Abstract
This experiment aimed to investigate the status of tomato plants in terms of sink or source-limitation of 2 cultivars of greenhouse tomato (Solanum lycopersicum L.), i.e., ‘Grandella’ and ‘Isabella’ under the greenhouse conditions of Iran and to improve the yield and plant growth by manipulating the sink-source balance. To this end, 4 treatments were applied: leaves were not pruned and fruits were pruned to one per truss (1F/3L), leaves were not pruned and fruits were pruned to two per truss (2F/3L), leaves were not pruned and fruits were pruned to three per truss (3F/3L) and no leaf and fruit pruning (control). The results showed that truss pruning reduced the sink demand and consequently, increased the amount of available assimilate for the growth of the remaining fruits or vegetative parts. The negative correlation between the leaf area index and the net assimilation rate and no significant difference in the net assimilation rate between different sink/source ratios showed that the excess leaf area index does not contribute in increasing the assimilate production and hence, total yield. Total fruit weight, harvest index, and the ratio of the ripe fruits to the total fruit led to the highest yield for control plants. No changes in chlorophyll, protein content and nitrate reductase activity were the evidence for the fact that sink/source ratio do not affect light-harvesting and light-utilizing components of photosynthesis. Since the individual weight of fruits increased with decreasing fruit number per trusses, the growth of individual fruits in both cultivars was source-limited and truss pruning can decrease this limitation. Future studies should be carried out to determine the best level of sink/source ratio that in addition to producing an acceptable amount of yield, meets the needs of consumers in the current stressful world by increasing the antioxidant and nutriceutical content of fruits.
Keyword: Carbohydrates, chlorophyll, fruit pruning, growth rate, net assimilation rate
Introduction
Plant growth and yield of crops are closely related to photosynthetic rate and assimilate partitioning. Photosynthetic rate strongly depend on environmental conditions including light, temperature, air humidity and carbon dioxide concentration (Hancock et al. 2014; Pastenes et al. 2014; Salazar-Parra et al. 2015) some internal conditions including the sink-source balance. For example, truss pruning in tomato plants (Matsuda et al. 2011) as well as girdling and fruit pruning in apple decreased the net photosynthetic rate (Zhou and Quebedeaux 2003; Pallas et al. 2018). Sink activity change canaffect the hormonal contents of plants, consequently affecting the stomatal and mesophyll resistance and hence, the photosynthetic activity (Hofius 2007). It can also accumulate starch and/or sugar in the source tissue, leading to inhibition of photosynthesis, a process called end-production inhibition (Araya et al. 2006; Nakano et al. 2000; Hofius 2007). Excess starch and sugars can decrease triose phosphate utilization and carboxylation rate of ribulose-1, 5-bisphosphate carboxylase (Rubisco) (Fabre et al. 2019). It can increase the resistance to diffusion of carbon dioxide into the leaf and prevent carbon dioxide diffusion into chloroplasts (Nakano et al. 2000; Andrade et al. 2019; Yan et al. 2011). It can also decrease light intensity within chloroplasts (Vasseur et al. 2011) and bind with the magnesium cation needed to activate Rubisco (Mayoral et al. 1985). Hofius in 2007 have reported that sugars serve as signaling molecules in the down-regulation of the photosynthetic genes.
Assimilate partitioning, another effective component of plant growth and yield, is mainly determined by the sink and source activity and may control the photosynthetic rate (Zhou and Quebedeaux 2003). Barzegar et al. (2013) have reported that when some fruits are removed from the melon plant, it invests the available assimilates into the remaining fruit or towards vegetative growth. The results of the Jorquera-Fontena et al. (2014) in blueberry showed that pruning of plants increased fruit weight. It means that plant growth and yield depend not only on assimilates production but also on the consumption of assimilates in sinks (Sonnewald and Fernie 2018).
Tomato is one of the most popular and widely consumed vegetable. It is considered as one of the very important crop due to its high economic and nutritional values. In tomato plants, it is possible to show that the removal of sink organs decreases the photosynthetic rate of the mature leaves (sink-limited plant) (Tanaka and Fujita 1974; Matsuda et al. 2011). On the other hand, the results of some studies have also shown that tomato truss pruning could increase the size of the remaining fruits, revealing that assimilate availability in the tomato plants is less than the assimilate demand and fruits do not have enough assimilating during their developmental stages (source-limited plant) (Heuvelink and Dorais 2005). Therefore, the investigation of each region and cultivar sho›uld be considered to obtain ideal growth and yield.
We hypothesize that the tomato plants exhibit a different status in terms of the sink- or source-limitation in their response of yield formation to different production region and cultivar. The objectives of the current study were to (a) investigate the status of tomato plants in terms of the sink or source-limitation under the greenhouse conditions of Iran, (b) improve the yield and yield components by manipulating the sink-source balance, (c) examine whether the response of these traits to sink-source balance is associated with genotype.
Materials and methods
Plant materials and greenhouse conditions
The study was carried out from November 2017 untill May 2018 in a greenhouse in Isfahan University of Technology (51.5320° E, 32.7191° N, 1650 h). The greenhouse was a venlo-type (Goldammer 2019) oriented north and south and covered with glass. The seeds of two F1 cultivars with medium fruit size of tomato consisted of ‘Grandella’ (produced by De Ruiter company, sensitive to tomato yellow leaf curl virus, high uniformity in fruit shape and size, 4–8 fruits per truss) and ‘Isabella’ (produced by PSS company, resistant to tomato yellow leaf curl virus, 4–6 fruits per truss) were sown into 105-cell plug trays filled with 80:20 (v/v) mixtures of cocopeat (Heylan cocopeat, Sir Lanka) and perlite (Siminkimia, Iran), in November 2017. Three-leaf stage seedlings were transplanted into 25 cm-diameter pots filled with a clay-loam soil (bulk density = 1.23 g cm−3, particle density = 2.63 g cm−3, total pores = 53.07% (v/v), pH 7.22, electrical conductivity = 0.840 ds m−1, N = 0.101%, P = 5.07 g kg−1, K = 92.16 g kg−1, Ca = 364 g kg−1, Mg = 46 g kg−1) at a density of 2.6 plants m−2 in January, 2018. The plants were grown under natural photoperiod, nutrition and chemical control for pests and diseases following the commercial practices. Irrigation was first applied every 3 days then it was followed every day as the plant growth increased with tap water (pH 7.14, electrical conductivity = 0.379 ds m−1). Irradiance was measured weekly above plants with a pyranometer (SKYE INSTRUMENTS LTD, United Kingdom) and radiation was converted into photosynthetic active radiation (PAR) (Gautier et al. 2001). The air temperature was measured with the max–min thermometer, in a daily manner (Dall'Amico and Hornsteiner 2006). The mean PAR and air temperature for the whole experimental duration (4 months) were 591.67 ± 15.15 µmol m−2 s−1 and 24.40 ± 0.33 °C, respectively.
