Abstract
Therapeutic options are limited for patients infected with Acinetobacter baumannii due to its multidrug-resistance profile. So, the search for new antimicrobials against this gram-negative bacterial pathogen has become a worldwide priority. The present study aimed to evaluate the effects of 1,10-phenanthroline (phen), 1,10-phenanthroline-5,6-dione (phendione), [Ag(phendione)2]ClO4 (Ag-phendione) and [Cu(phendione)3](ClO4)2·4H2O (Cu-phendione) on 26 carbapenemase-producing A. baumannii strains. The susceptibility to carbapenems was performed by detecting the metallo-beta-lactamase (MBL) genes by PCR and by determining the MIC. Also, disk diffusion method was applied to evaluate the susceptibility to other antimicrobial classes. The test compounds were evaluated on both planktonic- and biofilm-growing bacterial cells. The results revealed that all A. baumannii strains had the intrinsic blaoxa-51 gene, and at least one of the blaoxa-23 or blaoxa-24 genes. The geometric mean MIC and minimum bactericidal concentration (MBC) values, respectively, were as follows: Cu-phendione (1.56 and 2.30 μM), Ag-phendione (2.48 and 3.63 μM), phendione (9.44 and 9.70 μM), and phen (70.46 and 184.28 μM). The test compounds (at 0.5 × MIC) affected the biofilm formation and disrupted the mature biofilm, in a typically dose-dependent manner, reducing biomass and viability parameters. Collectively, silver and copper-phendione derivatives presented potent antimicrobial action against planktonic- and biofilm-forming cells of carbapenemase-producing A. baumannii.
Keywords: Acinetobacter baumannii; Carbapenemase; 1,10-Phenanthroline-5,6-dione; Metal-based compounds; Antimicrobial activity; Anti-biofilm action
Introduction
Acinetobacter baumannii is a gram-negative opportunistic bacterial pathogen able to cause human diseases, particularly associated with nosocomial infections, which presents high mortality rates [1]. Cross-transmission is the more important route in the maintenance of the infectious agent in the hospital environment and, consequently, in the occurrence of outbreaks related to A. baumannii [2]. The World Health Organization (WHO) has released a global priority list containing the main antimicrobial-resistant bacteria, aiming to highlight and to stimulate the researches around the world to focus on the development of new antimicrobial compounds and novel effective treatment strategies against such human pathogens. In this context, A. baumannii is at the top of the WHO priority list, being considered the most alarming among the multidrug-resistant (MDR) bacterial species. A. baumannii is endemic in several healthcare-associated settings, presenting a prevalence rate of more than 10% among gram-negative isolates, being particularly associated with pneumonia, septicemia, meningitis, and urinary tract infection [3].
The abilities of A. baumannii to survive on dry and inanimate surfaces, to form biofilm and to acquire antimicrobial resistance characteristics, without no doubt, contribute to its wide spread in the hospital environments and increase the likelihood of causing nosocomial infections [1]. This species produces an intrinsic β-lactamase, oxacillinase, represented by OXA-51. The genes encoding the blaoxa-51-like type β-lactamases are chromosomally located in all isolates of A. baumannii studied to date. The presence of carbapenemases is the most common cause of resistance to carbapenems, and the onset of metallo-β-lactamases (MBL) is becoming a therapeutic challenge [4]. The current therapeutic arsenal is usually ineffective for the control of infections caused by A. baumannii [1, 3].
Over the last years, metal-based drugs have been proven to be of great relevance due to their therapeutic principles and their pharmacological applications [5]. For instance, McCann et al. [6] synthesized three new derivatives from 1,10-phenanthroline (phen), which has been widely used as chelating agent and as molecular basis to synthesize new molecules with different bioactive properties: 1,10-phenanthroline-5,6-dione (phendione), [Ag(phendione)2]ClO4 (Ag-phendione) and [Cu(phendione)3](ClO4)2·4H2O (Cu-phendione) [7]. In vitro and in vivo experimental studies have revealed that phendione and its Ag+ and Cu2+ complexes have been presented the ability to alter the functioning and survival of a variety of microorganisms such as bacteria, fungi and protozoa [7, 8]. Corroborating these previous statements, Viganor et al. [8] showed that Ag-phendione and Cu-phendione presented potent antimicrobial effects against both planktonic- and biofilm-growing Pseudomonas aeruginosa cells, including carbapenem-resistant clinical strains recovered from different human anatomical sites. In a preceding study, Santos et al. [7] showed that both Ag-phendione and Cu-phendione were well-tolerated in in vivo tests using Galleria mellonella larvae and Swiss mice, which reinforces that these compounds may represent a new class of antimicrobial agents with potential therapeutic applications. Considering all these beneficial properties as well as the necessity to find new potential compounds against A. baumannii, herein, the effects of phen, phendione and its silver- and copper-derivatives were evaluated on planktonic proliferation and on biofilm-forming A. baumannii MDR strains.
