Abstract
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by prokaryotes. Here, the molecular characterization of aureocin 4181, a bacteriocin produced by Staphylococcus aureus 4181, a strain involved in bovine mastitis, is presented. Aureocin 4181 gene cluster (aurRID1CBAT) was mined from scaffold 15 of the draft genome of its producer strain. Three (AurABC) out of the four structural peptides of aureocin 4181 are identical to those of aureocin A70, except for AurD1 of aureocin 4181, which showed a conservative substitution of Leu29 to Phe29 when compared to AurD of aureocin A70. According to molecular mass determination and peptide sequencing, combined with genome sequencing data, aureocin 4181 is an N-formylated variant of aureocin A70. The analysis of its antimicrobial spectrum was extended to include strains of the two major contagious pathogens involved in bovine mastitis, S. aureus and Streptococcus agalactiae. Aureocin 4181 exhibited a striking activity against S. aureus, inhibiting most strains tested. Besides having a broader spectrum of activity, aureocin 4181 exhibited a stronger bacteriolytic action against the target strains and proved to be from two- to fourfold more active than aureocin A70 against S. aureus. Aureocin 4181 has potential to become an alternative drug for prevention and control of mastitic staphylococci, a pathogen that imposes a huge economic burden to dairy industry worldwide. It also represents the third four-component bacteriocin described in the literature, the second in staphylococci.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00315-z) contains supplementary material, which is available to authorized users.
Keywords: Aureocin 4181, Antimicrobial peptide, Bacteriocin, Staphylococcus aureus, Bovine mastitis
Introduction
Ribosomally synthesized bacterial antimicrobial peptides (AMPs) are collectively known as bacteriocins [1]. Many bacteriocins have potential biotechnological applications in both medical and veterinary areas due to their inhibitory activity against different bacterial genera and species, including pathogens [2, 3].
The bacteriocins produced by Gram-positive bacteria have been extensively studied for several decades. Their structural complexity and biochemical properties vary considerably, allowing their separation into different classes [4]. Most bacteriocins described to date belong to either class I (peptides with post-translationally modified amino acids) or class II (peptides without post-translationally modified amino acids) [1].
Bacteriocin-producer strains are generally protected from the effects of their own products by cognate immunity systems, whose expression is associated with bacteriocin production [1]. Generally, strains that produce either identical or similar bacteriocins exhibit cross-immunity, since the immune systems may be specific to related bacteriocins [5].
Aureocin A70 is a class II bacteriocin produced by S. aureus A70, a strain isolated from pasteurized commercial milk [6]. To date, it is the only bacteriocin described composed of four cationic and hydrophobic peptides, which initiate with a non-formylated methionine (Met) residue. These peptides, named AurA, AurB, AurC, and AurD, have relative molecular masses (Mr) ranging from 2797 to 3086 Da [7]. Differently from most class II bacteriocins that are produced as inactive precursors with a leader peptide, the four structural peptides of aureocin A70 are translated in their mature bioactive form. Aureocin A70 exhibits antagonistic activity only toward few species of Gram-positive bacteria, including strains of Listeria monocytogenes, an important foodborne pathogen. Therefore, aureocin A70 is a potential food biopreservative, exhibiting an antilisterial activity in skimmed milk [8]. The biosynthetic gene cluster involved in aureocin A70 production and immunity is encoded by plasmid pRJ6 [6].
To date, aureocin A70 production has only been described in staphylococcal isolates from Brazil and Argentina [9–13]. In the most comprehensive study about aureocin A70 dissemination, Ceotto et al. [13] tested 257 S. aureus strains isolated from bovine mastitis cases in Brazil for AMP production. Among 46 AMP+ strains, 34 produced a bacteriocin related to aureocin A70, based on the presence of the bacteriocin structural genes detected in all 34 strains by both PCR and DNA/DNA hybridization analyses.
S. aureus 4181 is one of these 34 AMP producers mentioned previously. Nevertheless, this strain exhibited a broader spectrum of inhibition when compared to S. aureus A70 [13], which led to the assumption that the AMP produced by S. aureus 4181, named aureocin 4181, might be an aureocin A70 structural variant. In the present study, the investigation of the spectrum of activity of both aureocins was extended to include strains of the two major contagious pathogens involved in bovine mastitis, S. aureus and Streptococcus agalactiae. Due to its biotechnological potential as a new antimicrobial to control mastitic S. aureus, aureocin 4181 was purified, characterized, and compared to aureocin A70. The biosynthetic gene cluster of aureocin 4181was also analyzed.
Materials and methods
Bacterial strains and culture conditions
Bacterial strains used in the present study are listed in Table 1. Staphylococcal, streptococcal, and M. luteus strains were grown at 37 °C in Brain-Heart Infusion (BHI; Difco, Sparks, MD, USA), Trypticase-Soy Broth (TSB; Difco), or GM17 broth [M17 (Difco) supplemented with 0.5% (w/v) glucose]. BHI and TSB media were supplemented with 5% (v/v) equine serum (Laborclin, Curitiba, PR, Brazil) for growth of streptococci. TSB medium was used to grow strains for DNA isolation, whereas BHI and GM17 media were used in bacteriocin assays, as described subsequently. The strains were stored in TSB with 40% glycerol (v/v) at − 20 °C until used. The media were supplemented with agar at 0.7% (w/v) or 1.5% (w/v), when required.
Table 1.
Strains used in this study
| Strain | Relevant characteristics, source, and plasmids | Reference |
|---|---|---|
| Staphylococcus aureus | ||
| A70 | Aureocin A70 producer; pRJ6 (7.9 kb) | [6] |
| A70 Bac− | Strain cured of pRJ6 and, therefore, of aureocin A70 production | [6] |
| 4181 | Aureocin 4181 producer; pRJ80 (8.3 kb) | [13] |
| 4181 Bac− | Strain cured of pRJ80 and, therefore, of aureocin 4181 production | This work |
| 30 Strains isolated from 15 different herds | Involved in bovine mastitis | This work |
| Micrococcus luteus ATCC 4698 | Indicator of bacteriocin production | [14] |
| Streptococcus spp. | ||
| 30 strains isolated from 15 different herds | Involved in bovine mastitis | This work |
Bac, bacteriocin
Sign –, absence of the phenotype
The strains of S. aureus, Streptococcus equinus, Streptococcus uberis, and S. agalactiae involved in bovine mastitis (Table S1) were isolated from 15 different herds located in the southeast region of Brazil, between 2002 and 2014. They were identified to the species level based on Gram staining and standard biochemical tests, as previously described [15], and on matrix-assisted laser desorption ionization/time-of-flight mass spectrometry (MALDI/TOF-MS) analysis performed according to Farias et al. [16].
