Abstract
Shigella flexneri has been a major public health problem in developing countries. This work analyzed the frequency of 16 virulence genes, the genotypic diversity, and the antimicrobial resistance profiles of 130 S. flexneri strains isolated in Brazil. The ipaH gene was found in all the 130 strains. The frequencies of the other genes were variable ial (88.5%), sigA (82.3%), iuc (74.6%), virA (73%), pic (72.3%), virF (57.7%), sat (48.5%), ipaBCD (37%), sen (36%), set1A (35.4%), sepA (30%), set1B (30%), virB (14%), icsA (10%), and ipgD (5.4%). A total of 57 (43.8%) strains were multidrug-resistant. ERIC-PCR grouped 96 of the strains into a single cluster with ≥ 70.4% of similarity, 75 of these strains presented a similarity ≥ 80.9%. PFGE grouped 120 of the strains into a single cluster with 57.4% of similarity and 82 of these strains presented a similarity ≥ 70.6%. In conclusion, the high frequency of some virulence genes reinforces the pathogenic potential of the strains studied. The high rates of MDR strains are alarming once it may lead to failure when antimicrobial treatment is necessary. Genotype techniques reveled a major cluster with high genetic similarity including S. flexneri strains from the different Brazilian states and distinct years of isolation, showing that they probably emerged from a common ancestor.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00332-y) contains supplementary material, which is available to authorized users.
Keywords: Shigella flexneri, Virulence genes, Antimicrobial resistance, ERIC-PCR, PFGE
Introduction
Shigellosis has been a major public health problem worldwide, particularly in developing countries where it is endemic [1, 2]. The World Health Organization (WHO) estimated an occurrence of approximately 191 million cases globally in 2010 [2]. Shigella spp. has been reported as one of the most common bacterial pathogens affecting children from one to 5 years of age in the world [3].
In Brazil, among the pathogens that cause gastroenteritis, Shigella spp. has been reported among the four most isolated bacteria of diarrheal feces, with a prevalence ranging from 8 to 10% in children under 1 year of age, and from 15 to 18% in children older than 2 years [4]. Specifically, the highest rates of isolation were reported in the Southeast (40%) and Northeast (34%) regions, while in the North, Central West and South regions, the rates are lower with a percentage of 13%, 10%, and 3%, respectively. The most frequently reported species in these cases has been S. flexneri (53%), with a higher incidence in Northeastern Brazil [1].
Shigellosis is transmitted by the ingestion of contaminated water and food or through fecal-oral contact and its capacity to cause disease depends on genes contained in an invasion plasmid pINV of 220 Kb, such as ipaH, ipaBCD, ial, sen, virA, virB, virF, icsA, sepA, and ipgD and on chromosomal genes, ipaH, iuc, sat, sigA, pic, set1A, and set1B [5].
Monitoring resistant Shigella spp. strains is essential for the effectiveness of therapy when it is necessary [6, 7]. In recent years, it has been observed that Shigella spp. strains are resistant to the most commonly used antibiotics in clinical therapy, such as tetracycline, chloramphenicol, trimethoprim-sulfamethoxazole, and ampicillin [8]. According to recent recommendations of the American Society for Infectious Diseases (IDSA), ceftriaxone—a third-generation cephalosporin, and the quinolones, ciprofloxacin, and norfloxacin—are drugs of choice to treat shigellosis. However, several strains of different species of the genus Shigella have become resistant to these antimicrobials in many countries [9, 10].
Regarding molecular typing methods to type S. flexneri, Pulsed-field gel electrophoresis (PFGE) and Enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) have been successfully used to study the genotypic diversity of these strains, among others methodologies [11–14].
In Brazil, most of the studies only investigated the occurrence of Shigella in different regions of the country, as well as the antimicrobial resistance profiles of the strains [12, 15–18]. Specifically, regarding S. flexneri, few studies have been performed in this country to characterize genotipically and phenotypically strains of this important pathogen [2, 12, 18, 19].
In light of this, the aims of this study were to analyze the genotypic diversity, the frequency of important virulence genes, and the antimicrobial resistance profiles of S. flexneri strains isolated from human diarrheal feces in the different states of Brazil over 34 years.
Material and methods
Bacterial strains
A total of 130 S. flexneri strains isolated from human diarrheal feces from 1983 to 2017 in Amapá and Pará States (Northern region), Bahia, Ceará, Maranhão, Pernambuco, Sergipe and Rio Grande do Norte States (Northeast region), Rio Grande do Sul (South region) and Minas Gerais, Rio de Janeiro, and São Paulo states (Southeast region) (Table 1). These strains were selected from the collections of the reference laboratories of the Oswald Cruz Institute of Rio de Janeiro (FIOCRUZ) and the Adolfo Lutz Institute of Ribeirao Preto (IAL-RP) in Brazil. They were systematically chosen to represent isolates from sporadic cases and outbreaks of the two collections of the reference laboratories mentioned above that occurred during different years and places of isolation. The S. flexneri strains were confirmed by biochemical and serological tests (Probac in Brazil).
Table 1.
