Abstract
Chronic lymphocytic leukemia (CLL) is associated with perturbed immune function and increased risk for second primary malignancies (SPM). Ibrutinib and acalabrutinib (BTKi) are effective therapies for CLL resulting in partial restoration of immune function. The incidence of and risk factors for SPM in CLL patients receiving BTKi are not yet characterized. We retrospectively determined the incidence of SPM in CLL patients treated with ibrutinib or acalabrutinib at our institution between 2009 and 2017, assessed for association between baseline characteristics and SPM incidence, and compared the observed to expected cancer incidence among age, sex, and year matched controls without CLL. After a median of 44 months follow-up, 64/691 patients (9%) were diagnosed with SPM (excluding non-melanoma skin cancer [NMSC]). The three-year cumulative incidence rate was 16% for NMSC and 7% for other SPM. On multivariable analysis, smoking was associated with increased SPM risk (HR 2.8 [95% CI: 1.6–4.8]) and higher baseline CD8 count was associated with lower SPM risk (HR 0.9 for 2-fold increase [95% CI: 0.8–0.9]). The observed over expected rate of SPM was 2.2 [95% CI: 1.7–2.9]. CLL patients treated with BTKi remain at increased risk for SPM, and secondary cancer detection is an important consideration in this population.
Keywords: second cancers, ibrutinib, acalabrutinib, chronic lymphocytic leukemia, immunosuppression
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in Western countries with a median age at diagnosis of 71 years (1). CLL is associated with a complex state of immune dysfunction, including hypo-gammaglobulinemia as well as impairment in T, NK, and neutrophil function and complement activation (2–6). Patients with CLL are at risk for morbidity and mortality related to progression of disease including Richter’s syndrome (RS), infectious complications, and an increased risk for second primary malignancies (SPM) (6–8).
Prior studies have established an increased risk for SPM relative to the general population in CLL patients among untreated patients and patients treated with chemo or chemo-immunotherapy (7, 9–16). Proposed etiologies for the increased SPM risk include shared environmental and occupational exposures, shared genetic risk factors, immune dysfunction secondary to CLL, and DNA damage from chemotherapy resulting in therapy associated myelodysplastic syndrome (tMDS) and acute myeloid leukemia (tAML) (8, 17, 18). While immunosuppression due to alkylating agents or PNA has been hypothesized as a risk factor for SPM, prior literature has not demonstrated an association between class of chemo or chemo-immunotherapy and overall SPM risk (7, 8, 19). Patterns of SPM may vary depending upon treatment, including the association between tAML and DNA damaging therapies mentioned previously, and a higher incidence of lung cancer seen in patients receiving cladribine compared to those treated with alkylating agents (19).
Novel targeted agents, including Bruton’s tyrosine kinase inhibitors (BTKi), have transformed the treatment paradigm for CLL in the past decade. Ibrutinib and acalabrutinib, both irreversible BTKi, are highly effective as therapy for both relapsed/refractory and previously untreated CLL and consequently have become widely used (20–23). Treatment of CLL with BTKi results in CLL disease control and associated partial restoration of humoral immunity and a decreasing infectious risk with ongoing response to treatment (24–26). As BTK also mediates signaling in myeloid immune cells (27–29), BTK inhibition has potentially deleterious effects on immune response, and opportunistic infections including invasive fungal infections have been reported in patients treated with ibrutinib (30–34). As immune dysfunction is central to the increased relative SPM risk in patients with CLL, the diverse effects of BTKi on immune response may change the risk for secondary malignancy. Second malignancies are an important contributor to morbidity and mortality in patients with CLL (35), and the incidence and patterns of SPM in CLL patients treated with BTKi have yet to be characterized. As this class of therapy is increasingly used, it is imperative to understand the rate of SPM during BTKi treatment for CLL.
Methods
Study Design
Patients with a diagnosis of CLL treated with either ibrutinib or acalabrutinib at The Ohio State University Comprehensive Cancer Center between December 1, 2009 and December 1, 2017 were identified retrospectively using electronic medical record prescription data after obtaining approval from the Institutional Review Board. The cutoff date for follow-up was October 30, 2018. The study was conducted in accordance with the Declaration of Helsinki. Patients were included if they were age 18 or older at start of ibrutinib or acalabrutinib treatment. Patients seen for initial consultation only were excluded, as were those with incomplete electronic medical records for the variables of interest, and those who were incarcerated at any time during treatment or follow-up.
Clinical data, including baseline clinical and demographic data at start of ibrutinib or acalabrutinib, were collected retrospectively from electronic medical records (EMR), as were serial clinical data regarding treatment discontinuation, second cancer diagnosis, second cancer management, and death. To identify second cancer diagnoses, progress notes, problem lists, and surgical pathology reports were reviewed in the EMR for each patient and additionally the EMR were queried using the search function terms “carcinoma”, “sarcoma”, “NMSC”, “SCC”, and “BCC”. For determining incidence of non-melanoma skin cancer (NMSC), operative and pathologic reports were obtained when available. Additionally, cases of patient reported NMSC, as documented in progress notes, without external confirmation were included. For other second invasive cancers, histologic diagnosis and staging were confirmed by review of pathology reports as well as operative reports and progress notes from referring providers, when applicable. Baseline laboratory values obtained within 30 days of start of BTKi therapy were recorded, including absolute CD4 and CD8 counts and natural killer cell count (defined as CD3-, CD16+ and CD56+) as determined by peripheral blood immuno-phenotyping performed as standard of care at our institution.
