Abstract
Aims
To determine the prevalence of potentially inappropriate medication (PIM) use at hospital admission and discharge, and the contribution to hospital admission among residential aged care facility residents with and without dementia.
Methods
We conducted a secondary analysis using data from a multihospital prospective cohort study involving consecutively admitted older adults, aged 75 years or older, who were taking 5 or more medications prior to hospital admission and discharged to a residential aged care facility in South Australia. PIM use was identified using the 2015 Screening Tool for Older Persons' Prescription and 2019 Beers criteria. An expert panel of clinicians with geriatric medicine expertise evaluated the contribution of PIM to hospital admission.
Results
In total, 181 participants were included, the median age was 87.5 years and 54.7% were female. Ninety‐one (50.3%) had a diagnosis of dementia. Participants with dementia had fewer PIMs, according to at least 1 of the 2 screening criteria, than those without dementia, at admission (dementia: 76 [83.5%] vs no dementia: 84 [93.3%], P = .04) and discharge (78 [85.7%] vs 83 [92.2%], P = .16). PIM use was causal or contributory to the admission in 28.1% of study participants (n = 45) who were taking at least 1 PIM at admission.
Conclusions
Over 80% of acutely admitted older adults took PIMs at hospital admission and discharge and for over a quarter of these people the admissions were attributable to PIM use. Hospitalisation presents an opportunity for comprehensive medication reviews, and targeted interventions that enhance such a process could reduce PIM use and related harm.
Keywords: Beers criteria, dementia, long term care, medication‐related hospitalisation, potentially inappropriate medication, residential aged care facility, STOPP criteria
What is already known about this subject
Medication‐related harm is the most common cause of preventable hospital admission in older people.
People living in residential aged care facilities are vulnerable to medication‐related harm from potentially inappropriate medication (PIM) use. However, evidence on PIM‐related hospital admission among residential aged care facility residents is limited.
What this study adds
PIM use was causal or contributory to 1/4 of hospital admissions among people with at least 1 PIM.
People with dementia had less PIMs, according to at least 1 of the 2 screening criteria (STOPP or Beers criteria), than those without dementia, at the time of hospital admission.
There was no significant difference in the use of at least 1 PIM from either of the 2 criteria between people with and without dementia at the time of hospital discharge.
1. INTRODUCTION
People with dementia are admitted to hospital more frequently than people without dementia (pooled relative risk of hospitalisation 1.4 [95% confidence interval 1.2–1.7]). 1 Dementia is associated with worse clinical outcomes during an inpatient stay, 2 including a higher incidence of delirium, longer hospital stay and greater decline in physical and cognitive function compared to those without dementia. 1 , 3
Medication‐related harm has been identified as a common cause of preventable hospital admission; up to 30% of hospital admissions are directly related to medication‐related problems in older adults. 4 The risk of harm due to polypharmacy and potentially inappropriate prescribing is becoming an increasing problem due to increased complexity of medicine use in an aging population. 4 , 5 , 6 Potentially inappropriate prescribing encompasses: (i) prescribing of potentially inappropriate medications (PIMs) where the risk associated with medicine use outweighs the clinical benefit; and (ii) potential prescribing omissions, which is the omission of clinically indicated medicines in patients with substantial life expectancy where no contraindications exist. 6 , 7 The most frequently utilised explicit screening criteria for PIMs in older people include the American Geriatric Society (AGS) Beers criteria, 8 and the Screening Tool of Older Person's Prescriptions/Screening Tool to Alert doctors to Right Treatment (STOPP/START) criteria. 9
People living in residential (long‐term) aged care facilities (RACFs) are at an increased risk of medication‐related harm from PIM use due to their advanced age, multimorbidity and high rates of polypharmacy. 10 Approximately half of all aged care residents in Australia have a diagnosis of dementia, 11 which places them at increased risk of harm due to PIMs, because medicines classified as PIMs such as benzodiazepines, antipsychotics, anticholinergics and the use of multiple central nervous system‐active medicines are associated with worse outcomes in people with dementia compared to those without dementia. 12
Results from previous studies regarding the impact of hospital admission on the reduction or increase in associated PIM use are conflicting. In a longitudinal study from Ireland involving 38 229 older adults in the primary care setting, the risk of using any PIM (defined by STOPP criteria) increased by 72% after hospital admission (adjusted odds ratio [AOR] 1.72 [1.63–1.84]). 13 Similar studies from Australia, Israel and Brazil found an increase in the prevalence of PIM use from admission to discharge, defined by the STOPP and Beers criteria, of between 4.3 and 6.7%. 14 , 15 , 16 In contrast, 3 other Australian studies found PIM use significantly reduced from the time of hospital admission to discharge, of a magnitude ranging from 9 to 15%. 17 , 18 , 19 In 1 further Spanish study, a cohort of 200 patients discharged from an acute geriatric unit had no change in PIM use from admission to discharge (68.5 vs 71.5%). 20 However, evidence is limited on the extent to which PIM use changes over time as well as the contribution of PIMs to hospital admission among older people with dementia compared with those without dementia.
The objective of this study was to compare the prevalence of PIM use at hospital admission and discharge, in RACF residents with and without dementia acutely admitted to hospital, and the contribution of PIMs to hospital admission.
2. METHODS
2.1. Design and setting
We re‐analysed data collected from a multicentre, prospective cohort study involving 5 major public hospitals in South Australia as part of a study to assess the potential for deprescribing for elderly hospitalised patients admitted from RACFs.
2.2. Study population
The study sample included consecutively admitted older adults, aged 75 years or older who were admitted with an acute illness managed by a general medicine ward, who were taking 5 or more regular medicines prior to admission, and who were discharged to a RACF. Participants undergoing palliative care were excluded from the study. Participants were enrolled from all 5 major public hospitals in South Australia over a 5‐week period between 5 June 2017 and 7 July 2017.
