SUMMARY
Reliable and consistent pluripotent stem cell reporter systems for efficient purification and visualization of motor neurons are essential reagents for the study of normal motor neuron biology and for effective disease modeling. To overcome the inherent noisiness of transgene-based reporters, we developed a new series of human induced pluripotent stem cell lines by knocking in tdTomato, Cre, or CreERT2 recombinase into the HB9 (MNX1) or VACHT (SLC18A3) genomic loci. The new lines were validated by directed differentiation into spinal motor neurons and immunostaining for motor neuron markers HB9 and ISL1. To facilitate efficient purification of spinal motor neurons, we further engineered the VACHT-Cre cell line with a validated, conditional CD14-GFP construct that allows for both fluorescence-based identification of motor neurons, as well as magnetic-activated cell sorting (MACS) to isolate differentiated motor neurons at scale. The targeting strategies developed here offer a standardized platform for reproducible comparison of motor neurons across independently derived pluripotent cell lines.
INTRODUCTION
Motor neurons are specialized cells that transmit signals from the central nervous system to muscles and other peripheral organs. Their importance is underscored by the progressive paralysis and high mortality rate associated with motor neuron degeneration in patients suffering from amyotrophic lateral sclerosis (ALS) (Hardiman et al., 2017; Salameh et al., 2015). Despite the fact that ALS was originally described by Jean-Martin Charcot 150 years ago and many disease-causing mutations have been identified over the past three decades, there are no cures and few treatment options for patients with ALS. With the advent of induced pluripotent stem cell (iPSC) technology (Takahashi et al., 2007) and optimized motor neuron differentiation protocols (Amoroso et al., 2013; Li et al., 2005; Maury et al., 2014), we can now access almost unlimited quantities of live human ALS motor neurons (Dimos et al., 2008).
The utility of in vitro-derived motor neurons as a tool to study cellular and molecular processes contributing to motor neuron pathology depends on our ability to visualize and purify motor neurons from mixed populations of cells generated during stem cell differentiation. In the past, many widely-used motor neuron reporter iPSC lines were generated by random insertion of transgenes coding for a fluorescent reporter under the control of motor neuron-specific HB9 (MNX1) or ISL1 enhancer sequences (Di Giorgio et al., 2007; Kim et al., 2015; Lewcock et al., 2007; Machado et al., 2014; Wichterle et al., 2002). These lines allowed for the purification of stem cell-derived motor neurons for genomic analyses, co-culture studies, drug screening, and for tracing motor neurons and their axonal projections upon transplantation in vivo (Di Giorgio et al., 2007; Lewcock et al., 2007; Machado et al., 2014; Peljto et al., 2010; Thams et al., 2019; Wichterle et al., 2002). Due to the random integration sites and variable copy numbers of the transgene, individual reporter lines show variable levels and patterns of reporter gene expression, making comparisons of motor neurons across independently-generated lines difficult to interpret.
Here we developed a more consistent reporter system based on CRISPR/Cas9 genome engineering. Besides targeting the HB9 gene, we also targeted SLC18A3 gene encoding vesicular acetylcholine transporter (VACHT), which is expressed in all cholinergic neurons. We generated lines in which a fluorescent reporter was inserted into and expressed directly from these loci, and lines in which reporter gene expression was amplified using the Cre-lox recombination system. In addition to fluorescent reporter systems, we developed a strategy to express a CD14 GPI-linked cell surface receptor protein in a motor neuron-specific manner, which is useful for large-scale purification of motor neurons by magnetic cell sorting (Machado et al., 2014). The targeting approaches and new cell lines generated and characterized in this study provide a refined tool for comparative analyses of human motor neurons isolated from diverse control and patient derived pluripotent stem cell line.
EXPERIMENTAL PROCEDURES
Cloning of guides and donors:
The 20-bp gRNAs were designed using the Deskgen online algorithm (https://www.deskgen.com). The sense and antisense sequences for the guide RNAs (gRNAs) were ordered as single-strand oligos, and cloned into the expression vector following the manufacturer instructions (Addgene cat. no. 41824) using In-Fusion HD mix (Takara). Table S1 lists the sequences of gRNAs used in this study.
VACHT guide RNAs were tested by directly performing the targeting on an iPSC line and analyzing which gRNA rendered a higher percentage of donor DNA insertion (data not shown). HB9 gRNAs were assayed by the T7 Endonuclease-I method: Two million SHSY5Y cells were electroporated in a Nucleofector 4D device (Lonza) using 7.5 μg of either guide and 2.5 μg of Cas9-mCherry plasmid using program CB-150 and the P3 Primary Cell 4D-Nucleofector® X Kit L (Cat # V4XP-3024, Lonza). Genomic DNA of the electroporated culture was extracted two days later, followed by amplification of the region of interest by PCR. The PCR products were gel-extracted using the Zymoclean Gel DNA Recovery kit (Zymo Research) following the manufacturer instructions. Heteroduplex formation was achieved by mixing targeted and un-targeted (wt) PCR products in equal parts and subjecting the mix to a gradual decrease in temperature, following the standardized protocol from New England Biolabs (https://www.neb.com/protocols/2014/08/11/determining-genome-targeting-efficiency-using-t7-endonuclease-i). The AAVS1 guide was previously published and was not tested before the actual targeting (Mali et al., 2013).