Years and states of isolation of the 130 Shigella flexneri strains isolated from diarrheal feces of humans with shigellosis from 1983 to 2017 in Brazil
| Year | States of isolation (no of strains) |
|---|---|
| 1983 | SP (10) |
| 1984 | SP (9) |
| 1985 | SP (7) |
| 1986 | SP (3) |
| 1987 | SP (4) |
| 1988 | SP (5) |
| 1989 | SP (4) |
| 1990 | SP (2) |
| 1991 | SP (2) |
| 1992 | SP (3) |
| 1993 | SP (3) |
| 1994 | SP (5) |
| 1995 | SP (3) |
| 1996 | SP (4) |
| 1997 | SP (5) |
| 1998 | SP (7) |
| 1999 | SP (1) |
| 2000 | SP (5) |
| 2001 | SP (1) |
| 2002 | SP (1) |
| 2003 | SP (3) |
| 2004 | SP (1) |
| 2005 | SP (1) |
| 2006 | SP (1) |
| 2010 | RS (2)/PA (2)/SP (1) |
| 2011 | RS (1)/RN (1) |
| 2012 | RJ (1)/RS (1)/PE (1)/RS (1)/MA (1) |
| 2013 | SP (5)/RN (1)/RJ (2)/PE (4)/RS (2)/MG (1) |
| 2014 | CE (2)/RS (3) |
| 2015 | AP (2)/RS (1)/MA (1)/PE (2) |
| 2017 | BA (1)/RS (1)/SE (1) |
AP, Amapá; BA, Bahia; CE, Ceará; MA, Maranhão; MG, Minas Gerais; PA, Pará; PE: Pernambuco; RJ, Rio de Janeiro; RN, Rio Grande do Norte; RS, Rio Grande do Sul; SE, Sergipe; SP, São Paulo
Detection of virulence genes by PCR
The detection of virulence genes was performed for the 130 S. flexneri strains studied (Table 1). The genomic DNA was extracted according to Campioni & Falcão (2014) [20]. The general PCR procedure was performed according to Falcão et al. (2006) [21]. Briefly, the conditions used for the PCR were as follows: initial incubation at 94 °C for 5 min; 35 cycles of 45 s at a specific temperature of each primer virulence gene (Table S1), 1 min at 72 °C and 15 min at 72 °C; and a final incubation 72 °C for 15 min. The primers used and the PCR conditions of each virulence gene investigated were described previously in the following references: ipaH and iuc [22]; ipaBCD [23]; ial [24]; set1A and set [25]; sen [26]; sat, sigA, pic, and sepA [27], virF [28], ipgD [29], virA [30], virB [31], and icsA [32]. A template PCR without DNA was used as a blank and Campylobacter jejuni ATCC 33291 was used as a negative control. The positive controls were S. flexneri ATCC 12022 and S. sonnei ATCC 25931. The PCR products were analyzed by agarose gel electrophoresis and visualized by UV light after staining with ethidium bromide (0.5 μg ml−1).
Antimicrobial resistance
The antimicrobial susceptibility test was performed for the 130 S. flexneri strains studied and listed in Table 1, using the disk diffusion method, following the guidelines described by the Clinical Laboratory Standards Institute–CLSI (2018). The antimicrobial agents tested were based on the CLSI (2018) [33] including those recommended for human treatment, and those described by de Paula et al. (2010) [17], Bastos & Loureiro (2011) [7], Nunes et al. (2012) [6], and Zhang et al. (2014) [34]. The antimicrobials tested were (μg per disc unless otherwise stated): nalidixic acid (30), ampicillin (10), gentamicin (10), amoxicillin-clavulanic acid (30), cefepime (30), cefotaxime (30), ciprofloxacin (5), levofloxacin (5), imipenem (10), ampicillin-sulbactam (20), trimethoprim-sulfamethoxazole (25), ceftazidime (30), chloramphenicol (30), tetracycline (30), ofloxacin (5), and piperacillin (100) (all from Oxoid). Escherichia coli ATCC 25922 was used as a quality control strain.
ERIC-PCR typing and analysis
The ERIC-PCR assay was performed for all 130 S. flexneri strains studied and listed in Table 1. The primers used were ERIC1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′), as described by Versalovic et al. (1991) [35]. Reactions without the DNA template were used as negative controls. The ERIC-PCR reactions were repeated twice for each strain to verify the reproducibility of the experiment. The ERIC-PCR products were resolved by 1.5% agarose gel electrophoresis, stained with ethidium bromide (0.5 μg ml−1) and revealed under UV light. The data obtained were analyzed using the Bionumerics 7.0 software package (Applied Maths). Only bands representing amplicons between 300 and 5000 bp in size were included in the analysis. A similarity dendrogram was constructed using the unweighted-pair group method (UPGMA) with the Dice similarity coefficient and a position tolerance of 1.5%. A standard molecular weight ladder (1 kb plus DNA Ladder; Invitrogen-Life Technologies) was included three times on each gel to normalize the images and to allow comparisons of the fingerprints on different gels.
PFGE typing and analysis
PFGE typing was performed using the standard CDC PulseNet protocol for Shigella spp. (Ribot et al., 2006) [36]. The S. flexneri strains listed in Table 1 were cultured in Trypticase Soy Agar (Kasvi) and incubated for 12–18 h at 37 °C. The cells were diluted in a cell suspension buffer (100 mM Tris, 100 mM EDTA; pH 8.0) to an O.D610 of 0.8–1.0 genomic DNA was digested with 50 U XbaI restriction enzyme (Thermo Scientific) at 37 °C for 2 h. Macrorestriction fragments were resolved by counter-clamped homogeneous electric Field electrophoresis in a CHEF-DRIII apparatus (Bio-Rad Laboratories). The pulse times were ramped from 2.2–54.2 s over 19 h, as described by Ribot et al. (2006) [36]. Salmonella Braenderup H9812 digested with 40 U XbaI at 37 °C for 2 h was used as a molecular mass standard and was included three times on each gel to normalize the images and to allow comparisons of the fingerprints over multiple gels. The gels were stained with ethidium bromide (0.5 μg ml−1) for 30 min and destained in distilled water for 60–80 min. The DNA fragments were then visualized under UV light. The genomic profile obtained with PFGE was analyzed using the Bionumerics 7.0 software package (Applied Maths). A similarity dendrogram was constructed using the unweighted-pair group method (UPGMA) with the Dice similarity coefficient and a position tolerance of 1.5%.
