Abstract
Background
Cutaneous warts are the commonest benign lesion produced by human papillomavirus. Lesions often regress spontaneously yet have a high rate of recurrence. They impair patients’ quality of life and carry the potential risk of cancer. Nowadays, Candida antigen immunotherapy has become an encouraging therapeutic modality for warts. We tried to assess the role of the complement pathway and T helper 1 immune response in clinical response to Candida antigen immunotherapy via complement component 3c (C3c) and tumor necrosis factor (TNF)-α, respectively.
Methods
A total of 44 patients with cutaneous warts were enrolled in the study. Patients were injected with Candida antigen at 2-week interval until complete clearance of the lesion or for a maximum of 5 sessions. Blood samples were collected before initiation and after completion of immunotherapy. C3 and C4 were measured using an automated turbidimetric method. Mannose-binding lectin (MBL), C3c, and TNF-α were measured using enzyme-linked immune sorbent assay.
Results
A total of 56.4%, 17.9%, and 25.7% of the patients showed complete, partial, and no response to immunotherapy, respectively. Lesions on the dorsum of the foot and sole showed significant clearance (p value = 0.037). All patients had no deficient C3, C4, and MBL serum levels. C3c and TNF-α serum levels were significantly higher in non-responder group (p value < 0.001 and < 0.001, respectively). C3c and TNF-α serum levels were strongly correlated in all the studied patients (r = 0.8, p value < 0.001).
Conclusions
Candida antigen immunotherapy is an effective therapeutic modality for cutaneous warts. C3c and TNF-α serum levels were higher in patients who failed to respond to immunotherapy.
Clinical trial registry number
NCT04399577, May 2020 “retrospectively registered”
Keywords: Candida antigen, Complement component 3c, Immunotherapy, Tumor necrosis factor-α, Warts
Introduction
Warts constitute the most frequent cutaneous and anogenital benign lesion of human papillomavirus (HPV) that infects epithelial cells of the skin and mucous membranes [1]. They often regress spontaneously after months or years; however, they also have a high rate of recurrence [2].
Early, through HPV infection, the innate immune system generates a pro-inflammatory state via recruitment of innate immune cells to clear out the infected cells, and consequently, launching an efficient adaptive immune response [3]. Therefore, the incompetent activation of an innate immune reaction and failure of priming an adaptive immunity can lead to a persistent viral infection that causes significant morbidity in a subset of individuals [4].
Nowadays, immunotherapy using Candida antigen injections has become an acceptable and effective therapeutic option with few side effects [5, 6]. Candida antigen provokes a delayed hypersensitivity reaction; thereby, the cell-mediated immune response could enhance the immune system to identify viral infection via induction of T helper (Th) 1 cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α which activate cytotoxic and natural killer cells to eradicate HPV infection [1, 7, 8]. Nonetheless, TNF-α has not been recognized in wart microenvironment [9]. Unlike IFN-γ and interleukin-10, TNF-α has not been investigated before in Candida antigen immunotherapy studies [1, 10, 11].
The basic components of Candida albicans (C. albicans) cell wall are β-glucans, chitin, and mannoproteins [12]. Different pattern recognition receptors, including Toll-like receptors (TLRs), are expressed on dendritic cells and have been implicated in recognition of C. albicans cell wall components and induction of innate immune responses [13, 14]. The regulation of the immune response against C. albicans appears to be mainly controlled by the balance between signals provoked by TLR2 and TLR4 [15].
The complement system is one of the key components of innate and adaptive immunity. It consists of nearly 30 different serum proteins with catalytic and regulating activities. Complement components are produced mainly by liver cells; however, other cells such as neutrophils, monocytes, macrophages, dendritic cells, and fibroblasts are also involved in their production [16]. Normally, complement components circulate in an inactive form in peripheral blood, and they become activated by three complement pathways: classic, alternative, and lectin pathways. Activation of the complement pathway can efficiently attract inflammatory cells and directly destroy cells via membrane attack complex. However, inappropriate complement activation can lead to adverse effects [17]. C. albicans is recognized by complement component 1q (C1q), C3, and mannose-binding lectin (MBL) components of the complement system [18]. In addition to triggering the lectin pathway, MBL is considered a recognition molecule of the innate immune system that enhances the complement-independent opsonophagocytosis [19]. Moreover, MBL deficiency appears to predispose to infectious diseases and influence the outcome of severe infections [20]. Since all the activation steps in the three different complement pathways converge on C3, C3 seems to be a good indicator of overall complement activation [21]. However, measuring the complement components is still problematic because of their aggressive nature and very short half-life [22, 23]. Therefore, it is useful to think of C3c, a stable activation product of C3, to assess the complement pathway activity.
