Abstract
Background
Adolescence is a period of high risk for the onset of depression, characterized by variability in symptoms, severity, and course. During adolescence, the neurocircuitry implicated in depression continues to mature, suggesting that it is an important period for intervention. Reflecting the recent emergence of ‘precision mental health’ – a person-centered approach to identifying, preventing, and treating psychopathology – researchers have begun to document associations between heterogeneity in features of depression and individual differences in brain circuitry, most frequently in resting-state functional connectivity (RSFC).
Methods
In this review, we present emerging work examining pre- and post-treatment measures of network connectivity in depressed adolescents; these studies reveal potential intervention-specific neural markers of treatment efficacy. We also review findings from studies examining associations between network connectivity and both types of depressive symptoms and response to treatment in adults, and indicate how this work can be extended to depressed adolescents. Finally, we offer recommendations for research that we believe will advance the science of precision mental health of adolescence.
Results
Nascent studies suggest that linking RSFC-based pathophysiological variation with effects of different types of treatment and changes in mood following specific interventions will strengthen predictions of prognosis and treatment response. Studies with larger sample sizes and direct comparisons of treatments are required to determine whether RSFC patterns are reliable neuromarkers of treatment response for depressed adolescents. Although we are not yet at the point of using RSFC to guide clinical decision-making, findings from research examining the stability and reliability of RSFC point to a favorable future for network-based clinical phenotyping.
Conclusions
Delineating the correspondence between specific clinical characteristics of depression (e.g., symptoms, severity, and treatment response) and patterns of network-based connectivity will facilitate the development of more tailored and effective approaches to the assessment, prevention, and treatment of depression in adolescents.
Keywords: depression, adolescence, connectivity, brain networks, heterogeneity, precision mental health
Depression in adolescence
The risk for experiencing Major Depressive Disorder (MDD) is highest during adolescence; indeed, nearly 15% of 12- to 17-year-olds experience at least one episode of MDD (Avenevoli, Swendsen, He, Burstein, & Merikangas, 2015). The adverse consequences of developing MDD in adolescence persist well into adulthood, including experiencing anxiety and recurrent episodes of depression, anxiety, and suicidal behaviors (Johnson, Dupuis, Piche, Clayborne, & Colman, 2018). Given the significant psychosocial toll of adolescent depression, there is an urgent need to identify and treat MDD as early in its progression as possible. Unfortunately, however, MDD goes undetected in 40% of adolescents, and those who do receive treatment often do not experience alleviation of symptoms (Michael & Crowley, 2002; Stein & Fazel, 2015).
A major factor that has hindered progress in identifying and treating adolescent depression is the considerable heterogeneity of this disorder. Depressed adolescents vary in the age at which they experience the onset of the disorder (Breslau et al., 2017), the types of symptoms with which they present (Chen et al., 2014), the course of their symptoms (Yaroslavsky, Pettit, Lewinsohn, Seeley, & Roberts, 2013), and their response to treatment (Mojtabai, Olfson, & Han, 2016). Adolescent females are at greater risk for the onset of depression than are their male counterparts, and also tend to show more severe symptoms that are stable and unremitting (Breslau et al., 2017). Forms of mood pathology in adolescent depression also vary. While some depressed adolescents exhibit symptoms consistent with the DSM-5 criteria for MDD (e.g., anhedonia, changes in sleep patterns, diminished mood), other depressed adolescents endorse diverse symptoms that are incongruous with traditional diagnostic criteria for MDD (e.g., anxiety, body dysmorphia, and vegetative symptoms; Blom et al., 2014). Comorbidity (e.g., with symptoms of anxiety) is an additional level of complexity that warrants attention in understanding the heterogeneity of depression in adolescence. In fact, 25–50% of adolescents with depression have been found to also meet diagnostic criteria for an anxiety disorder (Axelson & Birmaher, 2001; Costello et al., 2003; Garber & Weersing, 2010); further, an intervention that targets one psychiatric disorder may reduce the efficacy of treatment for another disorder (Curry et al., 2006). Finally, an estimated 20% of MDD patients go on to develop manic symptoms (Boschloo et al., 2014), underscoring the need to identify endophenotypes that may have varying levels of susceptibility to different types of symptoms and mood disturbances. Given this heterogeneity, it is unsurprising that a “one size fits all” treatment approach has not been effective for all depressed adolescents. For example, many adolescents receive antidepressants to treat MDD (Soria-Saucedo, Walter, Cabral, England, & Kazis, 2016); however, antidepressants alone are largely ineffective in treating their symptoms (Michael & Crowley, 2002). We argue here that examining associations between variability in the types, severity, and course of depressive symptoms and in treatment response, and individual differences in neurobiology (e.g., functional connectivity), will advance our knowledge of the specific treatments that are best suited to, and most effective for, adolescents who are experiencing MDD. This approach will also further our theoretical and empirical understanding of the neurobiological mechanisms underlying MDD.
Precision mental health of adolescence
Precision medicine refers to the practice of precisely tailoring treatments to subcategories of disease defined on the basis of differences in pathological components (e.g., observable symptom types, underlying neurobiology; Ashley, 2015; National Research Council, 2011). In precision medicine, data and analytics are used to classify heterogeneous individuals into subpopulations that differ in their biological make-up (e.g., genetics), susceptibility to disease (e.g., cancer), and response to treatment (e.g., chemotherapy). The broader goal of these initiatives is to improve quality of care by guiding the selection of treatment that is most effective for a given patient (Ginsburg & Phillips, 2018). Following this framework, we propose that differences in the symptoms, severity, prognosis, and treatment of depression in adolescents are associated with variation in the functional connectivity of brain networks. Harnessing the power of measuring heterogeneity in brain network connectivity as it relates to differences in characteristics of depression would advance the precision mental health of adolescence.
Properties of functional networks – i.e., collections of brain regions that co-activate to support shared functions – can be characterized using functional magnetic resonance imaging (fMRI) during a task or at rest (i.e., in the absence of stimuli). Researchers have posited that signal correlations between brain regions reflect a history of co-activation or structural connectedness, evidenced by studies showing that task-evoked functional connections are also detectable at rest (Dosenbach et al., 2007). We posit that measuring resting state functional connectivity (RSFC) of brain networks is a promising method for advancing the precision mental health of adolescence for several reasons. First, research suggests that organizational properties of functional networks at rest are reproducible across adolescents (Marek et al., 2019) and reflect stable, trait-like neurobiological signatures (Jalbrzikowski et al., 2019). Second, variability in functional connectivity has been shown to be largely attributable to individual difference characteristics and due less to day-to-day changes or task states (Gratton et al., 2018), suggesting that RSFC patterns reflect neural ‘fingerprints’ that can reliably reveal how adolescents differ from each other. Recent work also shows that patterns of RSFC predict differences in adolescents’ brain maturity and executive functioning (Cui et al., 2020). Finally, the intrinsic connectivity of particular brain networks, measured by resting state fMRI, has been found to be uniquely related both to specific symptoms (e.g., (Kühn et al., 2012) and to response to different forms of treatment (Brakowski et al., 2017).
In this paper, we review studies of depression and RSFC of brain networks in order to elucidate neurobiological factors that underlie differences in symptoms, course of disorder, and treatment response. We begin by recognizing that, regardless of neuroimaging modality, most studies examining neurobiological aspects of depression have used case-control designs in which (the mean of) a group of depressed persons on a particular metric is compared to (the mean of) a group of typical/healthy persons. In this approach, within-group heterogeneity is typically ignored or averaged; individual differences in symptoms and brain characteristics are generally not examined. However, research with depressed adults indicates that individual differences in RSFC can be used to identify specific neural patterns associated with both variability in symptom profiles and treatment response (Hou et al., 2018; Price, Gates, Kraynak, Thase, & Siegle, 2017a; Tokuda et al., 2018). Furthermore, evidence is now emerging from studies of depressed adolescents indicating that assessing variation in brain circuitry yields important information about different symptom types and severity (e.g., Rzepa & McCabe, 2018), symptom course (e.g., Connolly et al., 2017), and response to treatment (e.g., Klimes-Dougan et al., 2018a). These nascent findings suggest that RSFC patterns transcend traditional diagnostic boundaries and elucidate brain-symptom phenotypes that could be linked with tailored treatments, informing the precision mental health of adolescence. Additional research is needed, however, to identify the patterns of neural connectivity that predict which treatments will be successful for which subgroups of depressed adolescents.
The purpose of this review is to describe how examining heterogeneity in both brain network connectivity and depression can advance our understanding, and the person-centered treatment, of adolescent depression. We begin by summarizing findings of neurobiological alterations in adolescents with depression, typically reported in case-control studies. We first review findings of depression-relevant patterns of regional brain activation engaged by tasks, given that these studies provide foundational knowledge about the neurobiological and behavioral correlates of depression. We then describe findings from studies of RSFC indicating widespread alterations in neurocircuitry related to adolescent depression. We focus on RSFC because it reflects stable patterns of intrinsic connectivity between regions (Cole, Bassett, Power, Braver, & Petersen, 2014) and trait-like individual differences that are related to personality and neuropsychiatric disorders (Gratton et al., 2018). We then present emerging work examining pre- and post-treatment measures of network connectivity in adolescents with depression; these studies reveal potential intervention-specific neural markers of treatment efficacy. We also review findings from studies examining associations between network connectivity and both types of depression symptoms and response to treatment in adults and indicate how this work can be extended to the study of depressed adolescents. Finally, we offer recommendations for research that we believe is necessary to advance the science of precision mental health of adolescence in order to improve the well-being of adolescents.
The neurobiology of adolescent depression
Findings from case-control studies of the neurobiology of adolescent depression
Depressed adolescents have been found to be characterized by aberrant cognitive, affective, reward, and self-referential processing (e.g., Grahek, Shenhav, Musslick, Krebs, & Koster, 2019; Nejad, Fossati, & Lemogne, 2013). Researchers have used fMRI to elucidate neurobiological correlates of the anomalous patterns of information processing that have been documented in depressed individuals, although typically in case-control studies. Nevertheless, these group comparisons have provided important insights about region-specific and network-wide alterations in brain function that may underlie cognitive and behavioral deviations in depression. In this section, we first highlight key findings of depression-associated differences in regional activation from task-based neuroimaging studies of adolescents, and then discuss studies of RSFC that have assessed the intrinsic coordinated activity of multiple brain regions implicated in depression.