Bacteriocin bioassays
The antimicrobial activity against the target strains was investigated on solid medium by the agar-spot test, also known as deferred-antagonism assay, as described by Giambiagi-de Marval et al. [6]. Briefly, 7.0 log10 CFU of the producing strain grown in BHI were used to prepare the bacterial spots on the surface of a BHI agar plate (containing 20 mL of medium). After the growth of the producing cells followed by their killing by chloroform vapors, the medium was overlaid with 6.0 log10 CFU of the target strain in 3 mL of BHI soft agar. After overnight incubation at appropriate conditions, to minimize differences observed in the size of the inhibition zones due to normal fluctuations of the spot sizes, the antimicrobial activity was expressed in “real inhibition zones,” which represent the diameters of the spots formed by growth of the producer strain subtracted from the diameters of the clear inhibition zones, in millimeter.
Quantification of antimicrobial activity in bacteriocin units per mL (BU/mL) was determined by the microtiter plate assay performed as previously described [17], employing 100 μL of twofold serial dilutions of the bacteriocin prepared in GM17 (for aureocin 4181) or BHI broth (for aureocin A70) and 100 μL of an overnight culture of M. luteus ATCC 4698 diluted in the same broth to OD600 of 0.05. After incubation at 37 °C for 24 h, the OD600 of the bacterial growth observed in each well was measured and the BU/mL were defined as the reciprocal of the highest bacteriocin dilution required to obtain at least a 50% growth inhibition of the indicator culture when compared to the control grown in the absence of the bacteriocin, multiplied by 10.
The choice of M. luteus ATCC 4698 as the indicator strain used in antimicrobial activity assays was due to the fact that this strain proved to be the most sensitive target strain to both aureocins among all strains tested in the present investigation, exhibiting the largest real inhibition zones (Table S1).
The antimicrobial activity of aureocins 4181 and A70 was also tested in broth by microtiter plate assays as described previously, using eight bacterial strains involved in bovine mastitis randomly selected as target strains. All experiments were carried out at least in duplicate.
Curing of aureocin 4181 production by S. aureus 4181
Curing of plasmid pRJ80, which encodes aureocin 4181 production (see subsequent texts), was performed after tagging plasmid pRJ80 with Tn917-lac as described by Nascimento et al. [18]. Briefly, pTV32Ts, the thermosensitive delivery vector of Tn917-lac [carrying the genetic determinants for chloramphenicol resistance (CmR), the vector marker, and erythromycin resistance (EmR), the transposon marker], was transferred to S. aureus 4181 by transduction. Selection of CmR EmR transductants was performed at 32 °C. After growing the cells containing both pRJ80 and pTV32Ts for about 30 generations at 42 °C in the absence of selection, colonies that became CmS EmR were selected and tested for the presence of pRJ80::Tn917-lac and the ability to still produce aureocin 4181. One of such colony was then subjected to successive rounds of growth at 43 °C, followed by a screening of colonies that had lost the plasmid and, consequently, the ability to produce the bacteriocin. The antimicrobial activity exhibited by S. aureus 4181Bac− was investigated by tests performed both on solid medium and in broth as described previously, using M. luteus ATCC 4698 as the indicator strain.
Cross immunity between aureocins A70 and 4181
To test the cross immunity between both aureocins, the deferred-antagonism assay was performed employing S. aureus A70 and 4181 as bacteriocin producers and strains S. aureus A70, A70 Bac− (a derivative of strain A70 cured of pRJ6 and, therefore, of aureocin A70 production), 4181, and 4181 Bac− (a derivative of strain 4181 cured of pRJ80 and, therefore, of aureocin 4181 production) as targets.
Determination of the conditions for aureocin 4181 production
An 18-h broth culture of the bacteriocin-producer strain S. aureus 4181 was diluted 100 fold in 50 mL of BHI and GM17 broths, and incubated at 37 °C under shaking (120 rpm) for 24 h. At appropriate time intervals, samples were aseptically removed for determination of the antimicrobial activity (BU/mL).
Activity kinetics of aureocins 4181 and A70
The activity kinetics of aureocins 4181 and A70 against M. luteus ATCC 4698 was determined by the microtiter plate assay, as described previously [17], using partially purified aureocin preparations obtained by ammonium sulfate precipitation followed by cation-exchange chromatography (performed as described in the subsequent texts). For this analysis, 1024 BU of a given aureocin were incubated with 9.0 log10 CFU of the target strain in a 400 μL final volume. Two controls were included in these experiments, which are as follows: wells containing only 400 μL of GM17 or BHI broth and wells in which the target cells were incubated with the same volume of NaCl 0.5 M replacing the aureocin preparation (growth control). The culture OD600 was measured in intervals of 15 min, during incubation at 37 °C for 4 h in a microtiter plate reader (Bioscreen C; Oy Growth Curves AB Ltd., Helsinki, Finland). This experiment was performed in duplicate, using three wells for each condition tested. The growth curves generated were prepared using the Excel software, version 2010 (Microsoft Corporation, Redmont, WA, USA).
Bacteriocin purification
Aureocin 4181 was purified from a culture of S. aureus 4181 cultivated in 1 L of GM17, at 37 °C for 12 h, under shaking (180 rpm), as described by Ceotto et al. [17]. Briefly, after centrifugation, the culture supernatant was subjected to the following steps: (a) precipitation of proteins and peptides by ammonium sulfate (w/v; Merck Millipore, Darmstadt, Germany) at 65% saturation, carried out for 4 h at 4 °C; (b) cation-exchange chromatography (SP Sepharose Fast Flow; Amersham Biosciences do Brasil, São Paulo, SP) with bacteriocin elution in 20 mL of 0.5 M NaCl; and (c) two consecutive runs of reverse-phase high-performance liquid chromatography (HPLC) on an Äkta Purifier System (GE Healthcare; Chicago, IL, USA), employing a Resource RPC 1-mL column and a Sephasil Peptide C8 5-μm ST 4.6/250 column, respectively. A water-isopropanol gradient from 0 to 100% (v/v), containing 0.1% trifluoroacetic acid (TFA; v/v) was used to elute the peptides, whose antimicrobial activity was quantified in BU/mL by microtiter plate assays.
Partial purification of aureocins 4181 and A70 was performed by ammonium sulfate precipitation followed by cation exchange chromatography, as described previously. However, the culture of S. aureus A70 was grown in 1 L of BHI.
Bioautographic assay with partially purified preparations of both aureocins
Partially purified preparations of aureocins 4181 and A70 were also analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by a bioautographic assay, both performed according to the procedures described by Duarte et al. [19] with minor modifications. The polyacrylamide gel concentration was increased to 15% (w/v).
Molecular mass determination and amino acid sequencing
The Mr of the aureocin 4181 peptides recovered after two consecutive runs of HLPC was determined by MALDI/TOF-MS, employing the MALDI/TOF mass spectrometer Voyager-RP DE (Applied Biosystems, Foster City, CA, USA), and the amino acid sequence of each peptide was determined by MALDI/TOF/TOF mass spectrometry, as described by Brede et al. [20] and Peters et al. [21], respectively.
