Abstract
This study proposed that phage-enriched artemia could be a useful tool for transferring phage into the cultured fish (larvae or adult) as a feed, and introduce mode of phage administration and its safety in concern of tissue adaptation for efficient phage therapy in aquatic animals. First, whether Edwardsiella tarda phage (ETP-1) could attach or ingest by the artemia and optimum time period for the ETP-1 enrichment with artemia were investigated. ETP-1 dispersion, abundance and persistency, and zebrafish immune transcriptional responses and histopathology were evaluated after feeding the fish with ETP-1-enriched artemia. Hatched artemia nauplii (36 h) were enriched with 1.90 × 1011 PFUmL−1 of ETP-1, and maintained at 25 °C. The highest enrichment level was obtained after 4 h (3.00 × 109 PFUmL−1), and artemia were alive and active similar to control for 8 h. ETP-1 disseminated dose dependently to all the tissues rapidly (12 h). However, when feeding discontinued, it drastically decreased at day 3 with high abundance and persistency in the spleen (1.02 × 103) followed by the kidney (4.00 × 101) and the gut (1 × 101 PFUmL−1) for highest ETP-1-enriched artemia dose. In contrast, during continuous delivery of ETP-1-enriched artemia, ETP-1 detected in all the tissues (at day 10: gut; 1.90 × 107, kidney; 3.33 × 106, spleen; 5.52 × 105, liver; 6.20 × 104 PFUmL−1mg−1 tissues). Though the phage abundance varied, results indicated that oral fed ETP-1-enriched artemia disperse to the neighboring organs, even the absence of host as phage carrier. Non-significant differences of immune transcriptional and histopathology analysis between ETP-1-enriched artemia fed and controls suggest that no adverse apparent immune stimulation in host occurred, and use of ETP-1 at 1011 PFUmL−1 was safe. With further supportive studies, live artemia-mediated phage delivery method could be used as a promising tool during phage therapy against pathogenic bacteria to control aquatic diseases.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00324-y) contains supplementary material, which is available to authorized users.
Keywords: Artemia sp., ETP-1 phage, Phage therapy, Brine shrimp nauplii, Edwardsiella tarda, Phage delivery, Zebrafish
Introduction
The antibiotic usage is most commonly applied strategy against bacterial infection in aquaculture industry. At present, the use of such chemotherapy is problematic in aquaculture, because most of the economically important pathogenic strains are multi drug resistance to commonly use commercial antibiotics [1]. Moreover, it causes natural microbial imbalance and ecological and public health issues [1, 2]. Thus, bacterial disease outbreaks need to be managed carefully by eliminating or controlling only the problematic pathogenic bacteria, without disturbing the natural or beneficial microbes. Hence, alternative control strategies are essential to be developed without delay.
Phages are viruses that infect bacteria, and selectively eliminate or control their bacterial host, leaving natural microbes unaffected, thus, they are a promising alternatives for controlling multidrug resistant bacteria [3]. Phage therapy has attractive beneficial effects over traditional antibiotics therapy; thus, it has been applied for different fields including human and veterinary medicine, agriculture, and aquaculture with positive results [4]. Phages infect and replicate only in target bacterium, and no significant adverse effects cause to mammalian or human cells, thus, regarded as safe and have better tolerability. Moreover, administration is easier than antibiotics as it increases the phage concentration from the few dose of initial administration, and their action is limited to infected area and their usage is comparatively more economical. Due to the ubiquitous nature, new phages could be easily isolated against resistant or new pathogens and be used alone or in combination with antibiotics to treat the infections [3, 5]. Most importantly, as phages could be engineered easily with current cost effective DNA sequencing and synthesis technologies, this leads to overcome the issues arising in using antibiotic treatments [3]. Although, phage therapy demonstrates many benefits over antibiotic therapy, there are drawbacks, which delay the use of phages in pharmaceutical industry for human use [3]. However, phages can be particularly used on aquatic farmed species like shrimps that do not have specific immune system and thus no benefits from vaccination [6]. No activity against any given bacterial strain, chance of emergence of bacterial resistance against phages, contribution of phages for emergence of antibiotic resistance, diminishing the activity due to anti-bacteriophage immune response activation for phages, etc., are some of the factors that associate to limit the use of phage therapy in humans and animals [3, 5]. Moreover, due to the scarcity of the reliable evidences of safety on phage therapy, safety of the consumption of phage-treated fishes, and ecological impacts when excessive use of phages, most countries still not authorized phage therapy for industrial use. Hence, for efficient and safe phage therapy, it needs to be developed an appropriate formulation depending on the targeted bacteria, aquaculture species, targeted organs, and identifying an adequate phage shelf life [6].