The immune response studies in wart patients are important for understanding the local response to intralesional Candida antigen immunotherapy as well as for developing new therapeutic modalities. However, the failure to respond to Candida antigen immunotherapy in certain individuals is still being investigated. In the present study, we hypothesized that the intralesional injection of Candida antigen into cutaneous warts could trigger the classical, alternative, and lectin pathways of the complement system and stimulate TNF-α production via Th1 immune response. The study aimed to assess the activation of the complement system and Th1 immune response through the measurement of C3c and TNF-α serum levels, respectively, after Candida antigen immunotherapy in cutaneous warts.
Methods
This study was conducted at Immunology Research Laboratory, Microbiology and Immunology Department, Faculty of Medicine, Zagazig University from July 2017 to March 2019.
Subjects
This study included 44 patients from the attendants to the outpatient clinic of Dermatology and Andrology, Zagazig University Hospitals, Zagazig, Egypt. All patients had chronic multiple cutaneous recalcitrant or non-recalcitrant warts of different types, numbers, sites, and sizes. Recalcitrant warts were defined as warts of more than 2 years duration that failed to respond to two therapeutic modalities or more [1]. Patients were subjected to full history taking, clinical, and dermatological examination.
Exclusion criteria for this study were hypersensitivity to Candida antigen, acute febrile illness, immunosuppressive diseases or drugs, history of allergic skin disorders, meningitis or convulsions, pregnancy, lactation, and previous wart therapy in the last month before enrolment.
Candida antigen immunotherapy
Candida antigen (C. albicans 1:20 w/v 10 mL vial, Allergy Laboratories, INC. Oklahoma City, USA) was prepared by adding 0.1 mL of Candida antigen to 2.45 mL of 99.9% sterile glycerol and 2.45 mL of diluent composed of 0.4% phenol, 0.9% NaCl, and water. All patients were directly injected with 0.1 mL of 1/1000 solution of Candida antigen preparation into the mother wart, i.e., the earliest or the largest wart on the body. Each patient received the intralesional injections at 2-week intervals until complete clearance or for a maximum of five treatment sessions [24].
Assessment of clinical response
The response to immunotherapy was evaluated by the regression in the size of warts and the digital photographic comparisons at baseline and each visit. The response was considered complete if there was a 100% clearance of warts, partial if there was a 50–99% decrease in wart size, and no response if there was less than 50% decrease in wart size. Immediate and late adverse effects were also evaluated after each treatment session. Patients who showed positive response were further followed-up for 6 months after the fulfillment of treatment, and any recurrences were reported. Patients who did not show any response to the immunotherapy were offered alternative therapeutic options.
Blood sampling
A total volume of 3 mL peripheral blood was collected from each study participant before initiation and after fulfillment of Candida antigen immunotherapy. Blood samples were centrifuged without delay at 3000 rpm for 10 min. Serum was removed and stored at − 20 °C for the measurement of C3, C4, MBL, C3c, and TNF-α.
Quantitation of C3 and C4 serum levels
C3 and C4 serum levels were assayed before initiation of Candida antigen immunotherapy by using automated Sat 450 (Rome, Italy) turbidimetric method (Reactivos GPL, Barcelona, Spain). Normal ranges for C3 and C4 serum levels in our clinical laboratory are 0.90–1.80 mg/mL and 0.10–0.40 mg/mL, respectively.
Quantitation of MBL serum level
MBL serum level was measured before initiation and after fulfillment of Candida antigen immunotherapy by sandwich enzyme-linked immune sorbent assay (ELISA) according to the manufacturer’s company protocol (Quantikine® ELISA Human MBL; R&D Systems, Minneapolis, USA).
Quantitation of C3c and TNF-α serum levels
C3c and TNF-α serum levels were measured after fulfillment of Candida antigen immunotherapy by sandwich ELISA according to the manufactures’ company protocols (Human Complement Fragment 3c, Bioassay Technology Laboratory, Korea and Human TNF-α, INOVA, China).
Statistical analysis
Quantitative data were represented as mean value ± SD, median, and range. Associations between clinical responses, clinical, and laboratory variables were analyzed by Pearson chi-square (χ2) and Fisher’s exact tests. Mann–Whitney U and Kruskal-Wallis tests were used for calculation of the median difference between independent groups. Wilcoxon signed rank test was used for calculation of the median difference between the studied patients before and after Candida antigen immunotherapy. The correlation coefficient r was generated using Spearman’s correlation. All tests were two-tailed. Results were considered statistically significant when p values were equal to or less than 0.05. All analyses were performed using Statistical Package for the Social Sciences software version 24 (SPSS version 24, Inc., Chicago, IL, USA.).
Results
Of the 44 patients enrolled in the study, five had been excluded. Two of them did not meet the inclusion criteria, one declined to participate, and two had personal reasons. Those five patients were dropped from the study, and thus, we had 39 patients available for assessment at the end of the study. The present study included 33 females and six males who were treated by intralesional injection of Candida antigen. All patients had completed the five treatment sessions (Appendix Fig. 3). The baseline characteristics of the studied patients are demonstrated in Table 1.
Fig. 3.
