Abstract
Muscular dystrophies (MDs) are inherited disorders characterized by progressive muscle weakness. Previously, we have shown that resveratrol (3,5,4′-trihydroxy-trans-stilbene), an antioxidant and an activator of the protein deacetylase SIRT1, decreases muscular and cardiac oxidative damage and improves pathophysiological conditions in animal MD models. To determine whether resveratrol provides therapeutic benefits to patients with MDs, an open-label, single-arm, phase IIa trial of resveratrol was conducted in 11 patients with Duchenne, Becker or Fukuyama MD. The daily dose of resveratrol was 500 mg/day, which was increased every 8 weeks to 1000 and then 1500 mg/day. Primary outcomes were motor function, evaluated by a motor function measure (MFM) scale, muscular strength, monitored with quantitative muscle testing (QMT), and serum creatine kinase (CK) levels. Adverse effects and tolerability were evaluated as secondary outcomes. Despite the advanced medical conditions of the patients, the mean MFM scores increased significantly from 34.6 to 38.4 after 24 weeks of medication. A twofold increase was found in the mean QMT scores of scapula elevation and shoulder abduction. Mean CK levels decreased considerably by 34%. Diarrhoea and abdominal pain was noted in six and three patients, respectively. Resveratrol may provide some benefit to MD patients.
Subject terms: Drug discovery, Diseases, Medical research, Neurology
Introduction
Muscular dystrophies (MDs) are inherited myopathic disorders characterized by progressive muscle weakness and disability1. Mutations in a wide range of proteins, including the dystrophin-associated glycoprotein complex, which connects the myofibre cytoskeleton to the extracellular matrix, have been implicated in MDs1. Complete loss of the function of dystrophin causes Duchenne muscular dystrophy (DMD), the most common and lethal X-linked myopathy, whereas a partial loss of the function results in Becker muscular dystrophy (BMD), which has a milder phenotype1. Mutations in the FKTN gene cause Fukuyama congenital muscular dystrophy (FCMD), a fatal and the second most prevalent form in Japan2. Although novel therapeutic strategies based on stop codon readthrough or exon skipping have been developed3,4, the conventional and reliable medicine for DMD is glucocorticoids, which improve motor function and the quality of life. Glucocorticoids delay the median age of loss of mobility in DMD patients by 2.1–4.4 years5, but the medical condition of patients steadily deteriorates under medication. In addition, glucocorticoids have other adverse effects such as cushingoid features, weight gain, behavioural changes and growth delay5.
SIRT1, an NAD+-dependent protein deacetylase, is a mammalian homologue of Sir2 that prolongs lifespan when overexpressed in yeast, Caenorhabditis elegans, and Drosophila melanogaster6. SIRT1 deacetylates histones, various transcription factors and cytoplasmic proteins and plays critical roles in cell survival by reducing reactive oxygen species (ROS), inhibiting inflammation, and promoting mitochondrial function and autophagy6,7. SIRT1 overexpression in the skeletal muscles of mdx mice, an animal model of DMD, decreases serum creatine kinase (CK) levels, tissue fibrosis, and myofibril damage and increases their ability to run long distances compared with control mdx mice8.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a natural polyphenol found in grapes and red wine, has been shown to allosterically activate SIRT16,7. δ-Sarcoglycan is a component of the dystrophin glycoprotein complex, the defect of which leads to severe cardiomyopathy in hamsters. We found that SIRT1 induces antioxidative superoxide dismutase 2 (SOD2), and oral administration of resveratrol to δ-sarcoglycan-deficient TO-2 hamsters increases cardiac SOD2 levels, attenuates the deterioration of cardiac function and hypertrophy, and significantly extends the lifespan9. Resveratrol administration also improves the pathophysiological manifestations of dystrophin-deficient mdx mice. Resveratrol decreases oxidative stress levels in muscles, reduces cardiac and muscular fibrosis, increases the expression levels of myosin heavy chains and troponins, and inhibits cardiac hypertrophy10–12. Moreover, long-term administration of resveratrol improves cardiac and muscular function with a decrease in serum CK levels to one-third of those in untreated mdx mice11,12. Thus, resveratrol could be a useful medicine for treating MD patients. However, to date, there have been no clinical studies investigating this possibility. Because clinical studies show the relatively low toxicity of resveratrol13, we planned a pilot phase IIa study in MD patients to investigate its biological activity.
In the present study, we administered resveratrol to patients with DMD, BMD, or FCMD for 24 weeks. Since the diseases had progressed considerably in these patients, motor function, muscular strength, and CK levels were evaluated as primary outcomes. Adverse effects and tolerability were also evaluated as secondary outcomes.