Phage therapy efficacy depends upon the mode of phage administration and its efficacy of internalization [7]. The phage administration varies with the characteristics of the phage, nature of the infection, and fish species [6]. Many studies related to mode of phage administration and their positive and negative effects have been reported [8–13]. When animals are smaller and number is high, injection of phages may be laborious and time consuming [8]. Therefore, phage-incorporated diets (phage-coated feeds) have successfully used to treat fish in large quantities [9–11], which ensures the continuous delivery of phages in designated time period [8]. However, withstanding ability of the phages in high acidic and proteolytically active environment in the stomach is a challenge in oral delivery [8, 12, 13].
The brine shrimp (Artemia sp. Leach 1819) nauplii, the primitive form of crustacean of the class Brachiopoda, is used as a vector for transferring specific components such as essential nutrients (vitamins, fatty acids, etc.), vaccines, probiotics, antibiotics, and antimicrobial substances into cultured aquatic animals [14–19]. This is widely practiced worldwide in crustacean and finfish hatcheries to enhance the nutritional value (e.g., fatty acid content) of artemia, and the process called “artemia enrichment,” “boosting,” or “bioencapsulation” [14, 19]. With a similar approach, we hypothesize that enrichment of the artemia with phage and feeding it into the zebrafish could be used as a phage delivery method into the cultured larvae or adult fish. This could enhance the gut immune system, increase disease resistance, decrease the risk of disease outbreaks, and ultimately improve the overall health of the fish. However, a vulnerability could exist to destroy phages due to digestive enzymes or acidic environment of the gut, hence, it is important to identify phage abundance, persistency, and decay among tissues. Moreover, host immune responses upon phage administration are important to evaluate the safety and effectiveness of the treatment [20, 21]. Presumably, studies on administration of phage-enriched artemia as a fish feed have not been reported for fish pathogens like Edwardsiella sp. E. tarda is a Gram negative, short, rod shaped and also facultative anaerobic bacterium belongs to family Enterobacteriaceae, which causes Edwardsiellosis in many economically important fish species, and eventually leads to serious economic losses in aquaculture industry [22].
In the present study, we focused on to investigate whether E. tarda-infecting phage ETP-1 [23] could attach to the artemia or ingest by the artemia (ETP-1-enriched artemia). Moreover, ETP-1 distribution, abundance, persistency, or decay in zebrafish were investigated to find out whether the feeding of ETP-1-enriched artemia is an efficient phage incorporation method to fish. Additionally, to determine the tissue adaptation during internalization of phage to the organ tissues, immune transcriptional responses and histopathology changes were determined after ETP-1-enriched artemia diet was fed to zebrafish for 7 days.
Materials and methods
Bacteria strain and the bacteriophage
E. tarda strain and its infecting phage ETP-1 were previously isolated from seawater obtained from marine aquaculture farm and characterized in detail [23]. The bacteria strain stored at − 80 °C was streaked on a tryptic soy agar (TSA) plate and incubated over night at 25 °C. Subsequently, single colony was picked, inoculated in liquid culture, and incubated at 25 °C. Phage stored at 4 °C was checked for any bacterial contamination by plating on TSA plates.
Zebrafish husbandry
Disease-free healthy zebrafish (wild type, AB line) were maintained in an automated water recirculating system (Reverse Osmosis and UV treated) with a 14:10 h light:dark cycle at 28 + 1 °C and water conductivity at 500 + 50 μS/sm. The zebrafish fed with brine shrimp (Artemia sp.) on daily basis (4% of body weight diet per day), and pH, temperature, ammonia, dissolved oxygen, salinity, alkalinity, hardness, and conductivity of the water were checked on a regular basis.