Patterns of regional brain activation in adolescent depression
Converging findings indicate that depressed adolescents exhibit blunted neural response to reward cues, evidenced by dampened striatal activation during the anticipation and receipt of reward (O’Callaghan & Stringaris, 2019). Further, attenuated neural sensitivity to the receipt of reward has been found to be associated with lower positive affect (Forbes et al., 2009) and greater severity of symptoms (C. Insel et al., 2019) in depressed adolescents. Finally, high-risk youth who are resilient to depression have been found to exhibit greater activation in reward circuitry during anticipation of reward than do their counterparts who have experienced this disorder (Fischer et al., 2019). While neural activation during both reward anticipation and receipt has been shown to be altered in depressed adolescents, one meta-analysis showed that the striatum, insula, thalamus, and amygdala are recruited during anticipation of reward or loss, while orbitofrontal and prefrontal regions are recruited in response to reward outcome (Oldham et al., 2018). Thus, both the neural systems supporting motivational processes and those underlying value representations appear to be aberrant in adolescent depression.
Investigators have also documented deficits in cognitive control in depressed adolescents that contribute to dysregulated emotional reactivity, reduced processing speed, and compromised executive functioning (Rudolph et al., 2017; Sommerfeldt et al., 2016). Compared to healthy controls, depressed adolescents have been found to recruit cognitive and attention-orienting (i.e., frontocingulate and occipitoparietal) regions to a greater degree when they are required to inhibit responses in the presence of emotional distractors (Colich et al., 2017). Greater required engagement of these regions when faced with affective distractors suggests that depressed adolescents have insufficient top-down regulatory abilities, contributing to difficulties in managing negative emotions and persistent rumination (Joormann & Gotlib, 2010). Indeed, less engagement of prefrontal cortex (PFC) regions during an emotion regulation task has been found to be associated with greater severity of depressive symptoms in adolescents (Fitzgerald, Klumpp, Langenecker, & Phan, 2018). Depressed adolescents have also been found to exhibit abnormalities in the subgenual and dorsal subregions of the anterior cingulate cortex (ACC). Whereas the subgenual ACC is implicated in emotion regulation (Drevets et al., 2008), the dorsal ACC is posited to support goal-driven actions and error-monitoring (Luna et al., 2015; Velanova et al., 2008). During a stop-signal task, depressed adolescents exhibited greater subgenual ACC activity than did their healthy peers (Yang et al., 2009). Conversely, subgenual ACC activity during the viewing of fearful faces was inversely related to depression severity in adolescents (Hall et al., 2014). Findings regarding subgenual ACC activity in depressed adolescents suggest that atypical functioning is dependent on the context: whereas greater activity may be observed in cognitive tasks, lower activity may be observed in socioemotional contexts. With respect to the dorsal portion of the ACC, young women with a history of depression showed increases in both depressed mood and dorsal ACC activity following social evaluative feedback (Dedovic et al., 2016), although depressed youth have been found to exhibit hypoactivation in this region during executive functioning tasks (Miller et al., 2015). Unlike the subgenual ACC, self-monitoring-related dorsal ACC activity may be heightened in social contexts and lower in cognitive tasks in adolescent depression.
Depressed adolescents have also been found to exhibit aberrant neural responses to emotionally salient stimuli (e.g., emotional faces, social evaluation). For example, relative to healthy controls, depressed adolescents show greater activation in response to social rejection in emotion-processing regions, such as the amygdala, subgenual ACC, and insula (Silk et al., 2014). Depressed adolescents also show elevated amygdala activity to negative facial expressions and reduced activity to positive stimuli during a face-matching task (Redlich et al., 2018), suggesting that negatively valenced stimuli are particularly salient to depressed youth. Importantly, greater amygdala reactivity to emotional stimuli (e.g., faces) has been found to predict increases in the severity of adolescents’ depressive symptoms (Mattson, Hyde, Shaw, Forbes, & Monk, 2016).
Finally, depressed adolescents have been found to show aberrant activity in brain regions involved in self-referential processing and self-reflection, including the medial PFC (mPFC), insula, and posterior cingulate cortex (PCC) (Vilgis et al., 2018). Specifically, compared to their healthy peers, depressed adolescents exhibit greater activation in these regions, collectively referred to as cortical midline structures (Northoff et al., 2006), during rumination, a form of repetitive negative self-referential processing (Cooney, Joormann, Eugène, Dennis, & Gotlib, 2010). Moreover, activation in these regions is correlated positively with self-reported rumination and severity of depressive symptoms (Burkhouse et al., 2017; Vilgis et al., 2018). Depressed adolescents are also less able than are their nondepressed counterparts to suppress activity in brain regions involved in self-reflection in the presence of external cognitive demands (Han, Kim, Bae, Renshaw, & Anderson, 2016). Collectively, these findings highlight neural mechanisms that may contribute to the sustained negative self-focus that characterizes depressed individuals.
Studies of task-based regional brain activation provide foundational knowledge about key structures involved in the cognitive and affective anomalies generally exhibited by depressed adolescents. However, there is a growing appreciation that cognitive and affective processes are supported by the coordinated activity of multiple brain regions, or networks, rather than by the response of discrete brain regions to specific cues (van den Heuvel & Sporns, 2013). Thus, examining functional connectivity within and between networks in the absence of cues has allowed researchers to probe large-scale neural disruptions that may be related to depression broadly, as well as to specific symptoms (Bassett, Xia, & Satterthwaite, 2018).
Alterations in network connectivity in adolescent depression
Several resting-state networks have been identified in relation to adolescent depression. This research extends findings from task-based studies of regional activation, reviewed above, by linking behavioral manifestations of depression with alterations in networks, providing evidence of system-wide perturbations related to depression. We present findings from this research below.
The reward network (RN) comprises fronto-striatal regions involved in the processing of rewards (e.g., caudate, putamen, nucleus accumbens, and frontal regions) and is characterized by age-related increases over adolescence in within-network functional connectivity (Solé-Padullés et al., 2016). Although RN connectivity is lower in depressed than in nondepressed adults (Bai et al., 2018), one study found that depressed adolescents have higher RN RSFC than do nondepressed controls (Gabbay et al., 2013) and, in another study of children, stronger functional connectivity of the RN predicted greater risk for experiencing a depressive episode years later (Pan et al., 2017). Thus, depression-related alterations in the RN may be age-dependent, but in most age groups these alterations have been found to contribute to the anhedonia and loss of pleasure documented in MDD (Heshmati & Russo, 2015).
The cognitive control network (CCN) encompasses frontoparietal regions engaged during executive function and cognitive control processes (e.g., dorsolateral PFC, dorsal ACC, parietal cortex), supporting such functions as decision-making, working memory, and general top-down control (E. K. Miller & Cohen, 2001). Like the RN, the strength of functional connectivity within the CCN increases over adolescence (Sherman et al., 2014), supporting the integration of component processes involved in cognitive control, such as inhibitory control and working memory (Luna et al., 2015). Indeed, reduced activation, but increased coupling (i.e., co-activation or within-network connectivity), of CCN regions during task has been found to be associated with better cognitive control performance on a multi-source interference task in adolescents (Dwyer et al., 2014). Given the neurocognitive impairments reported in depressed adolescents and adults (Maalouf et al., 2011), it is plausible that the trajectory of CCN development is altered in the context of adolescent depression. In fact, weaker CCN connectivity has been documented both in depressed adolescents (Tang et al., 2018) and in adolescent daughters of depressed mothers (Clasen, Beevers, Mumford, & Schnyer, 2014), implicating anomalous development of CCN connectivity in the intergenerational transmission of risk for depression.
The affective limbic network (AN) includes brain regions involved in the processing and regulation of emotions (e.g., amygdala, hippocampus, insula, orbitofrontal cortex (OFC), and ventral ACC; Leppänen & Nelson, 2009). Functional connectivity between affective regions, such as the ACC and amygdala, during an emotion regulation task increases from childhood to adulthood (Perlman & Pelphrey, 2011). Given the roles of AN regions in emotion processing, it is not surprising that greater RSFC connectivity has been found within this network in depressed relative to nondepressed adolescents, likely underlying the negative mood and emotion dysregulation that characterizes this disorder (Pannekoek et al., 2014).
Network studies have identified regions of the AN as part of a larger salience network (SN), which, like the AN, shows protracted development in adolescence (Solé-Padullés et al., 2016). The SN, composed primarily of the anterior insula, amygdala, ventrolateral PFC, and dorsal ACC, is involved in external stimulus detection and task-switching, processing emotionally salient information, and generating emotional states (Seeley et al., 2007). As has been found for the AN, the SN is altered in adolescent depression, and stronger connectivity of SN regions predicts greater severity of depressive symptoms (Hulvershorn, Cullen, & Anand, 2011).
The default mode network (DMN; Raichle & Snyder, 2007) comprises a group of brain regions that show greater functional co-activity in the absence of stimuli, or during self-reflective states (e.g., precuneus, PCC, mPFC, inferior parietal cortex). These regions overlap with those referred to above as cortical midline structures. Activity in regions of the DMN is associated with internalized experiences, such as autobiographical memory, prospection, self-referential and introspective processing, and theory-of-mind reasoning (Spreng & Grady, 2010) – processes that have been found to be altered in depression (LeMoult, Kircanski, Prasad, & Gotlib, 2017). Importantly, researchers have documented elevated functional connectivity among regions of the DMN in depressed adolescents, both at rest and during an emotion identification task (Ho et al., 2015).
Finally, in addition to the depression-associated anomalies in within-network connectivity described above, several studies have found alterations in between-network connectivity in adolescent depression (Sacchet et al., 2016). For example, compared to healthy controls, depressed adolescents exhibit weaker RSFC between the amygdala and frontal regions of the CCN (Scheuer et al., 2017). This pattern of between-network RSFC in depressed adolescents has been found to reflect a reduced ability of the PFC to modulate hyper-responsivity of the amygdala (Perlman et al., 2012). Compared to healthy controls, depressed adolescents also exhibit stronger connectivity between the DMN and both the CCN and the SN (Sacchet et al., 2016). Given that decreased between-network connectivity between the DMN and CCN is related to better cognitive control performance in adolescence (Dwyer et al., 2014), and that increased connectivity between nodes of the CCN and SN is associated with improvements in inhibitory control (Marek, Hwang, Foran, Hallquist, & Luna, 2015), the atypical between-network connectivity patterns in depressed adolescents may underlie cognitive deficits in affective conditions (Joormann & Gotlib, 2010; Maalouf et al., 2011). Together, these case-control studies suggest that patterns of activation and co-activation (i.e., functional connectivity) of brain regions involved in cognitive, affective, self-referential, and reward processing are important neural markers of general and specific symptoms of adolescent (and adult) depression.