Plant treatments
Plants were trimmed to a single stem and all side shoots were removed at the 10–15 cm length stage. The main axis of a greenhouse tomato plant is made of repeating units of 3 leaves and one truss, so in this experiment fruits and expanded leaves were considered as sink and source of each unit, respectively. Applied sink/source treatments (Fig. 1) were: leaves were not pruned and fruits were pruned to one per truss (1F/3L), leaves were not pruned and fruits were pruned to 2 per truss (2F/3L), leaves were not pruned and fruits were pruned to three per truss (3F/3L), and no leaf and fruit pruning (control with the average of 5 fruits per truss for both cultivars) (Matsuda et al. 2011; Gautier et al. 2001). Fruit clusters were pruned when the first fruit reached 1 cm of their diameter (width), based on Matsuda et al. (2011). There were three replicates (each replication consisting of 3 plants) for each treatment in a completely randomized design (Ireland 2010).
Fig. 1.
Schematic diagram of the sink/source ratio. 1F/3L: leaves were not pruned and fruits were pruned to one per truss; 2F/3L: leaves were not pruned and fruits were pruned to two per truss; 3F/3L: leaves were not pruned and fruits were pruned to 3 per truss; Control: no leaf and fruit pruning
Plant growth and development analysis
The leaf generation, stem elongation and diameter expansion were studied for 6 weeks (0, 1, 2, 5, 6, 7 and 8 phonological growth stage) based on Meier (2001) (from the third week of February until the end of March) on all examined plants. The initial measurement was done two weeks after applying the treatments. Number of leaves over 2 cm in length were counted weekly and the leaf generation rate was calculated as the slope of the regression line fitted to the points representing the number of leaves per plant as a function of time. Stem length and diameter (in the middle of the third internode) were measured weekly using digital-caliper and meter tape, respectively. Stem elongation and diameter enlargement rate per plant were estimated as the slopes of the regression line were fitted to the points representing the length and diameter of stem per plant as a function of time, respectively (Gautier et al. 2001).
At harvest, fully ripe fruits were picked three times a week from 5 first trusses. The yield was determined for each cultivar per replicate in each treatment.
In May 2018, the plants were destructively harvested. Plant organs were separated and diameter, length, and fresh weight of the stem, leaf area, leaf fresh weight, root fresh weight and the fresh weight of before harvest-ripe fruits were measured. Separated organs of each plant were oven-dried at 80 °C for 1 week and their dry weights were measured (Ronga et al. 2019).
For each treatment, the rate of dry matter production per unit ground area, per time or crop growth rate (CGR), was calculated for the leaves, stem, root, before harvest-ripe fruits and ripe fruits between two measurement dates and days between measurements, as well as plant density. Leaf area was measured by analyzing the photos of leaves using Image J software. Leaf area index (LAI) was estimated according to the leaf area of each plant and plant density. The specific leaf area (SLA) was calculated by dividing the leaf area by the leaves' dry weight. The rate of dry matter production per unit leaf area, per unit time, or net assimilation rate (NAR), was estimated through dividing CGR by the average LAI of the two dates (Kumar et al. 2012). The ratio of before harvest-ripe fruits dry weight + ripe fruits dry weight to before harvest-ripe fruits dry weight + ripe fruits dry weight + Leaf dry weight + stem dry weight was calculated as the harvest index (HI) (Ronga et al. 2019).
Sample preparation for biochemical analysis
Leaf samples were taken from the leaves below and above the third truss. The samples were collected at three different developmental stages of fruits, i.e., the end of the cell division (15 days after treatment; DAT), cell expansion (30-DAT), and the beginning of ripening (45-DAT) (Gillaspy et al. 1993). The leaf samples were immediately frozen in liquid nitrogen and stored at − 80 °C until further use.
Chlorophyll content
500 mg of the samples were homogenized in 10 mL of 90% (v/v) acetone. The homogenate was centrifuged for 10 min at 10,000×g (Model Universal 320 R; Hettich-centrifuge; Germany) and the supernatant was transferred to a clean flask. Chlorophyll contents were measured at 645 and 663 nm using a spectrophotometer (model: UV-160A-SHIMADZU; Gholami Zali and Ehsanzadeh 2018).
Protein content and nitrate reductase activity
Extract protein contents were determined according to the method developed by Bradford (1976) with BSA as the standard protein. Enzyme activity was determined by putting 0.15 g of the leaf slice into a 5 mL measurement buffer containing 3 mM KNO3, 100 mM KH2PO4, 100 mM K2HPO4 and 2-propanol 50% (v/v). The tubes were incubated in water bath at 30 °C for 1 hr under dark conditions and then boiled for 5 min to deactivate the enzyme. Then, 2.5 mL of the sulfanilamide solution and 2.5 mL of 1-napthylethylenediamine hydrochloride were added to the tubes and the absorbance of each sample was measured at 540 nm. KNO2 was used as a standard and nitrate reductase activity was reported as nmol per µg protein per second (McCashin 2000).
Carbohydrate analysis
Total sugar was extracted using the protocol described by Mohamed et al. (2010) with some modifications. Accordingly, 100 mg of the samples were homogenized in 5 mL of 80% (v/v) ethanol and centrifuged for 10 min in 10,000×g. The extraction was repeated twice and the supernatants were collected and pooled. The pellet was stored at − 20 °C for starch determination. The supernatant volume was brought to 100 mL with distilled water. For colorimetric determination, 1 mL aliquot of the extract was mixed with 5 mL of anthrone reagent (0.2, w/v, in 95% cold sulfuric acid) in a test tube and mixed. The mixture was incubated in boiling water for 15 min and then kept on ice. Absorbance was read at 620 nm using a spectrophotometer. Glucose was used as the standard. Sugar content was reported in µmol per g fresh weight.
