Abstract
Aims
To investigate the decline of estimated glomerular filtration rate (eGFR) in patients with atrial fibrillation (AF) treated with vitamin K antagonists (VKAs) or non‐VKA oral anticoagulants (NOACs).
Methods
Multicentre prospective cohort study including 1667 patients with nonvalvular AF. The eGFR was assessed by the CKD‐EPI formula at baseline and during follow‐up. The primary endpoint of the study was the median annual decline of eGFR according to VKA (n = 743) or NOAC (n = 924) use. As secondary endpoints, we analysed the transition to eGFR <50 mL/min/1.73 m2 and the eGFR class worsening.
Results
Median age was 73.7 ± 9.1 years and 43.3% were women. VKA‐treated patients showed an eGFR decline of −2.11 (interquartile range [IQR] –5.68/−0.62), which was −0.27 (IQR –9.00/4.54, P < 0.001 vs VKAs), −1.21 (IQR –9.98/4.02, P = 0.004 vs VKAs) and −1.32 (IQR –8.70/3.99, P = 0.003 vs VKAs) in patients on dabigatran, rivaroxaban and apixaban, respectively.
Transition to eGFR <50 mL/min/1.73 m2 was lower in dabigatran‐ and apixaban‐treated patients: odds ratio (OR) 0.492, 95% confidence interval (CI) 0.298‐0.813, P = 0.006 and OR 0.449, 95% CI 0.276‐0.728, P = 0.001, respectively. A lower rate of eGFR class worsening was found in all groups of NOACs compared to VKAs. No difference between full and reduced dose of NOAC was found. Subgroup analysis showed that the association between NOAC and eGFR changes was markedly reduced in diabetic patients.
Conclusion
Patients prescribed NOACs showed a lower decline of renal function compared to those prescribed VKAs. This effect was partially lost in patients with diabetes.
Keywords: atrial fibrillation, warfarin, direct oral anticoagulants, anticoagulation treatment, renal failure
What is already known about this subject
Patients with atrial fibrillation (AF) have a decline of renal function over time.
Use of vitamin K antagonists (VKAs) has been associated to an accelerated decline of estimated glomerular filtration rate (eGFR).
What this study adds
AF patients on non–vitamin K antagonist oral anticoagulants (NOACs) showed a lower median decline of eGFR compared to those on VKAs.
Dabigatran and Apixaban were associated with a lower transition to eGFR <50 mL/min/1.73 m2 compared to VKAs.
1. INTRODUCTION
Atrial fibrillation (AF) and chronic kidney disease (CKD) are two highly prevalent and frequently coexisting diseases in elderly patients. 1 , 2 An analysis from the EURObservational Research Programme Atrial Fibrillation (EORP‐AF) Pilot Registry estimated that 47.2% of patients with AF have mild (estimated glomerular filtration rate [eGFR] <80 mL/min) and 17% moderate (eGFR <50 mL/min) CKD. 3
The presence of CKD in patients with AF has therapeutic and prognostic value, as CKD is associated with a lower quality of anticoagulation therapy with vitamin K antagonists (VKAs) 4 , 5 and increases the risk of both ischaemic and haemorrhagic complications. 6
In AF patients with CKD, the nonvitamin K antagonist oral anticoagulants (NOACs) are effective and safer than VKAs. 7 , 8 , 9 In particular, a recent metanalysis showed a lower risk of ischaemic stroke/systemic embolism in patients on NOACs as compared to those on VKAs among patients with mild (risk ratio [RR] 0.79, 95% confidence interval [CI] 0.68‐0.91) and moderate (RR 0.80, 95% CI 0.69‐0.92) CKD. 7 NOACs were also associated with lower major bleeding events among patients with mild (RR 0.86, 95% CI 0.77‐0.95) and moderate (RR 0.73, 95% CI 0.65‐0.82) CKD. 7
A clinical issue is represented by the modification of kidney function over time, thus a physiologic decline in eGFR of −0.55 and −0.33 mL/min/1.73 m2/year in males and females, respectively, has been described in the general population. 10 Concerning the AF population, previous studies showed that AF patients on treatment with VKAs have a significant risk of kidney function slope, 11 , 12 with a median annual decline in eGFR of −2.0 mL/min/1.73 m2 and 20‐30% of patients experiencing a rapid decline in renal function (loss >5 mL/min/year of eGFR). 11 , 12 Thus, an anticoagulant‐related nephropathy has been described for VKAs, especially for excessive anticoagulation (ie, International Normalized Ratio (INR) > 3.0), probably due to glomerular haemorrhages causing tubular lesions. 13 , 14
Recent studies have suggested that treatment with NOACs may favourably affect the modification of renal function in patients with AF, but this issue has been investigated essentially in the setting of randomized controlled clinical trials, 15 , 16 in which the characteristics of patients may not fully reflect those of patients managed in daily clinical practice. Prospective real‐world studies on this specific topic are lacking.
