Abstract
As loss of contractile function in heart disease could often be mitigated by increased cardiomyocyte number, expansion of cardiomyocyte endowment paired with increased vascular supply is a desirable therapeutic goal. Insulin-like growth factor 1 (IGF-1) administration increases fetal cardiomyocyte proliferation and heart mass, but how fetal IGF-1 treatment affects coronary growth and function is unknown. Near-term fetal sheep underwent surgical instrumentation and were studied from 127 to 134 d gestation (term=147 d), receiving either IGF-1 LR3 or vehicle. Coronary growth and function were interrogated using pressure-flow relationships, an episode of acute hypoxia with progressive blockade of adenosine receptors and nitric oxide synthase, and by modeling the determinants of coronary flow. The main findings were that coronary conductance was preserved on a per-gram basis following IGF-1 treatment, that adenosine and nitric oxide contributed to hypoxia-mediated coronary vasodilation similarly in IGF-1-treated and Control fetuses, and that the relationships between coronary flow and blood oxygen contents were similar between groups. We conclude that IGF-1-stimulated fetal myocardial growth is accompanied by appropriate expansion and function of the coronary vasculature. These findings support IGF-1 as a potential strategy to increase cardiac myocyte and coronary vascular endowment at birth.
Keywords: coronary autoregulation, adenosine, nitric oxide, angiogenesis, developmental origins, contracture
INTRODUCTION
Heart disease is the leading cause of death in the United States despite advanced medical and interventional therapies (1). The push to develop regenerative therapies for the adult myocardium has so far been unsuccessful because cardiac myocytes are non-proliferative after the perinatal period, and establishing a differentiated electrical syncytium from stem cells with necessary interstitial and vascular cells and structures has proved challenging (2). Young age has been found to be key to stimulating abundant, healthy proliferation of working cardiac myocytes and expansion of the coronary vasculature (3-10), therefore therapies to stimulate therapeutic myocardial expansion might first be successful in fetuses or infants at risk for heart failure. We have shown that insulin-like growth factor 1 (IGF-1) increases fetal myocyte proliferation and heart mass (11-13), and is a potential therapeutic agent to increase myocyte endowment and strengthen the perinatal heart (14-16). How IGF-1 affects coronary growth and function in the fetus is currently unknown.
New myocardium requires concomitant establishment of adequate vascular supply (17), as appropriate coronary vascularity is critical for life-long function and health (18-22). Inadequate coronary supply contributes to angina, cardiac syndrome X, impaired cardiac function, impaired healing, and cellular necrosis and apoptosis. Although regulation of vascular growth is a very complex process, IGF-1 is a good candidate for initiating coronary growth in the fetus as it stimulates endothelial and vascular smooth muscle growth and regulates vascular function in other models (23-26). Alternatively, new cardiac muscle may itself stimulate vascular growth in the IGF-1-treated fetus. Therefore, we hypothesized that vascular supply would grow to match myocardial growth and coronary function would be normal in these hearts.
The purpose of this study was to determine how IGF-1 treatment to increase fetal cardiac mass affects coronary growth and function in near-term fetal sheep. Three main experimental approaches were used to investigate coronary function in IGF-1-treated fetal sheep. First, we evaluated pressure-flow relationships before and after treatment to determine fundamental characteristics of coronary hemodynamics such as autoregulation and reserve (27). Second, at the end of the IGF-1 treatment, we created an episode of acute hypoxia to determine metabolic control of coronary flow and the signaling molecules responsible (27). Third, we simultaneously sampled arterial and coronary sinus blood under variable coronary flow conditions, including during spontaneous prelabor uterine contractions, to determine if IGF-1 changed variability in fetal coronary flow.
MATERIALS AND METHODS
Experimental model
Animal protocols were approved by the Institutional Animal Care and Use Committee, and conducted according to Guide for the Care and Use of Laboratory Animals (28). An experimental schematic is shown in Figure 1. Fasted ewes were given intramuscular atropine (7.5 mg) to control secretions, and anesthesia was induced with intravenous ketamine (400 mg; Fort Dodge Animal Health, Overland Park, KS, USA) and diazepam (10 mg; Abbott Laboratories, Abbot Park, IL, USA). Ewes were intubated and ventilated with oxygen (O2)-nitrous oxide (2:0.7) and isoflurane (1.5-2.0%). Fetal sheep were surgically instrumented with vascular catheters (Scientific Commodities, Lake Havasu City, AZ, USA), inflatable occluders (In Vivo Metric, Healdsburg, CA, USA) on the inferior vena cava and post-ductal thoracic aorta, and a transit-time flow probe (Transonic, Ithaca, NY, USA) on the circumflex coronary artery. At the conclusion of fetal surgery, ciprofloxacin (2 mg) and penicillin G (1,000,000 units) were injected into the amniotic sac. After abdominal closure, catheters were placed in the ewe’s main femoral artery (micropolyurethane tubing, 0.066 in inner diameter, 0.095 in outer diameter; Scientific Commodities, Lake Havasu City, AZ, USA) and trachea (standard bore extension set through a 3 mm punch biopsy hole; B. Braun Medical Inc, Bethlehem, PA, USA); penicillin G (1,000,000 units) was instilled into the subcutaneous space after placement of the tracheal catheter. Ewes received subcutaneous buprenorphine (0.3 mg; Bedford Laboratories, Bedford, OH, USA) immediately and twice daily for 2 days thereafter. Surgical recovery was 6.4±1.5 d.
Figure 1.
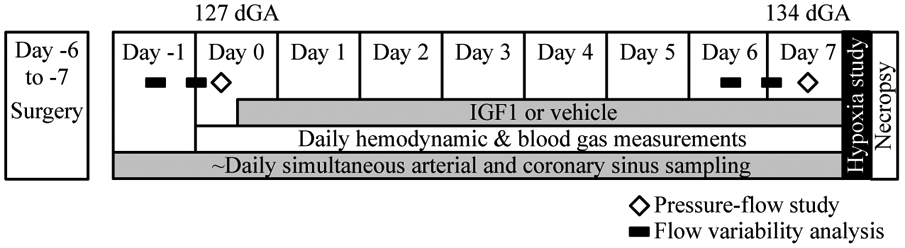
The experimental timeline.
Ewes were housed in metabolic pens that permitted standing or lying down at will, and received food and water ad libitum. Very low-flow heparinized lactated Ringer’s solution was infused into fetal catheters (Minipuls 3, Gilson, Middleton, WI, USA) to keep them open for continuous pressure recording. In-line transducers (Transpac, Abbott, Abbott Park, IL, USA) were connected to a bridge amplifier and recorder (PowerLab, ADInstruments, Colorado Springs, CO, USA) from which continuous hemodynamic data were recorded. Pressures were corrected daily for transducer voltage drift. Vascular pressures were normalized to intra-amniotic pressure. Heart rate was determined from the arterial waveform. Approximately an hour of early morning recorded hemodynamic data was averaged to determine daily hemodynamic parameters (Fig. 1). Arterial blood gas and content samples, and coronary sinus samples if available, were analyzed immediately on a Radiometer ABL 825 (Radiometer America, Cleveland, OH, USA). Fetuses received IGF-1 LR3 (6.6 μg hr−1 kg−1 based on estimated fetal weight at surgery and adjusted daily thereafter; Gropep Bioreagents, Thebarton, SA, Australia) or saline (volume matched, ~30 mL day−1) from 127 d gestational age (gestational length = 147 days) until 134 d gestational age (Fig. 1).
Thirty-three ewes and fetuses had surgery, of which 6 fetuses did not survive surgical recovery. At the beginning of the study period, 3 fetuses were noted to be spontaneously hypoxic and excluded, while 24 in good health were randomized to a study group. Of these, 20 completed the IGF-1 or vehicle treatment protocol. Litter size and fetal sex were randomized between treatment groups and not different. Seven fetuses were singletons and 13 were twins (only one of any twin pair was instrumented and studied). Eleven fetuses were male and 9 were female.
At the conclusion of the experiment, animals were humanely euthanized with an overdose of a commercial sodium pentobarbital solution, the fetuses weighed, and fetal tissues rapidly processed for analysis (Fig. 1). A catheter was placed in the coronary artery at the level of the flow probe and secured. Evan’s blue dye was instilled into the myocardium, and the area served by the artery dissected and weighed.