Discrimination index
The discriminatory power of PFGE and ERIC-PCR was assessed by Simpson’s diversity index, as described by Hunter & Gaston (1988) [37].
Results
Virulence genes
All the 130 strains studied (Table 1) presented the ipaH gene. The ial (88.5%), sigA (82.3%), iuc (74.6%), virA (73%), and pic (72.3%) genes were found in more than 70% of the strains studied. The ipaBCD (37%), sen (36%), set1A (35.4%), sepA (30%), and set1B (30%) of strains were studied. The sat and virF genes were found in 48.5% and 57.7% of the strains, respectively. The ipgD (5.4%), icsA (10%), and virB (14%) genes were found in less than 40% of the strains studied.
Antimicrobial resistance
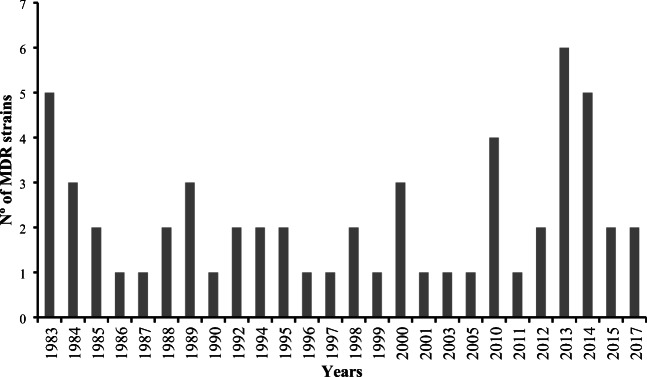
All the 130 S. flexneri strains studied (Table 1) were susceptible to the antimicrobials ciprofloxacin, levofloxacin, and ofloxacin. On the other hand, the highest resistance rates were against tetracycline observed in 74 (57%) strains, trimethoprim-sulfamethoxazole observed in 70 (54%) strains, and ampicillin found in 69 (53%) strains. Moreover, 56 (43%) strains were resistant to chloramphenicol (Table 2). The most frequent resistance profile found was tetracycline and trimethoprim-sulfamethoxazole (STX-TE), with 14 (10.7%) S. flexneri strains studied showing this profile. The frequency of resistance to all the 16 antimicrobials tested in the 130 S. flexneri strains studied is described in Table 2. Fifty-seven (43.8%) strains were multidrug-resistant (MDR) (Fig. 3).
Table 2.
Frequency of resistance to different antimicrobials in the 130 Shigella flexneri strains studied
| Antimicrobial classes | Antimicrobial | Total strains (%) |
|---|---|---|
| Carbapenems | IMP | 1 (0.7%) |
| Cephalosporins | FEP | 2 (1.5%) |
| CAZ | 4 (3%) | |
| CTX | 5 (4%) | |
| Quinolones | CIP | – |
| LVX | – | |
| OFLX | – | |
| NA | 2 (1.5%) | |
| Penincillins | PIP | 21 (16%) |
| AMP | 69 (53%) | |
| β-lactamase inhibitor combinations | AMC | 6 (4.6%) |
| SAM | 18 (14%) | |
| Aminoglycosides | CN | 2 (1.5%) |
| Tetracyclines | TE | 74 (57%) |
| Folate pathway inhibitors | SXT | 70 (54%) |
| Phenicols | C | 56 (43%) |
IMP, imipenem; FEP, cefepime; CAZ, ceftazidime; CTX, cefotaxime; CIP, ciprofloxacin; LVX: levofloxacin; OFLX, ofloxacin; NA, nalidixic acid; PIP, piperacillin; AMP, ampicillin; AMC, amoxicillin-clavulanic acid; SAM, ampicillin-sulbactam; CN, gentamicin; TE, tetracycline; SXT, trimethoprim-sulfamethoxazole; C, chloramphenicol
Fig. 3.
Frequency of the 57 multidrug-resistant (MDR) Shigella flexneri strains studied over 34 years
ERIC-PCR
The dendrogram generated by ERIC-PCR differentiated the 130 S. flexneri strains into 64 ERIC types and grouped the strains into two clusters designated ERIC-A and ERIC-B with 47.8% of similarity (Fig. 1). The ERIC-A cluster comprised 122 strains isolated in the Amapá, Ceará, Maranhão, Minas Gerais, Pará, Pernambuco, Rio Grande do Norte, Rio Grande do Sul, São Paulo, and Sergipe State between 1983 and 2017 with 61.1% of genetic similarity. Specifically, ERIC-A cluster comprised 96 strains with ≥ 70.4% of genetic similarity in a subcluster designated ERIC-A1. The ERIC-B cluster grouped eight strains isolated from the States of Rio Grande do Sul, Maranhão, Bahia, and São Paulo state from 1984 and 2017 with a genetic similarity ≥ 48.8% among each other.