To investigate this issue, we performed a prospective multicentre cohort study including a cohort of AF outpatients recruited from internal medicine and cardiology units treated with VKAs or NOACs, in whom variations of renal function were evaluated at baseline during follow‐up.
2. METHODS
An observational prospective multicentre study including AF outpatients recruited from five Italian centres of Internal Medicine and Cardiology: (a) I Clinica Medica, Atherothrombosis Center, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome; (b) Department of Cardiovascular, Respiratory, Nephrologic, Anesthesiologic and Geriatric Sciences, Division of Gerontology, Sapienza University of Rome; (c) Department of Medical and Surgical Sciences, University Magna Græcia of Catanzaro, (d) Cardiology and Cardiothoracic Surgery Department, San Raffaele University Hospital, Milan; and (e) Department of Experimental and Clinical Medicine, University of Florence.
All patients with nonvalvular AF aged >18 years who provided their consent and with a follow‐up of at least 1 year were included in the study. Exclusion criteria were the presence of valvular diseases (mechanical prosthetic heart valves or moderate‐severe mitral stenosis), chronic infectious diseases (ie, human immunodeficiency virus infection, hepatitis C virus, hepatitis B virus) or autoimmune systemic diseases. Subjects were also excluded from the study if they had active cancer or liver failure (eg, cirrhosis). At baseline, anthropometric data as well as comorbidities and concomitant therapies were collected. The presence of cardiovascular risk factors was defined as previously described. 17
At baseline, serum creatinine (mg/dL) was obtained for all patients. The estimated glomerular filtration rate (eGFR) was calculated using the CKD‐EPI formula. 18 Patients were classified in eGFR categories according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines 19 : normal eGFR (>90 mL/min/1.73 m2, Stage G1), mildly decreased eGFR (89‐60 mL/min/1.73 m2, Stage G2), moderately decreased eGFR (59‐30 mL/min/1.73 m2, Stage G3), and severely decreased eGFR (<30 mL/min/1.73 m2, Stage G4). A second serum creatinine was collected during follow‐up at a median of 1.1 (interquartile range [IQR] 1.0‐2.5, min. 6 months, max. 10 years) years.
2.1. Study endpoints
The primary endpoint of the study was the difference in the median annual decline of eGFR between VKAs and NOAC (dabigatran, rivaroxaban and apixaban) treated patients. As secondary endpoints we analysed the transition to eGFR <50 mL/min/1.73 m2 and the eGFR class worsening according to KDIGO 2012 classification.
The local ethical boards approved the study protocol. The study was conducted according to the principles embodied in the Declaration of Helsinki. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
2.2. Statistical analysis
Categorical variables are reported as counts (percentage). Continuous variables are expressed as mean ± standard deviation (SD) or median and IQR (25th‐75th percentile), as appropriate. Two‐sided t‐tests or the Wilcoxon rank sum test, depending on the shape of the distribution curve, were used to compare means. The Pearson chi‐square test was used to compare proportions. Bivariate analysis was performed with Pearson's linear correlation. Appropriate nonparametric tests (Mann‐Whitney U test and Spearman rank correlation test) were employed for all the other variables. Comparisons among groups were performed with ANOVA or Kruskal Wallis tests.
We calculated the annual absolute decline of the eGFR expressed as the difference between the two eGFR values (follow‐up eGFR – basal eGFR/years of follow‐up). For patients with a second creatinine collected after 1 year, we assumed a linear extrapolation for eGFR decline, as we excluded patients with acute kidney failure.
We performed a multivariable logistic regression analysis to calculate the adjusted odds ratios (ORs) of factors associated with the annual decline of kidney function (below median).
The threshold value of 50 mL/min/1.73 m2 was chosen as an endpoint given that it represents one criterion for dose reduction of NOACs.
The multivariate analysis was determined, including all variables that could potentially affect renal function: pattern of AF (persistent/permanent vs paroxysmal AF), age ≥75 years, female sex, arterial hypertension, diabetes mellitus, history of myocardial infarction/coronary heart disease, heart failure, history of stroke/transient ischemic attack, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, β blockers, calcium channel antagonists, statins, antiplatelets.
Subgroup analysis by NOAC dose and the presence of diabetes was performed.
All tests were two‐tailed and analyses were performed using computer software packages (SPSS‐25.0, SPSS Inc.). Only P values <0.050 were considered as statistically significant.
3. RESULTS
3.1. Baseline characteristics
The study cohort was composed of 1667 AF patients. Of these, 743 were on VKAs (590 on warfarin and 153 on acenocumarol) and 924 were on NOACs. Of these, 280 were on dabigatran, 299 on rivaroxaban and 345 on apixaban (n = 345).
The median age of patients was 73.7 ± 9.1 years and 43.3% were women (Table 1). Patients treated with dabigatran (P = 0.041), rivaroxaban (P < 0.001) and apixaban (P < 0.001) were older than those treated with VKAs (Table 1). No difference was found regarding arterial hypertension or anti‐hypertensive drug use among groups. Patients on dabigatran were less likely to use statins, and antiplatelet drugs were more frequently associated with VKAs than NOACs (Table 1).