End Points and Statistical Analysis
The incidence of second invasive cancers seen in our cohort of patients was compared with that in the general population as standardized incidence ratios (SIR). SIR were calculated as the ratio of the observed to the expected number of SPM diagnosed following initiation of ibrutinib or acalabrutinib therapy. For patients with multiple SPM, only the first secondary invasive malignancy diagnosis was considered for SIR calculation. Data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute were used to determine expected incidence, based on cases of the same sex and year of diagnosis and similar age at diagnosis (36). In addition to SIR, 95% confidence intervals (CI) were calculated assuming a Poisson distribution for the observed incidence (37).
Cumulative incidence rates (CIR) of NMSC, RS and SPM (excluding RS and NMSC) at three years from BTKi start date were estimated using the cumulative incidence function, treating death as the competing risk; patients without event were censored at last follow-up. Overall survival (OS) was measured from start of ibrutinib or acalabrutinib treatment to death from any cause, censoring survivors at time of last follow-up. BTKi treatment duration was calculated from BTKi initiation to the time of stopping treatment, patients who remained on therapy were censored at time of last follow-up. The method of Kaplan-Meier was used to estimate OS and BTKi treatment duration. The impact of SPM on OS was evaluated by proportional hazards model treating the occurrence of SPM as a time-dependent covariate. Clinical variables were associated with the outcome of SPM occurrence (excluding NMSC and RS) using proportional sub-distributional hazards model treating death as the competing risk. Univariable models were first fit for each clinical variable. Covariates with a significance level of p<0.2 from univariable analysis were further screened using a backward selection procedure until the final model contained only prognostic variables with p<0.05. Statistical analysis was performed using SAS software 9.4. Rate sessions in SEER*Stat software were used to generate expected incidence rates (38).
Results
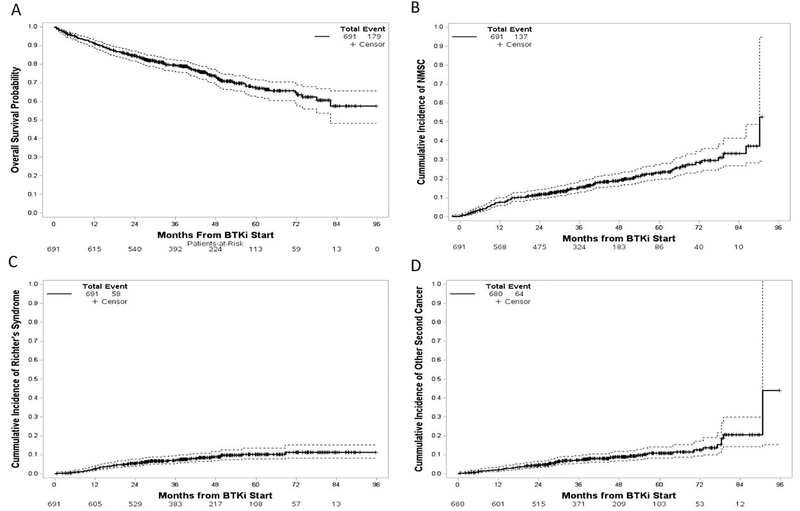
Six-hundred ninety one patients were identified who met inclusion criteria with baseline characteristics at start of BTKi treatment displayed in Table 1. The majority of patients were previously treated with PNA therapy (55%) as well as alkylating chemotherapy (60%), with a median of two prior lines of therapy. Treatment was with ibrutinib in 545 patients (79%) and acalabrutinib in 146 patients (21%). 136 patients (20%) were treatment naïve prior to ibrutinib or acalabrutinib treatment (Suppl Table 1). The median follow-up was 44 months (range 0.7–95.7). During follow-up, 346 patients discontinued ibrutinib or acalabrutinib after a median duration of treatment of 46 months (95% CI: 43–53). Reasons for treatment discontinuation included progressive disease (n=150), toxicity or intolerance (n=119), patient and/or provider preference or alternative treatment (n=51), and second malignancy (n=26). Twenty-six patients subsequently resumed BTKi therapy, among whom nine discontinued again with the remaining 17 continuing BTKi treatment at last follow-up. OS is displayed in Figure 1A; 179 deaths occurred with an estimated three year OS of 79% (95% CI: 76–82%). Causes of death included progression of CLL in 57 (32%), RS in 45 (25%), SPM in 24 (13%), infection in 23 (13%), cardiovascular disease in seven (4%), and other causes in 23 (13%) patients. Among deaths due to SPM, four deaths were in patients with SPM diagnosed prior to start of BTKi with subsequent progression while receiving BTKi and 20 deaths were in patients with SPM diagnosed after starting BTKi treatment. The most common SPM diagnoses leading to death were lung cancer in five patients, tMDS/tAML in five patients, and Merkel cell carcinoma in three patients (Suppl Table 2).