All donors were cloned into the same promoterless pcDNA3.1 backbone, except for the AAVS1 donor backbone for the loxSTOPlox-tdTomato targeting, which was purchased from Addgene (22212). The loxSTOPlox-tdTomato cassette was PCR-amplified from Addgene 22799. The loxSTOP-lox-CD14-GFP donor was made by cloning CD14-IRES-GFP from a plasmid generously donated by Dr Ivo Lieberam (Machado et al., 2014) into a pcDNA3.1 backbone containing the homology arms of AAVS1. For the rest of donors, homology arms to targeted loci were amplified from genomic DNA from control iPSC lines, and cloning was performed by In-Fusion. A list of primers used in cloning and genotyping is shown in Table S2.
Cell maintenance:
iPSCs were routinely culture in feeder-free conditions using mTeSR1 media (Stem Cell Technologies) and plates coated with matrigel (Corning). Passaging was performed by washing the cultures twice with 0.5 mM EDTA, followed by mechanical dislodging and trituration. Cells were seeded in media containing 10 μM ROCK inhibitor for 24 hours to increase survival. Media was replenished every two days.
Electroporation of iPSCs and clone isolation:
Cell media was changed at least one hour prior to electroporation to media containing ROCK inhibitor. Subsequently, cells were washed twice with 0.5 mM EDTA and dislodged from the plate by pipetting. Two million cells were then span down and the pellet resuspended in electroporation solution containing 7.5 μg gRNA, 2.5 μg Cas9mCherry, 10–20 μg donor, 18 μl Supplement and 82 μl P3 solution (Lonza). Suspension was then transferred to a Nucleofector 4D electroporation cuvette and electroporated using the program CB-150. Following electroporation, cells were seeded in mTeSR1 + ROCK inhibitor onto one well of a 6-well plate (10 cm2) coated with matrigel.
Cells were washed with EDTA and triturated 24–30 hours after electroporation, followed by resuspension in FACS media (10 % KSR, 10 μM ROCKI in Mg- and Ca-free PBS) and passing through a 40 μm strainer. The single-cell suspension was then centrifuged and resuspended in 200–500 μl of FACS media. FACS was performed against mCherry on a Bio-Rad S3e sorter. The purified population was seeded in matrigel-coated plates with mTeSR1 + ROCKI at a cell density of 500 cells per cm2.
In the case of the AAVS1 targeting, cells were seeded in a well of a 6-well plate after electroporation as described above, and the antibiotic selection was started 2–3 days later with puromycin at 0.5 μg/ ml. Selection media was changed for 3–4 consecutive days. After antibiotic withdrawal, media was changed every two days.
Cells were allowed to grow into colonies for 7–10 days. Clones were picked by pipetting in an IVF hood and each of them was split into two replica wells of two individual 96-well plates, one of which would be used for genotyping, while the other is used for culture expansion.
Genomic DNA extraction and genotyping of clones:
Two to four days after clone isolation, one of the replica 96-well plates was washed with 100 μl of Mg- and Ca-free PBS per well, followed by incubation in lysis buffer at 60°C for 30 minutes and a 5-minute incubation at 95C to inactivate the proteinase K. Cell lysis buffer contains 100 mM NaCl, 10 mM Tris-HCl pH 8.0, 25 mM EDTA, 0.5 % SDS, and proteinase K at 200 μg/ml.
Serial dilutions of random clones were used to optimize the amount of lysate to be used in the genotyping prior to the genotyping of all clones. Genotyping was performed using KAPA HiFi 2x mix (KAPA Biosystems). Genotyping reactions were designed so an upward shift of the wt size due to exogenous DNA insertion was apparent, using primers outside the homology arm regions contained in the donor plasmid. If the mutant allele was too large to amplify using this approach, one sense primer outside and one antisense primer inside the donor DNA sequence were used.
Motor neuron differentiation:
The protocol used to differentiate iPSCs to motor neurons follows that published by the Nedelec group (Maury et al., 2015). Cell media was changed 1 hour before starting the differentiation. Cells were then dislodged mechanically from the matrigel-coated plates after two washes with 0.5 mM EDTA. Cells were differentiated in suspension at a starting density of 25,000 – 100,000 cells per ml. Embryoid bodies (EBs) were dissociated on day 16 as follows: EBs were collected in conical tubes and let settle by gravity. After two 5-minute PBS washes, EBs were incubated in 0.25 % trypsin/EDTA at 37°C for 15 minutes, vortexing every 5 minutes. After trypsin digestion, reaction was stopped with an equal volume of FBS and the EBs were dissociated by trituration. The culture was then passed through a 40 μm strainer to remove clumped cells and centrifuged at 1,200 rpm for 5 minutes. The single-cell suspension was then resuspended in appropriate seeding media (Neurobasal medium, 1× glutamine, 1× NEAA, 1× B27, 10 ng/ml GDNF, 10 ng/ml BDNF, 10 ng/ml IGF-1, 10 ng/ml CNTF, 2.27 μM ascorbic acid, and 1 μM UFdU) or FACS media (described above) if applicable. Plates for seeding mixed motor neuron cultures were coated with poly-D-lysine and mouse Laminin. Motor neuron media was changed every 2–3 days (UFdU was withdrawn in all these feedings).