Heterogeneity of the neurobiology of adolescent depression
Although case-control RSFC studies have provided important information about anomalous patterns of neural connectivity associated with clinical features of depression, this approach assumes, at least implicitly, homogeneity within groups and overlooks individual differences in connectivity that may be associated with specific clinical features (Seghier & Price, 2018). Importantly, in a study of brain structure in patients with schizophrenia and bipolar disorder, Wolfers et al. (2018) found that no individual matched the “average patient,” and argued that group-level differences masked biological and individual heterogeneity. These ‘group-averaged’ approaches may explain why researchers have not yet reliably identified robust biomarkers of the course of depression or response to treatment. Similarly, variations in behavioral and symptom data are often overlooked, despite well-documented heterogeneity in the developmental course, symptom profiles, symptom severity, treatment response, and biological correlates of depression in youth.
To date, only a small number of studies have examined the relation between functional connectivity and individual differences in specific characteristics of depression in adolescents. This emerging work suggests that variability in adolescents’ functional connectivity relates to their current symptoms and the severity of their depression, as well as to subsequent changes in their symptoms (i.e., prognosis). For example, RSFC between the caudate (part of the RN) and dorsolateral PFC (part of the CCN), as well as within the DMN, has been found to be positively correlated with symptom severity in depressed adolescents (Ho et al., 2015). Patterns of RSFC have also been found to predict future depressive symptoms. For example, adolescents with weaker initial AN-CCN connectivity exhibit increasing severity of depressive symptoms over time (Scheuer et al., 2017); in contrast, adolescents with higher baseline AN-DMN connectivity have greater reductions in symptoms months later (Connolly et al., 2017).
Symptom-specific network alterations have also been found in adolescent depression. For example, higher levels of anhedonia have been associated with lower DMN connectivity with the CCN (Rzepa & McCabe, 2018), and higher levels of dysphoria have been linked with lower connectivity between the amygdala (part of the AN) and hippocampus (of the DMN) (Cullen et al., 2014) in depressed adolescents. Finally, RSFC patterns in depressed youth vary as a function of the age of onset of depression: whereas earlier onset is associated with greater amygdala (part of the AN) connectivity with the DMN, later onset is related to greater amygdala connectivity with the CCN (Clark et al., 2018). In sum, examining individual differences in RSFC within samples of depressed adolescents, rather than making comparisons between groups of depressed adolescents and groups of control participants, can yield insight both about the heterogeneity of the severity and course of depression, and about profiles of symptoms in this disorder.
Emerging work and future directions: toward a precision mental health of adolescence
Given the associations documented between patterns of RSFC and individual variation in characteristics of depression (e.g., severity, symptoms, course), researchers are beginning to harness the translational potential of network neuroscience, bridging basic research with clinical applications, to identify brain connectivity signatures that may be associated with various treatment responses. In this section, we describe nascent work linking initial levels and changes in network connectivity in depressed adolescents with treatment effects. To date, most studies of the associations among patterns of brain connectivity, subtypes of depression, and treatment outcomes have been of adults. While a small number of studies have examined baseline and post-treatment connectivity in adolescents, we do not yet know whether depressed adolescents can be classified into subtypes based on their clinical characteristics, brain network patterns, and treatment response. We describe results from the few existing studies of adolescents below, followed by work with adults as examples of the potential benefits of examining depression and brain network heterogeneity together in order to increase our understanding of, and improve the effectiveness of treatments for, adolescent depression from the perspective of precision mental health.
Preliminary network-based translational research in adolescent depression
Although several metrics of connectivity may be informative (e.g., task-based functional connectivity, diffusion imaging based structural connectivity), one approach to meet the objective of precision mental health is to identify pre-treatment RSFC signatures that are associated with effective interventions for subtypes of depression. As we describe below, a small number of studies have focused specifically on amygdala connectivity; their findings suggest not only that specific pre-treatment neural signatures can aid in predicting the effectiveness of treatments for depression, but further, that different treatments lead to changes in connectivity in among specific networks, and these changes in RSFC from pre- to post-treatment are related to symptom improvement.
In one study, depressed adolescent patients completed a resting-state fMRI scan before and after five sessions of Cognitive Behavioral Therapy (CBT; Straub et al., 2017). As expected, relative to controls, patients initially exhibited stronger RSFC between the subgenual ACC and amygdala (both regions of the AN) that weakened following successful CBT. Further, depressed adolescents had weaker CCN-SN connectivity than did controls at baseline; RSFC between these networks increased in the depressed adolescents following CBT. Importantly, improvement in symptoms from pre- to post-CBT was correlated with changes in RSFC, suggesting that this type of treatment in depressed adolescents leads to changes in connectivity between the CCN and SN, as well as within the AN. In addition, pre-treatment amygdala connectivity predicted response to CBT; specifically, depressed adolescents who exhibited greater connectivity between the amygdala and the CCN or SN (i.e., neural signatures more similar to those of healthy controls) had greater clinical improvement. These findings suggest that in depressed adolescents, CBT alters aberrant connectivity between the CCN and AN (i.e., emotion-regulatory processes), as well as anomalous patterns of activation in regions within these networks. However, the study sample was small (N=38) and composed primarily of females (N=30). Future studies with larger samples are needed to clarify whether the documented network alterations are specific to CBT or, alternatively, if these or similar changes also result from other forms of therapy and/or medications.
Two studies have shown that connectivity between the AN and CCN in depressed adolescents predicts response to selective serotonin reuptake inhibitors (SSRIs), a commonly prescribed antidepressant medication. Klimes-Dougan et al. (2018) showed that adolescents with stronger baseline RSFC between the amygdala (AN) and regions of the CCN exhibited symptom improvement after eight weeks of treatment with an SSRI; in contrast, adolescents with stronger connectivity between the amygdala and right precentral gyrus (i.e., two AN nodes) did not improve. Similarly, Cullen et al. (2016) showed that response to SSRIs was associated with increased connectivity between the amygdala and frontal cortex (i.e., AN-CCN) and with decreased connectivity between the amygdala and precuneus (i.e., AN-DMN) from pre- to post-treatment. These studies, while promising for the precision mental health of adolescence, are limited by sample sizes with significant variability in age, lack of information about the type or dose of SSRIs, and no control or placebo groups to assess whether changes in connectivity might be attributed to moderators and confounding variables. Future studies should use randomized control treatment (RCT) designs with larger samples to track the relation between changes in functional connectivity and treatment type and dose.
Despite these limitations, both CBT and antidepressants have been shown to affect AN-CCN connectivity; further, depressed adolescents with more ‘normative’ patterns of RSFC are more likely to show symptom improvement following treatment. Although connectivity of the AN, particularly the amygdala, may represent a neural signature that is not treatment-specific, these studies suggest there are differential network changes that result from specific treatments. For example, depressed adolescents showed improved (decreases in) connectivity between the DMN and AN following CBT (Straub et al., 2017). While amygdala-DMN connectivity was also shown to decrease following SSRI treatment, SSRIs were further shown to improve (strengthen) connectivity between the amygdala and CCN (Cullen et al., 2016). As we noted above, weaker amygdala-CCN connectivity predicts increasing severity of depressive symptoms (Scheuer et al., 2017); treatment with SSRIs may specifically target lower AN-CCN connectivity, strengthen regional coupling, and halt the progression of symptoms. Thus, RSFC patterns, particularly those involving the amygdala, may be neurobiological predictors of SSRI and CBT treatment-related improvements in adolescent depression.
It is important that we understand which adolescents may be more or less amenable to different types of treatments. To this end, we should work to identify subgroups of patients that share biological markers that predict their response to particular treatments. To accomplish this goal, researchers must develop comprehensive algorithms to describe discrete brain-symptom phenotypes and examine whether these ‘neurophysiological’ subtypes of depression respond differentially to various types of treatment. Although research examining these questions to date has been limited to adults, these studies serve as examples for future research with depressed adolescents. We describe these studies below.
Network-based precision mental health research in depression: examples from adult studies
By modeling network metrics from RSFC data, studies of depressed adults have not only provided valuable information about the neural correlates of putative subtypes of this disorder (e.g., Price, Gates, et al., 2017), but have also demonstrated that these subtypes differ in subsequent clinical outcomes and response to treatment (e.g., Drysdale et al., 2017; Tokuda et al., 2018). Brain network connectivity can be modeled to detect associations between system-wide patterns of functional connectivity and dimensions of symptom sets. For example, Maglanoc et al. (2018) clustered neural data from a large sample of depressed adults based on types and severity of symptoms, and obtained five subtypes that differed in connectivity of fronto-temporal regions and in symptom profiles. Interestingly, fronto-temporal network connectivity was not associated with total severity of symptoms, suggesting that different patterns of RSFC reflect specific characteristics of depression.
Examining individual variation in network connectivity has the potential to provide clinically relevant information about depression subtypes that is typically overlooked in traditional between-group comparisons. For example, Price and colleagues (2017) identified two subgroups of depressed individuals defined by the similarities in their patterns of RSFC in brain regions documented in previous studies to be associated with depression (including regions in the AN, CCN, and DMN). The larger subgroup was characterized by a pattern of heightened DMN connectivity, consistent with findings from a previous study of depressed adults (Sambataro, Wolf, Pennuto, Vasic, & Wolf, 2014). The smaller subgroup was characterized by stronger functional connectivity between subcortical areas involved in emotion processing and threat detection; this subgroup also had more females than males and a higher proportion of patients with recurrent depression and comorbid anxiety (Price et al., 2017b). Importantly, the two subgroups had unique patterns of functional connectivity and clinical profiles (e.g., symptoms, etiology, severity), suggesting that brain networks reflect the heterogeneity of depression and transcend diagnostic boundaries that typify case-control comparisons. Of course, it is unclear whether these depression subgroups are sample-dependent; it is possible in a larger sample that a greater number of subgroups with varying brain-symptom phenotypes would be detected, or that no differentiated subgroups would be found.