Fig. 1.

Dendrogram representing genetic relationships among 130 Shigella flexneri strains based on ERIC-PCR fingerprints. Similarity (%) between patterns was calculated by using the Dice index and is represented by the numbers beside the nodes. The data were sorted by the UPGMA method. States abbreviations: AP: Amapá; BA: Bahia; CE: Ceará; MA: Maranhão; MG: Minas Gerais, PA: Pará; PE: Pernambuco; RJ: Rio de Janeiro; RN: Rio Grande do Norte; RS: Rio Grande do Sul; SE: Sergipe; SP: São Paulo. Antibiotics abbreviations: IMP: Imipenem; FEP: Cefepime; CAZ: Ceftazidime; CTX: Cefotaxime; NA: Nalidixic acid; PIP: Piperacillin; AMP: Ampicillin; AMC: Amoxicillin-clavulanic acid; SAM: Ampicillin-sulbactam; CN: Gentamicin; TE: Tetracycline; SXT: Trimethoprim-sulfamethoxazole; C: Chloramphenicol
PFGE
The dendrogram generated by PFGE grouped the 130 S. flexneri strains into two clusters designated PFGE-A and PFGE-B (Fig. 2). The PFGE-A cluster comprised 120 strains isolated from the states of Amapá, Bahia, Ceará, Maranhão, Minas Gerais, Pernambuco, Rio de Janeiro, Rio Grande do Norte, Rio Grande do Sul, São Paulo and Sergipe, and states between 1983 and 2017 with 57.4% of genetic similarity. Specifically, 74 strains of this cluster presented a similarity above 70.6% among each other. The PFGE-B cluster comprised 10 strains isolated from the states of São Paulo, Pará, and Rio Grande do Sul between 1984 and 2011 with ≥ 65.6% of genetic similarity.
Fig. 2.

Dendrogram representing genetic relationship among 130 Shigella flexneri strains based on PFGE fingerprints. Similarity (%) between patterns was calculated by using the Dice index and is represented by the numbers beside the nodes. The data were sorted by the UPGMA method. States abbreviations: AP: Amapá; BA: Bahia; CE: Ceará; MA: Maranhão; MG: Minas Gerais, PA: Pará; PE: Pernambuco; RJ: Rio de Janeiro; RN: Rio Grande do Norte; RS: Rio Grande do Sul; SE: Sergipe; SP: São Paulo. Antibiotics abbreviations: IMP: imipenem; FEP: cefepime; CAZ: ceftazidime; CTX: cefotaxime; NA: nalidixic acid; PIP: piperacillin; AMP: ampicillin; AMC: Amoxicillin-clavulanic acid; SAM: ampicillin-sulbactam; CN: gentamicin; TE: tetracycline; SXT: trimethoprim-sulfamethoxazole; C: chloramphenicol
Discrimination index
The DI was of 0.98 for ERIC-PCR and 0.99 for PFGE.
Discussion
The present study searched for the frequency of 16 virulence genes and verified the antimicrobial resistance profile against 16 drugs in 130 S. flexneri strains isolated from human diarrheal feces in different states of Brazil over a 34-year period. Moreover, the genotypic diversity of these strains was verified by ERIC-PCR and PFGE.
We observed a high frequency of virulence genes among the 130 S. flexneri strains studied. All the 130 strains presented the ipaH gene. The ial, sigA, iuc, virA, and pic genes were found in more than 70% of the strains studied. Moreover, sat and virF genes were found in 48.5% and 57.7% of the strains, respectively. On the other hand, ipaBCD, ipgD, icsA, virB, sepA, set1B, set1A, and sen genes were found in less than 40% of the strains studied.
Similarly to our results, other studies conducted in Peru, Bangladesh, China, and Iran also found the ipaH gene in 100% of the S. flexneri strains studied. However, the frequencies of other genes were variable and different from the ones of this present study [38].
Of note, the frequencies of some virulence genes, such as sat, pic, and sepA genes were higher in other studies performed by Souza et al. (2013), Lluque et al. (2015) and Shahnaij et al. (2018) that analyzed S. flexneri strains from Brazil, Peru, and Bangladesh [18, 38]. It is important to mention that a high frequency of the sepA gene was observed in the S. flexneri strains of the studies above mentioned, which is an important indicator of increased clinical severity once this gene was associated to intense abdominal pain and was the major predictor in virulence genes combinations associated with bloody stools [2]. Moreover, Boisen et al. (2012) evaluated the combinations of virulence genes associated with enteroaggregative E. coli infections, and found a strong association between the presence of the sepA gene and diarrhea [39]. Furthermore, Frank et al. (2011) observed that the sepA gene was present in the genome of the Shiga-toxin producing E. coli strain related to a large outbreak of uremic hemolytic syndrome in Europe [40].
The frequencies of the plasmidial genes such as ipaH, ipaBCD, ial, sen, sepA, icsA, virB, virF, ipgD, and virA were different among the strains studied. The regulation of the virulence plasmid of Shigella is induced at 37 °C and repressed at 30 °C [41]. A possible explanation for this variation found is that the strains in this study are from a collection of bacteria maintained at room temperature in a solid medium, and thus this instability in the storage environment could have provided mutations and differences in the populations studied, as well as the loss of the virulence plasmid. Another explanation for this is the plasmid plasticity that naturally occurs during the evolution of these strains, through the migration of mobile genetic elements such as transposons or insertion sequences (ISs) [42].