Starch content was determined using the perchloric acid method as described by Robin et al. (1991). For starch hydrolyzation, 5 mL of 35% (v/v) perchloric acid was added to the pellet obtained during sugar extraction and mixed on a shaker (model: Gerber GmbH) set at 200 rpm for 15 min. The homogenate was centrifuged for 5 min at 10,000×g and the supernatant was transferred to a clean flask. The residue was mixed with another 5 mL of 35% (v/v) perchloric acid to hydrolyze the remaining starch. The resulting supernatant was added to the previous one and brought to 100 mL with distilled water. For colorimetric determination, 1 mL aliquot of the extract was mixed with 5 mL of the anthrone reagent. The mixture was incubated in boiling water for 15 min and then put on ice. The absorbance was read at 620 nm. Glucose was used as a standard. Starch content was reported as µmol of Glc produced per g fresh weight.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) based on a split-plot factorial experiment for the characteristics measured at different fruit developmental stages or a factorial experiment for other characteristics using the SAS software (Version 9.0; SAS Institute, Cary, NC, USA) (Wilson et al. 2006). The least significant difference (LSD) test was used to separate means at a probability value of P ≤ 0.05. Principal component analysis (PCA) was performed for each cultivar × sink/source ratio treatment of different replications using the RStudio software (Version 3.5.2, Eggshell Igloo) (Patel et al. 2017).
Results
Plant growth and yield
Reducing the sink/source ratio significantly increased stem growth (Fig. 2). Stem diameter and diameter enlargement rate of the 1F/3L treatment were 9.94 and 44.91% greater than those of the control (Fig. 2a, b). Stem length and stem elongation rate in 1F/3L and 2F/3L were greater than those of 3F/3L and the control (Fig. 2c, d). The stem fresh and dry weight increased significantly with decreasing sink/source ratio by 72.55%, 44.50% and 16.75% for the fresh weight and 75.28%, 48.04% and 22% for dry weight in comparison to the control, for 1F/3L, 2F/3L, and 3F/3L, respectively (Fig. 2e, f). The ‘Isabella’ stem diameter, diameter enlargement rate, and the fresh and dry weight were significantly higher than those of ‘Grandella’. On the other hand, ‘Grandella’ had a longer stem with higher elongation rate in comparison to ‘Isabella’ (Fig. 2).
Fig. 2.
Stem diameter a, diameter enlargement rate b, stem length c, stem elongation rate d, stem fresh weight e, and stem dry weight (f) of tomato plants under different sink demand treatments for two tomato greenhouse cultivars. Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
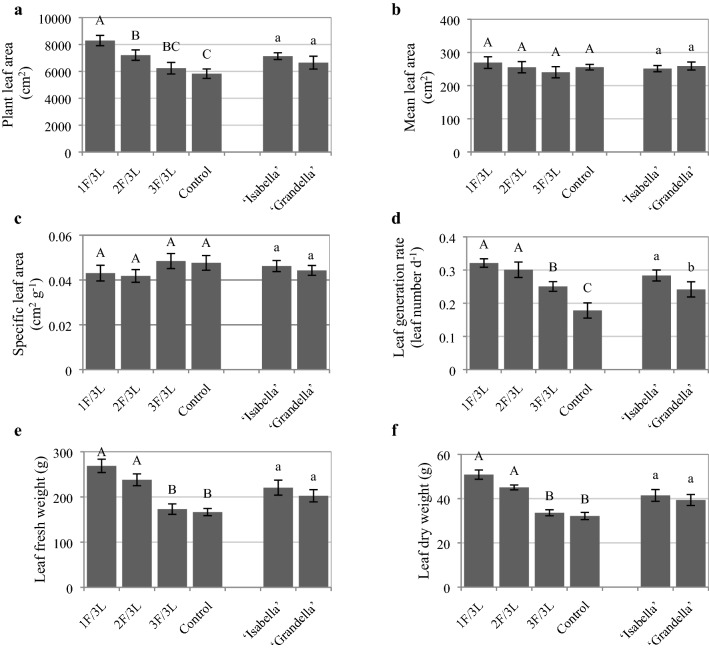
The results showed that decreasing the sink/source ratio increased the leaf area per plant and had no effect on the mean leaf area of each leaf and SLA (Fig. 3a, b, c). The plant leaf area of 1F/3L was the highest (42.22% higher than the control) (Fig. 3a). Leaf generation rate in 1F/3L and 2F/3L were greater than those of 3F/3L and control (80.24% and 68.88%, in comparison to the control, respectively) (Fig. 3d). The leaf generation rate of ‘Isabella’ was significantly greater than that of ‘Grandella’ (17.35%) (Fig. 3d). The greatest leaves fresh and dry weights were detected in the 1F/3L and 2F/3L treatments (Fig. 3e, f).
Fig. 3.
Plant leaf area a, mean leaf area b, specific leaf area c, leaf generation rate d, leaf fresh weight e, and leaf dry weight (f) of tomato plants under different sink demand treatments for two tomato greenhouse cultivars. Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
The root fresh and dry weights were the highest in 1F/3L and the lowest in the control (Fig. 4).
Fig. 4.
Root fresh weight a, and root dry weight b of tomato plants under different sink demand treatments for two tomato greenhouse cultivars. Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
The greatest yield of five first trusses before harvest-ripe and ripe fruit weight (total fruit weight) and the ripe fruit/total fruit ratio of both cultivars were detected in the control plants (Fig. 5a, b, c, d, f), but the individual weight of the 1F/3L fruits was the greatest (49.00% higher than that of the control) (Fig. 5e).
Fig. 5.
Ripe fruit fresh weight of five first trusses a, ripe fruit dry weight of five first trusses b, before harvest-ripe and ripe fruit fresh weight c, before harvest-ripe and ripe fruit dry weight d, individual fruit fresh weight e, and ripe fruit/total fruit f of tomato plants under different sink demand treatments for two tomato greenhouse cultivars. Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
Truss pruning increased dry matter allocation to root, stem, and leaves, thus globally increasing the vegetative dry matter (Fig. 6).
Fig. 6.