Research conducted with depressed adults shows that different patterns of RSFC are associated not only with heterogeneous presentations of depression, but also with remission from depression following treatment. One large study (N=1,188) identified four neurophysiological subtypes of depressed adults based on different patterns of RSFC of limbic (i.e., AN) and fronto-striatal (i.e., RN) networks (Drysdale et al., 2017). The four subtypes shared a neuroanatomical presentation of alterations in connectivity of the insula, OFC, ventromedial PFC, fronto-amygdala, RN, and various subcortical areas, regions that have previously been implicated in depression (Greicius et al., 2007). They differed, however, both in other patterns of RSFC and in their clinical symptom profiles. For example, two subtypes exhibited stronger RN connectivity than did the other two subtypes, along with higher levels of anhedonia and alterations in psychomotor behavior. Importantly, the four subtypes also differed in their response to treatment with TMS; specifically, the subtype characterized by reduced connectivity within fronto-amygdala and OFC areas showed the greatest reduction in severity of depressive symptoms in response to TMS (Drysdale et al., 2017). This significant interaction of neurophysiology-based depression subtype and treatment may be due to the match of the location of the aberrant connectivity (among frontal regions) and the TMS target (the dorsomedial PFC). The characterization of depression biotypes in this study based on connectivity and the match between patients’ brain-symptom phenotypes and favorable treatment response illustrates the improvement in therapeutics that is possible with a precision mental health approach. A recent attempt to replicate this study (identifying distinct connectivity-based subtypes of depression), however, was unsuccessful with a smaller sample (N=187) (Dinga et al., 2019); thus, the validity of these biotypes of adult depression is unclear. Dinga and colleagues (2019) recommended that researchers examine symptoms of depression in relation to continuous measures of RSFC, rather than attempt to group patients into discrete biotypes, a recommendation consistent with our view that testing group differences may mask important inter-individual variation in symptoms and neurobiology. Drysdale et al.’s findings nevertheless underscore the heterogeneous presentation of depression at multiple levels (i.e., neurobiology and symptoms) and highlight the potential utility of examining RSFC in developing more effective treatments.
Similar to Drysdale et al., (2017), Tokuda et al. (2018) clustered clinical and RSFC data in depressed adults and found that functional connectivity between the angular gyrus (AG), a hub of the DMN, and other DMN regions differed among the three identified depression subtypes and predicted each subtype’s response to SSRIs. Specifically, depressed patients in a subtype that exhibited lower AG-DMN connectivity showed reductions in depressive symptoms following treatment with SSRIs; in contrast, patients in a subtype characterized by higher AG-DMN connectivity did not show a reduction in symptoms. These findings highlight the importance of considering alterations in functional connectivity of connector hub regions, particularly in the DMN, in elucidating heterogeneous symptom profiles and guiding specialized approaches to treatment. Like most research in the nascent field of precision mental health, Tokuda and colleagues’ work characterizing disparate depression subtypes was conducted with a relatively small sample of participants (N=134), and it is possible that their findings will not replicate in future studies with larger samples.
Other findings in adults further support the formulation that examining RSFC patterns can be useful in developing more effective treatments for depression. A recent review indicated that connectivity within and between the CCN, AN, DMN, and visual networks predicts response to TMS and antidepressants (Dichter, Gibbs, & Smoski, 2015). For example, heightened DMN and reduced CCN connectivity was found to be associated with symptom improvement following antidepressants, whereas greater subcallosal cortex connectivity was associated with response to TMS. Similarly, lower RN connectivity and stronger anhedonic symptoms have been shown to predict less responsiveness to TMS placed at the dorsomedial PFC, suggesting that patients with greater RN dysfunction require either TMS that is targeted to different locations or other forms of therapy altogether (Downar et al., 2014). In addition, higher connectivity within the DMN and between the DMN and CCN has been shown to predict response to sertraline, an antidepressant (Chin Fatt et al., 2019). Taken together, these findings with depressed adults suggest that heterogeneous symptom profiles are associated with variations in RSFC, and that specific neural markers may forecast treatment effectiveness.
Placebos have been associated with symptom improvement (although to a lesser degree than have antidepressants) in depressed adolescents (Locher et al., 2017) and adults (Cipriani et al., 2018). As is the case with traditional treatments (e.g., antidepressants), researchers have also found that neural heterogeneity is related to inter-individual variability in placebo response. Greater recruitment of the lateral PFC has been shown to link depressed patients’ expectations of mood improvement to actual mood improvement following administration of a placebo antidepressant (Peciña et al., 2018). Greater baseline RSFC of the salience network has also been shown to predict depressed patients’ responses to placebo (Sikora et al., 2016).
Many of the studies reviewed above (particularly those with adolescents) have relatively small samples, and their findings may not be replicated at other sites. Research suggests that resting state fMRI studies with fewer than 80 participants (or 40 per group) have minimal power and a lower likelihood of obtaining results that reflect “true” effects (Chen et al., 2018). Large sample sizes are required when attempting to identify subtypes of depression and treatment-associated neural alterations, particularly when studying a developmental sample given the modifications in neural circuitry described above that occur during adolescence.
On the feasibility of network-based phenotyping
Despite promising initial findings, existing research attempting to link RSFC patterns with symptoms and treatment outcomes continues to be limited in methodology and applicability. Below, we outline hurdles in the study of RSFC patterns in the context of heterogeneity in adolescent depression, and describe opportunities for effectively overcoming these challenges.
Study design
As we noted above, most studies involve small numbers of participants and do not test dose effects, other treatment modalities, or nonspecific treatment factors (e.g., assessing patient expectations and adherence to treatment). However, large samples are needed to detect individual differences in treatment response, particularly if multiple treatments are being directly compared. Multi-site studies are critical in overcoming this obstacle, although there are other difficulties to consider with this approach. For example, recent work suggests that while findings concerning adolescents’ brain network patterns are similar across sites, types of MRI scanners influence measures of connectivity in different ways (Marek et al., 2019). In working to resolve these discrepancies, researchers have used traveling-subject datasets to develop novel harmonization methods that reduce multi-site bias (Yamashita et al., 2019). In addition, treatment protocols should be standardized across sites to minimize inter-site heterogeneity in study procedures.
Data acquisition and processing
Several limitations should be considered in evaluating the utility of resting fMRI in precision mental health. First, fMRI has good spatial resolution but relatively low temporal resolution, limiting its ability to detect differences in granular signal fluctuations that may be related to symptoms and treatment response. Second, fMRI is prone to signal dropout and spatial distortion due to magnetic susceptibility (e.g., at air and fluid interfaces with brain tissue) and motion caused by pulsation of cerebrospinal fluid and blood, breathing, and general head movements (Duyn, 2013). Although the majority of variation in RSFC measures across individuals has not been linked to head motion, the effects of motion on network measures are systematic and wide-reaching. Depending on the network, motion may artificially amplify or reduce connectivity estimates (Van Dijk et al., 2012). Further, it is unclear what the best methods are for correcting the impact of motion on quantitative estimates of RSFC (Parkes et al., 2018). Fortunately, considerable progress is being made towards ensuring that neuroimaging processing pipelines are becoming standardized and are rigorously tested for optimization (Esteban et al., 2019; Pervaiz et al., 2020).
Third, datasets with large numbers of participants afford the ability to identify subgroups within samples and require relatively small amounts of data within persons (e.g., a 5–10 minute fMRI scan while at rest). However, the ability to reliably map individual connectivity patterns is dependent on the amount of data available within that individual (Gordon et al., 2017). One study demonstrated improvements in reliability of individual data by collecting more than 25 minutes of resting fMRI; further, the reliability of functional connectivity fingerprints (i.e., divergence of an individual from the population) continued to improve even after four hours of measurement (Anderson et al., 2011). Although individual mapping improves within-person estimates of network measures, the time and financial costs of collecting hours of resting fMRI makes this approach impractical, particularly in a clinical setting. However, issues with individual-level reliability speak to the need for more sophisticated methods of data acquisition, processing, and analysis that would improve the signal-to-noise ratio in fMRI data (Welvaert & Rosseel, 2013). For example, recent work suggests that removing volumes where subjects are sleepy (measured via physiological recordings) robustly improves RSFC reliability (Wang et al., 2017). Currently, work using densely sampled individuals may provide foundational insights to potential targets for precision mental health (e.g., Sylvester et al., 2020); however, examining treatment effects in larger datasets with less densely measured participants may reveal broader subcategories of adolescent depression.
Reproducibility and implementation
Perhaps the largest obstacle to making progress in the field of precision mental health involves the question of the reproducibility of findings. Reproducible experiments and results ensure the credibility of research; they rely on analytic transparency, standardized guidelines for research practice and analytic approaches (especially for neuroimaging), and the use of adequately-powered samples (Picciotto, 2018; Poldrack, 2019). We recommend that future studies follow standardized methodological guidelines to examine pre-treatment neural signatures that may be associated with baseline symptom characteristics, clearly state and/or share data processing and analysis steps, and when possible, use large samples (or moderate sizes with multiple time-points) of depressed individuals. In addition, it is important that findings from these studies be replicated in independent samples in order to establish the usefulness of RSFC measurements in planning tailored interventions. Certainly, attempts to replicate without positive results should not be ignored; indeed, we believe that a comprehensive understanding of the strengths and limitations of precision mental health must include reports of both successful and unsuccessful replication attempts (e.g., Dinga et al., 2019).
Although we are not yet at the point of using RSFC to guide clinical decision-making, we believe that findings from research examining the stability and reliability of RSFC point to a favorable future for network-based clinical phenotyping. Large multi-dataset studies show not only that the organization of functional networks is reproducible across studies (Marek et al., 2019), but further, that individual differences are dominant sources of variance in measures of connectivity (Gratton et al., 2018). By continuing to examine patterns of RSFC in relation to symptom dimensions and treatment responses, we will increase our ability to intervene effectively in adolescent depression. Characterizing developmental changes related to symptoms and the brain are important first steps in achieving this goal.
The future of the precision mental health of adolescence
Research with depressed adults has provided a critical foundation for precision mental health, identifying and characterizing neurobiological features that are associated with distinct treatment benefits. It is important to recognize, however, that depression-relevant functional brain connectivity matures and becomes “re-wired” in adolescence (Solé-Padullés et al., 2016); consequently, it is during this developmental period that we have the potential to offset the emergence of depressive symptoms and minimize long-term abnormalities in functional brain networks through targeted treatment should individuals manifest symptoms Modeling metrics of brain connectivity in adolescents can help to refine a precision mental health approach in order to improve assessment and treatment of adolescent depression through a person-centered lens. At this time, we do not know how, when, and for whom alterations in neural networks emerge that are related to depressive symptoms. We urge researchers to take a precision mental health approach to the study of depression in adolescents; we believe that this approach will facilitate the development of more effective tailored interventions for depression in this age group. Specifically, there is a need for studies with adolescents that delineate the associations between specific neurobiological signatures and associated profiles of depressive symptoms, and elucidate how adolescents with varying brain-symptom phenotypes respond differentially to various treatments (Figure 1). Researchers have begun to address both of these goals with depressed adults; it is critical that we extend these investigations to the study of depressed adolescents in order to create tailored combinations of existing treatments or develop new pharmacological and therapeutic interventions that target networks of interest.