CreERT2 clones were treated with Neurobasal media containing 1 μM 4-OH-tamoxifen for 15 minutes at 37°C every day from differentiation day 7 to differentiation day 17 (1 day post-seeding). If applicable, cells were later treated every two days with 200 nM 4-OH-tamoxifen.
Live imaging:
Live imaging was performed on ZEISS microscope Axio Observer Z1. Imaging media contained M199 (Invitrogen, 11043–023), 1x Glutamine (Gibco), 1× beta-mercaptoethanol (Chemicon, ES-007-E), 0.4 % D-glucose (Sigma), 200 μM Sodium Pyruvate (Gibco), 10 mM HEPES (Gibco), 1× NEAA. Cells were routinely imaged on day 2 and day 9 post-dissociation. The exposure time for endogenously tagged reporters and Cre/CreERT2 reporters was always maintained at 420 ms and 6 ms, respectively.
Live imaging of the CD14 line required a prior step of live staining. Cells were blocked by washing twice with DMEM/ 10 % FBS, followed by incubation with mouse CD14 antibody (MCA596EL, Table S3) for 30 minutes at 37°C, followed by three DMEM/10 % FBS washes and a 30-minute incubation at 37°C with an anti-mouse Alexa 546 secondary (Life Technologies, A11126, Table S3), followed by three washes with DMEM/10 % FBS.
MACS:
For MN purification by MACS from 10 ml of suspension culture, EBs were collected in a 15 ml Falcon tube, span down, and then washed with Mg- and Ca-free PBS. Subsequently, EBs were treated with 0.05 % trypsin for 15 minutes at 37°C. EBs were then triturated and the trypsin reaction blocked by an equal volume of FBS. The suspension was passed through a 40 μm strainer and washed twice by centrifugation with MACS wash buffer (3 mM MgCl2 in L15 media). Then, cells were washed in MACS sorting buffer (0.5 % BSA and 3 mM MgCl2 in Mg- and Ca-free PBS). The cell pellet was resuspended in 500 μl of MACS sorting buffer containing 5 μg/ml of CD14 antibody (MCA596EL, Table S3). Incubation with anti-CD14 was performed for 30 minutes at 4°C. The antibody was washed by centrifugation in MACS sorting buffer, and the cell pellet was then resuspended in 1.2 ml of sorting buffer. The suspension was passed through an equilibrated column mounted on a metallic stage and containing anti-mouse IgG microbeads. After passing the suspension, the column was washed with sorting buffer three times. The column was then dismounted from the stage and CD14-captured cells were eluted with 2 ml of sorting buffer. Pure population was subsequently seeded in MN media or subjected to FACS analysis.
Immunocytochemistry:
Cells were fixed at day 2 and 9 post-dissociation with 4 % PFA for 10–15 minutes and washed with 0.1 % TX-100/PBS. Cells were blocked with 10 % donkey serum/PBS for 1 hour. Primary antibodies were diluted in 3 % donkey serum/0.1 % TX100/PBS, and staining was performed overnight at 4°C. Incubation with secondary antibodies was performed in 3 % donkey serum/0.1 % TX100/PBS at room temperature for 1 hour. A list of antibodies is shown in Table S3.
RESULTS
Transgenic motor neuron reporters based on HB9 or ISL1 regulatory regions play an essential role in studies of motor neuron function and in motor neuron disease modeling (Bos et al., 2019; Di Giorgio et al., 2008; Di Giorgio et al., 2007; Hoang et al., 2018; Jacko et al., 2018; Lewcock et al., 2007; Sternfeld et al., 2017; Thams et al., 2019). However, expression of randomly-integrated transgenes is often influenced by the positional effect of integration sites (data not shown), resulting in variable patterns of reporter expression across independently generated reporter cell lines. Additionally, most cranial motor neurons do not express HB9 (Arber et al., 1999). This limits the utility of HB9-reporter cell lines for disease modeling, as cranial motor neurons are an ideal control population for spinal motor neurons given their increased resistance to degeneration in ALS (An et al., 2019; Kanning et al., 2010; Kaplan et al., 2014).
To develop a more reproducible and broadly-applicable reporter system, we turned to targeted genome engineering. In addition to HB9, we selected VACHT (SLC18A3) as a reporter gene, since it is expressed in all cholinergic cells, including all mature spinal and cranial motor neurons. We designed guide RNAs (gRNAs) to target the C-terminus of the HB9 gene with a vector that introduces a P2A sequence and a reporter gene in front of the STOP codon (Figure 1A, Table S1). The VACHT gene was targeted by gRNAs at its N-terminal domain with a construct carrying the reporter gene followed by the IRES sequence that was inserted after the ATG translation start site (Figure 1A). The P2A and IRES sequences facilitate bicistronic expression of the targeted gene and the reporter from the same allele. To ensure that these strategies were applicable across independently-derived iPSC lines, we targeted a control NCRM1 line (CRMi003-A) generated by NIH-CRM (https://hpscreg.eu/cell-line/CRMi003-A), a control FA011 line derived by the Columbia Stem Cell Core (ND50004, TALSCTRL15.12), and an ALS iPSC line NS013 carrying SOD1A4V mutation (NH50164, Stem Cell Core, Eleanor and Lou Gehrig ALS Center at Columbia University). Individual nucleofected cells were expanded, genotyped, and clones that underwent homologous recombination in one or both alleles were selected for further characterization of reporter gene expression (see Table 1 for descriptions, targeting efficiencies, and zygosities of the studied lines).