TABLE 1.
Baseline characteristics according to anticoagulant drugs
| Overall (n = 1667) | VKAs (n = 743) | Dabigatran (n = 280) | Rivaroxaban (n = 299) | Apixaban (n = 345) | P value | |
|---|---|---|---|---|---|---|
| Age (years) | 73.7 ± 9.1 | 72.0 ± 8.9 | 73.2 ± 8.8* | 75.1 ± 9.6** | 76.7 ± 8.4** | <0.001 a |
| Age ≥75 years (%) | 51.2 | 40.5 | 53.6 | 57.2 | 67.0 | <0.001 b |
| Women (%) | 43.3 | 41.5 | 38.9 | 48.8 | 46.1 | 0.045 b |
| Persistent/permanent AF (%) | 59.3 | 55.5 | 62.4 | 68.6 | 57.1 | 0.001 b |
| CHA2DS2 VASc score | 3.5 ± 1.5 | 3.4 ± 1.5 | 3.4 ± 1.4 | 3.7 ± 1.5 ∧ | 3.7 ± 1.5∧∧ | <0.001 a |
| eGFR (mL/min/1.73 m2) | 70.9 [55.7‐85.8] | 72.2 [56.1‐88.0] | 74.2 [61.0‐87.7] | 65.1 [51.3‐85.2] † | 68.3 [54.5‐83.3] †† | <0.001 c |
| CKD classes (%) | <0.001 b | |||||
| G1 ≥90 mL/min/1.73 m2 | 20.0 | 21.8 | 21.1 | 20.1 | 15.4 | |
| G2 89‐60 mL/min/1.73 m2 | 48.1 | 48.6 | 57.5 | 38.5 | 47.8 | |
| G3‐G4 <60 mL/min/1.73 m2 | 31.9 | 29.6 | 21.4 | 41.5 | 36.8 | |
| Arterial hypertension (%) | 87.5 | 87.1 | 87.0 | 87.9 | 88.4 | 0.921 b |
| Diabetes mellitus (%) | 24.0 | 23.1 | 24.4 | 30.5 | 20.0 | 0.016 b |
| History of MI/CHD (%) | 18.3 | 20.3 | 15.1 | 16.9 | 17.7 | 0.214 b |
| Heart failure (%) | 15.8 | 18.2 | 12.0 | 17.6 | 11.9 | 0.012 b |
| History of stroke/TIA (%) | 15.3 | 10.8 | 21.1 | 15.8 | 20.0 | <0.001 b |
| ACE inhibitors (%) | 36.0 | 36.9 | 36.2 | 37.9 | 32.5 | 0.457 b |
| ARBs (%) | 37.3 | 39.4 | 32.9 | 37.5 | 36.2 | 0.263 b |
| β blockers (%) | 48.3 | 49.9 | 47.1 | 50.2 | 44.1 | 0.278 b |
| Calcium channel antagonists (%) | 25.6 | 28.0 | 25.4 | 21.7 | 23.8 | 0.158 b |
| Statins (%) | 43.5 | 47.8 | 36.4 | 40.8 | 42.3 | 0.006 b |
| Anti‐platelets (%) | 14.3 | 17.8 | 11.4 | 11.7 | 11.3 | 0.004 b |
Abbreviations: ACE, angiotensin converting enzyme; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; CHD, coronary heart disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; TIA, transient ischemic attack; VKAs, vitamin K antagonists.
P = 0.041 vs VKAs.
P < 0.001 vs VKAs.
P = 0.005 vs VKAs.
P = 0.002 vs VKAs.
P = 0.010 vs VKAs.
P = 0.019 vs VKAs.
ANOVA test.
χ2 test.
Kruskal‐Wallis test.
Basal eGFR was 70.9 (55.7‐85.8; mL/min/1.73 m2); only 20.0% of patients had a normal renal function at baseline, while 48.1% had a mild CKD and 31.9% a moderate CKD (ie, <60 mL/min/1.73 m2; Table 1 and Figure 1).
FIGURE 1.