The published literature from Brazil contains some studies that searched for the presence of a few numbers of virulence genes in strains of S. flexneri isolated from a unique region of the country. However, none of them included strains isolated for a long period in Brazil [2, 16, 18]. The present study demonstrated the high frequency of some important virulence genes in the strains isolated during 34 years in the different states of Brazil highlighting the pathogenic potential of those strains.
The increase of antimicrobial resistance in Shigella strains has been reported worldwide [6, 14, 43]. In this work, 116 (89.2%) S. flexneri strains studied were resistant to at least one of the antimicrobials tested (Table 2). We observed high rates of resistance to the clinically used antibiotics such as tetracycline (57%), trimethoprim-sulfamethoxazole (54%), ampicillin (53%), and chloramphenicol (43%) among the strains studied. This is in accordance with other studies from other parts of the world. Vubil et al. (2018) observed a high level of antimicrobial resistance to ampicillin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole among the isolates analyzed, being S. flexneri much more resistant compared with all the other species [8].
This scenario has led to the use of new-generation antibiotics such as fluoroquinolones, although resistance to these agents has also been reported [44]. In our study, we did not find resistance to fluoroquinolones. However, we found a low number of strains (1.5%) resistant to nalidixic acid (Table 2). Studies have been shown that resistance to nalidixic acid may lead to reduced susceptibility to fluoroquinolones [6, 17, 18].
Furthermore, we found that 43.8% of the 130 strains studied were MDR (Fig. 3). The study of Vubil et al. (2018) with Shigella spp. strains isolated in Mozambique showed that the majority of the MDR isolates were from S. flexneri (47.7%) compared with 7.4% from other species [8]. In Brazil, Sidrim et al., 1998, studied the resistance of 26 S. flexneri strains isolated in the Northeast of Brazil and observed that all strains studied were MDR [45]. Penatti et al. (2007) showed that 90% of the S. flexneri strains isolated in Campinas, State of São Paulo, Brazil, were MDR [46].
The rapid emergence of S. flexneri MDR strains is largely due to their ability to acquire and disseminate exogenous genes associated with mobile genetic elements such as transposons, integrons, plasmids, and other genomic islands [47]. Surveillance programs are needed to monitor antimicrobial resistance and to provide information to guide clinical management.
In the present work, the genotypic diversity of the 130 strains of S. flexneri was assessed by ERIC-PCR and PFGE. The dendrogram generated with ERIC-PCR shows that the ERIC-A1 subcluster comprised 96 strains (73.8%) with a similarity ≥ 70.4% isolated from the States of Pará, Rio Grande do Sul, Rio Grande do Norte, Rio de Janeiro, São Paulo, Sergipe, Amapá, and Pernambuco, between 1984 and 2017. Specifically, 75 strains of this subcluster presented a similarity above 80.9% among them. This fact suggests that besides the geographic distance among those strains; they showed to be genetically related and descending from a common ancestor. In contrast, Penatti et al., 2007 studied S. flexneri isolated from the metropolitan area of Campinas, State of São Paulo, Brazil [46], using ERIC-PCR and found that and all strains were grouped into three main groups, without a predominant cluster [46].
To our knowledge, there are few studies that genetically characterized S. flexneri strains [48–50]. Specifically, only two used ERIC-PCR to type S. flexneri strains [46, 50], and just one of these studies was from Brazil [46]. In general, studies had shown that the S. flexneri strains isolated over the years in other countries, grouped into different clusters without a prevalent subtype. Kosek et al. (2012) typed 97 S. flexneri strains from Peru by ERIC-PCR and the isolates were divided into nine different clusters [13]. Zamanlou et al. (2018) observed the 73 S. flexneri strains isolated from feces of patients with gastroenteritis in Iran, divided into six major clusters and 41 subclusters [50]. In the study of Penatti et al. (2007) all the S. flexneri strains from the southern of Brazil were grouped in three clusters by ERIC-PCR with 30% of genetic similarity among them [46].
Additionally, the dendrogram generated by the PFGE data grouped most of the strains in one major cluster, demonstrating a common ancestor. The PFGE-A cluster grouped 120 strains, among which, 74 were present in the PFGE-A1 subcluster with more than 70% of similarity among them (Fig. 2). Therefore, the PFGE results may suggest that majority of the S. flexneri strains studied descended from a common ancestor and that these strains have prevailed and have been contaminating humans for 34 years in the different states of Brazil.
Data generated by the PFGE analyzes in our study corroborate with the findings from some other studies that also demonstrated that a great number of S. flexneri strains from other countries were grouped in a single cluster, indicating that the strains descend from a common ancestor [8, 51]. Shahnaij et al. (2018) grouped the strains from Bangladesh in a single cluster with a similarity of 80% and this cluster was divided into four subclusters with a similarity of 85% [51].
There are few studies that molecularly characterized S. flexneri using the PFGE technique in Brazil and these studies are not recent. Lima et al. (1997) used PFGE to analyze the molecular epidemiology of S. flexneri strains isolated in Fortaleza, Brazil [52]. The data obtained demonstrated the persistence of certain PFGE patterns as well as the acquisition of multiple antibiotic resistance plasmids. The other study that presented a more recent data is the one of Angellini et al. (2009) that used PFGE to investigate the molecular epidemiology of 119 strains of S. sonnei and S. flexneri isolated from shigellosis cases that occurred in São Paulo State, Brazil [12]. The results indicated the existence of just a few clusters for both species, and the prevalence of specific genotypes in these cities, suggesting the successful adaptation of them.