Figure 1. Direct targeting of VACHT and HB9 loci with tdTomato.
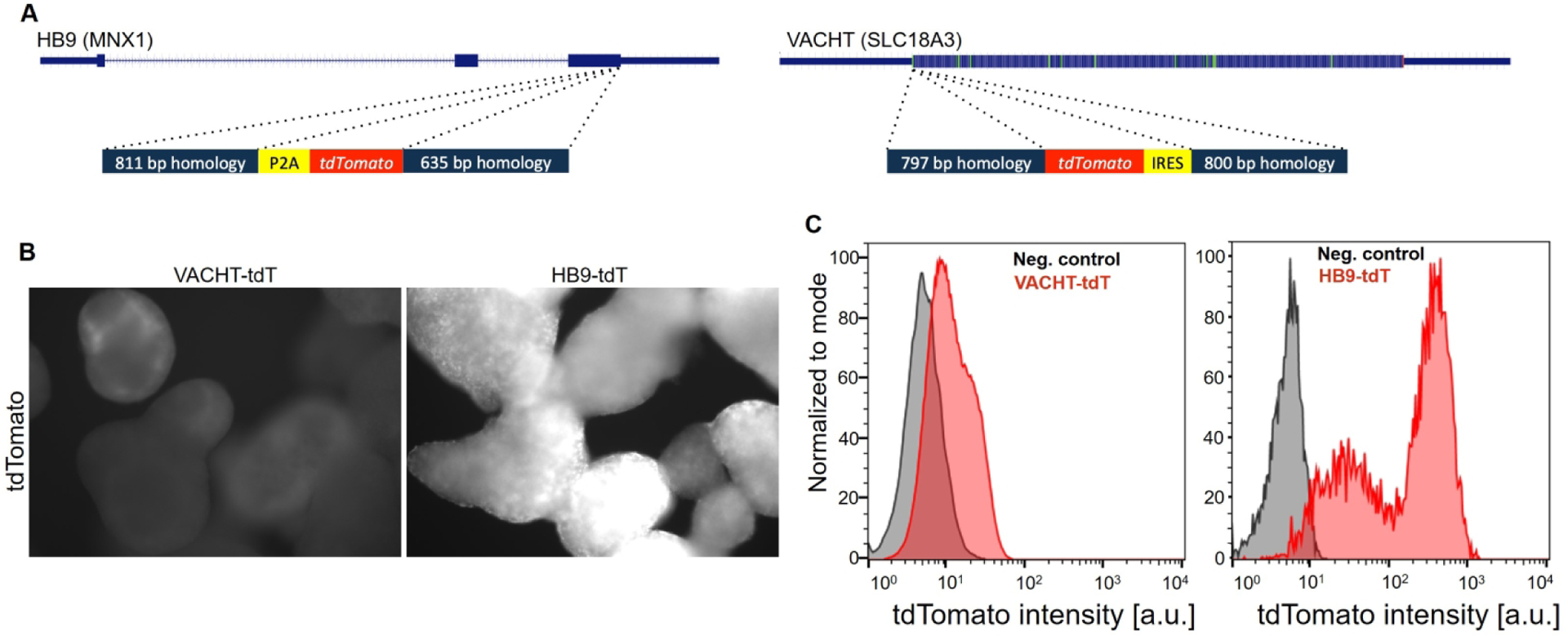
A: schematic of targeted genes and the donor targeting DNA. B: Fluorescence imaging of tdTomato expression in embryoid bodies on differentiation day 16 (500 ms acquisition time). C: Flow cytometry histograms showing fluorescence intensity (fluorescence arbitrary units – a.u.) of VACHT-tdTomato and HB9-tdTomato lines on day 16. Reporter lines are shown in red, control cells in grey.
Table 1. List of the generated iPSC lines.
The lines identified with NINDS ID are deposited at RUCDR Infinite Biologics at Rutgers University and are available upon request (https://stemcells.nindsgenetics.org/) from this distribution facility. The remaining lines (marked as CUSCCF) are distributed by the Columbia University Stem Cell Core Facility. Note that the NS013 parental line is highly sensitive to electroporation, explaining the small number of surviving clones available for analysis.