Materials and methods
Chemicals
The clinically available antimicrobial agents, gentamicin (GEN), ciprofloxacin (CIP), levofloxacin (LEV), tobramycin (TOB), amikacin (AMI), and trimethoprim/sulfamethoxazole (SUT), were purchased from Sensidisc (Diagnósticos Microbiológicos Especializados - DME, São Paulo, Brazil). Meropenem (MPM), imipenem (IPM), phen, crystal violet, menadione, 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[phenylamine] carbonyl]-2H-tetrazolium hydroxide (XTT), dimethyl sulfoxide (DMSO), AgNO3, and CuSO4·5H2O were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Luria-Bertani (LB) and blood agar (BA) were purchased from Merck (Darmstadt, Germany). Mueller-Hinton Agar (MHA) and Mueller-Hinton II broth (MHB-ca) was purchased from Becton, Dickinson and Company (BD, Sparks, MD, USA). Phendione, Ag-phendione, and Cu-phendione were prepared in accordance with the methods described in the literature [9]. All other reagents were of analytical grade.
Bacterial strains
Twenty-six non-duplicated A. baumannii strains collected from patients hospitalized in 10 Brazilian hospitals in 2014 were included in this study. Strains were obtained from distinct infection sites: tracheal aspirate (n = 6), tracheal secretion (n = 5), sacral wound swab (n = 3), urine (n = 2), fragment of bone tissue (n = 2), catheter tip (n = 2), fragment of the gluteal region (n = 1), bronchoalveolar lavage (n = 1), swab for cystostomy (n = 1), left lower limb wound swab (n = 1), nasal swab (n = 1), and abdominal tissue (n = 1). Each A. baumannii strain used in the present study came from only one patient, and it was representative of different clones as assessed by PFGE genotyping [10]; consequently, each bacterial strain was isolated from only one anatomical site regarding each individual. The strains were identified by VITEK® 2 automated system. The reference strain of Pseudomonas aeruginosa ATCC 27853 and a clinical strain of P. aeruginosa (09HC), whose susceptibility profiles to MPM, IPM, phen, phendione, Ag-phendione, and Cu-phendione were previously defined by Viganor et al. [8] were used herein as quality control of the antimicrobial tests.
Determination of MIC and MBC of the carbapenems
The MIC and MBC for both MPM and IPM were evaluated by microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) standard protocol [11], using a 96-well microtiter plate with MHB-ca.
PCR for detection of prevalent OXA carbapenemases
The multiplex polymerase chain reaction (PCR) assay was performed to verify the presence of blaoxa-23-like (501 bp), blaoxa-24-like (246 bp), and blaoxa-58-like (599 bp) β-lactamase encoding genes plus intrinsic blaoxa-51-like (353 bp), as previously described [12]. The amplification conditions were briefly described as follows: initial denaturation at 94 °C for 5 min, 30 cycles at 94 °C for 25 s, followed by a cycle at 52 °C for 40 s and a cycle at 72 °C for 50 s, and a final elongation at 72 °C for 6 min. The PCR products were analyzed by electrophoresis with 1.4% agarose gels with 0.5× Tris–borate–EDTA (TBE; 89 mM Tris, 89 mM boric acid, 2 mM EDTA, pH 8.0) running buffer at 100 V for 1–2 h, then stained with 0.5 mg/mL of ethidium bromide and detected under UV transillumination (Bio Rad Gel Doc 2000 with UV Trans Iluminator).