2.3. Data collection
Data were collected prospectively during the admission by a clinical pharmacist, using a standardised data extraction form comprising sociodemographic characteristics, a complete list of medicines taken at admission and on discharge, reasons for admission, comorbidities and pathology test results. Dementia diagnosis and diagnoses for other chronic health conditions were extracted from the medical history noted during the course of the admission (including any new diagnosis during the admission). All medication changes from the time of admission to discharge were noted. These changes included medications that were ceased, changed from regular use to when required or from when required to regular use, medicines with the dose decreased or increased as well as newly started medicines at discharge were recorded. Medications were coded according to the World Health Organisation (WHO) Anatomical Therapeutic Chemical classification system. 21 Ethics approval was obtained via the Southern Adelaide Clinical Human Research Ethics Committee, reference number HREC/17/SAC/368 and the University of South Australia human research ethics committee.
2.4. PIM use at hospital admission and discharge
Use of PIMs at the time of hospital admission and discharge was defined according to 2 internationally validated indicators, the STOPP version 2 9 and the 2019 AGS Beers Criteria. 8 STOPP version 2 criteria is a set of 80 criteria encompassing PIMs in patients aged 65 years and above that should be avoided. Eighteen of the original 80 STOPP criteria could not be applied due to insufficient data and so were excluded from the study. The AGS Beers criteria categorises PIM use into 5 categories: PIMs to avoid for older adults, medications to avoid in older adults with specific diseases or syndromes, medications to avoid or the dosage of which should be adjusted based on renal function, and clinically important drug–drug interactions and drugs to be used with caution in older adults. The Beers criteria category drugs to be used with caution in older adults (table 4 in the 2019 Beers criteria) was not included in the analysis because the authors of the Beers criteria note that, although these medicines should be used with caution in older adults, there is currently insufficient evidence to officially designate these medicines as PIMs. 22 The other 4 Beers criteria categories were used in this study. The prevalence of PIM use was determined by dividing the number of people with use of at least 1 PIM by the total number of people in the study. We did not report the number of Beers criteria per person as some medicines appear in >1 of the 4 categories of Beers criteria included in the study, meaning that reporting of the number of Beers criteria met per person would result in double counting of these medicines. We calculated the number of STOPP criteria per person as published in the 2015 update. We also measured the composite PIM exposure prevalence, defined as PIM exposure according to at least 1 of the 2 screening criteria: Beers or STOPP criteria. Prevalence of PIM use was assessed for both RACF residents with and without dementia, at hospital admission and at discharge. Differences in the prevalence of PIM use were compared for people with and without dementia. We calculated the total number of unique medicines per person as well as the number of regular medicines taken at admission and discharge based on Anatomical Therapeutic Chemical codes (at the fifth level). Regular medicines comprised medicines taken on a regular basis, excluding when required medicines. For the purpose of this study, dementia refers to people with diagnosis of any form of dementia or cognitive impairment, as recorded by the clinical team.
TABLE 4.
Potentially inappropriate medication (PIM) use prevalence as per Screening Tool of Older Person's Prescriptions (STOPP) criteria at hospital admission vs discharge
| Characteristics | People with dementia, n = 91 | People without dementia, n = 90 | ||
|---|---|---|---|---|
| Admission | Discharge | Admission | Discharge | |
| ≥1 PIM | 71 (78.0) | 72 (79.1) | 79 (87.8) | 77 (85.6) |
| Median number (IQR) of STOPP criteria | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (2–3) |
| Most common PIMs | ||||
| Any duplicate drug class prescription | 14 (15.4) | 10 (11.0) | 12 (13.3) | 10 (11.1) |
| Loop diuretics without evidence of heart failure, liver failure, nephrotic syndrome or renal failure | 13 (14.3) | 14 (15.4) | 23 (25.6) | 27 (30.0) |
| Benzodiazepines for ≥4 weeks | 21 (23.1) | 20 (22.0) | 22 (24.4) | 18 (21.0)* |
| Use of medicines with anticholinergic properties | 32 (35.2) | 41 (45.1)* | 20 (22.2) | 18 (20.0) |
| High dose PPI for >8 weeks | 29 (31.9) | 25 (27.5)* | 43 (47.8) | 43 (47.8) |
| Benzodiazepines use with history of falls or fractures | 16 (17.6) | 18 (19.8) | 9 (10.0) | 7 (7.8)* |
| Antipsychotics use with history of falls or fractures | 13 (14.3) | 19 (20.9)* | 2 (2.2) | 1 (1.1) |
| Use of regular opioids without concomitant laxative | 9 (9.9) | 10 (11.0) | 14 (15.6) | 12 (13.3) |
| Long‐acting opioids without short‐acting opioids for break‐through pain | 10 (11.0) | 10 (11.0) | 16 (17.8) | 16 (17.8) |
| Concomitant use of 2 or more drugs with anticholinergic properties | 7 (7.7) | 6 (6.6) | 3 (3.3) | 2 (2.2) |
*P < .05;
IQR: interquartile range; PPI: proton pump inhibitors
2.5. PIM‐related hospital admissions
To determine the extent to which PIM use was associated with the reason for hospital admission, the medications, medical conditions and pathology results for study participants with PIMs identified at hospital admission were reviewed by an expert clinical panel of clinicians with geriatric medicine expertise (one medical doctor and 3 pharmacists). Each member of the panel independently evaluated the potential for these PIMs to have contributed to the admission. In rating the potential for PIM use to have contributed to the hospital admission, each expert panel member independently used the WHO–Uppsala Monitoring Centre criteria for causality assessment. 23 PIMs with a causal connection or contribution to the main reason for admission were defined as those assessed as having a certain, probable or possible causal relationship as defined by the WHO–Uppsala Monitoring Centre criteria. Where there was disagreement about the contribution of the PIM to the reason for admission (n = 18 cases), a fourth expert panel member with expertise in clinical pharmacy blindly and independently evaluated these cases to resolve discrepancies.