| New cell line | Parental line | mutation | NINDS ID | Donor type | Puromycin selection | Clones genotyped | Homozygous | Heterozygous | Zygosity of studied clones |
|---|---|---|---|---|---|---|---|---|---|
| HB9-tdT | FA011 | WT | NH50286 | Undigested plasmid | No | 61 | 2 (3%) | 9 (15%) | homozygous |
| HB9-CreERT2 | NS013#11-AAVS1-LSL-tdT | SOD1A4V | CU-SCCF | PCR product | No | 72 | 4 (5%) | 2 (3%) | homozygous |
| VAChT-tdT | NS013#11 | SOD1A4V | CU-SCCF | Linearized plasmid | No | 6 | 0 | 1 (17%) | heterozygous |
| VAChT-Cre/tdT | NCRM1-AAVS1-LSL-tdT | WT | NH50275 | Linearized plasmid | No | 9 | 1 (11%) | 0 | homozygous |
| NS013#11-AAVS1-LSL-tdT | NS013#11 | SOD1A4V | CU-SCCF | Undigested plasmid | Yes | 1 | 0 | 1 (100%) | N/A |
| NCRM1-AAVS1-LSL-tdT | NCRM1 | WT | NH50285 | Undigested plasmid | Yes | 7 | 0 | 7 (100%) | N/A |
| VACHT-Cre | FA011 | WT | NH50162 | Undigested plasmid | No | 12 | 1 (8%) | 0 | N/A |
| VACHT-Cre/CD14-GFP | VACHT-Cre | WT | CU-SCCF | Undigested plasmid | Yes | 27 | 5 (19%) | 10 (37%) | homozygous |
Verified and karyotyped (Figure S5) targeted clones were differentiated into spinal motor neurons using an optimized differentiation protocol (Figure S6) (Maury et al., 2014). We detected widespread expression of the reporter gene in HB9-tdTomato lines on day 16 of differentiation. Meanwhile, we detected only a weak fluorescent signal in differentiating VACHT-tdTomato lines, as observed by live-imaging of embryoid bodies prior to dissociation (Figure 1B). To evaluate the intensity of reporters more quantitatively and to assess whether the lines could be used for motor neuron purification, we dissociated embryoid bodies on day 16 and subjected them to fluorescent activated cell sorting (FACS). FACS analysis revealed that HB9-tdTomato lines expressed the reporter at levels that readily allowed for the separation of positive and negative cells (Figure 1C). In contrast, VACHT-tdTomato-positive cells could not be cleanly separated from the negative population (Figure 1C). To examine whether VACHT-tdTomato fluorescence would increase with maturation, we plated dissociated cells on laminin coated dishes and live-imaged them 2 days (DIV16+2) and 9 days (DIV16+9) later. We observed that the VACHT-tdTomato reporter lines became brighter, reaching levels comparable to the intensity of the HB9-tdTomato line by day 9 post plating (Figure S1).
Considering the relatively weak reporter gene expression in directly targeted HB9 and VACHT loci, we explored whether the Cre-lox based recombination system would yield more stable and intense reporter gene expression. We first engineered an iPSC line with a CAGGS-lox-STOP-lox-tdTomato cassette in the AAVS1 safe harbor site (Mali et al., 2013). To activate expression of tdTomato specifically in motor neurons, we performed a second round of engineering during which we introduced Cre recombinase into the VACHT or HB9 loci, using the above-described targeting strategy (Figure 2A). Even low level of Cre recombinase expression driven by the endogenous HB9 or VACHT promoters is sufficient to excise the STOP cassette and activate expression of tdTomato under the control of a strong and ubiquitous CAGGS promoter in these lines. Indeed, when differentiated into motor neurons, the VACHT-Cre/tdTomato cells exhibited ~100 fold brighter tdTomato expression compared to VACHT-tdTomato cells (~3000 vs ~30 fluorescence units, Figure 2C, 1C). The difference in fluorescence intensity was further confirmed by live imaging of dissociated and plated reporter cells, where the exposure times had to be adjusted from 420 ms to 6 ms in order to image the VACHT-Cre/tdTomato line (Figure S1).
Figure 2. Motor neuron reporters based on the Cre-lox recombination system.

A: schematic of targeting performed in HB9, VACHT, and AAVS1 loci. B: Fluorescence imaging of tdTomato signal in embryoid bodies on dissociation day 16 (500 ms acquisition time). C: Flow cytometry histograms showing reporter fluorescence (fluorescence arbitrary units – a.u.) of dissociated cultures on day 16. Reporter lines are shown in red, control cells in grey.
Examination of the HB9-Cre/tdTomato cell line revealed a premature induction of fluorescence as soon as differentiation day 3 (data not shown). Considering that this is before motor neurons are first generated, we concluded that transient, low-level activation of HB9 gene transcription at the onset of differentiation likely results in the premature reporter gene activation. To rectify this problem, we developed a cell line in which Cre activity can be conditionally regulated by tamoxifen treatment (HB9-CreERT2) (Figure 2A). Accordingly, no fluorescent cells were observed when this line was differentiated in the absence of tamoxifen. Treatment of differentiating cells with tamoxifen starting on day 7 resulted in the appearance of fluorescent cells in differentiating embryoid bodies at the time of postmitotic motor neuron differentiation (Figure 2B). Although the intensity of individual cells was as strong as in the VACHT-Cre cell line (and ~25 fold brighter than tdTomato in HB9-tdTomato cells, Figure 1C, 2C), many fewer fluorescent cells were detected by flow cytometry analysis, indicating that tamoxifen-controlled, CreERT2-driven recombination might be less efficient (Figure 2C).