Genome sequencing of S. aureus 4181, mining, and in silico analyses of the gene cluster involved in aureocin 4181 production
After growing S. aureus 4181 for 18 h at 37 °C in 5 mL of TSB broth, its genomic DNA was extracted using the GenElute™ Bacterial Genomic DNA Kit (Sigma-Aldrich Brasil Ltda, São Paulo, SP, Brazil) according to the manufacturer’s instructions. The Nextera® XT DNA Sample Preparation Kit (Illumina Brasil Produtos de Biotecnologia Ltda, São Paulo, SP, Brazil) was used to prepare the sequencing library following the manufacturer’s recommendations. Whole-genome sequencing and de novo assembly of reads were performed using the Illumina MiSeq system and the software A5-miseq pipeline (https://sourceforge.net/projects/ngopt/), respectively. The Rapid Annotation using Subsystems Technology (http://rast.nmpdr.org) was employed for genome annotation. The single scaffold corresponding to a plasmid replicon (scaffold 15) was detected by the presence of a gene encoding a plasmid Rep protein. The bacteriocin gene cluster was then mined from this scaffold by visual inspection. The presence of additional gene clusters encoding AMP on the bacterial genome was investigated using the online platforms BACTIBASE (http://bactibase.hammamilab.org), BAGEL4 (http://bagel.molgenrug.nl), and antiSMASH bacterial version (https://antismash.secondarymetabolites.org).
Similarities between proteins encoded by the gene clusters of aureocins 4181 and A70 were investigated by using the BLASTp tool (http://blast.ncbi.nlm.nih.gov/). The alignments between the genes and proteins involved in aureocins 4181 and A70 production were performed using the Clustal Omega tool (https://www.ebi.ac.uk/Tools/msa/clustalo/). The detection of putative σA promoters and ribosome binding sites (RBS) upstream of each gene from aureocin 4181 gene cluster was performed by visual inspection. The presence of putative transcription terminator sites downstream of each gene was investigated by visual inspection as well. Prediction of pI and Mr of each structural peptide of aureocin 4181 was done by the ProtParam tool (http://web.expasy.org/compute pi/).
GenBank accession number
The GenBank accession number for the whole sequence of plasmid pRJ80 is MK796167.
Results
Antimicrobial activity of aureocins 4181 and A70 on solid medium
The investigation of the antimicrobial spectra exhibited by the producing strains of aureocins 4181 and A70 was extended to include 30 S. aureus and 30 Streptococcus spp. strains involved in bovine mastitis in Brazil. Due to the large number of strains to be tested, the assays with all strains were only performed on solid medium by the deferred-antagonism assay. The producing strains of aureocins 4181 and A70 exhibited different spectra of activity toward them (Table S1). The producer of aureocin 4181 inhibited 24 (80%) staphylococcal strains and only five (16.7%) streptococcal strains. All inhibition zones observed for streptococci were turbid, contrasting to those of S. aureus that were clear. The producer of aureocin A70 inhibited only 11 (36.7%) staphylococcal strains, whose inhibition zones were also turbid. No streptococcal strain was inhibited by S. aureus A70 on solid medium. The formation of turbid inhibition zones is suggestive of the presence of a heterogeneous population containing aureocin resistant cells.
Antimicrobial activity of both aureocins against staphylococci and streptococci in broth
The inhibitory activity of partially purified preparations of aureocins 4181 and A70 against eight representative strains randomly chosen among both mastitic staphylococci and streptococci was compared. Both aureocin preparations exhibited the same titer (2560 BU/mL) against the control strain M. luteus ATCC 4698, indicating that similar bacteriocin units of each aureocin were present in each preparation.
When these partially purified preparations were tested against the eight strains (Table 2), all of them proved to be from two to fourfold more sensitive to aureocin 4181 than to aureocin A70. Moreover, S. aureus proved to be more sensitive to aureocin 4181 than streptococci requiring lower BU to reach at least a 50% inhibition of the bacterial growth in the presence of the bacteriocin. These results suggest that aureocin 4181 is more active than aureocin A70, especially against S. aureus.
Table 2.
Activity of partially purified preparations of aureocins 4181 and A70 against mastitic pathogens in broth
| Mastitic pathogens | BU/mLa required for inhibition by | Effectiveness | |
|---|---|---|---|
| Aureocin A70 | Aureocin 4181 | ||
| S. aureus 4801 | 80 | 20 | 4 |
| S. aureus 4802 | 80 | 20 | 4 |
| S. aureus 4807 | 80 | 20 | 4 |
| S. aureus 4819 | 160 | 80 | 2 |
| S. agalactiae 4810 | 640 | 320 | 2 |
| S. agalactiae 4824 | > 640 | 640 | ≥ 2 |
| S. agalactiae 4828 | 640 | 320 | 2 |
| S. agalactiae 4923 | > 640 | 640 | ≥ 2 |
aMeans of two independent experiments which gave identical results
The numbers represent the minimal BU/mL required to inhibit at least 50% of the bacterial growth, when compared with the control with no aureocin added
Activity kinetics of aureocins 4181 and A70 against micrococci and staphylococci
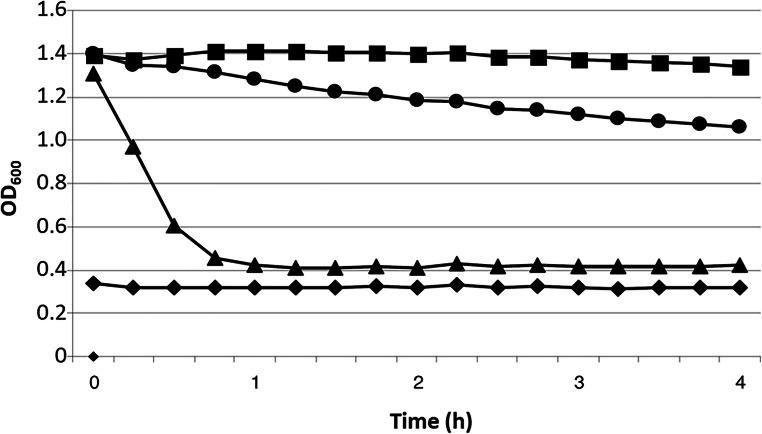
The activity kinetics of partially purified preparations of aureocins 4181 and A70 (both at 1024 BU) against M. luteus ATCC 4698 were compared and they are shown in Fig. 1. In the presence of aureocin 4181, the bacterial culture OD600 dropped drastically from 1.3 to 0.4 in the first hour of incubation at 37 °C, suggesting the occurrence of a strong cell lysis. On the contrary, the culture OD600 decayed subtly from 1.4 to ~ 1.1 along the time in the presence of aureocin A70, reaching the smallest value only by the end of the experiment (4 h), suggesting that this target strain was only partially lysed by this bacteriocin.
Fig. 1.
Activity kinetics of partially purified preparations of aureocins 4181 and A70 against M. luteus ATCC 4698. (■) M. luteus ATCC 4698 grown in the absence of bacteriocin (control); (▲) M. luteus ATCC 4698 grown in the presence of aureocin 4181 (1024 BU); (●) M. luteus ATCC 4698 grown in the presence of aureocin A70 (1024 BU); and (♦) GM17 broth (blank). The numbers represent the means of two independent experiments in which three wells of a microtiter plate were used for each condition tested
A bacteriolytic activity against two representative strains of S. aureus involved in bovine mastitis was also observed, even when only 256 BU of aureocin 4181were tested (Fig. S1). The lysis caused by aureocin A70 was again clearly weaker.