Results
Patients and protocol
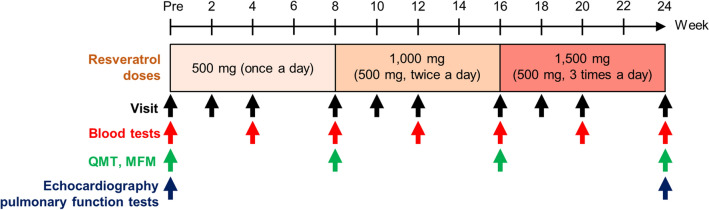
The mean ages of eleven DMD, BMD, and FCMD patients were 21.0 ± 9.7 years (range, 12–39 years), 33.5 ± 8.3 years (range, 23–46 years), and 14.5 ± 2.5 years (range, 12–17 years), respectively, and the mean age of all participants was 24.4 ± 11.1 years (range, 12–46 years) (Table 1). One FCMD patient was female, and the remaining ten participants were male. One DMD patient and three patients with BMD were ambulatory. The other seven patients were unable to walk and needed a wheelchair; therefore, they were assigned a grade of 9 on the Vignos scale14. Resveratrol was orally administered daily to all participants at an initial dose of 500 mg/day for 8 weeks (Fig. 1). The dose was then increased every 8 weeks to 1000 and finally to 1500 mg/day, similar to dosages of resveratrol administered to healthy subjects and patients with cancer or metabolic diseases13. All patients were assessed for motor function, muscle strength, and blood examinations. Mean scores of muscle function measure (MFM), quantitative muscle testing (QMT), and serum CK levels of participants before resveratrol medication are shown in Table 2.
Table 1.
Participants characteristics.
| Type | Age, years | Sex | Weight (kg) | Genetic testing | Other diagnostic basis | Vignos scale | Brooke scale | |
|---|---|---|---|---|---|---|---|---|
| 1 | Duchenne | 12 | Male | 35.9 | Deletion of exon 18 | Grade 4 | Grade 2 | |
| 2 | 14 | Male | 27.5 | Deletion of exons 44–47 | Family history | Grade 9 | Grade 4 | |
| 3 | 17 | Male | 45.8 | Deletion of exon 11 | Grade 9 | Grade 6 | ||
| 4 | 23 | Male | 47.7 | Not available | Biopsy | Grade 9 | Grade 6 | |
| 5 | 39 | Male | 49.8 | Not available | Family history | Grade 9 | Grade 6 | |
| 6 | Becker | 23 | Male | 64.0 | Deletion of exons 45–48 | Grade 2 | Grade 1 | |
| 7 | 31 | Male | 71.0 | Deletion of exon 3 | Grade 9 | Grade 6 | ||
| 8 | 34 | Male | 64.5 | No copy number variation | Biopsy | Grade 3 | Grade 1 | |
| 9 | 46 | Male | 64.0 | Deletion of exons 45–48 | Biopsy | Grade 6 | Grade 2 | |
| 10 | Fukuyama | 12 | Female | 26.9 | Heterozygous for the founder haplotype | Brain MRI | Grade 9 | Grade 6 |
| 11 | 17 | Male | 22.6 | Homozygous for the founder haplotype | Biopsy | Grade 9 | Grade 6 |
Figure 1.
Protocol for resveratrol administration. Daily doses of resveratrol are shown, and visits along with blood and physical examinations are indicated by arrows.
Table 2.
Mean MFM scale and QMT scores of participants before resveratrol administration.
| Type of muscular dystrophy | Total (N = 11) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duchenne (N = 5) | Becker (N = 4) | Fukuyama (N = 2) | ||||||||||
| Number (%) | Range | Mean (SD) | Number (%) | Range | Mean (SD) | Number (%) | Range | Mean (SD) | Number (%) | Range | Mean (SD) | |
| MFM scale | 5 (100) | 2–63 | 22.2 (21.5) | 4 (100) | 38–85 | 64.5 (16.9) | 2 (100) | 2–10 | 6 | 11 (100) | 2–85 | 34.6 (29.3) |