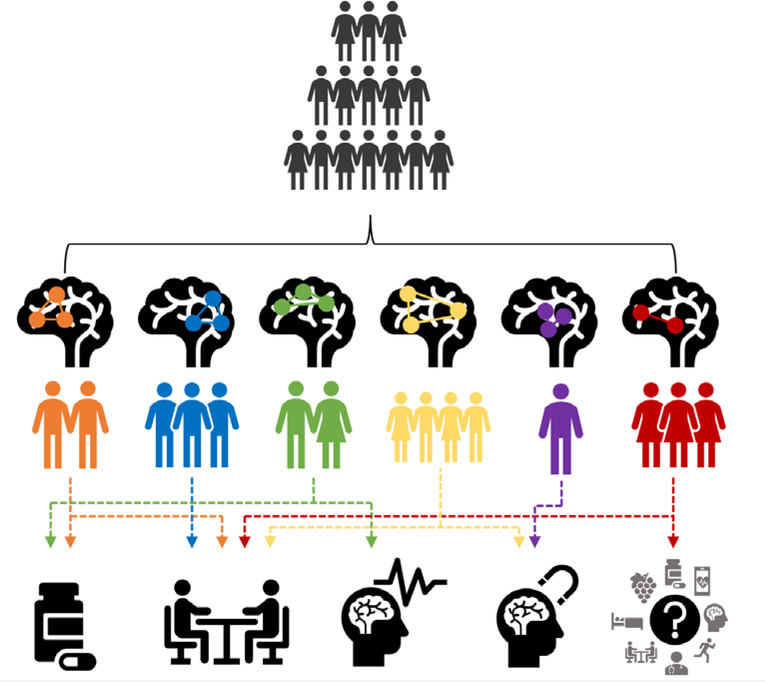
Figure 1.
A schematic of precision mental health of adolescence
The goal of precision mental health of adolescence is to identify the optimal intervention(s) for depressed youth by associating treatment response with neurophenotypes – for example, based on brain network heterogeneity. Resting-state fMRI data may help us attain this goal. Initial findings suggest that patterns of resting-state functional connectivity (RSFC) cross traditional diagnostic boundaries and may elucidate brain-symptom phenotypes that could inform tailored treatments. In this figure, we convey how depressed adolescents may differ in patterns of RSFC, and how those neural signatures may elucidate individual differences in response to various treatments (e.g., antidepressant medication, psychotherapy, electroconvulsive therapy, transcranial magnetic stimulation, and other forms of treatment).
The goal of parsing diagnostic groups to identify biomarkers that may aid in improving the understanding and treatment of adolescent depression via precision mental health is aligned with the National Institute of Mental Health’s Research Domain Criteria (RDoC) project (Insel et al., 2010). Proponents of the RDoC have outlined the advantages of understanding the pathophysiology of psychiatric disorders in order to guide diagnosis and treatment selection, rather than relying solely on traditional symptom-based clinical decisions. In this perspective, the ultimate goal of the RDoC is “precision medicine for psychiatry […] based on a deeper understanding of the biological and psychosocial basis” of disorders (page 396, Insel, 2014). Research conducted using the RDoC framework could provide complementary evidence for individual differences in brain networks and symptoms. For example, a study seeking to identify the shared and unique neural patterns associated with depressive severity and anhedonia (a variable of interest in the RDoC) found that RSFC of the dorsomedial PFC dissociated these features (Rzepa & McCabe, 2018). As described above, Dichter and colleagues (Dichter et al., 2015) found that connectivity within the CCN predicted response to antidepressants and TMS. Atypical CCN connectivity may underlie anhedonia and relate to treatment response for depressed individuals with anhedonia. We are now at the beginning stages of precision mental health endeavors. Linking RSFC-based pathophysiological variation with effects of different types of treatment and changes in mood following specific interventions will eventually yield stronger predictions of prognosis and treatment response, as envisioned by the RDoC.
In particular, it is important to examine patterns of brain connectivity as they develop over the course of adolescence in order to use precision mental health approaches to identify vulnerable adolescents as early as possible. Adopting a longitudinal approach, we can measure developing neurobiological signatures that may, over time, contribute to or be shaped by depression (Gotlib & Ordaz, 2016; Guyer, Pérez-Edgar, & Crone, 2018); we can also capture heterogeneity in depression as it emerges. At this point, despite a growing recognition that depression is a disorder of brain circuits (Williams, 2017), there are few longitudinal studies of the relation between within-person changes in network function or structure and depression in adolescents. Although individuals with internalizing psychopathology (e.g., depression and anxiety) have been found to have age-related alterations in functional network connectivity (Burkhouse et al., 2019), researchers have not yet related longitudinal within-person changes in connectivity to changes in symptoms of depression over adolescence. One study showed that individual differences in RSFC patterns are related to past and current internalizing symptoms in adolescents and also predict future symptoms, suggesting that specific patterns of functional brain connectivity are a vulnerability factor for depressive symptoms across this developmental period (Chahal et al., in review). In addition, Shapero et al. (2019) found that abnormalities in CCN and DMN network connectivity predict the onset of depression in adolescence, suggesting that neural anomalies associated with depression are present even before clinical symptoms appear. With additional longitudinal research, we can advance our understanding of which adolescents may require (and benefit from) early intervention, working to slow or halt the progression of depression. Large, open-access fMRI databases, such as those available via OpenfMRI (Poldrack et al., 2013) and the Human Connectome Project (Glasser et al., 2016), could be used to understand network connectivity changes that signal the onset of symptoms and whether certain neural signatures predict depression symptom trajectories. Data from the Adolescent Brain and Cognitive Development study (Volkow et al., 2018) will be an invaluable resource for benchmarking developmental deviations that predict depression and represent biomarkers of subtypes based on a very large sample of youth assessed over time. Indeed, precision mental health seeks to identify the most effective treatments at the optimal time (Abrahams, 2008).
It is important to recognize that although we argue resting fMRI is at the forefront of advancing precision mental health, we are not limited to RSFC in measuring variations in brain metrics that may be associated with the heterogeneous symptoms, course, and treatment response in adolescent depression. Other metrics that capture system-wide function and structure in the brain could similarly be used to examine whether neurobiological profiles in adolescent depression are associated with response to different forms of treatments. For example, diffusion tensor imaging allows researchers to quantify structural connectivity by examining the neuroanatomical WM tracts that connect brain regions. In this context, microstructural properties of the cingulum bundle (a WM tract traversing regions of the limbic system) have been found to predict remission following antidepressant treatment in depressed adults (Korgaonkar, Williams, Song, Usherwood, & Grieve, 2014). Further, variability in depression course throughout adolescence has been shown to predict later differences in WM connectivity (Rajpreet Chahal et al., 2020). In addition, task-based fMRI allows researchers to measure connectivity between brain regions and may be a useful supplement to RSFC in order to understand how brain regions co-activate in the conditions that require cognitive and affective processes known to be affected in psychiatric disorders. Indeed, pretreatment striatal activity during a monetary reward task has been shown to be associated with levels of depression severity following CBT in depressed adolescents (Forbes et al., 2010). Ultimately, multimodal approaches that examine multiple sources of neural data (e.g., combined fMRI and EEG) will allow researchers to more deeply phenotype associations among the brain, symptoms, and response to treatment.
The precision mental health approach can also be extended to a range of other mental health difficulties that emerge in adolescence, such as anxiety, stress, or disruptive behavioral disorders. Individual differences in RSFC in adolescents diagnosed with these psychiatric conditions may be associated with response to treatment; to date, however, such brain-treatment associations have been examined primarily in adults. For example, adults with social anxiety disorder (SAD) who exhibited stronger negative RSFC between the amygdala and ventrolateral PFC (e.g., AN-CCN) were more likely to respond to CBT (K. S. Young et al., 2019). Similarly, response to CBT was predicted more accurately by amygdala RSFC than by a clinical measure of the initial severity of SAD (Whitfield-Gabrieli et al., 2016). Finally, researchers have identified changes in network connectivity that are related to successful response to CBT for both panic disorder (Neufang et al., 2018) and bipolar disorder (Ellard et al., 2018). Taken together, this work supports the formulation that individuals diagnosed with neuropsychiatric disorders may be treated with greater precision and more effectively by integrating measures of RSFC (or structural connectivity) with profiles of clinical symptoms and by determining the optimal time in development to administer targeted treatments.
One example of how understanding heterogeneous neural profiles may aid in the development of more effective treatments is real-time fMRI neurofeedback. Depressed participants are able to upregulate or dampen the activity and connectivity of regions involved in emotional processing (e.g., amygdala) by focusing on memories or imagery while viewing their fMRI signals. Following this technique, patients show reduced depression symptoms and long-term changes in the brain (Young et al., 2018). Another study found that depressed men exhibit greater functional connectivity between the amygdala and prefrontal areas following neurofeedback training; further, this connectivity change was positively associated with symptom improvement (Zotev 2011). Depressed adults also show reductions in SN response to negative stimuli, accompanied by decreases in negative emotional responses, following real-time neurofeedback (Hamilton et al., 2016). Although neurofeedback may be an effective noninvasive neural intervention, it is not clear whether certain connectivity signatures are predictive of regulation success. The ability to focally target neural connections is a strength of neurofeedback training that could be utilized to more effectively treat heterogeneous depressed patients in whom RSFC architecture has been mapped.
Conclusions
In conclusion, patterns of RSFC show promise as neuromarkers that may one day guide the prescription of optimally tailored treatments for depressed adolescents. To advance this potential, there are three main goals that we believe should guide the integration of network neuroscience with precision mental health of adolescence. First, it is essential that we link heterogeneous clinical symptom profiles with distinct signatures of brain connectivity in order to identify meaningful and reliable subtypes, or continuous brain-symptom associations, in adolescent depression. Second, it is important that we assess differential treatment response of adolescents based on these heterogeneous brain-symptom indicators, with the goal of guiding interventions and helping to predict adolescents’ prognoses. Finally, it is critical that we measure the unfolding neurodevelopmental mechanisms of depression in order to inform the optimal timing of interventions. Attaining these goals necessitates that researchers use state-of-the-art statistical models, neuroimaging analysis programs, and evidence-based treatments that will allow them to probe these questions. Measuring network connectivity as it develops and determining when symptom-related alterations in networks emerge are crucial next steps in developing more effective predictions of prognosis and treatment. Adopting these approaches will allow us, ultimately, to improve the lives of adolescents with mental health difficulties.
Key points.
Onset of depression peaks during adolescence; the disorder is heterogeneous with respect to symptoms, course, severity, and response to treatment.
