Abstract
Aims
Several addictovigilance studies have described the off‐label use of morphine sulfate (MS) for nonchronic pain in opioid use disorder (OUD) patients as an alternative to conventional opioid substitution treatments (OSTs). This study primarily sought to compare the incidence of unintentional opioid‐related overdose in the year following the prescription initiation in off‐label MS users, compared to OST‐maintained patients.
Methods
Sequential cohorts of OUD patients who were regularly dispensed MS, buprenorphine, or methadone, between 1 April 2012 and 31 December 2014, were retrospectively identified using the French nationwide healthcare data system. The incidence of overdoses, deaths, doctor shopping, and complications of a viral, bacterial or thrombotic nature, was compared using the Cox regression method.
Results
Overall, 1075, 20 834 and 9778 OUD patients without chronic‐pain were included in the MS, buprenorphine, and methadone cohorts, respectively. Overdose incidence was 3.8 (P < .01 [95% confidence interval (CI): 2.1–6.8]) and 2.0 (P = .02 [95%CI: 1.1–3.6]) higher in the MS cohort vs buprenorphine and methadone, respectively. Death incidence was 9.1 (P < .01 [95%CI: 3.2–25.9]) and 3.9 (P < .01 [95%CI: 1.4–10.7]) higher in the MS cohort vs buprenorphine and methadone, respectively. The incidences of other associated risks were significantly higher in the MS group vs OSTs, except for hepatitis C viral infection and thrombotic complications.
Conclusion
This first French comprehensive nationwide study reveals increasing overdose, death, bacterial infection, abuse and diversion risks when off‐label MS is initiated as alternative to OST. These results question the relevance of prescribing MS as a safe opioid maintenance treatment, considering its health risk profile.
Keywords: abuse, addiction, misuse, opioid, overdose
1.
What is already known about this subject
In France, slow‐release oral morphine sulfate is at times prescribed off‐label to patients with opioid use disorder after failure or undesirable effects with conventional treatments (methadone or buprenorphine).
Previous field studies have shown that prescribed oral morphine sulfate is commonly diverted by intravenous injection at high doses, without knowledge of this practice's associated risks.
What this study adds
Comprehensive data confirm the field findings and clarify the extent of off‐label morphine sulfate use in an opioid‐dependent setting.
The increased risk of overdose and death found in the year following the initiation of morphine sulfate in opioid use disorder patients prevents this molecule to be considered a safe alternative to conventional treatments.
2. INTRODUCTION
Over the past decade, we have noticed a rise in pharmacovigilance signals associated with increased opioid‐related overdose and death in developed countries. These alerts, initially focused on North America, have gradually spread to most industrialized countries, thus becoming a global public health concern. 1 , 2 , 3 , 4 , 5 These signals reveal a worrying increase in unintentional intoxications linked to prescribed opioid analgesic overuse, causing many deaths. 6 , 7 This has led the US Public Health Agency to declare this situation a national health emergency. 8 While oxycodone is likely to be the most often implicated drug in the USA, other opioid analgesics, such as fentanyl or morphine sulfate (MS), are involved in the worldwide spread. 2 , 5 , 9
In France, MS is available in rapid‐ and slow‐release oral formulations indicated for severe acute or resistant chronic pain, especially cancer pain. In several European countries like Austria, Luxembourg, Slovenia, Bulgaria and Switzerland, a slow‐release oral morphine (SROM) formulation was approved for managing opioid use disorders (OUD). 10 In France, SROM is at times prescribed off‐label, outside its analgesic recommendations. MS may then constitute an alternative to opioid substitution treatment (OST) for a minority of OUD patients with insufficient efficacy or undesirable events following conventional OST: high‐dose buprenorphine (HDB) or methadone (MTD). These patients are sometimes more relieved by MS. However, as the French monitoring centre for drugs and drug addiction has shown, some OUD patients may misuse the prescribed MS without the prescriber's knowledge. This misuse corresponds more to a recreational addictive behaviour than a substitute purpose. Between 2003 and 2006, this recreational use increased from 3.2 to 9.0% among users of risk reduction centres, while the proportion of patients using MS as an alternative to OST remained stable during this period. 11 While diverted MS use in OUD patients is not entirely new, this phenomenon remained limited until 2011. 12 At that time, for some opioid‐dependent drug users, a shortage in heroin led to a reduction in its quality/price ratio and transition to prescribed or black‐market MS, for which the quality was constant. 13 Indeed, by drug users, MS is considered as more easily injectable than MTD, procuring a greater high effect than HDB, which some patients are unable to discontinue despite maintenance treatment. 14
Several studies have investigated SROM formulations as an OST, yielding heterogeneous results. Two meta‐analyses were unable to identify sufficient data in the literature to assess the use of MS as OST due to a lack of good‐quality studies. 10 , 15 No data were found on the risks associated with SROM, especially when administered in an unconventional manner.
Although very few data are so far available pertaining to the MS use in OUD in France, it occurs that, in most cases, oral MS is diverted for intravenous injection. 16 , 17 Initial data from the first French supervised drug‐injection facility in Paris reported that 47.6% of intravenous drug users were shown to inject oral formulations of MS (Skenan). 18 This finding has meanwhile been confirmed by the results of various field surveys, where 71–93% of MS users in OUD settings reported injecting the drug. 17 , 19 This widespread diversion of the initial administration route has become a current public health problem. According to a survey of drug users, the effect of intravenous MS injection is likely to be shorter than that of heroin, resulting in increased injections and the risk of complications, such as overdosing, viral and bacterial infections, and thrombosis. 20
Most developed countries worldwide are currently facing a sharp increase in overdosing and deaths associated with prescribed opioid analgesics, which are gradually diverted from their original indications. In France, these MS off‐label prescriptions to OUD patients are performed without knowledge of the associated risks, related to both the direct effect of the opioid or its route of administration.
This study primarily sought to compare the incidence of unintentional opioid‐related overdose in an OUD cohort, during the year following the prescription initiation of continuous and regular off‐label MS, compared to that of HDB or MTD‐maintained patients.
3. METHODS
In the absence of formal guidelines for pharmaco‐epidemiological studies, the TREND and RECORD (extension of the existing STROBE guidelines) statements were applied to report the study findings. 21 , 22 , 23
3.1. Study plan
This was a population‐based retrospective cohort study of nonchronic pain OUD patients treated with MS, HDB or MTD, between 2012 and 2015, using anonymous data collection from the exhaustive French health insurance database.
3.2. Data source
Data were extracted from the French Nationwide Healthcare Data System (SNDS). This database, widely employed for public health and pharmacoepidemiological purposes, covers 98.8% of the French population, namely more than 66 million people, comprising exhaustive anonymous individual information on administrative, medical and pharmacy data. 24 Anonymous identifiers link reimbursement data and hospital admissions, providing discharge diagnoses using the International Statistical Classification of Diseases, 10th revision (ICD‐10), and the death registry.
