, depending on context and reasons for nonadherence to therapy. 53 Most importantly, shared decisions about the initiation of treatment, discussing the potential risks and benefits of statin therapy, and shared management plans are essential to improve adherence rates and quality of medicines use. 20 , 26 We will be discussing the implications of our findings with key stakeholders in Scotland as a basis for debating possible future interventions that could be instigated to improve prescribing and adherence rates.
4.1. Strengths and limitations
This is a population‐based study assessing the association of statin intensity with the entire medication‐taking behaviour process, hence providing unprecedented insight into adherence to statin treatment. Considering that access to health care is universal and electronic health records cover the entire population, some confounding factors present in other settings, such as missing out on sections of the population based on incomplete health insurance coverage or inaccessibility of treatment due to copayments, are not present in Scotland. Furthermore, PIS and SMR01 have previously been used for research, and data validity and accuracy have been established. 54
Nevertheless, this study has several limitations. First, study data were obtained from NHS Scotland information systems, which have been implemented for administrative purposes; hence, not all desirable data were available. Details about diagnoses and the indication for drug treatment, for instance, are not available in PIS. Consequently, the indication for the use of statins (ie, primary or secondary prevention) was estimated based on hospital records, potentially affecting the accuracy of findings based on stratification by the presence or absence of prior CVD. In addition, due to the lack of primary care data, the Charlson Comorbidity Index was calculated using hospital discharge records and since some comorbidities might not have been captured, Charlson scores might have been underestimated. Data regarding the number of medications taken by patients, occasionally used as a proxy for comorbidities but also an important factor when assessing adherence in itself, were also not available for analysis, with potential implications for the interpretation of results. Furthermore, data related to clinical or behavioural factors such as lipid control and other potentially confounding lifestyle variables (including smoking habits, diet and physical activity) were unavailable. Consequently, reasons for discontinuation of treatment (which could have been based on a decision made by the prescriber rather than the patient, particularly among low‐risk patients being treated with low or moderate intensity statins) were unknown. Second, the lack of standardised definitions and methods for measuring adherence, discontinuation and persistence could hamper comparison of findings across studies, but this is a well‐recognised issue in drug utilisation research. 34 , 54 To accommodate the range of admissible gaps used in other studies, we conducted sensitivity analyses. Apart from changes in absolute numbers of patients discontinuing treatment, overall trends and differences between patients with and without prior CVD, or between levels of statin intensity, were not affected. Furthermore, based on applied definitions and calculations, adherence for individual patients could have been underestimated/discontinuation might have been overestimated if they intermittently received statin prescriptions for a different intensity, but the impact on overall findings is likely to be small due to small numbers of observed instances. Finally, an important limitation is the underlying assumption that all dispensed medicines were taken by patients, which is not always the case. Although findings could have been impacted if patients continued to fill in prescriptions despite not actually taking the drugs, this limitation is difficult to address, especially considering the size of the study cohort. As with all observational research, unmeasured confounding cannot be ruled out.
5. CONCLUSIONS
The study findings indicate higher long‐term persistence and adherence among high‐intensity statin users compared to moderate intensity, thus not supporting our initial concerns that high‐intensity statin therapy might impede patients' persistence and adherence due to higher risks of side effects. However, the study findings reinforce current beliefs that long‐term persistence and adherence to treatment with statins remain a challenge in the prevention of CVD, as this potentially leads to suboptimal treatment outcomes. Further research, potentially analysing laboratory data in combination with adverse events, is urgently needed to address these issues. Furthermore, steps need to be undertaken to encourage persistence and adherence in Scotland, particularly among patients without prior CVD where statins have been prescribed. We will be following up on this with key stakeholders to plan next steps given the continual high rates of CVD in Scotland.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
All authors contributed to the study concept and design. TM: data management, data analysis, drafting of the manuscript; RDN: data analysis, manuscript review; AK: data management, manuscript review. All authors contributed to the interpretation of the results and were involved in critically revising the text. All authors read and approved the final version of the manuscript.
Supporting information
Supporting Information Table S1 Baseline characteristics of new statin users, full cohort, by level of intensity
Supporting Information Figure S1 Kaplan‐Meier curve of time to first treatment discontinuation, full cohort; admissible gap 60 days
Supporting Information Figure S2 Kaplan‐Meier curves of time to first treatment discontinuation, full cohort, by level of intensity; admissible gap 60 days
ACKNOWLEDGEMENTS
FA Acurcio acknowledges support from FAPEMIG, the Minas Gerais State Research Foundation, Brazil; RCRM Nascimento acknowledge support from CAPES, the Brazilian Federal Agency for Support and Evaluation of Graduate Education, Brazil. There was no additional funding for this study.
Rezende Macedo do Nascimento RC, Mueller T, Godman B, et al. Real‐world evaluation of the impact of statin intensity on adherence and persistence to therapy: A Scottish population‐based study. Br J Clin Pharmacol. 2020;86:2349–2361. 10.1111/bcp.14333
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from NHS Scotland. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from NHS National Services Scotland, Information Services Division, with the permission of the Public Benefit and Privacy Panel for Health and Social Care.