To probe whether reporter gene expression is activated specifically under motor neuron differentiation conditions, we cultured the VACHT-Cre/tdTomato cell line in the absence of smoothened agonist (SAG), which is required for efficient motor neuron induction. Omission of SAG resulted in embryoid bodies largely devoid of fluorescent cells on day 16, indicating that majority of reporter expressing cells are likely motor neurons (Figure 3A,B). To evaluate the identity of reporter-expressing cells more directly, we performed a series of immunocytochemical studies. Dissociated and re-plated day 16 embryoid bodies differentiated in the presence of SAG were fixed two or nine days post-plating and stained with antibodies against the motor neuron specific markers HB9 and ISL1. In each of the examined lines we observed that >70% of the tdTomato-positive cells expressed HB9, and >80% of the tdTomato-positive cells expressed ISL1 (Figure 3C–E, Figure S2). The proportion of reporter cells expressing HB9, but not ISL1, was significantly lower in VACHT-based reporter lines compared to the HB9-based reporters (77 % compared to 95 %) (Figure 3D, S2), consistent with the notion that not all spinal motor neuron subtypes express HB9 (Arber et al., 1999) and indicating that VACHT reporter lines capture a more diverse set of spinal motor neurons than HB9 reporter lines. The percentage of HB9-positive or ISL1-positive motor neurons that express the reporter was also very high, with more than 80% of HB9 or ISL1 positive cells expressing tdTomato reporter in the VACHT-Cre, VACHT-tdTomato and HB9-tdTomato lines by day 9 post-plating. In contrast, only 42% of HB9 or ISL1 positive motor neurons expressed the fluorescent reporter in the HB9-CreERT2 cell line, demonstrating that the tamoxifen-regulated CreERT2 recombinase is less efficient in activating the tdTomato reporter.
Figure 3. Immunocytochemical characterization of tdTomato expressing cells.

A: VACHT-Cre/tdTomato cells differentiated in the presence or absence of SAG, immunostained with antibodies against tdTomato (red) and a mixture of antibodies against HB9 and Isl1 (green) on day 16+2. B: tdTomato reporter expression was analyzed by flow cytometry on day 16 of differentiation of VACHT-Cre/tdTomato cells in the presence or absence of SAG. C: immunocytochemistry of DIV16+2 mixed population cultures stained against motor neuron markers HB9 (blue) and ISL1 (green). D: percentage of tdTomato+ cells expressing HB9 in DIV16+2 differentiated HB9-based and VACHT-based reporter lines (boxes represent 25th-75th percentiles; whiskers delimit highest and lowest values; black line across boxes = median; red dot = mean, p- value determined by t-test, n=6). E: percentage of HB9+ cells expressing tdTomato on DIV 16+2 and 16+9 [boxes represent 25th-75th percentiles; whiskers delimit highest and lowest values; black line across boxes = median; red dot = mean; p-value determined by pairwise t-tests between HB9-CreERT2 cell line and the other cell lines combined for both timepoints, n=6].
Although the VACHT-Cre reporter shows high specificity for motor neurons on day 16+2, we observed a trend toward an increased number of tdTomato-positive cells that did not express HB9 or ISL1 by day 16+9 (Figure S3). This suggests that the specificity of the VACHT reporter for spinal motor neurons decreases with time in culture, possibly due to the appearance of cholinergic interneurons. We reasoned that this could be rectified by purifying VACHT-positive reporter cells on day 16 of differentiation, when most fluorescent cells express the motor neuron markers HB9 and/or ISL1. Indeed, most FACS purified fluorescent cells cultured for 9 days maintained expression of motor neuron markers HB9 or ISL1 (Figure S4). Together, these findings confirm the fidelity of HB9- and VACHT-based reporters on day 16 of differentiation and justify their use as a proxy for motor neuron analysis, purification, and quantification.
The need to purify VACHT reporter cell lines on day 16 prompted us to investigate alternative approaches to FACS, which is impractical for projects that demand large quantities of motor neurons. We modified the VACHT-Cre cell line with a CAGGS-lox-STOP-lox-CD14-IRES-GFP transgene inserted into the AAVS1 safe harbor site. This line expresses a GPI-linked cell surface receptor CD14 that is compatible with large-scale purification of motor neurons by magnetic cell sorting (MACS) (Machado et al., 2014). MACS purification of day 16 dissociated cultures resulted in an enrichment of GFP positive cells from 81.4 % in the input to 99.8 % in the eluate (Figure 4A,B). Immunostaining validated that the majority of purified neurons expressed motor neuron markers HB9 and/or ISL1 (Figure 4C). While MACS was effective in purifying cells expressing high levels of CD14-GFP, it failed to efficiently isolate cells with low GFP expression (compare input, flow through and eluate in Figure 4B). In summary, the combination of VACHT-Cre with a CD14 reporter offers a powerful system for large-scale preparations of purified human motor neurons, but further optimizations of the MACS conditions are warranted to increase the overall motor neuron yield.
Figure 4. MACS purification of VACHT-Cre/CD14 motor neurons.