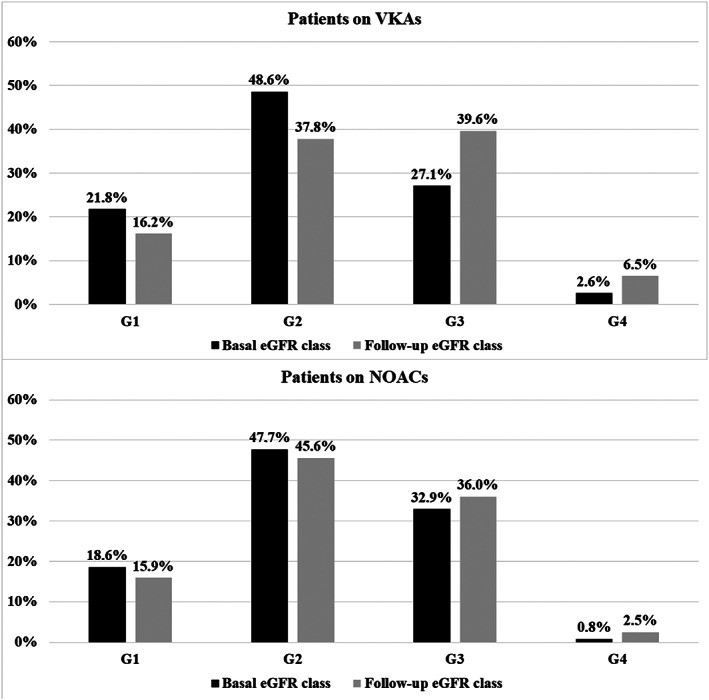
Distribution of patients among eGFR classes according to treatment with vitamin K (VKA) or nonvitamin K oral anticoagulants (NOACs) at baseline and during follow‐up. G1, eGFR >90 mL/min/1.73 m2; G2, eGFR 89‐60 mL/min/1.73 m2; G3, eGFR 59‐30 mL/min/1.73 m2; G4, eGFR <30 mL/min/1.73 m2
In particular, patients on rivaroxaban and apixaban showed a significantly lower baseline renal function compare to VKAs, with a higher proportion of patients in the G3‐G4 eGFR class (Table 1).
3.1.1. Decline of renal function
The median annual decline of eGFR was −2.11 (IQR –5.68/−0.62) mL/min/1.73 m2 with VKAs, −0.27 (IQR –9.00/4.54) mL/min/1.73 m2 with dabigatran, −1.21 (IQR –9.98/4.02) mL/min/1.73 m2 (P < 0.001 vs VKAs) with rivaroxaban (P = 0.004 vs VKAs) and −1.32 (IQR –8.7/3.99) mL/min/1.73 m2 with apixaban (P = 0.003 vs VKAs) (Figure 2; Kruskall‐Wallis P < 0.001).
FIGURE 2.
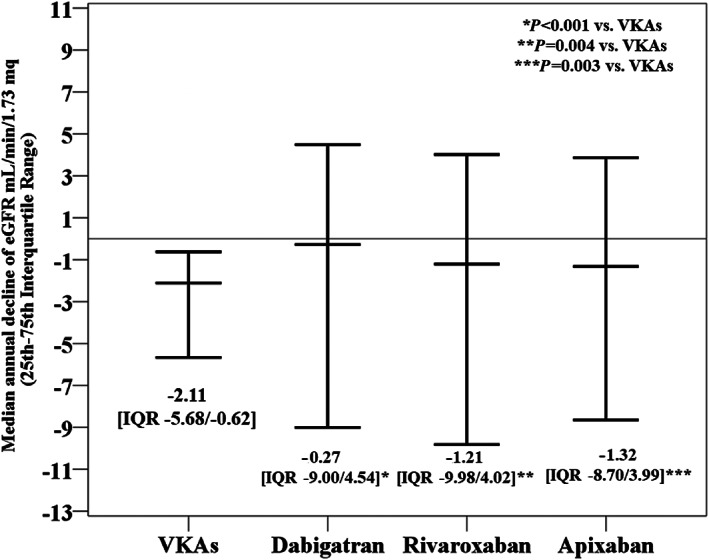
Median annual change in eGFR according to anticoagulant drugs
When we divided patients according to the median decline of eGFR, we found that dabigatran and rivaroxaban were inversely associated with an annual decline below the median (Figure 3), while a marginal statistical significance was found for apixaban.
FIGURE 3.
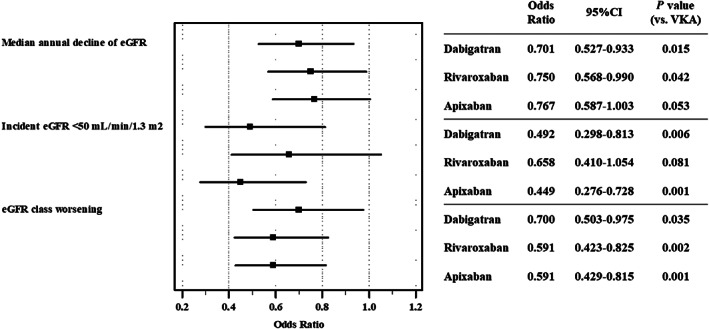
Adjusted OR for each direct oral anticoagulant drug for prespecified endpoints as compared to VKAs. Adjusted for pattern of atrial fibrillation, age ≥75 years, female sex, arterial hypertension, diabetes mellitus, history of myocardial infarction/coronary heart disease, heart failure, history of stroke/transient ischemic attack, angiotensin converting enzyme inhibitor, angiotensin receptor blockers, β blockers, calcium channel antagonists, statins, antiplatelets
For the complete list of covariates see Supporting Information Table S1.