Administrative data comprise individual information on the year of birth, sex, date of death, long‐term diseases, free supplemental health insurance, as granted to the unemployed and people with low‐income. This latter information can consequently be used as a substitute for low‐income status. In the French healthcare system, 30 major chronic diseases have been designated as long‐term diseases (LTDs), including diabetes mellitus, cancer, or psychiatric diseases, representing surrogates for comorbidity assessment. Pharmacy data comprise anonymous pharmacy identifiers and exhaustive claims for all reimbursed medicines dispensed in retail pharmacies (quantities supplied, dates of prescription, and dispensation) including opioid analgesics and conventional OSTs. Medicines are identified by their anatomical therapeutic chemical (ATC) codes. Medical data comprise anonymous doctor identifiers and prescribers' specialties. In addition, data on hospital admissions are recorded during the patient hospital stays, with coded diagnoses available based on the ICD‐10.
3.3. Data availability statement
Study approval was obtained from the French National Institute for Health Data (INDS, No. 176) and French Data Protection Agency (CNIL, No. 1946535), which prohibit the data from being disclosed. As a result, research data are not shared by the authors but can be requested from the INDS (website: https://www.indsante.fr). The programming code can be made available in raw form on request to the corresponding author.
3.4. Participants and study data
The lack of recommendations pertaining to MS as a validated OST implies its exclusive dispensing in pharmacies. Only control patients with OST dispensed in pharmacies, available in the database, could be included in the study, representing over 90% of substituted patients in France. 25 The remaining patients were given their treatments directly in addictology delivery centres. In accordance with the OST prescription recommendations, the study included all patients aged 15 years or older, male or female, who received the drug of interest at least once between 1 April 2012 and 31 December 2014, with no dispensing during the 3 months prior to inclusion, with the aim to recruit only incident patients. Data were available up to 31 December 2015, allowing for a theoretical follow‐up of at least 1 year for each patient (see Supporting information Figure S1).
The index date was defined as the first date of a continuous 90‐day sequence, during which the treatment was regularly dispensed, i.e. with <35 days between each pharmacy dispensation. This 35‐day threshold corresponds to the French legal obligations on narcotic drugs, which limit the prescription and dispensation of opioid medicines to a maximum of 28 days, without any possibility of renewal without a new prescription, to which 7 days were added in order to avoid recording a prescription discontinuation incorrectly. Patients who met these criteria for at least 90 days were considered regular and continuous substance users and could thus be included.
Strong opioid dispensations were identified using the ATC codes N02AA01 and N02AA51, and HDB or MTD by the ATC codes N07 BC01 and N07 BC02, respectively. The date of each dispensation was collected so as to determine the regularity of use. All the ICD‐10 codes used to select patients according to inclusion and exclusion criteria have been detailed in Table S1.
Patients suffering from cancer or receiving palliative care were excluded, as well as chronic‐pain condition patients identified through:
specific ICD‐10 codes from hospital discharge reports or LTDs for chronic pain or a rheumatic condition for which MS is recommended in France 26 ;
identification of care dispensed in pain clinics;
identification of a continuous analgesic prescription other than MS for at least 3 months, was considered as reflecting the management of chronic pain.
OUD patients were defined:
as having been dispensed conventional OSTs (HDB or MTD) at least once;
on the basis of hospital discharge reports or chronic conditions for an opioid‐related disorder ICD‐10 code.
Based on our criteria, regular MS users without a diagnosis of chronic pain or opioid use disorders were subjected to further analysis. The youngest (≤37 years) of these users, receiving the highest doses (≥550 mg/day), had a profile of OUDs rather than chronic pain patients, according to data from a previous French field study. 12 They were therefore included in the OUD MS cohort.
Human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus (HCV) infections were identified from either hospital discharge diagnoses or LTDs. The most common injection‐related infectious complications in drug addicts 27 , 28 , 29 and thrombotic complications (arterial and venous thrombosis, upper and lower limbs) 30 , 31 , 32 were selected according to literature data and identified based on hospital discharge diagnoses.
Data relating to unintentional opioid intoxication, major psychiatric conditions (LTD‐23), alcohol‐dependence (by its specific treatments 33 : disulfiram, acamprosate, naltrexone and nalmefene), as well as data on anxiolytic benzodiazepine (BZD, ATC code N05BA) dispensations were additionally collected.
For control cohorts, patients receiving BHD, MTD or MS dispensations within the 3 months preceding the index date were excluded from the study in an effort to recruit only incident conventional OST patients, excluding those who were in transition from using MS to regular conventional OST.
Cohort 1 comprised all OUD incident patients starting a continuous sequence of MS between 1 April 2012 and 31 December 2014. Cohorts 2 and 3 included all opioid‐maintained incident patients starting a continuous sequence of HDB or MTD, respectively, between 1 April 2012 and 31 December 2014, without MS dispensation within the 3 months before the index date. The absence of chronic pain will no longer be mentioned, since chronic pain patients were systematically excluded from the study.
3.5. Doctor shopping
Doctor shopping behaviour (DSB) is defined as the practice of obtaining overlapping prescriptions for a drug of interest from 1 or more physicians and multiple different pharmacies in an effort to get an increased amount of prescribed medication. 34 , 35 This behaviour is usually associated with a high level of misuse, abuse and diversion. 36 , 37 , 38 , 39 , 40 The threshold of at least 1 day of overlap, at least 2 different prescribers, and at least 3 different pharmacies was chosen, for it has been applied in previous research, which mainly assessed DSB pertaining to analgesic opioids. 41 , 42
3.6. Outcome measures
The primary outcome was the incidence of unintentional opioid overdosing in the MS cohort vs OST cohorts 1 year after the patient had become a regular user (i.e. 1 year and 3 months after the index date). Secondary outcomes were the 1‐year incidence of all‐cause death, DSB and injection‐related complications (HCV seroconversion, bacterial infections and thrombosis).
3.7. Statistical analyses
The data were expressed as frequency and associated percentage for categorical variables and as mean ± standard deviation or median and interquartile range for quantitative variables, depending on their statistical distribution (normality studied using the Shapiro–Wilk test).
Comparisons between cohorts were carried out using the χ2 test or Fisher's exact test for categorical data, as appropriate; for continuous variables, analyses of variances were performed, with the Kruskal–Wallis test employed when normality was rejected.
Survival analyses were performed to assess the delay between the occurrence of complications associated with MS misuse and the prescription initiation. Death, cessation of continuous and regular dispensations, or appearance of MS and OST concurrent prescriptions constituted censorship criteria. The appearance of 1 of these censorship criteria resulted in the study being stopped for the patient concerned. Otherwise, survival analyses were pursued until the end of the year following regular MS or OST use. Survival curves were plotted using the Kaplan–Meier method. The same method was applied to analyse time‐to‐death, doctor shopping, HCV seroconversion, and infectious or thrombotic complications.
Crude incidences with 95% confidence intervals (95%CI) were calculated per 100 000 persons per year, for each of the study's objectives.
Multivariate analyses were performed using the Cox model to analyse MS and OST exposure in relation with opioid intoxication, death, DSB, HCV, and infectious or thrombotic complications after adjusting for age, sex, socioeconomic status, chronic alcohol consumption, concurrent BZD use (defined as receiving at least 1 BZD delivery during the period in which the prescribed opioid was regularly delivered), as well as major chronic somatic or psychiatric comorbid disease. These Cox's proportional risk regression results are expressed as hazard ratios with their 95% confidence interval.