Effect of different sink demand treatments and tomato greenhouse cultivar on the distribution of plant parts. Vertical bars denote standard error (SE)
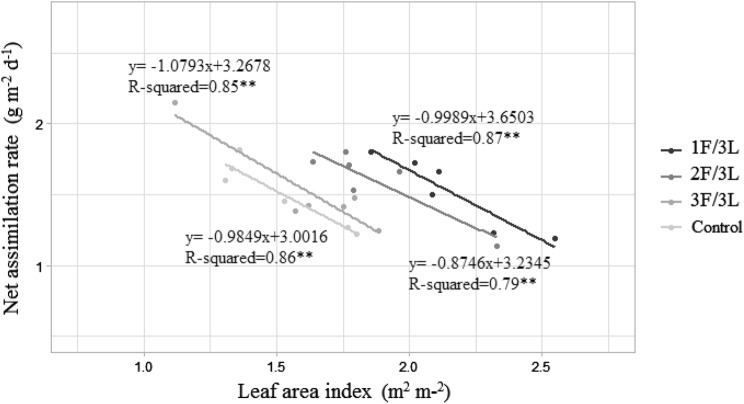
There was a significant difference in LAI and CGR among treatments. This was such that both tend to increase as the sink/source ratio was decreased (Fig. 7a, b); while NAR was statistically the same among different sink/source ratio treatments (Fig. 7c). There was however, significant inverse relationships between LAI and NAR for each treatment (Fig. 8). HI trend to decrease as the sink/source ratio was reduced (Fig. 7d).
Fig. 7.
Leaf area index a, crop growth rate b, net assimilation rate c, and harvest index d of tomato plants under different sink demand treatments or 2 tomato greenhouse cultivars. Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
Fig. 8.
The relationship between net assimilation rate and leaf area index of different sink/source ratio
Chlorophyll content
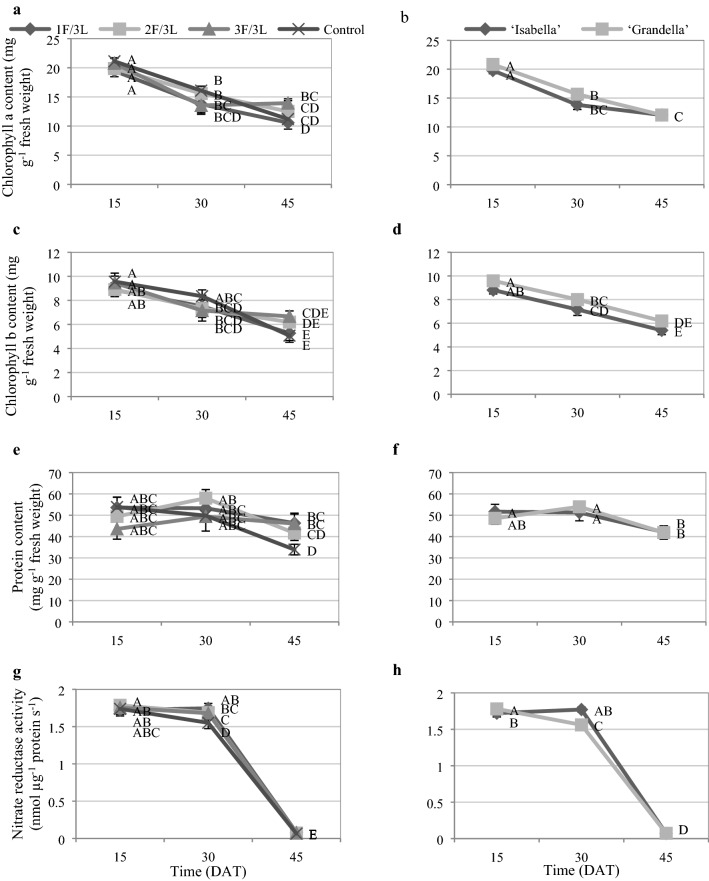
There was no significant difference in terms of chlorophyll content between sink/source ratio treatments and the examined cultivars (Fig. 9a, b). The chlorophyll content of the treatments and the two examined cultivars were decreased with the progress of time (Fig. 10a, b, c, d).
Fig. 9.
Chlorophyll a content a, chlorophyll b content b, protein content c, and nitrate reductase activity d of tomato plants under different sink demand treatments for two tomato greenhouse cultivars (mean of 3 sampling times). Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
Fig. 10.
Chlorophyll a content a and b, chlorophyll b content c and d, protein content e and f, and nitrate reductase activity g and h content pattern during the experiment. Mean values followed by the same letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
Protein content and nitrate reductase activity
Sink/source ratio treatments and the examined cultivars did not affect the protein content and nitrate reductase activity (Fig. 9c, d). The protein content in almost all examined treatments and both cultivars was constant with the elapse of time until 30-DAT and then decreased until 45-DAT (Fig. 10e, f). The nitrate reductase activity in both examined cultivars and all sink/source ratios was significantly decreased from 30 to 45-DAT (Fig. 10g, h).
Carbohydrate content
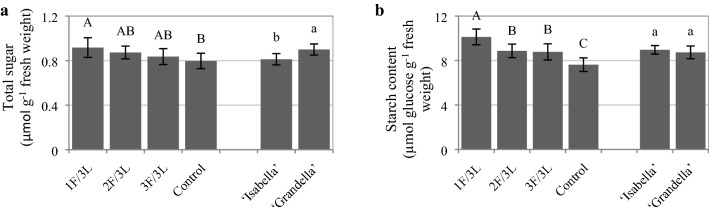
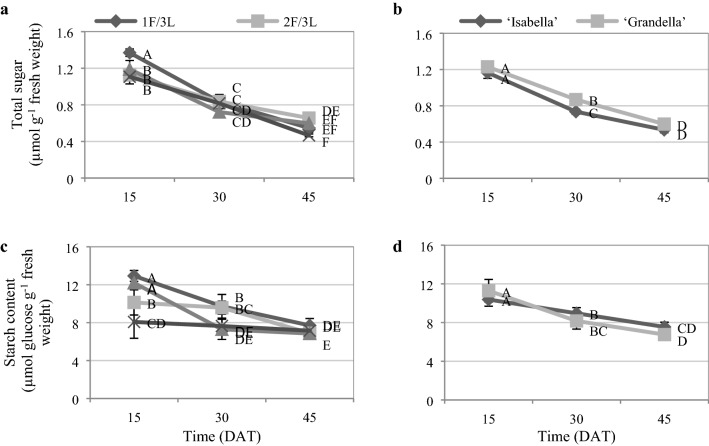
Reducing the sink/source ratio significantly increased the total sugar and starch content of leaves (Fig. 11). The highest total sugar and starch contents were detected in the presence of 1F/3L (15.11% and 32.76% greater than those of the control, respectively). The total sugar and starch contents of almost all treatments and both cultivars were decreased significantly with the progress of time (Fig. 12).
