Abstract
Aims
We performed the retrospective analysis to clarify the significance of drug monitoring for mycophenolic acid (MPA), the active form of mycophenolate mofetil (MMF), in prophylaxis for graft‐vs‐host disease (GVHD) in cord blood transplantation.
Methods
We retrospectively analysed the data of 46 patients who underwent first cord blood transplantation and received GVHD prophylaxis with tacrolimus plus MMF. MPA levels were measured on days 7 and 21, and 24‐hour areas under the curve (AUC0–24) were estimated.
Results
The engraftment and 3‐year overall survival rates of all patients were 94% and 78%, respectively. The cumulative incidence of sepsis before engraftment was higher in patients with AUC0–24 on day 7 of >60 μg h/mL than in other patients (33 vs 6%, P = .02). The cumulative incidence of grade II–IV acute GVHD was higher in patients with AUC0–24 on day 21 of ≤30 μg h/mL than in other patients (80 vs 50%, P = .04). The cumulative incidence of human herpesvirus 6 reactivation was higher in patients with AUC0–24 on day 21 of ≤48 μg h/mL (median) than in other patients (50 vs 19%, P = .03).
Conclusion
Blood level of MPA was associated with risk of acute GVHD and infection. A prospective trial evaluating the benefit of personalized MMF dosing using MPA levels is needed.
Keywords: cord blood transplantation, drug monitoring, mycophenolic acid
What is already known about this subject
Low level of mycophenolic acid after allogeneic haematopoietic stem cell transplantation is associated with high incidence of acute graft‐vs‐host disease.
What this study adds
Low level of mycophenolic acid after cord blood transplantation is associated with high incidence of acute graft‐vs‐host disease and human herpesvirus 6 reactivation, whereas a high level of mycophenolic acid is associated with high incidence of sepsis before engraftment.
1. INTRODUCTION
Graft‐vs‐host disease (GVHD) is a major cause of morbidity and mortality after allogeneic haematopoietic stem cell transplantation (allo‐SCT). The combination of calcineurin inhibitors with short‐course methotrexate after allo‐SCT has been standard GVHD prophylaxis for >20 years. 1 , 2 Following the successful use of mycophenolate mofetil (MMF) in the USA and Europe, the use of MMF with calcineurin inhibitor in cord blood transplantation (CBT) has recently increased in Japan to promote neutrophil recovery. 3 , 4 Wide interpatient variability in blood levels of mycophenolic acid (MPA), the active form of MMF, has been reported even after administering the same dose of MMF. 5 , 6 In kidney transplantation, therapeutic monitoring of MPA is reported to be important, with recommended target ranges for areas under the curve (AUCs) of 30–60 μg h/mL. AUCs <30 μg h/mL are associated with transplanted kidney rejection, whereas AUCs >60 μg h/mL are associated with infection and myelosuppression. 6 , 7 Similarly, there are some reports on drug monitoring for MPA in bone marrow or peripheral blood stem cell transplantation. 5 , 8 , 9 , 10 , 11 However, significance of drug monitoring for MPA in CBT was not fully elucidated. 12 In 2015, we reported that a low blood AUC of MPA on day 21 was associated with a high incidence of acute GVHD in the analysis of 24 patients who received CBT. 13 Based on this previous result, we started to increase the dose of MMF in patients with low MPA AUCs on day 7. In 2018, the number of patients increased sufficiently to perform univariate and multivariate analyses, and the observation times became long enough to evaluate the 3‐year overall survival (OS). This retrospective analysis was performed to clarify the association between MPA AUCs and CBT outcomes.
2. METHODS
2.1. Data collection
We retrospectively analysed the data of 46 patients who underwent their first CBT and received GVHD prophylaxis with tacrolimus plus MMF in our institute between October 2011 and July 2016. Tacrolimus was continuously and intravenously administered from day −1. Blood levels of tacrolimus were measured at least 3 times per week and were maintained at 12–15 ng/mL. After neutrophil engraftment, tacrolimus was orally administered, with trough levels maintained at 5–10 ng/mL. The patients received 10 mg/kg of oral MMF every 8 hours from day −1 to day 30 as inpatient treatment. Blood samples were collected immediately before, and 1, 2 and 4 hours after the morning administration of MMF on days 7 and 21. Total MPA levels in the plasma were measured using the enzyme multiplied immunoassay technique 14 , 15 or the Roche Total Mycophenolic Acid assay 16 (C0, C1, C2 and C4, respectively), and C8 (implying the trough level of the next administration) was assumed to be equal to the C0 values based on the results of previous studies. 9 , 11 , 17 , 18 The 8‐hour area under the curve (AUC0–8) was determined using the linear trapezoidal method, and the 24‐hour AUC (AUC0–24) was calculated as 3 times the AUC0–8. This method is frequently used in the setting of MMF administration 3 times daily (every 8 h) 9 , 18 because MPA levels peak within 2 hours from administration and decrease linearly after 4 hours. 17 , 19 After 2014, the dose of MMF for patients with MPA AUC0–24 of <30 μg h/mL on day 7 was increased. The dose of those patients from days 8–30 was calculated as follows: [dose of MMF before day 7] × 40/[AUC0–24 on day 7]. We analysed the effect of MPA AUC0–24 on transplant outcomes. The cut‐off values of AUC0–24 were set to 30 and 60 μg h/mL, which were the same as the values used for kidney transplantation. 6 We also set the cut‐off value as the median. To evaluate human herpesvirus 6 (HHV‐6) and cytomegalovirus (CMV) reactivation, peripheral blood was collected at least once weekly until 90 days after transplantation. The HHV‐6 DNA copy number was measured using real‐time polymerase chain reaction methods, as described previously. 20 The present study was approved by the institutional ethics committee of the Graduate School of Medicine, Kyoto University. All patients provided consent to participate in the present study.