Table 1:
Baseline Patient Characteristics at Start of BTK Inhibitor Treatment
| Characteristic | Median [range] or Number (%) | Number Evaluable |
|---|---|---|
| Age | 64 [24–91] | 691 |
| Months From Diagnosis to Start of BTK Inhibitor | 75.8 [0.7–393.5] | 691 |
| Male sex | 484 (70%) | 691 |
| Race | 691 | |
| White | 649 (94%) | |
| Black | 31 (4%) | |
| BTK Inhibitor | 691 | |
| Ibrutinib | 545 (79%) | |
| Acalabrutinib | 146 (21%) | |
| Body Mass Index | 27 [17–52] | 691 |
| ECOG Performance Status 0 or 1 | 655 (95%) | 685 |
| Previously Untreated | 136 (20%) | 691 |
| Prior Lines of Therapy | 2 [0–18] | 691 |
| Prior Alkylating Chemotherapy | 413 (60%) | 691 |
| Prior Purine Nucleoside Analog | 377 (55%) | 691 |
| Prior Anti-CD20 Monoclonal Ab | 522 (76%) | 691 |
| Prior HCT | 22 (3%) | 691 |
| Smoking History | 301 (44%) | 689 |
| Platelet (1,000/μL) | 110 [7–432] | 689 |
| IgG (mg/dL) | 588 [0–3460] | 609 |
| CD4 absolute (mg/dL) | 943 [4–11274] | 595 |
| CD8 absolute (mg/dL) | 636.5 [5–10168] | 580 |
| NK absolute (mg/dL) | 258 [1–4454] | 517 |
| Deletion 11q22 | 204 (30%) | 680 |
| Trisomy 12 | 141 (21%) | 680 |
| Deletion 13q14 | 353 (52%) | 681 |
| Deletion 17p13 | 215 (32%) | 681 |
| IgVH unmutated | 451 (77%) | 586 |
BTK- Bruton’s tyrosine kinase, ECOG- Eastern Cooperative Oncology Group, Ab- antibody, HCT- hematopoietic cell transplant, NK- natural killer cell count, IgVH- immunoglobulin variable heavy chain gene
Figure 1:
(a) Overall survival from start of BTKi treatment. (b) Estimated cumulative incidence of non-melanoma skin cancer. (c) Estimated cumulative incidence of Richter’s syndrome. (d) Estimated cumulative incidence of other second primary malignancy. Survival and cumulative incidence depicted by solid lines with 95% confidence estimates depicted by dashed lines.
Second Cancer Incidence and Comparison with SEER Data
A total of 137 patients (20%) were diagnosed with one or more NMSC during follow-up after starting BTKi. Among patients with NMSC, 59 (43%) were diagnosed with squamous cell carcinoma (SCC), 60 (44%) were diagnosed with basal cell carcinoma (BCC), 15 (11%) were diagnosed with both SCC and BCC, two were diagnosed with Trichilemmal cystic carcinoma, and in one patient the NMSC histology was not documented. Three patients, all with SCC, developed metastatic disease during follow-up, with two deaths due to metastatic disease. The three-year CIR of NMSC was 16% (95% CI: 13–19%) (Figure 1B and Table 2).
Table 2:
Cumulative Incidence Rate of Second Cancers
| Cumulative Incidence Rate % (95% CI) | NMSC | Secondary Invasive Malignancy (Excluding NMSC) |
|---|---|---|
| 6 month | 3.2 (2.1–4.7) | 1.0 (0.5–2.1) |
| 1 year | 7.4 (5.6–9.5) | 1.9 (1.1–3.2) |
| 2 year | 11.4 (9.2–14.0) | 4.5 (3.1–6.3) |
| 3 year | 15.4 (12.7–18.3) | 7.1 (5.3–9.3) |
| 4 year | 19.0 (15.9–22.4) | 8.9 (6.7–11.5) |
| 5 year | 22.9 (19.0–26.9) | 10.8 (8.0–14.0) |
CI- confidence interval, NMSC- non-melanoma skin cancer
RS was diagnosed in 58 patients, including 49 (85%) with Diffuse Large B cell histology, six patients with Hodgkin’s lymphoma (10%), two patients with prolymphocytic leukemia transformation, and one patient with plasmablastic lymphoma. The three-year CIR for RT was 7% (95% CI: 5–9%) (Figure 1C).
Excluding NMSC and RS, invasive SPM were diagnosed in 64 patients (9%), including five patients with two separate primary invasive cancers diagnosed during follow-up. Additionally, five patients were diagnosed with pre-invasive cancers including one case of ductal carcinoma in situ, two cases of melanoma in situ, and two cases of high-grade urothelial carcinoma in situ. In secondary invasive cancers occurring at least twice in our cohort, we compared the incidence in our cohort with the expected incidence in patients without CLL matched on year of diagnosis, age at diagnosis, and sex using SEER Program data (Table 3). The overall incidence of SPM was 2.2 (95% CI: 1.7–2.9) times as high as that expected in the general population, with significantly increased risk for lung cancer, melanoma, bladder cancer, Merkel cell carcinoma, and salivary gland cancer (Table 3). Other second primary malignancy sites occurring only once included tongue, thyroid, uterine, colon, anal, brain (glioblastoma multiforme), unknown primary (squamous histology), langerhans cell histiocytosis (BRAF V600E mutated), essential thrombocytosis (calreticulin exon 9 mutated), and histiocytic sarcoma. The median time from initiation of BTKi to diagnosis of SPM was 26 months (range: 0.4–91). The three-year CIR of invasive SPM was 7% (95% CI: 5–9%) (Figure 1D and Table 2). Among patients with SPM diagnosed after starting BTKi (excluding NMSC), the risk of death was 40% higher in comparison to patients without SPM (HR 1.4 [95% CI: 0.9–2.1], p=0.11).