Explanation of hemodynamic parameters
Cardiac work is heart rate multiplied by the difference between mean arterial pressure and mean venous pressure, and is expressed as mmHg beat min−1.
Coronary conductance is the relationship between coronary flow and perfusion pressure, normalized to weight of myocardium perfused, expressed as mL min−1 g−1 mmHg−1.
Coronary reserve is the degree to which, at a given pressure, flow can be increased through vasodilation, which allows metabolic activity to rise above baseline. It is expressed as fold difference. Reserve was calculated based on flow at each fetus’ resting pressure on the day of study, with and without adenosine.
O2 delivery is the O2 brought to the myocardium in arterial blood. It is calculated as coronary flow multiplied by CaO2 (where arterial O2 content = CaO2). We express it per work as mL g−1 beat−1 mmHg−1.
O2 consumption is the O2 taken up by the myocardium. It is calculated as coronary flow multiplied by the difference between CaO2 and CvO2 (where coronary sinus O2 content = CvO2), and expressed as mL min−1 g−1. We also expressed it per cardiac work as mL g−1 mmHg−1 beat−1.
O2 extraction ratio is the proportion of oxygen that is brought to the myocardium which is taken up. It is calculated as the CaO2 and CvO2 difference divided by CaO2, and is expressed as a percent. We also discuss the raw value of O2 extracted from arterial blood, expressed as mL dL−1.
Coronary pressure-flow relationship
On days 0 (prior to initiation of IGF-1 or vehicle treatment) and 7 (Fig. 1), the relationship between perfusion pressure and circumflex coronary flow was measured (Fig. 2). Heart rate was controlled with propranolol (1 mg kg−1) and atropine (0.5 mg kg−1). Over approximately 10 seconds, arterial pressure was ramped up by progressive inflation of the aortic occluder, or down by progressive inflation of the occluder on the inferior vena cava. After releasing the occluder, pressures, heart rate and flow were allowed to return to normal before repeating the pressure ramp to achieve a total of three reproducible ramps up and three down. A dose-response relationship was then established between adenosine (5 mg mL−1), infused into the coronary circulation via the left atrial catheter, and coronary flow. For most fetuses, an infusion of 478 μg min−1 adenosine caused maximal coronary flow without a change in systemic pressure. Pressure-flow relationships were again determined with adenosine.
Figure 2. Coronary pressure-flow relationship example.
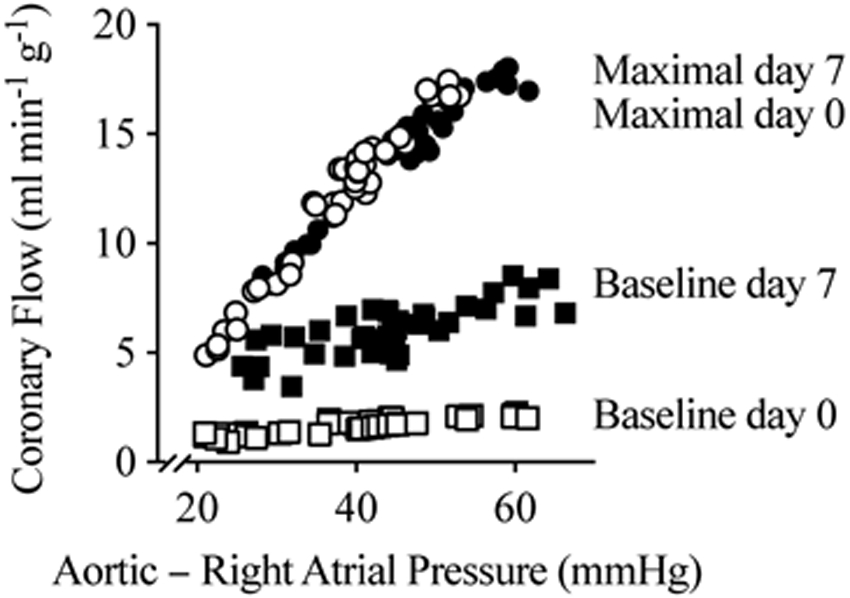
A pressure-flow relationship example from an IGF-1-treated fetal sheep. At day 0 (127 d gestational age [GA]) and at day 7 (134 dGA, term=147 dGA) after chronic IGF-1 treatment, autonomic responses were blocked and vascular occluders were used to transiently increase or decrease coronary driving pressure. Then a maximally-vasodilating dose of adenosine was administered to the coronary circulation, and the pressure manipulations were repeated. The slope of each relationship is the coronary conductance, and the difference between the slopes at a given pressure, on a given day, is the coronary reserve.
Coronary flows were corrected to mass of myocardium perfused in each animal. On day 7, the mass was measured directly by staining with Evan’s blue and dissection, as described above. For day 0, mass was imputed from previously published heart growth rates from the same herd (5), using the assumptions that the ratio of myocardium represented by the stained weight to total heart weight was proportional on days 0 and 7, and that the IGF-1-treated group had an average heart weight on day 0.
Coronary metabolic adaptation during acute hypoxia
On day 7, following the pressure-flow relationship study, an acute hypoxia study was conducted in the 10 Control fetuses and 7 IGF-1-infused fetuses (Fig. 1) of ewes with functional tracheal catheters. Heart rate continued to be controlled with propranolol (1 mg kg−1) and atropine (0.5 mg kg−1). Maternal and fetal hypoxia were caused by infusion of nitrogen gas into a maternal tracheal catheter in the awake, calmly resting ewe. Maternal and fetal blood gases were measured every 5 minutes, while fetal hemodynamics were continuously recorded. Once a stable plane of hypoxia was reached (half normal maternal saturation), it was maintained throughout the experiment (54±7 min). Theophylline, to block adenosine receptors (AR), was given as a bolus followed by continuous infusion into the right atrium at three increasing rates (10 mg + 0.25 mg min−1, 20 mg + 0.75 mg min−1, 30 mg + 1.5 mg min−1). Nitric oxide synthase (NOS) was blocked with Nω-Nitro-L-arginine methyl ester (L-NAME), also given as a bolus followed by continuous infusion (30 mg + 6 mg min−1). As the results were the same whether L-NAME was given first or last, the data are presented as progressive blockade bins regardless of order. Some values are missing in some analyses as two ewes did not complete the full hypoxia experiment (because e.g. their tracheal catheter dislodged) or because fetal coronary sinus catheters stopped functioning.
Simultaneous analysis of arterial and coronary sinus blood during variable coronary flow
Two approaches were used to analyze mediators of fetal coronary flow. The variability of fetal coronary flow was analyzed over approximately 6 hours starting at 9 am or 9 pm on days −1 and 6 (to avoid confounding by the experimental manipulations on days 0 and 7; Fig. 1). Elevations from baseline flow in the same sampling period were manually identified, and characteristics of the elevations were noted and compared with immediately adjacent baseline periods.
An analysis was undertaken to understand the relationships between putative mediators, including IGF-1 treatment, and coronary flow. Simultaneous arterial and coronary sinus blood samples were drawn at various times, and analyzed together with the hemodynamic values recorded from the same seconds during which the samples were drawn. Sampling periods included during spontaneous flow variations in the normal, conscious animal, as well as during experimental interventions such as during maximal coronary vasodilation with adenosine, during acute hypoxia, and during acute hypoxia with AR and NOS blockade. The relationship between coronary flow and these factors were explored by mixed effects gamma regression.
Statistical analysis
Coronary pressure-flow conductances were calculated using linear regression. Coronary hemodynamic measurements during the period of maternal nitrogen breathing were tested for the presence of a linear trend. Daily blood gas and hemodynamic values, and those obtained during the hypoxia study, and coronary conductances were analyzed by two-way repeated measures analysis of variance (ANOVA), or two-way mixed effects analysis (if there were missing values), followed, if justified, by the Holm-Šidák multiple comparisons test. Spontaneous variability of coronary flow was analyzed by three-way mixed model ANOVA. Differences from a hypothetical value were assessed by one-sample t-test for normally distributed data or Wilcoxon signed rank test otherwise. Mixed effects gamma regression with a logarithmic link function was used to investigate the relationship between coronary flow and putative regulators for data where AR and NOS blockade were absent. A gamma model was selected due to the continuous, positive, and right-skewed dependent variable and provided better fit for these data than log transformation. A random intercept term was included to address clustering of observations within sheep. Variable selection for fixed effects was conducted with backward selection based on Bayesian information criterion. Variables cardiac work, CaO2, CvO2, myocardial lactate production, intracoronary adenosine, maternal hypoxia, IGF-1 treatment, systolic pressure load, and study day were considered for inclusion as were interaction terms between CaO2 and CvO2 as well as IGF-1 treatment and study day. All continuous predictors were mean-centered and scaled; quadratic terms were also evaluated to allow for curvilinear relationships. Model diagnostics were used to assess model fit, violations of model assumptions, and identification of influential observations. Coefficients and bootstrapped 95% confidence intervals were estimated from the final model along with the intraclass coefficient for clustered Gamma-distributed data (29). For analyses other than the mixed effects model, significance was determined at P<0.05; exact P-values are given for values of 0.1 or less. Data are displayed as mean ± SD. Statistical analyses were carried out using Prism (GraphPad Software, La Jolla, CA, USA, Version 8.3.0 for Mac OS X) and SAS (SAS Institute Inc., Cary, NC, USA, Version 9.4).