3.1.2. Decline of the eGFR below 50 mL/min/1.73 m2
The characteristics of patients with an eGFR above or below 50 mL/min at baseline are reported in Table 2. Patients with an eGFR <50 mL/min were older, more likely women, with persistent/permanent AF and higher comorbidity burden as compared with those with eGFR ≥50 mL/min (Table 2).
TABLE 2.
Characteristics of patients with eGFR above and below 50 mL/min at baseline
| eGFR ≥50 mL/min (n = 1383) | eGFR <50mL/min (n = 284) | P value | |
|---|---|---|---|
| Age (years) | 72.8 ± 9.2 | 78.2 ± 7.1 | <0.001 a |
| Women (%) | 41.6 | 51.8 | 0.002 b |
| Persistent/permanent AF (%) | 58.0 | 65.6 | 0.020 b |
| CHA2DS2 VASc score | 3.4 ± 1.5 | 4.2 ± 1.4 | <0.001 a |
| Arterial hypertension (%) | 86.2 | 93.7 | <0.001 b |
| Diabetes mellitus (%) | 23.1 | 28.6 | 0.056 b |
| History of MI/CHD (%) | 17.1 | 24.1 | 0.007 b |
| Heart failure (%) | 13.6 | 26.4 | <0.001 b |
| History of stroke/TIA (%) | 14.8 | 17.7 | 0.239 b |
| ACE inhibitors (%) | 34.9 | 41.7 | 0.035 b |
| ARBs (%) | 37.1 | 38.4 | 0.687 b |
| β blockers (%) | 47.6 | 51.8 | 0.216 b |
| Calcium channel antagonists (%) | 25.2 | 27.1 | 0.502 b |
| Statins (%) | 43.0 | 46.1 | 0.325 b |
| Anti‐platelets (%) | 14.0 | 15.5 | 0.515 b |
Abbreviations: ACE, angiotensin converting enzyme; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; TIA, transient ischemic attack.
Student t‐test.
χ2 test.
This analysis was restricted to 1383 patients with a baseline eGFR >50 mL/min/1.73 m2.
During follow‐up, 174 (12.6%) patients showed a decline in eGFR below 50 mL/min/1.73 m2. Of these, 96 (55.2%) were in the VKA group and 78 (44.8%) were in the NOAC group: 24 in the dabigatran group, 28 in the rivaroxaban group and 26 in the apixaban group.
Multivariable logistic regression analysis showed that dabigatran and apixaban were associated with a lower rate of eGFR decline, while rivaroxaban did not reach statistical significance (Figure 3).
In addition, calcium channel antagonists (OR 1.641, 95% CI 1.145‐2.351, P = 0.007) and age ≥75 years (OR 2.644, 95% CI 1.852‐3.774, P < 0.001) were associated with a higher rate of eGFR decrease. For the complete list of covariates see Supporting Information Table S2.
3.1.3. eGFR class worsening
The distribution of patients into eGFR classes at baseline and during follow‐up is shown in Figure 1.
In the whole cohort, 411 (24.7%) of patients passed to a lower eGFR class. Of these 360 (87.6%) changed only one class, while 26 (6.3%) decreased two or more classes.
The proportion of patients with moderate CKD significantly increased in patients treated with VKAs as compared to those on NOACs (+12.5% vs +3.1%, respectively; Figure 1).
Overall, the number of patients showing a worsening in renal class was higher in the VKA group compared to the NOAC group (P = 0.002; Figure 4).
FIGURE 4.
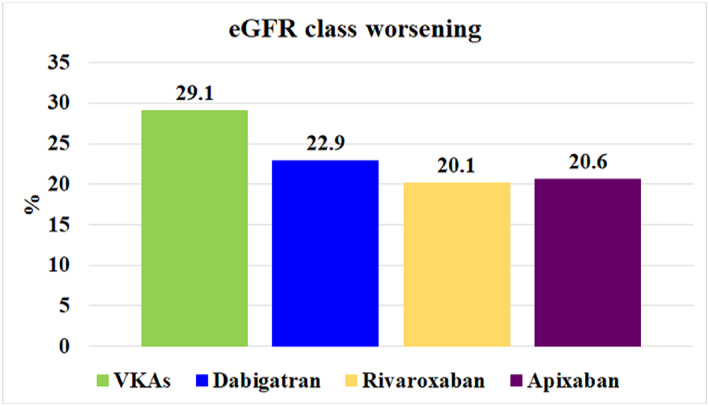
Proportion of patients showing eGFR class worsening according to anticoagulation treatment
Multivariable logistic regression analysis showed that all three NOACs compared to VKAs were associated with a reduced rate of eGFR class worsening (Figure 3).
For the complete list of covariates see Supporting Information Table S3.