The ERIC-PCR and PFGE methodologies were efficient and discriminated in a very similar way the S. flexneri strains studied in accordance with the discrimination index (DI), indicating that even monomorphic organisms such as Shigella can be well discriminated by these techniques. In addition, both methods provided consistent and concordant epidemiological information (Figs. 1 and 2). It is important to mention that no differences were observed in the frequency of the virulence genes and/or in the resistance profiles according to the clusters of ERIC-PCR and PFGE.
In conclusion, the high frequency of some virulence genes reinforces the pathogenic potential of the strains studied. The high rates of MDR strains are alarming once it may lead to failure when antimicrobial treatment is necessary. The high genetic similarity observed among a great number of the S. flexneri strains studied circulating in this country may suggest that they descended from a common ancestor. Altogether, these results contributed for a better characterization of S. flexneri strains isolated in Brazil.
Electronic supplementary material
(DOCX 17 kb)
(DOCX 42 kb)
Funding information
We thank Sao Paulo Research Foundation (FAPESP) (Process number 2014/ 13029-0 and 2016/2716-3) and CAPES for financial support (Finance Code 001). During the course of this work, J. C. G. was supported by National Council for Scientific and Technological Development (CNPq) and J. P Falcão recieved a productive fellowship (CNPq) (Process number 303475/2015-3 and 304399/2018-3).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lima IF, Havt A, Lima AA. Update on molecular epidemiology of Shigella infection. Curr Opin Gastroenterol. 2015;31:30–37. doi: 10.1097/MOG.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros PHQS, Lima AM, Guedes MM, Havt A, Bona MD, Rey LC, Soares AM, Guerrant RL, Weigl BH, Lima IFN. Molecular characterization of virulence and antimicrobial resistance profile of Shigella species isolated from children with moderate to severe diarrhea in northeastern Brazil. Diagn Microbiol Infect Dis. 2018;90:198–205. doi: 10.1016/j.diagmicrobio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.The HC, Thanh DP, Holt KE, Thomson NR, Baker S. The genomic signatures of Shigella evolution, adaptation and geographical spread. Nat Rev Microbiol. 2016;14:235–250. doi: 10.1038/nrmicro.2016.10. [DOI] [PubMed] [Google Scholar]
- 4.Brasil (2019) Surtos de doenças transmitidas por alimentos no Brasil. Ministério da Saúde do Brasil. http://portalarquivos.saude.gov.br/images/pdf/2016/dezembro/09/Apresentacao-Surtos-DTA-2016.pdf. Accessed 25 Nov 2019
- 5.Ferreira LG, Campos LC, Martinez MB (2015) Shigella. In: Trabulsi LR, Alterthum F (eds) Microbiologia, 6th edn. Atheneu, São Paulo, pp 343–350
- 6.Nunes MR, Magalhães PP, Penna FJ, Nunes JM, Mendes EN. Diarrhea associated with Shigella in children and susceptibility to antimicrobials. J Pediatr. 2012;88:125–128. doi: 10.2223/JPED.2131. [DOI] [PubMed] [Google Scholar]
- 7.Bastos FC, Loureiro EC. Antimicrobial resistance of Shigella spp. isolated in the state of Pará, Brazil. Rev Soc Bras Med Trop. 2011;44:607–610. doi: 10.1590/s0037-86822011005000051. [DOI] [PubMed] [Google Scholar]
- 8.Vubil D, Balleste-Delpierre C, Mabunda R, Acácio S, Garrine M, Nhampossa T, Alonso P, Mandomando I, Vila J. Antibiotic resistance and molecular characterization of shigella isolates recovered from children aged less than 5 years in Manhiça, southern Mozambique. Int J Antimicrob Agents. 2018;51:881–887. doi: 10.1016/j.ijantimicag.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, Hennessy T, Griffin PM, DuPont H, Sack RB, Tarr P, Neill M, Nachamkin I, Reller LB, Osterholm MT, Bennish ML, Pickering LK, America IDSo Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–351. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 10.Poirel L, Cattoir V, Nordmann P. Plasmid-mediated quinolone resistance; interactions between human, animal, and environmental ecologies. Front Microbiol. 2012;3:24. doi: 10.3389/fmicb.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee TM, Chang CY, Chang LL, Chen WM, Wang TK, Chang SF. One predominant type of genetically closely related Shigella sonnei prevalent in four sequential outbreaks in school children. Diagn Microbiol Infect Dis. 2003;45:173–181. doi: 10.1016/s0732-8893(02)00524-2. [DOI] [PubMed] [Google Scholar]
- 12.Angelini M, Stehling EG, Moretti ML, da Silveira WD. Molecular epidemiology of Shigella spp strains isolated in two different metropolitam areas of southeast Brazil. Braz J Microbiol. 2009;40:685–692. doi: 10.1590/S1517-838220090003000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosek M, Yori PP, Gilman RH, Vela H, Olortegui MP, Chavez CB, Calderon M, Bao JP, Hall E, Maves R, Burga R, Sanchez GM. Facilitated molecular typing of Shigella isolates using ERIC-PCR. Am J Trop Med Hyg. 2012;86:1018–1025. doi: 10.4269/ajtmh.2012.11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seribelli AA, Frazão MR, Medeiros MIC, Falcão JP. Molecular and phenotypic characterization of strains of Shigella sonnei isolated over 31 years suggests the circulation of two prevalent subtypes in São Paulo state, Brazil. J Med Microbiol. 2016;65:666–677. doi: 10.1099/jmm.0.000290. [DOI] [PubMed] [Google Scholar]