| QMT pinch (N) | 5 (100) | 0.5–2.4 | 11.1 (0.7) | 4 (100) | 0.3–4.9 | 3.8 (2.1) | 2 (100) | 0.1–0.5 | 0.3 | 11 (100) | 0.1–4.9 | 1.9 (2.0) |
| QMT grip (kgf) | 3 (60) | 0.8–4.8 | 2.3 (1.8) | 4 (100) | 1.3–25.5 | 12.7 (9.5) | 0 (0) | – | – | 7 (64) | 0.8–25.5 | 8.3 (8.9) |
| QMT scapula elevation (kgf) | 4 (80) | 0.9–8.6 | 3.3 (3.1) | 4 (100) | 5.3–6.8 | 6.0 (0.6) | 2 (100) | 1.4–1.4 | 1.4 | 10 (91) | 0.9–8.6 | 4.0 (2.7) |
| QMT shoulder abducttion (kgf) | 1 (20) | – | 3.0 (–) | 4 (100) | 3.5–6.2 | 4.7 (1.0) | 0 (0) | – | – | 5 (45) | 3.0–6.2 | 4.4 (1.1) |
| QMT elbow flexion (kgf) | 1 (20) | – | 1.6 (–) | 4 (100) | 0.6–6.2 | 5.1 (3.0) | 1 (50) | – | 1.1 | 6 (55) | 0.6–6.2 | 3.9 (3.1) |
| QMT elbow extension (kgf) | 1 (20) | – | 2.8 (–) | 4 (100) | 3.1–9.2 | 5.7 (2.5) | 0 (0) | – | – | 5 (45) | 2.8–9.2 | 5.1 (2.5) |
| QMT hip flexion (kgf) | 1 (20) | – | 5.2 (–) | 4 (100) | 4.3–10.1 | 7.7 (2.2) | 0 (0) | – | – | 5 (45) | 4.3–10.1 | 7.2 (2.2) |
| QMT knee extension (kgf) | 2 (40) | 2.0–2.5 | 5.2 (–) | 4 (100) | 1.3–11.4 | 6.0 (3.9) | 0 (0) | – | – | 6 (55) | 1.3–11.4 | 4.8 (3.6) |
| QMT ankle dorsiflexion (kgf) | 1 (20) | – | 5.2 (–) | 4 (100) | 3.2–7.9 | 5.4 (2.2) | 1 (50) | – | 1.4 | 6 (55) | 1.4–7.9 | 4.4 (2.4) |
| Creatine kinase (unit/l) | 5 (100) | 200–9518 | 2522 (3518) | 4 (100) | 431–2807 | 1601 (1020) | 2 (100) | 840–1981 | 1411 | 11 (100) | 200–9518 | 1985 (2512) |
Motor function
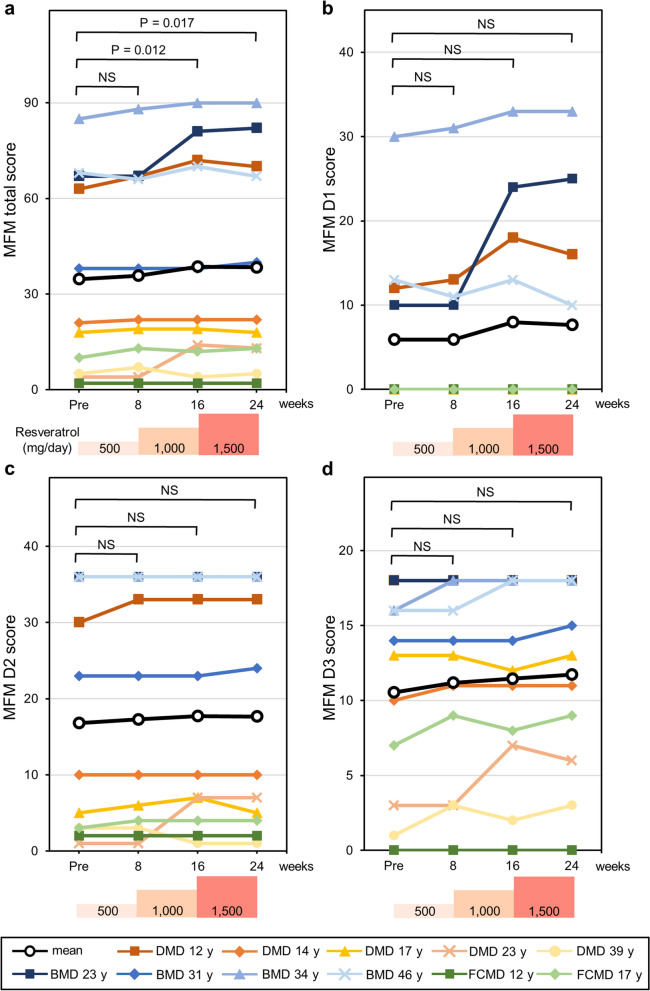
The MFM scale is a sensitive and reliable method for evaluating motor function in neuromuscular diseases, including muscular dystrophy15. Patients’ motor functions were evaluated by the MFM scale scores on 32 items in three dimensions. The mean MFM total score of all participants before resveratrol administration was 34.6 ± 29.3 (range, 2–85). MFM scores after 16 and 24 weeks of medication were 38.5 ± 31.7 (range, 2–90) (p = 0.012) and 38.4 ± 31.3 (range, 2–90) (p = 0.017), respectively (Fig. 2a). Thus, long-term administration of resveratrol significantly increased MFM scores by 10%. Sub-score analyses of D1, D2, and D3 showed increasing tendencies, but these did not meet the threshold of statistical significance (Fig. 2b–d), suggesting that resveratrol does not improve muscle function specifically but instead affects the whole body. The Brooke and Vignos scale grades did not change during this study.
Figure 2.
Motor function scores following resveratrol administration. (a) Total score, (b) D1 sub-score in standing position and transfers, (c) D2 sub-score in axial and proximal motor function, and (d) D3 sub-score in distal motor function of each patient are measured with the MFM scale before (Pre) and at 8, 16, and 24 weeks. Black lines show mean values. Average values at each visit were compared with those before medication and were analysed using ANOVA. Post hoc analysis was performed using Dunnett’s test. P values < 0.05 are indicated. NS: not significant.