RESULTS
Effects of IGF-1 on fetal physiology and growth
IGF-1 or the vehicle solution was given intravenously to fetal sheep for 7 days, between gestational days 127 and 134 (Fig. 1). Heart rate decreased with gestational age in Control fetuses as expected, but was elevated by IGF-1 treatment (Table 1). Coronary flow, both raw flow and flow normalized myocardial mass of the flow probe distribution area, was greatly increased by IGF-1 treatment, but it was also highly variable. Partial pressure of carbon dioxide (CO2) in arterial blood was elevated by IGF-1 treatment (Table 2), while partial pressure of O2 was decreased. Consequently, CaO2 was decreased. Circulating arterial glucose levels were lower following IGF-1 treatment. IGF-1 did not have any statistically significant effect on coronary sinus blood gas or chemistry values (Table 2).
Table 1.
Daily study hemodynamic values.
| Day of study | P-value | ||||
|---|---|---|---|---|---|
| 0 | 7 | Interaction | Day | Group | |
| Arterial pressure (mmHg) | |||||
| Control | 41.6 ± 0.5 | 43.9 ± 0.8 | NS | 0.029 | NS |
| IGF-1 | 41.3 ± 3.3 | 42.3 ± 4.3 | |||
| Venous pressure (mmHg) | |||||
| Control | 2.9 ± 0.6 | 2.7 ± 0.8 | NS 0.061 |
NS | 0.003 |
| IGF-1 | 3.3 ± 1.1 | 4.3 ± 1.3 | |||
| Heart rate (beats min−1) | |||||
| Control | 173 ± 19 | 152 ± 13† | 0.002 | - | - |
| IGF-1 | 175 ± 16 | 196 ± 25*† | |||
| Circumflex flow (mL min−1) | |||||
| Control | 7.6 ± 4.0 | 8.3 ± 3.4 | 0.017 | - | - |
| IGF-1 | 5.4 ± 1.4 | 18.9 ± 17.2*† | |||
| Circumflex flow (mL min−1 g−1) | |||||
| Control | 1.6 ± 0.8 | 1.5 ± 0.7 | 0.008 | - | - |
| IGF-1 | 1.0 ± 0.2 | 2.7 ± 1.9*† | |||
Control n=12; IGF-1-treated n=8. Data analyzed by 2-way repeated measures ANOVA.
Different within-day from Control
within-group from day 0 (P<0.05). Data shown as mean ± SD.
Table 2.
Daily study blood gas and chemistry values.
| Day of Study | P-value | ||||
|---|---|---|---|---|---|
| 0 | 7 | Interaction | Day | Group | |
| Fetal arterial blood | |||||
| pH | |||||
| Control | 7.341 ± 0.031 | 7.321 ± 0.040 | NS | NS 0.070 |
NS |
| IGF-1 | 7.338 ± 0.017 | 7.324 ± 0.025 | |||
| CO2 partial pressure (mmHg) | |||||
| Control | 51.9 ± 3.4 | 51.8 ± 2.9 | 0.002 | - | - |
| IGF-1 | 51.0 ± 2.3 | 54.1 ± 2.0† | |||
| O2 partial pressure (mmHg) | |||||
| Control | 19.6 ± 3.1 | 19.6 ± 2.0 | 0.026 | - | - |
| IGF-1 | 20.2 ± 1.7 | 17.0 ± 2.4† | |||
| Total Hemoglobin (g dL−1) | |||||
| Control | 10.1 ± 1.6 | 10.4 ± 2.3 | 0.042 | - | - |
| IGF-1 | 10.6 ± 1.4 | 9.6 ± 1.9 | |||
| O2 content (mL dL−1) | |||||
| Control | 7.2 ± 1.1 | 7.1 ± 1.5 | 0.013 | - | - |
| IGF-1 | 7.8 ± 1.4 | 5.3 ± 1.7*† | |||
| Glucose (mM) | |||||
| Control | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.011 | - | - |
| IGF-1 | 1.0 ± 0.3 | 0.7 ± 0.2† | |||
| Lactate (mM) | |||||
| Control | 1.5 ± 0.3 | 1.5 ± 0.3 | NS | NS | NS |
| IGF-1 | 1.5 ± 0.3 | 2.7 ± 2.8 | |||
| Hematocrit (%) | |||||
| Control | 34.6 ± 6.0 | 35.2 ± 7.5 | NS 0.07 |
NS | NS |
| IGF-1 | 36.4 ± 4.9 | 33.2 ± 6.8 | |||
| Fetal coronary sinus blood | |||||
| pH | |||||
| Control | 7.322 ± 0.035 | 7.292 ± 0.052 | NS | NS | NS |
| IGF-1 | 7.308 ± 0.017 | 7.304 ± 0.025 | |||
| CO2 partial pressure (mmHg) | |||||
| Control | 55.8 ± 2.4 | 58.0 ± 3.8 | NS | NS 0.061 |
NS 0.054 |
| IGF-1 | 58.7 ± 2.6 | 60.0 ± 2.2 | |||
| O2 partial pressure (mmHg) | |||||
| Control | 12.9 ± 2.0 | 11.7 ± 1.4 | NS | 0.015 | NS |
| IGF-1 | 11.7 ± 1.9 | 10.6 ± 2.2 | |||
| O2 content (mL dL−1) | |||||
| Control | 3.5 ± 1.0 | 2.8 ± 1.1 | NS | 0.022 | NS |
| IGF-1 | 2.9 ± 1.1 | 2.2 ± 0.9 | |||
| Glucose (mM) | |||||
| Control | 0.8 ± 0.2 | 0.6 ± 0.2 | NS | 0.001 | NS |
| IGF-1 | 0.8 ± 0.3 | 0.5 ± 0.2 | |||
| Lactate (mM) | |||||
| Control | 1.3 ± 0.2 | 1.2 ± 0.5 | NS | NS | NS |
| IGF-1 | 1.0 ± 0.3 | 2.5 ± 3.1 | |||
Arterial values, Control n=12 (except O2 saturation n=10) and IGF-1-treated n=8. Coronary sinus values, Control n=9 and IGF-1 n=8 on day 0, and both groups n=7 on day 7. Arterial values analyzed by 2-way repeated measures ANOVA. Coronary sinus values analyzed by mixed-effects analysis. If justified, further analysis was by the Holm-Šidák test.
Different within-day from Control
within-group from day 0 (P<0.05). Data shown as mean ± SD.
At the end of the treatment period, body weight was not significantly different between Control and IGF-1-treated fetuses (Table 3), but heart weight was 20% greater in the IGF-1 group. There was a trend for all cardiac chamber walls to be heavier in IGF-1-treated fetuses, although these differences individually only reached statistical significance for the right ventricular free wall and interventricular septum.
Table 3.
Necropsy weights.
| Control | IGF-1 | P-value | |
|---|---|---|---|
| Body (kg) | 3.9±0.8 | 4.4±0.4 | NS |
| Heart (g) | 24.0±5.7 | 30.5±4.4 | 0.013 |
| Left ventricular freewall (g) | 6.1±1.5 | 7.7±1.9 | NS 0.061 |
| Right ventricular freewall (g) | 5.8±1.8 | 8.2±1.2 | 0.005 |
| Interventricular septum (g) | 4.7±1.3 | 6.1±1.3 | 0.036 |
| Atrial freewall and septum (g) | 3.9±1.3 | 5.1±0.9 | NS 0.056 |
Control n=12, except cardiac components n=9; IGF-1 n=8. Groups compared by Student’s unpaired t-test.