2.6. Data analysis
Descriptive statistics (numbers and percentages for categorical data, means [± standard deviation] and medians with interquartile range [IQR] for continuous variables) were used to describe baseline characteristics of participants with and without dementia. Paired and unpaired t‐tests, Wilcoxon 2‐sample tests, χ2 tests or Fisher's exact tests were used to test differences in proportions between the 2 groups concerning sociodemographic and clinical characteristics. Prevalence of PIM use at the time of hospital admission and discharge was compared using χ2 test and McNemar statistics for people with and without dementia. The proportion of people whose hospital admission was attributed to PIMs by the expert panel was calculated. The change from admission to discharge in the use of PIMs, psychotropic medicines, hyperpolypharmacy (defined as the regular use of 10 or more unique medicines) and PIMs related to fall‐increasing medicines was assessed using mixed‐effects logistic regression analysis (the GLIMMIX procedure), adjusted for age, sex, length of hospital stay and age‐adjusted Charlson comorbidity index. This model allows for clustering within all 5 hospitals and within subjects (repeated measures at admission and discharge). Statistical significance was set at P‐value <.05. All analyses were performed using Statistical Analysis System (SAS) software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
2.7. Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
A total of 181 participants were included in the study (Figure 1), of whom, 91 (50.3%) participants had a diagnosis of dementia (Table 1). The median age at admission was 87.0 years (IQR 81.7–91) for people with dementia, and 88.4 years (IQR 82.9–92.1) for those without dementia. The most common primary diagnoses at admission were pneumonia/lower respiratory tract infections (n = 45, 24.9%), falls (n = 25, 13.8%) and cardiovascular conditions (n = 21, 11.6%). There was no difference in median duration of hospital stay in people with and without dementia (10 days for people with dementia vs 8.5 days for those without dementia, P = .85) or median age‐adjusted Charlson comorbidity index (6 vs 7, P = .15, Wilcoxon 2‐sample test).
FIGURE 1.
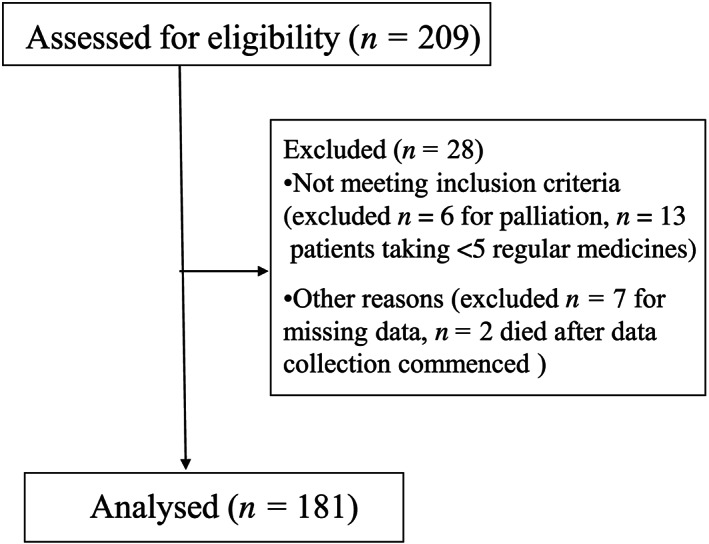
Study flow chart
TABLE 1.
Study participant demographic and clinical characteristics (n (%) unless otherwise stated)
| All | With dementia | Without dementia | P‐value | |
|---|---|---|---|---|
| n | 181 | 91 (50.3) | 90 (49.7) | |
| Age (y), median (IQR) | 87 (81.7–90.9) | 88.4 (82.9–92.1) | .12 | |
| Female | 99 (54.7) | 47 (51.7) | 52 (57.8) | .41 |
| RACF resident prior to admission | 135 (74.6) | 67 (73.6) | 68 (75.6) | .57 |
| Length of hospital stay (d), median (IQR) | 10 (5–18) | 8.5 (5–20) | .85 | |
| Comorbidity status | ||||
| Age‐adjusted Charlson comorbidity index, median (IQR) | 6 (5–8) | 7 (6–8) | .15 | |
| IHD/MI | 71 (39.2) | 29 (31.9) | 42 (46.7) | .04 |
| CCF | 39 (21.5) | 14 (15.4) | 25 (27.8) | .04 |
| Diabetes mellitus | 43 (23.8) | 23 (25.3) | 20 (22.2) | .63 |
| Stroke/TIA | 58 (32.0) | 28 (30.8) | 30 (33.3) | .71 |
| COPD | 45 (24.9) | 18 (19.8) | 27 (30.0) | .11 |
| GORD | 60 (33.1) | 24 (26.4) | 36 (40.0) | .05 |
| Osteoporosis | 58 (32.0) | 30 (33.0) | 28 (31.1) | .96 |
| Renal function at admission, mL/min | ||||
| ≥60 | 12 (6.6) | 10 (11.0) | 2 (2.2) | .01 |
| 30–59 | 88 (48.6) | 50 (55.0) | 38 (42.2) | |
| 15–29 | 62 (34.3) | 24 (26.4) | 38 (42.2) | |
| <15 | 19 (10.5) | 7 (7.7) | 12 (13.3) | |
| Admission diagnosis | ||||
| Exacerbation of known co‐morbidity | 100 (55.2) | 46 (50.6) | 54 (60.0) | .20 |
| New condition | 81 (44.8) | 45 (49.5) | 36 (40.0) | |
| Specific admission diagnoses | ||||
| Pneumonia/lower respiratory tract infection | 45 (24.9) | 29 (31.9) | 16 (17.8) | .03 |
| Falls | 25 (13.8) | 13 (14.3) | 12 (13.3) | .85 |
| Cardiovascular related | 19 (10.5) | 7 (7.7) | 14 (15.6) | .10 |
| Sepsis | 13 (7.2) | 6 (6.6) | 7 (7.8) | .76 |
| Fracture | 9 (5.0) | 2 (2.2) | 7 (7.8) | .08 |
| COPD exacerbation | 9 (5.0) | 3 (3.3) | 6 (6.7) | .30 |
| Urinary tract infection | 10 (5.5) | 5 (5.5) | 5 (5.6) | .99 |
| Dementia related admission | 11 (6.1) | 11 (12.1) | ‐ | ‐ |
IQR: interquartile range; SD: standard deviation; RACF: residential aged care facility; IHD/MI: ischaemic heart disease/myocardial infarction; CCF: congestive cardiac failure; COPD: chronic obstructive pulmonary disease; GORD: gastro‐oesophageal reflux disease; TIA: transient ischaemic attack.