The results' reliability was reinforced by 2 falsification analyses, performed on 2 criteria independent of the study objectives, the comorbidities expected in these populations and the treatments involved. The control events selected were the incidence of asthma and primary hypertension. In the absence of any difference, these falsification analyses make it possible to affirm that the significant differences obtained for the study objectives do not result from a selection bias not controlled by adjustment methods between cohorts.
All statistical analyses were performed using SAS‐9.3 (SAS Institute, USA) and STATA‐14.1 (StataCorp, USA).
4. RESULTS
Between April 2012 and December 2014, 1075 OUD patients were included in the MS cohort, with 20 834 HDB‐maintained patients and 9778 MTD‐maintained patients in the second and third cohorts, respectively (see flowchart in Figure 1). The median length of time each cohort received continuous and regular treatment according to our criteria was comparable between MS (234 days) and buprenorphine (259 days), yet higher for MTD patients (451.5 days; Table 1).
FIGURE 1.
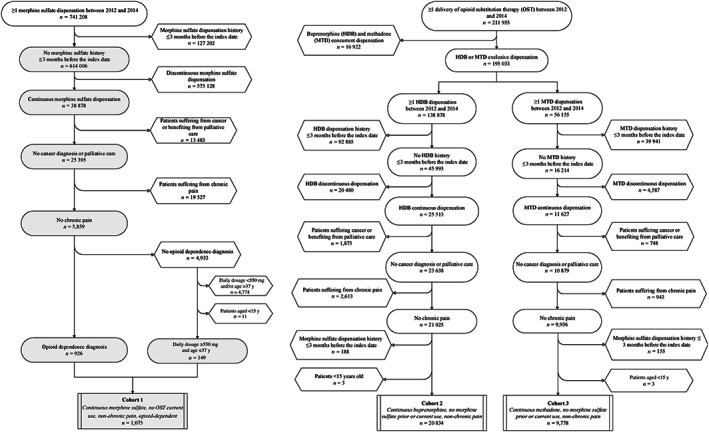
Flowchart of morphine sulfate, buprenorphine and methadone cohorts
TABLE 1.
Characteristics of patients included in the morphine sulfate and control cohorts
| Cohort 1 Continuous morphine sulfate, no OST current use, opioid‐dependent | Cohort 2 Continuous buprenorphine, no morphine sulfate prior or current use | Cohort 3 Continuous methadone, no morphine sulfate prior or current use | P‐value | |
|---|---|---|---|---|
| n = 1075 | n = 20 834 | n = 9778 | ||
| Age (y), | ||||
| Mean ± SD | 34.7 ± 8.7 | 34.5 ± 9.1 | 33.5 ± 8.2 | <.0001 |
| Sex, % (n) | ||||
| Male | 76.6 (823) | 79.0 (16 453) | 71.8 (7018) | <.0001 |
| Low‐income status, % (n) | 44.3 (476) | 26.7 (5556) | 24.2 (2361) | <.0001 |
| History of HIV, % (n) | 2.0 (21) | 0.5 (100) | 0.6 (58) | <.0001 |
| History of HBV, % (n) | 0.8 (9) | 0.2 (49) | 0.3 (25) | <.0001 |
| History of HCV, % (n) | 13.4 (144) | 3.5 (724) | 4.9 (479) | <.0001 |
| Mental health disorders, % (n) | 19.7 (212) | 9.0 (1873) | 9.6 (938) | <.0001 |
| Alcohol dependence, % (n) | 18.1 (194) | 9.4 (1958) | 7.0 (687) | <.0001 |
| Concurrent benzodiazepines prescription, % (n) | 22.1 (238) | 28.6 (5961) | 27.6 (2698) | <.0001 |
| Median daily dose, mg [IQR] | 553.0 [250.1–881.0] | 9.5 [5.4–16.6] | 46.7 [30.2–68.2] | |
| Median length of treatment, days [IQR] | 234.0 [142.0–436.0] | 259.0 [148.0–503.0] | 451.5 [233.0–787.0] | <.0001 |
HIV: human immunodeficiency virus; HBV: hepatitis B virus; HCV: hepatitis C virus; IQR: interquartile range; OST: opioid substitution therapy; SD: standard deviation.
4.1. Patient characteristics
The sociodemographic and health characteristics of the patients included in the 3 cohorts are shown in Table 1. No significant difference was observed for age (P = .5) and sex (P = .07) between MS and HDB patients. Compared to HDB and MTD patients, MS patients were more frequently low‐incomers (P < .01) and had more comorbidities (P < .01) consisting of mental health disorders, alcohol dependence, HIV, HCV, and hepatitis B virus complications, yet lower concurrent BZD prescriptions (P < .01).
4.2. Hospital admissions for unintentional opioid overdose (Figure 2)
FIGURE 2.
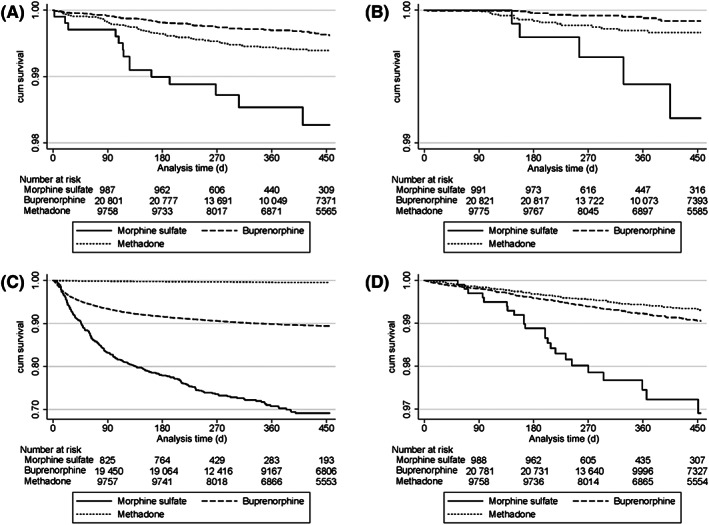
Kaplan–Meier survival curves for the primary (A, overdose related hospitalization) and statistically significant secondary outcomes (B, all‐cause mortality; C, doctor shopping behaviour; D, bacterial infection related hospitalization)
The overall crude opioid overdose incidence was 4.3 (95%CI: 2.5–7.2), 0.9 (95%CI: 0.7–1.1) and 1.4 (95%CI: 1.1–1.9) per 100 000 patient‐years (/100 000 p‐y) in the MS, HDB and MTD cohorts, respectively (Table 2). When adjusted, the 1‐year overdose risk in the cohort starting continuous MS was 3.8 higher compared to HDB (P < .01 [95%CI: 2.1–6.8]) and 2.0 vs MTD (P = .02 [95%CI: 1.1–3.6]).
TABLE 2.