REFERENCES
- 1. WHO . Cardiovascular diseases (CVDs) Geneva, Switzerland: World Health Organisation; 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2. ISD Scotland . Scottish heart disease statistics Edinburgh, UK: NHS national services Scotland; 2017. Available from: https://www.isdscotland.org/Health-Topics/Heart-Disease/Publications/2017-02-21/2017-02-21-Heart-Disease-Report.pdf
- 3. National Records of Scotland . Projected population of Scotland Edinburgh, UK; 2017. Available from: https://www.nrscotland.gov.uk/files//statistics/population-projections/2016-based-scot/pop-proj-2016-scot-nat-pop-pro-pub.pdf
- 4. Scottish Intercollegiate Guidelines Network . SIGN 149 Risk estimation and the prevention of cardiovascular disease. Healthcare Improvement Scotland: Edinburgh, UK; 2017. [Google Scholar]
- 5. Rabar S, Harker M, O'Flynn N, Wierzbicki AS. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: summary of updated NICE guidance. BMJ: Brit Med J. 2014;349:g4356. [DOI] [PubMed] [Google Scholar]
- 6. Heart Protection Study Collaborative Group . MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20 536 high‐risk individuals: a randomised placebo controlled trial. The Lancet. 2002;360(9326):7‐22. [DOI] [PubMed] [Google Scholar]
- 7. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267‐1278. [DOI] [PubMed] [Google Scholar]
- 8. O'Brien EC, Wu J, Schulte PJ, et al. Statin use, intensity, and 3‐year clinical outcomes among older patients with coronary artery disease. Am Heart J. 2016;173:27‐34. [DOI] [PubMed] [Google Scholar]
- 9. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;2013(1):CD004816‐CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. NICE . Cardiovascular disease: risk assessment and reduction, including lipid modification London, UK: National Institute for Health and Care Excellence; 2016. Available from: https://www.nice.org.uk/guidance/cg181
- 11. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37(39):2999‐3058. [DOI] [PubMed] [Google Scholar]
- 12. Cholesterol TReatment Trialists' Collaboration (CTT) , Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39(2):e38‐e81. [DOI] [PubMed] [Google Scholar]
- 14. Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin. 2016;32(6):631‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. The Lancet. 2010;375(9716):735‐742. [DOI] [PubMed] [Google Scholar]
- 16. Virani SS, Woodard LD, Akeroyd JM, Ramsey DJ, Ballantyne CM, Petersen LA. Is high‐intensity statin therapy associated with lower statin adherence compared with low‐ to moderate‐intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guidelines. Clin Cardiol. 2014;37(11):653‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shang P, Liu GG, Zheng X, et al. Association between medication adherence and 1‐year major cardiovascular adverse events after acute myocardial infarction in China. J am Heart Assoc. 2019;8(9):e011793‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940‐2948. [DOI] [PubMed] [Google Scholar]
- 20. Bates TR, Connaughton VM, Watts GF. Non‐adherence to statin therapy: a major challenge for preventive cardiology. Expert Opin Pharmacother. 2009;10(18):2973‐2985. [DOI] [PubMed] [Google Scholar]
- 21. Latry P, Molimard M, Dedieu B, Couffinhal T, Bégaud B, Martin‐Latry K. Adherence with statins in a real‐life setting is better when associated cardiovascular risk factors increase: a cohort study. BMC Cardiovasc Disord. 2011;11(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ofori‐Asenso R, Jakhu A, Zomer E, et al. Adherence and persistence among statin users aged 65 years and over: a systematic review and meta‐analysis. J Gerontol Series A. 2017;73(6):813‐819. [DOI] [PubMed] [Google Scholar]
- 23. Chen S‐T, Huang S‐T, Shau W‐Y, et al. Long‐term statin adherence in patients after hospital discharge for new onset of atherosclerotic cardiovascular disease: a population‐based study of real world prescriptions in Taiwan. BMC Cardiovasc Disord. 2019;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oforio‐Asenso R, Ilomäki J, Tacey M, et al. Predictors of first‐year nonadherence and discontinuation of statins among older adults: A retrospective cohort study. Br J Clin Pharmacol. 2019;85(1):227‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long‐term statin therapy? Curr Atheroscler Rep. 2013;15(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vodonos A, Ostapenko I, Toledano R, et al. Statin adherence and LDL cholesterol levels. Should we assess adherence prior to statin upgrade? Eur J Intern Med. 2015;26(4):268‐272. [DOI] [PubMed] [Google Scholar]
- 28. Wake M, Oh A, Onishi Y, Guelfucci F, Shimasaki Y, Teramoto T. Adherence and persistence to hyperlipidemia medications in patients with atherosclerotic cardiovascular disease and those with diabetes mellitus based on administrative claims data in Japan. Atherosclerosis. 2019;282:19‐28. [DOI] [PubMed] [Google Scholar]