Cross immunity between aureocins A70 and 4181
The aureocins produced by S. aureus A70 and 4181 did not inhibit the producing strains S. aureus A70 and 4181, but displayed antagonistic activity toward S. aureus A70 Bac− and 4181 Bac−, strains cured of the plasmids carrying either the aureocin A70 or aureocin 4181 gene cluster, respectively (Table S2), and, therefore, of immunity. Such findings confirmed the presence of cross immunity and, therefore, of relatedness between both aureocins.
Analyses of aureocin 4181 gene cluster
In a previous study, a strong homology was shown by DNA-DNA hybridization experiments between an amplicon carrying the aureocin A70 structural genes, used as probe, and the single plasmid present in S. aureus 4181 [13]. This result suggested that the bacteriocin produced by S. aureus 4181 is related to aureocin A70 and encoded by a plasmid DNA. Therefore, in this study, the genome of this strain was sequenced in order to mine the gene cluster involved in aureocin 4181 production. The draft genome sequencing data will be published elsewhere. Strain 4181 proved to be a methicillin-sensitive S. aureus strain (MSSA) that belongs to the MLST type ST-126, which is prevalent among MSSA isolates associated with bovine mastitis in Brazil [22]. Scaffold 15 (8289 bp) proved to correspond to the single plasmid DNA, named pRJ80, found in S. aureus 4181, as it contains a rep gene that codes for a Rep protein with a high similarity (from 74 to 97%) to proteins involved in replication initiation of bacteriocinogenic plasmids of S. aureus (data not shown). Moreover, genes involved in plasmid mobilization and an oriT region were also found in scaffold 15, as well as the gene cluster involved in aureocin 4181 production. All these findings are consistent with scaffold 15 being the plasmid DNA associated to aureocin 4181 production.
pRJ80 involvement in aureocin 4181 production was conclusively shown by curing pRJ80::Tn917-lac (a derivative of pRJ80 carrying a transposon insertion in a region which neither affects bacteriocin production nor immunity) during growth of its host strain at high temperature. The cured derivative gave rise to very small inhibition zones against M. luteus ATCC 4698 on solid medium (Fig. S2d) and no detectable antimicrobial substance was found in its culture supernatant subjected to ammonium sulfate precipitation and a tenfold concentration (Fig. S3b).
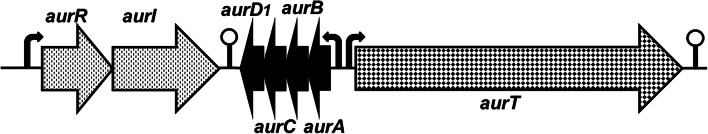
DNA sequence analysis revealed the presence in pRJ80 of seven genes related to aureocin 4181 production (aurRID1CBAT), arranged in three transcriptional units (Fig. 2) preceded by a putative σA-dependent promoter (Fig. 2 and Table 3). Candidate RBS at appropriated distances (5 to 11 bp) were also found upstream of all genes (Table 3). So transcription and translation of the genes are expected to occur, which is in accordance with the presence of the bacteriocin in the culture supernatant of its producing strain, as shown below. Inverted repeats resembling putative rho-independent stem-and-loop transcription terminators were detected downstream of aurT (with 42 bp; ΔG = − 29.7 kcal/mol) and in the intergenic region between aurI and aurD1 (with 32 bp; ΔG = − 13.7 kcal/mol).
Fig. 2.
Gene cluster involved in production of aureocin 4181. The arrows indicate putative promoters, and the lollipop symbols represent putative transcription terminators
Table 3.
Genes found in the aureocin 4181 gene cluster
| Gene (bp) | Putative promoter | Putative RBS | Gene product | Product size (aa) | Similarity to aureocin A70 proteins or peptides | Identity/similarity (%) | Putative function |
|---|---|---|---|---|---|---|---|
| aurT (1716) | + | + | AurT | 571 | AurT | 100 | ABC transporter |
| aurA (96) | + | + | AurA | 31 | AurA | 100 | Peptide A from aureocin 4181 |
| aurB (93) | – | + | AurB | 30 | AurB | 100 | Peptide B from aureocin 4181 |
| aurC (96) | – | + | AurC | 31 | AurC | 100 | Peptide C from aureocin 4181 |
| aurD1 (96) | – | + | AurD1 | 31 | AurD | 97/100 | Peptide D1 from aureocin 4181 |
| aurR (210) | + | + | AurR | 69 | AurR | 100 | Transcription regulator |
| aurI (417) | – | + | AurI | 138 | AurI | 100 | Immunity protein |
aa, amino acid
bp, base pairs
RBS, ribosome binding site
+, presence; −, absence
Six genes involved in aureocin 4181 production were identical to the corresponding genes of the aureocin A70 gene cluster (Table 3). The aureocin 4181 structural genes, aurABCD1, encode four related peptides that are small (30–31 amino acid residues), cationic and hydrophobic. Except for aurD1, the remaining structural genes of aureocin 4181 share 100% identity to the structural genes of aureocin A70 (Fig. S4). When compared to aurD, aurD1 shows a transversion A ➔ T at position 87, leading to a conservative substitution in the amino acid sequence of AurD1, Leu29 to Phe29 (Table 4). Based on the DNA sequence of their genes, the Mr of the aureocin 4181 peptides was predicted (Table 4).
Table 4.
Components of aureocins 4181 and A70
| Bacteriocin (reference) | Peptide | Relative molecular mass (Da) | Amino acid sequence (number of aa) | |
|---|---|---|---|---|
| Predicted from gene sequencea | Detected by MALDI/TOF-MS | |||
| Aureocin 4181 (this work) | AurA | 2924.5 | 2951.5 ± 1.5 | fMGKLAIKAGKIIGGGIASALGWAAGEKAVGK (31)b |
| AurB | 2797.3 | 2824.4 ± 1.5 | fMGAVAKFLGKAALGGAAGGATYAGLKKIFG (30)b | |
| AurC | 2954.6 | 2983.6 ± 1.5 | fMGALIKTGAKIIGSGAAGGLGTYIGHKILGK (31)b | |
| AurD1 | 3120.8 | 3147.7 ± 1.5 | fMGAVIKVGAKVIGWGAASGAGLYGLEKIFKK (31)b | |
| Aureocin A70 [7] | AurA | 2924.5 | 2927.3 ± 1.5 | MGKLAIKAGKIIGGGIASALGWAAGEKAVGK (31)c |
| AurB | 2797.3 | 2795.7 ± 1.5 | MGAVAKFLGKAALGGAAGGATYAGLKKIFG (30)c | |
| AurC | 2954.6 | 2954.8 ± 1.5 | MGALIKTGAKIIGSGAAGGLGTYIGHKILGK (31)c | |
| AurD | 3086.8 | 3087.7 ± 1.5 | MGAVIKVGAKVIGWGAASGAGLYGLEKILKK (31)c | |
aa, amino acid
Da, daltons
f, formylation of the methionine residue
aPredicted Mr calculated by the ProtParam tool
bThe amino acid sequence determined for the aureocin 4181 peptides by MALDI/TOF/TOF mass spectrometry is shown in bold
cThe underlined amino acid sequences in the aureocin A70 peptides are also found in the structural peptides of aureocin 4181
Determination of the conditions for aureocin 4181 production
Aureocin 4181 production was investigated in two different broths, BHI and GM17 (Fig. S5). Aureocin 4181 production was first detected after 4 h of incubation at 37 °C in both media. However, the bacteriocin activity obtained in BHI (40 BU/mL) was half of that achieved in GM17 (80 BU/mL). The highest production of aureocin 4181 (640 BU/mL) was observed after 8 h of bacterial growth in GM17 broth and remained unchanged until 12 h of growth. On the other hand, in BHI medium, the maximum bacteriocin activity (160 BU/mL) was observed from 6 to 12 h of bacterial growth. The aureocin 4181 activity declined to 80 BU/mL at 24 h of bacterial growth in both culture media. The maximum aureocin 4181 production (640 BU/mL) in GM17 was confirmed during growth of its producing strain for bacteriocin purification (Table S3).