CONSORT flow diagram. A single-arm, open-label clinical trial evaluating disease response following treatment with Candida antigen immunotherapy in patients with cutaneous warts
Table 1.
Baseline characteristics of the studied patients
| Variable | N = 39 | Percent |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 24.8 ± 12.2 | |
| Range | 9–45 | |
| Gender | ||
| Female | 33 | 84.6 |
| Male | 6 | 15.4 |
| Wart site | ||
| Face | 6 | 15.4 |
| Dorsum of hands | 12 | 30.8 |
| Forearm | 9 | 23.1 |
| Dorsum of foot | 9 | 23.1 |
| Sole | 3 | 7.7 |
| Wart size | ||
| < 1 cm | 24 | 61.5 |
| > 1 cm | 15 | 38.5 |
| Recalcitrance | ||
| No | 22 | 56.4 |
| Yes | 17 | 43.6 |
| Response | ||
| Complete | 22 | 56.4 |
| Partial | 7 | 17.9 |
| No | 10 | 25.7 |
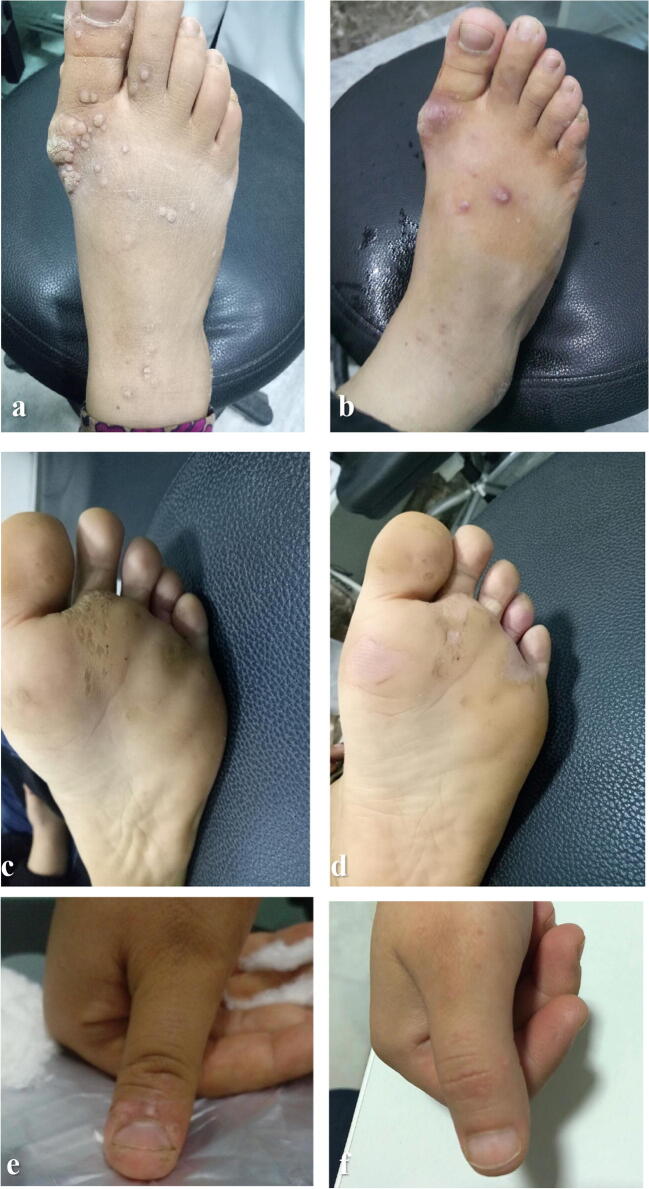
In the present work, 22 patients (56.4%) showed complete clearance of warts, seven patients (17.9%) showed a partial response, and 10 patients (25.7%) showed no response. Age or gender did not affect the clinical response to Candida antigen immunotherapy (p value = 0.14 and 0.23, respectively). The complete clinical response was significantly observed with common warts at the dorsum of the foot and sole (p value = 0.037) (Fig. 1). No significant relationship was found between the clinical response to Candida antigen immunotherapy and both the size and the recalcitrance of warts (p value = 0.3 and 0.2, respectively) (Table 2).
Fig. 1.
Clinical response of multiple cutaneous warts to Candida antigen immunotherapy. Multiple warts on the dorsum of foot: a before and b after immunotherapy. Multiple plantar warts: c before and d after immunotherapy. Multiple warts on the dorsum of thumb finger: e before and f after immunotherapy
Table 2.