Antimicrobial susceptibility
Antimicrobial susceptibility test was performed and interpreted according to the protocol recommended by CLSI [11] by means of disk diffusion method to six antimicrobials: GEN (10 μg), CIP (5 μg), LEV (5 μg), TOB (10 μg), AMI (30 μg), and SUT (1.25/23.75 μg). Bacterial strains that displayed resistance to three or more antimicrobial classes were classified as MDR [13].
MICs and MBCs of phen, phendione, and its metal-based compounds against planktonic bacterial growth
The MIC and MBC values of the test compounds (phen, phendione, Ag-phendione, and Cu-phendione) as well as simple salts (AgNO3 and CuSO4·5H2O) were determined by microdilution method according to Viganor et al. [8] using a 96-well microtiter plate with MHB-ca containing 1.56–200 μg/mL of each compound (starting from a 20 mM solution in DMSO). In all cases, the lowest concentration of each compound that inhibited the bacterial growth, as ascertained by the absence of visible turbidity in each well, was considered the MIC. The geometric mean MIC (GM-MIC) was calculated, since this measure more realistically reflects the results of sets of numbers whose values change exponentially, considering the amount of strains in each concentration [14]. Wells containing MHB-ca plus bacterial suspension, MHB-ca plus test compound solutions, MHB-ca plus DMSO, MHB-ca plus DMSO plus bacterial suspension, and MHB-ca only were used as controls. An aliquot of 10 μL of wells showing no growth were subcultured into nutrient agar plates for the MBC determination of each test compound.
Effects of test compounds on biofilm formation
For this assay, the bacterial strains were seeded on nutrient agar and incubated for 24 h at 35 ± 2 °C. Subsequently, the cells were spiked in LB broth and incubated for 24 h at 35 ± 2 °C. Suspensions of bacterial strains were prepared in turbid LB broth corresponding to the 0.5 McFarland standard (1.5 × 108 CFU/mL). Then, 100 μL of each suspension was distributed in a 96-well polystyrene plate, which was incubated for 24 h at 35 ± 2 °C in the absence or in the presence of phen and its derivatives at a concentration of 0.5 × MIC. Thereafter, the supernatants were collected and the wells were washed three times with 200 μL of saline. To evaluate the biofilm biomass, 100 μL of violet crystal solution (0.3% w/v) was added to the wells, and after 15 min, the wells were washed with running water to remove the excess of the dye. Finally, after drying the plates, the violet crystal adsorbed on the biofilm was diluted by the addition of 100 μL of 70% ethanol with 10% isopropyl alcohol. The absorbance was measured spectrophotometrically using a Thermomax Molecular Device microplate reader at 540 nm [8, 15]. The metabolic activity after treatment with phen, phendione, and its derivatives was evaluated by the reduction of 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[phenylamine) carbonyl]-2H-tetrazolium (XTT) as previously described by Viganor et al. [8]. After the biofilm formation period, in the presence and absence of equivalent concentrations, the supernatants were removed and the biofilm was washed 3 times with PBS for removal of the non-adherent cells. Then, 158 μL of PBS, 40 μL of XTT (1 mg/mL) and 2 μL of menadione (4 mM) were added. The plate was incubated for 3 h at 35 ± 2 °C under the light. At the end of the incubation, XTT reduction was measured at 490 nm for the evaluation of metabolically active cells [8, 15].
Effects of test compounds on the mature biofilm
Bacterial strains were seeded in BA and incubated for 24 h at 35 ± 2 °C. Then, bacterial suspensions were made at 0.5 McFarland standard turbidity in LB broth. Subsequently, 100 μL of the suspension was distributed into 96-well polystyrene plates. After the biofilm formation (24 h), the compounds were added to the wells at different concentrations (1.56–25 μg/mL). The systems were incubated for another 24 h at 35 ± 2 °C. At the end of the incubation period, staining was performed with crystal violet and XTT and biofilm viability was measured as previously described [8, 15].
Statistics
The experiments were carried out in biological triplicates. Data were expressed as mean ± standard deviation. The results were evaluated through analysis of variance (ANOVA), with a statistical significance level of 95% (p < 0.05). The software used for analysis was GraphPad Prism 7.