Muscle strength
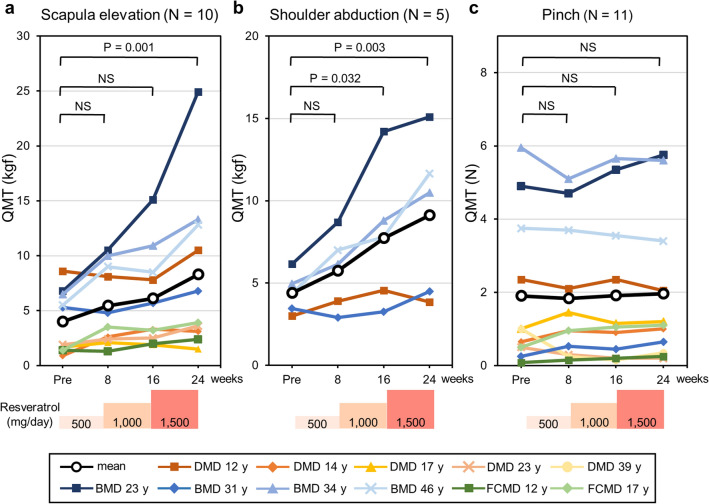
To determine whether muscle strength was affected by resveratrol, we evaluated the muscle strength of each patient. Strength was monitored with quantitative muscle testing (QMT), which is a reliable method for evaluating the muscle strength of ambulatory and non-ambulatory MD patients16–18. Nine different QMT scores were measured, but in some patients, certain items could not be scored due to their severe muscle weakness or contracture. For example, a 39-year-old patient with DMD could be evaluated only for pinch strength. The monitored activities and number of patients assessed were as follows: scapula elevation, 10; shoulder abduction, 5; pinch, 11; elbow extension, 5; elbow flexion, 6; hip flexion, 5; knee extension, 6; ankle dorsiflexion, 6; and grip, 7 (Table 2, Fig. 3, Supplementary Fig. S1). Scapula elevation was measured in ten participants, including three non-ambulatory patients. The administration of resveratrol for 24 weeks increased the mean QMT score of scapula elevation from 4.0 ± 2.7 kg-force (kgf) (range, 0.9–8.6 kgf) to 8.3 ± 6.9 kgf (range, 1.5–24.9 kgf) (p = 0.001) (Fig. 3a), while the mean shoulder abduction strength measured in one DMD patient and four patients with BMD increased from 4.4 ± 1.1 kgf (range, 3.0–6.2 kgf) to 9.1 ± 4.3 kgf (range, 3.9–15.1 kgf) (p = 0.003) (Fig. 3b). QMT scores of elbow extension, elbow flexion, hip flexion, knee extension, and ankle dorsiflexion in some patients were markedly improved by resveratrol, but the differences were not significant (Supplementary Fig. S1a–e). Resveratrol had no effect on pinch strength between the thumb and forefinger (Fig. 3c) or on grip strength (Supplementary Fig. S1f).
Figure 3.
Muscle strength following resveratrol administration. (a–c) Time course of muscle strength for (a) scapula elevation (N = 10), (b) shoulder abduction (N = 5), and (c) pinch (N = 11) measured by quantitative muscle testing (QMT) using a hand-held dynamometer and pinch strength metre before (Pre) and 8, 16, and 24 weeks after resveratrol administration. The daily dose of each patient is also shown, except that one patient with FCMD (FCMD 12 y) received 1000 instead of 1500 mg/day. Black lines show mean values. Average values at each visit were compared with those before medication and were analysed using ANOVA. Post hoc analysis was performed using Dunnett’s test. P values < 0.05 are indicated. NS: not significant.
Serum CK levels
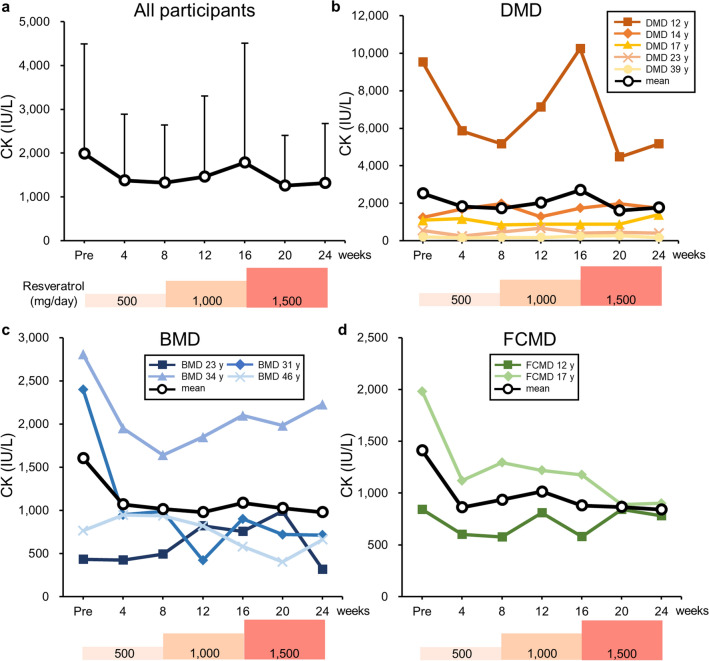
Serum CK levels in patients were examined once every 4 weeks (Fig. 4a–d). The mean CK levels were 1985 ± 2511 IU/L (range, 200–9518 IU/L) before resveratrol administration and 1315 ± 1357 IU/L (range, 166–5167 IU/L) after 24 weeks of medication. Thus, a 34% decrease in mean CK levels was achieved by resveratrol, but the difference was not statistically significant (p = 0.148) (Fig. 4a).
Figure 4.
Serum creatine kinase (CK) levels following resveratrol administration. (a) Mean serum CK levels of all participants with standard deviation. (b–d) Change in CK activity in each patient with (b) Duchenne, (c) Becker, or (d) Fukuyama congenital muscular dystrophy. Black lines show the mean CK levels.