- 15.Orlandi PP, Silva T, Magalhães GF, Alves F, de Almeida Cunha RP, Durlacher R, da Silva LH. Enteropathogens associated with diarrheal disease in infants of poor urban areas of Porto Velho, Rondônia: a preliminary study. Mem Inst Oswaldo Cruz. 2001;96:621–625. doi: 10.1590/s0074-02762001000500005. [DOI] [PubMed] [Google Scholar]
- 16.Silva T, Nogueira PA, Magalhães GF, Grava AF, Silva LH, Orlandi PP. Characterization of Shigella spp. by antimicrobial resistance and PCR detection of ipa genes in an infantile population from Porto Velho (Western Amazon region), Brazil. Mem Inst Oswaldo Cruz. 2008;103:731–733. doi: 10.1590/s0074-02762008000700017. [DOI] [PubMed] [Google Scholar]
- 17.de Paula CM, Mercedes PG, do Amaral PH, Tondo EC. Antimicrobial resistance and PCR-ribotyping of Shigella responsible for foodborne outbreaks occurred in southern Brazil. Braz J Microbiol. 2010;41:966–977. doi: 10.1590/S1517-838220100004000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa M, Mendes EN, Collares GB, Péret-Filho LA, Penna FJ, Magalhães PP. Shigella in Brazilian children with acute diarrhoea: prevalence, antimicrobial resistance and virulence genes. Mem Inst Oswaldo Cruz. 2013;108:30–35. doi: 10.1590/s0074-02762013000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Cruz CB, de Souza MC, Serra PT, Santos I, Balieiro A, Pieri FA, Nogueira PA, Orlandi PP. Virulence factors associated with pediatric shigellosis in Brazilian Amazon. Biomed Res Int. 2014;2014:539697–539699. doi: 10.1155/2014/539697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campioni F, Falcão JP. Genotypic diversity and virulence markers of Yersinia enterocolitica biotype 1A strains isolated from clinical and non-clinical origins. APMIS. 2014;122:215–222. doi: 10.1111/apm.12126. [DOI] [PubMed] [Google Scholar]
- 21.Falcão JP, Falcão DP, Pitondo-Silva A, Malaspina AC, Brocchi M. Molecular typing and virulence markers of Yersinia enterocolitica strains from human, animal and food origins isolated between 1968 and 2000 in Brazil. J Med Microbiol. 2006;55:1539–1548. doi: 10.1099/jmm.0.46733-0. [DOI] [PubMed] [Google Scholar]
- 22.Kingombe CI, Cerqueira-Campos ML, Farber JM. Molecular strategies for the detection, identification, and differentiation between enteroinvasive Escherichia coli and Shigella spp. J Food Prot. 2005;68:239–245. doi: 10.4315/0362-028x-68.2.239. [DOI] [PubMed] [Google Scholar]
- 23.Faruque SM, Khan R, Kamruzzaman M, Yamasaki S, Ahmad QS, Azim T, Nair GB, Takeda Y, Sack DA. Isolation of Shigella dysenteriae type 1 and S. flexneri strains from surface waters in Bangladesh: comparative molecular analysis of environmental Shigella isolates versus clinical strains. Appl Environ Microbiol. 2002;68:3908–3913. doi: 10.1128/aem.68.8.3908-3913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel G, Giron JA, Valmassoi J, Schoolnik GK. Multi-gene amplification: simultaneous detection of three virulence genes in diarrhoeal stool. Mol Microbiol. 1989;3:1729–1734. doi: 10.1111/j.1365-2958.1989.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 25.Fasano A, Noriega FR, Maneval DR, Chanasongcram S, Russell R, Guandalini S, Levine MM. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Invest. 1995;95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farfán MJ, Garay TA, Prado CA, Filliol I, Ulloa MT, Toro CS. A new multiplex PCR for differential identification of Shigella flexneri and Shigella sonnei and detection of Shigella virulence determinants. Epidemiol Infect. 2010;138:525–533. doi: 10.1017/S0950268809990823. [DOI] [PubMed] [Google Scholar]
- 27.Boisen N, Ruiz-Perez F, Scheutz F, Krogfelt KA, Nataro JP. Short report: high prevalence of serine protease autotransporter cytotoxins among strains of enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2009;80:294–301. doi: 10.4269/ajtmh.2009.80.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal M, Kruger E, Durán C, Lagos R, Levine M, Prado V, Toro C, Vidal R. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43:5362–5365. doi: 10.1128/JCM.43.10.5362-5365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan R, Lumb B, Ryan D, Reeves PR. Molecular evolution of large virulence plasmid in Shigella clones and enteroinvasive Escherichia coli. Infect Immun. 2001;69:6303–6309. doi: 10.1128/IAI.69.10.6303-6309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villalobo E, Torres A. PCR for detection of Shigella spp. in mayonnaise. Appl Environ Microbiol. 1998;64:1242–1245. doi: 10.1128/AEM.64.4.1242-1245.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller D, Greune L, Heusipp G, Karch H, Fruth A, Tschäpe H, Schmidt MA. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl Environ Microbiol. 