Bioautographic assay with partially purified preparations of both aureocins
To confirm the presence of AMPs in the culture supernatant of S. aureus 4181 with a molecular mass similar to those of the aureocin A70 peptides, partially purified preparations of both aureocins were subjected to a bioautographic assay after SDS-PAGE. These preparations were obtained using the best conditions previously determined for maximum aureocin production. Figure S6 shows the results of this analysis. A single inhibition zone (Fig. S6b) was observed against M. luteus ATCC 4698, in a region of the gel corresponding to peptides with Mr (around 3.0 kDa) expected for aureocins 4181 or A70.
Purification and molecular characterization of aureocin 4181
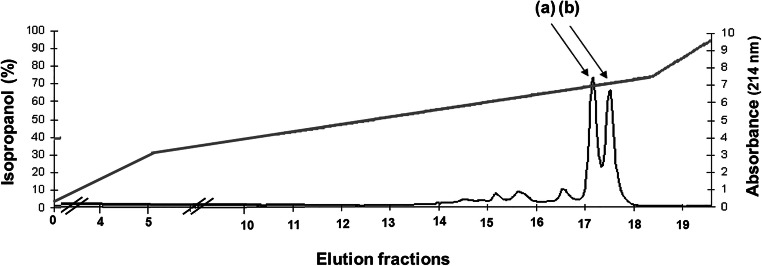
This bacteriocin was purified from the supernatant of a 1-L culture of S. aureus 4181 cultivated in GM17 for 12 h. After ammonium sulfate precipitation, followed by cation exchange chromatography and two runs of reverse-phase HPLC, aureocin 4181 was eluted as two peaks at approximately 65% isopropanol (Fig. 3), and the yield of antimicrobial activity in each purification step is presented in Table S3. Fractions 17 and 18 from the second run of the reverse-phase HPLC showed the highest antimicrobial activity (16,000 BU/mL in each fraction), measured by microtiter plate assays, and were used for mass spectrometry analysis and amino acid sequencing. Both fractions contained a mixture of compounds (Fig. 4). The Mr determined for the four components of aureocin 4181 were as follows: 2951.5 ± 1.5 Da (for AurA), 2824.4 ± 1.5 Da (for AurB), 2983.6 ± 1.5 Da (for AurC), and 3147.7 ± 1.5 Da (for AurD1). However, the theoretical monoisotopic masses of the gene-derived peptides (Table 4) were ~ 28 Da less than the masses determined by the mass spectrometry analysis of the peptides obtained after HLPC. When these peptides were submitted to amino acid sequencing by MALDI/TOF/TOF mass spectrometry, only internal sequences, corresponding to amino acid sequences predicted from each structural gene, were determined (Table 4).
Fig. 3.
Elution of aureocin 4181 in a gradient of water and isopropanol (0 to 100%) containing 0.1% trifluoroacetic acid, during purification in the HPLC column Sephasil Peptide C8 (2nd run), monitored at 214 nm. The arrows indicate fractions 17 (a) and 18 (b) that showed antimicrobial activity and that were used to determine the Mr of the peptides. The gray line represents the isopropanol concentration
Fig. 4.
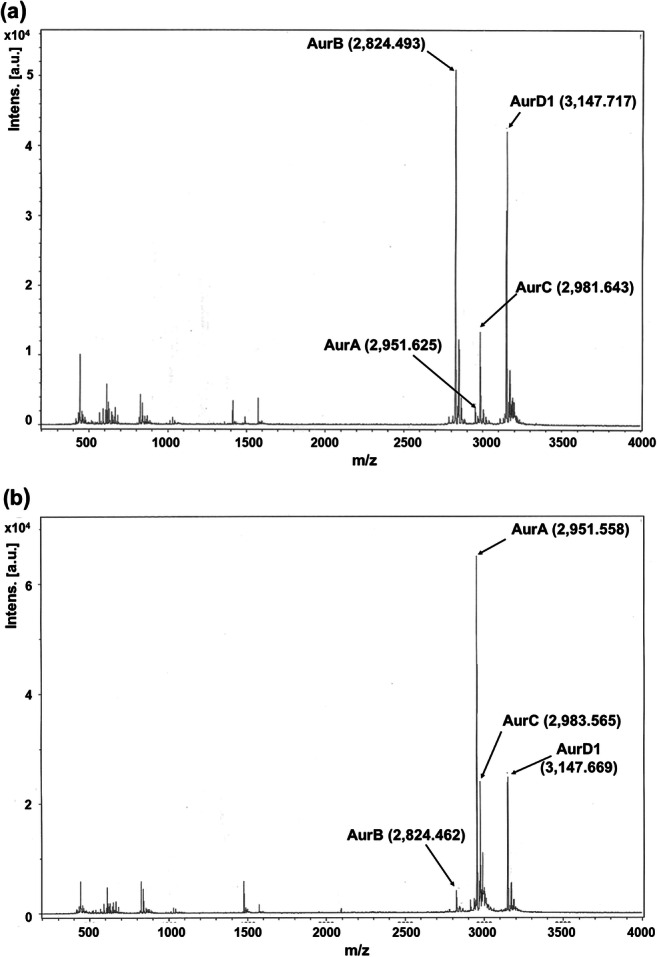
MALDI/TOF-MS performed with fractions 17 (a) and 18 (b) from the second run of the reverse-phase HPLC eluted in 65% isopropanol. The Mr of each peptide is given in Da
Discussion
After genome sequencing and several in silico analyses, a single gene cluster involved in AMP production was found in the genome of S. aureus 4181. Therefore, this strain produces a single AMP, aureocin 4181, which is responsible for its antagonistic activity toward Gram-positive bacteria. Curing of pRJ80, the single plasmid found in S. aureus 4181 and associated to aureocin 4181 production, corroborated this finding as the cured derivative lost the ability to secrete any detectable antimicrobial substance in its culture supernatant.