2.2. Definition
OS was defined as the time from transplantation to death, and patients who remained alive at the time of the last follow‐up were censored. Complete haematological remission (CR) was defined based on morphology for all the patients, except for those with lymphoma; for lymphoma patients, it was based on positron emission tomography–computed tomographic imaging. 21 , 22 The relapse date of patients who did not achieve CR before and after SCT was defined as day 1. The patients were divided into 2 groups according to the conditioning regimen: myeloablative conditioning (MAC) or reduced‐intensity conditioning. MAC and reduced‐intensity conditioning were defined as proposed by Giralt et al 23 and Bacigalupo et al, 24 respectively. Pre‐engraftment immune reaction (PIR) was characterized by the presence of at least 3 of the following symptoms with no direct consequences of infection or adverse effects of medication 6 or more days before engraftment, as described previously: a high fever (>38.5°C), skin eruptions, body weight gain >5% of baseline, or peripheral oedema. 25 , 26 HHV‐6 reactivation was defined as plasma HHV‐6 DNA level ≥104 copies/mL. CMV reactivation was defined as the detection of at least 2 CMV pp65 antigen‐positive cells per 50 000 leucocytes.
2.3. Data analysis
Descriptive statistics were used to summarize the variables related to patient demographics and transplantation characteristics. The probability of the OS time was estimated according to the Kaplan–Meier method. 27 To evaluate the influences of confounding factors on OS, log‐rank tests were used for the univariate analysis. 28 The competing risk regression model was used for the multivariate analysis of acute GVHD. 29 The cumulative incidences of engraftment, relapse and GVHD were calculated, and death without events was considered a competing risk. Landmark analysis was performed to evaluate the effect of MPA level on days 7 and 21, with the landmark day set to days 7 and 21. Mann–Whitney U tests were used as nonparametric tests for 2 groups. Correlation of AUC and single point concentration was assessed by Spearman's correlation coefficient test. 30 The results are expressed as hazard ratio and their 95% confidence intervals (CIs). All tests were 2 sided, and P‐values <.05 were considered to indicate statistical significance. All statistical analyses were performed using Stata (version 13.0, Stata Corporation) and EZR (Saitama Medical Center, Jichi Medical University), a graphical user interface for R (The R Foundation for Statistical Computing, version 2.3.0). 31
2.4. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
3.1. Patient characteristics
The median observation time of the survivors was 1352 (range: 103–2487) days. The median age was 50 (range: 20–66) years. The percentages of patients diagnosed with acute myeloid leukaemia, acute lymphoid leukaemia, myelodysplastic syndrome and malignant lymphoma were 43, 13, 15 and 17%, respectively. The percentage of patients with CR was 52%. Fifty‐two percent of patients received MAC and 93% received total body irradiation (TBI; Table 1).
TABLE 1.
Patient characteristics
| Number (%) or median (range) | ||
|---|---|---|
| Age at SCT (y) | 50 (20–66) | |
| Sex | M/F | 29 (63) /17 (37) |
| Disease | AML/MDS | 20 (43) /17 (37) |
| ALL | 6 (13) | |
| NHL | 8 (17) | |
| CML/MPN | 1 (2) /1 (2) | |
| ATL | 1 (2) | |
| AA | 2 (4) | |
| PS at SCT | 0/1 | 23 (50) /14 (30) |
| 2–3 | 9 (20) | |
| Serum albumin at SCT (g/dL) | 3.2 (1.9–4.2) | |
| HLA mismatch | 0/1/2 | 1 (2) /11 (24) /10 (22) |
| (A, B, C and DRB1 alleles) | 3/4/5 | 11 (24) /11 (24) /2 (4) |
| Disease status | CR/PR | 24 (52) /2 (4) |
| At SCT | Others | 20 (43) |
| Days from diagnosis | < 90 | 6 (13) |
| To SCT | 90–180 | 14 (30) |
| >180 | 26 (57) | |
| Conditioning | MAC | 24 (52) |
| RIC | 22 (48) | |
| TBI | 0 | 3 (7) |
| 3–4 | 21 (46) | |
| 10–12 | 22 (48) | |
| Total nuclear cell count (×107/kg) | 2.47 (1.94–4.27) | |
| CD34+ cell count (×105/kg) | 0.57 (0.18–1.91) |
AA: aplastic anaemia; ALL: acute lymphoblastic leukaemia; AML: acute myeloidleukaemia; ATL: adult T cell leukaemia/lymphoma; CML: chronic myeloid leukaemia; CR: complete remission; F: female; HLA: human leucocyte antigen; M: male; MAC: myeloablative conditioning; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm; NHL: non‐Hodgkin lymphoma; PR: partial remission; PS: performance status; RIC: reduced‐intensity conditioning; SCT: stem cell transplantation; TBI: total body irradiation.