Overall crude incidences per 100 000 patients‐years, by cohort
| Cohort 1 Continuous morphine sulfate, no OST current use, opioid‐dependent | Cohort 2 Continuous buprenorphine, no morphine sulfate prior or current use | Cohort 3 Continuous methadone, no morphine sulfate prior or current use | P‐value | ||||
|---|---|---|---|---|---|---|---|
| Incidence | 95% CI | Incidence | 95% CI | Incidence | 95% CI | ||
| Unintentional opioid overdose | 4.3 | [2.5–7.2] | 0.9 | [0.7–1.1] | 1.4 | [1.1–1.9] | <.0001 |
| All‐cause mortality | 1.5 | [0.6–3.6] | 0.2 | [0.1–0.3] | 0.4 | [0.2–0.7] | <.0001 |
| Doctor shopping behaviour | 106.0 | [94.2–119.4] | 31.1 | [29.8–32.4] | 1.2 | [0.9–1.6] | <.0001 |
| Hepatitis C virus diagnosis | 5.5 | [3.5–8.7] | 2.5 | [2.1–2.9] | 3.1 | [2.6–3.7] | .0015 |
| Bacterial infection | 7.0 | [4.7–10.6] | 2.2 | [1.8–2.5] | 1.6 | [1.2–2.0] | <.0001 |
| Thrombotic complication | 13.3 | [9.8–17.9] | 8.1 | [7.4–8.8] | 8.4 | [7.5–9.4] | .0068 |
OST: opioid substitution therapy; 95% CI: 95% confidence interval.
4.3. All‐cause mortality (Figure 2)
The overall crude all‐cause mortality incidence was 1.5 (95%CI: 0.6–3.6), 0.2 (95%CI: 0.1–0.3), and 0.4 (95%CI: 0.2–0.7)/100 000 p‐y in the MS, HDB and MTD cohorts, respectively (Table 2). When adjusted, the 1‐year all‐cause mortality risk in the cohort starting continuous MS was 9.1 greater compared to HDB (P < .01 [95%CI: 3.2–25.9]) and 3.9 vs MTD (P < .01 [95%CI: 1.4–10.7]).
4.4. DSB (Figure 2)
The overall crude DSB incidence was 106.0 (95%CI: 94.2 119.4), 31.1 (95%CI: 29.8–32.4) and 1.2 (95%CI: 0.9–1.6) /100 000 p‐y in MS, HDB and MTD cohorts, respectively (Table 2). When adjusted, the 1‐year DSB in the cohort starting continuous MS was 2.9 more frequent compared to HDB (P < .01 [95%CI: 2.6–3.3]) and 66.8 vs MTD (P < .01 [95%CI: 48.5–91.9]).
4.5. Complications arising from intravenous injection
The overall crude HCV seroconversion incidence (Supporting information Figure S2) was 5.5 (95%CI: 3.5 8.7), 2.5 (95%CI: 2.1–2.9) and 3.1 (95%CI: 2.6–3.7) /100 000 p‐y in MS, HDB and MTD cohorts, respectively (Table 2). When adjusted, the 1‐year HCV seroconversion risk in the cohort starting continuous MS was 1.6 greater compared to HDB (P = .06 [95%CI: 1.0–2.6]) and 1.1 vs MTD (P = .7 [95%CI: 0.7–1.8]).
The overall crude incidences of hospitalizations for bacterial infection (Figure 2) were 7.0 (95%CI: 4.7 10.6), 2.2 (95%CI: 1.8–2.5) and 1.6 (95%CI: 1.2–2.0) /100 000 p‐y in MS, HDB, and MTD cohorts, respectively (Table 2). When adjusted, 1‐year risk of hospitalizations for bacterial infection in cohort starting continuous MS was 2.8‐times greater compared to HDB (P < .01 [95%CI: 1.8–4.4]) and 3.6 vs MTD (P < .01 [95%CI: 2.2–5.9]).
The overall crude incidences of thrombotic‐related hospitalizations (Supporting information Figure S2) were 13.3 (95%CI: 9.8 17.9), 8.1 (95%CI: 7.4–8.8), and 8.4 (95%CI: 7.5–9.4) /100 000 p‐y in the MS, HDB, and MTD cohorts, respectively (Table 2). When adjusted, the 1‐year risk of thrombotic‐related hospitalizations in the cohort starting continuous MS was 1.4‐times greater compared to HDB (P = .06 [95%CI: 1.0–1.9]) and 1.3 vs MTD (P = .14 [95%CI: 0.9–1.8]).
4.6. Falsification analysis
When adjusted, the 1‐year asthma risk in the cohort starting continuous MS was 1.0 compared to HDB (P = .97 [95%CI: 0.3–3.3]) and 1.1 vs MTD (P = .83 [95%CI: 0.3–3.8]). The 1‐year primary hypertension risk in the cohort starting continuous MS was 1.6 greater compared to HDB (P = .32 [95%CI: 0.6–3.8]) and 1.3 vs MTD (P = .56 [95%CI: 0.5–3.3]).
5. DISCUSSION
This is the first comprehensive population‐based study to be conducted nationwide in France, which focuses on the opioid‐overdose incidence in OUD patients, in the year following regular off‐label MS use. Our results reveal an increased risk of overdosing that requires hospital care in this population compared to control patients who initiate conventional OST. This increased risk questions off‐label MS safety, because it does not allow for a dose reduction similar to that described in the literature for patients who initiate conventional OSTs. 43 , 44 This higher risk can be partly explained by the high prevalence of intravenous MS use, as well as common association with alcohol abuse disorders and concomitant BZD prescriptions, with well‐established risks reported in the literature. 45 , 46 Our results regarding BZD prescriptions are consistent with European data, 47 but we found a lower BZD prescription prevalence in the MS cohort compared to controls. This contrasts with the increased prevalence detected in the MS cohort of mental health and alcohol use disorders, for which BZDs are commonly employed. The psychotropic effect of MS has been demonstrated though an improvement in mood and anxiety, sleep quality and general well‐being scores revealed in studies evaluating MS as an OST alternative. 48 , 49 , 50 , 51 , 52 , 53 By contrast, the guidelines for OUD management recommend a multidisciplinary approach, integrating psychiatric comorbidities. Because the management of the latter commonly involves the prescription of BZD, this may contribute to the increased prevalence of BZD use observed in the control cohorts. 54 , 55
This study uncovered a significantly higher incidence of all‐cause mortality in MS patients compared to controls. The low mortality rate observed in the control cohorts should encourage offering OUD patients 1 of the 2 validated OSTs rather than MS as first‐line treatment, in order to reduce their risk of death. However, this low occurrence of overdosing and death during our study period should not encourage us to hastily conclude that France is not yet, or will never been, affected by this problem. The latest European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) report clearly indicates an increase in prescribed opioids being involved in overdose‐deaths, currently affecting northern European countries, in a young male population similar to that of our study. 56 In the EMCDDA report, the average age of the deceased patients was 39 years, therefore higher than that of our included patients, which may mean that the risk of death from a morphine overdose may appear later in the follow‐up, not in the year following initiation, unlike conventional OSTs where the risk is increased at the beginning of treatment, 57 especially for MTD.