A: Immunostaining of differentiated VACHT-Cre/CD14-GFP cells purified by MACS sorting with antibodies against the CD14 epitope (red) and GFP (green). B: flow cytometry assessment of GFP-positive population before (top histogram) and after (bottom histogram) MACS-mediated purification performed on day 16. C: Immunostaining of MACS purified cells for a combination of motor neuron markers HB9 and ISL1 (red).
DISCUSSION
A standardized motor neuron reporter system is a prerequisite for comparisons of motor neuron function and survival across diverse control and patient-derived induced pluripotent cell lines. To identify the best targeting strategy and the best choice of reporter genes, we generated a series of CRISPR-targeted iPSC lines and compared the specificity, intensity, and fidelity of the reporter gene expression in motor neurons. We focused on the HB9 gene, which, in the central nervous system, is expressed almost exclusively in spinal motor neurons (Arber et al., 1999) and has been deployed to generate a number of transgenic motor neuron reporter cell lines. Additionally, we selected the VACHT gene that, although less specific to motor neurons, is expressed not only in spinal motor neurons, but also in cranial motor neurons. Another benefit of VACHT reporters is that expression of this gene is maintained at high levels in mature motor neurons, while HB9 expression is downregulated over time.
To generate reporter cell lines, we knocked-in either a fluorescent reporter gene, or a Cre recombinase that activates reporter gene expression from the AAVS1 locus. We note that the NS013 line was highly sensitive to electroporation, significantly limiting the number of recovered clones available for genotyping analysis (Table 1). Of the lines tested, two stand out as particularly useful for motor neuron studies. The HB9-tdTomato reporter cell line offers a one-step engineering approach that results in reporter gene expression compatible with FACS purification and live imaging under higher magnification. Interestingly, we did not see significant downregulation or loss of reporter expression over the 9-day culture of postmitotic motor neurons, indicating that this reporter might be even useful for longer-term survival experiments. The limitations of this line are that the fluorescence is not strong enough to perform live imaging of motor neurons under low magnification or under low-intensity illumination, and that the reporter is not expressed in a subset of spinal and majority of cranial motor neurons.
In order to generate brighter reporter lines we turned to a Cre-lox system for indelible and amplified labeling of motor neurons. Unexpectedly, the Hb9-Cre line exhibited significant reporter activation in progenitors during early stages of stem cell differentiation. This is reminiscent of the broad activation of reporters in the tail bud mesoderm of HB9-Cre mouse embryos. We rectified this problem by generating a tamoxifen-inducible HB9-CreERT2 reporter cell line. While this line faithfully recapitulated reporter gene expression in motor neurons, the efficiency of recombination was relatively low, with only 30–40% of motor neurons effectively labeled.
In contrast, the VACHT-Cre/tdTomato reporter system is activated efficiently and specifically in the majority of motor neurons. Importantly, the intensity of the Cre-based reporter expression is two orders of magnitude higher compared to the HB9-tdTomato line, making it an ideal reagent for live imaging of motor neurons. Additionally, this reporter is expressed in all cholinergic cells, including cranial motor neurons, allowing for direct comparison of spinal and cranial motor neurons differentiated from a single pluripotent cell line. In more mature cultures, the VACHT-Cre reporter is activated in additional cells besides motor neurons, which may limit the utility of this reporter system for studying long-term motor neuron function and survival. We demonstrate that this “non-specificity” can be eliminated by purifying reporter-expressing nascent motor neurons using FACS on day 16 of differentiation.
While all of the reporter lines generated here are compatible with FACS purification of motor neurons, we also developed a reporter line compatible with large-scale purification of motor neurons by magnetic sorting. For this purpose, we generated a variation of the lox-STOP-lox-tdTomato reporter by substituting the tdTomato for CD14-IRES-GFP. Motor neurons differentiated from this line could be sorted by either FACS using GFP or MACS using CD14, depending on the preference or resources available to the researcher. MACS purification is faster and gentler on cells, which might result in improved plating efficiency and motor neuron survival. Although it has not been directly tested in this study, increased plating efficiency would be an important benefit when setting up large-scale screening assays.
In summary, our study identified two reporter systems that allow for the convenient and reproducible study of human stem cell derived motor neurons. Direct targeting of the HB9 locus results in a reliable reporter system for motor neuron purification and analysis, while the amplified VACHT-Cre system is ideal for live imaging studies, when combined with purification of motor neurons on day 16 of differentiation. Finally, large-scale preparations of motor neurons can be accomplished by combining the VACHT-Cre reporter system with a magnetically-sortable surface reporter, such as CD14. The reporter systems described here can serve as standardized tools for comparison of motor neurons across different human iPSC and embryonic stem cell lines, facilitating efficient and reproducible studies of motor neuron function and disease modeling.