3.2. Outcomes of all patients
The cumulative incidence of neutrophil engraftment was 94%. A median of 25 (range: 11–43) days was required for neutrophil engraftment (Figure 1A). Engraftment failure was seen in 3 patients, and they had risks for engraftment failure; 1 patient had high tumour burden, another patient received weak conditioning (fludarabine 125 mg/m2 plus melphalan 80 mg/m2 without TBI) and the other patient transplanted the minimum count of CD34+ cells (0.18 × 105/kg) in this cohort. The cumulative incidences of PIR, grade II–IV acute GVHD, grade III–IV acute GVHD, limited chronic GVHD, and extensive chronic GVHD were 30, 63, 11, 15 and 13%, respectively. The 3‐year OS, cumulative incidence of relapse and nonrelapse mortality rates were 78% (95% CI: 62–87%), 20% (95% CI: 10–33%) and 11% (95% CI: 4–22%), respectively. After excluding 2 patients with aplastic anaemia, we divided patients with respect to disease status at SCT. The 3‐year OS, cumulative incidence of relapse, and nonrelapse mortality rates of patients in CR (n = 24) were 82, 22 and 13%, (95% CI: 59–93, 8–41 and 3–30%), respectively. The 3‐year OS, cumulative incidence of relapse, and nonrelapse mortality rates of patients in non‐CR (n = 20) were 70% (95% CI: 45–85%), 20% (95% CI: 6–40%) and 10% (95% CI: 2–28%), respectively (Figure 1B–D). Next, we divided the patients with respect to the number of human leucocyte antigen (HLA) mismatches for graft‐vs‐host direction. The cumulative incidence of grade II–IV acute GVHD in patients who underwent CBT with >2 HLA antigen mismatches in the HLA‐A, B, C and DRB1 loci (n = 15) was 80% (95% CI: 46–94%), which was significantly higher than that of the other patients (48%, 95% CI: 30–65%, n = 31; P = .02; Figure 1E). The cumulative incidence of grade II–IV acute GVHD in patients who underwent CBT with more than 2 HLA allele mismatches in the HLA‐A, B, C and DRB1 loci (n = 24) was 75% (95% CI: 51–88%), which was significantly higher than that of the other patients (41%, 95% CI: 20–61%, n = 22; P = .02; Figure 1F).
FIGURE 1.
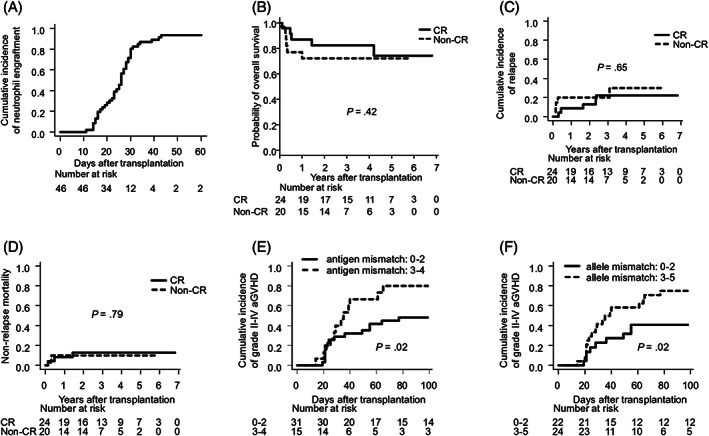
Use of mycophenolate mofetil enabled high neutrophil engraftment and feasible overall survival rates. (A) Cumulative incidence of engraftment. (B) Kaplan–Meier curves of overall survival of patients in CR and non‐CR at transplantation. (C) Cumulative incidence of relapse and (D) nonrelapse mortality of patients in CR and non‐CR. (E) Cumulative incidence of grade II–IV graft‐vs‐host disease and the number of HLA antigen mismatches (A, B, C, and DRB1). (F) Cumulative incidence of grade II–IV graft‐vs‐host disease and the number of HLA allele mismatches (A, B, C and DRB1). aGVHD: acute graft‐vs‐host disease; CR: complete remission; HLA: human leukocyte antigen
3.3. Impact of MPA AUCs on outcomes
None of the cases discontinued MMF administration, except for 1 case of death before day 30. No patients experienced MMF‐related severe toxicities such as diarrhoea and myelosuppression. The AUC0–24 of MPA on day 7 (MPA7) was 49.4 ± 24.7 μg h/mL (mean ± standard deviation). There was no association between MPA7 and the incidence of PIR, engraftment rate, engraftment speed or acute GVHD after day 7 (data not shown). In the landmark analysis on day 7, the rate of sepsis between day 7 and engraftment in patients with MPA7 > 60 μg h/mL was 33% (95% CI: 9–60%), which was higher than that in the other patients (6%, 95% CI: 1–18%; P = .02; Figure 2A). The AUC0–24 of MPA on day 21 (MPA21) was 47.7 ± 20.3 μg h/mL. After excluding 3 cases of graft failure, we divided patients with respect to MPA21. In the landmark analysis on day 21, the cumulative incidence of grade II–IV acute GVHD after day 21 in patients with MPA21 ≤ 30 μg h/mL was 80% (95% CI: 34–96%), which was higher than that in the other patients (50%, 95% CI: 31–67%; P = .04; Figure 2B). In the landmark analysis on day 21, the cumulative incidence of HHV‐6 reactivation after day 21 in patients with MPA21 ≤48 μg h/mL (median) was 50% (95% CI: 28–68%), which was higher than that in the other patients (19%, 95% CI: 6–38%; P = .03; Figure 2C). Only 2 patients developed HHV‐6 encephalitis, and there was no significant association between the development of HHV‐6 encephalitis and the blood level of MPA (data not shown). In the analysis of OS, the cumulative incidence of relapse, chronic GVHD, and reactivation of cytomegalovirus, no significant association was found with the blood level of MPA (data not shown).