Fig. 11.
Total sugar a, and starch b content of tomato plants under different sink demand treatments for two tomato greenhouse cultivars (mean of three sampling times). Mean values ± standard error (SE) for each variable with the same upper-case (for sink/source ratios) or lower-case (for cultivars) letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
Fig. 12.
Total sugar a, b and starch c, d content pattern during the experiment. Mean values followed by the same letters are not significantly different at P ≤ 0.05 by the least significant difference (LSD) test
PCA
To explore the interrelationships among different treatments and the measured characteristics, PCA was performed on all treatments and the measured characteristics. Results of PCA revealed that the first and second components, together explained 80.7% of the total variation (Fig. 13). PC1 and PC2 could explain 67.3 and 13.4% of the variance, respectively (Fig. 13). The PCA related to the measured characteristics could classify different cultivars and treatments into separate groups. ‘Grandella’ × control (GC), ‘Isabella’ × control (IC), ‘Grandella’ × 3F/3L (G3F/3L), and ‘Isabella’ × 3F/3L (I3F/3L) were positively associated with a high value of before harvest-ripe and ripe fruit weight, HI, ripe fruit/total fruit ratio, and chlorophyll a content (Fig. 13). There was a positive correlation between ripe fruit/total fruit ratio, before harvest-ripe and ripe fruit weight, HI, and chlorophyll a content and between individual fruit fresh weight and starch and total sugar contents (Fig. 13).
Fig. 13.
Principal component analysis showing the relationship between measured variables. G1F/3L: ‘Grandella’ × 1F/3L; G2F/3L: ‘Grandella’ × 2F/3L; G3F/3L: ‘Grandella’ × 3F/3L; GC: ‘Grandella’ × control; I1F/3L: ‘Isabella’ × 1F/3L; I2F/3L: ‘Isabella’ × 2F/3L; I3F/3L: ‘Isabella’ × 3F/3L; IC: ‘Isabella’ × control; FW: fresh weight; DW; dry weight; BH-ripe: before harvest-ripe fruits; Leaf gen rate: leaf generation rate; LAI; leaf area index; LA; leaf area; NAR: net assimilation rate; CGR; crop growth rate
Discussion
The reduction of the sink/source ratio contributed to large plants with large fruits (Figs. 2, 3, 4, and 5). Truss pruning reduced the sink demand and consequently increased the amount of the available assimilates for the growth of the remaining fruit or vegetative parts (stem, leaves, and root). These results were, therefore in agreement with those of Gautier et al. (2001) on tomato, and Barzegar et al. (2013) and De Queiroga et al. (2008) on melons. However, the decreased sink/source ratio reduced the portion of dry matter distributed into the fruits, and this was compensated by the increase of the vegetative growth (Fig. 6).
The decreased sink/source ratio increased the total mass of the leaves with a significant increase in the leaf area (Fig. 3), which agreed with Hurd et al. (1979). However, these were in contrast to the results obtained by Yasumura (2009) and Gautier et al. (2001). Leaf area of the plants results from the appearance of new leaves, their expansion, and senescence (Johnson and Thornley 2006). Reducing the sink/source ratio increased the leaf generation rate, but it did not affect the mean individual leaf area. So, in the present study, just the leaf generation rate contributed to the large leaf area of the plants. It seems that under reduced sink/source ratio shoot-apex, as another sink, received more assimilates and grew rapidly, so the leaf generation rate and consequently the inflorescence generation rate (inflorescence generation rate in greenhouse tomatoes is about one–third of the rate of leaf generation rate) could be increased. SLA, defined as the ratio of total leaf area to total leaf dry weight, has been shown as one of the leaf characteristic reflecting the thickness of the leaves. Thick leaves (plants with low SLA) due to limited diffusion of light and carbon dioxide to the site of carboxylation show a low photosynthetic capacity (Gulias et al. 2003). Heuvelink and Dorais (2005) reported that reducing the sink/source ratio of tomato plants can reduce light absorption and photosynthesis by increasing leaves thickness. The manipulated sink/source ratio in the present study did not affect SLA (Fig. 3c). These results indicate that under the present experimental conditions, manipulation of the sink/source ratio did not alter the plant conditions to affect the leaf thickness to regulate light absorption. Reduced sink/source ratio also increased the stem and root growth (Figs. 2 and 4). The increasing trend of stem and root growth under the low sink/source ratio, as reported by Yasumura (2009) and Jiang et al. (2017), could distribute a part of the excess carbohydrates.
CGR and LAI tend to increase as the sink/source ratio decreased (Fig. 7). Fruit removal in the study of Matsuda et al. (2011) changed LAI, which was like the results of the present study. LAI represented plant leaf photo interception which highly influenced biomass and yield production (Firouzabadi et al. 2015). Campillo et al. (2010) also stated that the intercepted light increased with the rise in LAI until 3–4 m2 m−2 by the greenhouse tomato plant canopy. The maximum amount of LAI in the present study was 2.16 m2 m−2 (Fig. 7). As the soil nutrition, climate and other factors like location and management practices influence LAI (Al Mamun Hossain et al. 2017), the current study can conclude that the optimal LAI under our experimental condition was much less than that shown in Campillo et al. (2010). The negative correlation was observed between LAI and NAR (Fig. 8) and no significant difference in the rate of dry matter production per unit leaf area per unit time (NAR) between different sink/source ratios (Fig. 7c) showed that because of increased size and number of other organs by decreasing sink/source ratio, there was high maintenance respiration which could not be captured in NAR. Lakchamenkumar and Guru (2014) stated that CGR and NAR increase at a higher amount of received radiation In the present study NAR remains constant and CGR, which is the product of NAR and LAI, increased by decreasing the sink/source ratio. It showed the increased CGR is just related to high LAI that could not influence the amount of NAR because of high maintenance respiration.