The mean number of unique medicines taken by all participants increased from 10 at admission to 11 at discharge (P < .0001). When stratified by people with and without dementia, there was a similar temporal change in medicine use from admission to discharge (Table 2). People with dementia had a mean increase of 0.95 ± 2.05 new medicines from admission to discharge, which was not significantly different to people without dementia (0.98 ± 2.12, P = .92). People with dementia used a significantly lower number of medicines at both admission and discharge, compared to those without dementia (Table 2).
TABLE 2.
Comparison of medication use at hospital admission vs discharge (n (%) unless otherwise stated)
| Admission | Discharge | |||
|---|---|---|---|---|
| Dementia | No dementia | Dementia | No dementia | |
| Total number of unique medications, mean (±SD) | 9.5 (±3.5) | 10.9 (±3.4) * | 10.5 (±3.6) | 11.8 (±3.5) * |
| Number of regular medicines, mean (±SD) | 8.8 (±3.2) | 10.1 (±3.2) * | 10.2 (±3.4) | 11.4(±3.5) * |
| Hyperpolypharmacy a | 36 (39.6) | 47 (52.2) | 45 (49.5) | 60 (66.7)* |
| Proportion receiving any psychotropic medicine | 58 (63.7) | 50 (55.6) | 64 (70.3) | 49 (54.4)* |
| Proportion receiving: | ||||
| Antipsychotics | 24 (26.4) | 6(6.7)* | 36 (39.6) | 6 (6.7)* |
| Antidepressants, | 41 (45.1) | 33(36.7) | 41 (45.1) | 33 (36.7) |
| Anxiolytics/hypnotics | 31(34.1) | 33(36.7) | 40 (44.0) | 31 (34.4) |
| Anti‐dementia medicines | 8(8.8) | ‐ | 9 (9.9) | ‐ |
*P < .05;
SD: standard deviation; a defined as the regular use of 10 or more unique medicines
3.1. Prevalence of PIM use at admission and discharge
There was no difference in the prevalence of STOPP or Beers criteria PIM use in people with dementia compared to those without dementia on admission or at discharge (Table 3 and 4). At the time of admission, the prevalence of exposure to at least 1 PIM from either of the 2 criteria was lower in people with dementia (n = 76, 83.5%) than in those without dementia (n = 84, 93.3%; P = .04). At the time of discharge from hospital, 86% of people with dementia used at least 1 PIM, which was not significantly different to the prevalence in people without dementia 92.2% (P = .16).
TABLE 3.
Potentially inappropriate medication (PIM) use prevalence as per the 2019 beers criteria at hospital admission vs discharge
| Characteristics | People with dementia, n = 91 | People without dementia, n = 90 | ||
|---|---|---|---|---|
| Admission | Discharge | Admission | Discharge | |
| ≥1 PIM | 72 (79.1) | 77 (84.6) | 73 (81.1) | 77 (85.6) |
| Most common PIMs | ||||
| Independent of diagnosis or condition | ||||
| Antidepressants | 4 (4.4) | 3 (3.3) | 10 (11.1) | 7 (7.8) |
| Antipsychotics | 24 (26.4) | 36 (39.6)* | 4 (4.4) | 5 (5.6) |
| Benzodiazepines & related drugs | 32 (35.2) | 42 (46.2)* | 33 (36.7) | 31 (34.4) |
| Proton pump inhibitors | 38 (41.8) | 33 (36.3)* | 47 (52.2) | 46 (51.1) |
| Clinically important drug–drug interactions | ||||
| Any combination of 3 or more CNS‐active medicines | 28 (30.8) | 33 (36.3)* | 27 (30.0) | 27 (30.0) |
| Concurrent use of opioids and benzodiazepines | 18 (19.8) | 22 (24.2)* | 22 (24.4) | 23 (25.6) |
| Concurrent use of opioids and pregabalin | 6 (6.6) | 9 (9.9) | 9 (10.0) | 9 (10.0) |
| Use of medicines with strong anticholinergic properties | 17 (18.7) | 15 (16.5) | 15 (16.7) | 11 (12.2)* |
| Considering disease and syndrome interactions | ||||
| Antiepileptics with history of falls or fractures | 10 (11.0) | 9 (9.9) | 4 (4.4) | 2 (2.2) |
| Antipsychotics with history of falls or fractures | 13 (14.3) | 19 (20.9)* | 2 (2.2) | 1 (1.1) |
| Benzodiazepines with history of falls or fractures | 16 (17.6) | 18 (19.8) | 9 (10.0) | 7 (7.8)* |
| Antidepressants with history of falls or fractures | 16 (17.6) | 15 (16.5) | 12 (13.3) | 12 (13.3) |
| Opioids with history of falls or fractures | 17 (18.7) | 22 (24.2)* | 17 (18.7) | 24 (26.7)* |
| Medications that should be avoided or dose reduced with decreased kidney function | ||||
| Spironolactone | 5 (5.5) | 7 (7.7) | 6 (6.7) | 4 (4.4) |
P < .05;
CNS: central nervous system
The most common Beers criteria PIMs at admission were long‐term use of proton pump inhibitors (41.8% in those with dementia and 52.2% in those without), use of benzodiazepines and related medicines (35.2% in those with dementia and 36.7% in those without), use of 3 or more CNS‐active medicines (30.8% in those with dementia and 30% in those without), and concurrent use of opioids and benzodiazepines (19.8% in those with dementia and 24.4% in those without; Table 3).