Relation between clinical response to Candida antigen immunotherapy and different clinical and laboratory variables
| Variable | Response (N = 39) | Test of significance | P value | ||
|---|---|---|---|---|---|
| Complete n = 22 (%) | Partial n = 7 (%) | No n = 10 (%) | |||
| Age (years) | The Kruskal-Wallis test | 0.14 | |||
| Mean ± SD | 25.9 ± 12.2 | 16.7 ± 9.8 | 26.4 ± 13.3 | ||
| Median | 25.5 | 13.0 | 21.0 | ||
| Range | 11.0–45.0 | 9.0–35.0 | 13.0–45.0 | ||
| Gender | Fisher’s exact | 0.23 | |||
| Female | 17 (77.3) | 6 (85.7) | 10 (100) | ||
| Male | 5 (22.7) | 1 (14.3) | 0 (0.0) | ||
| Wart site | 0.037* | ||||
| Face | 3 (13.6) | 1 (14.3) | 2 (20.0) | ||
| Dorsum of hands | 3 (13.6) | 4 (57.1) | 5 (50.0) | ||
| Forearm | 4 (18.2) | 2 (28.6) | 3 (30.0) | ||
| Dorsum of foot | 9 (40.9) | 0 (0.0) | 0 (0.0) | ||
| Sole | 3 (13.6) | 0 (0.0) | 0 (0.0) | ||
| Wart size | 0.3 | ||||
| < 1 cm | 15 (68.2) | 3 (42.9) | 8 (80.0) | ||
| > 1 cm | 7 (31.8) | 4 (57.1) | 2 (20.0) | ||
| Recalcitrance | 0.15 | ||||
| Yes | 7 (68.2) | 5 (71.4) | 5 (50.0) | ||
| No | 15 (31.8) | 2 (28.6) | 5 (50.0) | ||
| C3 serum level (mg/mL) | The Kruskal-Wallis test | 0.1 | |||
| Mean ± SD | 1.52 ± 0.4 | 1.20 ± 0.28 | 1.44 ± 0.14 | ||
| Median | 1.75 | 1.19 | 1.48 | ||
| Range | 0.89–1.90 | 0.94–1.59 | 1.19–1.59 | ||
| C4 serum level (mg/mL) | 0.1 | ||||
| Mean ± SD | 0.26 ± 0.08 | 0.24 ± 0.09 | 0.31 ± 0.05 | ||
| Median | 0.27 | 0.25 | 0.31 | ||
| Range | 0.07–0.36 | 0.15–0.38 | 0.25–0.38 | ||
| MBL serum levela (μg/mL) | 0.3 | ||||
| Mean ± SD | 1.8 ± 1.1 | 1.8 ± 0.6 | 1.2 ± 1.0 | ||
| Medianc | 1.9 | 1.5 | 1.2 | ||
| Range | 0.3–3.3 | 0.8–2.6 | 0–2.4 | ||
| MBL serum levelb (μg/mL) | 0.1 | ||||
| Mean ± SD | 1.9 ± 1.3 | 2.1 ± 0.8 | 1.0 ± 0.8 | ||
| Medianc | 1.4 | 1.9 | 0.8 | ||
| Range | 0.2–3.4 | 1.4–3.2 | 0.1–1.9 | ||
| C3c serum level (ng/mL) | 0.001* | ||||
| Mean ± SD | 5.1 ± 3.0 | 13.5 ± 10.0 | 17.8 ± 6.2 | ||
| Median | 4.3 | 14.5 | 21.2 | ||
| Range | 1.9–11.5 | 3.3–24.2 | 3.5–22.1 | ||
| C3c/C3 index | < 0.001* | ||||
| Mean ± SD | 3.6 ± 2.2 | 12.0 ± 9.7 | 12.7 ± 4.9 | ||
| Median | 2.9 | 11.7 | 14.1 | ||
| Range | 1.1–9.0 | 2.2–25.7 | 2.2–18.1 | ||
| TNF-α serum level (pg/mL) | < 0.001* | ||||
| Mean ± SD | 18.1 ± 22.5 | 79.5 ± 77.8 | 186.9 ± 84.3 | ||
| Median | 12.5 | 50.6 | 224.2 | ||
| Range | 0.0–74.1 | 1.0–187.3 | 37.5–309.1 | ||
*Significant difference
aMBL serum level before Candida antigen immunotherapy
bMBL serum level after Candida antigen immunotherapy
cNon-significant difference in MBL serum level before and after Candida antigen immunotherapy by Wilcoxon signed-rank test (p = 0.3)
Relation between the clinical response and C3, C4, MBL, C3c, and TNF-α serum levels
C3 and C4 serum levels were within the normal range in all the studied patients. The relation between MBL serum level, before and after Candida antigen immunotherapy, and the clinical response was non-significant (p value = 0.1 and 0.3, respectively). Candida antigen immunotherapy did not induce a significant boost in MBL serum level in all patients (p value = 0.3) (Table 2).
C3c serum level, C3c/C3 index, and TNF-α serum level showed significant difference among the clinical groups (p value = < 0.001, < 0.001 and 0.001, respectively) (Table 2). Moreover, a strong statistical correlation was existed between C3c/C3 index and TNF-α serum levels (r = 0.8, p value < 0.001) (Fig. 2 and Appendix Fig. 3).
Fig. 2.