Higashid and Heuvelink (2009) reported that the increase in yield or the rise of HI might be ascribed to a change in the sink-source balance. In the present study, the total fruit weight followed the descending order of control > 3F/3L > 2F/3L > 1F/3L (Fig. 5). The highest total fruit weight in the control plants might be related to three main traits including ripe fruit weight, HI, and the ratio of the ripe fruits to the total fruits (Fig. 13). Improvement in yield was not correlated with leaf carbohydrate content as shown in the PCA (Fig. 13). This showed that increased yield is associated with translocation of carbohydrates toward sinks. Assimilate distribution into the fruit in tomato strongly depends on the number of fruits per truss. Fruit pruning increased the available assimilate for the remaining fruits and individual fruit weight was affected (Gautier et al. 2001). On the other hand, Ronga et al. (2019) have reported that the primary yield components of tomato are fruit number and fruit weight. As 1F/3L plants showed the highest individual fruit weight and lowest total fruit weight (Fig. 5), the current study could conclude that the number of fruits per plant, rather than individual fruit weight could be mainly responsible for regulating tomato yield under the present experimental conditions. The PCA analysis also supports this conclusion (Fig. 13).
Altering the sink-source balance by truss pruning treatments had no influence on chlorophyll and protein content (Fig. 9a, b, c). Chlorophyll and soluble protein participate as the light-harvesting and light-utilizing components of the photosynthetic machinary in leaves, respectively (Kim et al. 2009). The insignificant influence of the sink/source ratio on chlorophyll and soluble protein contents showed that the sink/source ratio did not affect light-harvesting and light-utilizing components of photosynthesis. These results have also been confirmed by Yasumura (2009) and Petridis et al. (2020) in the case of chlorophyll and protein contents, and Matsuda et al. (2011) in the case of the chlorophyll content. Nitrite reductase is closely associated with photosynthetic electron transport and can slow down or even stop under situations less supportive for photosynthesis (Lea et al. 2004). The results, therefore, showed that nitrate reductase activity was unaffected by truss pruning (Fig. 9d). This is another piece of evidence showing that applied sink/source ratio did not affect light-harvesting and light-utilizing components of photosynthesis under the current experimental conditions.
Development of tomato fruits proceeds through four stages; fruit set, intensive cell division, followed by cell expansion during which fruit volume increases and the final ripening stage. The fruit sink activity changes along developmental stages (Gillaspy et al. 1993). On the other hand, adjacent leaves have a more important role in providing assimilates (Barzegar et al. 2013) and may be affected by fruit development. To obtain further understanding regarding the biochemical status of leaves in response to sink activity changes of fruits along the developmental stages, adjacent leaves of the third truss were subjected for the study. The current study results showed that chlorophyll and protein contents of leaves were degraded from 15 to 34-DAT and 30 to 45-DAT, respectively. These degradations were almost same in the investigated sink/source ratios and cultivars (Fig. 10a, b, c, d, e, f). It is well known that photosynthetic rate changes with leaf age. It is generally high in young expanded leaves and decreases with the leaf senescence (Bielczynski et al. 2017). The same decline in chlorophyll and protein contents in different sink/source ratios with the elapse of time suggested that chlorophyll and protein decompositions could be done independently of the sink/source ratio. The nitrate reductase activity of the two cultivars at all sink/source ratios were decreased significantly from 30 to 45-DAT (Fig. 10g, h). As low sugar represses the nitrate reductase gene expression and activity (Klein et al. 2000), likely the reduction in sugar content of leaves (Fig. 12) due to transfer to the fruits has led to a decrease in the activity of this enzyme. On the other hand, the reduction in protein content from 30 to 45-DAT can also exacerbate nitrate reductase activity.
Reduced sink/source ratio caused carbohydrate accumulation in leaves (Fig. 9) which agreed with Nakano et al. (2000), Matsuda et al. (2011), and Zhou and Quebedeaux (2003). It has been proposed that the sink/source ratio regulates the balance between production and utilization of assimilates. Zhou and Quebedeaux (2003) and Chikov et al. (2015) reported that the accumulation of carbohydrates in the source leaves leads to a decrease of photosynthetic rates through the suppressed expression of genes coding the photosynthetic system. Based on the present results, neither chlorophyll nor protein content (as components that effect on photosynthesis) was affected by treatments. So, the current study can conclude that in the current experimental conditions, the levels of carbohydrates accumulated in the leaves were not high enough to suppress the light-harvesting components of the photosynthetic machinery. But, because of the same NAR under different treatments, while LAI increased with decreasing sink/source ratio, it can be concluded that besides maintenance respiration the accumulation of carbohydrates in the leaf blade has affected other components of the photosynthesis.
Formed photo-assimilates in the leaves were transferred (as sucrose) through phloem into sinks, where these photo-assimilates can be used for growth and development or stored (Zhang et al. 2020). The decline of the carbohydrates content in leaf blades along fruit developmental stages (Fig. 12) showed the increasing demand of sink activity with elapse of time that could lead to the mobilization of some of the stored carbohydrates from the leaves blade to the developing sinks. The concentration of the starch in 3F/3L and control treatments was significantly lower than others at 30-DAT, which show higher sink demand of 3F/3L and control treatments at that stage.
Conclusion
Altering the sink/source ratio changed the distribution of assimilates between vegetative and reproductive organs. Under the present experimental conditions, extra carbohydrates that cannot be used for growth remained in leaves to keep the net assimilation rate constant while the leaf area index increased by decreasing the sink/source ratio. The response of tomato plants to sink/source ratio in the present experimental condition was not cultivar specific and since the individual weight of fruits increased with decreasing fruit number per trusses, the growth of individual fruits in both cultivars was source-limited. The source-limitation in tomato plants occurred during all examined sink developmental stages. The practical implication of this study is that truss pruning of both examined cultivars can help farmers to increased individual fruit weight. Also, it is possible that increasing the rate of inflorescence formation and continuous inflorescence production by reducing the number of fruits per truss may increase yield, which requires further studies. Future studies should be carried out to determine the best level of sink/source ratio that in addition to producing an acceptable amount of yield, meets the needs of consumers in the current stressful world by increasing the antioxidant and nutriceutical compounds in fruits.
Abbreviations
- ANOVA
Analysis of variance
- CGR
Crop growth rate
- DAT
Days after treatment
- LAI
Leaf area index
- LSD
Least significant difference
- NAR
Net assimilation rate
- PAR
Photosynthetic active radiation
- PCA
Principal component analysis
- SLA
Specific leaf area
Author contributions
LA, MG and MM proposed, organized, planned and carried out the experiment. Further, LA wrote the manuscript. Finally, all authors commented and contributed to the preparation of the final manuscript.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Leila Aslani, Email: l.aslani@ag.iut.ac.ir.