Our results have revealed an increased DSB risk in the MS cohort compared to the controls, particularly affecting MTD‐maintained patients. This is to be accounted for, in France, by the very low DSB scores for MTD compared to buprenorphine, because of a more flexible prescription framework, rendering it more accessible despite its easier diversion in form of snorting or injections. 58 DSB reflects the user's need to obtain ever‐increasing doses of drugs in order to maintain their psychoactive effects and additional efficacy, in spite of the habituation that develops during regular substance exposure. It has been shown that a high DSB score correlates with an increased risk of death and overdose. 37 , 40 , 58 , 59 The high scores found in France could hint towards an increase in misuse of prescribed opioids, similar to that observed in the USA in the late 1990's, prior to the emergence of the current peak of overdoses and deaths. 39 , 60
The risk of bacterial infection was shown to be increased in the MS cohort compared to the controls. This result is in line with the persistent use of the intravenous route, as suspected in previous French field studies, for both recreational and substitutive MS. 12 , 17 The first‐line prescription of a conventional OST is likely to reduce the practice of intravenous injections, 61 , 62 , 63 , 64 without always allowing its total abandonment. This can be explained by the susceptibility to addictive behaviour, which is less accessible to drug therapy. 65 , 66 , 67 , 68 , 69 , 70 Our study demonstrated no significant between‐group difference in hepatitis C virus or thrombotic events reported in the 3 groups. This nonsignificant difference is to be balanced by the observation that our study analyses are based on the diagnostic codes assigned at the end of hospitalization. For these 2 risks, complications that were serious enough to lead to hospitalization as recorded in the database might have occurred later in the course of the disease and not necessarily in the year following the beginning of management. In any case, associated infectious comorbidities must be detected and promptly treated throughout the follow‐up.
These results are instrumental in defining lines for both improving practices and reducing risks. To reduce overdoses and deaths, assessing these risks according to each user's practices appears essential, as well as improving the information provided to the patients and their families as for the clinical signs to be searched for and rescue action to be taken. These measures require the widespread prescription of naloxone rescue kits, as well as the training of users and persons likely to be exposed to overdoses to reduce life‐threatening risks pending assistance. 71 , 72 , 73 In addition, referring these complex patients to specialized addictology centres rather than primary care facilities would provide them with the opportunity to benefit from a supervised and fractioned treatment dispensation, along with regular global and multidisciplinary follow‐up, comprising medical, psychological, educational therapy workshops, and social support, on account of their significant precariousness. 72 , 73 , 74
The systematic use of a prescription monitoring programme, such as those used in the USA, would enable us to follow the treatment pathway in an effort to reduce medical nomadism and overlapping prescriptions. 75 , 76 , 77 , 78 The protocolization of care with the appointment of a referring prescribing physician, in agreement with the social security medical officer, would in addition reinforce the fight against medical nomadism.
As for preventing intravenous injections, the use of abuse‐deterrent formulations, thus limiting misuse, would help control the administration route of the prescribed drug. 79 , 80 , 81 However, intravenous injections can represent a behavioural addiction that is difficult to overcome for some patients. Therefore, stopping it abruptly cannot be imposed due to the risk of seeing them return to heroin. 82 , 83 To maintain these patients in care, it should be possible to offer them a transitional stage, namely the prescription of a galenic formulation adapted to the intravenous route, which they could administer under medical supervision in a lower‐risk consumption room, with dispensation of free sterile injection kits.
For the interpretation of these results, the limitations and strengths of the study design must be considered. Concerning the general methodology, a conventional observational study with field recruitment would have been able to only include a small proportion of relevant patients, particularly in a small and marginalized population, such as that of our study. Given this case scenario, the risk would then be to create unrepresentative cohorts, leading to erroneous results. The SNDS renders it possible to achieve greater exhaustiveness compared to field studies, although with certain limitations. One of the main study limitations is the inherent nature of the data collected corresponding to reimbursement figures. With such a design, it is possible to only include patients benefiting from pharmacies dispensing without recruiting those who illegally buy the drugs on the black market from drug dealers, potentially being the most marginalized. Additionally, the SNDS includes inpatient data. Thus, information on overdosing managed by emergency medical assistance services without hospitalization (i.e. a stay of <24 hours in hospital) was not considered. Regarding mortality, as the database does not contain the cause of death, it was therefore impossible to precisely know whether death was directly related to the substance or its use. Nevertheless, these limitations are only associated with an underestimation of the cohorts' sizes and risks, to which MS users are likely to be exposed.
The strengths of the study stem from its ability to recruit large numbers of people so as to achieve near comprehensiveness nationwide. These large cohorts increase the reliability of the results obtained, as compared to previously published French field studies. These previous studies do attest to the good validity of our algorithm, enabling us to recruit comparable patients in terms of age, sex‐ratio and daily dosage, despite the restrictive selection criteria and unavailability of data from the database for maximally 10% of patients who directly benefit from their OST dispensation in an addictology centre. 12 , 17 In OST cohorts, age, sex‐ratio and daily dosage were consistent with a recent French pharmaco‐epidemiological study 84 and quite comparable among the 3 OUD cohorts. The falsification analyses conducted reinforce the reliability of the results obtained, showing that the differences observed between cohorts are not the result of selection bias. The cumulative high doses found in the MS cohort were comparable to those retrieved in previous clinical studies evaluating MS as maintenance therapy vs MTD. 50 , 52 , 85 , 86 , 87
6. CONCLUSION
The results of this first national pharmaco‐epidemiological study demonstrate an increased risk of overdose, death, DSB, and bacterial infections within the year following the off‐label MS initiation in OUD patients.
The increased risks revealed here, as well as the global context of overdosing and deaths linked to prescribed opioids, must attract prescriber vigilance and remind us that MS off‐label use should be a third‐line treatment, administered only after failure of or major intolerance to buprenorphine and MTD. To limit complications and misuse, prescribing should be performed within a protocolized, multidisciplinary and supervised management setting in specialized addictology centres rather than in primary care services. Finally, these results raise the question as to whether we should propose an adapted injectable substitution, which is safer than a diverted oral galenic formulation, to be self‐administered by the user under medical supervision, in lower‐risk consumption rooms.
ACKNOWLEDGEMENTS
This study was part of the POMA project (Prescription Opioids Misuse Assessment in chronic pain patients), funded by the French National Agency for Medicines and Health Products Safety (ANSM: Agence Nationale de Sécurité du Médicament et des Produits de Santé – Grant number 20145013). The financial sponsor of this work played no role in the design and conduct of the study, or in the data collection, management, analysis, or interpretation. The sponsor had no role in the preparation or review of the manuscript or in the decision to submit.
COMPETING INTERESTS
G.B. received sponsorship to attend scientific meetings and speaker honoraria from Indivior. The other authors have no competing interests to declare.
CONTRIBUTORS
Conceptualization and methodology: C.B., J.D., M.R., C.C., N.A. Software and data extraction: C.B., J.D. Formal analysis: C.B., M.R. Validation and supervision: G.B., C.C., N.A. Writing of manuscript: C.B. Revision of manuscript: C.B., J.D., M.R., H.P., G.B., C.C., N.A. Project administration and funding acquisition: A.E., D.A., N.A.