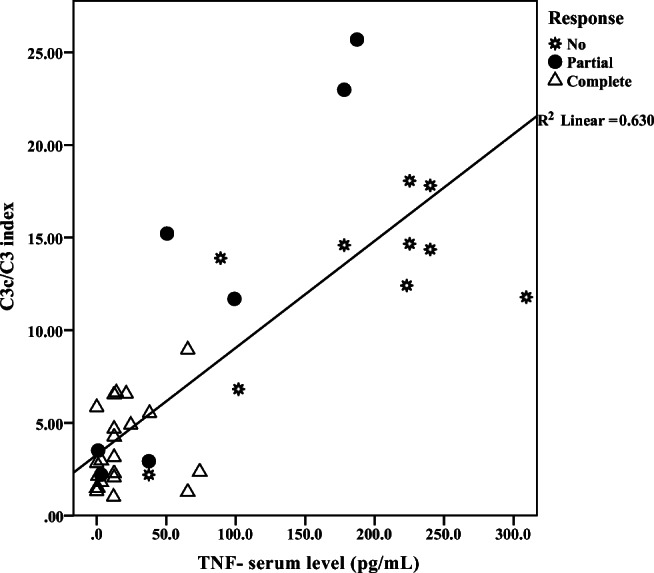
Positive correlation between C3c/C3 index and TNF-α r = 0.8, p value <0.001
Follow-up period
During the 6 months follow-up period, no recurrences of warts were reported in any of the patients who showed complete response to Candida antigen immunotherapy, and none of the non-responders showed any further reduction in the size or the number of warts.
Discussion
Candida antigen immunotherapy has become a promising, effective, and safe therapeutic option for warts [25]. The present work could achieve a complete resolution of warts in more than half of the patients enrolled in the study (56.4%) which is comparable to that reported by Signore [26] (51%) and Horn et al. [5] (52%) but higher than that reported by Alikhan et al. [27] (39%). Consonant with previous studies [6, 24], the clinical variables of the patients, including age, gender, and recalcitrance and size of warts did not affect their clinical outcomes. However, the successful clearance observed in lesions on the dorsum of foot and sole was remarkable.
The accompanying extermination of non-injected warts distant from the injected mother wart might support an induced systemic immune response against HPV infection [24, 28]. Nevertheless, the mechanisms that underlie the variation in the immunologic responses of the patients to this therapeutic modality remain to be determined. In the present study, we tried to evaluate the complement pathways and TNF-α after intralesional injection of Candida antigen in common warts.
All the study participants had normal C3, C4, and MBL serum levels; therefore, no deficiency in these complement proteins could be identified and correlated to the response to Candida antigen immunotherapy. MBL deficiency was defined before by plasmatic protein levels below 0.5 µg/mL [29]. In the present study, Candida antigen immunotherapy could not achieve a significant rise in the MBL serum level, a finding that agrees to a recent report [6]. However, MBL2 gene structural polymorphisms solely could be responsible for the defective function of MBL despite its normal serum level [6, 30].
In the present work, an interesting finding was the significant-high C3c serum level in non-responders. One possible explanation is the duration of the inflammatory process. It, partly, depends on whether a successful resolution and elimination of the initiating agent have occurred. Moreover, the inflammatory reaction may continue from stimuli that initiate a low-grade and asymptomatic response [31]. In this context, the activation of the complement system generates anaphylatoxins that interact with their receptors expressed on neutrophils, macrophages, and mast cells [32]. Furthermore, the recognition of Candida antigen is mediated through multiple pattern recognition receptors expressed on these cells [33]. However, to prevent the individual variations of C3 serum level from influencing the evaluation of complement activation, a C3c/C3 index was calculated [17], yet the C3c/C3 index was significantly highest in non-responders.
In this study, we observed that TNF-α serum level was significantly higher in non-responders. Since TNF-α is a substantial Th1 cytokine that plays a significant role in body defense against viral infection, the low TNF-α serum level in responders might be antithetical. However, its involvement has been considered in some types of cancers [34, 35]. TNF-α is a pleiotropic cytokine. Besides, being cytotoxic to tumor cells under certain conditions, TNF-α can boost tumor-promoting inflammation and angiogenesis [35]. It is now believed that persistent inflammation is a major detrimental factor for tumorigenesis, and growing evidence explicates that TNF-α has fundamental roles in this process [34, 35]. In this context, the authors proposed that the duration of the inflammatory process could be a possible explanation for the high TNF-α serum level in non-responders. On the other hand, the lower TNF-α serum level in responders might be attributed to the demise in the cytokine level after a successful priming of Th1 immune response and eradication of HPV infection. Furthermore, TNF-α is recognized by two receptors: TNF receptor 1 (TNFR1), ubiquitously expressed, and TNFR2, mainly expressed on immune cells. The interaction between TNF-α and TNFRs results in activation of at least four signaling pathways: proapoptotic and antiapoptotic pathways. The decision between TNF-α mediated-life and death is under the control of nuclear factor kappa B activity [36]. The balance between proapoptotic and antiapoptotic signaling can be shifted by activation of TNFR2 to promote cell adhesion, migration, proliferation, survival, and angiogenesis [37]. One of the HPV immune evasion strategies is that keratinocytes displaying HPV early proteins show extensive changes in the expression of proteins implicated in apoptosis regulation/execution, including TNFRs. Moreover, there was evidence that TNFR1 was downregulated and may be trapped in the cytoplasm of keratinocytes expressing HPV early proteins [38]. Virtually, it seems that the pro- and antiapoptotic TNF-α responses depend on its local concentration as well as its expression site [34]. For these reasons, further studies are encouraged to measure TNF-α before each treatment session and after immunotherapy to assess the alterations in TNF-α production in responders and non-responders. Moreover, the expression of TNF receptors and the activation of TNF-α signaling pathways in wart tissue should be evaluated to study the local immune responses.