Brine shrimp hatching and maintenance
Brine shrimp eggs (cysts) were purchased from INVE Aquaculture, Inc. Salt Lake City, UT 84104, USA, and hatching process was conducted according to manufactures instructions with some modifications. In brief, brine shrimp hatchery tank was filled with 2 L of artificial seawater (32 g L−1) using sea salt (Chonnam Shinan-gun, Rep. of Korea) and added 5 g of cysts per 2 L seawater (2.5 g cyst L−1 seawater). A vigorous circulation was maintained from the bottom of the tank using an air pump. The water temperature was maintained at 28 °C, and the hatchery was kept under the continuous natural light. The filtered seawater salinity and pH were 32–35 ppt and 8.0, respectively. The brine shrimp cysts were then left to hatch for 36 h. Once the artemia were hatched, air pump was removed, and the culture was allowed to settle for 5 min. Nauplii were collected with the help of tap at the bottom of the hatcher. Collected nauplii were strained to a net (350-μm nylon mesh), and rinsed them into a container with zebrafish system water. The waste water from the hatching system was disinfected with hypochlorite (bleach) and disposed.
Preliminary experiment of ETP-1 enrichment with artemia
In vivo feeding of ETP-1 to artemia
The following procedure was used to determine whether artemia could be enriched with ETP-1. Six mililiters of hatched artemia were enriched with 25 mL of 1.90 × 1011 PFUmL−1 ETP-1 by immersion in 10-cm petri dish and kept at room temperature (RT, 25 °C). Another 6 mL of artemia was immersed in 25 mL of SM buffer in another 10-cm petri dish in similar manner. Since we used two sampling points (2 and 4 h), similar set of plates were prepared additionally. At 2 and 4 h later, artemia samples were filtered, and supernatants were taken as Sup1. Then the artemia was washed with 50 mL of SM buffer, and centrifuged at 3500 rpm for 10 min at 4 °C. Supernatant was collected to petri dish and identified as wash 1 (W1). Another 50 mL of SM buffer was added to the artemia in the tube, and washed by centrifuging with similar conditions and filtered. Filtrate was identified as wash 2 (W2). By this time, most of the artemia has died, and those were homogenized using mortar and pestle to become paste. Then, 1 mL of SM buffer was added, and filtered by the 350-μm nylon net followed by 0.2-μm filter. Filtrate was identified as F1, and assumed that it consist of artemia that had ingested or attached ETP-1. Standard soft agar assay was performed [23] in series of dilutions 101–108 for the Sup1, W1, W2, and F1 at both 2 and 4 h to determine the presence of ETP-1.
Additionally, another experiment was conducted to find out changes of the artemia survival over the time due to the ETP-1 enrichment. In three separate 10-cm petri dishes, 1 mL of 36-h hatched dense artemia (25,000 artemia mL−1) were placed. To each plate, 8 mL of artificial seawater, SM buffer, and 1011 PFU mL−1 filtered ETP-1 was added. From each plate, 75 μL of artemia was added to wells of 24-well plate in triplicates, and 500 μL of respective fresh treatment media was added. The plate was incubated at 25 °C for minimum of 16 h for enrichment process. The phage survival was checked at 0, 8, 12, and 16 h post enrichment (hpe) under sterio microscopy (Leica S8 AP0, Leica Microsystems, Wetzlar, Germany) with LED light source (Leica, KL 300 LED, Leica Microsystems, Wetzlar, Germany). Images of the condition of artemia were taken by Moticam Pro 205A microscope camera (Motic Deutschland GmbH, Germany) connected to the microscope. To quantify the survival of the artemia over the enrichment time period, arbitrary index was introduced considering their movement as surviving (active), moderately active, and dead artemia. Number of artemia belongs to each index were counted in each representative treatment samples at 0, 8, 12, and 16 hpe in triplicates. The percentage (%) of artemia belongs to each category in sample was calculated as: artemia % = (number of artemia belongs to particular index (Ai)/total number of artemia in the sample counted (At)) × 100, where, Ai = either active, moderately active, or dead artemia; At = total of active, moderately active, and dead artemia. After confirming the presence of phages in the F1 and optimum time for the enrichment, ETP-1-enriched artemia diet was prepared.