Emerging research suggests that differences in characteristics of adolescent depression (e.g., symptoms and treatment response) are associated with variations in neurocircuitry, particularly in resting state functional connectivity (RSFC) of depression-relevant brain networks.
We argue that elucidating the concordance between RSFC of brain networks and features of depression will facilitate the identification of biomarkers of adolescent depression and expedite progress in developing more effective and tailored approaches to assessment, prevention, and intervention for this disorder.
We review emerging research that highlights the clinical and translational potential of examining individual differences in network connectivity and depression, and propose directions for research that will advance our understanding and treatment of adolescent depression from a Precision Mental Health perspective.
Acknowledgements
Preparation of this manuscript was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001860 and linked award TL1 TR001861 (R.C.), and by the National Institute of Mental Health grants R37-MH101495 (I.H.G.), R03-MH116519 (A.E.G.), and F32MH120975 (R.C.). The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Abrahams E (2008). Right Drug—Right Patient—Right Time: Personalized Medicine Coalition. Clinical and Translational Science, 1(1), 11–12. 10.1111/j.1752-8062.2008.00003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, & Yurgelun-Todd D (2011). Reproducibility of single-subject functional connectivity measurements. AJNR. American Journal of Neuroradiology, 32(3), 548–555. 10.3174/ajnr.A2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley EA (2015). The precision medicine initiative: A new national effort. JAMA, 313(21), 2119–2120. 10.1001/jama.2015.3595 [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Swendsen J, He J-P, Burstein M, & Merikangas KR (2015). Major Depression in the National Comorbidity Survey–Adolescent Supplement: Prevalence, Correlates, and Treatment. Journal of the American Academy of Child & Adolescent Psychiatry, 54(1), 37–44.e2. 10.1016/j.jaac.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson DA, & Birmaher B (2001). Relation between anxiety and depressive disorders in childhood and adolescence. Depression and Anxiety, 14(2), 67–78. [DOI] [PubMed] [Google Scholar]
- Bai T, Zu M, Chen Y, Xie W, Cai C, Wei Q, Ji G-J, Tian Y, & Wang K (2018). Decreased Connection Between Reward Systems and Paralimbic Cortex in Depressive Patients. Frontiers in Neuroscience, 12 10.3389/fnins.2018.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Xia CH, & Satterthwaite TD (2018). Understanding the Emergence of Neuropsychiatric Disorders With Network Neuroscience. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(9), 742–753. 10.1016/j.bpsc.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom EH, Forsman M, Yang TT, Serlachius E, & Larsson J-O (2014). Latent Classes of Symptoms related to Clinically Depressed Mood in Adolescents. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology, 2(1), 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Spijker AT, Hoencamp E, Kupka R, Nolen WA, Schoevers RA, & Penninx BWJH (2014). Predictors of the onset of manic symptoms and a (hypo)manic episode in patients with major depressive disorder. PloS One, 9(9), e106871 10.1371/journal.pone.0106871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakowski J, Spinelli S, Dörig N, Bosch OG, Manoliu A, Holtforth MG, & Seifritz E (2017). Resting state brain network function in major depression – Depression symptomatology, antidepressant treatment effects, future research. Journal of Psychiatric Research, 92, 147–159. 10.1016/j.jpsychires.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, & Miller E (2017). Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Translational Psychiatry, 7(5), e1139 10.1038/tp.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Jacobs RH, Peters AT, Ajilore O, Watkins ER, & Langenecker SA (2017). Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cognitive, Affective & Behavioral Neuroscience, 17(2), 394–405. 10.3758/s13415-016-0486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Stange JP, Jacobs RH, Bhaumik R, Bessette KL, Peters AT, Crane NA, Kreutzer KA, Fitzgerald K, Monk CS, Welsh RC, Phan KL, & Langenecker SA (2019). Developmental changes in resting-state functional networks among individuals with and without internalizing psychopathologies. Depression and Anxiety, 36(2), 141–152. 10.1002/da.22864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R, Weissman DG, Hallquist MN, Robins RW, Hastings PD, & Guyer AE (in review). Neural Connectivity Biotypes: Associations with Internalizing Problems throughout Adolescence. Psychological Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal Rajpreet, Weissman DG, Marek S, Rhoads SA, Hipwell AE, Forbes EE, Keenan K, & Guyer AE (2020). Girls’ Brain Structural Connectivity in Late Adolescence Relates to History of Depression Symptom. Journal of Child Psychology and Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yu J, Zhang L, Li X, & Zhang J (2014). Etiological heterogeneity of symptom dimensions of adolescent depression. PsyCh Journal, 3(4), 254–263. 10.1002/pchj.62 [DOI] [PubMed] [Google Scholar]
- Chen X, Lu B, & Yan C-G (2018). Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Human Brain Mapping, 39(1), 300–318. 10.1002/hbm.23843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin Fatt CR, Jha MK, Cooper CM, Fonzo G, South C, Grannemann B, Carmody T, Greer TL, Kurian B, Fava M, McGrath PJ, Adams P, McInnis M, Parsey RV, Weissman M, Phillips ML, Etkin A, & Trivedi MH (2019). Effect of Intrinsic Patterns of Functional Brain Connectivity in Moderating Antidepressant Treatment Response in Major Depression. American Journal of Psychiatry, 177(2), 143–154. 10.1176/appi.ajp.2019.18070870 [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, & Geddes JR (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. The Lancet, 391(10128), 1357–1366. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Konduru N, Kemp A, Bray S, Brown EC, Goodyear B, & Ramasubbu R (2018). The impact of age of onset on amygdala intrinsic connectivity in major depression. Neuropsychiatric Disease and Treatment, 14, 343–352. 10.2147/NDT.S145042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Beevers CG, Mumford JA, & Schnyer DM (2014). Cognitive control network connectivity in adolescent women with and without a parental history of depression. Developmental Cognitive Neuroscience, 7, 13–22. 10.1016/j.dcn.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, & Petersen SE (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83(1), 238–251. 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Ho TC, Foland-Ross LC, Eggleston C, Ordaz SJ, Singh MK, & Gotlib IH (2017). Hyperactivation in Cognitive Control and Visual Attention Brain Regions During Emotional Interference in Adolescent Depression. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 2(5), 388–395. 10.1016/j.bpsc.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, Simmons AN, & Yang TT (2017). Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. Journal of Affective Disorders, 207, 86–94. 10.1016/j.jad.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugène F, Dennis EL, & Gotlib IH (2010). Neural correlates of rumination in depression. Cognitive, Affective & Behavioral Neuroscience, 10(4), 470–478. 10.3758/CABN.10.4.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, & Angold A (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60(8), 837–844. 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- Cui Z, Li H, Xia CH, Larsen B, Adebimpe A, Baum GL, Cieslak M, Gur RE, Gur RC, Moore TM, Oathes DJ, Alexander-Bloch AF, Raznahan A, Roalf DR, Shinohara RT, Wolf DH, Davatzikos C, Bassett DS, Fair DA, … Satterthwaite TD (2020). Individual Variation in Functional Topography of Association Networks in Youth. Neuron, 0(0). 10.1016/j.neuron.2020.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Vu DP, Westlund Schreiner M, Mueller BA, Eberly LE, Camchong J, Westervelt A, & Lim KO (2016). Neural Correlates of Antidepressant Treatment Response in Adolescents with Major Depressive Disorder. Journal of Child and Adolescent Psychopharmacology, 26(8), 705–712. 10.1089/cap.2015.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, & Lim KO (2014). Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–1147. 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry J, Rohde P, Simons A, Silva S, Vitiello B, Kratochvil C, Reinecke M, Feeny N, Wells K, Pathak S, Weller E, Rosenberg D, Kennard B, Robins M, Ginsburg G, March J, & TADS Team. (2006). Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). Journal of the American Academy of Child and Adolescent Psychiatry, 45(12), 1427–1439. 10.1097/01.chi.0000240838.78984.e2 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Slavich GM, Muscatell KA, Irwin MR, & Eisenberger NI (2016). Dorsal Anterior Cingulate Cortex Responses to Repeated Social Evaluative Feedback in Young Women with and without a History of Depression. Frontiers in Behavioral Neuroscience, 10 10.3389/fnbeh.2016.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Gibbs D, & Smoski MJ (2015). A Systematic Review of Relations between Resting-State functional-MRI and Treatment Response in Major Depressive Disorder. Journal of Affective Disorders, 172, 8–17. 10.1016/j.jad.2014.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinga R, Schmaal L, Penninx BWJH, van Tol MJ, Veltman DJ, van Velzen L, Mennes M, van der Wee NJA, & Marquand AF (2019). Evaluating the evidence for biotypes of depression: Methodological replication and extension of Drysdale et al. (2017). NeuroImage: Clinical, 22, 101796 10.1016/j.nicl.2019.101796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, Bakker N, Blumberger DM, Daskalakis ZJ, Kennedy SH, Flint AJ, & Giacobbe P (2014). Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biological Psychiatry, 76(3), 176–185. 10.1016/j.biopsych.2013.10.026 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, & Trimble M (2008). The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectrums, 13(8), 663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, … Liston C (2017). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23(1), 28–38. 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyn J (2013). MR Susceptibility Imaging. Journal of Magnetic Resonance (San Diego, Calif. : 1997), 229, 198–207. 10.1016/j.jmr.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DB, Harrison BJ, Yücel M, Whittle S, Zalesky A, Pantelis C, Allen NB, & Fornito A (2014). Large-scale brain network dynamics supporting adolescent cognitive control. The Journal of Neuroscience, 34(42), 14096–14107. 10.1523/JNEUROSCI.1634-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard KK, Gosai AG, Bernstein EE, Kaur N, Sylvia LG, Camprodon JA, Dougherty DD, Nierenberg AA, & Deckersbach T (2018). Intrinsic functional neurocircuitry associated with treatment response to transdiagnostic CBT in bipolar disorder with anxiety. Journal of Affective Disorders, 238, 383–391. 10.1016/j.jad.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, & Gorgolewski KJ (2019). fMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1), 111–116. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AS, Ellwood-Lowe ME, Colich NL, Cichocki A, Ho TC, & Gotlib IH (2019). Reward-circuit biomarkers of risk and resilience in adolescent depression. Journal of Affective Disorders, 246, 902–909. 10.1016/j.jad.2018.12.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, Klumpp H, Langenecker S, & Phan KL (2018). Transdiagnostic neural correlates of volitional emotion regulation in anxiety and depression. Depression and Anxiety 10.1002/da.