Supplementary Material
Highlights:
New set of human HB9 and VACHT targeted iPSC reporter lines for purification of motor neurons and other cholinergic neurons
The Cre-lox amplification of reporter expression is compatible with live-imaging and long-term culture studies
Cre based reporters expressing CD14 receptor are useful for motor neuron purification at scale
ACKNOWLEDGEMENTS
H.W. will be forever grateful to Tom Jessell for sparking his interest in spinal cord and motor neuron biology, for scientific rigor and intellectual clarity one can only strive to replicate, and for building one of the most vibrant and stimulating neuroscience communities at Columbia University. We would like to thank all the members of the Columbia Stem Cell Core Facility for their valuable input and their work on iPSC line derivations. We are also grateful to the Eleanor and Lou Gehrig ALS Center at Columbia University for supplying de-identified human biopsies for iPSC generation. We would like to thank Ivo Lieberam (King’s College, London) for providing the CD14-IRES-GFP plasmid, and Mike Kissner for his assistance with FlowJo software. H.W. holds an endowed chair from Jerry and Emily Spiegel. This work was made possible thanks to the financial support of Project ALS, Target ALS, Biogen and NIH R21NS109661.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amoroso MW, Croft GF, Williams DJ, O’Keeffe S, Carrasco MA, Davis AR, Roybon L, Oakley DH, Maniatis T, Henderson CE, et al. (2013). Accelerated High-Yield Generation of Limb-Innervating Motor Neurons from Human Stem Cells. J Neurosci 33, 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D, Fujiki R, Iannitelli DE, Smerdon JW, Maity S, Rose MF, Gelber A, Wanaselja EK, Yagudayeva I, Lee JY, et al. (2019). Stem cell-derived cranial and spinal motor neurons reveal proteostatic differences between ALS resistant and sensitive motor neurons. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, and Sockanathan S (1999). Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659–674. [DOI] [PubMed] [Google Scholar]
- Bos PH, Lowry ER, Costa J, Thams S, Garcia-Diaz A, Zask A, Wichterle H, and Stockwell BR (2019). Development of MAP4 Kinase Inhibitors as Motor Neuron-Protecting Agents. Cell Chem Biol 26, 1703–1715 e1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, and Eggan KC (2008). Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell 3, 637–648. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, and Eggan K (2007). Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10, 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221. [DOI] [PubMed] [Google Scholar]
- Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, and van den Berg LH (2017). Amyotrophic lateral sclerosis. Nat Rev Dis Primers 3, 17071. [DOI] [PubMed] [Google Scholar]
- Hoang PT, Chalif JI, Bikoff JB, Jessell TM, Mentis GZ, and Wichterle H (2018). Subtype Diversification and Synaptic Specificity of Stem Cell-Derived Spinal Interneurons. Neuron 100, 135–149 e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacko M, Weyn-Vanhentenryck SM, Smerdon JW, Yan R, Feng H, Williams DJ, Pai J, Xu K, Wichterle H, and Zhang C (2018). Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron 97, 853–868 e856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, and Henderson CE (2010). Motor neuron diversity in development and disease. Annu Rev Neurosci 33, 409–440. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Spiller KJ, Towne C, Kanning KC, Choe GT, Geber A, Akay T, Aebischer P, and Henderson CE (2014). Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 81, 333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Park C, Jeong Y, and Song MR (2015). Functional Diversification of Motor Neuron-specific Isl1 Enhancers during Evolution. PLoS Genet 11, e1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock JW, Genoud N, Lettieri K, and Pfaff SL (2007). The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 56, 604–620. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, and Zhang SC (2005). Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 23, 215–221. [DOI] [PubMed] [Google Scholar]
- Machado CB, Kanning KC, Kreis P, Stevenson D, Crossley M, Nowak M, Iacovino M, Kyba M, Chambers D, Blanc E, et al. (2014). Reconstruction of phrenic neuron identity in embryonic stem cell-derived motor neurons. Development 141, 784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, and Church GM (2013). RNA-guided human genome engineering via Cas9. Science 339, 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury JJ, El Farran CA, Ng D, Loh YH, Bi X, Bardor M, and Choo AB (2015). RING1B O-GlcNAcylation regulates gene targeting of polycomb repressive complex 1 in human embryonic stem cells. Stem Cell Res 15, 182–189. [DOI] [PubMed] [Google Scholar]
- Maury Y, Come J, Piskorowski RA, Nouzha S-M, CHevaleyre V, Peschanski M, Martinat C, and Nedelec S (2014). Systematic combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nature Biotechnology 33(1), 89–96. [DOI] [PubMed] [Google Scholar]
- Peljto M, Dasen JS, Mazzoni EO, Jessell TM, and Wichterle H (2010). Functional diversity of ESC-derived motor neuron subtypes revealed through intraspinal transplantation. Cell Stem Cell 7, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh JS, Brown RH Jr., and Berry JD (2015). Amyotrophic Lateral Sclerosis: Review. Semin Neurol 35, 469–476. [DOI] [PubMed] [Google Scholar]
- Sternfeld MJ, Hinckley CA, Moore NJ, Pankratz MT, Hilde KL, Driscoll SP, Hayashi M, Amin ND, Bonanomi D, Gifford WD, et al. (2017). Speed and segmentation control mechanisms characterized in rhythmically-active circuits created from spinal neurons produced from genetically-tagged embryonic stem cells. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Thams S, Lowry ER, Larraufie MH, Spiller KJ, Li H, Williams DJ, Hoang P, Jiang E, Williams LA, Sandoe J, et al. (2019). A Stem Cell-Based Screening Platform Identifies Compounds that Desensitize Motor Neurons to Endoplasmic Reticulum Stress. Mol Ther 27, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, and Jessell TM (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