Table 3-.
Secondary Invasive Cancers, Observed Versus Expected Incidence
| Cancer Type | Observed | Expected | SIR | 95% CI |
|---|---|---|---|---|
| Overall | 64 | 28.62 | 2.2 | 1.7–2.9 |
| Lung | 14 | 4.07 | 3.4 | 1.9–5.8 |
| Prostate | 9 | 6.66 | 1.4 | 0.6–2.6 |
| Melanoma | 7 | 1.31 | 5.3 | 2.2–11 |
| Bladder | 5 | 1.34 | 3.7 | 1.2–8.7 |
| Kidney | 3 | 1.03 | 2.9 | 0.6–8.5 |
| MDS/AML | 4 | 0.75 | 5.3 | 1.5–13.7 |
| Breast | 3 | 2.32 | 1.3 | 0.3–3.8 |
| Merkel Cell Carcinoma | 3 | 0.04 | 75 | 15.5–219.2 |
| Non-Hodgkin’s lymphoma | 2 | 1.19 | 1.7 | 0.2–6.1 |
| Esophageal | 2 | 0.3 | 6.7 | 0.8–24.1 |
| Salivary gland | 2 | 0.07 | 28.6 | 3.5–103.2 |
SIR- standardized incidence rate, CI- confidence interval, MDS- myelodysplastic syndrome, AML- acute myeloid leukemia
Risk Factors for SPM Diagnosis
We next sought to determine the association between baseline clinical characteristics and diagnosis of SPM. Univariable analysis (Table 4) was performed and demonstrated an association between smoking (HR 2.6, 95% CI: 1.6–4.3, p=0.0002) and a higher risk of SPM diagnosis. A lower likelihood of SPM diagnosis was found in patients with higher baseline CD4 (HR 0.9 for two-fold increase, 95% CI: 0.8–1.0, p=0.02) and CD8 (HR 0.9 for two-fold increase, 95% CI: 0.8–1.0, p=0.0007) counts. Aside from deletion 11q by FISH (HR 1.9, 95% CI: 1.2–3.1, p=0.01), no other clinical variables were associated with risk for SPM in the univariable model including age, gender, prior lines of therapy, prior PNA, or prior alkylating chemotherapy. When comparing treatment-naive to previously treated patients, no association was seen between prior treatment and risk for second malignancy, and similarly no association was seen between patients treated with ibrutinib versus acalabrutinib and SPM incidence. On multivariable analysis (Table 4), smoking remained strongly associated with higher SPM risk (HR 2.8, 95% CI: 1.6–4.8, p=0.0003), higher baseline CD8 count continued to be associated with lower SPM risk (HR 0.9, 95% CI: 0.8–0.9, p<0.0001), and higher baseline platelet count showed an association with increased risk for SPM which reached statistical significance (HR 1.1, 95% CI: 1.0–1.2, p=0.03).
Table 4-.
Univariable and Multivariable Analysis of Risk Factors for Second Primary Invasive Malignancy
| Variable | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
| Hazard Ratio | p-value | Hazard ratio | p-value | |
| Age (5-year increase) | 1.07 (0.95–1.20) | 0.27 | --- | --- |
| Sex (male vs female) | 1.04 (0.62–1.76) | 0.88 | --- | --- |
| Baseline Performance Status | 1.03 (0.65–1.65) | 0.89 | --- | --- |
| BMI (1 unit increase) | 0.98 (0.93–1.02) | 0.28 | --- | --- |
| Prior Lines Treatment | 0.94 (0.83–1.05) | 0.27 | --- | --- |
| Prior Treatment vs Untreated | 0.92 (0.48–1.77) | 0.80 | --- | --- |
| Prior Purine Nucleoside Analog | 0.95 (0.58–1.55) | 0.83 | --- | --- |
| Prior Alkylating Chemotherapy | 0.73 (0.45–1.20) | 0.22 | --- | --- |
| Prior Anti-CD20 mAb | 0.90 (0.50–1.63) | 0.72 | --- | --- |
| Smoking (ever vs never) | 2.61 (1.56–4.34) | 0.0002 | 2.76 (1.60–4.76) | 0.0003 |
| BTK Inhibitor (Acalabrutinib vs Ibrutinib) | 0.55 (0.25–1.19) | 0.13 | --- | --- |
| Platelets, 30 unit increase | 1.08 (0.99–1.17) | 0.09 | 1.10 (1.01–1.2) | 0.03 |
| IgG (2-fold increase) | 1.09 (0.80–1.48) | 0.59 | --- | --- |
| CD4 absolute (2-fold increase) | 0.90 (0.82–0.98) | 0.02 | --- | --- |
| CD8 absolute (2-fold increase) | 0.89 (0.83–0.95) | 0.0007 | 0.87 (0.81–0.93) | <0.0001 |
| NK absolute (2-fold increase) | 0.97 (0.90–1.04 | 0.38 | --- | --- |
| Deletion 11q22 | 1.88 (1.16–3.07) | 0.01 | --- | --- |
| Trisomy 12 | 1.09 (0.61–1.93) | 0.78 | --- | --- |
| Deletion 17p13 | 1.00 (0.61–1.66) | 1.00 | --- | --- |
| Deletion 13q14 | 1.13 (0.69–1.83) | 0.63 | --- | --- |
| IgVH (mutated vs unmutated) | 0.55 (0.25–1.21) | 0.14 | --- | --- |
CI- confidence interval, BMI- body mass index, mAb- monoclonal antibody, BTK- Bruton’s tyrosine kinase, NK- natural killer cell count, IgVH- Immunoglobulin variable heavy chain gene
Specific Disease Characteristics and Management
The most commonly observed invasive SPM in our cohort of patients was lung cancer, with 14 cases including 12 cases of Non-Small Cell Lung cancer (NSCLC), one case of small cell lung cancer, and one case of well-differentiated neuroendocrine cancer (clinicopathologic information in Suppl Table 3). Two of 12 patients diagnosed with NSCLC were never smokers. Five of 12 cases of NSCLC were stage I at diagnosis, with four patients undergoing surgical resection and one patient treated with stereotactic radiation therapy. All cases of stage I cancer were diagnosed following abnormal results on scheduled imaging studies in patients enrolled in therapeutic clinical trials. After a median follow-up of 11 months among surviving patients, five of 14 patients had died (deaths attributed to metastatic lung cancer) with a median survival of 6 months from diagnosis. Of five patients with stage I NSCLC, four were alive with no evidence of disease at last follow-up, while one died due to metastatic carcinoma.