3.2. Subgroup analysis
3.2.1. Full versus reduced dose of NOACs
Among the 924 patients on NOACs, 477 (51.6%) patients were taking a reduced dose. The median annual difference of eGFR was not different between full and reduced NOAC groups (P = 0.165). Logistic regression analysis showed no association between reduced dose and eGFR class worsening (univariate OR 1.063, 95% CI 0.774‐1.458, P = 0.706).
Conversely, an increased rate of eGFR from above to below 50 mL/min was found in patients on a reduced dose of NOACs (n = 766, univariate OR 2.516, 95% CI 1.541‐4.109, P < 0.001).
3.2.2. Patients with and without diabetes
In the whole cohort, 24% of patients had diabetes. The proportion of diabetic patients was higher in the group of patients on rivaroxaban (Table 1). When we investigated the association between NOAC use and renal endpoints according to the presence of diabetes, we found that the use of NOAC was generally not significantly associated with eGFR changes in the group of patients with diabetes (Table 3). The only significant association was for apixaban against incident eGFR <50 mL/min (Table 3).
TABLE 3.
Adjusted OR for NOAC use according to each renal endpoint in patients with and without diabetes
| With diabetes | Without diabetes | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Median annual decline of eGFR | ||||
| Dabigatran | 0.621 (0.345‐1.117) | 0.112 | 0.734 (0.528‐1.020) | 0.065 |
| Rivaroxaban | 0.777 (0.455‐1.329) | 0.357 | 0.741 (0.534‐1.029) | 0.073 |
| Apixaban | 0.591 (0.326‐1.073) | 0.084 | 0.822 (0.606‐1.113) | 0.205 |
| Incident eGFR <50 mL/min | ||||
| Dabigatran | 0.406 (0.146‐1.125) | 0.083 | 0.527 (0.292‐0.952) | 0.034 |
| Rivaroxaban | 0.677 (0.285‐1.610) | 0.378 | 0.599 (0.334‐1.073 | 0.085 |
| Apixaban | 0.261 (0.081‐0.839) | 0.024 | 0.497 (0.288‐0.857) | 0.012 |
| eGFR class worsening | ||||
| Dabigatran | 1.127 (0.578‐2.198) | 0.725 | 0.611 (0.415‐0.898) | 0.012 |
| Rivaroxaban | 0.789 (0.412‐1.511) | 0.475 | 0.539 (0.363‐0.800) | 0.002 |
| Apixaban | 0.690 (0.327‐1.453) | 0.329 | 0.567 (0.396‐0.813) | 0.002 |
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio.
4. DISCUSSION
In this prospective multicentre cohort study, we showed that AF patients prescribed NOACs disclosed a lower median annual decline of eGFR, less eGFR class worsening and a lower transition from eGFR above to below 50 mL/min/1.73 m2 compared to patients treated with VKAs.
In our study the median annual decline of eGFR in patients on VKAs was −2 mL/min, which is slightly lower than that observed in the warfarin arms of clinical trials. Thus, in the post hoc analysis of Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY), patients on warfarin showed a decline in creatinine clearance of −3.68 (±0.24) mL/min 15 and −4.3 ± 14.6 mL/min in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) trial. 16
In addition, we found a lower decline of renal function compared with AF patients included in the RE‐LY and ROCKET trials. 15 , 16 Reasons for this difference may be several, including the fact that we used eGFR rather than creatinine clearance to estimate renal function and the characteristics of patients included in the studies. For instance, in the ROCKET AF trial, patients on rivaroxaban had a decline of −3.5 ± 15.1 mL/min, which was significantly lower than in warfarin‐treated patients (P < 0.001), but still higher than that observed in our study (median −1.21 mL/min). 16 However, our patients presented with fewer comorbidities, including diabetes (30 vs 40%), heart failure (18 vs 62%) and history of cerebrovascular events (16 vs 55%) compared to the ROCKET AF cohort. 16
In the RE‐LY trial, patients on dabigatran 110 mg/day showed a decline in eGFR of −2.57 (±0.24) mL/min (P = 0.0009 vs warfarin) and those on 150 mg/day showed a decline in eGFR of −2.46 (±0.23) mL/min (P = 0.0002 vs warfarin). 15 Conversely, in our study, the renal function of patients treated with dabigatran was essentially stable at 1 year. A relevant difference between the two studies relies on the baseline eGFR class distribution: in our study a higher proportion of patients had normal renal function (eGFr ≥90 mL/min) at baseline compared to RE‐LY patients (20 vs 7.5%), with fewer patients presenting with stage 3‐5 CKD (21.4 vs >40% in the RE‐LY). 15 This is of concern as a baseline low eGFR class is a risk factor for future decline of renal function. 12
Another retrospective study showed that NOACs were globally associated with a lower risk of ≥30% loss of eGFR (Hazard Ratio (HR) 0.77, 95% CI 0.66‐0.89, P < 0.001) compared to warfarin, with dabigatran and rivaroxaban showing the most favourable profile for renal outcomes. 20
We also analysed other markers of renal functional decrease not previously investigated in AF patients treated with NOACs, such as eGFR class worsening and eGFR passing from above to below 50 mL/min. We found that almost 25% of patients changed eGFR classes during follow‐up. This finding is clinically relevant and suggests that one in four patients will pass to a lower eGFR class with most patients changing one class of eGFR and 6.3% of patients decreasing by two or more classes. In particular, we found a significantly lower proportion of patients on NOACs showing an eGFR class worsening as compared to those on VKAs. Thus, 29.1% of patients on VKAs changed to a lower eGFR class as compared to 20.6%, 20.1% and 22.9% of patients on apixaban, rivaroxaban and dabigatran, respectively.