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, & Dahl RE (2009). Altered Striatal Activation Predicting Real-World Positive Affect in Adolescent Major Depressive Disorder. The American Journal of Psychiatry, 166(1), 64–73. 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, & Dahl RE (2010). Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience, 10(1), 107–118. 10.3758/CABN.10.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, & Milham MP (2013). Striatum-based circuitry of adolescent depression and anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 628–641.e13. 10.1016/j.jaac.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, & Weersing VR (2010). Comorbidity of Anxiety and Depression in Youth: Implications for Treatment and Prevention. Clinical Psychology : A Publication of the Division of Clinical Psychology of the American Psychological Association, 17(4), 293–306. 10.1111/j.1468-2850.2010.01221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, & Phillips KA (2018). Precision Medicine: From Science to Value. Health Affairs (Project Hope), 37(5), 694–701. 10.1377/hlthaff.2017.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JLR, Auerbach EJ, Behrens TEJ, Coalson TS, Harms MP, Jenkinson M, Moeller S, Robinson EC, Sotiropoulos SN, Xu J, Yacoub E, Ugurbil K, & Van Essen DC (2016). The Human Connectome Project’s neuroimaging approach. Nature Neuroscience, 19(9), 1175–1187. 10.1038/nn.4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, & Dosenbach NUF (2017). Precision functional mapping of individual human brains. Neuron, 95(4), 791–807.e7. 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, & Ordaz S (2016). The Importance of Assessing Neural Trajectories in Pediatric Depression. JAMA Psychiatry, 73(1), 9–10. 10.1001/jamapsychiatry.2015.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahek I, Shenhav A, Musslick S, Krebs RM, & Koster EHW (2019). Motivation and cognitive control in depression. Neuroscience and Biobehavioral Reviews, 102, 371–381. 10.1016/j.neubiorev.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, Nelson SM, Coalson RS, Snyder AZ, Schlaggar BL, Dosenbach NUF, & Petersen SE (2018). Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron, 98(2), 439–452.e5. 10.1016/j.neuron.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, & Schatzberg AF (2007). Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry, 62(5), 429–437. 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Pérez-Edgar K, & Crone EA (2018). Opportunities for Neurodevelopmental Plasticity From Infancy Through Early Adulthood. Child Development, 89(3), 687–697. 10.1111/cdev.13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LMJ, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, Mueller BA, Lim KO, & Cullen KR (2014). An fMRI study of emotional face processing in adolescent major depression. Journal of Affective Disorders, 168, 44–50. 10.1016/j.jad.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Bagarinao E, Chang C, Mackey S, Sacchet MD, & Gotlib IH (2016). Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Research: Neuroimaging, 249, 91–96. 10.1016/j.pscychresns.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Kim SM, Bae S, Renshaw PF, & Anderson JS (2016). A failure of suppression within the default mode network in depressed adolescents with compulsive internet game play. Journal of Affective Disorders, 194, 57–64. 10.1016/j.jad.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Heshmati M, & Russo SJ (2015). Anhedonia and the brain reward circuitry in depression. Current Behavioral Neuroscience Reports, 2(3), 146–153. 10.1007/s40473-015-0044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, Frank G, Max JE, Wu J, Chan M, Tapert SF, Simmons AN, & Yang TT (2015). Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biological Psychiatry, 78(9), 635–646. 10.1016/j.biopsych.2014.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Gong L, Zhi M, Yin Y, Zhang Y, Xie C, & Yuan Y (2018). Distinctive pretreatment features of bilateral nucleus accumbens networks predict early response to antidepressants in major depressive disorder. Brain Imaging and Behavior, 12(4), 1042–1052. 10.1007/s11682-017-9773-0 [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, & Anand A (2011). Toward dysfunctional connectivity: A review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior, 5(4), 307–328. 10.1007/s11682-011-9134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel C, Glenn CR, Nock MK, & Somerville LH (2019). Aberrant striatal tracking of reward magnitude in youth with current or past-year depression. Journal of Abnormal Psychology, 128(1), 44–56. 10.1037/abn0000389 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Insel TR (2014). The NIMH Research Domain Criteria (RDoC) Project: Precision Medicine for Psychiatry. American Journal of Psychiatry, 171(4), 395–397. 10.1176/appi.ajp.2014.14020138 [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Liu F, Foran W, Calabro F, Roeder K, Devlin B, & Luna B (2019). Cognitive and default mode networks support developmental stability in functional connectome fingerprinting through adolescence. BioRxiv. 10.1101/812719 [DOI] [Google Scholar]
- Johnson D, Dupuis G, Piche J, Clayborne Z, & Colman I (2018). Adult mental health outcomes of adolescent depression: A systematic review. Depression and Anxiety, 35(8), 700–716. 10.1002/da.22777 [DOI] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion, 24(2), 281–298. 10.1080/02699930903407948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Westlund Schreiner M, Thai M, Gunlicks-Stoessel M, Reigstad K, & Cullen KR (2018a). Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: A preliminary study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 194–202. 10.1016/j.pnpbp.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Westlund Schreiner M, Thai M, Gunlicks-Stoessel M, Reigstad K, & Cullen KR (2018b). Neural and neuroendocrine predictors of pharmacological treatment response in adolescents with depression: A preliminary study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 194–202. 10.1016/j.pnpbp.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Korgaonkar MS, Williams LM, Song YJ, Usherwood T, & Grieve SM (2014). Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. The British Journal of Psychiatry: The Journal of Mental Science, 205(4), 321–328. 10.1192/bjp.bp.113.140376 [DOI] [PubMed] [Google Scholar]
- Kühn S, Vanderhasselt M-A, De Raedt R, & Gallinat J (2012). Why ruminators won’t stop: The structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorders, 141(2), 352–360. 10.1016/j.jad.2012.03.024 [DOI] [PubMed] [Google Scholar]
- LeMoult J, Kircanski K, Prasad G, & Gotlib IH (2017). Negative Self-Referential Processing Predicts the Recurrence of Major Depressive Episodes. Clinical Psychological Science: A Journal of the Association for Psychological Science, 5(1), 174–181. 10.1177/2167702616654898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, & Nelson CA (2009). Tuning the developing brain to social signals of emotions. Nature Reviews. Neuroscience, 10(1), 37–47. 10.1038/nrn2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, Kessler RC, & Kossowsky J (2017). Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Psychiatry, 74(10), 1011–1020. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, & Chahal R (2015). An integrative model of the maturation of cognitive control. Annual Review of Neuroscience, 38, 151–170. 10.1146/annurev-neuro-071714-034054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf FT, Brent D, Clark L, Tavitian L, McHugh RM, Sahakian BJ, & Phillips ML (2011). Neurocognitive impairment in adolescent major depressive disorder: State vs. trait illness markers. Journal of Affective Disorders, 133(3), 625–632. 10.1016/j.jad.2011.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglanoc LA, Landrø NI, Jonassen R, Kaufmann T, Córdova-Palomera A, Hilland E, & Westlye LT (2018). Data-Driven Clustering Reveals a Link Between Symptoms and Functional Brain Connectivity in Depression. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, & Luna B (2015). The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biology, 13(12), e1002328 10.1371/journal.pbio.1002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Nielsen AN, Wheelock MD, Miller RL, Laumann TO, Earl E, Foran WW, Cordova M, Doyle O, Perrone A, Miranda-Dominguez O, Feczko E, Sturgeon D, Graham A, Hermosillo R, Snider K, Galassi A, Nagel BJ, … Dosenbach NUF (2019). Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Developmental Cognitive Neuroscience, 40, 100706 10.1016/j.dcn.2019.100706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WI, Hyde LW, Shaw DS, Forbes EE, & Monk CS (2016). Clinical neuroprediction: Amygdala reactivity predicts depressive symptoms 2 years later. Social Cognitive and Affective Neuroscience, 11(6), 892–898. 10.1093/scan/nsw018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael KD, & Crowley SL (2002). How effective are treatments for child and adolescent depression?: A meta-analytic review. Clinical Psychology Review, 22(2), 247–269. 10.1016/S0272-7358(01)00089-7 [DOI] [PubMed] [Google Scholar]
- Miller CH, Hamilton JP, Sacchet MD, & Gotlib IH (2015). Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry, 72(10), 1045–1053. 10.1001/jamapsychiatry.2015.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, & Cohen JD (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, & Han B (2016). National Trends in the Prevalence and Treatment of Depression in Adolescents and Young Adults. Pediatrics, 138(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. (2011). Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. National Academies Press (US) http://www.ncbi.nlm.nih.gov/books/NBK91503/ [PubMed] [Google Scholar]
- Nejad AB, Fossati P, & Lemogne C (2013). Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience, 7, 666 10.3389/fnhum.2013.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Geiger MJ, Homola GA, Mahr M, Schiele MA, Gehrmann A, Schmidt B, Gajewska A, Nowak J, Meisenzahl-Lechner E, Pham M, Romanos M, Akhrif A, & Domschke K (2018). Cognitive-behavioral therapy effects on alerting network activity and effective connectivity in panic disorder. European Archives of Psychiatry and Clinical Neuroscience. 10.1007/s00406-018-0945-8 [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, & Panksepp J (2006). Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- O’Callaghan G, & Stringaris A (2019). Reward Processing in Adolescent Depression Across Neuroimaging Modalities. Zeitschrift Für Kinder- Und Jugendpsychiatrie Und Psychotherapie, 1–7. 10.1024/1422-4917/a000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, & Lorenzetti V (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping, 39(8), 3398–3418. 10.1002/hbm.24184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PM, Sato JR, Salum GA, Rohde LA, Gadelha A, Zugman A, Mari J, Jackowski A, Picon F, Miguel EC, Pine DS, Leibenluft E, Bressan RA, & Stringaris A (2017). Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disorder in a Longitudinal Community-Based Sample. The American Journal of Psychiatry, 174(11), 1112–1119. 10.1176/appi.ajp.2017.17040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff SJA, Meens PHF, van den Bulk BG, Jolles DD, Veer IM, van Lang NDJ, Rombouts SARB, van der Wee NJA, & Vermeiren RRJM (2014). Aberrant resting-state functional connectivity in limbic and salience networks in treatment—Naïve clinically depressed adolescents. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(12), 1317–1327. 10.1111/jcpp.12266 [DOI] [PubMed] [Google Scholar]