Serum resveratrol concentrations
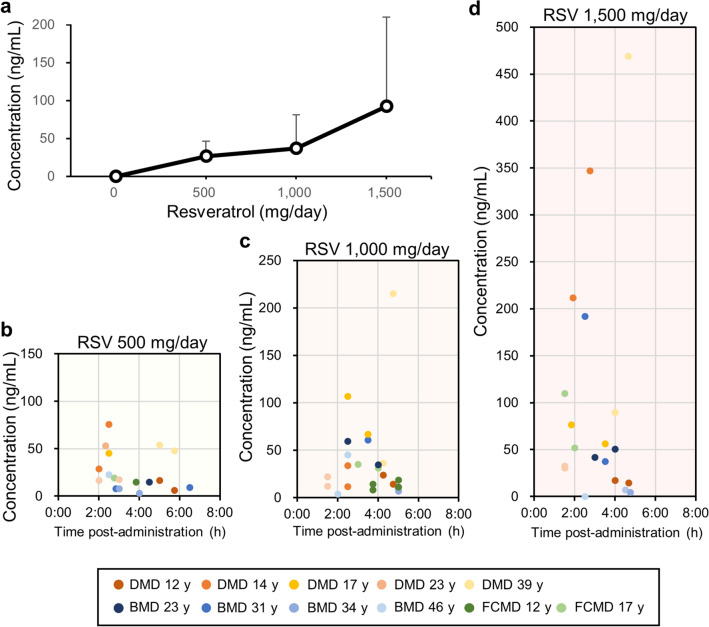
Serum resveratrol concentrations in all patients were examined every 4 weeks. Levels of serum resveratrol increased in a dose-dependent manner, and the mean resveratrol levels at doses of 500, 1000, and 1500 mg/day were 26.4 ± 20.0 ng/mL (range, 2.6–75.9 ng/mL), 36.9 ± 44.7 ng/mL (range, < 1.0–215.0 ng/mL), and 92.2 ± 117.7 ng/mL (range, < 1.0–469.0 ng/mL), respectively (Fig. 5a). Serum resveratrol concentration and the time interval for blood draw after ingestion in each patient are shown in Fig. 5b–d.
Figure 5.
Serum resveratrol concentration at doses of 500, 1000, and 1500 mg/day. (a) Mean serum resveratrol levels of all participants with standard deviation (N = 11). (b–d) Serum resveratrol level and time from resveratrol ingestion to blood drawing in each patient are shown by a dot. RSV: resveratrol.
Adverse events
Grade 1 diarrhoea was noted in four patients (Table 3). Grade 2 diarrhoea was found in two patients administered 1500 mg/day resveratrol. These two patients needed to reduce the dosage from 1500 to 1000 mg/day. One patient resumed a dose of 1500 mg/day after several days, whereas the other patient (FCMD 12 y) was maintained at the dose of 1000 mg/day. Grade 1 abdominal pain was observed in three patients. Two cases of upper respiratory tract infection and one case of pneumonia were observed during the study. Among them, two patients were temporarily hospitalized but left the hospital soon afterwards. During hospitalization, they continued to take resveratrol on schedule. Transient alopecia, erythema multiforme, and acneiform rash were observed in one patient each. All participants continued to take resveratrol for 24 weeks. Resveratrol did not affect blood markers of liver and renal function (Supplementary Table S1).
Table 3.
Adverse events following resveratrol administration.
| Symptom | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total (%) |
|---|---|---|---|---|---|---|
| Diarrhoea | 4 | 2 | 6 (54.5) | |||
| Abdominal pain | 3 | 3 (27.3) | ||||
| Upper respiratory infection | 1 | 1 | 2 (18.2) | |||
| Lung infection | 1 | 1 (9.1) | ||||
| Alopecia | 1 | 1 (9.1) | ||||
| Erythema multiform | 1 | 1 (9.1) | ||||
| Rash acneiform | 1 | 1 (9.1) |
The numbers of patients are indicated.
Discussion
Resveratrol has been used in humans, and previous data show that its toxicity is limited13. Since resveratrol ameliorated pathology and improved the function of MD animal models, we planned this study as a pilot study.
Resveratrol improved motor function and muscle power in the proximal muscles of participants. However, the distal muscles of patients were unaffected (Fig. 3c, Supplementary Fig. S1f). One patient with BMD (BMD 23 y) experienced relief from muscle pain during resveratrol administration, which may have contributed to the improvement in motor function. The observed increase in patients’ muscle strength following resveratrol treatment was consistent with findings in mdx mice10,12, in which approximately fivefold increases in slow-fibre-type myosin heavy chain and troponin gene transcripts were detected in the biceps femoris muscles10. Resveratrol binds the N-terminal region of the SIRT1 protein, resulting in the allosteric activation of SIRT1 activity7. SIRT1 deacetylates and activates peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), which switches muscle types from fast to slow fibres6,7,19. Indeed, transgenic mice overexpressing PGC-1α19 or SIRT18 show muscle type conversion from fast- to slow-twitch fibres. PGC-1α transgenic mice also show increased resistance to muscular fatigue19. Thus, the beneficial effects of resveratrol on the muscles of MD patients may involve PGC-1α activation by SIRT1. In mdx mice, serum CK levels were decreased by the overexpression of SIRT1 in the muscles8 and by the long-term administration of resveratrol12. Recently, we have shown that skeletal muscle-specific SIRT1-knockout mice have a fragile muscle cell plasma membrane and that SIRT1 is involved in membrane resealing after cell injury20. In the present study, mean CK levels were decreased by resveratrol, although the change was non-significant. We noted that patients with low serum CK levels, i.e., less than 2000 IU/L, showed little response to resveratrol treatment, which could be caused by the lack of sufficient muscle mass. Resveratrol administration at early disease stages may afford great benefits to MD patients.
Oxidative stress plays a key role in the development of MD pathology21. Resveratrol decreases ROS levels by inducing the expression levels of the antioxidant enzyme superoxide dismutase9, suppressing superoxide-generating NADPH oxidase10, and eliminating damaged mitochondria, a major cellular source of ROS22, via mitochondrial autophagy, known as mitophagy11,12,23,24. These antioxidative functions of resveratrol are mediated at least in part by the deacetylation and activation of Forkhead box O transcription factors and PGC-1α via SIRT1 activation6,7.