Different from Control (P<0.05). Data shown as mean ± SD.
Coronary pressure-flow relationships
Coronary pressure-flow relationship experiments were conducted to explore fundamental characteristics of coronary hemodynamics. The slopes of the conductance relationship, per gram of myocardium, were significantly greater during adenosine-mediated hyperemia than during autoregulation (baseline). Neither at baseline nor during hyperemia were conductances different between groups on day 0 (Fig. 3A) or 7 (Fig. 3B). Although not significantly different between groups, coronary reserve was reduced within the IGF-1-treated group by 43% on day 7 compared to day 0 (Fig. 3C). Coronary reserve is calculated from coronary autoregulation relationships obtained under autonomic blockade.
Figure 3. Coronary pressure-flow relationships.
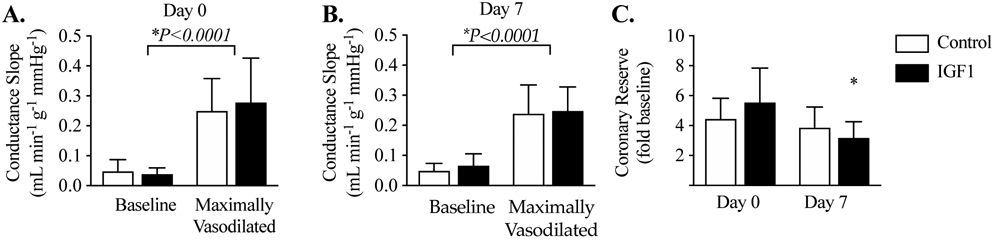
The coronary conductance, normalized to weight of myocardium perfused, was not different between groups at resting “baseline” flow, or maximal hyperemia on A) day 0, or B) day 7 of IGF-1 or vehicle treatment. C) Coronary reserve was reduced within the IGF-1 group between days 0 and 7. Control n=12, IGF-1 n=8. Data were analyzed by 2-way repeated measures ANOVA followed, if justified, by the Holm-Šidák multiple comparisons test (P<0.05). Different from *same-group day 0. Data are shown as mean ± SD.
Coronary metabolic adaptation during acute hypoxia
On the last experimental day, animals were subject to a period of acute hypoxia during autonomic blockade to determine coronary metabolic adaptation and regulatory roles for endogenous adenosine and nitric oxide. Maternal inspired O2 was reduced by infusion of nitrogen into the trachea, depressing maternal arterial O2 levels by about half (Table 4). Hypoxia was maintained at this level by nitrogen breathing for the remainder of the experiment (less than an hour).
Table 4.
Acute hypoxia experiment blood gas and chemistry values.
| AR & NOS Blockade | Interaction | Time | Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Hypoxia | + | ++ | +++ | ++++ | P-value | P-value | P-value | |
| Fetal arterial blood | |||||||||
| pH | |||||||||
| Control | 7.285 ± 0.041† | 7.262 ± 0.047 | 7.247 ± 0.047† | 7.231 ± 0.050† | 7.201 ± 0.064† | 7.194 ± 0.067† | NS | <0.0001 | NS |
| IGF-1 | 7.263 ± 0.052† | 7.222 ± 0.054 | 7.188 ± 0.073† | 7.166 ± 0.081† | 7.144 ± 0.087† | 7.092 ± 0.092† | |||
| CO2 partial pressure (mmHg) | |||||||||
| Control | 54 ± 3† | 50 ± 4 | 49 ± 5 | 48 ± 5 | 48 ± 5 | 47 ± 3† | NS | <0.0001 | 0.003 |
| IGF-1 | 59 ± 4† | 57 ± 7 | 57 ± 6 | 56 ± 6 | 56 ± 6 | 56 ± 5 | |||
| O2 partial pressure (mmHg) | |||||||||
| Control | 18.2 ± 1.9† | 10.7 ± 1.1 | 10.8 ± 1.1 | 11.4 ± 1.1 | 11.7 ± 1.3† | 12.5 ± 1.4† | <0.0001 | - | - |
| IGF-1 | 15.1 ± 2.8 | 11.1 ± 1.2 | 11.7 ± 1.7 | 12.0 ± 1.6 | 12.5 ± 2.0 | 12.2 ± 1.5 | |||
| Hemoglobin, total (g dL−1) | |||||||||
| Control | 10.7 ± 1.8† | 11.7 ± 2.1 | 11.8 ± 1.9 | 11.9 ± 2.1 | 11.9 ± 1.9 | 12.4 ± 2.2† | NS | <0.001 | NS |
| IGF-1 | 10.0 ± 2.0† | 10.7 ± 2.3 | 11.0 ± 3.0 | 10.9 ± 2.7 | 10.6 ± 2.3 | 10.6 ± 2.5† | |||
| O2 content (mL dL−1) | |||||||||
| Control | 6.4 ± 1.1† | 2.6 ± 0.4 | 2.5 ± 0.3 | 2.8 ± 0.4 | 2.9 ± 0.6 | 3.2 ± 0.8 | <0.0001 | - | - |
| IGF-1 | 4.0 ± 1.8 | 2.1 ± 0.4 | 2.2 ± 0.5 | 2.2 ± 0.6 | 2.3 ± 0.6 | 2.2 ± 0.6 | |||
| Glucose (mM) | |||||||||
| Control | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.3 | 1.3 ± 0.2† | 1.2 ± 0.3† | 0.098 | <0.0001 | 0.024 |
| IGF-1 | 0.6 ± 0.2 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.3† | 0.7 ± 0.3† | 0.8 ± 0.4† | |||
| Lactate (mM) | |||||||||
| Control | 1.9 ± 0.4† | 4.8 ± 1.6 | 5.5 ± 1.9† | 6.3 ± 1.9† | 7.4 ± 2.6† | 8.4 ± 2.6† | NS | <0.0001 | 0.03 |
| IGF-1 | 4.0 ± 4.1† | 7.3 ± 3.5 | 9.0 ± 3.6† | 10.2 ± 4.1† | 11.3 ± 4.1† | 11.9 ± 5.0† | |||
| Hematocrit (%) | |||||||||
| Control | 36 ± 6† | 39 ± 7 | 39 ± 7 | 40 ± 7 | 40 ± 7 | 41 ± 7† | NS | <0.0001 | NS |
| IGF-1 | 33 ± 7† | 37 ± 8 | 37 ± 8 | 38 ± 8 | 38 ± 8 | 38 ± 9† | |||
| Fetal coronary sinus blood | |||||||||
| pH | |||||||||
| Control | 7.253 ± 0.041† | 7.243 ± 0.028 | 7.152 ± 0.051† | NS 0.052 |
<0.0001 | NS 0.072 |
|||
| IGF-1 | 7.244 ± 0.061† | 7.181 ± 0.042 | 7.050 ± 0.065† | ||||||
| CO2 partial pressure (mmHg) | |||||||||
| Control | 62 ± 3 | 54 ± 5 | 55 ± 2 | NS | 0.027 | 0.03 | |||
| IGF-1 | 63 ± 4 | 62 ± 7 | 61 ± 5 | ||||||
| O2 partial pressure (mmHg) | |||||||||
| Control | 12.4 ± 0.9† | 6.8 ± 1.5 | 6.1 ± 1.2 | NS | <0.0001 | NS | |||
| IGF-1 | 10.4 ± 2.2† | 6.3 ± 0.6 | 6.1 ± 1.1 | ||||||
| O2 content (mL dL−1) | |||||||||
| Control | 3.2 ± 0.4† | 1.2 ± 0.2 | 0.9 ± 0.2 | 0.042 | - | - | |||
| IGF-1 | 2.0 ± 0.9 *† | 0.8 ± 0.1 * | 0.7 ± 0.1† | ||||||
| Glucose (mM) | |||||||||
| Control | 0.58 ± 0.08 | 0.72 ± 0.16 | 1.08 ± 0.10 | 0.029 | - | - | |||
| IGF-1 | 0.57 ± 0.27 | 0.65 ± 0.31 | 0.72 ± 0.34† | ||||||
| Lactate (mM) | |||||||||
| Control | 1.52 ± 0.64† | 4.98 ± 1.72 | 9.38 ± 2.15† | NS | <0.0001 | NS | |||
| IGF-1 | 4.07 ± 4.49† | 8.02 ± 3.49 | 12.60 ± 4.24† | ||||||
| Maternal arterial blood | |||||||||
| O2 partial pressure (mmHg) | |||||||||
| Control | 107.6 ± 10.9† | 40.3 ± 6.0 | 42.7 ± 4.4 | 39.3 ± 11.4 | 42.5 ± 8.2 | 41.2 ± 12.4 | NS | <0.0001 | NS |
| IGF-1 | 102.7 ± 7.3† | 42.0 ± 7.5 | 46.3 ± 5.5 | 40.7 ± 9.0 | 47.0 ± 8.0 | 38.8 ± 7.4 | |||
| O2 saturation (%) | |||||||||
| Control | 100 ± 1† | 58 ± 14 | 64 ± 11 | 55 ± 22 | 63 ± 12 | 57 ± 24 | NS | <0.0001 | NS |
| IGF-1 | 98 ± 3† | 60 ± 16 | 69 ± 13 | 58 ± 17 | 69 ± 11 | 56 ± 15 | |||
Arterial and maternal values Control n=10, except last dose n=9; IGF-1 n=7, except last dose n=6. Coronary sinus values Control n=6, except last dose n=4; IGF-1 n=6, except last dose n=5. Not significant (NS). Arterial values analyzed by 2-way repeated measures ANOVA. Coronary sinus values analyzed by mixed-effects analysis. If justified, further analysis was by the Holm-Šidák test.