- 29. Bellows BK, Olsen CJ, Voelker J, Wander C. Antihyperlipidemic medication treatment patterns and statin adherence among patients with ASCVD in a managed care plan after release of the 2013 ACC/AHA guideline on the treatment of blood cholesterol. J Manag Care Spec Pharm. 2016;22(8):892‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deshpande S, Quek RGW, Forbes CA, et al. A systematic review to assess adherence and persistence with statins. Curr Med Res Opin. 2017;33(4):769‐778. [DOI] [PubMed] [Google Scholar]
- 31. Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm. 2014;36(1):55‐69. [DOI] [PubMed] [Google Scholar]
- 32. Elseviers M, Wettermark B, Almarsdottir AB, et al. Drug Utilization Research: Methods and Applications. Hoboken, NJ: John Wiley & Sons; 2016. [Google Scholar]
- 33. Alvarez‐Madrazo S, McTaggart S, Nangle C, Nicholson E, Bennie M. Data resource profile: the Scottish National Prescribing Information System (PIS). Int J Epidemiol. 2016;45(3):714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leporowski A, Godman B, Kurdi A, et al. Ongoing activities to optimize the quality and efficiency of lipid‐lowering agents in the Scottish National Health Service: influence and implications. Expert Rev Pharmacoecon Outcomes Res. 2018;18(6):655‐666. [DOI] [PubMed] [Google Scholar]
- 36. NHS Greater Glasgow & Clyde . Who we are, what we do Glasgow, UK; 2019. Available from: https://www.nhsggc.org.uk/about-us/who-we-are-what-we-do/
- 37. National Records of Scotland . Mid‐year population estimates. Edinburgh, UK; 2020. Available from: https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/population/population-estimates/mid-year-population-estimates
- 38. Scottish Health Survey . Dashboard. Edinburgh, UK; 2020. Available from: https://scotland.shinyapps.io/sg-scottish-health-survey/
- 39. ISD Scotland . Data dictionary a ‐ Z: CHI number. Edinburgh, UK; 2019. Available from: https://www.ndc.scot.nhs.uk/Dictionary-A-Z/Definitions/index.asp?ID=128&Title=CHI%20Number
- 40. Parker MM, Moffet HH, Adams A, Karter AJ. An algorithm to identify medication nonpersistence using electronic pharmacy databases. J Am Med Inform Assoc. 2015;22(5):957‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7‐8):1280‐1288. [DOI] [PubMed] [Google Scholar]
- 42. Scottish Government . The Scottish Index of Multiple Deprivation, Edinburgh, UK; 2019. Available from: https://www2.gov.scot/Topics/Statistics/SIMD
- 43. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 44. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 45. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for statistical computing. 2019.
- 46. Ripley B, Lapsley M. RODBC: ODBC database access. 2019.
- 47. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins – a study‐level network meta‐analysis of 246 955 participants from 135 randomised, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6(4):390‐399. [DOI] [PubMed] [Google Scholar]
- 48. Kronish IM, Ross JS, Zhao H, Muntner P. Impact of hospitalization for acute myocardial infarction on adherence to statins among older adults. Circ Cardiovasc Qual Outcomes. 2016;9(4):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472‐483. [DOI] [PubMed] [Google Scholar]
- 50. Armstrong SO, Little RA. Cost effectiveness of interventions to improve adherence to statin therapy in ASCVD patients in the United States. Patient Prefer Adherence. 2019;13:1375‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rocque R, Leanza Y. A systematic review of patients' experiences in communicating with primary care physicians: intercultural encounters and a balance between vulnerability and integrity. PLoS ONE. 2015;10(10):e0139577‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Healthcare Improvement Scotland . iHUB: Scottish Patient Safety Programme. Edinburgh, UK; 2020. Available from: https://ihub.scot/improvement-programmes/scottish-patient-safety-programme-spsp/spsp-medicines-collaborative/pharmacotherapy/
- 53. Oñatibia‐Astibia A, Malet‐Larrea A, Larrañaga B, et al. Tailored interventions by community pharmacists and general practitioners improve adherence to statins in a Spanish randomized controlled trial. Health Serv Res. 2019;54(3):658‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mueller T, Alvarez‐Madrazo S, Robertson C, Bennie M. Use of direct oral anticoagulants in patients with atrial fibrillation in Scotland: applying a coherent framework to drug utilisation studies. Pharmacoepidemiol Drug Saf. 2017;26(11):1378‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Baseline characteristics of new statin users, full cohort, by level of intensity
Supporting Information Figure S1 Kaplan‐Meier curve of time to first treatment discontinuation, full cohort; admissible gap 60 days
Supporting Information Figure S2 Kaplan‐Meier curves of time to first treatment discontinuation, full cohort, by level of intensity; admissible gap 60 days
Data Availability Statement
The data that support the findings of this study are available from NHS Scotland. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from NHS National Services Scotland, Information Services Division, with the permission of the Public Benefit and Privacy Panel for Health and Social Care.