Furthermore, 174 (12.6%) patients had an eGFR transitioning from above to below 50 mL/min. We found that age (ie, >75 years) and patients using calcium channel blockers were more likely to pass below 50 mL/min, whilst treatment with dabigatran and apixaban was associated with a lower rate of eGFR decline.
The significant proportion of patients passing to a lower eGFR class during follow‐up, and in particular passing from above to below 50 mL/min, should be regarded to as a clinically important issue for the management of anticoagulant therapy. Thus, these patients should be closely monitored during follow‐up, as according to European Heart Rhythm Association (EHRA) practical guide patients transitioning to eGFR <50 mL/min will require a dose reduction of rivaroxaban and edoxaban, while for dabigatran and apixaban a dose reduction may be considered according to bleeding risk and the patient's profile. 21
Finally, we analysed the effect according to the dosage of NOACs and the presence of diabetes. We found no apparent difference between full and reduced dose of NOACs for renal endpoints, suggesting that also reduced dose of NOAC may be useful in this context when appropriately prescribed. Regarding patients with diabetes, who are the subgroup of patients at highest risk of kidney failure, we found that the effect of NOAC was markedly reduced, as the association of NOAC use with almost all renal endpoints became non‐significant. This result suggests that other factors, such as glycaemic control, may play a more prominent role in kidney function protection in patients with AF.
Altogether these findings have clinical implications. The difference in absolute annual decline of eGFR between VKAs and NOACs may be relevant considering the risk of end‐stage renal disease (ESRD) associated with eGFR decline. Thus, it is estimated that for each −2 mL/min of eGFR, there is a risk of ESRD of HR 2.71 (95% CI 2.08‐3.53). 19 Furthermore, worsening of renal function has been shown to be associated with an increased risk of thromboembolic and bleeding events in AF patients. 22
Regarding anticoagulation treatment, previous findings showed that NOACs should be preferred over VKAs in AF patients with worsening CKD because they are safe and effective in this subgroup of patients. 23 Our results reinforce the favourable effect of NOACs in AF patients with CKD, highlighting that NOACs are also associated with a slower decline of renal function over time.
The limitations of the study include the study design, as this was not a randomized trial aimed at comparing the efficacy of NOACs versus warfarin regarding renal function modifications. Thus, a cause‐effect relationship cannot be established, only an association can be deduced. Furthermore, we included only Caucasian patients, thus the generalizability of our results to other populations is uncertain. Finally, decline of renal function was evaluated only by two points of creatinine during a median follow‐up of 1 year. A more accurate evaluation of renal function would include the presence of albuminuria, which was not measured in this study, and a longer follow‐up. Indeed, we assumed a linear decline of eGFR for patients with a second creatinine collected after 1 year. Nevertheless, in the absence of acute events causing a sudden worsening of kidney function (such as acute kidney failure due to drugs), it is clinically reasonable to assume such a linear change of renal function. Finally, we cannot exclude that NOAC patients who were previously treated with VKAs may have a different course of eGFR compared to NOAC‐naive patients.
In conclusion, in this prospective study the use of NOACs was associated with a lower decline of renal function in patients with nonvalvular AF. What we observed seems to be a class effect, with few differences among NOACs depending on the endpoint considered. We found no apparent difference between full and reduced dose of NOACs but the favourable association between NOAC use and eGFR decline was markedly reduced in patients with diabetes.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
D.P.: analysis and interpretation of data, drafting of manuscript, guarantor of the paper. E.E.: acquisition of data, analysis and interpretation of data. G.Y.H.L.: analysis and interpretation of data, critical revision. A.S.: analysis and interpretation of data, drafting of manuscript. F.P.: analysis and interpretation of data, critical revision. F.M.: acquisition of data, analysis and interpretation of data. C.G.: analysis and interpretation of data, drafting of manuscript. R.M.: analysis and interpretation of data, drafting of manuscript. M.B.: acquisition of data, analysis and interpretation of data. F.V.: guarantor of the paper, analysis and interpretation of data, critical revision. P.P.: guarantor of the paper, analysis and interpretation of data, critical revision.