ETP-1 enrichment with artemia for zebrafish feeding
For the ETP-1 enrichment experiment, four enrichment groups were designed as follows: 1st, ETP-1 1011; 2nd, ETP-1 108; 3rd, ETP-1 105 PFU mL−1; and 4th, SM buffer-immersed artemia (control). Each group consisting, 2 mL of 36-h post hatched dense artemia culture transferred into 10-cm petri dishes and each differently supplemented with 8 mL of 105, 108, and 1011 PFUmL−1 filtered ETP-1. To the fourth group, 8 mL of SM buffer was added. Then, all the plates were kept minimum 4 h at 25 °C to enrich with ETP-1. After incubation, excess phage solution was drained and washed with distilled water. This enriched artemia-ETP-1 mixture was considered as a diet per day. The fish were fed thrice a day and after each feeding, the remaining was kept at 4 °C for next feeding.
ETP-1-enriched artemia feeding to adult zebrafish
This experiment was conducted to determine once the phage was administered to the zebrafish, whether ETP-1-enriched artemia fed fish have the ability to sustain the phages in their gut and neighboring organs, assuming that all the phage-enriched artemia has ingested by the fish. For the feeding experiment, forty adult zebrafish (0.25 + 0.05 g) were transferred in to four tanks (4.50 L; 10 fish per tank) and four groups were designed as described in the above. Normal artemia feeding tank was separately maintained routinely at our fish culture facility. Prior to experiment, fish were acclimatized for 3 days giving the normal artemia diet daily (4% of body weight), and maintained at 25 °C (phage growing at 25 °C). Routinely given normal artemia diet to the zebrafish was stopped prior to the experiment. Fish were fed with the different concentrations of phage-enriched artemia diets thrice a day, for 3 days using 1 mL dropper. Control fish was fed with SM buffer-enriched artemia diet in similar manner. To reduce the accumulation of excess ammonia due to uneaten feed and fecal matter, after 2–3 h from the final feed daily, half of the water in the tank was removed partially and replaced with system water. After 3 days of continuous feeding, the phage-enriched artemia feeding was stopped, and for next 7 days, normal artemia diet was continued. Twelve hours before the tissue collection, feeding was discontinued and tissues were collected from fish at 12 h, 3, and 7 days post phage enriched artemia feeding (n = 3).
Another experiment was conducted to feed ETP-1-enriched artemia continuously to zebrafish for 10 days. Eighty-four adult zebrafish (0.25 + 0.05 g) were divided into 6 tanks (4.50 L) to make 2 groups (14 fish/tank, triplicate per group) namely, group 1; ETP-1 1011 PFUmL−1 and group 2; SM buffer immersed artemia feeding (control). As described previously, fish were fed with ETP-1-enriched artemia, thrice a day, and continued for 10 days. Similarly, control fish were fed with SM buffer enriched artemia diet. At day 1, 4, 7, and 10, the tissues from zebrafish (n = 3) were collected to determine the phage distribution, abundance, and persistency or decay in ETP-1-enriched artemia fed zebrafish.
Sample preparation for qualitative and quantitative analysis of ETP-1 in fish organ tissues
To check whether ETP-1-enriched artemia had been ingested by zebrafish and spread to organ tissues, the gut, kidney, spleen, and liver were collected. To evaluate the phage retention time in those tissues over the period of time, the presence of phage and phage abundance were analyzed qualitatively and quantitatively by the modified method of Christiansen et al. [8]. For tissue sampling, 3 fish were taken from each tank. Fish were anesthetized, and the gut, kidney, spleen, and liver were extracted and put into 450 μL of SM buffer and 5 μL of chloroform containing Eppendorf tubes. Weight of the whole fish and total tissue weight were separately recorded. Each tissue was homogenized using hand-held homogenizer for 1 min at 20 kHz and vortexed. Samples were centrifuged at 12000 rpm at 4 °C for 5 min, and then filtered using 200-μm filters and kept the supernatant at 4 °C until testing the presence of phages and quantification. Additionally, 450 μL of water removed from each tank were tested for the presence of phages and phage abundance (n = 3).