FIGURE 2.
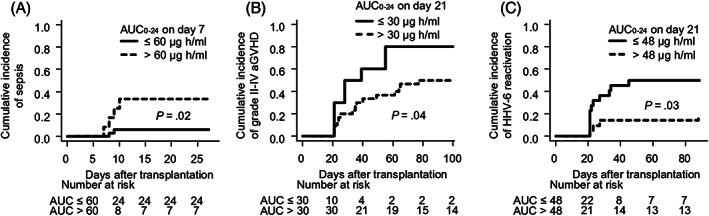
AUC0–24 of MPA affected the incidence of sepsis, grade II–IV acute GVHD, and reactivation of human herpesvirus 6. (A) AUC0–24 on day 7 and cumulative incidence of sepsis before neutrophil engraftment. (B) AUC0–24 on day 21 and cumulative incidence of grade II–IV graft‐vs‐host disease. (C) AUC0–24 on day 21 and cumulative incidence of reactivation of human herpesvirus 6. AUC0–24: 24‐hour area under the curve; MPA: mycophenolic acid; aGVHD: acute graft‐vs‐host disease
Next, we surveyed the causes of low MPA levels. The MPA21 of patients with diarrhoea on day 21 was significantly lower than that of patients without diarrhoea (35.8 vs 54.5 μg h/mL, P = .006; Figure 3A). Patients with serum albumin levels <3 g/dL on day 21 tended to have low MPA21 (37.8 vs 53.8 μg h/mL, P = .10; Figure 3B).
FIGURE 3.
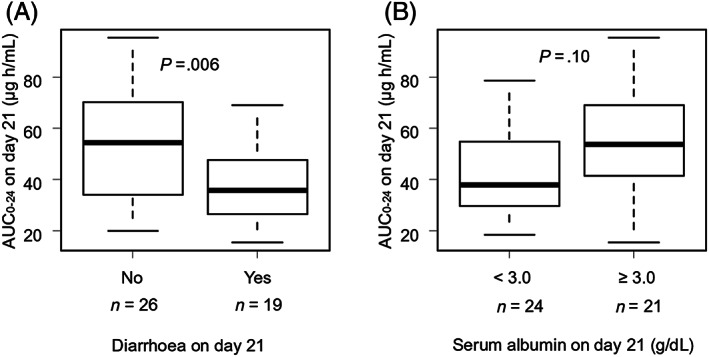
Diarrhoea and poor nutrition decreased AUC0–24 of MPA on day 21. (A) Diarrhoea and box plot of AUC0–24 on day 21. (B) Serum albumin levels and box plot of AUC0–24 on day 21. AUC0–24: 24‐hour area under the curve; MPA: mycophenolic acid
We assessed the efficacy of the dose escalation of MMF. After 2014, the MPA7 of 8 patients was <30 μg h/mL and the MMF dose was escalated after day 7. In these patients, the MPA21 of 6 patients was higher than their MPA7; the MPA21 of 1 patient who had moderate diarrhoea was less than MPA7 and 1 patient died before day 21 (Figure 4A). The cumulative incidence of grade II–IV acute GVHD of these 8 patients was 43%, which was lower than the incidence of all patients. The MPA7 of 13 patients was 30–60 μg h/mL and the dose of MMF was not escalated after day 7. Among these patients, 9 and 4 patients had MPA21 less and more than their MPA7s, respectively (Figure 4B). The cumulative incidence of grade II–IV acute GVHD of these 13 patients was 78%, which was higher than the incidence of all patients.
FIGURE 4.