Cardiomyopathy and subsequent heart failure is a major cause of death in MD patients. Resveratrol suppresses cardiac hypertrophy and fibrosis in TO-2 hamsters9 and mdx mice25, which may be mediated by the deacetylation and ubiquitin-dependent degradation of transcriptional coactivator p300 via SIRT1 activation25. In the present study, we could not detect any improvement in echocardiographic parameters or levels of plasma brain natriuretic peptide (BNP), a marker of cardiomyopathy. This may be because all participants showed normal BNP levels (< 18.4 pg/ml), and only 2 patients had a reduced ejection fraction of less than 50% (Supplementary Table S1). Longer-term administration of resveratrol may be necessary to reveal the cardiac function of resveratrol in MD patients.
Resveratrol is rapidly absorbed and yields peak serum concentration at 1 h post dose13. In the present study, the levels of serum resveratrol concentration in MD patients (Fig. 5b–d) were consistent with those in healthy volunteers13. Six (54.5%) and three (27.3%) patients had diarrhoea and abdominal pain, respectively (Table 3). These symptoms were transient and tolerable in participants except for the female patient (FCMD 12 y), who suffered from severe diarrhoea at the dose of 1500 mg resveratrol. The weight of the patient was only 26.5 kg, and she could not continue the dose. Similar to our study, Brown et al. has reported mild to moderate gastrointestinal symptoms in healthy volunteers who ingested resveratrol at doses of 2500 and 5000 mg/day26. Two patients contracted transient grade 3 upper respiratory and lung infections, and one patient suffered from a grade 2 upper respiratory infection. We believe that these events were unrelated to resveratrol since all three patients had repeatedly suffered from respiratory infections before the clinical trial and recovered immediately while taking resveratrol.
We found that administration of resveratrol to mdx mice with the highest dose (4 g/kg food) is more effective in increasing myofibre cross-section area than lower doses of 0.4 g and 0.04 g/kg food12. However, a moderate dose of resveratrol (0.4 g/kg food) also decreases the number of fibres with central nuclei, the number of fine fibres, and serum creatine kinase levels. Additionally, it significantly improves motor function in mdx mice12. Resveratrol at 0.4 g/kg food was estimated to be approximately 50 mg/kg body weight/day10. The mean resveratrol level at 1000 mg/day was 36.9 ± 44.7 ng/mL (range, < 1.0–215.0 ng/mL) (Fig. 5a), which is consistent with the data of Almeida et al.27. The administration of resveratrol to healthy adult subjects at 150 mg six times/day for 13 doses resulted in a mean plasma concentration of 63.8 ng/mL and a half-life of resveratrol of 2–5 h27. Resveratrol at 36.9 ng/mL is equal to 160 nM. Because 160 nM resveratrol was 1/68 of the concentration needed for twofold activation of SIRT1 enzymatic activity in vitro28, the activation of SIRT1 by resveratrol in the present study did not seem strong. However, other studies show the physiological effects of resveratrol even in small amounts13,27. The administration of resveratrol at 150 mg/day for 30 days significantly decreased plasma glucose, insulin, triglycerides and leptin in healthy obese men29. Using muscular biopsy, Timmers et al. showed that resveratrol activates AMPK activity, increases SIRT1 and PGC1α levels, and promotes mitochondrial respiration activity in the muscle29. Based on these findings, we suggest that resveratrol at a dose of 25–50 mg/kg body weight/day is sufficient to treat MD patients. The dosing period required to detect an effect of resveratrol seems to be more than 4 months for MD patients.
Glucocorticoids have been shown to prolong the survival of patients with DMD5. In the present study, glucocorticoids were treated in only one patient with DMD (DMD 12 y), who showed increased muscle strength and motor function without any adverse effects during resveratrol administration. Recently, novel methods to treat DMD have been developed. Ataluren is a stop codon readthrough drug for DMD based on nonsense mutations3, and is currently being evaluated in a post-approval safety study in Europe. Eteplirsen is a morpholino antisense oligomer that induces the skipping of exon 51 of the dystrophin gene4, and accelerated approval for this drug was obtained from the U.S. Food and Drug Administration in 2016. The use of these kinds of therapies is expected to increase, although they have some limitations30,31. Given its unique mode of action and relatively mild adverse effects, resveratrol could combine with glucocorticoids or recent novel medicines such as ataluren and eteplirsen.
A limitation of the present study was that it was not a randomized, double-blind, placebo-controlled trial. We could not analyse an untreated group because the number of patients was limited, and three types of MD patients with various clinical conditions were recruited. In the case of rare diseases, phase I studies for cancer and phase IIa studies for other diseases have been executed without any control32–36. These trials were planned because of problems in recruiting patients, non-uniformity in patients’ disease conditions, the use of an investigational drug that has never been administered in humans, no prior information on dose levels, difficulty in preparing a control group or difficulty in obtaining the agreement of patients to use a placebo. Usually, if some possibility concerning effectiveness of the drug is found in a phase IIa study, a phase IIb clinical study is carried out to identify the effect and dose of the investigational drug by a randomized and double-blind study.