Mahdiyeh Gholami, Email: mah.gholami@iut.ac.ir.
Mostafa Mobli, Email: mobli@iut.ac.ir.
Mohammad Reza Sabzalian, Email: sabzalian@iut.ac.ir.
References
- Al Mamun Hossain SH, Wang L, Chien T. Leaf area index assessment for tomato and cucumber growing period under different water treatment. Plant Soil Environ. 2017;63:461–467. [Google Scholar]
- Andrade D, Covarrubias MP, Benedetto G, Pereira EG, Almeida AM. Differential source-sink manipulation affects leaf carbohydrate and photosynthesis of early and late-harvest nectarine varieties. Theor Exp Plant Physiol. 2019;31:341–356. [Google Scholar]
- Araya T, Noguchi K, Terashima I. Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant Cell Physiol. 2006;47:644–652. doi: 10.1093/pcp/pcj033. [DOI] [PubMed] [Google Scholar]
- Barzegar T, Badeck FW, Delshad M, Kashi AK, Berveiller D, Ghashghaie J. 13C-labelling of leaf photoassimilates to study the sink-source relationship in two Iranian melon cultivars. Sci Hortic. 2013;151:157–164. [Google Scholar]
- Bielczynski LW, Laqcki MK, Hoefnggels I, Gambin A, Croce R. Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiol. 2017;175:1634–1648. doi: 10.1104/pp.17.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the determination of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campillo C, Garcia MI, Daza C, Prieto MH. Study of a nondestructive method for estimating the leaf area index in vegetable crop using digital image. HortScience. 2010;45:1459–1463. [Google Scholar]
- Chikov VI, Akhtyamova GA, Batasheva SN, Mikhailov AL, Khamidullina LA, Timofeeva OA. Effect of silencing of the apoplastic invertase gene on photosynthesis in tomato. Russ J Plant Physiol. 2015;62:39–44. [Google Scholar]
- Dall'Amico M, Hornsteiner M. A simple method for estimating daily and monthly mean temperature from daily minima and maxima. Int J Climatol. 2006;26:1929–1936. [Google Scholar]
- Fabre D, Dingkuhn M, Yin X, Clement-Vidal A, Roques S, Soutiras A, Luquet D. Genotypic variation in source and sink traits affects the response of photosynthesis and growth to elevated atmospheric CO2. Plant Cell Environ. 2019;43:579–593. doi: 10.1111/pce.13693. [DOI] [PubMed] [Google Scholar]
- Firouzabadi AG, Raeini-Sarjaz M, Shahnazari A, Zareabaneh H. Non-destructive estimation of sunflower leaf area and leaf area index under different water regime managements. Arch Agron soil Sci. 2015;61:1357–1367. [Google Scholar]
- Gautier H, Guichard S, Tchamitchian M. Modulation of competition between fruits and leaves by flower pruning and water fogging, and consequence on tomato leaf and fruit growth. Ann Bot. 2001;88:645–652. [Google Scholar]
- Gholami Zali A, Ehsanzadeh P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind Crops Prod. 2018;111:133–140. [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldammer T. Greenhouse management: a guide to operations and technology. Centreville, Virginia: Apex publishers; 2019. [Google Scholar]
- Gulias J, Flexas J, Mus M, Cifre J, Lefi E, Medrano H. Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non-endemic Mediterranean species. Ann Bot. 2003;92:215–222. doi: 10.1093/aob/mcg123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RD, Morris WL, Ducreux LJ, Morris JA, Usman M, Verrall SR, Fuller J, Simpson CG, Zhang R, Hedley PE, Taylor MA. Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell Environ. 2014;37:439–450. doi: 10.1111/pce.12168. [DOI] [PubMed] [Google Scholar]
- Heuvelink E, Dorais M. Crop growth and yield. In: Heuvelink E, editor. Tomatoes. Wallingford: CABI Publishing; 2005. pp. 81–144. [Google Scholar]
- Higashid T, Heuvelink E. Physiological and morphological changes over 50 years in yield components in tomato. J Am Soc Hortic Sci. 2009;134:460–465. [Google Scholar]
- Hofius D, Bornke FAJ. Photosynthesis, carbohydrate metabolism and source-sink relations. In: Vreugdenhil D, Bradshaw J, Gebhardt C, Govers F, Taylor M, MacKerron D, Ross H, editors. Potato biology and biotechnology. Amsterdam: Elsevier; 2007. pp. 257–285. [Google Scholar]
- Hurd RG, Gay AP, Mountifeld AC. The effect of partial flower removal on the relation between root, shoot and fruit growth in the indeterminate tomato. Ann Appl Biol. 1979;93:77–89. [Google Scholar]
- Ireland C. Experimental statistics for agriculture and horticulture. UK: Writtle College; 2010. [Google Scholar]
- Jiang G, Johkan M, Hohjo M, Tsukagoshi S, Ebihara M, Nakaminami A, Maruo T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci Hortic. 2017;222:221–229. [Google Scholar]
- Johnson IR, Thornley J. Vegetative crop growth model incorporating leaf area expansion and senescence, and applied to grass. Plant Cell Environ. 2006;6:721–729. [Google Scholar]
- Jorquera-Fontena E, Alberdi M, Franck N. Pruning severity affects yield, fruit load and fruit and leaf traits of ‘Brigitta’ blueberry. J Soil Sci Plant Nutr. 2014;14:855–868. [Google Scholar]
- Kim EH, Li XP, Razeghifard R, Anderson JM, Niyogi KK, Pogson BJ, Chow WS. The multiple roles of light-harvesting chlorophyll a/b-protein complex define structure and optimize function of Arabidopsis chloroplasts: a study using two chlorophyll b less mutants. Biochim Biophys Acta Bioenergetics. 2009;1787:973–984. doi: 10.1016/j.bbabio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Klein D, Morcuende R, Stitt M, Krapp A. Regulation of nitrate reductase expression in leaves by nitrate and nitrogen metabolism is completely overridden when sugars fall below a critical level. Plant Cell Environ. 2000;23:863–871. [Google Scholar]