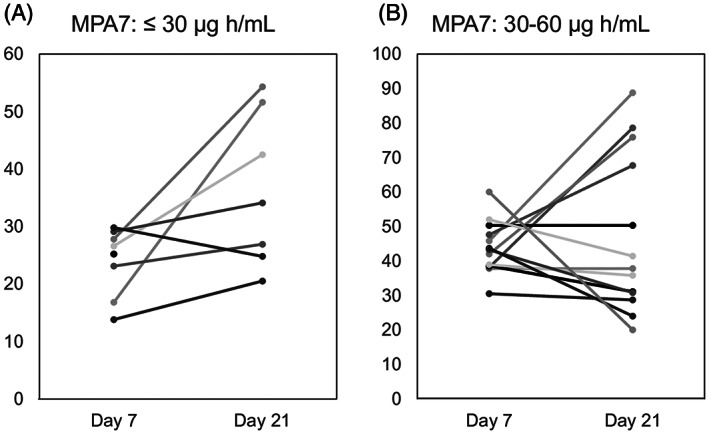
Dose escalation for low AUC0–24 on day 7 increased the AUC0–24 on day 21. (A) AUC0–24 of MPA on days 7 and 21 among patients with MPA7 (AUC0–24 of MPA on day 7) <30 μg h/mL. The MMF dose of these patients was escalated after day 7. (B) AUC0–24 on days 7 and 21 of patients with MPA7 of 30–60 μg h/mL. The MMF dose of these patients was not escalated after day 7. AUC0–24: 24‐hour area under the curve; MPA: mycophenolic acid; MMF: mycophenolate mofetil
3.4. Correlation between AUC0–24 and single point concentration
To analyse whether we could reduce the number of blood collections, we investigated the correlation between AUC0–24 and single point concentration of MPA. We found that there was stronger correlation between AUC0–24 and C2 (correlation coefficient = 0.737, P = 8.69 × 10−17), compared with C0, C1 and C4 (Figure S1A‐D). However, there was no significant association between C2 on day 7 and the incidence of sepsis before engraftment, and between C2 on day 21 and the incidence of grade II‐IV acute GVHD or HHV‐6 reactivation (data not shown).
3.5. Univariate and multivariate analyses for grade II–IV acute GVHD
After excluding the patients with graft failure and the patients who developed grade II‐IV acute GVHD before day 21, we performed univariate and multivariate analyses for grade II–IV acute GVHD after day 21. In the univariate analysis, HLA allele mismatch, non‐CR at SCT and MPA level were associated with a high incidence of grade II–IV acute GVHD (Table 2). Multivariate analysis revealed that non‐CR at SCT was associated with a high incidence of grade II–IV acute GVHD (Table 3).
TABLE 2.
Univariate analysis for grade II–IV acute GVHD
| Variables | Number | HR | 95% CI | P | |
|---|---|---|---|---|---|
| Age at SCT (y) | |||||
| < 50 | 20 | 1 | |||
| ≥50 | 20 | 1.55 | 0.72–3.35 | .27 | |
| PS at SCT | |||||
| 0–1 | 34 | 1 | |||
| 2–4 | 6 | 0.56 | 0.16–1.90 | .35 | |
| HCT‐CI at SCT | |||||
| 0–1 | 31 | 1 | |||
| >2 | 9 | 0.6 | 0.22–1.63 | .31 | |
| Sex mismatch | |||||
| Match, M to F | 34 | 1 | |||
| F to M | 6 | 0.99 | 0.61–1.62 | .98 | |
| HLA allele mismatch | |||||
| 0–2 | 20 | 1 | |||
| 3–5 | 20 | 2.26 | 1.05–4.86 | .04 | |
| Disease status at SCT | |||||
| CR | 22 | 1 | |||
| Non‐CR | 18 | 1.29 | 1.16–1.45 | <.001 | |
| Days from diagnosis to SCT | |||||
| 0–180 | 16 | 1 | |||
| > 180 | 24 | 1.01 | 0.58–1.75 | .98 | |
| Conditioning | |||||
| MAC | 22 | 1 | |||
| RIC | 18 | 0.92 | 0.41–2.07 | .85 | |
| TBI | |||||
| No | 2 | 1 | |||
| Yes | 38 | 2.41 | 0.31–18.55 | .40 | |
| AUC0–24 of MPA on day 21 | |||||
| ≤30 μg h/mL | 10 | 1 | |||
| >30 μg h/mL | 30 | 0.38 | 0.15–0.91 | 0.03 | |
| CD34+ cell count | |||||
| ≤0.57 × 105/kg | 1 | ||||
| >0.57 × 105/kg | 1.27 | 0.62–2.60 | 0.51 | ||
AUC0‐24: 24‐hour area under the curve; CI: confidence interval;CR: complete remission; F: female; GVHD: graft‐vs‐host disease; HCT‐CI: comorbidity index of haematopoietic cell transplantation; HLA: human leucocyte antigen; HR: hazard ratio; M: male; MAC: myeloablative conditioning; MPA: mycophenolic acid;PS: performance status; SCT: stem cell transplantation; RIC: reduced‐intensity conditioning; TBI: total body irradiation.
TABLE 3.
Multivariate analysis for grade II–IV acute GVHD
| Variables | Number | HR | 95% CI | P | |
|---|---|---|---|---|---|
| HLA allele mismatch | |||||
| 0–2 | 20 | 1 | |||
| 3–5 | 20 | 2.14 | .96–4.79 | .06 | |
| Disease status at SCT | |||||
| CR | 22 | 1 | |||
| Non‐CR | 18 | 1.26 | 1.07–1.48 | .006 | |
| AUC0–24 of MPA on day 21 | |||||
| ≤30 μg h/mL | 10 | 1 | |||
| >30 μg h/mL | 30 | 0.66 | 0.24–1.78 | .41 | |
AUC0‐24: 24‐hour area under the curve; CI: confidence interval; CR: complete remission; GVHD: graft‐vs‐host disease; HLA: human leucocyte antigen; HR: hazard ratio; MPA: mycophenolic acid; SCT: stem cell transplantation.