The strains S. aureus A70 and 4181 were shown to produce related bacteriocins, although both strains were not genetically related. Pulsed-field gel electrophoresis analysis of their genomic DNA digested with SmaI showed that both strains belong to quite distinct pulsotypes [23]. Although closely related to aureocin A70, aureocin 4181 displayed a broader spectrum of activity than aureocin A70 toward both S. aureus and Streptococcus spp. isolates from bovine mastitis in tests performed on solid medium. Aureocin 4181 was able to inhibit 80% and 16.7% of the staphylococcal and streptococcal strains tested, respectively. On the other hand, aureocin A70 could only inhibit 36.7% of the staphylococcal strains, with the presence of resistant cells in the inhibition zones, and no Streptococcus spp. strain was inhibited by this bacteriocin.
In a previous study, Coelho et al. [15] tested the inhibitory activity of aureocin A70 toward a different group of staphylococcal and streptococcal strains involved in bovine mastitis, finding out that this bacteriocin possesses a poor activity against these microorganisms. Aureocin A70 was able to inhibit 23.6% (39 out of 165) of the S. aureus strains and only 1.4% (1 out of 74) of the S. agalactiae strains tested. The data obtained in this study corroborate these findings.
The better activity of aureocin 4181, when compared to aureocin A70, was also confirmed by microtiter plate assays using partially purified preparations of each bacteriocin. When equal BU of both aureocins were individually tested against eight mastitic strains, aureocin 4181 was shown to be from two to fourfold more active than aureocin A70. S. aureus proved to be more sensitive to aureocin 4181 than S. agalactiae. However, M. luteus ATCC 4698 was the most sensitive target strain to both aureocins. Furthermore, the lytic activity of aureocin 4181 over the latter strain was stronger than that displayed by aureocin A70. Aureocin 4181 also proved to be more bacteriolytic against strains of S. aureus involved in bovine mastitis than aureocin A70.
Taken together, these results show that aureocin 4181 possesses a broader spectrum of inhibitory activity and a stronger bacteriolytic activity when compared to aureocin A70. Although the antagonistic activity of aureocin 4181 varied among the target strains, it is well known that the susceptibility of bacterial strains to a given bacteriocin is not uniform, varying depending on the bacterial genus and species, and even among different strains of a given species [24].
These data also suggest the potential application of aureocin 4181 in the veterinary area. S. aureus is one of the major pathogens involved in bovine mastitis, not only in Brazil [15] but also in many countries [25]. Although antibiotic therapy in lactating and dry cows is the main strategy used to prevent and treat bovine mastitis currently [26], emergence of drug resistance among mastitic pathogens makes urgent the search for new therapeutic options to overcome this resistance [26]. In this scenario, bacteriocins, such as aureocin 4181, emerge as potential drugs to be used to prevent and treat bovine mastitis, reducing the huge economic losses to the dairy industry caused by S. aureus infections worldwide.
GM17 broth favored aureocin 4181 production. Similarly to aureocin A70 [27], the maximal production of aureocin 4181 (640 BU/mL) was observed toward the end of the log phase of growth, after 8 h of incubation in this broth. After 12 h of growth, aureocin 4181 recovery decreased drastically, suggesting the occurrence of proteolytic degradation of this bacteriocin during the stationary phase of growth. In a previous study, the AMP produced by S. aureus 4181 was shown to be sensitive to proteolytic enzymes [13], similarly to aureocin A70 [6].
Both aureocins A70 and 4181 are encoded by plasmid DNAs of 7904 bp (pRJ6) [28] and 8289 bp (pRJ80), respectively. Both gene clusters comprise seven genes, arranged in the same transcriptional units, composed of aurT, aurABCD/D1, and aurRI. Most genes involved in production of aureocins 4181 and A70 are identical, except for aurD1 and, therefore, they encode identical products playing the same roles. aurT encodes a 571-aa single-component ABC transporter involved in aureocin A70 export [7]. aurR codes for an intrinsic transcription regulator, which belongs to HTH-XRE family, being involved in reduction of aureocin A70 production on solid medium, but not in broth [29]. AurI is a membrane protein involved in self-protection against aureocin A70, being co-expressed with AurR in pRJ6 [30]. The full immunity phenotype to aureocin A70 seems to depend on translational coupling involving aurR and aurI [30]. This phenomenon is expected to occur in relation to aureocin 4181 as well.
Bacteriocin regulation is generally a well-elaborated mechanism that controls the bacteriocin locus expression, activating or suppressing the peptide production. Quorum sensing is the most common regulatory mechanism of bacteriocin production [1]. However, it has been shown that aureocin A70 production is regulated by a much more complex system that involves, at least, the participation of three factors: the intrinsic transcription regulator AurR, the alternative σB factor and the Φ11 regulator cI [29]. Due to the high identity between the gene clusters of aureocins A70 and 4181, the production of the latter bacteriocin may be subjected to the same complex regulation.
The operon aurABCD1 found in pRJ80 encodes four related cationic peptides, the structural components of aureocin 4181. The aurABC genes of pRJ6 and pRJ80 are identical and so are their products. aurD1 shows a single transversion, leading to replacement of Leu29 by Phe29 in AurD1. Based on the sequence of the structural genes, the Mr of the aureocin 4181 peptides were predicted as follows: 2924.5 Da for AurA, 2797.3 Da for AurB, 2954.6 Da for AurC, and 3120.8 Da for AurD1. To confirm them, aureocin 4181 was purified using the best conditions for its production investigated in the present study and the Mr of each peptide was determined by MALDI/TOF-MS.
All four components of aureocin 4181 were shown to be produced and exported as mature peptides, like those of aureocin A70 [7]. No post-translationally modified amino acid was found in the peptides. However, a mass increment of ~ 28 Da was observed for all of them, consistent with the presence of an N-formyl group in the first Met residue of each peptide of aureocin 4181. Therefore, the formylation of the four-component peptides of aureocin 4181 was inferred from these data, as also done in relation to garvicin KS [31].
Only internal sequences of the structural peptides of aureocin 4181 were determined by MALDI/TOF/TOF mass spectrometry and all proved to be identical to internal amino acid sequences of the aureocin A70 peptides. The amino acid substitution found in AurD1 could not be confirmed by amino acid sequence as the C-terminal of the peptide was not sequenced. However, the relative Mr determined for AurD1 (3147.7 ± 1.5 Da) by MALDI/TOF-MS agrees with the theoretical mass of the N-formylated form of the aurD1 gene-derived peptide (3148.8 Da).
The individual inhibitory activity of each chemically synthesized peptide of aureocin A70 was tested on solid medium against different bacterial genera [14]. Except for AurD, which did not possess any antimicrobial activity by itself, AurA, AurB, and AurC exhibited antibacterial activity against M. luteus when tested alone (60 μg). When Listeria innocua and S. aureus strains were used as targets, inhibition was only observed when all four peptides were combined together (25 μg of each peptide). Based on the high identity found between the peptides of aureocins A70 and 4181, all four peptides of aureocin 4181 must be required for S. aureus inhibition as well. However, this assumption awaits further confirmation. It can also be assumed that the conservative amino acid substitution carried by AurD1 does not interfere in the antimicrobial activity of aureocin 4181. Nisin Z, a natural variant of nisin A, carries a single non-conservative amino acid substitution, His27 to Asp27, which does not affect its inhibitory activity [32].