We repetitively measured the MFM and QMT scores of the participants. Some studies have reported that exercises increase the motor function and muscle strength of MD patients37. For example, the knee flexor maximum voluntary contraction torque of the limb-girdle in Becker and facioscapulohumeral MD patients increased by 13% after a 12-week resistance training programme38. However, two meta-analyses of exercise interventions on muscle strength in MD patients showed no evidence of improvement in muscle strength and in endurance39. Because the average time required for MFM has been reported to be 36 min (range 8–75 min)15, measuring MFM and QMT scores once every 8 weeks in the present study was unlikely to improve motor function of the patients. Since participants are aware of exercise interventions, the two meta-analyses39 also suggest that placebo effects of interventions are minimal on MD patients. Moreover, average muscle strength in DMD patients treated with placebo shows similar decline with that of untreated patients during administration for 6 months40. Because mean QMT scores of scapula elevation and shoulder abduction increased by twofold 24 weeks after resveratrol administration (Fig. 3a,b), it was less likely that the efficacy of resveratrol was a placebo effect.
As mentioned above, our study was a phase IIa study to investigate the possibility of the use of resveratrol in MD patients. To determine whether resveratrol could provide benefit for MD patients and be used as a symptomatic medication for MDs, a phase IIb study of resveratrol in a multi-centre trial with randomized, double-blind, and parallel-group comparison design will be necessary. An evaluation of the long-term effects of resveratrol on MD patients in a uniform cohort, such as age-matched young DMD patients, would be benefical.
Methods
Clinical study design
This study was an open-label, single-arm, phase IIa trial. The protocol was approved by the Institutional Review Board (IRB) on May 12, 2014 and registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR Unique ID: UMIN000014836) on August 12, 2014. This trial was conducted in accordance with the Declaration of Helsinki and was carried out at Sapporo Medical University Hospital from June 2014 to September 2015. Patient inclusion criteria were as follows: (1) DMD, BMD, or FCMD diagnosed based on clinical manifestations, family history, pathological findings of muscle biopsy, and/or genetic examination; and (2) minimum age of 12 years. Patients with a history of hypersensitivity to resveratrol were excluded. The daily dose of resveratrol was 500 mg/day, which was increased every 8 weeks to 1000 and then to 1500 mg/day. During the 24 weeks of medication, motor activity and blood were examined four and seven times, respectively (Fig. 1). Echocardiography and pulmonary function tests were performed prior to administration of resveratrol and after 24 weeks of medication (Fig. 1).
Reagents and evaluation methods
Resveratrol (TransMax) was purchased from Biotivia (New York, NY, USA). Physical examinations and blood draws were performed as shown in Fig. 1. Motor function and muscle strength were evaluated by well-trained physical therapists prior to the initiation of resveratrol administration and at 8, 16, and 24 weeks. Motor function was estimated with the MFM scale15. The total score was calculated by adding the individual scores of 32 items and ranged from 0 to 96. The generic grading at each item is as follows: 0, does not initiate movement or starting position cannot be maintained; 1, partially completes the exercise; 2, completes the exercise with compensations, slowness or obvious clumsiness; and 3, completes the exercise with a standard pattern. MFM total score and D1 (standing position and transfers), D2 (axial and proximal motor function), and D3 (distal motor function) sub-scores were analysed15. Muscle strength was evaluated by quantitative muscle testing (QMT)16–18. QMT was performed using a hand-held dynamometer (μTas F-1; ANIMA Co., Tokyo, Japan), squeeze dynamometer (Smedley Dynamometer TTM-YO II; Tsutsumi Co., Tokyo, Japan), and pinch strength metre (SPR-641; SAKAI Medical Co., Tokyo, Japan). Pinch, grip, scapula elevation, shoulder abduction, elbow flexion, elbow extension, hip flexion, knee extension, and ankle dorsiflexion were measured. Ejection fraction (EF), percent fractional shortening (%FS), and left ventricular end-diastolic diameter (LVDd) were measured with echocardiography. Pulmonary function tests and blood were examined at Sapporo Medical University Hospital, and resveratrol concentration was measured by liquid chromatography–tandem mass spectrometry at Toray Research Center (Tokyo, Japan). Adverse events were classified, and their degree of severity was determined according to the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0.
Statistical analysis
As the primary endpoint, the MFM score, QMT score, and CK value were evaluated as continuous variables. All data and average values at each visit were plotted. Average values at each visit were compared with those before medication and were analysed using ANOVA. Post hoc analysis was performed using Dunnett’s test. P < 0.05 was considered statistically significant. For the QMT score, participants in whom the baseline value of each item could not be measured were excluded. Statistical analyses were performed using SPSS v.22 software (IBM, Armonk, NY, USA).
Ethics declarations
Study approval The protocol was approved by the IRB of Sapporo Medical University Hospital on May 12, 2014. The protocol was then slightly modified twice and approved by the IRB on Sep. 18, 2014 and Jan. 28, 2015. Written informed consent was obtained from all participants or their legal guardians.
Supplementary information
Acknowledgements
This study was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology for Translational Research (Seed A-25); the Japanese Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers, 15K08312 and 18K06965); the Setsuro Fujii Memorial, the Osaka Foundation for Promotion of Fundamental Medical Research; and the Hiroshige Kondo Foundation. We thank Shun Shimohama, Tomihiro Imai, Shin Hisahara (Department of Neurology, Sapporo Medical University School of Medicine) and Tomoyuki Dobata (Toseikai Healthcare Corporation, Life-Long Care Clinic for Disabled People) for the referral of patients.