Results
Confirmation of carbapenems’ susceptibility
Our results confirmed that all the A. baumannii strains were resistant to both carbapenems. The strains showed MIC values for MPM ranging from 16 to 64 μg/mL and for IPM varying from 32 to 128 μg/mL (Table 1). All the A. baumannii strains harbored at least one of the evaluated carbapenemase gene. The intrinsic blaOXA-51-like gene was detected in all 26 clinical strains studied, while 24 (92.3%) were positive for the blaOXA-23-like gene and 2 (7.7%) were positive for the blaOXA-24-like gene. Conversely, none of the strains had the blaOXA-58-like gene.
Table 1.
Effects of test compounds on planktonic growth of A. baumannii clinical strains
| Compounds | Range (μg/mL) | MIC50 (μg/mL) | MIC90 (μg/mL) | GM-MIC, μg/mL (μM) | GM-MBC, μg/mL (μM) |
|---|---|---|---|---|---|
| MPM | 16–64 | 32 | 64 | 44.42 (ND) | ND |
| IPM | 32–128 | 64 | 128 | 61.54 (ND) | ND |
| Phen | 12.5–25 | 12.5 | 12.5 | 12.98 (70.46 μM) | 36.53 (184.28 μM) |
| Phendione | 1.562–6.25 | 1.562 | 1.562 | 1.98 (9.44 μM) | 2.04 (9.70 μM) |
| Ag-phendione | 1.562 | 1.562 | 1.562 | 1.562 (2.48 μM) | 2.28 (3.63 μM) |
| Cu-phendione | 1.562 | 1.562 | 1.562 | 1.562 (1.63 μM) | 2.22 (2.30 μM) |
| AgNO3 | 6.25–12.5 | 6.25 | 6.25 | 6.73 (39.6 μM) | ND |
| CuSO4·5H2O | > 200 | > 200 | > 200 | ND | ND |
MIC50 and MIC90, correspond to the minimum concentration of test compound required to inhibit 50% and 90% of the clinical bacterial strains, respectively
MPM meropenem, IPM imipenem, phen 1,10-phenanthroline, phendione 1,10-phenanthroline-5,6-dione, Ag-phendione [Ag(phendione)2]ClO4, Cu-phendione [Cu(phendione)3](ClO4)2·4H2O, GM-MIC geometric mean of MIC, GM-MBC geometric mean of MBC, ND not determined
Antimicrobial susceptibility profiles
The disk diffusion method was performed to ascertain the susceptibility profiles to other six antimicrobials belonging to different classes. All strains were resistant to CIP and LEV, both antimicrobials belong to the quinolone class. The resistance percentages for the remaining tested antimicrobials were as follows: 80.9% for SUT, 76.9% for GEN, 73.1% for TOB, and 50% for AMI. Our results pointed out that all studied Brazilian clinical strains of A. baumannii showed high percentages of antimicrobial resistance. Corroborating this finding, 80.7% (21/26) A. baumannii strains were resistant to at least two other classes of antimicrobials beyond β-lactams, being classified as MDR strains.
MICs and MBCs of phen, phendione, and its metal-based compounds against planktonic bacterial growth
The results of antimicrobial susceptibility test for phen, phendione, Ag-phendione, and Cu-phendione are summarized in Table 1. Both Cu-phendione (GM-MIC = 1.63 μM) and Ag-phendione (GM-MIC = 2.48 μM) were extremely effective in inhibiting planktonic bacterial growth followed by phendione (GM-MIC = 9.44 μM) and phen (GM-MIC = 70.46 μM). The silver salt (AgNO3) showed moderate growth inhibition activity (GM-MIC = 39.6 μM), while the copper salt (CuSO4·5H2O) was considered inactive. Aqueous DMSO, which was used as solvent for all test compounds, did not interfere with the bacterial growth (data not shown). The MBC values were also determined, showing the following potency pattern: Cu-phendione (GM-MBC = 2.30 μM) > Ag-phendione (GM-MBC = 3.63 μM) > phendione (GM-MBC = 9.70 μM) > phen (GM-MBC = 184.28 μM).