4. DISCUSSION
In the present study, GVHD prophylaxis with tacrolimus and MMF enabled a high incidence of neutrophil engraftment and prolonged OS compared to those reported in a nationwide survey in Japan. 4 , 32 A previous report indicated that 3000 mg/d of MMF was associated with delayed neutrophil engraftment in CBT, 33 but there was no evidence of an association between the dose of MMF and the rate of neutrophil engraftment. TBI is reportedly necessary for enhanced engraftment in CBT. 34 We administered MMF at 30 mg/kg/d, and 93% of patients received 3–12 Gy of TBI, which may have improved the incidence of neutrophil engraftment. Randomized studies are required to determine the optimal dose of MMF and the best conditioning regimen.
The cumulative incidence of grade II–IV acute GVHD in the present study was higher than that in previous reports. 4 , 32 The main reason for this result might be the high percentage of patients who underwent CBT with more than 2 antigen or allele mismatches in the HLA‐A, B, C and DRB1 loci. In Japan, HLA mismatches are generally evaluated with HLA‐A, B and DRB1 antigens, and fewer than 3 mismatches were permitted 35 ; our institute evaluated HLA mismatches in the same way. Our results suggest that HLA mismatch in CBT should be evaluated with the HLA‐A, B, C and DRB1 alleles to avoid acute GVHD.
In the present study, we observed that the blood levels of MPA fluctuated widely among patients after CBT. The kinetics of MMF are influenced by gastrointestinal damage caused by conditioning, loss of appetite, decreased oral intake, and other drug interactions. 36 Change from oral to intravenous MMF may be 1 strategy to stabilize blood level 37 ; however, intravenous MMF is not available in Japan.
The appropriate level of MPA in CBT is still not confirmed. We suggest that the AUC0–24 on day 7 should be <60 μg h/mL to avoid sepsis before engraftment and that the AUC0–24 on day 21 should be >30 μg h/mL to prevent acute GVHD. This range is similar to the adequate AUC0–24 used in kidney transplantation. 6 , 7
CBT is 1 risk factor of HHV‐6 encephalitis, 38 and epidemiologic evidence indicates the association between HHV‐6 reactivation and acute GVHD. 39 In the present study, the cumulative incidence of HHV‐6 reactivation was higher in patients with AUC0–24 on day 21 of ≤48 μg h/mL (median) than in the other patients. Therefore, maintaining a high level of MPA and suppressing graft‐vs‐host reaction may prevent HHV‐6 reactivation.
Based on these results, we hypothesize that MPA level should be changed before and after engraftment. In kidney transplantation, high MPA level is associated with myelosuppression and infection. 7 Similarly, MPA level in CBT should not be high to prevent bacterial infection before engraftment. After engraftment, alloreactive T cells become activated and excessive activation of alloreactive T cells leads to acute GVHD and delays immune reconstitution. Acute GVHD and delay of immune recovery cause HHV‐6 reactivation. 39 , 40 Moreover, treatment for GVHD also favours HHV‐6 reactivation, as described with other herpes viruses. 41 Therefore, MPA level should be high to suppress alloreactive T cells and prevent acute GVHD and HHV‐6 reactivation. These are hypotheses and should be further explored.
After 2014, we escalated the dose of MMF of patients with low blood levels of MPA on day 7 and succeeded in reducing the incidence of grade II–IV acute GVHD in those patients. However, the levels on day 21 in some patients without dose escalation fell below the levels measured on day 7 and many of them developed grade II–IV acute GVHD. More frequent monitoring of MPA and dose adjustment of MMF is necessary, especially for patients with diarrhoea or poor nutrition, whose blood levels of MPA tend to be low.
We focused on AUC and collected blood from patients 4 times per day. To proceed to multicentre study, we analysed whether we could make the measurement simpler. As a result, there was no significant association between single point concentration and the incidence of acute GVHD or infection. Thus, AUC is a better marker to predict the risk of acute GVHD and infection compared with single‐point concentration, and 4 blood collections per day is recommended.
Multivariate analysis revealed that non‐CR at SCT was associated with the development of grade II–IV acute GVHD, while a low MPA AUC0–24 on day 21 was not. In some patients with non‐CR at SCT, the physicians decided to maintain low levels of MPA, expecting a graft‐vs‐tumour effect of cord blood. This was the limitation of this small‐scale retrospective study.
The present study also had additional limitations. We measured whole‐blood levels of MPA. Levels of unbound form of MPA should be measured to evaluate the direct effect of MMF on lymphocytes. 42 Moreover, we did not survey the single‐nucleotide polymorphism for the genes coding inosine‐5‐monophosphate dehydrogenase (IMPDH). IMPDH is an enzyme inhibited by MPA; single‐nucleotide polymorphisms in those genes influence the activity of IMPDH and suppress lymphocyte proliferation. 43 , 44 , 45 In addition, our results should be interpreted with caution because of the small sample size and the heterogeneous patient and transplant backgrounds.
In conclusion, blood AUCs of MPA affected the incidence of grade II–IV acute GVHD, sepsis before engraftment, and HHV‐6 reactivation in CBT. A multicentre prospective study is required to clarify the appropriate range of MPA AUCs.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
H.M., J.K., Y.A. and T.K. designed the research, organized the project, and drafted the manuscript. H.M. and J.K. gathered the data and performed the statistical analysis. T.S., M.H., T.Y., K.Y., K.M. and A.T.K. contributed to the data analysis and writing of the manuscript. All authors approved the final version of the manuscript for submission for publication.