Different from same-timepoint Control
same-group Hypoxia timepoint. Data shown as mean ± SD.
Fetal CaO2 decreased but was similar between the Control and IGF-1 groups after onset of maternal hypoxia (Fig. 4A). CvO2 decreased 60% in both groups with onset of hypoxia (Fig. 4B), while maximal AR and NOS blockade during hypoxia decreased CvO2 by a further 40%. The CaO2-CvO2 difference was reduced ~50% by onset of hypoxia, and was ~28% less for IGF-1-treated fetuses overall (Fig. 4C). The percent of O2 extracted from fetal arterial blood by the heart was similar between groups, rising from 53% at baseline to 68% at maximal AR and NOS blockade during hypoxia (Fig. 4D).
Figure 4. Blood O2 contents and coronary hemodynamics during acute hypoxia on study day 7.
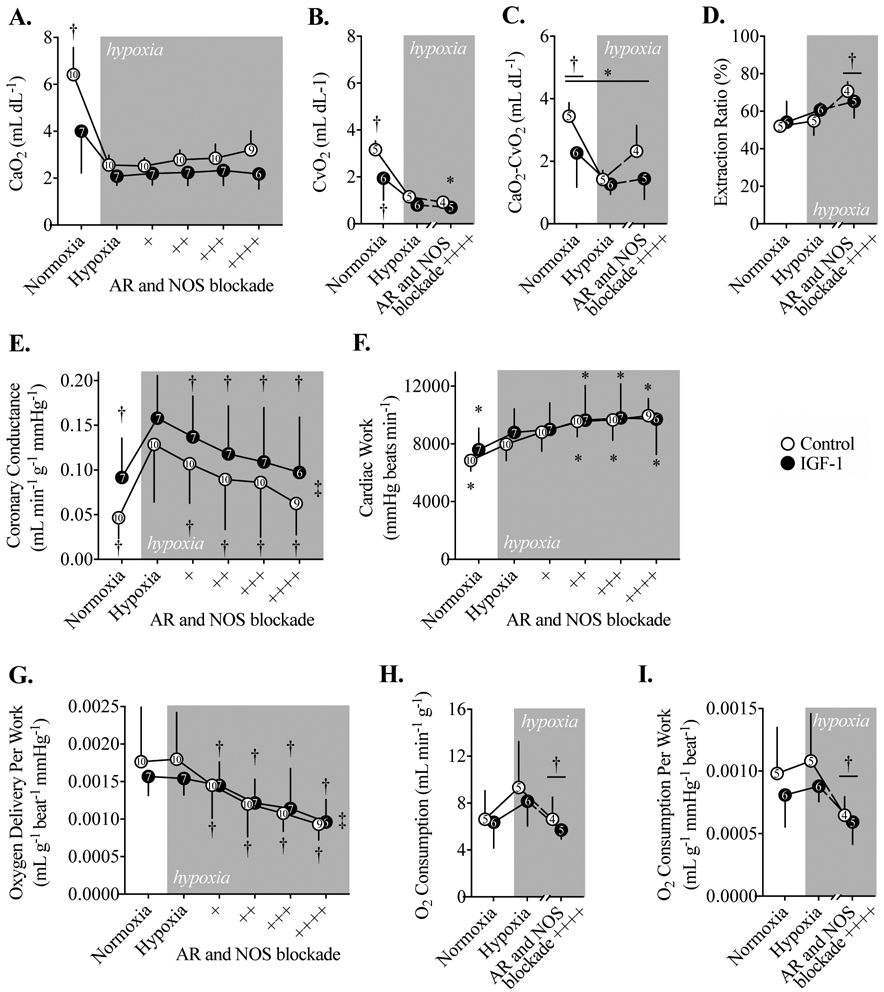
A) Arterial O2 content (CaO2) in Control and IGF-1-treated fetal sheep during acute hypoxia, and with adenosine receptor (AR) and nitric oxide synthase (NOS) blockade. B) Coronary sinus O2 content (CvO2) during acute hypoxia and maximum AR and NOS blockade. C) The difference between CaO2 and CvO2, which is the O2 extracted by the myocardium. D) Coronary extraction ratio, the percent of O2 extracted from arterial blood by the myocardium. E) Coronary conductance during acute hypoxia. F) Cardiac work, calculated as the double product. G) Myocardial O2 delivered as a function of cardiac work during acute hypoxia, and with AR and NOS blockade. H) Myocardial O2 consumption, and I) myocardial O2 consumption per work. Number of animals is shown within symbols. Different from *Control, †Hypoxia timepoint within-group by the Holm-Šidák multiple comparisons test following a significant interaction term by 2-way mixed effects analysis (a symbol and bar denotes differences at this level), and significant ‡linear trend across columns (P<0.05). Data are shown as mean ± SD.
Coronary conductance was not significantly different by treatment group, despite an apparent tendency to be greater with IGF-1 treatment (Fig. 4E). Similarly, there were no differences between the IGF-1-treated and Control groups with respect to cardiac work, myocardial O2 delivery per work, O2 consumption, and O2 consumption per work (Fig. 4F-I). As fetal CaO2 fell with the onset of maternal nitrogen breathing, fetal coronary flow increased (Table 5) and coronary conductance more than doubled in both groups (Fig. 4E). Each successive dose of AR or NOS blockade reduced conductance by ~15%. The reduction in conductance did not impair cardiac work, indeed, work increased with onset of hypoxia and with each successive dose of AR and NOS blockade (Fig. 4F). Consequently, O2 delivery (the amount of O2 presented to the myocardium by arterial blood relative to cardiac work), which remained the same with onset of hypoxia, was reduced ~15% by each successive dose of AR or NOS blockade (Fig. 4G). Further, O2 consumption, which remained the same with onset of hypoxia, was substantially reduced after maximal AR and NOS blockade (Fig. 4H-I). This was true whether O2 consumption was expressed as a raw value (Fig. 4H) or normalized to cardiac work (Fig. 4I).
Table 5.