Effects of phen, phendione, and its metal-based compounds on the biofilm
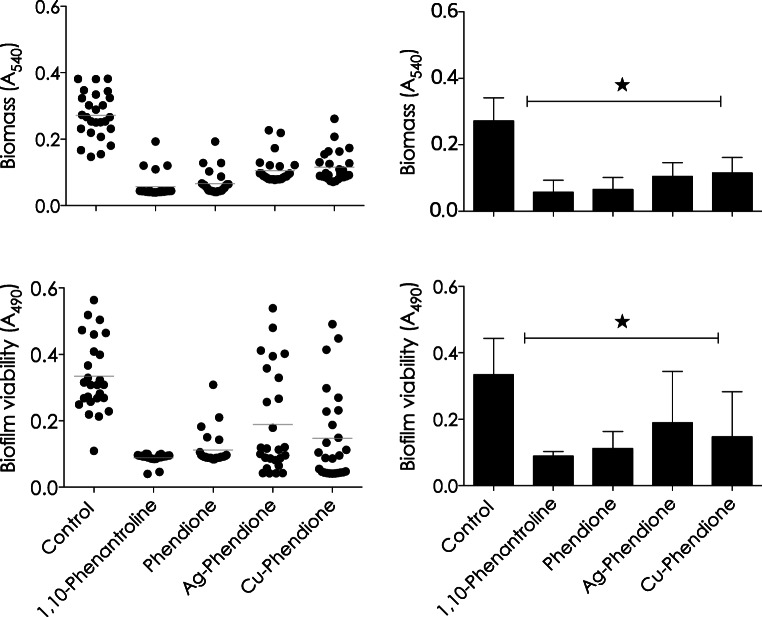
The effect of tested compounds on biofilm formation by 26 A. baumannii strains were analyzed in terms of total biomass and cellular metabolic activity. For these experiments, bacterial cells were incubated with the compounds at 0.5 × MIC value during the whole period of biofilm formation (24 h) on a polystyrene surface. Phen, phendione, Ag-phendione and Cu-phendione were able to inhibit the biofilm formation, reducing the biomass by approximately 79%, 76%, 61%, and 57%, respectively (Fig. 1a and b), and the metabolic activity by around 73%, 66%, 43%, and 56%, respectively (Fig. 1c and d).
Fig. 1.
Effects of phen, phendione, Ag-phendione, and Cu-phendione on biofilm formation by 26 A. baumannii clinical strains. Biofilm biomass was measured by crystal violet incorporation and absorbance value at 540 nm (a and b), and cellular viability was quantified by XTT and absorbance value at 490 nm (c and d). The distribution of biofilm biomass (a) and viability (c) in each clinical strain regarding each analyzed system was plotted. In parallel, the values representing the mean ± standard deviation concerning biofilm biomass (b) and viability (d) for each analyzed system are also shown. The gray lines in a and c indicate the arithmetic mean of the production of biofilm in each system. Stars denote a significant difference between untreated and treated bacterial cells (P < 0.05; one-way ANOVA, Dunnett’s multiple comparison test)
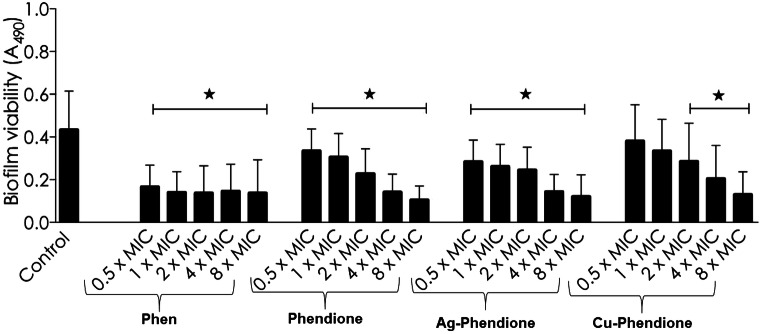
The ability of tested compounds to kill the A. baumannii cells embedded inside the mature biofilm structure was also assessed. To this goal, different concentrations of phen, phendione, Ag-phendione, and Cu-phendione were added to the 24-h mature biofilm of A. baumannii followed by an additional incubation of 24 h. Phendione and its metal-based derivatives were able to reduce the biofilm viability in a typically dose-dependent manner (Fig. 2). In this set of experiments, Cu-phendione was the most potent compound, presenting the lowest concentration required to reduced 50% of biofilm viability (IC50 = 13.54 μM), followed by Ag-phendione (IC50 = 22.05 μM) and phendione (IC50 = 34.58 μM) (Fig. 2).