2007;73:3380–3390. doi: 10.1128/AEM.02855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lett MC, Sasakawa C, Okada N, Sakai T, Makino S, Yamada M, Komatsu K, Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989;171:353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CLSI . Performance standards for antimicrobial susceptibility testing. CLSI Supplement M100. Wayne: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 34.Zhang J, Jin H, Hu J, Yuan Z, Shi W, Yang X, Xu X, Meng J. Antimicrobial resistance of Shigella spp. from humans in Shanghai, China, 2004-2011. Diagn Microbiol Infect Dis. 2014;78:282–286. doi: 10.1016/j.diagmicrobio.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- 37.Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/JCM.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lluque A, Mosquito S, Gomes C, Riveros M, Durand D, Tilley DH, Bernal M, Prada A, Ochoa TJ, Ruiz J. Virulence factors and mechanisms of antimicrobial resistance in Shigella strains from periurban areas of Lima (Peru) Int J Med Microbiol. 2015;305:480–490. doi: 10.1016/j.ijmm.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boisen N, Scheutz F, Rasko DA, Redman JC, Persson S, Simon J, Kotloff KL, Levine MM, Sow S, Tamboura B, Toure A, Malle D, Panchalingam S, Krogfelt KA, Nataro JP. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J Infect Dis. 2012;205:431–444. doi: 10.1093/infdis/jir757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, Wadl M, Zoufaly A, Jordan S, Kemper MJ, Follin P, Müller L, King LA, Rosner B, Buchholz U, Stark K, Krause G, Team HI. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 41.Schuch R, Maurelli AT. Virulence plasmid instability in Shigella flexneri 2a is induced by virulence gene expression. Infect Immun. 1997;65:3686–3692. doi: 10.1128/IAI.65.9.3686-3692.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilla G, McVicker G, Tang CM. Genetic plasticity of the Shigella virulence plasmid is mediated by intra- and inter-molecular events between insertion sequences. PLoS Genet. 2017;13:e1007014. doi: 10.1371/journal.pgen.1007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-Torralba A, García-Esteban C, Alós JI. Enteropathogens and antibiotics. Enferm Infecc Microbiol Clin. 2018;36:47–54. doi: 10.1016/j.eimc.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Azmi IJ, Khajanchi BK, Akter F, Hasan TN, Shahnaij M, Akter M, Banik A, Sultana H, Hossain MA, Ahmed MK, Faruque SM, Talukder KA. Fluoroquinolone resistance mechanisms of Shigella flexneri isolated in Bangladesh. PLoS One. 2014;9:e102533. doi: 10.1371/journal.pone.0102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sidrim JJ, Moreira JL, Paixão GC, Lima SB, Filho RE, Rocha MF, Lima AA. Multiple antibiotic resistance mediated by R plasmid in Shigella flexneri strains isolated in the northeast of Brazil. Rev Soc Bras Med Trop. 1998;31:263–270. doi: 10.1590/s0037-86821998000300003. [DOI] [PubMed] [Google Scholar]
- 46.Penatti MP, Hollanda LM, Nakazato G, Campos TA, Lancellotti M, Angellini M, Brocchi M, Rocha MM, Dias da Silveira W. Epidemiological characterization of resistance and PCR typing of Shigella flexneri and Shigella sonnei strains isolated from bacillary dysentery cases in Southeast Brazil. Braz J Med Biol Res. 2007;40:249–258. doi: 10.1590/s0100-879x2007000200012. [DOI] [PubMed] [Google Scholar]
- 47.Rowe-Magnus DA, Guerout AM, Mazel D. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol Microbiol. 2002;43:1657–1669. doi: 10.1046/j.1365-2958.2002.02861.x. [DOI] [PubMed] [Google Scholar]
- 48.Ud-Din AI, Wahid SU, Latif HA, Shahnaij M, Akter M, Azmi IJ, Hasan TN, Ahmed D, Hossain MA, Faruque AS, Faruque SM, Talukder KA. Changing trends in the prevalence of Shigella species: emergence of multi-drug resistant Shigella sonnei biotype g in Bangladesh. PLoS One. 2013;8:e82601. doi: 10.1371/journal.pone.0082601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Cheng Y, Yang H, Hu L, Cheng J, Ye Y, Li J. Characterization of extended-spectrum β-lactamase genes of Shigella flexneri isolates with fosfomycin resistance from patients in China. Ann Lab Med. 2017;37:415–419. doi: 10.3343/alm.2017.37.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamanlou S, Rezaee MA, Aghazadeh M, Ghotaslou R, Nave HH, Khalili Y. Genotypic diversity of multidrug resistant Shigella species from Iran. Infect Chemother. 2018;50:29–37. doi: 10.3947/ic.2018.50.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahnaij M, Latif HA, Azmi IJ, Amin MB, Luna SJ, Islam MA, Talukder KA. Characterization of a serologically atypical Shigella flexneri Z isolated from diarrheal patients in Bangladesh and a proposed serological scheme for Shigella flexneri. PLoS One. 2018;13:e0202704. doi: 10.1371/journal.pone.0202704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lima AA, Sidrim JJ, Lima NL, Titlow W, Evans ME, Greenberg RN. Molecular epidemiology of multiply antibiotic-resistant Shigella flexneri in Fortaleza, Brazil. J Clin Microbiol. 1997;35:1061–1065. doi: 10.1128/JCM.35.5.1061-1065.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17 kb)
(DOCX 42 kb)