Of nine patients diagnosed with prostate cancer, seven were diagnosed by routine PSA screening (clinicopathologic characteristics in Suppl Table 4). Melanoma was diagnosed in seven patients after initiation of BTKi therapy (six stage IA and one stage IB) and was managed in all cases with surgical resection; no melanoma recurrences were observed during follow-up. Melanoma was diagnosed during preventive dermatologic evaluation in five patients. Bladder cancer was diagnosed in five patients, including muscle invasive bladder cancer in two patients and non-muscle invasive bladder cancer in three patients. Evaluation and diagnosis followed abnormal imaging findings or microscopic hematuria in two cases and the presence of symptoms in three cases. Four of five patients with bladder cancer had a prior smoking history. Three cases of kidney cancer, all stage I, were diagnosed; two of three cases were diagnosed following abnormal results on routine imaging.
Secondary hematologic malignancies were diagnosed in 11 patients, including two patients for whom the hematologic malignancy represented a third primary cancer (Suppl Table 5). Five patients were diagnosed with tMDS and one patient was diagnosed with tAML, with prior alkylating and/or PNA therapy in all cases (median time to diagnosis 9.9 years after first alkylating therapy, range 1.4– 17 years). Five of six patients with tMDS/tAML had died, with a median survival of two months from diagnosis. Two cases of T cell non-Hodgkin’s lymphoma were diagnosed, including one case of panniculitis like cutaneous T cell lymphoma occurring in a patient receiving ibrutinib as first line therapy.
A total of 10 patients were treated with the PD-1 inhibitors nivolumab or pembrolizumab for secondary malignancies while continuing ibrutinib or acalabrutinib (Suppl Table 6). One immune related adverse event was noted, a case of grade 3 pneumonitis in a patient with metastatic SCC receiving ibrutinib and nivolumab, with resolution following cessation of both agents. Five patients were treated for SPM with platinum-based chemotherapy either as adjuvant treatment or for metastatic disease while continuing BTKi treatment. A median of four cycles of chemotherapy were given (range 3–7) with no serious adverse events recorded, and no instances of discontinuation of BTKi or chemotherapy due to toxicity.
Discussion
The development of effective targeted agents, including BTKi, has transformed the management of CLL and altered the natural disease course for high-risk patients. Given the remarkable improvements in disease control and consequently survival, other competing long-term health risks including secondary malignancies take on increasing importance (39). Prior studies regarding second cancer incidence in CLL patients have primarily included patients treated prior to the development of BTKi therapy, and rates of SPM in patients treated with BTKi therapies are not well characterized. Impaired immune surveillance mediated by malignant CLL cells appears to contribute to the increased SPM risk in CLL patients, and given that treatment with BTKi leads to effective and in many cases durable CLL disease control, we sought to determine if rates of SPM remain elevated in CLL patients receiving these therapies.