Qualitative detection of ETP-1
Spot test was carried out to examine the presence of ETP-1 in the gut, kidney, spleen, and liver. Lawn of E. tarda indicator strain was prepared in the BHI agar with 1% NaCl plates. In brief, 4 mL of melted and cooled down to 45 °C soft agar (BHI broth with 0.4% agar) tubes were prepared and to that, 200 μL of bacteria in the exponential growth phase (OD 600 nm, 0.2–0.3) was added. Mixture was slightly vortexed and immediately poured onto BHI with 1% NaCl agar plate and incubated 10 min at 25 °C to solidify. Filtered tissue samples were tenfold serially diluted up to six (106), and 5 μL from each were spotted on to plates containing E. tarda lawn. Plates were then incubated overnight at 25 °C in the incubator. Following day, total lysis or the single plaques at the site of the sample added were checked, and at least one of such results was found; it was considered as positive for the phage.
Quantitative detection of ETP-1
Weight-specific ETP-1 abundance in the tissue samples was tested using spot test assay as described above. The countable plaque numbers were counted directly from the plates, which were subjected to spot assay for qualitative analysis, and for remaining samples, serial dilution and spot tests were repeated for quantifying the phages.
Immune gene expression analysis of ETP-1-enriched artemia-fed adult zebrafish
mRNA expression of 6 immune genes (cxcl-8a, il-1β, tnf-α, il-6, il-10, and sod-1) were analyzed in zebrafish gut at 7 days post ETP-1-enriched artemia feeding. ETP-1-enriched artemia was administered to twenty-four zebrafish (mean weight; 0.55 ± 0.05 g, n = 8/tank, in triplicates), continuously for 7 days, while the control group of twenty-four zebrafish (n = 8/tank, in triplicates) were fed with same amount of SM buffer-immersed artemia. Water was renewed daily, and fresh ETP-1-enriched artemia was added on daily basis. Gut tissue (n = 3) was extracted at 7 days post ETP-1-enriched artemia feeding, snap frozen in liquid nitrogen, and stored in − 80 °C. RNA isolation, cDNA synthesis, and quantitative real-time (qRT)-PCR to evaluate relative mRNA expression levels of the target genes (primer list; Table S1) were carried out as described previously [23, 24], and gene expression data were analyzed according to the 2−ΔΔCt method [25].
Histological analysis of ETP-1-enriched artemia-fed zebrafish gut
For the histology analysis, 7 days post ETP-1-enriched artemia feeding and SM buffer-immersed artemia-fed fish (n = 3) were selected from the experiment setup described in immune gene expression analysis. Fish were euthanized using an overdose of buffered tricaine (Sigma-Aldrich, USA), and gut tissues were collected and preserved in 10% neutral buffered formalin (NBF) for 24 h. Then formalin-fixed gut was processed (Leica TP1020 Semi-enclosed Benchtop Tissue Processor, Germany), embedded (Leica EG1150 Tissue Embedding Center, Germany), and sectioned into 4 μm-thickness (Leica RM2125 microtome, Germany). The tissue samples were stained by standard hematoxylin and eosin method (H&E) and examined under light microscope (Leica 3000 LED, Wetzlar, Germany) and the images were captured by LEICA DCF450-C camera.
Statistical analysis
Data of ETP-1-enriched artemia feeding-experiments were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni posttest. Additionally, one-way ANOVA followed by Tukey’s multiple comparison test was performed to find statistical significance between treatment effects, days, and tissues. The data are shown as means + standard deviation (SD) in triplicate experiments. Gene expression data were analyzed by unpaired t test between ETP-1-enriched artemia-fed and-control gut tissues, and error bars represent means + standard error (SE). All the analyses were done by GraphPad Prism software version 6 for Windows (GraphPad Software Inc. USA) and P < 0.05 was considered for indicating statistical significance.