- Parkes L, Fulcher B, Yücel M, & Fornito A (2018). An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage, 171, 415–436. 10.1016/j.neuroimage.2017.12.073 [DOI] [PubMed] [Google Scholar]
- Peciña M, Heffernan J, Wilson J, Zubieta JK, & Dombrovski AY (2018). Prefrontal expectancy and reinforcement-driven antidepressant placebo effects. Translational Psychiatry, 8(1), 1–11. 10.1038/s41398-018-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell-Sills L, & Yang TT (2012). Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. Journal of Affective Disorders, 139(1), 75–84. 10.1016/j.jad.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, & Pelphrey KA (2011). Developing connections for affective regulation: Age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology, 108(3), 607–620. 10.1016/j.jecp.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz U, Vidaurre D, Woolrich MW, & Smith SM (2020). Optimising network modelling methods for fMRI. NeuroImage, 116604 10.1016/j.neuroimage.2020.116604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M (2018). Analytical Transparency and Reproducibility in Human Neuroimaging Studies. Journal of Neuroscience, 38(14), 3375–3376. 10.1523/JNEUROSCI.0424-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA (2019). The Costs of Reproducibility. Neuron, 101(1), 11–14. 10.1016/j.neuron.2018.11.030 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Barch DM, Mitchell J, Wager T, Wagner AD, Devlin JT, Cumba C, Koyejo O, & Milham M (2013). Toward open sharing of task-based fMRI data: The OpenfMRI project. Frontiers in Neuroinformatics, 7 10.3389/fninf.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Gates K, Kraynak TE, Thase ME, & Siegle GJ (2017a). Data-Driven Subgroups in Depression Derived from Directed Functional Connectivity Paths at Rest. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(13), 2623–2632. 10.1038/npp.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Gates K, Kraynak TE, Thase ME, & Siegle GJ (2017b). Data-driven subgroups in depression derived from directed functional connectivity paths at rest. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 42(13), 2623–2632. 10.1038/npp.2017.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, & Snyder AZ (2007). A default mode of brain function: A brief history of an evolving idea. NeuroImage, 37(4), 1083–1090. 10.1016/j.neuroimage.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Redlich R, Opel N, Bürger C, Dohm K, Grotegerd D, Förster K, Zaremba D, Meinert S, Repple J, Enneking V, Leehr E, Böhnlein J, Winters L, Froböse N, Thrun S, Emtmann J, Heindel W, Kugel H, Arolt V, … Dannlowski U (2018). The Limbic System in Youth Depression: Brain Structural and Functional Alterations in Adolescent In-patients with Severe Depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 43(3), 546–554. 10.1038/npp.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Davis MM, & Monti JD (2017). Cognition-emotion interaction as a predictor of adolescent depressive symptoms. Developmental Psychology, 53(12), 2377–2383. 10.1037/dev0000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepa E, & McCabe C (2018). Anhedonia and depression severity dissociated by dmPFC resting-state functional connectivity in adolescents. Journal of Psychopharmacology (Oxford, England), 32(10), 1067–1074. 10.1177/0269881118799935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet MD, Ho TC, Connolly CG, Tymofiyeva O, Lewinn KZ, Han LK, Blom EH, Tapert SF, Max JE, Frank GK, Paulus MP, Simmons AN, Gotlib IH, & Yang TT (2016). Large-Scale Hypoconnectivity Between Resting-State Functional Networks in Unmedicated Adolescent Major Depressive Disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(12), 2951–2960. 10.1038/npp.2016.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Wolf ND, Pennuto M, Vasic N, & Wolf RC (2014). Revisiting default mode network function in major depression: Evidence for disrupted subsystem connectivity. Psychological Medicine, 44(10), 2041–2051. 10.1017/S0033291713002596 [DOI] [PubMed] [Google Scholar]
- Scheuer H, Alarcón G, Demeter DV, Earl E, Fair DA, & Nagel BJ (2017). Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psychiatry Research. Neuroimaging, 266, 35–41. 10.1016/j.pscychresns.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, & Price CJ (2018). Interpreting and Utilising Intersubject Variability in Brain Function. Trends in Cognitive Sciences, 22(6), 517–530. 10.1016/j.tics.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapero BG, Chai XJ, Vangel M, Biederman J, Hoover CS, Whitfield-Gabrieli S, Gabrieli JDE, & Hirshfeld-Becker DR (2019). Neural markers of depression risk predict the onset of depression. Psychiatry Research: Neuroimaging, 285, 31–39. 10.1016/j.pscychresns.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M, Heffernan J, Avery ET, Mickey BJ, Zubieta J-K, & Peciña M (2016). Salience Network Functional Connectivity Predicts Placebo Effects in Major Depression. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 1(1), 68–76. 10.1016/j.bpsc.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, & Dahl RE (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798–1807. 10.1093/scan/nst175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Padullés C, Castro-Fornieles J, de la Serna E, Calvo R, Baeza I, Moya J, Lázaro L, Rosa M, Bargalló N, & Sugranyes G (2016). Intrinsic connectivity networks from childhood to late adolescence: Effects of age and sex. Developmental Cognitive Neuroscience, 17, 35–44. 10.1016/j.dcn.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeldt SL, Cullen KR, Han G, Fryza BJ, Houri AK, & Klimes-Dougan B (2016). Executive Attention Impairment in Adolescents With Major Depressive Disorder. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 45(1), 69–83. 10.1080/15374416.2015.1072823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Saucedo R, Walter HJ, Cabral H, England MJ, & Kazis LE (2016). Receipt of Evidence-Based Pharmacotherapy and Psychotherapy Among Children and Adolescents With New Diagnoses of Depression. Psychiatric Services (Washington, D.C.), 67(3), 316–323. 10.1176/appi.ps.201500090 [DOI] [PubMed] [Google Scholar]
- Spreng RN, & Grady CL (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22(6), 1112–1123. 10.1162/jocn.2009.21282 [DOI] [PubMed] [Google Scholar]
- Stein K, & Fazel M (2015). Depression in young people often goes undetected. The Practitioner, 259(1782), 17–22, 2–3. [PubMed] [Google Scholar]
- Straub J, Metzger CD, Plener PL, Koelch MG, Groen G, & Abler B (2017). Successful group psychotherapy of depression in adolescents alters fronto-limbic resting-state connectivity. Journal of Affective Disorders, 209, 135–139. 10.1016/j.jad.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Yu Q, Srivastava AB, Marek S, Zheng A, Alexopoulos D, Smyser CD, Shimony JS, Ortega M, Dierker DL, Patel GH, Nelson SM, Gilmore AW, McDermott KB, Berg JJ, Drysdale AT, Perino MT, Snyder AZ, Raut RV, … Dosenbach NUF (2020). Individual-specific functional connectivity of the amygdala: A substrate for precision psychiatry. Proceedings of the National Academy of Sciences. 10.1073/pnas.1910842117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Lu L, Zhang L, Hu X, Bu X, Li H, Hu X, Gao Y, Zeng Z, Gong Q, & Huang X (2018). Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine, 36, 436–445. 10.1016/j.ebiom.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda T, Yoshimoto J, Shimizu Y, Okada G, Takamura M, Okamoto Y, Yamawaki S, & Doya K (2018). Identification of depression subtypes and relevant brain regions using a data-driven approach. Scientific Reports, 8(1). 10.1038/s41598-018-32521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, & Sporns O (2013). An anatomical substrate for integration among functional networks in human cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(36), 14489–14500. 10.1523/JNEUROSCI.2128-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, & Buckner RL (2012). The Influence of Head Motion on Intrinsic Functional Connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, & Luna B (2008). Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex (New York, N.Y.: 1991), 18(11), 2505–2522. 10.1093/cercor/bhn012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgis V, Gelardi KL, Helm JL, Forbes EE, Hipwell AE, Keenan K, & Guyer AE (2018). Dorsomedial Prefrontal Activity to Sadness Predicts Later Emotion Suppression and Depression Severity in Adolescent Girls. Child Development, 89(3), 758–772. 10.1111/cdev.13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, Pérez-Stable EJ, Riley WT, Bloch MH, Conway K, Deeds BG, Dowling GJ, Grant S, Howlett KD, Matochik JA, Morgan GD, Murray MM, Noronha A, Spong CY, … Weiss SRB (2018). The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental Cognitive Neuroscience, 32, 4–7. 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Han J, Nguyen VT, Guo L, & Guo CC (2017). Improving the Test-Retest Reliability of Resting State fMRI by Removing the Impact of Sleep. Frontiers in Neuroscience, 11 10.3389/fnins.2017.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welvaert M, & Rosseel Y (2013). On the definition of signal-to-noise ratio and contrast-to-noise ratio for FMRI data. PloS One, 8(11), e77089 10.1371/journal.pone.0077089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, Reynolds GO, Hofmann SG, Pollack MH, & Gabrieli JDE (2016). Brain connectomics predict response to treatment in social anxiety disorder. Molecular Psychiatry, 21(5), 680–685. 10.1038/mp.2015.109 [DOI] [PubMed] [Google Scholar]
- Williams LM (2017). Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: A theoretical review of the evidence and future directions for clinical translation. Depression and Anxiety, 34(1), 9–24. 10.1002/da.22556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T, Doan NT, Kaufmann T, Alnæs D, Moberget T, Agartz I, Buitelaar JK, Ueland T, Melle I, Franke B, Andreassen OA, Beckmann CF, Westlye LT, & Marquand AF (2018). Mapping the Heterogeneous Phenotype of Schizophrenia and Bipolar Disorder Using Normative Models. JAMA Psychiatry. 10.1001/jamapsychiatry.2018.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Yahata N, Itahashi T, Lisi G, Yamada T, Ichikawa N, Takamura M, Yoshihara Y, Kunimatsu A, Okada N, Yamagata H, Matsuo K, Hashimoto R, Okada G, Sakai Y, Morimoto J, Narumoto J, Shimada Y, Kasai K, … Imamizu H (2019). Harmonization of resting-state functional MRI data across multiple imaging sites via the separation of site differences into sampling bias and measurement bias. PLOS Biology, 17(4), e3000042 10.1371/journal.pbio.3000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Lansing AE, Wu J, Brown GG, & Paulus MP (2009). Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport, 20(4), 440–444. 10.1097/WNR.0b013e3283262e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Pettit JW, Lewinsohn PM, Seeley JR, & Roberts RE (2013). Heterogeneous trajectories of depressive symptoms: Adolescent predictors and adult outcomes. Journal of Affective Disorders, 148(0), 391–399. 10.1016/j.jad.2012.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Zotev V, Phillips R, Misaki M, Drevets WC, & Bodurka J (2018). Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry and Clinical Neurosciences, 72(7), 466–481. 10.1111/pcn.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, LeBeau RT, Niles AN, Hsu KJ, Burklund LJ, Mesri B, Saxbe D, Lieberman MD, & Craske MG (2019). Neural connectivity during affect labeling predicts treatment response to psychological therapies for social anxiety disorder. Journal of Affective Disorders, 242, 105–110. 10.1016/j.jad.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]