The most common STOPP related PIMs at admission were use of medicines with anticholinergic properties (35.2% in those with dementia and 22.2% in those without), use of high dose PPIs for longer than 8 weeks (31.9% in those with dementia and 47.8% in those without), and use of benzodiazepines for longer than 4 weeks (23.1% in those with dementia and 24.4% in those without; Table 4).
Among people with dementia, there was a significant increase from admission to discharge in the use of medicines with anticholinergic properties (STOPP‐related PIM, 35 vs 45%, P < .01) and benzodiazepines and related drugs (Beers' PIM, 35 vs 46.2%, P < .01). A similar trend was observed in the use of antipsychotics (STOPP/Beers' PIM, 14.3 vs 21%, P = .014) and opioids (Beers' PIM, 18.9 vs 24.2%, P = .025) in people with dementia with history of falls or fracture. In addition, there was a significant reduction in the use of PPIs from admission to discharge in people with dementia (Beers' PIM, 41.8 vs 36.3%, P < .01). For participants without dementia, the use of opioids in people with history of falls or fracture significantly increased from admission to discharge (18.7 vs 26.7%, P = .02), while the use of benzodiazepines in people without dementia with a history of falls or fracture was significantly decreased from admission to discharge (STOPP/Beers' PIM, 10 vs 7.8%, P < .01; Table 3 and 4).
3.2. Change in number of people with use of at least 1 PIM from admission to discharge
After adjusting for covariates, there was no statistically significant difference in the odds of receiving at least 1 Beers or STOPP criteria PIM at discharge compared to admission for participants with or without dementia (Table 5).
TABLE 5.
Change in medicine use from hospital admission to discharge
| Characteristics | AOR (95% CI) a , P‐value | |
|---|---|---|
| With dementia | Without dementia | |
| Hyperpolypharmacy | 1.95 (0.90–4.23), .09 | 2.38 (1.14–4.98), .02 |
| Psychotropic medicines useb | 1.52 (0.72–3.19), .27 | 0.93 (0.43–2.01), .85 |
| PIM use defined by beers Criteriac | 1.57 (0.67–3.69), .29 | 1.58 (0.61–4.14), .34 |
| PIM use defined by STOPP Criteriac | 1.09 (0.48–2.44), .87 | 0.75 (0.26–2.18), .60 |
| Fall‐related STOPP criteria PIM usec | 1.28 (0.57–2.86), .55 | 0.61 (0.20–1.93), .40 |
| Fall‐related beers criteria PIM usec | 1.44 (0.67–3.10), .34 | 1.43 (0.62–3.32), .40 |
AOR shown for time‐periods (hospital discharge vs admission), adjusted for age, sex, length of hospital stay, age‐adjusted Charlson Comorbidity Index; b excluding antidementia medicines; c modelling the probability that patient is exposed to at least 1 PIM.
There was no significant change in the odds of taking at least 1 fall‐risk increasing PIM (STOPP/Beers criteria) from admission to discharge among the whole cohort, after adjusting for covariates. However, analysis by dementia status showed people with dementia had a significant increase in the odds of being exposed to at least 1 fall‐risk‐increasing PIM (STOPP criteria) from admission to discharge when compared to those without dementia, (AOR 3.18 [1.31–7.74], P = .01). This was not significant when applying the Beers criteria for fall‐risk‐increasing PIMs (AOR 1.83 (0.83–4.04), P = .13).
3.3. PIM‐related hospital admissions
The expert panel assessment found that PIM use was causal or contributory to the admission in 45 study participants (i.e. 28.1% of participants who had at least 1 Beers/STOPP criteria PIM at admission). Among those exposed to PIMs at admission, 23 (30.3%) participants with dementia experienced PIM‐related hospitalisation compared with 22 (26.2%) participants without dementia (P = .57). Of the 45 admissions where the PIM was a causal or contributory factor, 29 admissions (64.4%) were attributable to PIMs in both the STOPP and Beers criteria, 11 (24.4%) were attributable to PIMs in the 2019 Beers criteria only, and 5 (11.1%) were attributable to PIMs in the STOPP criteria only. Falls (with or without fracture) while receiving fall‐risk‐increasing PIM (benzodiazepines, opioids, vasodilators and antipsychotics) were the most common PIM‐related event considered causal or contributory to the admission, occurring in 25(55.5%) of the 45 admissions (Figure 2).
FIGURE 2.
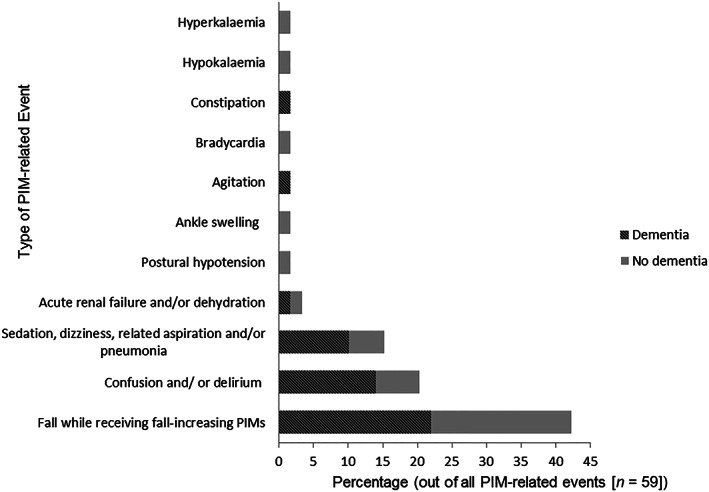
Percentage and types of potentially inappropriate medication (PIM)‐related events causal or contributory to the admission in the study cohort
4. DISCUSSION
Results of our study highlight that PIM use is common among acutely hospitalised RACF residents with polypharmacy. Residents with dementia had less PIM at the time of hospital admission according to at least 1 of the 2 screening criteria than those without dementia. There was no significant change in the prevalence of STOPP or Beers criteria PIM use between discharge and admission for patients with or without dementia.