In CLL patients treated with ibrutinib or acalabrutinib, we observed an overall rate of SPM 2.2 times the expected rate in the general population, including 3.4 times the expected rate for lung cancer, which was the most common SPM. While NMSC are not captured in SEER and thus SIR rates for NMSC could not be calculated, NMSC were frequently observed with a CIR of 15% at 3 years, which is similar to the observed NMSC rate in a prior single center series in CLL patients before the era of BTKi therapy (40). Regarding the overall rate of SPM reported in prior literature prior to the use of BTKi, registry studies have reported a range in the magnitude of increased risk, with SIR ranging from 1.2–2.2 in studies including both treated and untreated CLL patients (12, 13, 41, 42). The present study, which included only patients requiring CLL treatment seen at a tertiary care CLL center with a younger median age than the general CLL population, represents a select population not directly comparable to registry studies. The SIR for SPM observed in this study is similar to that reported following treatment with FCR (SIR 2.4) and similar to the reported rate in a large cohort of CLL patients followed at a single tertiary center prior to the use of BTKi (SIR 2.2) (7, 16). We observed an increased incidence specifically of lung cancer, melanoma, and bladder cancer relative to the general population. An increased risk for melanoma in CLL patients has been consistently reported, with SIR ranging from 1.9–7.7 in prior studies (7, 9, 12, 13, 41, 42). A higher than expected incidence of lung cancer in CLL patients has been observed in many but not all prior studies; including a SIR of 1.6 reported by Schollkopf et al, SIR of 2.2 reported by Royle et al, and SIR of 2.4 reported by Zheng et al (12, 13, 42). An increased risk for bladder cancer has not been consistently observed in prior studies, although two prior studies noted higher than expected incidence (Royle et al SIR 2.4, Zheng et al SIR 1.8) (12, 42).
Our study, which included both relapsed and treatment naïve patients prior to start of BTKi, showed no association between prior lines of treatment and overall SPM incidence. However, all cases of tMDS or tAML were seen in patients previously treated with cytotoxic agents, and tMDS/tAML was the joint leading cause of cancer related death in this cohort, with dismal outcomes following diagnosis consistent with prior reports (18). We did not observe a difference in SPM incidence between patients treated with ibrutinib versus those treated with acalabrutinib, although the proportion of patients receiving acalabrutinib was relatively small (21%). Our findings in CLL patients treated with BTKi are thus consistent with findings in patients treated with chemo or chemo-immunotherapy, which have found no association between class of treatment and overall SPM risk and a linear CIR throughout the duration of follow-up (7, 8, 16, 39). While the observational nature of this study limits determination of the mechanism of this persistently increased SPM risk, possible hypotheses include persistent CLL induced immune dysfunction, shared genetic risk factors, or suppressed innate immune response due to BTK inhibition.
Carcinogenic exposures leading to epithelial damage combined with impaired immune surveillance and response to injury appear to contribute to the increased SPM risk in patients with CLL. In the present study as well as prior studies of SPM incidence in patients with CLL, increased rates of lung cancer, bladder cancer and melanoma were seen compared with age and sex matched controls, and a high cumulative incidence of NMSC was seen with a higher proportion of SCC to BCC than would be expected in the general population (41, 42). Environmental exposures are unlikely to be the primary explanation for the increased SPM rates relative to the general population in this cohort or in CLL patients in general, given the lack of association between tobacco use or UV exposure and CLL incidence (43, 44). Impaired immune surveillance has been linked to increased risk for these malignancies in patients with HIV infection and patients who have undergone solid organ transplant, and low CD4 count and CD4/CD8 ratio is associated with increased lung cancer risk among HIV patients (45–47). Our study further supports an association between immune response and SPM risk among CLL patients, with higher baseline CD8 count prior to BTKi therapy associated with a decreased risk for SPM, which remained significant on multivariable analysis. While functional T cell analysis was not available in this study, we hypothesize that higher baseline absolute CD8 count is associated with less impairment in immune surveillance leading to lower SPM incidence. To our knowledge, the recognition of a correlation between baseline CD4 and CD8 count and SPM risk in a CLL population is a novel observation and builds upon previous associations in other patient populations between quantitative and qualitative deficits in lymphocyte function and risk for invasive malignancy (46, 48–51).
Given the increased incidence of SPM in patients with CLL, management of patients with concurrent CLL and a separate primary malignancy is a challenging and frequently encountered clinical scenario. Monoclonal antibodies targeting the PD-1/PD-L1 axis are established systemic therapies for a variety of malignancies including NSCLC, melanoma, bladder cancer, and Merkel cell carcinoma. Ibrutinib and acalabrutinib have been studied in combination with PD-1 inhibitors in patients with lymphoid malignancies, with the combination appearing feasible without prohibitive toxicity in phase II studies (52–54). Consistent with prospective trial observations, no unexpected safety signals were seen in our real-world cohort of 10 patients who received PD-1 inhibitor therapy for treatment of SPM while continuing ibrutinib or acalabrutinib. Similarly, although the number of patients treated was small, we observed no unexpected toxicity or treatment discontinuation when BTKi treatment was continued during systemic cytotoxic chemotherapy for SPM.
The strengths of our study include the large cohort size, inclusion and comparison of patients treated with both ibrutinib and the more selective BTKi acalabrutinib, and the availability of detailed baseline and follow-up clinical information. Our study does have important limitations. Data regarding the incidence of second malignancies were obtained retrospectively and it is possible that the incidence may be under-estimated through incomplete records or loss to follow-up, particularly for NMSC in patients treated outside of our medical system. Conversely, early detection due to the close nature of follow-up and frequent imaging of the patients included in this cohort, particularly among patients enrolled in prospective trials, may have resulted in ascertainment bias with early detection of second malignancies resulting in an apparent higher second cancer incidence over the relatively short period of follow-up. Additionally, the proportion of patients in our cohort receiving BTKi as initial therapy was relatively small. Further study is warranted to better characterize the SPM risk in patients receiving frontline BTKi given the expanding use in this indication. This study was not designed to evaluate whether ibrutinib or acalabrutinib treatment is associated with an increased SPM risk as all patients were treated with BTKi, and further study should be considered to directly compare rates of SPM between BTKi and alternative classes of CLL therapy.