Results and discussion
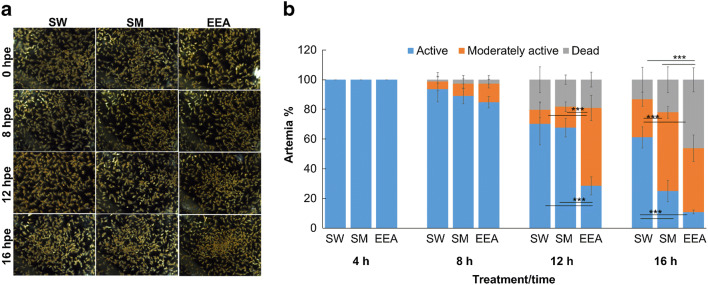
In this study, ETP-1-enriched artemia was developed as a feed, which could be used as an effective live tool for transferring phage into the cultured larvae or adult zebrafish to improve fish overall health. First, by investigating enrichment levels of artemia, we demonstrated that ETP-1 could enrich with artemia. The ETP-1-enriched nauplii were harvested after 2 and 4 h, and we found that the highest enrichment level (P < 0.05) was at 4 hpe (3.00 × 109 PFUmL−1) compared to at 2 hpe (7.60 × 107 PFUmL−1). SM immersed artemia had zero phages. Figure 1 shows the microscopic observations and quantification analysis of artemia survival conditions after enriched in seawater, SM buffer and ETP-1 at different time points at 25 °C. We observed that ETP-1-enriched artemia were alive or active as seawater and SM buffer-enriched artemia until 8 h, and with the time passes, their activity had declined (Fig. 1a). Moreover, when quantified the survival percentage, it clearly confirmed that phage-enriched artemia were active (> 80%) as seawater or SM buffer-enriched artemia until 8 h (P > 0.05) (Fig. 1b). They gradually became inactive and initiated the mortality, showing 52% moderately active and 19% dead artemia at 12 hpe (P < 0.05), respectively. Eventually, at 16 hpe, the survived percentage was dropped sharply (10%), and the dead artemia were increased (> 40%) compared to 4 and 8 h post ETP-1-enriched artemia. In parallel, both the seawater and SM buffer-enriched artemia survived percentage was also dropped at 16 h compared to 4 and 8 h post enriched artemia (P < 0.05). Hence, we selected suitable time period for enrichment as 4 h, because, 100% survival was observed at 4 h post enriched artemia that kept at 25 °C. Even though, this procedure alone is premature to establish for a certain whether the artemia had ingested the phage; however, phage either ingested or attached to the artemia was reflected by the soft agar assay, indicating that ETP-1-enriched artemia contained phages. Thus, we suggest that ETP-1-enriched artemia for 4 h, and giving by orally to zebrafish would be a novel technique for phage delivery.
Fig. 1.
Survival of ETP-1-enriched artemia at different time points at 25 °C. (a) Images of survival condition (qualitative) and (b) survival percentage (quantitative) of ETP-1-enriched artemia under microscopic observation. For the quantitative analysis, arbitrary index was given to count number of artemia as “survived,” “moderately active,” and “dead” and artemia percentage was calculated for the each category as described in methodology. Error bars represent the + SD of the means of triplicate experiments. Magnification of images: × 12.5. SW; seawater-immersed artemia, SM;, SM buffer-immersed artemia, EEA; ETP-1-enriched artemia
The qualitative and quantitative analysis results of phage dispersion and persistency upon oral administration of three phage concentrations enriched with artemia for 3 days continuously, and then discontinued feeding represented in Tables 1 and 2, respectively. Data resulted quick spread of phages to all the organ tissues by 12 h (P < 0.05), except in the spleen of 105 PFUmL−1 of phage fed artemia (Table 1). It is obvious that none of the fish that fed with artemia immersed in SM buffer contained ETP-1 in their tissues throughout the experiment period (data not shown). The detection was significantly high at 12 h as the phage enriched concentration increased (P < 0.05), and the highest abundance was in the gut (105; 1.57 × 107, 108; 9.91 × 108, 1011; 2.66 × 109 PFU mg−1 tissues) and kidney (105; 3.69 × 107, 108; 7.60 × 108, 1011; 3.21 × 109 PFU mg−1 tissues) in all the concentrations (Table 2). However, the persistency of ETP-1 drastically decreased (P < 0.05) over the time in all the concentrations tested, especially