- Kumar U, Singh P, Boote KJ. Chapter two-effect of climate change factors on processes of crop growth and development and yield of groundnut (Arachis hypogaea L.) Adv Agron. 2012;116:41–69. [Google Scholar]
- Lakchamenkumar P, Guru SK. Growth indices of yield variability in wheat (Triticum aesitivum L.) under varying degree of shade. J Hill Agric. 2014;5:525–530. [Google Scholar]
- Lea US, Ten Hoopen F, Provan F, Kaiser WM, Meyer C, Lillo C. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta. 2004;219:59–65. doi: 10.1007/s00425-004-1209-6. [DOI] [PubMed] [Google Scholar]
- Matsuda R, Suzuki K, Nakano A, Higashide T, Takaichi M. Responses of leaf photosynthesis and plant growth to altered source-sink balance in a Japanese and a Dutch tomato cultivar. Sci Hortic. 2011;127:520–527. [Google Scholar]
- Mayoral ML, Plaut Z, Reinhold L. Effect of translocation-hinding procedures on source leaf photosynthesis in cucumber. Plant Physiol. 1985;77:712–717. doi: 10.1104/pp.77.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. Growth stages of mono-and dicotyledonous plants: BBCH-Monograph. Berlin: Open agrar repositorium; 2001. [Google Scholar]
- McCashin BG (2000) Association for biology laboratory education: induction of nitrate reductase in plant shoots. Department of biological sciences. The university of Alberta. Edmonton https://www.zoo.utoronto.ca/able
- Mohamed HB, Vadel AM, Genus JMC, Khemira H. Biochemical changes in dormant grapevine shoot tissues in response to chilling: possible role in dormancy release. Sci Hortic. 2010;124:440–447. [Google Scholar]
- Nakano H, Muramatsu S, Makino A, Mae T. Relationship between the suppression of photosynthesis and starch accumulation in the pod-removed bean. Aust J Plant Physiol. 2000;27:167–173. [Google Scholar]
- Pallas B, Bluy S, Ngao J, Martinez S, Clement-Vidal A, Kelner JJ, Costes E. Growth and carbon balance are differently regulated by tree and shoot fruiting contexts: an integrative study on apple genotypes with contrasted bearing patterns. Tree Physiol. 2018;38:1395–1408. doi: 10.1093/treephys/tpx166. [DOI] [PubMed] [Google Scholar]
- Patel SN, Patil HE, Popat RC. Genetic diversity study in finger millet (Eleusine coracana L.) genotypes: a multivariate analysis approach. Int J Pure Appl Biosci. 2017;5:183–189. [Google Scholar]
- Pastenes C, Villalobos L, Rios N, Reyes F, Turgeon R, Franck N. Carbon partitioning to berries in water stressed grapevines: the role of active transport in leaves and fruits. Environ Exp Bot. 2014;107:154–166. [Google Scholar]
- Petridis A, Kaay J, Sungurtas J, Verrall SR, McCallum S, Graham J, Hancock RD. Photosynthetic plasticity allows blueberry (Vaccinium corymbosum L.) plants to compensate for yield loss under conditions of high sink demand. Environ Exp Bot. 2020;174:10431. [Google Scholar]
- Queiroga RCF, Puiatti M, Fontes PCR, Cecon PR. Yield and quality of muskmelon fruits varying fruit and leaf numbers per plant. Hortic Bras. 2008;26:209–215. [Google Scholar]
- Robin R, Cathy LR, Steven KO, Keith RF, Daniel MD, William LB. Starch determination by perchloric acid vs enzymes: evaluating the accuracy and precision of six colorimetric methods. J Agrc Food Chem. 1991;39:2–11. [Google Scholar]
- Ronga D, Francia E, Rizza F, Badeck FW, Garadonia F, Montevecchi G, Pecchioni N. Changes in yield components, morphological, physiological and fruit quality traits in processing tomato cultivated in Italy since the 1930’s. Sci Hortic. 2019;257:108726. [Google Scholar]
- Salazar-Parra C, Aranjuelo I, Pascual I, Erice G, Sanz-Saez A, Aguirreolea J, Sanchez-Diaz M, Irigoyen JJ, Araus JL, Morales F. Carbon balance, partitioning and photosynthetic acclimation in fruit-bearing grapevine (Vitis vinifera L. cv. Tempranillo) grown under simulated climate change (elevated CO2, elevated temperature and moderate drought) scenarios in temperature gradient greenhouses. J Plant Physiol. 2015;174:97–109. doi: 10.1016/j.jplph.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Fernie AR. Next-generation strategies for understanding and influencing source-sink relations in crop plants. Curr Opin Plant Biol. 2018;43:63–70. doi: 10.1016/j.pbi.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Fujita K. Nutrio-physiological studies on the tomato plant IV: source-sink relationship and structure of the source-sink unit. Soil Sci Plant Nutr. 1974;20:305–315. [Google Scholar]
- Vasseur F, Pantin F, Vile D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011;34:1563–1576. doi: 10.1111/j.1365-3040.2011.02353.x. [DOI] [PubMed] [Google Scholar]
- Wilson C, Liu X, Lesch SM, Suarez DL. Growth response of major USA cowpea cultivars II: effect of salinity on leaf gas exchange. Plant Sci. 2006;170:1095–1101. [Google Scholar]
- Yan ST, Li XD, Li WD, Fan PG, Duan W, Li SH. Photosynthesis and chlorophyll flurescence response to low sink demand of tubers and roots in Dahlia pinnata source leaves. Biol Plant. 2011;55:83–89. [Google Scholar]
- Yasumura Y. The effect of altered sink-source relations on photosynthetic traits and matter transport during the phase of reproductive growth in the annual herb Chenopodium album. Photosynthetica. 2009;47:263–270. [Google Scholar]
- Zhang M, Dongyuan S, Zuirong N, Jixuan Y, Xiaolei Z, Kang X. Effects of combined organic/inorganic fertilizer application on growth, photosynthetic characteristics, yield and fruit quality of Actinidia chinesis cv ‘Hongyang’. Glob Ecol Conserv. 2020;22:e00997. [Google Scholar]
- Zhou R, Quebedeaux B. Changes in photosynthesis carbohydrate metabolism in mature apple leaves in response to whole plant source-sink manipulation. J Am Soc Hortic Sci. 2003;128:113–119. [Google Scholar]