Despite the limitations, this study has important implications. Establishing that patients with CLL treated with BTKi continue to experience increased risk for SPM relative to the general population supports increased awareness of this risk and further development of guidelines for screening to provide early detection. While SPM diagnosis was associated with a trend towards increased risk for death, this risk may be modifiable with early detection for many solid organ malignancies, and good outcomes were observed among patients with early stage melanoma or NSCLC diagnosed following routine imaging or comprehensive skin exam respectively. Lung cancer was the most common SPM observed, and we noted a relatively high proportion of early stage cases following scheduled imaging performed in patients enrolled on clinical trials. Establishing specific secondary cancer screening guidelines for cancers with increased incidence in patients with CLL such as lung cancer and melanoma should be considered. We observed an association between quantitative increase in baseline CD8+ lymphocyte count and decreased risk for second cancer, further supporting impaired immune surveillance as a primary factor in the development of invasive malignancy in CLL patients and identifying quantitative changes in lymphocyte count as a potential biomarker for SPM risk. Given the importance of disease related immune dysfunction to second cancer risk among patients with CLL, further study is warranted to determine with longer follow-up whether SPM incidence rates may decrease over time in patients achieving prolonged responses to BTKi therapy and to assess for association between depth of response and SPM rates in an era of expanding novel treatment approaches allowing for greater depth of response than generally achieved with single agent BTKi therapy.
Supplementary Material
Acknowledgments
Competing Interests: KAR has consulted for Acerta Pharma, AstraZeneca, and Pharmacyclics, has received travel funding from Astra Zeneca, and has received research funding from Genentech, AbbVie, and Janssen. SAB has consulted for Pharmacyclics and Janssen. MRG has served on an advisory committee for Acerta Pharma. SMJ has received research funding from Pharmacyclics. JCB has consulted for Janssen, received travel support from TG Therapeutics, Janssen, Pharmacyclics, and Gilead, served on speakers bureau for Gilead, Pharmacyclics, Janssen, Novartis, and TG Therapeutics, and received research funding from Gilead, Acerta, BeiGene, Janssen, Genentech, Pharmacyclics, and TG Therapeutics. JAW has consulted for Janssen, Pharmacyclics, AstraZeneca, and Arqule and has received research funding from Janssen, Pharmacyclics, Loxo, and Arqule. DAB, YH, JLF, ASR, DHO, EMB, and KJM have no relevant competing interests.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–89. [DOI] [PubMed] [Google Scholar]
- 2.Dasanu CA. Intrinsic and treatment-related immune alterations in chronic lymphocytic leukaemia and their impact for clinical practice. Expert Opin Pharmacother. 2008;9(9):1481–94. [DOI] [PubMed] [Google Scholar]
- 3.Perri RT, Kay NE. Abnormal T cell function in early-stage chronic lymphocytic leukemia (CLL) patients. Am J Hematol. 1986;22(1):55–61. [DOI] [PubMed] [Google Scholar]
- 4.Schlesinger M, Broman I, Lugassy G. The complement system is defective in chronic lymphatic leukemia patients and in their healthy relatives. Leukemia. 1996;10(9):1509–13. [PubMed] [Google Scholar]
- 5.Alvarez-Mon M, Casas J, Laguna R, Jorda J, Durantez A. Clinical signification of natural killer activity in B-cell chronic lymphocytic leukemia. Eur J Haematol. 1987;38(3):268–73. [DOI] [PubMed] [Google Scholar]
- 6.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–81. [DOI] [PubMed] [Google Scholar]
- 7.Tsimberidou AM, Wen S, McLaughlin P, O’Brien S, Wierda WG, Lerner S, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(6):904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer C, Langerbeins P, Bahlo J, Cramer P, Fink AM, Pflug N, et al. Effect of first-line treatment on second primary malignancies and Richter’s transformation in patients with CLL. Leukemia. 2016;30(10):2019–25. [DOI] [PubMed] [Google Scholar]
- 9.Hisada M, Biggar RJ, Greene MH, Fraumeni JF, Jr., Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98(6):1979–81. [DOI] [PubMed] [Google Scholar]
- 10.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232(3):267–9. [PubMed] [Google Scholar]
- 11.Mellemgaard A, Geisler CH, Storm HH. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur J Haematol. 1994;53(4):218–22. [DOI] [PubMed] [Google Scholar]
- 12.Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105(7):1076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121(1):151–6. [DOI] [PubMed] [Google Scholar]
- 14.Greene MH, Hoover RN, Fraumeni JF, Jr. Subsequent cancer in patients with chronic lymphocytic leukemia--a possible immunologic mechanism. J Natl Cancer Inst. 1978;61(2):337–40. [PubMed] [Google Scholar]
- 15.Travis LB, Curtis RE, Hankey BF, Fraumeni JF, Jr., Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84(18):1422–7. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56(6):1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam CC, Ma ES, Kwong YL. Therapy-related acute myeloid leukemia after single-agent treatment with fludarabine for chronic lymphocytic leukemia. Am J Hematol. 2005;79(4):288–90. [DOI] [PubMed] [Google Scholar]
- 18.Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20(18):3878–84. [DOI] [PubMed] [Google Scholar]