Supporting information
FIGURE S1 Study diagram for standard morphine sulfate patients
FIGURE S2 Kaplan–Meier survival curves for nonstatistically significant secondary outcomes (a: hepatitis C virus diagnosis; b: hospitalizations for thrombotic complication)
TABLE S1 International classification of diseases (10th revision) selection codes
Bertin C, Delorme J, Riquelme M, et al. Risk assessment of using off‐label morphine sulfate in a population‐based retrospective cohort of opioid‐dependent patients. Br J Clin Pharmacol. 2020;86:2338–2348. 10.1111/bcp.14082
The authors confirm that the PI for this paper is Prof. Authier Nicolas, and that he had direct clinical responsibility for patients and databases.
REFERENCES
- 1. Fareed A, Stout S, Casarella J, Vayalapalli S, Cox J, Drexler K. Illicit opioid intoxication: diagnosis and treatment. Subst Abuse Res Treat. 2011;5:17‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Novak SP, Håkansson A, Martinez‐Raga J, Reimer J, Krotki K, Varughese S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry. 2016;16(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giraudon I, Lowitz K, Dargan PI, Wood DM, Dart RC. Prescription opioid abuse in the UK. Br J Clin Pharmacol. 2013;76(5):823‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roxburgh A, Hall WD, Dobbins T, et al. Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug Alcohol Depend. 2017;179:291‐298. [DOI] [PubMed] [Google Scholar]
- 5. Martins SS, Sampson L, Cerdá M, Galea S. Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. Am J Public Health. 2015;105(11):e29‐e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618‐627. [DOI] [PubMed] [Google Scholar]
- 7. Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999‐2006. NCHS Data Brief. 2009;22:1‐8. [PubMed] [Google Scholar]
- 8. Lancet T. The opioid crisis in the USA: a public health emergency. The Lancet. 2017;390(10107):2016. [DOI] [PubMed] [Google Scholar]
- 9. Martins SS, Ghandour LA. Nonmedical use of prescription drugs in adolescents and young adults: not just a Western phenomenon. World Psychiatry. 2017;16(1):102‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jegu J, Gallini A, Soler P, Montastruc J‐L, Lapeyre‐Mestre M. Slow‐release oral morphine for opioid maintenance treatment: a systematic review. Br J Clin Pharmacol. 2011;71(6):832‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cadet‐Taïrou A, Gandilhon M, Toufik A, Evrard I. Phénomènes émergents liés aux drogues en 2005 ‐ Septième rapport national du dispositif TREND. OFDT [Internet]. Saint‐Denis: OFDT; 2007 Jan [cited 2016 Dec 10]. Available from: http://www.ofdt.fr/publications/collections/rapports/rapports‐d‐etudes/rapports‐detudes‐ofdt‐parus‐en‐2007/phenomenes‐emergents‐lies‐aux‐drogues‐en‐2005‐septieme‐rapport‐national‐du‐dispositif‐trend‐janvier‐2007/ (Archived at www.webcitation.org/6w6KEplam)
- 12. Peyriere H, Eiden C, Micallef J, Lapeyre‐Mestre M, Faillie J‐L, Blayac J‐P. Slow‐release oral morphine sulfate abuse: results of the postmarketing surveillance systems for psychoactive prescription drug abuse in France. Eur Addict Res. 2013;19(5):235‐244. [DOI] [PubMed] [Google Scholar]
- 13. Lahaie E, Cadet‐Taïrou A. Héroïne ‐ composition, prix, connaissances des usagers. mai 2014 ‐ OFDT [Internet]. Saint Denis: OFDT; 2014 May [cited 2016 Dec 11]. Available from: http://www.ofdt.fr/publications/collections/rapports/rapports‐d‐etudes/rapports‐detudes‐ofdt‐parus‐en‐2014/heroine‐composition‐prix‐connaissances‐des‐usagers‐mai‐2014/ (Archived at http://www.webcitation.org/6w6KPLpG0)
- 14. Cadet‐Taïrou A, Gandilhon M. Morphine sulphate consumption by French drug users: Recent trends (2012–2013) [Internet]. Saint Denis: OFDT; 2014 Jul [cited 2018 Aug 14]. Available from: https://bdoc.ofdt.fr/doc_num.php?explnum_id=20011 (Archived at http://www.webcitation.org/71iPBwMWQ)
- 15. Ferri M, Minozzi S, Bo A, Amato L. Slow‐release oral morphine as maintenance therapy for opioid dependence. Cochrane Database Syst Rev. 2013;6:CD009879. [DOI] [PubMed] [Google Scholar]
- 16. Nordmann S, Pradel V, Lapeyre‐Mestre M, et al. Doctor shopping reveals geographical variations in opioid abuse. Pain Physician. 2013;16(1):89‐100. [PubMed] [Google Scholar]
- 17. Peyriere H, Nogue E, Eiden C, et al. Evidence of slow‐release morphine sulfate abuse and diversion: epidemiological approaches in a French administrative area. Fundam Clin Pharmacol. 2016;30(5):466‐475. [DOI] [PubMed] [Google Scholar]
- 18. Avril E. Premier bilan de l'ouverture de la première salle de consommation à moindre risque [Internet]. 2017. [cited 2017 Feb 13]. Available from: http://www.addictoscope.fr/cgi‐bin/pb.pl?x=fa36d1f5b9c9d763fe979eb648a520d358593702ae36cd2e9509087f08c2869fda57fa684f58e67d0bc6336b5c6f3fdc48c05ddefc62e1f10de661a0b83e7c3f8d9c1f539d7e566f626ab18047fbc4cfce44dee781fac188b0f299c38950e84a008cbc44f067d194eb250dc1f1318050a78380152adfaaa30593ef3e5d9704e9
- 19. Cadet‐Taïrou A, Gandilhon M, Lahaie E, Chalumeau M, Coquelin A. Drogues et usages de drogues en France ‐ État des lieux et tendances récentes 2007–2009 [Internet]. OFDT; 2010. Jan [cited 2016 Dec 10]. Available from: http://www.ofdt.fr/BDD/publications/docs/epfxacq1.pdf (Archived at http://www.webcitation.org/6wOIOYGOL)
- 20. Schléret Y, Olivier R, Balteau S, Bailly F, Monzel M. Rapport de site Trend‐Metz (Lorraine), 2008. [Internet]. ORSAS Lorraine; 2009 Jun [cited 2016 Dec 11]. Available from: http://www.ofdt.fr/ofdt/fr/trend/metz08.pdf (Archived at http://www.webcitation.org/6w6LHvcrX)
- 21. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely‐collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Des Jarlais DC, Lyles C, Crepaz N. TREND group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholls SG, Quach P, von Elm E, et al. The REporting of studies conducted using observational routinely‐collected health data (RECORD) statement: methods for arriving at consensus and developing reporting guidelines. PLOS One. 2015;10(5):e0125620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954‐962. [DOI] [PubMed] [Google Scholar]
- 25. Brisacier A‐C, Collin C. Les traitements de substitution aux opiacés en France: données récentes [Internet]. OFDT; 2014 Oct [cited 2018 Aug 14] p. 6. Report No.: 94. Available from: https://www.ofdt.fr/BDD/publications/docs/eftxabua.pdf (Archived at http://www.webcitation.org/71iP4y9Bf)
- 26. Moisset X, Trouvin A‐P, Tran V‐T, et al. Utilisation des opioïdes forts dans la douleur chronique non cancéreuse chez l'adulte. Recommandations françaises de bonne pratique clinique par consensus formalisé (SFETD). Douleurs Eval Diagn Trait. 2016;17(3):145‐160. [DOI] [PubMed] [Google Scholar]