Fig. 2.
Effects of phen, phendione, Ag-phendione, and Cu-phendione on mature biofilm formed by A. baumannii clinical strains. The values represent the mean ± standard deviation concerning biofilm viability for each analyzed compound. Stars denote a significant difference between untreated and treated bacterial cells (P < 0.05; one-way ANOVA, Dunnett’s multiple comparison test)
Discussion
Acinetobacter baumannii is a human opportunistic pathogen, whose infections are notoriously difficult to treat due to the intrinsic and acquired antimicrobial resistance often limiting the effective therapeutic options. The bacterial persistence in the hospital environments exposes it to the constant selective pressure imposed by the antimicrobials, which, in addition to its remarkable ability to acquire resistance, led to the emergence of MDR strains [16]. To worsen this scenario, carbapenem-resistant A. baumannii strains have a high potential for nosocomial dissemination [2]. In these context, we decided to select strains of A. baumannii resistant to carbapenems, since this antibacterial class is often used as “last-line agents” or “antibiotics of last resort” when patients with infections become gravely ill or are suspected of harboring resistant bacteria [17].
All the strains selected for this study proved to be phenotypically resistant to carbapenem, and they harbored at least one carbapenemase gene. The carbapenem resistance strategies in A. baumannii include the production of MBLs, mainly β-lactamase class D (type OXA), which is responsible for this phenotype [18]. In our study, the blaoxa-51 gene was detected in all strains along with blaoxa-23 or blaoxa-24 genes. The subgroups of carbapenem-hydrolyzing OXAs, such as the OXA-23, OXA-24, OXA-51, and OXA-58 subgroups, are considered prevalent in A. baumannii [1, 19]. These enzymes are a major worldwide concern, because they present the possibility that all isolates of A. baumannii should be able to become resistant to carbapenems [20].
In addiction of the carbapenems’ susceptibility profile, the knowledge of the susceptibility of the strains to other antimicrobial classes, mainly in relation to the existence of other resistance mechanisms, are important data for a better evaluation of the antimicrobial activity of the compounds derived from phen, highlighting the possibility of different resistance mechanisms presented by these strains interfere with the action of these compounds, e.g., the efflux systems or change of target site related to resistance to fluoroquinolones and aminoglycoside, respectively [21]. In this context, we evidenced a high rate of MDR among the strains of A. baumannii selected for this study highlight of the existence of different mechanism of resistance.
The searching for new compounds with anti-A. baumannii action is urgently required. However, due to the lack perspective regarding the release of new antimicrobials in the global market, this need is far from to be achieved. In order to contribute in this avenue, we investigated the possible antimicrobial activity of phen and phendione-based compounds on carbapenem-resistant A. baumannii strains. Our results showed that phendione and its Ag+ and Cu2+ complexes showed bactericidal action on planktonic-growing A. baumannii cells in concentrations < 10 μM, with Cu-phendione having the most powerful action. These compounds were already tested on another gram-negative, ubiquitous and multidrug-resistant bacterium, P. aeruginosa [8]. Interestingly, the MICs of phendione and its metal-derivatives were at least 4 times lower for A. baumannii when compared to P. aeruginosa [8].
In general, our results demonstrated that the low concentrations of Ag-phendione and Cu-phendione exhibit a potent antimicrobial effect against A. baumannii planktonic cells. The low MIC values found here contrast with the elevated concentrations calculated to kill G. mellonella larvae and Swiss mice in in vivo experiments, reinforcing that these compounds were well tolerated by these model organisms [22]. These data are relevant, because in a process of developing new drugs it is crucial to evaluate and select those that have a balanced efficacy/safety profile. Thus, efficacy and safety data generated in vitro and in vivo can be used to calculate a clinical therapeutic index of a drug candidate at an early stage [23]. Therefore, the results obtained in our study reinforce the antimicrobial potential of phendione-based compounds, particularly Cu-phendione, stimulating the determination of important and crucial pharmacodynamic and phamacocynetic properties of these promising compounds.