A nation‐wide Danish study of RACF residents with dementia (n = 19 248) and without dementia (n = 30 632), found PIM use was less frequent in residents with dementia than in those without dementia (PIMs defined by the Danish red‐yellow‐green list, 50.7 vs 54.2%, P < .001; PRISCUS PIMs list: 27.7 vs 33.7, P < .001), 24 which is consistent with our findings. Similarly, a previous systematic review of the use of PIMs in older adults living in nursing homes found dementia was associated with a decrease in PIM use. 25 Our finding that people with dementia had a marginally lower, but not statistically significant, level of comorbidity, and were taking a lower number of unique medicines than in those without dementia further supports this, as prior evidence shows that there is a linear relationship between number of medicines used, multimorbidity and PIM use. 26
In our study, there was a non‐significant increase in the odds of taking at least 1 Beers criteria PIM at discharge for both RACF residents with and without dementia, while there was a small but non‐significant reduction in STOPP criteria PIM use in those without dementia. Previously published data on the change in PIM prevalence from hospital admission to discharge in RACF residents with and without dementia is limited. However, some studies have compared PIM use at admission and discharge among older adults in general and people with dementia in particular. These studies had mixed results, with no significant difference from admission to discharge in 1 study, 20 a significant reduction in PIM prevalence between admission and discharge in 3 studies 17 , 18 , 19 and an increase in PIM prevalence from admission to discharge in 3 others. 14 , 15 , 16 In an Australian study involving 277 people with dementia who had unplanned hospital admission, 96% of the participants were exposed to at least 1 Beers PIM at admission and, prevalence was reduced to 87% at discharge. 19 In contrast, our study found no significant difference in Beers PIM use between admission and discharge. The reason for this difference may be that in our study we only included RACF residents receiving polypharmacy, and included additional categories of PIMs, such as PIMs dependent on diseases/conditions.
This study also examined whether PIM use was causal or contributory to the hospital admission. The panel of experts adjudicated that over a quarter of patient admissions among those taking at least 1 PIM was attributable to the use of PIMs. Half of PIMs with a causal or contributory relationship to admission involved a fall while receiving fall‐risk‐increasing PIMs and the next most common PIM related events were confusion and/or delirium. The prevalence of PIM‐related hospitalisation in our study is higher than reported in an earlier Australian study involving 534 older hospital in‐patients, which found that 6% of all hospital admissions were due to use of a STOPP criteria PIM. 16 Similarly, an Irish study of 715 older patients admitted with acute illness found PIM use was casual or contributory to hospital admission in 11.5% and 6% of patients with STOPP and Beers criteria PIMs respectively. 27 In both studies, falls associated with use of PIMs was the commonest PIM‐related event considered causal or contributory to the admission, in line with our study. In addition, a study from the US involving 600 older adults admitted with acute illness found that 158 (26.3%) participants experienced 329 adverse drug events (ADEs); 219 of these 329 ADEs (66.6%) were causal or significantly contributory to hospital admission. Of the 329 ADEs, 51.7% and 20.4% ADEs involved STOPP and Beers criteria PIMs respectively, P < .01. 28 These differences may be due to a number of reasons. First, in our study, we included older adults, who were taking 5 or more medicines prior to admission. In contrast, in other studies not all participants were exposed to polypharmacy, with an Irish study having only 48% of participants prescribed 5 or more medicines 27 and in a US study, 2/3 were prescribed 6 or more medicines. 28 In addition, half of participants in our study had dementia, compared to the other studies, where between 10–19.5% of participants had a history of chronic cognitive impairment or dementia. 27 , 28 There were higher rates of PIM use in our study, with participants taking 1 or more STOPP (83%) or Beers criteria PIM (80.1%), compared with 54.8% participants taking 1 or more STOPP PIMs in the other Australian study 16 ; participants taking 1 or more STOPP (35%) or Beers criteria PIMs (25%) 27 ; patients taking 1 or more STOPP (56.2%) or Beers criteria PIMs (28.8%). 28 Consistent with our findings, a 2019 Malaysian study of 301 older inpatients, found that PIM use in 24% of patients with ≥1 STOPP criteria PIM (n = 105) was plausibly linked with preventable ADEs, which were considered causal or contributory to the admission. 29 However, the rate of STOPP criteria PIM use on admission was higher in our study (83%) than in the Malaysian study (39.5%).
In this study, we also looked at the change from admission to discharge in the use of fall‐risk‐increasing PIMs. Results of our study show a significant increase in the odds of taking at least 1 fall‐related STOPP criteria PIM in people with dementia compared to those without dementia on discharge, despite a large body of evidence indicating people with dementia are particularly vulnerable to poor outcomes associated with a fall. 30 This may be related to a significant increase in the use of psychotropic medicines on discharge in people with dementia (70.3%) than in those without dementia (54.4%). It is recommended that people with dementia on fall‐risk‐increasing PIMs with a history of falls should have their medication reviewed, and if possible discontinued to reduce their risk of falling, which is a major cause of morbidity and mortality in dementia. Prior studies examining the factors for psychotropic prescribing in people with dementia have largely focused on RACFs, although evidence is limited in acute care settings. In a qualitative study from the UK, old age psychiatrists believe there is a lack of viable alternatives and pressure from nurses, care home staff and families to prescribe antipsychotics for behavioural and psychological symptoms of dementia. 31 Future studies should explore the reasons for the observed increase in the use of PIMs of fall‐risk‐increasing medicines in people with dementia in acute care setting.