Acute hypoxia experiment hemodynamics.
| AR & NOS Blockade | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Hypoxia | + | ++ | +++ | ++++ | Interaction | Time | Group | |
| Fetal arterial pressure (mmHg) | |||||||||
| Control | 43.8 ± 2.9† | 48.8 ± 5.8 | 52.1 ± 6.7 | 53.3 ± 6.2 | 51.5 ± 5.0 | 53.5 ± 5.3† | NS | <0.0001 | NS |
| IGF-1 | 42.9 ± 2.9† | 46.5 ± 5.8 | 47.4 ± 6.6 | 50.0 ± 10.3 | 49.7 ± 7.6 | 51.3 ± 6.9† | |||
| Fetal venous pressure (mmHg) | |||||||||
| Control | 2.4 ± 2.0 | 2.6 ± 2.2 | 2.7 ± 2.2 | 2.8 ± 2.2 | 1.9 ± 1.8† | 1.4 ± 0.9† | NS | 0.009 | NS |
| IGF-1 | 3.4 ± 1.1 | 3.5 ± 1.1 | 2.6 ± 0.8 | 2.6 ± 1.0 | 2.6 ± 1.2† | 1.9 ± 0.7† | |||
| Fetal heart rate (beats min−1) | |||||||||
| Control | 166 ± 22 | 173 ± 17 | 180 ± 28 | 192 ± 31 | 196 ± 29 | 192 ± 23 | NS | 0.022 | NS 0.086 |
| IGF-1 | 191 ± 23 | 204 ± 14 | 200 ± 23 | 203 ± 23 | 207 ± 29 | 195 ± 31 | |||
| Fetal circumflex coronary flow (mL min−1) | |||||||||
| Control | 10.0 ± 3.8† | 30.5 ± 12.3 | 27.6 ± 9.9† | 22.6 ± 9.8† | 21.5 ± 9.8† | 17.1 ± 5.9† | NS | <0.0001 | 0.035 |
| IGF-1 | 27.2 ± 16.8† | 43.5 ± 18.4 | 39.6 ± 15.5† | 36.8 ± 18.4† | 35.4 ± 21.0† | 34.8 ± 24.3† | |||
| Fetal circumflex coronary flow per myocardial mass (mL min−1 g−1) | |||||||||
| Control | 1.9 ± 0.9† | 5.9 ± 2.8 | 5.3 ± 2.1† | 4.4 ± 2.3† | 4.1 ± 2.4† | 3.2 ± 1.4† | NS | <0.0001 | NS |
| IGF-1 | 3.6 ± 1.8† | 6.7 ± 1.7 | 5.9 ± 1.3† | 5.3 ± 1.6† | 4.8 ± 1.9† | 4.5 ± 2.2† | |||
Not significant (NS). Control n=10, except last timepoint n=9; IGF-1 n=7, except last timepoint n=6. Data analyzed by 2-way mixed effects analysis and, if justified, by the Holm-Šidák test.
Different from same-timepoint Control
same-group Hypoxia timepoint. Data shown as mean ± SD.
Variable coronary flow
Coronary flow in the fetal sheep was variable. Often, spontaneous transient increases in fetal coronary flow immediately followed an increase in amniotic pressure (Fig. 5). The magnitude of these contractions did not differ by IGF-1 treatment, gestational age, or time of day, and were ~0.6 mmHg higher during a high coronary flow episode than during normal coronary flow (P<0.001 different from 0 mmHg difference). Although amniotic pressure was not greater during all periods of increased coronary flow, it was greater in 70% of those periods (P<0.001 different than 50%, which would be expected by chance). Between ~126 d gestational age and ~133 d gestational age, time spent in periods of high coronary flow increased from 22% to 29% (P<0.001). This was due to an increase in episode frequency from 1.4 per hour to 1.7 per hour (P=0.037) rather than episode duration (9.8 ± 2.8 min), which was unaffected by age, treatment, or time of day.
Figure 5. Example of spontaneous transient increases in circumflex coronary flow in a fetal sheep.
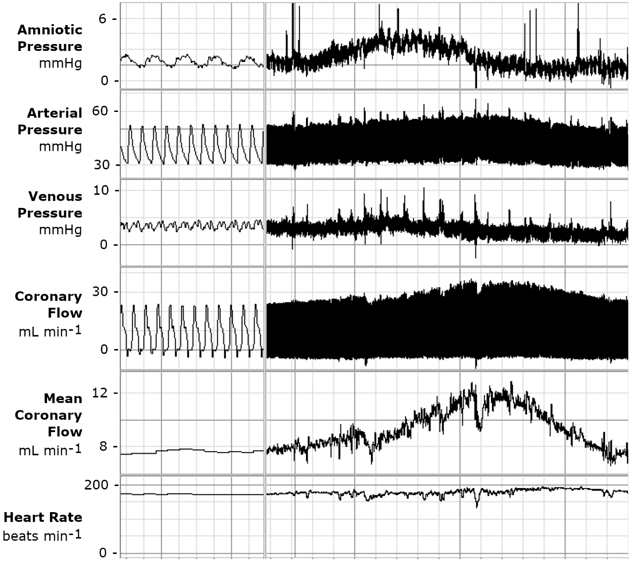
A LabChart window displaying (top-bottom) fetal amniotic pressure, arterial pressure (corrected for amniotic pressure), venous pressure (corrected for amniotic pressure), raw coronary flow, mean coronary flow, and heart rate in a fetus of an awake, calmly resting ewe. In this example, fetal coronary flow rises, following an elevation in amniotic compartment pressure (peak-peak interval approximately 4.5 min). Flow is elevated for approximately 11 minutes. Note that perfusion pressure and work did not change, as both arterial and venous pressures followed amniotic pressure, and heart rate did not increase. Time scale for right window, 1 division = 0.5 s; on left 1 division = 60 s.
Factors potentially contributing to changes in coronary flow were explored by visualizing data from simultaneously acquired blood samples. CvO2, sampled from the coronary sinus, reflects the myocardial O2 level, and under normal conditions, during variable flows, it closely follows CaO2 (Fig. 6A). In a visual representation of the relationship between coronary flow and CaO2, it can be seen that most values from the Control and IGF-1-treated groups at rest, during hypoxia, and during hypoxia with AR and NOS blockade display a curvilinear relationship in which flow increases as O2 content falls (Fig. 6B). During adenosine treatment, values were shifted to both a higher CaO2, and flow rate. A similar relationship appears to hold between coronary flow and CvO2, although here it can be seen that in some cases adenosine administration increases CvO2 to values higher than normal (Fig. 6C). This relationship appeared to persist when the fetal heart was subjected to acute hypoxia alone as well as with AR and NOS blockade. However, hyperemia induced by intracoronary adenosine administration appeared to shift the relationship such that CvO2 increased at a greater rate as CaO2 increased. When coronary flow is plotted against the difference of CaO2 and CvO2, all physiological states and treatment groups measured exhibit a curvilinear relationship relating lower extracted O2 to higher flow (Fig. 6D). In these data visualizations, the distribution of data points from IGF-1 treated and Control fetuses appear similar.
Figure 6. O2 contents and coronary flows obtained during simultaneous sampling of arterial and coronary sinus blood, and measurements of hemodynamics.
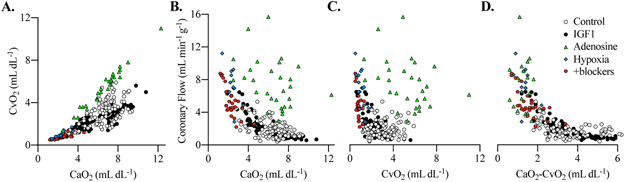
A) The relationship between arterial O2 content (CaO2) and coronary sinus O2 content (CvO2). Coronary flow as a function of B) CaO2 and C) CvO2. D) Coronary flow as a function of the difference in between CaO2 and CvO2. Individual measurements are plotted as raw data.
These factors, and others, which contributed to changes in coronary flow were explored by mixed effects regression (Table 6). Supporting the data visualization, IGF-1 treatment was not associated with variation in coronary flow. In this exploratory analysis, intracoronary adenosine, cardiac work, CvO2, and CaO2 linear and squared terms were significantly associated with increased coronary flow (Table 6). Of the endogenous factors, CvO2 exerted the strongest influence on fetal coronary flow followed by cardiac work and CaO2. Experimentally imposed intracoronary adenosine, which acts directly on the coronary vasculature, had a strong impact on coronary flow. Net myocardial lactate production, used to estimate anaerobic glycolysis, was not significantly associated with fetal coronary flow nor were experimental factors, maternal hypoxia, systolic pressure load, or study day.
Table 6.
Results from mixed effects gamma regression of fetal coronary flow regulation.a
| Variable | Exp(β) | (95% Confidence Interval) |
|---|---|---|
| Intracoronary adenosine | 2.69 | (2.16 – 3.77) |
| Cardiac workb | 1.16 | (1.11 – 1.20) |
| Coronary sinus O2 contentb | 1.32 | (1.11 – 1.51) |
| Arterial O2 contentb | 0.49 | (0.45 – 0.56) |
| Squared arterial O2 contentb | 1.12 | (1.09 – 1.20) |
Intraclass correlation coefficient = 0.210, indicating 21.0% of the total variation is accounted for by sheep fetus
Variables are mean-centered and scaled to represent a one unit change.