Acinetobacter baumannii has the ability to form biofilm on inert surfaces, contributing to the ecological success of this pathogen in the hospital environment [24]. Also, the A. baumannii biofilm lifestyle permits the pathogen to survive for long periods even in an abiotic surface, which increases its probability of causing nosocomial infections and outbreaks [24], representing an important virulence attribute [25]. Biofilm is a well-known structure of resistance against both chemical and physical stressors, which is produced by various microorganisms, including human pathogenic bacteria [1, 26]. The present study demonstrated that all test compounds significantly reduced the biofilm formation, considering both biomass and metabolic activity, in A. baumannii isolates on polystyrene surface at concentrations up to 10 times the MIC calculated to the planktonic growth. In addition, phendione, Ag-phendione, and Cu-phendione were able to significantly reduce the viability of bacterial cells even when embedded in a mature biofilm structure. Similar results were reported by Viganor et al. [8] to biofilm formed by P. aeruginosa, in which phendione and its Ag+ and Cu+2 complexes were able to disrupt the mature biofilm in a typically dose-dependent fashion. The model of disarticulation of mature biofilms by chelating agents, such as phen and phendione, is consolidated on bacterial biofilms. In this context, Tay et al. [27] showed that phendione eradicated the biofilm formation in Enterococcus faecalis suggesting a mechanism of chelating action of essential metals (Zn2+, Fe2+, Ca2+, Cu2+, Co2+, Mn2+, and Ni2+) for bacterian metabolism. In P. aeruginosa, the negatively charged extracellular DNA (eDNA) interacts with cations, such as Mg2+ and Ca2+, stabilizing the extracellular matrix [28]. So, the chelating agents sequester the cations from eDNA, leading to its destabilization [29], leading to its disarticulation. It would be interesting to further explore the interference mechanisms of these compounds in the biofilm formation, which can open new windows to use these compounds as an anti-virulence (anti-biofilm) strategy in order to combat carbapenemase-producing A. baumannii.
In conclusion, our results pointed out that phendione-based compounds, particularly Cu-phendione, presented potent antimicrobial action against both planktonic- and biofilm-forming cells of A. baumannii MDR strains, including carbapenemase-producing ones, indicating that these compounds may represent a novel class of antimicrobial agents with potential therapeutic applications.
Acknowledgments
The authors thank the Public Health Central Laboratory of Espírito Santo (LACEN) for supplying the A. baumanni strains.
Funding information
This study was supported by Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES), Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Financial code 001).
Compliance with ethical standards
Ethical considerations
The submission to an ethics committee not was required, because the strains used in this study were selected from a bacterial collection stored in the LACEN.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee CR, Lee JH, Park M, Park KS, Bae IK, Kim YB, Cha CJ, Jeong BC, Lee SH. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment. Options. Front Cell Infect Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:5–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2017) Global priority list of antibiotic-resistant bacteria to guide research, diskovery, and development of new antibiotics. http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed 30 Nov 2019
- 4.Wang TH, Leu YS, Wang NY, Liu CP, Yan TR. Prevalence of different carbapenemase genes among carbapenem-resistant. Acinetobacter baumannii blood isolates in Taiwan. Antimicrob Resist Infect Control. 2018;7:123. doi: 10.1186/s13756-018-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warra AA (2011) Transition metal complexes and their application in drugs and cosmetics. J Chem Pharm Res 3:951–958. http://www.jocpr.com/articles/transition-metal-complexes-andtheir-application-in-drugs-and-cosmetics--a-review.pdf. Accessed 01 Dec 2019
- 6.McCann M, Kellett A, Kavanagh K, Devereux M, Santos AL. Deciphering the antimicrobial activity of phenanthroline chelators. Curr Med Chem. 2012;19:2703–2714. doi: 10.2174/092986712800609733. [DOI] [PubMed] [Google Scholar]
- 7.Santos AL, Sodre CL, Valle RS, Silva BA, Abi-Chacra EA, Silva LV, Souza-Goncalves AL. Antimicrobial action of chelating agents: repercussions on the microorganism development, virulence and pathogenesis. Curr Med Chem. 2012;19:2715–2737. doi: 10.2174/092986712800609788. [DOI] [PubMed] [Google Scholar]