Supporting information
FIGURE S1 Correlation between AUC0–24 and single point concentration. Correlation between AUC0–24 and (A) C0, (B) C1, (C) C2 and (D) C4. AUC0–24: 24‐hour area under the curve. R: correlation coefficient.
ACKNOWLEDGEMENTS
The authors thank Emi Furusaka, Tomoko Okuda and Megumi Oka for their expert data management and the haematopoietic stem cell transplantation team members of Kyoto University Hospital for their dedicated care of the patients.
Muranushi H, Kanda J, Arai Y, et al. Drug monitoring for mycophenolic acid in graft‐vs‐host disease prophylaxis in cord blood transplantation. Br J Clin Pharmacol. 2020;86:2464–2472. 10.1111/bcp.14354
The authors confirm that the Principal Investigator for this paper is Hiroyuki Muranushi and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729‐735. [DOI] [PubMed] [Google Scholar]
- 2. Storb R, Deeg HJ, Pepe M, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft‐versus‐host disease in patients given HLA‐identical marrow grafts for leukemia: long‐term follow‐up of a controlled trial. Blood. 1989;73(6):1729‐1734. [PubMed] [Google Scholar]
- 3. Uchida N, Wake A, Nakano N, et al. Mycophenolate and tacrolimus for graft‐versus‐host disease prophylaxis for elderly after cord blood transplantation: a matched pair comparison with tacrolimus alone. Transplantation. 2011;92(3):366‐371. [DOI] [PubMed] [Google Scholar]
- 4. Terakura S, Kuwatsuka Y, Yamasaki S, et al. GvHD prophylaxis after single‐unit reduced intensity conditioning cord blood transplantation in adults with acute leukemia. Bone Marrow Transplant. 2017;52(9):1261‐1267. [DOI] [PubMed] [Google Scholar]
- 5. Giaccone L, McCune JS, Maris MB, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106(13):4381‐4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw LM, Figurski M, Milone MC, Trofe J, Bloom RD. Therapeutic drug monitoring of mycophenolic acid. Clin J Am Soc Nephrol. 2007;2(5):1062‐1072. [DOI] [PubMed] [Google Scholar]
- 7. Kuypers DR, Le Meur Y, Cantarovich M, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010;5(2):341‐358. [DOI] [PubMed] [Google Scholar]
- 8. Haentzschel I, Freiberg‐Richter J, Platzbecker U, et al. Targeting mycophenolate mofetil for graft‐versus‐host disease prophylaxis after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2008;42(2):113‐120. [DOI] [PubMed] [Google Scholar]
- 9. Wakahashi K, Yamamori M, Minagawa K, et al. Pharmacokinetics‐based optimal dose prediction of donor source‐dependent response to mycophenolate mofetil in unrelated hematopoietic cell transplantation. Int J Hematol. 2011;94(2):193‐202. [DOI] [PubMed] [Google Scholar]
- 10. Jacobson P, Rogosheske J, Barker JN, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78(5):486‐500. [DOI] [PubMed] [Google Scholar]
- 11. McDermott CL, Sandmaier BM, Storer B, et al. Nonrelapse mortality and mycophenolic acid exposure in nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(8):1159‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harnicar S, Ponce DM, Hilden P, et al. Intensified Mycophenolate Mofetil dosing and higher mycophenolic acid trough levels reduce severe acute graft‐versus‐host disease after double‐unit cord blood transplantation. Biol Blood Marrow Transplant. 2015;21(5):920‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arai Y, Kondo T, Kitano T, et al. Monitoring mycophenolate mofetil is necessary for the effective prophylaxis of acute GVHD after cord blood transplantation. Bone Marrow Transplant. 2015;50(2):312‐314. [DOI] [PubMed] [Google Scholar]
- 14. Beal JL, Jones CE, Taylor PJ, Tett SE. Evaluation of an immunoassay (EMIT) for mycophenolic acid in plasma from renal transplant recipients compared with a high‐performance liquid chromatography assay. Ther Drug Monit. 1998;20(6):685‐690. [DOI] [PubMed] [Google Scholar]
- 15. Brunet M, Oppenheimer F, Martorell J, et al. Mycophenolic acid monitoring: evaluation of the EMIT MPA immunoassay in kidney and lung transplantation. Transplant Proc. 1999;31(6):2275‐2276. [DOI] [PubMed] [Google Scholar]
- 16. Parant F, Ranchin B, Gagnieu MC. The Roche Total mycophenolic acid® assay: an application protocol for the ABX Pentra 400 analyzer and comparison with LC‐MS in children with idiopathic nephrotic syndrome. Pract Lab Med. 2017;7:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Hest RM, Doorduijn JK, de Winter BC, et al. Pharmacokinetics of mycophenolate mofetil in hematopoietic stem cell transplant recipients. Ther Drug Monit. 2007;29(3):353‐360. [DOI] [PubMed] [Google Scholar]
- 18. Jacobson P, El‐Massah SF, Rogosheske J, et al. Comparison of two mycophenolate mofetil dosing regimens after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44(2):113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H, Mager DE, Sandmaier BM, Maloney DG, Bemer MJ, McCune JS. Population pharmacokinetics and dose optimization of mycophenolic acid in HCT recipients receiving oral mycophenolate mofetil. J Clin Pharmacol. 2013;53(4):393‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. J Infect Dis. 2006;193(1):68‐79. [DOI] [PubMed] [Google Scholar]