DISCUSSION
We showed in this study that when we gave IGF-1 to increase fetal cardiac mass by stimulating cardiomyocyte proliferation, we also proportionally increased coronary vascular supply, and coronary auto-regulation remained normal. IGF-1-treated fetuses responded normally to acute hypoxia by increasing coronary conductance and myocardial O2 extraction, and had a normal O2 delivery rate per cardiac work. IGF-1 treatment did not change the adenosine- and nitric oxide-dependence of the coronary conductance response, blockade of which reduced both coronary conductance and thus myocardial O2 consumption. These results showing normal coronary regulation at rest and during hypoxia in IGF-1-treated fetuses demonstrate the potential of IGF-1 as a therapeutic agent to augment healthy myocardial growth in the late-term fetus.
Regulation of coronary growth
We did not determine in this study if the coronary vascular growth induced by IGF-1 was via direct action on vascular cells, but a direct effect is possible given the known vascular role of IGF-1. It is known to play a significant role in angiogenesis through promotion of endothelial migration and tube formation, expansion of endothelial progenitor cells (23, 30-33), and induction of vascular smooth muscle proliferation (34). IGF-1 deficiency contributes to hypertension-associated microvascular rarefaction (35). While direct action on endothelial and smooth muscle cells is a possible means of coronary vascular growth during fetal IGF-1 treatment, it is notable that coronary vascular growth closely matched myocardial growth. Using a published growth model from the same flock (5), we calculate that the Control fetuses put on 3.7 g heart weight over the treatment period, while the IGF-1-treated fetuses put on 10.2 g, indicating a growth rate 2-3 fold greater. Despite this, coronary conductance per gram of myocardium was similar between IGF-1-treated and Control fetuses during maximal hyperemia, indicating anatomical equivalence in the vascular-myocardial relationships.
Although coronary function was similar between Control and IGF-1-treated fetuses, we did note that coronary reserve was reduced within the IGF-1 group between 0 and 7 days (Fig. 3C). This may reflect an increase in utilization of reserve at rest (increased coronary flow) to offset the slight reduction in arterial CaO2 following IGF-1 treatment. Thus, while there was an increase in coronary growth to match myocardial growth, IGF-1-stimulated coronary growth was not sufficient to also offset the slight decrease in arterial O2 found in those fetuses.
Regulation of coronary function
CaO2 does not vary appreciably in healthy adults at rest, while spontaneous CaO2 variation in the at-rest fetus likely reflects changes in placental perfusion, for example during non-laboring uterine contractions. Two primary mechanisms enable the adult myocardium to obtain enough O2 to support contraction when arterial O2 levels decrease or exercise increases myocardial O2 demand: increased coronary flow and increased myocardial O2 extraction (36, 37). We found similar mechanisms regulate fetal coronary flow. During experimental hypoxia fetuses responded with both substantially increased coronary flow (Table 5) and decreased CvO2 (increased O2 extraction) (Fig. 4D). The coronary conductance response depended on endogenous adenosine and nitric oxide signaling blockade (Fig. 4E). In response to decreased coronary flow during AR and NOS blockade, O2 extraction increased (Fig. 4D).
Spontaneous prelabor uterine contractions are brief, periodic episodes that cause mild relative hypoxia and stimulate fetal activity and maturation (38-41). In addition to investigating coronary flow regulation during experimental hypoxia, we studied these episodes as they represent normally-occurring episodes of fetal hypoxia and variation in coronary flow (Fig. 6, Table 6). IGF-1 treatment was not found to be a contributing factor to coronary flow variation. We found that regardless of treatment, the strongest endogenous predictor of fetal coronary flow is CvO2 (Fig. 6B, Table 6), which reflects the myocardial O2 level. Together with the results of blockade during experimental hypoxia, these data illustrate the essential relationship between intra-cardiomyocyte O2 and coronary vasodilation, communicated by adenosine and NO. To the best of our knowledge, we are the first to demonstrate that the fetal heart relies on endogenous adenosine and nitric oxide for metabolic coronary adaptation.
An apparent paradox
During the acute hypoxia experiment at the end of the treatment period, the relationship between cardiac work and myocardial O2 consumption was not the tightly controlled relationship we expected after AR and NOS blockade (Fig. 4I). There are some possibilities to explain this finding. Our estimation of cardiac work was heart rate multiplied by arterial pressure less venous pressure. It is possible that true cardiac work is not captured by this measurement. For example, it does not include factors such as the rate of contraction and relaxation, which may have been reduced through blockade of AR (42). It has also been found that nitric oxide acts directly at the mitochondrial complex to improve myocardial O2 efficiency (43), and in contrast to our findings we would expect to see reduced efficiency if L-NAME was an influencing factor. Another explanation is that a large component of O2 use in the fetus is devoted to growth, which can be 1.5-5 fold basal O2 consumption in mammalian cells (44). Oxygen free radical formation also uses oxygen taken up from arterial blood. The apparent paradox may thus be explained if cell growth ceases abruptly in response to reduced O2 availability, or if mitochondrial efficiency increases. However, if the O2 consumption attributable to growth was so apparent in our measurements, we would have expected baseline O2 consumption per work to be higher in the IGF-1 treatment group (Fig. 4I) as IGF-1 increased cardiac growth rate more than 2-fold (Table 3).
Other changes due to IGF-1 treatment
IGF-1 treatment reduced O2 and glucose, and increased CO2 levels, in blood sampled from the fetal ascending aorta, similar to other reports in which IGF-1 was given to healthy fetuses (11, 45). Consistent with a known action of IGF-1, the decrease in circulating glucose has been attributed to increased glucose uptake into tissues (45). In that study, fetal oxygen and glucose uptake, and placental perfusion, were maintained with IGF-1 infusion, suggesting that nutrient transfer to the fetus was unimpaired. Consequently, it is unlikely that the changes in fetal arterial blood gases and glucose represent reduced placental efficiency. Although blood returning from the placenta is preferentially distributed to the left ventricle (46) and ejected into the aorta, fetal arterial blood is mixed with venous blood. Therefore, the changes in blood gases and glucose in IGF-1-treated fetuses may represent a greater admixture of venous blood in the left ventricle, but more likely represent metabolic changes in fetal tissues reducing O2 and glucose, and increasing CO2, in the blood returning to the heart from the body.
CONCLUSIONS
In this study we found that IGF-1 treatment in the late fetal period increases coronary vascular growth in parallel with myocardial growth, and that coronary function in IGF-1-treated fetal hearts appears normal during experimental and spontaneous hypoxia. CvO2 is the strongest endogenous fetal variable determining coronary flow. We also determined that endogenous adenosine and nitric oxide contribute to the fetal coronary vascular response to acute hypoxia. Interestingly, in both normal and IGF-1-treated fetuses, O2 consumption per cardiac work decreased substantially in the context of acute hypoxia with progressive AR and NOS blockade with no reduction in cardiac work.
Acknowledgements
Supported by NICHD NIH R01HD071068, P01HD034430, and NHLBI R01HL142483, and T32HL094294.
The authors would like to acknowledge the technical assistance of Loni Socha, Daniel Kamna and Isa Lindgren.