Supporting information
Supporting Information Table S1 Multivariable logistic regression analysis of factors associated with median annual eGFR decline (below median)
Supporting Information Table S2 Multivariable logistic regression analysis of factors associated with decline from above to below 50 mL/min/1.73 m2
Supporting Information Table S3 Multivariable logistic regression analysis of factors associated with class worsening
Pastori D, Ettorre E, Lip GYH, et al. Association of different oral anticoagulants use with renal function worsening in patients with atrial fibrillation: A multicentre cohort study. Br J Clin Pharmacol. 2020;86:2455–2463. 10.1111/bcp.14350
Daniele Pastori, Evaristo Ettorre and Gregory Y. H. Lip equal contribution
Francesco Violi and Pasquale Pignatelli, joint senior authorship
Dr Daniele Pastori is the Principal Investigator of the study.
ATHERO‐AF study group members: Mirella Saliola, Danilo Menichelli, Marco Antonio Casciaro and Vito Menafra.
REFERENCES
- 1. Odutayo A, Wong CX, Hsiao AJ, et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 2. Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: Reasons for geographic and racial differences in stroke (REGARDS) study. Circ Arrhythm Electrophysiol. 2011;4(1):26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boriani G, Laroche C, Diemberger I, et al. Glomerular filtration rate in patients with atrial fibrillation and 1‐year outcomes. Sci Rep. 2016;6:30271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonde AN, Lip GYH, Kamper AL, et al. Renal function, time in therapeutic range and outcomes in warfarin‐treated atrial fibrillation patients: a retrospective analysis of nationwide registries. Thromb Haemost. 2017;117(12):2291‐2299. [DOI] [PubMed] [Google Scholar]
- 5. Esteve‐Pastor MA, Rivera‐Caravaca JM, Roldan‐Rabadan I, et al. Relation of renal dysfunction to quality of anticoagulation control in patients with atrial fibrillation: the FANTASIIA registry. Thromb Haemost. 2018;118(2):279‐287. [DOI] [PubMed] [Google Scholar]
- 6. Apostolakis S, Guo Y, Lane DA, et al. Renal function and outcomes in anticoagulated patients with non‐valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013;34:3572‐3579. [DOI] [PubMed] [Google Scholar]
- 7. Zou R, Tao J, Shi W, et al. Meta‐analysis of safety and efficacy for direct oral anticoagulation treatment of non‐valvular atrial fibrillation in relation to renal function. Thromb Res. 2017;160:41‐50. [DOI] [PubMed] [Google Scholar]
- 8. Li YG, Pastori D, Lip GYH. Fitting the right non‐vitamin K antagonist oral anticoagulant to the right patient with non‐valvular atrial fibrillation: an evidence‐based choice. Ann Med. 2018;50:288‐302. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen PB, Lane DA, Rasmussen LH, et al. Renal function and non‐vitamin K oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: a systemic review and meta‐regression analysis. Clin Res Cardiol. 2015;104:418‐429. [DOI] [PubMed] [Google Scholar]
- 10. Halbesma N, Kuiken DS, Brantsma AH, et al. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol: JASN. 2006;17(9):2582‐2590. [DOI] [PubMed] [Google Scholar]
- 11. Roldan V, Marin F, Fernandez H, et al. Renal impairment in a "real‐life" cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding). Am J Cardiol. 2013;111(8):1159‐1164. [DOI] [PubMed] [Google Scholar]
- 12. Violi F, Pastori D, Perticone F, et al. Relationship between low ankle‐brachial index and rapid renal function decline in patients with atrial fibrillation: a prospective multicentre cohort study. BMJ Open. 2015;5:e008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation‐related nephropathy. J Thromb Haemost: JTH. 2016;14(3):461‐467. [DOI] [PubMed] [Google Scholar]
- 14. Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin‐related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):181‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE‐LY trial. J Am Coll Cardiol. 2015;65(23):2481‐2493. [DOI] [PubMed] [Google Scholar]
- 16. Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On‐treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134(1):37‐47. [DOI] [PubMed] [Google Scholar]
- 17. Pastori D, Lip GYH, Farcomeni A, et al. Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non‐vitamin K antagonist oral anticoagulants. Int J Cardiol. 2018;264:58‐63. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group M. evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825‐830. [DOI] [PubMed] [Google Scholar]
- 20. Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621‐2632. [DOI] [PubMed] [Google Scholar]
- 21. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330‐1393. [DOI] [PubMed] [Google Scholar]
- 22. Fauchier L, Bisson A, Clementy N, et al. Changes in glomerular filtration rate and outcomes in patients with atrial fibrillation. Am Heart J. 2018;198:39‐45. [DOI] [PubMed] [Google Scholar]
- 23. Miyazawa K, Pastori D, Lip GYH. Changes in renal function in patients with atrial fibrillation: efficacy and safety of the non‐vitamin K antagonist oral anticoagulants. Am Heart J. 2018;198:166‐168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Multivariable logistic regression analysis of factors associated with median annual eGFR decline (below median)
Supporting Information Table S2 Multivariable logistic regression analysis of factors associated with decline from above to below 50 mL/min/1.73 m2
Supporting Information Table S3 Multivariable logistic regression analysis of factors associated with class worsening