- 21. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working Group for Diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642‐4649. [DOI] [PubMed] [Google Scholar]
- 22. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giralt S, Ballen K, Rizzo D, et al. Reduced‐intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyakoshi S, Yuji K, Kami M, et al. Successful engraftment after reduced‐intensity umbilical cord blood transplantation for adult patients with advanced hematological diseases. Clin Cancer Res. 2004;10(11):3586‐3592. [DOI] [PubMed] [Google Scholar]
- 26. Kishi Y, Kami M, Miyakoshi S, et al. Early immune reaction after reduced‐intensity cord‐blood transplantation for adult patients. Transplantation. 2005;80(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 27. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457‐481. [Google Scholar]
- 28. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163‐170. [PubMed] [Google Scholar]
- 29. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496‐509. [Google Scholar]
- 30. Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15(1):72‐101. [PubMed] [Google Scholar]
- 31. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terakura S, Wake A, Inamoto Y, et al. Exploratory research for optimal GvHD prophylaxis after single unit CBT in adults: short‐term methotrexate reduced the incidence of severe GvHD more than mycophenolate mofetil. Bone Marrow Transplant. 2017;52(3):423‐430. [DOI] [PubMed] [Google Scholar]
- 33. Okamura A, Shimoyama M, Ishii S, et al. Delayed neutrophil engraftment in cord blood transplantation with intensive administration of mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant. 2011;46(1):148‐149. [DOI] [PubMed] [Google Scholar]
- 34. Nakasone H, Fuji S, Yakushijin K, et al. Impact of total body irradiation on successful neutrophil engraftment in unrelated bone marrow or cord blood transplantation. Am J Hematol. 2017;92(2):171‐178. [DOI] [PubMed] [Google Scholar]
- 35. Kanda J, Atsuta Y, Wake A, et al. Impact of the direction of HLA mismatch on transplantation outcomes in single unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(2):247‐254. [DOI] [PubMed] [Google Scholar]
- 36. Okamura A, Yamamori M, Shimoyama M, et al. Pharmacokinetics‐based optimal dose‐exploration of mycophenolate mofetil in allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2008;88(1):104‐110. [DOI] [PubMed] [Google Scholar]
- 37. Li H, Mager DE, Bemer MJ, et al. A limited sampling schedule to estimate mycophenolic acid area under the concentration‐time curve in hematopoietic cell transplantation recipients. J Clin Pharmacol. 2012;52(11):1654‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P. HHV‐6 encephalitis in umbilical cord blood transplantation: a systematic review and meta‐analysis. Bone Marrow Transplant. 2013;48(4):574‐580. [DOI] [PubMed] [Google Scholar]
- 39. Pichereau C, Desseaux K, Janin A, et al. The complex relationship between human herpesvirus 6 and acute graft‐versus‐host disease. Biol Blood Marrow Transplant. 2012;18(1):141‐144. [DOI] [PubMed] [Google Scholar]
- 40. Greco R, Crucitti L, Noviello M, et al. Human herpesvirus 6 infection following Haploidentical transplantation: immune recovery and outcome. Biol Blood Marrow Transplant. 2016;22(12):2250‐2255. [DOI] [PubMed] [Google Scholar]
- 41. Ljungman P, Aschan J, Lewensohn‐Fuchs I, et al. Results of different strategies for reducing cytomegalovirus‐associated mortality in allogeneic stem cell transplant recipients. Transplantation. 1998;66(10):1330‐1334. [DOI] [PubMed] [Google Scholar]
- 42. Frymoyer A, Verotta D, Jacobson P, Long‐Boyle J. Population pharmacokinetics of unbound mycophenolic acid in adult allogeneic haematopoietic cell transplantation: effect of pharmacogenetic factors. Br J Clin Pharmacol. 2013;75(2):463‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Yang JW, Zeevi A, et al. IMPDH1 gene polymorphisms and association with acute rejection in renal transplant patients. Clin Pharmacol Ther. 2008;83(5):711‐717. [DOI] [PubMed] [Google Scholar]
- 44. Sombogaard F, van Schaik RH, Mathot RA, et al. Interpatient variability in IMPDH activity in MMF‐treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenet Genomics. 2009;19(8):626‐634. [DOI] [PubMed] [Google Scholar]
- 45. McCune JS, Storer B, Thomas S, McKiernan J, Gupta R, Sandmaier BM. Inosine monophosphate dehydrogenase Pharmacogenetics in hematopoietic cell transplantation patients. Biol Blood Marrow Transplant. 2018;24(9):1802‐1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Correlation between AUC0–24 and single point concentration. Correlation between AUC0–24 and (A) C0, (B) C1, (C) C2 and (D) C4. AUC0–24: 24‐hour area under the curve. R: correlation coefficient.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.