The present findings have implications for improving health outcomes by preventing medication‐related harm in older adults and people with dementia. One‐quarter of hospital admissions for patients taking at least 1 PIM were attributed to PIM exposure. It is possible that interventions targeting PIM use, which is modifiable, could lead to improved health outcomes in older adults and people with dementia. Furthermore, although hospitalisation could enable the opportunity for focused medication review and subsequent reduction in prescribing of PIMs, our result suggests missed opportunities to improve medicines use during this time. Future studies are warranted to generate evidence on risk factors that drive prescribing of PIMs in older adults and people with dementia so tailored interventions can be designed to reduce PIM use. In South Australia, there are several hospital‐based initiatives to achieve continuity in medicines management. These include ward‐based clinical pharmacy services such as medication review and reconciliation (on admission, during admission and on discharge) through MedMAP (medication management plan), preadmission clinic and emergency department pharmacist services (some public hospitals) 32 which help to reduce PIM use in people with dementia. However, data regarding the extent to which these services are provided to people with dementia are limited. Published systematic reviews have shown additional interventions that could help to optimise medicines use for people with dementia. 33 These include deprescribing interventions, comprehensive geriatric assessment and consultations, multidisciplinary case conferencing and training of nursing staff for PIM use reduction in people with dementia. 33
The strengths of this study are the use of most recent version of the STOPP (2015 version 2) and Beers (2019) criteria to identify PIM use, use of data from a multicentre prospective cohort study from 5 major public hospitals, and the involvement of expert panels in the assessment of PIM‐related events associated with hospital admissions. Study limitations included restriction to a cohort of older inpatients who were taking at least 5 medicines prior to admission, and this study sample may not be representative of an entire RACF resident population, although polypharmacy is common among RACF residents. Thus, the prevalence of PIM use should be interpreted in view of the study cohort characteristics, older adults (≥ 75 years) who were taking multiple medicines (5 or more medicines). Some causality ratings were rated as unassessable/unclassifiable and these may have been assessed as probable, possible or certain if sufficient information with regard to history of present illness (e.g. aggression, aspiration), and time of medication initiation (some benzodiazepines and opioids) were available. Similarly, had this information been available (e.g. what happened/how did the patient fall, blood pressure reading just after fall), some cases rated as probable or possible may have been re‐assessed as certain. We have enrolled consecutively admitted older adults over a 5‐week period, and therefore the impact of seasonal variation in hospital admissions was not accounted in this study. Another limitation was the relatively small sample size, which may have precluded the ability to detect any statistically significant differences in PIM use between groups and from admission and discharge. It may be that statistically significant differences would have emerged with a larger sample size, which has the potential to limit the generalisability of our findings.
5. CONCLUSION
Our findings indicate that a substantial proportion of acutely hospitalised aged care residents with and without dementia and/or cognitive impairment received PIMs at hospital admission and discharge. Among participants exposed to PIMs, over a quarter of hospital admissions were attributable to PIM use. Tailored interventions to reduce PIM use and associated harm among aged care residents are warranted.
COMPETING INTERESTS
There are no competing interest to declare.
CONTRIBUTORS
All authors have made substantial contributions to the study. T.C.E. contributed to the study conception and design, analysis and interpretation of data, and drafting the manuscript. G.R., T.A.N., M.H.G., D.M. and L.M.K.E. contributed to the study conception and design, and interpretation of data and revised the manuscript critically for important intellectual content. All authors have approved the final manuscript.
AOR: adjusted odds ratio; CI: confidence interval
ACKNOWLEDGEMENTS
T.C.E. is supported by an Australian Government Research Training Program Scholarship. L.M.K.E. and T.A.N. are the recipients of NHMRC‐ARC Dementia Research Development Fellowships (L.M.K.E. Grant identification number APP1101788, TAN Grant identification number APP1103860). The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of NHMRC.