The statistical correlation between C3c/C3 index and TNF-α serum level might assume a reciprocal interplay between the complement activation and TNF-α production. It is well known that cytokines activate the complement system and increase C3 production [39]. In previous work, TNF-α and IFN-γ synergistically amplified the C3 production by cultured human epidermal keratinocytes through different pathways, raising evidence that the outermost layer of the skin is not merely a barrier, but also a rich source of C3 [40]. On the other hand, TNF-α release in a complement-dependent manner has been proved by several studies of autoimmune and inflammatory disorders [41–43], giving stronger evidence that the C3 and TNF-α are interlinked.
In this work, the injected Candida antigen is composed of C. albicans cell wall or zymosan. The basic components of C. albicans cell wall are β-glucans, chitin, and mannoproteins. Zymosan is a protein-carbohydrate complexes derived from fungal cell walls [12]. Tada et al. reported a close similarity between fungal mannan and lipopolysaccharide (LPS) receptor and signaling pathway. Moreover, mannan derived from C. albicans induced TNF-α production in an LPS binding protein-, CD14-, and TLR4-dependent and TLR2-independent manner. TLR4 can strongly induce pro-inflammatory cytokines like TNF-α and type I IFNs which stimulate the subsequent production of Th1 cytokines like IFN-γ [44]. On the other hand, TLR2-mediated induction of pro-inflammatory cytokines has a weaker effect than TLR4 [45]. Moreover, the activation of TLR2 induces Th2 immune response due to failure of induction of interleukin-12 and Th1 type IFN-γ [46]. However, the observation that anti-TLR2 monoclonal antibody-mediated inhibition of TNF-α production induced by zymosan suggested that the activity of C. albicans cell walls is not solely due to mannan. The complex structure of glucan and mannan in C. albicans cell wall could be responsible for TNF-α production higher than that induced by mannan fraction alone [47]. Consequently, further in vitro assessment of TNF-α production together with TLRs expression and signaling, induced by a distinct cell wall component, could bring behind the scene roles of polysaccharide and protein fractions of C. albicans cell wall to the spotlight. Moreover, it is important to note that the relatively small number of the participants and the heterogenicity in their age and gender might need further research in a larger and more homogenous group of patients.
In conclusion, Candida antigen immunotherapy achieved a successful clearance of HPV-induced multiple, recurrent, and recalcitrant cutaneous warts in more than 50% of the patients. Nevertheless, the assessment of C3c and TNF-α after immunotherapy revealed an elevated serum levels in the non-responders to treatment compared to the responders.
Acknowledgments
We are grateful to the patients and their families for their cooperation with the trial.