Most bacteriocins are synthesized as inactive precursor peptides, whose leader peptides are removed after translation to generate the mature and bioactive bacteriocins [1]. Few bacteriocins, like both aureocins discussed in this manuscript, are leaderless, being already translated as mature peptides. All bacteriocins with more than two components and some two-component bacteriocins as well belong to this latter group and, therefore, are generally formylated [7, 31, 33]. Aureocin A70 and some two-component enterocins are exceptions [7, 33].
Formylated bacteriocins have been shown to possess a broad spectrum of antimicrobial activity, inhibiting strains of distantly related bacterial genera, as observed for aureocin A53 [34], Bac Sp222 [35] and garvicin KS [31]. Therefore, aureocin 4181 behaves as expected, as it has shown antagonistic activity toward strains of M. luteus, S. agalactiae, S. aureus, and several species of coagulase-negative staphylococci that are not inhibited by aureocin A70 (unpublished data). Once aureocins 4181 and A70 have almost the same primary structure, the differences between their spectrum of action and bacteriolytic activity are possibly due to the presence of the N-formyl group found in aureocin 4181 peptides and its absence from aureocin A70. In accordance with this assumption, the formylated two-component enterocin L50 was shown to be more potent than its non-formylated variant. Formylation of the peptides EntL50A and EntL50B increased their activity eight fold and two fold, respectively [33].
Previous studies have shown that aureocin A70 is neither hemolytic nor toxic to eukaryotic cells, being heat resistant (at 65 and 80 °C for 15 min and at 100 °C for 5 min), sensitive to gastric juice and bile salts, and quite stable when kept at 25, 4, and − 20 °C [8]. This bacteriocin could also adsorb to and be released from films used for food bioactive packaging [8]. Due to the high similarities between both aureocins, aureocin 4181 is expected to share these same advantageous properties for an antimicrobial agent that might be used, for example, incorporated within intramammary teat seals, as done with lacticin 3147 [36], although further experiments are required to confirm them.
In conclusion, S. aureus 4181 produces a natural N-formylated variant of aureocin A70 with a broader spectrum of antimicrobial activity and a stronger bacteriolytic action. Therefore, aureocin 4181 represents the third four-component class II bacteriocin reported in the literature, after aureocin A70 [7] and cereucin H [31]. This bacteriocin differs from aureocin A70 not only by the presence of an N-formyl group in each peptide, but also by a single conservative substitution in the amino acid sequence of AurD1. Aureocin 4181 also represents a new alternative antimicrobial agent for prevention and control of bovine mastitis, as its broad spectrum of antibacterial activity comprises several mastitic S. aureus strains.
Electronic supplementary material
(DOC 1672 kb)
Acknowledgments
SLSMB and GSA were recipients of scholarships from CAPES/Brazil (Finance Code 001) and PIBIC/UFRJ, respectively. PCF is recipient of a fellowship from FAPERJ/Brazil. Genome sequencing was performed at the University of the State of Rio de Janeiro (UERJ), Brazil.
Funding information
This work was supported by a research program funded by the National Council for Scientific and Technological Development (CNPq, 303.602/2015-5), Brazil, to MCFB.
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Acedo JZ, Chiorean S, Vederas JC, van Belkum MJ. The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol Rev. 2018;42:805–828. doi: 10.1093/femsre/fuy033. [DOI] [PubMed] [Google Scholar]
- 2.Cotter PD, Ross RP, Hill C. Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 3.Newstead LL, Varjonen K, Nuttall T, Paterson GK. Staphylococcal-produced bacteriocins and antimicrobial peptides: their potential as alternative treatments for Staphylococcus aureus infections. Antibiotics. 2020;9:40. doi: 10.3390/antibiotics9020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coelho MLV, Duarte AFS, Bastos MCF. Bacterial labionin-containing peptides and sactibiotics: unusual types of antimicrobial peptides with potential use in clinical settings (a review) Curr Top Med Chem. 2017;17:1177–1198. doi: 10.2174/1568026616666160930144809. [DOI] [PubMed] [Google Scholar]
- 5.Diep DB, Skaugen M, Salehian Z, Holo H, Nes IF. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc Natl Acad Sci U S A. 2007;104:2384–2389. doi: 10.1073/pnas.0608775104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giambiagi-de Marval M, Mafra MA, Penido EGC, Bastos MCF. Distinct groups of plasmids correlated with bacteriocin production in Staphylococcus aureus. J Gen Microbiol. 1990;136:1591–1599. doi: 10.1099/00221287-136-8-1591. [DOI] [PubMed] [Google Scholar]
- 7.Netz DJA, Sahl H-G, Marcolino R, Nascimento JS, Oliveira SS, Soares MB, Bastos MCF. Molecular characterisation of aureocin A70, a multi-peptide bacteriocin isolated from Staphylococcus aureus. J Mol Biol. 2001;311:939–949. doi: 10.1006/jmbi.2001.4885. [DOI] [PubMed] [Google Scholar]
- 8.Fagundes PC, Farias FM, Santos OCS, Paz JAS, Ceotto-Vigoder H, Alviano DS, Romanos MTV, Bastos MCF. The four-component aureocin A70 as a promising agent for food biopreservation. Int J Food Microbiol. 2016;237:39–46. doi: 10.1016/j.ijfoodmicro.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Gamon MR, Moreira EC, Oliveira SS, Teixeira LM, Bastos MCF (1999) Characterization of a novel bacteriocin-encoding plasmid found in clinical isolates of Staphylococcus aureus. Antonie van Leeuwenhoek 75:233–243 [DOI] [PubMed]
- 10.Nascimento JS, Santos KRN, Gentilini E, Sordelli D, Bastos MCF. Phenotypic and genetic characterization of bacteriocin-producing strains of Staphylococcus aureus involved in bovine mastitis. Vet Microbiol. 2002;85:133–144. doi: 10.1016/S0378-1135(01)00476-X. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento JS, Fagundes PC, Brito MAVP, Bastos MCF. Production of bacteriocins by coagulase-negative staphylococci involved in bovine mastitis. Vet Microbiol. 2005;106:61–71. doi: 10.1016/j.vetmic.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Brito MAVP, Somkuti GA, Renye JA. Production of antilisterial bacteriocins by staphylococci isolated from bovine milk. J Dairy Sci. 2011;94:1194–1200. doi: 10.3168/jds.2010-3849. [DOI] [PubMed] [Google Scholar]