- 8.Viganor L, Galdino ACM, Nunes APF, Santos KR, Branquinha MH, Devereux M, Kallett A, McCann M, Santos ALS. Anti-Pseudomonas aeruginosa activity of 1,10-phenanthroline-based drugs against both planktonic- and biofilm-growing cells. J Antimicrob Chemother. 2016;71:128–134. doi: 10.1093/jac/dkv292. [DOI] [PubMed] [Google Scholar]
- 9.McCann M, Santos ALS, Silva BA, Romanos MTV, Pirro AS, Devereux M, Kavanag K, Fichtner I, Kallett A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicol Res. 2012;1:47–54. doi: 10.1039/c2tx00010e. [DOI] [Google Scholar]
- 10.Durmaz R, Otlu B, Koksal F, Hosoglu S, Ozturk R, Ersoy Y, Elif A, Canan GN, Ahmet C (2009) The optimization of a rapid pulsed-field gel electrophoresis protocol for the typing of Acinetobacter baumannii, Escherichia coli and Klebsiella spp. Jpn J Infect Dis 62:372–377. https://www.ncbi.nlm.nih.gov/pubmed/19762987. Accessed 01 Dec 2019 [PubMed]
- 11.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial disk susceptibility tests. 13. Wayne: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 12.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SGB, Livermore DM. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 14.Manikandan S. Measures of central tendency: the mean. J Pharmacol Pharmacother. 2011;2:140–142. doi: 10.4103/0976-500X.81920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi L, Li H, Zhang C, Liang B, Li J, Wang L, Du X, Liu X, Qiu S, Song H. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiol. 2016;7:483. doi: 10.3389/fmicb.2016.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lob SH, Hoban DJ, Sahm DF, Badal RE. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int J Antimicrob Agents. 2016;47:317–323. doi: 10.1016/j.ijantimicag.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Nowak P, Paluchowska P. Acinetobacter baumannii: biology and drug resistance role of carbapenemases. Folia Histochem Cytobiol. 2016;4:61–74. doi: 10.5603/FHC.a2016.0009. [DOI] [PubMed] [Google Scholar]
- 18.June CM, Vallier BC, Bonomo RA, Leonard DA, Powers RA. Structural origins of oxacillinase specificity in class d β-lactamases. Antimicrob Agents Chemother. 2014;58:333–341. doi: 10.1128/AAC.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labarca JA, Salles MJC, Seas C, Guzmán-Blanco M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbiol. 2016;42:276–292. doi: 10.3109/1040841X.2014.940494. [DOI] [PubMed] [Google Scholar]
- 20.Evans BA, Amyes SG. OXA β-lactamases. Clin Microbiol Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyne S, Courvalin P, Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother. 2011;55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann M, Coyle B, McKay S, McCormack P, Kavanagh K, Devereux M, McKee V, Kinsella P, O'Connor R, Clynes M. Synthesis and X-ray crystal structure of [Ag(phendio)2]ClO4 (phendio = 1,10-phenanthroline-5,6-dione) and its effects on fungal and mammalian cells. Biometals. 2004;17:635–645. doi: 10.1007/s10534-004-1229-5. [DOI] [PubMed] [Google Scholar]
- 23.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 24.Runci F, Bonchi C, Frangipani E, Visaggio D, Visca P. Acinetobacter baumannii biofilm formation in human serum and disruption by gallium. Antimicrob Agents Chemother. 2017;61:01563–01516. doi: 10.1128/AAC.01563-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca I, Espinal P, Vila-Farrés X, Vila J. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front Microbiol. 2012;3:148. doi: 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wi YM, Patel R. Understanding biofilms and novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin N Am. 2018;32:915–929. doi: 10.1016/j.idc.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay CX, Quah SY, Lui JN, Yu VSH, Tan KS. Matrix metalloproteinase inhibitor as an antimicrobial agent to eradicate Enterococcus faecalis biofilm. J Endod. 2015;41:858–863. doi: 10.1016/j.joen.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Lewenza S. Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front Microbiol. 2013;4:21. doi: 10.3389/fmicb.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavaliere R, Ball JL, Turnbull L, Whitchurch CB. The biofilm matrix destabilizers, EDTA and DNase I, enhance the susceptibility of nontypeable Hemophilus influenzae biofilms to treatment with ampicillin and ciprofloxacin. Microbiologyopen. 2014;3:557–567. doi: 10.1002/mbo3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]