Appendix
Author’s contribution
All the named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki of 1964 and in compliance with clinical practices and local regulatory requirements. This study was approved by the institutional review board (IRB) Zagazig University Faculty of Medicine. Prior to enrollment, written informed consent was obtained from competent adult patients and from the legal representatives of patients younger than 21 years of age.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nofal A, Marei A, Amer A, Amen H. Significance of interferon gamma in the prediction of successful therapy of common warts by intralesional injection of Candida antigen. Int J Dermatol. 2017;56(10):1003–1009. doi: 10.1111/ijd.13709. [DOI] [PubMed] [Google Scholar]
- 2.Aldahan AS, Mlacker S, Shah VV, Kamath P, Alsaidan M, Samarkandy S, Nouri K. Efficacy of intralesional immunotherapy for the treatment of warts: a review of the literature. Dermatol Ther. 2016;29(3):197–207. doi: 10.1111/dth.12352. [DOI] [PubMed] [Google Scholar]
- 3.Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5(11):2624–2642. doi: 10.3390/v5112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(2):S15–S21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida, and Trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141(5):589–594. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 6.Nasr M, Marie A, Boghdadi G, Elsaid R, Salah E (2019) Role of mannose binding lectin in response to candida antigen immunotherapy of warts. J Dermatol Treat:1–5. 10.1080/09546634.2019.1662365 [DOI] [PubMed]
- 7.La'Pelusa A, Rorex J, Weir NM, Travers JB. An aberrant reaction to Candida albicans antigen used for recalcitrant warts successfully treated with oral prednisone. JAAD Case Rep. 2018;4(3):242–244. doi: 10.1016/j.jdcr.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaheen AA, Mansour SA, Gerges MA, Marei AM, El-Hady HIA. Human papilloma virus genotypes and induced protein-10 as predictors for the clinical response to Candida antigen immunotherapy of warts. Egypt J Med Microbiol. 2019;28(2):129–135. [Google Scholar]
- 9.Brustin R, Toledano M, Geffen T, Goona R, Hochberg M, Kreisberg B, Murad S, Pitcovski J. Immune modulation and treatment of human papilloma virus-related warts with energetics of living systems acupuncture. Med Acupunct. 2017;29(3):145–154. doi: 10.1089/acu.2017.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boghdadi G, El Shahaway AA. Correlation between interferon-gamma production in vitro and clinical response to immunotherapy in recalcitrant genital warts. Egypt J Med Microbiol. 2017;38(5793):1–7. [Google Scholar]
- 11.Nofal A, Elkot R, Nofal E, Mazen M. Combination therapy versus monotherapy in the treatment of recalcitrant warts: a clinical and immunological study. J Cosmet Dermatol. 2019;18(5):1448–1455. doi: 10.1111/jocd.12848. [DOI] [PubMed] [Google Scholar]
- 12.Gow NA, Van De Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10(2):112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arana DM, Prieto D, Román E, Nombela C, Alonso-Monge R, Pla J. The role of the cell wall in fungal pathogenesis. Microb Biotechnol. 2009;2(3):308–320. doi: 10.1111/j.1751-7915.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabry HH, Hamed AM, Salem RM, Marei AM, El Sebaey RM. Peripheral blood toll-like receptor 4 correlates response to candida immunotherapy of warts. Dermatol Ther. 2018;31(5):e12691. doi: 10.1111/dth.12691. [DOI] [PubMed] [Google Scholar]
- 15.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6(1):67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 16.Kabut J, Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A. Levels of complement components iC3b, C3c, C4, and SC5b-9 in peritoneal fluid and serum of infertile women with endometriosis. Fertil Steril. 2007;88(5):1298–1303. doi: 10.1016/j.fertnstert.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 17.Frey A, Ertl G, Angermann C, Hofmann U, Störk S, Frantz S. Complement C3c as a biomarker in heart failure. Mediat Inflamm. 2013;2013:1–7. doi: 10.1155/2013/716902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellis V, De Seta F, Crovella S, Bossi F, Bulla R, Guaschino S, Radillo O, Garred P, Tedesco F. Mannose binding lectin and C3 act as recognition molecules for infectious agents in the vagina. Clin Exp Immunol. 2005;139(1):120–126. doi: 10.1111/j.1365-2249.2005.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammad NM, El Badawy NE, Ghramh HA. Al Kady LM (2018) mannose-binding lectin: a potential therapeutic candidate against Candida infection. Biomed Res Int. 2018;2018:1–8. doi: 10.1155/2018/2813737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitzeneder S, Seidel M, Förster-Waldl E, Heitger A. Mannan-binding lectin deficiency—good news, bad news, doesn't matter? Clin Immunol. 2012;143(1):22–38. doi: 10.1016/j.clim.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Nesargikar P, Spiller B, Chavez R. The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol. 2012;2(2):103–111. doi: 10.1556/EuJMI.2.2012.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumura N, Nomura M, Tada T, Kameko M, Moriyama T, Katsuyama T, Kanai M. Effects of sample storage on serum C3 assay by immunonephelometry. Clin Lab Sci. 1990;3(1):54–57. [Google Scholar]