Author contributions
K.K., A.T., Y.H., and H.T. designed the study. K.K., S.F., K.N., N.T., N.K., T.S., and K.H. executed the study. N.K. and T.S. measured muscle strength and motor function. K.K., M.M., Y.M.I., S.H., A.K., Y.K., Y.H., and H.T. analysed the data. K.K., Y.H., and H.T. wrote the manuscript. All authors read and approved the final version of the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yoshiyuki Horio, Email: horio@sapmed.ac.jp.
Hiroyuki Tsutsumi, Email: tsutsumi@saiseikai-midori.jp.
Supplementary information
is available for this paper at 10.1038/s41598-020-77197-6.
References
- 1.Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CM, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1489–1498. doi: 10.1016/S0140-6736(17)31611-2. [DOI] [PubMed] [Google Scholar]
- 4.Cirak S, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: An open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald CM, et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: A prospective cohort study. Lancet. 2018;391:451–461. doi: 10.1016/S0140-6736(17)32160-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonkowski MS, Sinclair DA. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalkiadaki A, et al. Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS Genet. 2014;10:e1004490. doi: 10.1371/journal.pgen.1004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanno M, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori YS, et al. Resveratrol ameliorates muscular pathology in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. J. Pharmacol. Exp. Ther. 2011;338:784–794. doi: 10.1124/jpet.111.183210. [DOI] [PubMed] [Google Scholar]
- 11.Kuno A, et al. Resveratrol ameliorates mitophagy disturbance and improves cardiac pathophysiology of Dystrophin-deficient mdx mice. Sci. Rep. 2018;8:15555. doi: 10.1038/s41598-018-33930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebori R, et al. Resveratrol decreases oxidative stress by restoring mitophagy and improves the pathophysiology of Dystrophin-deficient mdx mice. Oxid. Med. Cell Longev. 2018;2018:9179270. doi: 10.1155/2018/9179270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cottart CH, Nivet-Antoine V, Beaudeux JL. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014;58:7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 14.Vignos PJ, Spencer GE, Archibald KC. Management of progressive muscular dystrophy in childhood. JAMA. 1963;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 15.Bérard C, et al. A motor function measure for neuromuscular diseases. Construction and validation study. Neuromuscul. Disord. 2005;15:463–470. doi: 10.1016/j.nmd.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Stuberg WA, Metcalf WK. Reliability of quantitative muscle testing in healthy children and in children with Duchenne muscular dystrophy using a hand-held dynamometer. Phys. Ther. 1988;68:977–982. doi: 10.1093/ptj/68.6.977. [DOI] [PubMed] [Google Scholar]
- 17.Connolly AM, et al. Outcome reliability in non-ambulatory boys/men with Duchenne muscular dystrophy. Muscle Nerve. 2015;51:522–532. doi: 10.1002/mus.24346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escolar DM, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara D, et al. SIRT1 deficiency interferes with membrane resealing after cell membrane injury. PLoS ONE. 2019;14:e0218329. doi: 10.1371/journal.pone.0218329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 22.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morselli E, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuno A, et al. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. J. Biol. Chem. 2013;288:5963–5972. doi: 10.1074/jbc.M112.392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown VA, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida L, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009;53(Suppl 1):S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 28.Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 29.Timmers S, et al. Calorie restriction-like effects of 30 days of Resveratrol (resVidaTM) supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesselheim AS, Avorn J. Approving a problematic muscular dystrophy drug: Implications for FDA policy. JAMA. 2016;316:2357–2358. doi: 10.1001/jama.2016.16437. [DOI] [PubMed] [Google Scholar]
- 31.Wood CL, Cheetham T. Treatment of Duchenne muscular dystrophy: First small steps. Lancet. 2017;390:1467–1468. doi: 10.1016/S0140-6736(17)31669-0. [DOI] [PubMed] [Google Scholar]
- 32.Riess JW, et al. A phase IIa study repositioning desipramine in small cell lung cancer and other high-grade neuroendocrine tumors. Cancer Treat. Res. Commun. 2020;23:100174. doi: 10.1016/j.ctarc.2020.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebastian M, et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 2019;68:799–812. doi: 10.1007/s00262-019-02315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syková E, et al. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: Results of phase I/IIa clinical trial. Cell Transplant. 2017;26:647–658. doi: 10.3727/096368916X693716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai YA, et al. Treatment of spinocerebellar ataxia with mesenchymal stem cells: A phase I/IIa clinical study. Cell Transplant. 2017;26:503–512. doi: 10.3727/096368916X694373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva EC, et al. Motor function measure scale, steroid therapy and patients with Duchenne muscular dystrophy. Arq. Neuropsiquiatr. 2012;70:191–195. doi: 10.1590/S0004-282X2012000300007. [DOI] [PubMed] [Google Scholar]
- 37.Umphred, D. A. & Lazoro, R. T. Neurological Rehabilitation, 6th ed (eds. Roller, M. & Burton, G.) 563–565 (Elsevier Health Sciences, 2012).
- 38.Bostock EL, et al. The effects of resistance exercise training on strength and functional tasks in adults with Limb-Girdle, Becker, and Facioscapulohumeral dystrophies. Front Neurol. 2019;10:1216. doi: 10.3389/fneur.2019.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gianola S, et al. Efficacy of muscle exercise in patients with muscular dystrophy: A systematic review showing a missed opportunity to improve outcomes. PLoS ONE. 2013;8:e65414. doi: 10.1371/journal.pone.0065414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griggs RC, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Arch Neurol. 1991;48:383–388. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.