- 19.Robak T, Blonski JZ, Gora-Tybor J, Kasznicki M, Konopka L, Ceglarek B, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur J Cancer. 2004;40(3):383–9. [DOI] [PubMed] [Google Scholar]
- 20.Woyach JA, Blachly JS, Rogers KA, Bhat SA, Jianfar M, Lozanski G, et al. Acalabrutinib plus Obinutuzumab in Treatment-Naive and Relapsed/Refractory Chronic Lymphocytic Leukemia. Cancer Discov. 2020;10(3):394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond DA, Woyach JA. Targeting BTK in CLL: beyond Ibrutinib. Curr Hematol Malig Rep. 2019;14:197–205. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131(17):1910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126(19):2213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132(18):1985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiedler K, Sindrilaru A, Terszowski G, Kokai E, Feyerabend TB, Bullinger L, et al. Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood. 2011;117(4):1329–39. [DOI] [PubMed] [Google Scholar]
- 29.Lougaris V, Baronio M, Vitali M, Tampella G, Cattalini M, Tassone L, et al. Bruton tyrosine kinase mediates TLR9-dependent human dendritic cell activation. J Allergy Clin Immunol. 2014;133(6):1644–50 e4. [DOI] [PubMed] [Google Scholar]
- 30.Ahn IE, Jerussi T, Farooqui M, Tian X, Wiestner A, Gea-Banacloche J. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128(15):1940–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–9. [DOI] [PubMed] [Google Scholar]
- 32.Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin Infect Dis. 2018;67(5):687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors Targeting Immune Signaling Pathways. Clin Infect Dis. 2018;66(1):140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, El Boghdadly Z, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33:2527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leuk Lymphoma. 2004;45(3):507–13. [DOI] [PubMed] [Google Scholar]
- 36.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence- SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 (Sub 2000–2016) <Katrina/Rita Population Adjustment> -Linked to County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. [Google Scholar]
- 37.Sahai H, Khurshid A. Confidence-Intervals for the Mean of a Poisson-Distribution - a Review. Biometrical J. 1993;35(7):857–67. [Google Scholar]
- 38.Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 8.3.5.
- 39.Mulligan SP, Shumack S, Guminski A. Chronic lymphocytic leukemia, skin and other second cancers. Leuk Lymphoma. 2019;60(13):3104–6. [DOI] [PubMed] [Google Scholar]
- 40.Mansfield AS, Rabe KG, Slager SL, Schwager SM, Call TG, Brewer JD, et al. Skin cancer surveillance and malignancies of the skin in a community-dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia. J Oncol Pract. 2014;10(1):e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton LM, Curtis RE, Linet MS, Bluhm EC, Tucker MA, Caporaso N, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28(33):4935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng G, Chattopadhyay S, Sud A, Sundquist K, Sundquist J, Forsti A, et al. Second primary cancers in patients with acute lymphoblastic, chronic lymphocytic and hairy cell leukaemia. Br J Haematol. 2019;185(2):232–9. [DOI] [PubMed] [Google Scholar]
- 43.Cahoon EK, Pfeiffer RM, Wheeler DC, Arhancet J, Lin SW, Alexander BH, et al. Relationship between ambient ultraviolet radiation and non-Hodgkin lymphoma subtypes: a U.S. population-based study of racial and ethnic groups. Int J Cancer. 2015;136(5):E432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton LM, Hartge P, Holford TR, Holly EA, Chiu BC, Vineis P, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph). Cancer Epidemiol Biomarkers Prev. 2005;14(4):925–33. [DOI] [PubMed] [Google Scholar]
- 45.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. [DOI] [PubMed] [Google Scholar]
- 46.Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4(2):e67–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–31. [DOI] [PubMed] [Google Scholar]
- 48.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–9. [DOI] [PubMed] [Google Scholar]
- 49.Kesselring A, Gras L, Smit C, van Twillert G, Verbon A, de Wolf F, et al. Immunodeficiency as a risk factor for non-AIDS-defining malignancies in HIV-1-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2011;52(12):1458–65. [DOI] [PubMed] [Google Scholar]
- 50.Dutta A, Uno H, Lorenz DR, Wolinsky SM, Gabuzda D. Low T-cell subsets prior to development of virus-associated cancer in HIV-seronegative men who have sex with men. Cancer Causes Control. 2018;29(11):1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asgari MM, Ray GT, Quesenberry CP, Jr., Katz KA, Silverberg MJ. Association of Multiple Primary Skin Cancers With Human Immunodeficiency Virus Infection, CD4 Count, and Viral Load. JAMA Dermatol. 2017;153(9):892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6(2):e67–e78. [DOI] [PubMed] [Google Scholar]
- 53.Jain N, Basu S, Thompson P, Ohanian M, Ferrajoli A, Pemmaraju N, et al. Nivolumab Combined with Ibrutinib for CLL and Richter Transformation: A Phase II Trial. Blood. 2016;128(22):59. [Google Scholar]
- 54.Witzig TE, Maddocks K, De Vos S, Lyons RM, Edenfield J, Sharman J, et al. Phase 1/2 Trial of Acalabrutinib Plus Pembrolizumab in Relapsed/ Refractory Diffuse Large B-cell Lymphoma. J Clin Oncol. 2019;37(Suppl abstr 7519):7519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.