- 27. Dally S, Thomas G, Danan M. Candidiasis in heroin abusers. Br Med J Clin Res Ed. 1983;287(6404):1549. [PMC free article] [PubMed] [Google Scholar]
- 28. Tunkel AR, Pradhan SK. Central nervous system infections in injection drug users. Infect Dis Clin. 2002;16(3):589‐605. [DOI] [PubMed] [Google Scholar]
- 29. Brettle RP. Infection and injection drug use. J Infect. 1992;25(2):121‐131. [DOI] [PubMed] [Google Scholar]
- 30. Cornford CS, Mason JM, Inns F. Deep vein thromboses in users of opioid drugs: incidence, prevalence, and risk factors. Br J Gen Pract. 2011;61(593):e781‐e786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Del Giudice P. Cutaneous complications of intravenous drug abuse. Br J Dermatol. 2004;150(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 32. Kwiatkowska W, Knysz B, Gąsiorowski J, Witkiewicz W. Deep vein thrombosis of the lower limbs in intravenous drug users. Postepy Hig Med Doswiadczalnej Online. 2015;69:510‐520. [DOI] [PubMed] [Google Scholar]
- 33. Rolland B, Paille F, Gillet C, et al. Pharmacotherapy for alcohol dependence: the 2015 recommendations of the French alcohol society, issued in partnership with the European Federation of Addiction Societies. CNS Neurosci Ther. 2016;22(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sansone RA, Sansone LA. Doctor shopping. Innov Clin Neurosci. 2012;9(11–12):42‐46. [PMC free article] [PubMed] [Google Scholar]
- 35. Cepeda MS, Fife D, Berwaerts J, Friedman A, Yuan Y, Mastrogiovanni G. Doctor shopping for medications used in the treatment of attention deficit hyperactivity disorder: shoppers often pay in cash and cross state lines. Am J Drug Alcohol Abuse. 2015;41(3):226‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chenaf C, Kabore J‐L, Delorme J, et al. Codeine shopping behavior in a retrospective cohort of chronic noncancer pain patients: incidence and risk factors. J Pain. 2016;17(12):1291‐1301. [DOI] [PubMed] [Google Scholar]
- 37. Peirce GL, Smith MJ, Abate MA, Halverson J. Doctor and pharmacy shopping for controlled substances. Med Care. 2012;50(6):494‐500. [DOI] [PubMed] [Google Scholar]
- 38. Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Assessing opioid shopping behaviour: a large cohort study from a medication dispensing database in the US. Drug Saf. 2012;35(4):325‐334. [DOI] [PubMed] [Google Scholar]
- 39. McDonald DC, Carlson KE. Estimating the prevalence of opioid diversion by “doctor shoppers” in the United States. PLoS ONE. 2013;8(7):e69241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Z, Wilsey B, Bohm M, et al. Defining risk of prescription opioid overdose: pharmacy shopping and overlapping prescriptions among long‐term opioid users in medicaid. J Pain. 2015;16(5):445‐453. [DOI] [PubMed] [Google Scholar]
- 41. Cepeda MS, Fife D, Vo L, Mastrogiovanni G, Yuan Y. Comparison of opioid doctor shopping for Tapentadol and oxycodone: a cohort study. J Pain. 2013;14(2):158‐164. [DOI] [PubMed] [Google Scholar]
- 42. Cepeda MS, Fife D, Chow W, Mastrogiovanni G, Henderson SC. Opioid shopping behavior: how often, how soon, which drugs, and what payment method. J Clin Pharmacol. 2013;53(1):112‐117. [DOI] [PubMed] [Google Scholar]
- 43. Clausen T, Waal H, Thoresen M, Gossop M. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction. 2009;104(8):1356‐1362. [DOI] [PubMed] [Google Scholar]
- 44. Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross‐registry study. Drug Alcohol Depend. 2008;94(1–3):151‐157. [DOI] [PubMed] [Google Scholar]
- 45. Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125(1–2):8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lavie E, Fatséas M, Denis C, Auriacombe M. Benzodiazepine use among opiate‐dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug Alcohol Depend. 2009;99(1–3):338‐344. [DOI] [PubMed] [Google Scholar]
- 48. Eder H, Jagsch R, Kraigher D, Primorac A, Ebner N, Fischer G. Comparative study of the effectiveness of slow‐release morphine and methadone for opioid maintenance therapy. Addiction. 2005;100(8):1101‐1109. [DOI] [PubMed] [Google Scholar]
- 49. Vasilev GN, Alexieva DZ, Pavlova RZ. Safety and efficacy of Oral slow release morphine for maintenance treatment in heroin addicts: a 6‐month open noncomparative study. Eur Addict Res. 2006;12(2):53‐60. [DOI] [PubMed] [Google Scholar]
- 50. Kastelic A, Dubajic G, Strbad E. Slow‐release oral morphine for maintenance treatment of opioid addicts intolerant to methadone or with inadequate withdrawal suppression. Addiction. 2008;103(11):1837‐1846. [DOI] [PubMed] [Google Scholar]
- 51. Bond AJ, Reed KD, Beavan P, Strang J. After the randomised injectable opiate treatment trial: post‐trial investigation of slow‐release oral morphine as an alternative opiate maintenance medication. Drug Alcohol Rev. 2012;31(4):492‐498. [DOI] [PubMed] [Google Scholar]
- 52. Mitchell TB, White JM, Somogyi AA, Bochner F. Slow‐release oral morphine vs methadone: a crossover comparison of patient outcomes and acceptability as maintenance pharmacotherapies for opioid dependence. Addiction. 2004;99(8):940‐945. [DOI] [PubMed] [Google Scholar]
- 53. Winklbaur B, Jagsch R, Ebner N, Thau K, Fischer G. Quality of life in patients receiving opioid maintenance therapy. A comparative study of slow‐release morphine vs methadone treatment. Eur Addict Res. 2008;14(2):99‐105. [DOI] [PubMed] [Google Scholar]
- 54. Nicholls L, Bragaw L, Ruetsch C. Opioid dependence treatment and guidelines. J Manag Care Pharm. 2010;16(1):14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. The Lancet. 1999;353(9148):221‐226. [DOI] [PubMed] [Google Scholar]
- 56. European Drug Report 2018: Trends and Developments [Internet]. Lisbon: EMCDDA; 2018 Jun p. 96. (EMCDDA, editor. European Drug Report). Available from: http://www.emcdda.europa.eu/publications/edr/trends‐developments/2018 (Archived at http://www.webcitation.org/77Ct6nNrs)
- 57. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta‐analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Delorme J, Chenaf C, Kabore J‐L, et al. Incidence of high dosage buprenorphine and methadone shopping behavior in a retrospective cohort of opioid‐maintained patients in France. Drug Alcohol Depend. 2016;162:99‐106. [DOI] [PubMed] [Google Scholar]