- 13.Ceotto H, Nascimento JS, Brito MAVP, Bastos MCF. Bacteriocin production by Staphylococcus aureus involved in bovine mastitis in Brazil. Res Microbiol. 2009;160:592–599. doi: 10.1016/j.resmic.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Bastos MCF, Ceotto H, Coelho MLV, Nascimento JS. Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr Pharm Biotechnol. 2009;10:38–61. doi: 10.2174/138920109787048580. [DOI] [PubMed] [Google Scholar]
- 15.Coelho MLV, Nascimento JS, Fagundes PC, Madureira DJ, Oliveira SS, Brito MAVP, Bastos MCF. Activity of staphylococcal bacteriocins against Staphylococcus aureus and Streptococcus agalactiae involved in bovine mastitis. Res Microbiol. 2007;158:625–630. doi: 10.1016/j.resmic.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Farias FM, Nascimento JS, Santos OCS, Bastos MCF. Study of effectiveness of staphylococcins in biopreservation of Minas fresh (Frescal) cheese with a reduced sodium content. Int J Food Microbiol. 2019;304:19–31. doi: 10.1016/j.ijfoodmicro.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Ceotto H, Brede DA, Salehian Z, Nascimento JS, Fagundes PC, Nes IF, Bastos MCF. Aureocins 4185, bacteriocins produced by Staphylococcus aureus 4185: potential application in food preservation. Foodborne Pathog Dis. 2010;7:1255–1262. doi: 10.1089/fpd.2010.0578. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento JS, Coelho MLV, Ceotto H, Potter A, Fleming LR, Salehian Z, Nes IF, Bastos MCF. Genes involved in immunity to and secretion of aureocin A53, an atypical class II bacteriocin produced by Staphylococcus aureus A53. J Bacteriol. 2012;194:875–883. doi: 10.1128/JB.06203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duarte AFS, Ceotto-Vigoder H, Barrias EM, Souto-Padrón TCBS, Nes IF, Bastos MCF. Hyicin 4244, the first sactibiotic described in staphylococci, exhibits an anti-staphylococcal biofilm activity. Int J Antimicrob Agents. 2018;51:349–356. doi: 10.1016/j.ijantimicag.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Brede DA, Faye T, Johnsborg O, Odegard I, Nes IF, Holo H. Molecular and genetic characterization of propionicin F, a bacteriocin from Propionibacterium freudenreichii. Appl Environ Microbiol. 2004;70:7303–7310. doi: 10.1128/AEM.70.12.7303-7310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters EC, Horn DM, Tully DC, Brock A. A novel multifunctional labeling reagent for enhanced protein characterization with mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2387–2392. doi: 10.1002/rcm.517. [DOI] [PubMed] [Google Scholar]
- 22.Silva NCC, Guimarães FF, Manzi MP, Budri PE, Gómez-Sanz E, Benito D, Langoni H, Rall VLM, Torres C. Molecular characterization and clonal diversity of methicillin-susceptible Staphylococcus aureus in milk of cows with mastitis in Brazil. J Dairy Sci. 2013;96:6856–6862. doi: 10.3168/jds.2013-6719. [DOI] [PubMed] [Google Scholar]
- 23.Ceotto H, Dias RCS, Nascimento JS, Brito MAVP, Giambiagi-deMarval M, Bastos MCF (2012) Aureocin A70 production is disseminated amongst genetically unrelated Staphylococcus aureus involved in bovine mastitis. Lett Appl Microbiol 54:455–461 [DOI] [PubMed]
- 24.Jack RW, Tagg JR, Ray B. Bacteriocins of Gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/MMBR.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruegg PL. A 100-year review: mastitis detection, management, and prevention. J Dairy Sci. 2017;100:10381–10397. doi: 10.3168/jds.2017-13023. [DOI] [PubMed] [Google Scholar]
- 26.Gomes F, Henriques M. Control of bovine mastitis: old and recent therapeutic approaches. Curr Microbiol. 2015;72:377–382. doi: 10.1007/s00284-015-0958-8. [DOI] [PubMed] [Google Scholar]
- 27.Nascimento JS, Abrantes J, Giambiagi-deMarval M, Bastos MCF. Growth conditions required for bacteriocin production by strains of Staphylococcus aureus. World J Microbiol Biotechnol. 2004;20:941–947. doi: 10.1007/s11274-004-3626-x. [DOI] [Google Scholar]
- 28.Coelho MLV, Ceotto H, Madureira DJ, Nes I, Bastos MCF. Mobilization functions of the bacteriocinogenic plasmid pRJ6 of Staphylococcus aureus. J Microbiol. 2009;47:327–336. doi: 10.1007/s12275-009-0044-7. [DOI] [PubMed] [Google Scholar]
- 29.Coelho MLV, Fleming LR, Bastos MCF. Insights into aureocin A70 regulation: participation of regulator AurR, alternative transcription factor σB and phage ϕ11 regulator cI. Res Microbiol. 2016;167:90–102. doi: 10.1016/j.resmic.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Coelho MLV, Coutinho BG, Santos OCS, Nes IF, Bastos MCF. Immunity to the Staphylococcus aureus leaderless four-peptide bacteriocin aureocin A70 is conferred by AurI, an integral membrane protein. Res Microbiol. 2014;165:50–59. doi: 10.1016/j.resmic.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Ovchinnikov KV, Chi H, Mehmeti I, Holo H, Nes IF, Diep DB. Novel group of leaderless multipeptide bacteriocins from Gram-positive bacteria. Appl Environ Microbiol. 2016;82:5216–5224. doi: 10.1128/AEM.01094-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Vos WM, Mulders JWM, Siezen RJ, Hugenholtz J, Kuipers OP. Properties of nisin Z and distribution of its gene, nisZ, in Lactococcus lactis. Appl Environ Microbiol. 1993;59:213–218. doi: 10.1128/AEM.59.1.213-218.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanchi H, Hammami R, Fernandez B, Kourda R, Ben Hamida J, Fliss I. Simultaneous production of formylated and nonformylated enterocins L50A and L50B as well as 61A, a new glycosylated durancin, by Enterococcus durans 61A, a strain isolated from artisanal fermented milk in Tunisia. J Agric Food Chem. 2016;64:3584–3590. doi: 10.1021/acs.jafc.6b00700. [DOI] [PubMed] [Google Scholar]
- 34.Netz DJA, Pohl R, Beck-Sinckinger AG, Selmer T, Pierik AJ, Bastos MCF, Sahl H-G. Biochemical characterization and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J Mol Biol. 2002;319:745–756. doi: 10.1016/S0022-2836(02)00368-6. [DOI] [PubMed] [Google Scholar]
- 35.Wladyka B, Piejko M, Bzowska M, Pieta P, Krzysik M, Mazurek L, Guevara-Lora I, Bukowski M, Sabat AJ, Friedrich AW, Bonar E, Miedzobrodzki J, Dubin A, Ma P. A peptide factor secreted by Staphylococcus pseudintermedius exhibits properties of both bacteriocins and virulence factors. Sci Rep. 2015;5:14569. doi: 10.1038/srep14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan MP, Flynn J, Hill C, Ross RP, Meaney WJ. The natural food grade inhibitor, lacticin 3147, reduced the incidence of mastitis after experimental challenge with Streptococcus dysgalactiae in nonlactating dairy cows. J Dairy Sci. 1999;82:2208–2114. doi: 10.3168/jds.S0022-0302(99)75519-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 1672 kb)