Abbreviations:
- IGF-1
insulin-like growth factor 1
- AR
adenosine receptors
- NOS
nitric oxide synthase
- L-NAME
Nω-Nitro-L-arginine methyl ester
- CaO2
arterial O2 content
- CvO2
coronary sinus O2 content
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on, E., Prevention Statistics, C., and Stroke Statistics, S. (2018) Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Chien KR, Frisen J, Fritsche-Danielson R, Melton DA, Murry CE, and Weissman IL (2019) Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol 37, 232–237 [DOI] [PubMed] [Google Scholar]
- 3.Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, and Lumbers ER (2003) Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat. Rec. A Discov. Mol. Cell. Evol. Biol 274, 952–961 [DOI] [PubMed] [Google Scholar]
- 4.Jonker SS, and Louey S (2016) Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment. J. Endocrinol 228, R1–R18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonker SS, Louey S, Giraud GD, Thornburg KL, and Faber JJ (2015) Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. FASEB J 29, 4346–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huttenbach Y, Ostrowski ML, Thaller D, and Kim HS (2001) Cell proliferation in the growing human heart: MIB-1 immunostaining in preterm and term infants at autopsy. Cardiovasc. Pathol 10, 119–123 [DOI] [PubMed] [Google Scholar]
- 7.Kim HD, Kim DJ, Lee IJ, Rah BJ, Sawa Y, and Schaper J (1992) Human fetal heart development after mid-term: morphometry and ultrastructural study. J. Mol. Cell. Cardiol 24, 949–965 [DOI] [PubMed] [Google Scholar]
- 8.Porrello ER, Widdop RE, and Delbridge LM (2008) Early origins of cardiac hypertrophy: does cardiomyocyte attrition programme for pathological ‘catch-up’ growth of the heart? Clin. Exp. Pharmacol. Physiol 35, 1358–1364 [DOI] [PubMed] [Google Scholar]
- 9.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, and Frisen J (2009) Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flanagan MF, Aoyagi T, Currier JJ, Colan SD, and Fujii AM (1994) Effect of young age on coronary adaptations to left ventricular pressure overload hypertrophy in sheep. J Am Coll Cardiol 24, 1786–1796 [DOI] [PubMed] [Google Scholar]
- 11.Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, and Thornburg KL (2003) Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R1481–1489 [DOI] [PubMed] [Google Scholar]
- 12.Chattergoon NN, Louey S, Stork PJ, Giraud GD, and Thornburg KL (2014) Unexpected maturation of PI3K and MAPK-ERK signaling in fetal ovine cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol 307, H1216–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonker SS, and Louey S (2016) Endocrine and other physiologic modulators of perinatal cardiomyocyte endowment. J Endocrinol 228, R1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonker SS, Kamna D, Loturco D, Kailey J, and Brown LD (2018) IUGR impairs cardiomyocyte growth and maturation in fetal sheep. J Endocrinol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonker SS, Louey S, and Roselli CE (2018) Cardiac myocyte proliferation and maturation near term is inhibited by early gestation maternal testosterone exposure. Am. J. Physiol. Heart Circ. Physiol 315, H1393–H1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilburn AN, Giraud GD, Louey S, Morgan T, Gandhi N, and Jonker SS (2018) Systemic arterial hypertension but not IGF-I treatment stimulates cardiomyocyte enlargement in neonatal lambs. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R1038–R1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulombe KL, Bajpai VK, Andreadis ST, and Murry CE (2014) Heart regeneration with engineered myocardial tissue. Annu. Rev. Biomed. Eng 16, 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pries AR, and Reglin B (2017) Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur. Heart J 38, 478–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao Y, Liu Y, Liu J, and Kang YJ (2018) The Association Between Myocardial Fibrosis and Depressed Capillary Density in Rat Model of Left Ventricular Hypertrophy. Cardiovasc. Toxicol 18, 304–311 [DOI] [PubMed] [Google Scholar]
- 20.Campbell DJ (2015) Letter by Campbell Regarding Article, “Coronary Microvascular Rarefaction and Myocardial Fibrosis in Heart Failure With Preserved Ejection Fraction”. Circulation 132, e205. [DOI] [PubMed] [Google Scholar]
- 21.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, and Redfield MM (2015) Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131, 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charytan DM, Skali H, Shah NR, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Bibbo CR, Hainer J, Dorbala S, Blankstein R, and Di Carli MF (2018) Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 93, 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach LA (2015) Endothelial cells and the IGF system. J Mol Endocrinol 54, R1–13 [DOI] [PubMed] [Google Scholar]
- 24.Shuang T, Fu M, Yang G, Wu L, and Wang R (2018) The interaction of IGF-1/IGF-1R and hydrogen sulfide on the proliferation of mouse primary vascular smooth muscle cells. Biochem. Pharmacol 149, 143–152 [DOI] [PubMed] [Google Scholar]
- 25.Zhu B, Zhao G, Witte DP, Hui DY, and Fagin JA (2001) Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology 142, 3598–3606 [DOI] [PubMed] [Google Scholar]
- 26.Jia G, Cheng G, and Agrawal DK (2006) Differential effects of insulin-like growth factor-1 and atheroma-associated cytokines on cell proliferation and apoptosis in plaque smooth muscle cells of symptomatic and asymptomatic patients with carotid stenosis. Immunol. Cell Biol 84, 422–429 [DOI] [PubMed] [Google Scholar]
- 27.van de Hoef TP, Nolte F, Rolandi MC, Piek JJ, van den Wijngaard JP, Spaan JA, and Siebes M (2012) Coronary pressure-flow relations as basis for the understanding of coronary physiology. J. Mol. Cell. Cardiol 52, 786–793 [DOI] [PubMed] [Google Scholar]
- 28.National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), and National Academies Press (U.S.) (2011) Guide for the care and use of laboratory animals. pp. xxv, 220 p, National Academies Press,, Washington, D.C. [Google Scholar]
- 29.Nakagawa S, Johnson PCD, and Schielzeth H (2017) The coefficient of determination R(2) and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, and Bates DO (2008) Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci 121, 3487–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Back K, Islam R, Johansson GS, Chisalita SI, and Arnqvist HJ (2012) Insulin and IGF1 receptors in human cardiac microvascular endothelial cells: metabolic, mitogenic and anti-inflammatory effects. J Endocrinol 215, 89–96 [DOI] [PubMed] [Google Scholar]
- 32.Chisalita SI, and Arnqvist HJ (2004) Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab 286, E896–901 [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Jiang R, Yue Q, and Peng H (2017) MicroRNA-29 regulates myocardial microvascular endothelial cells proliferation and migration in association with IGF1 in type 2 diabetes. Biochem Biophys Res Commun 487, 15–21 [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Ying Z, Qi X, Shi Y, and Tang Q (2015) MicroRNA-1 regulates the proliferation of vascular smooth muscle cells by targeting insulin-like growth factor 1. Int J Mol Med 36, 817–824 [DOI] [PubMed] [Google Scholar]
- 35.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, and Csiszar A (2016) Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 38, 273–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaijser L, Grubbstrom J, and Berglund B (1990) Coronary circulation in acute hypoxia. Clin. Physiol 10, 259–263 [DOI] [PubMed] [Google Scholar]
- 37.Goodwill AG, Dick GM, Kiel AM, and Tune JD (2017) Regulation of Coronary Blood Flow. Compr Physiol 7, 321–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson SS, Johnson SL, Bacher LF, Wood JR, Wong CH, Robinson SR, Smotherman WP, and Nathanielsz PW (1996) Contractile activity of the uterus prior to labor alters the temporal organization of spontaneous motor activity in the fetal sheep. Dev Psychobiol 29, 667–683 [DOI] [PubMed] [Google Scholar]
- 39.Shinozuka N, Yen A, and Nathanielsz PW (2000) Increased myometrial contracture frequency at 96–140 days accelerates fetal cardiovascular maturation. Am J Physiol Heart Circ Physiol 278, H41–49 [DOI] [PubMed] [Google Scholar]
- 40.Llanos AJ, Court DJ, Block BS, Germain AM, and Parer JT (1986) Fetal cardiorespiratory changes during spontaneous prelabor uterine contractions in sheep. Am J Obstet Gynecol 155, 893–897 [DOI] [PubMed] [Google Scholar]
- 41.Sunderji SG, El Badry A, Poore ER, Figueroa JP, and Nathanielsz PW (1984) The effect of myometrial contractures on uterine blood flow in the pregnant sheep at 114 to 140 days’ gestation measured by the 4-aminoantipyrine equilibrium diffusion technique. Am J Obstet Gynecol 149, 408–412 [DOI] [PubMed] [Google Scholar]
- 42.Riksen NP, Smits P, and Rongen GA (2011) The cardiovascular effects of methylxanthines. Handb Exp Pharmacol, 413–437 [DOI] [PubMed] [Google Scholar]
- 43.Massion PB, Moniotte S, and Balligand JL (2001) Nitric oxide: does it play a role in the heart of the critically ill? Curr Opin Crit Care 7, 323–336 [DOI] [PubMed] [Google Scholar]
- 44.Wagner BA, Venkataraman S, and Buettner GR (2011) The rate of oxygen utilization by cells. Free Radic. Biol. Med 51, 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harding JE, Liu L, Evans PC, and Gluckman PD (1994) Insulin-like growth factor 1 alters feto-placental protein and carbohydrate metabolism in fetal sheep. Endocrinology 134, 1509–1514 [DOI] [PubMed] [Google Scholar]
- 46.Anderson DF, Faber JJ, Morton MJ, Parks CM, Pinson CW, and Thornburg KL (1985) Flow through the foramen ovale of the fetal and new-born lamb. J. Physiol 365, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]