- 23.Garred P, Mollnes T, Lea T. Quantification in enzyme-linked immunosorbent assay of a C3 neoepitope expressed on activated human complement factor C3. Scand J Immunol. 1988;27(3):329–335. doi: 10.1111/j.1365-3083.1988.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 24.Marei A, Nofal A, Alakad R, Abdel-Hady A (2019) Combined bivalent human papillomavirus vaccine and Candida antigen versus Candida antigen alone in the treatment of recalcitrant warts. J Cosmet Dermatol [DOI] [PubMed]
- 25.Nofal A, Salah E, Nofal E, Yosef A. Intralesional antigen immunotherapy for the treatment of warts: current concepts and future prospects. Am J Clin Dermatol. 2013;14(4):253–260. doi: 10.1007/s40257-013-0018-8. [DOI] [PubMed] [Google Scholar]
- 26.Signore RJ. Candida albicans intralesional injection immunotherapy of warts. Cutis. 2002;70(3):185–192. [PubMed] [Google Scholar]
- 27.Alikhan A, Griffin JR, Newman CC. Use of Candida antigen injections for the treatment of verruca vulgaris: a two-year Mayo Clinic experience. J Dermatol Treat. 2016;27(4):355–358. doi: 10.3109/09546634.2015.1106436. [DOI] [PubMed] [Google Scholar]
- 28.Majid I, Imran S. Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: a study. Indian J Dermatol. 2013;58(5):360–365. doi: 10.4103/0019-5154.117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean MM, Flower RL, Eisen DP, Minchinton RM, Hart DN, Vuckovic S. Mannose-binding lectin deficiency influences innate and antigen-presenting functions of blood myeloid dendritic cells. Immunology. 2011;132(2):296–305. doi: 10.1111/j.1365-2567.2010.03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammad NM, El Badawy NE, Nasr AM, Ghramh HA, Al 1Kady LM (2018) Mannose-binding lectin gene polymorphism and its association with susceptibility to recurrent vulvovaginal candidiasis. Biomed Res Int 2018, 2018, 1, 8 [DOI] [PMC free article] [PubMed]
- 31.Collins T (1999) Acute and chronic inflammation. Cotran RS, V. Kumar, Collins T. Robbins pathologic basis of disease, 6th ed Pennsylvania: WB Saunders
- 32.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa M, Coleman HN, Wang X, Daniels J, Sikes J, Nagarajan UM. IL-12 secretion by Langerhans cells stimulated with Candida skin test reagent is mediated by dectin-1 in some healthy individuals. Cytokine. 2014;65(2):202–209. doi: 10.1016/j.cyto.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:1–19. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse BW, Scott KF, Russell PJ. Paradoxical roles of tumour necrosis factor-alpha in prostate cancer biology. Prostate Cancer. 2012;2012:1–8. doi: 10.1155/2012/128965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fas SC, Baumann S, Zhu JY, Giaisi M, Treiber MK, Mahlknecht U, Krammer PH, Li-Weber M. Wogonin sensitizes resistant malignant cells to TNFα-and TRAIL-induced apoptosis. Blood. 2006;108(12):3700–3706. doi: 10.1182/blood-2006-03-011973. [DOI] [PubMed] [Google Scholar]
- 37.Urschel K, Cicha I. TNF-α in the cardiovascular system: from physiology to therapy. Internat J Interferon Cytokine Med Res. 2015;7:9–25. [Google Scholar]
- 38.Cabeça TK, de Mello AA, Andrette R, de Souza LV, Morale MG, Aguayo F, Termini L, Villa LL, Lepique AP, Boccardo E. HPV-mediated resistance to TNF and TRAIL is characterized by global alterations in apoptosis regulatory factors, dysregulation of death receptors, and induction of ROS/RNS. Int J Mol Sci. 2019;20(1):198. doi: 10.3390/ijms20010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon B, Kim HR, Kim H, Chung DK (2016) In vitro and in vivo downregulation of C3 by lipoteichoic acid isolated from lactobacillus plantarum K8 suppressed cytokine-mediated complement system activation. FEMS Microbiol Lett 363(14) [DOI] [PubMed]
- 40.Terui T, Ishii K, Ozawa M, Tabata N, Kato T, Tagami H. C3 production of cultured human epidermal keratinocytes in enhanced by IFNγ and TNFα through different pathways. J Invest Dermatol. 1997;108(1):62–67. doi: 10.1111/1523-1747.ep12285633. [DOI] [PubMed] [Google Scholar]
- 41.Nymo S, Niyonzima N, Espevik T, Mollnes TE. Cholesterol crystal-induced endothelial cell activation is complement-dependent and mediated by TNF. Immunobiology. 2014;219(10):786–792. doi: 10.1016/j.imbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Tan Y, Zhang J, Zou L, Deng G, Xu X, Wang F, Ma Z, Zhang J, Zhao T. C5aR, TNF-α, and FGL2 contribute to coagulation and complement activation in virus-induced fulminant hepatitis. J Hepatol. 2015;62(2):354–362. doi: 10.1016/j.jhep.2014.08.050. [DOI] [PubMed] [Google Scholar]
- 43.Di Muzio G, Perricone C, Ballanti E, Kroegler B, Greco E, Novelli L, Conigliaro P, Cipriani P, Giacomelli R, Perricone R. Complement system and rheumatoid arthritis: relationships with autoantibodies, serological, clinical features, and anti-TNF treatment. Int J Immunopathol Pharmacol. 2011;24(2):357–366. doi: 10.1177/039463201102400209. [DOI] [PubMed] [Google Scholar]
- 44.Toshchakov V, Jones BW, Perera P-Y, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ. TLR4, but not TLR2, mediates IFN-β–induced STAT1α/β-dependent gene expression in macrophages. Nat Immunol. 2002;3(4):392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 45.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69(3):1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276(40):37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 47.Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, Ohno N, Tamura H, Ki S, Akashi S. Saccharomyces cerevisiae-and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14-and toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46(7):503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.