- 59. King NB, Fraser V, Boikos C, Richardson R, Harper S. Determinants of increased opioid‐related mortality in the United States and Canada, 1990–2013: a systematic review. Am J Public Health. 2014;104(8):e32‐e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Han H, Kass PH, Wilsey BL, Li C‐S. Increasing trends in schedule II opioid use and doctor shopping during 1999–2007 in California. Pharmacoepidemiol Drug Saf. 2014;23(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strathdee SA, Stockman JK. Epidemiology of HIV among injecting and non‐injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep. 2010;7(2):99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. The Lancet. 2008;372(9651):1733‐1745. [DOI] [PubMed] [Google Scholar]
- 63. Nelson P, Mathers B, Cowie B, et al. The epidemiology of viral hepatitis among people who inject drugs: results of global systematic reviews. Lancet. 2011;378(9791):571‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hickman M, Martin NK, European Monitoring Centre for Drugs and Drug Addiction . Hepatitis C among drug users in Europe: epidemiology, treatment and prevention. Luxembourg: Publications Office; 2016 [cited 2017 May 27]. Available from: www.emcdda.europa.eu/system/files/publications/2953/TDXD16002ENN_final_web.pdf (Archived at http://www.webcitation.org/6w6N0BZs0)
- 65. Winstock AR, Lea T, Sheridan J. Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. Int J Drug Policy. 2008;19(6):450‐458. [DOI] [PubMed] [Google Scholar]
- 66. Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addict Abingdon Engl. 2005;100(2):197‐205. [DOI] [PubMed] [Google Scholar]
- 67. Guichard A, Lert F, Calderon C, et al. Illicit drug use and injection practices among drug users on methadone and buprenorphine maintenance treatment in France. Addiction. 2003;98(11):1585‐1597. [DOI] [PubMed] [Google Scholar]
- 68. Vidal‐Trecan G, Varescon I, Nabet N, Boissonnas A. Intravenous use of prescribed sublingual buprenorphine tablets by drug users receiving maintenance therapy in France. Drug Alcohol Depend. 2003;69(2):175‐181. [DOI] [PubMed] [Google Scholar]
- 69. Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti JP. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96(2):267‐272. [DOI] [PubMed] [Google Scholar]
- 70. Moratti E, Kashanpour H, Lombardelli T, Maisto M. Intravenous misuse of buprenorphine: characteristics and extent among patients undergoing drug maintenance therapy. Clin Drug Investig. 2010;30(Suppl 1):3‐11. [DOI] [PubMed] [Google Scholar]
- 71. Strang J, Powis B, Best D, et al. Preventing opiate overdose fatalities with take‐home naloxone: pre‐launch study of possible impact and acceptability. Addiction. 1999;94(2):199‐204. [DOI] [PubMed] [Google Scholar]
- 72. Heavey SC, Burstein G, Moore C, Homish GG. Overdose education and naloxone distribution program attendees: who attends, what do they know, and how do they feel? J Public Health Manag Pract JPHMP. 2018;24(1):63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013; 346: f174. 10.1136/bmj.f174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huhn AS, Garcia‐Romeu AP, Dunn KE. Opioid overdose education for individuals prescribed opioids for pain management: randomized comparison of two computer‐based interventions. Front Psych. 2018;9:34 10.3389/fpsyt.2018.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Davis CS, Pierce M, Dasgupta N. Evolution and convergence of state Laws governing controlled substance prescription monitoring programs, 1998‐2011. Am J Public Health. 2014;104(8):1389‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff Proj Hope. 2016;35(6):1045‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Katz N, Panas L, Kim M, et al. Usefulness of prescription monitoring programs for surveillance—analysis of schedule II opioid prescription data in Massachusetts, 1996–2006. Pharmacoepidemiol Drug Saf. 2010;19(2):115‐123. [DOI] [PubMed] [Google Scholar]
- 78. Fink DS, Schleimer JP, Sarvet A, et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: a systematic review. Ann Intern Med. 2018;168(11):783‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hale ME, Moe D, Bond M, Gasior M, Malamut R. Abuse‐deterrent formulations of prescription opioid analgesics in the management of chronic noncancer pain. Pain Manag. 2016;6(5):497‐508. [DOI] [PubMed] [Google Scholar]
- 80. Moorman‐Li R, Motycka CA, Inge LD, Congdon JM, Hobson S, Pokropski B. A review of abuse‐deterrent opioids for chronic nonmalignant pain. Pharm Ther. 2012;37(7):412‐418. [PMC free article] [PubMed] [Google Scholar]
- 81. Katz NP, Adams EH, Chilcoat H, et al. Challenges in the development of prescription opioid abuse‐deterrent formulations. Clin J Pain. 2007;23(8):648‐660. [DOI] [PubMed] [Google Scholar]
- 82. Simon K, Worthy SL, Barnes MC, Tarbell B. Abuse‐deterrent formulations: transitioning the pharmaceutical market to improve public health and safety. Ther Adv Drug Saf. 2015;6(2):67‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cicero TJ, Ellis MS, Surratt HL. Effect of abuse‐deterrent formulation of OxyContin. N Engl J Med. 2012;367(2):187‐189. [DOI] [PubMed] [Google Scholar]
- 84. Kernisant M, Delorme J, Kabore J‐L, et al. Trend in buprenorphine and methadone shopping behavior in France from 2004 to 2014. Presse Med (Paris, France: 1983). 2016;45(12 Pt 1):e369-e375. [DOI] [PubMed] [Google Scholar]
- 85. Mitchell TB, White JM, Somogyi AA, Bochner F. Comparative pharmacodynamics and pharmacokinetics of methadone and slow‐release oral morphine for maintenance treatment of opioid dependence. Drug Alcohol Depend. 2003;72(1):85‐94. [DOI] [PubMed] [Google Scholar]
- 86. Beck T, Haasen C, Verthein U, et al. Maintenance treatment for opioid dependence with slow‐release oral morphine: a randomized cross‐over, non‐inferiority study vs methadone. Addiction. 2014;109(4):617‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hämmig R, Köhler W, Bonorden‐Kleij K, et al. Safety and tolerability of slow‐release oral morphine vs methadone in the treatment of opioid dependence. J Subst Abuse Treat. 2014;47(4):275‐281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Study diagram for standard morphine sulfate patients
FIGURE S2 Kaplan–Meier survival curves for nonstatistically significant secondary outcomes (a: hepatitis C virus diagnosis; b: hospitalizations for thrombotic complication)
TABLE S1 International classification of diseases (10th revision) selection codes
Data Availability Statement
Study approval was obtained from the French National Institute for Health Data (INDS, No. 176) and French Data Protection Agency (CNIL, No. 1946535), which prohibit the data from being disclosed. As a result, research data are not shared by the authors but can be requested from the INDS (website: https://www.indsante.fr). The programming code can be made available in raw form on request to the corresponding author.