Eshetie TC, Roberts G, Nguyen TA, Gillam MH, Maher D, Kalisch Ellett LM. Potentially inappropriate medication use and related hospital admissions in aged care residents: The impact of dementia. Br J Clin Pharmacol. 2020;86:2414–2423. 10.1111/bcp.14345
The authors confirm that the PI for this paper is Greg Roberts and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from Greg Roberts. Restrictions apply to the availability of these data, which requires ethics approvals from the Southern Adelaide Clinical Human Research Ethics Committee. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Shepherd H, Livingston G, Chan J, et al. Hospitalisation rates and predictors in people with dementia: a systematic review and meta‐analysis. BMC Med. 2019;17(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Australian Commission on Safety and Quality in Health Care . Evidence for the safety and quality issues associated with the care of patients with cognitive impairment in acute care settings: a rapid review. Sydney: The Commission; 2013. [Google Scholar]
- 3. Fogg C, Griffiths P, Meredith P, Bridges J. Hospital outcomes of older people with cognitive impairment: an integrative review. Int J Geriatr Psychiatry. 2018;33(9):1177‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leendertse AJ, Van Den Bemt PMLA, Poolman JB, et al. Preventable hospital admissions related to medication (HARM): cost analysis of the HARM study. Value Health. 2011. 1//;14(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 5. O'Connor MN, Gallagher P, O'Mahony D. Inappropriate prescribing [journal article]. Drugs Aging. 2012;29(6):437‐452. [DOI] [PubMed] [Google Scholar]
- 6. O'Mahony D, O'Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2014;16:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher P, O'Connor M, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clinical Pharmacology & Therapeutics. 2011;89(6):845‐854. [DOI] [PubMed] [Google Scholar]
- 8. American Geriatrics Society . American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. [DOI] [PubMed] [Google Scholar]
- 9. O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson NM, March LM, Sambrook PN, Hilmer SN. Medication safety in residential aged‐care facilities: a perspective. Ther Adv Drug Saf. 2010;1(1):11‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Australian Institute of Health and Welfare . Dementia among aged care residents: first information from the Aged Care Funding Instrument. Canberra: AIHW; 2011. [Google Scholar]
- 12. Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case‐control study. BMJ. 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pérez T, Moriarty F, Wallace E, et al. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ. 2018;363:k4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frankenthal D, Lerman Y, Lerman Y. The impact of hospitalization on potentially inappropriate prescribing in an acute medical geriatric division [journal article]. Int J Clinic Pharm. 2015. February 01;37(1):60‐67. [DOI] [PubMed] [Google Scholar]
- 15. Juliano ACDSRS, Lucchetti ALG, Silva JTSD, et al. Inappropriate prescribing in older hospitalized adults: a comparison of medical specialties. J am Geriatr Soc. 2018;66(2):383‐388. [DOI] [PubMed] [Google Scholar]
- 16. Chróinín DN, Neto HM, Xiao D, et al. Potentially inappropriate medications (PIMs) in older hospital in‐patients: prevalence, contribution to hospital admission and documentation of rationale for continuation. Australas J Ageing. 2016;35(4):262‐265. [DOI] [PubMed] [Google Scholar]
- 17. Chang WT, Kowalski SR, Sorich W, Alderman CP. Medication regimen complexity and prevalence of potentially inappropriate medicines in older patients after hospitalisation [journal article]. Int J Clinic Pharm. 2017. August 01;39(4):867‐873. [DOI] [PubMed] [Google Scholar]
- 18. Doody HK, Peterson GM, Watson D, Castelino RL. Retrospective evaluation of potentially inappropriate prescribing in hospitalized patients with renal impairment. Curr Med Res Opin. 2015. 2015/03/04;31(3):525‐535. [DOI] [PubMed] [Google Scholar]
- 19. Kable A, Fullerton A, Fraser S, et al. Comparison of potentially inappropriate medications for people with dementia at admission and discharge during an unplanned admission to hospital: results from the SMS dementia study. Healthcare. 2019;7(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutiérrez‐Valencia M, Izquierdo M, Malafarina V, et al. Impact of hospitalization in an acute geriatric unit on polypharmacy and potentially inappropriate prescriptions: a retrospective study. Geriatr Gerontol Int. 2017;17(12):2354‐2360. [DOI] [PubMed] [Google Scholar]
- 21. WHO Collaborating Centre for Drug Statistics Methodology . Anatomical Therapeutic Chemical Code Classification Index with Defined Daily Doses Oslo. Norwegian Institute of Public Health; 2016. Available from https://www.whocc.no/atc_ddd_index/. [Google Scholar]
- 22. Panel BTAGSBCUE . American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J am Geriatr Soc. 2015;63(11):2227‐2246. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organisation . The use of the WHO‐UMC system for standardised casecausality assessment [cited 2019 Aug 11]. Available from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf
- 24. Kristensen RU, Norgaard A, Jensen‐Dahm C, et al. Polypharmacy and potentially inappropriate medication in people with dementia: a Nationwide study. J Alzheimers Dis. 2018;63(1):383‐394. [DOI] [PubMed] [Google Scholar]
- 25. Morin L, Laroche M‐L, Texier G, Johnell K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J am Med Direc Assoc. 2016. 9/1/;17(9):862.e1‐862.e9. [DOI] [PubMed] [Google Scholar]
- 26. Clague F, Mercer SW, McLean G, et al. Comorbidity and polypharmacy in people with dementia: insights from a large, population‐based cross‐sectional analysis of primary care data. Age Ageing. 2016;13:2016. [DOI] [PubMed] [Google Scholar]
- 27. Gallagher P, O'Mahony D. STOPP (screening tool of older Persons' potentially inappropriate prescriptions): application to acutely ill elderly patients and comparison with Beers' criteria. Age Ageing. 2008;37(6):673‐679. [DOI] [PubMed] [Google Scholar]
- 28. Hamilton H, Gallagher P, Ryan C, Byrne S, O'Mahony D. Potentially inappropriate medications defined by stopp criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013‐1019. [DOI] [PubMed] [Google Scholar]
- 29. Fahrni ML, Azmy MT, Usir E, Aziz NA, Hassan Y. Inappropriate prescribing defined by STOPP and START criteria and its association with adverse drug events among hospitalized older patients: a multicentre, prospective study. PLOS One. 2019;14(7):e0219898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shaw FE. Falls in cognitive impairment and dementia. Clin Geriatric Med. 2002. 2002/05/01/;18(2):159‐173. [DOI] [PubMed] [Google Scholar]
- 31. Wood‐Mitchell A, James IA, Waterworth A, Swann A, Ballard C. Factors influencing the prescribing of medications by old age psychiatrists for behavioural and psychological symptoms of dementia: a qualitative study. Age Ageing. 2008;37(5):547‐552. [DOI] [PubMed] [Google Scholar]
- 32. Government of South Australia . Continuity in Medication Management – A Handbook for South Australian Hospitals. 2019.
- 33. Shafiee Hanjani L, Long D, Peel NM, Peeters G, Freeman CR, Hubbard RE. Interventions to optimise prescribing in older people with dementia: a systematic review. Drugs Aging. 2019. 03;36(3):247‐267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from Greg Roberts. Restrictions apply to the availability of these data, which requires ethics approvals from the Southern Adelaide Clinical Human Research Ethics Committee. The data are not publicly available due to privacy or ethical restrictions.