zero phages in all the tissues were observed after 3 and 7 days in 105 and at 7 days in 108 PFUmL−1 fed artemia. Infective phages were observed only in gut tissue by 3 days at 108 (7.29 mg−1tissues), whereas at 1011, significantly high (P < 0.05) infective phages were observed in the spleen (1.02 × 101 mg−1 tissues), gut (1.01 × 101 mg−1 tissues), and kidney (4.00 × 101 mg−1 tissues) compared to 105 and 108 PFUmL−1 enriched organ tissues. According to Christiansen et al., [8] we suggest that administration of high ETP-1 concentration is important to distribute phage to all the internal organs via circulatory system. However, it was observed that even at the highest concentration (1011 PFUmL−1), the infective phages were observed at very low levels in the gut (1 mg−1 tissues) and kidney (1.95 mg−1 tissues) tissues until 7 days, but the values were not significant (P > 0.05) between tissues. As suggested by other researchers, observed reduction in ETP-1 persistency or phage decay among tissues could be due to assemblage of factors such as the use of filtrating systems by higher organisms for removal of phages, production of phage neutralization antibodies, and irreversible adsorption to fish tissues [8, 13]. We reported previously, that ETP-1 could withstand (40% survival) at the pH 3 up to 1 h in vitro, and completely loss the infectivity at ≤ pH 2 and ≥ 11 [23]. Thus, it could expect the stability issues of the phages while transporting through high acidic and proteolytic activity environments during oral delivery. Hence, even though the phage abundance was high at initial stages, loss of some number of phages, especially from the gut could be obvious. In this study, we have not introduced host bacteria (E. tarda) to the fish. As pointed out previously [26], this study demonstrated, although the duration that the ETP-1 retained at organs were relatively short, even the absence of host as phage carrier, ETP-1 could disperse to the neighboring organs, indicating that ETP-1-enriched artemia given by oral delivery as a phage therapy could be effective at the stage of systemic infection.
Table 1.
Qualitative analysis of phage dispersion and persistency upon oral feeding of ETP-1-enriched artemia
| Sampling time | Tissues | Oral feeding | ||
|---|---|---|---|---|
| 105 PFUmL−1 | 108 PFUmL−1 | 1011 PFUmL−1 | ||
| 12 h | Spleen | – | ++ | ++ |
| Gut | ++++++++ | ++++++++++ | +++++++++++ | |
| Kidney | ++++++++ | +++++++++ | +++++++++++ | |
| Liver | ++ | ++++ | +++++ | |
| 3 days | Spleen | – | – | + |
| Gut | – | ++ | ++ | |
| Kidney | – | – | ++ | |
| Liver | – | – | – | |
| 7 days | Spleen | – | – | – |
| Gut | – | – | + | |
| Kidney | – | – | + | |
| Liver | – | – | – | |
A “+” sign shows the presence of phages in the tissues. “−” sign indicates absence of phages.
Phages were examined by spotting 5 μL of sample on to the E. tarda host strain in triplicate
If one or more of the triplicate showed phages, the sample was considered as positive
Number of “+” indicates the tenfold value per dissolved whole tissue volume (457.5 μL)
Original stock concentration used to enrich artemia for 3 days feeding were 2.00 × 105, 2.00 × 108, and 2.00 × 1011 PFUmL−1
Table 2.
Quantitative analysis of phage dispersion and persistency upon oral feeding of ETP-1-enriched artemia
| Sampling time | Tissue | Phage dispersion | ||
|---|---|---|---|---|
| PFU mg tissue−1 | ||||
| 105 PFUmL−1 | 108 PFUmL−1 | 1011 PFUmL−1 | ||
| 12 h | Spleen | 0 | 9.48 × 103 + 1.20 × 102 | 1.71 × 102 + 1.20 × 101 |
| Gut | 1.57 × 107 + 1.03 × 102a | 9.91 × 108 + 1.23 × 103b | 2.66 × 109 + 3.33 × 107c | |
| Kidney | 3.69 × 107 + 2.00 × 103a | 7.60 × 108 + 2.01 × 103b | 3.21 × 109 + 2.31 × 107c | |
| Liver | 0 | 2.79 × 103 + 3.56 × 101 | 1.81 × 104 + 1.20 × 102 | |
| 3 days | Spleen | 0 | 0a | 1.02 × 103 + 3.10 × 101b |
| Gut | 0 | 7.29a | 1.01 × 101 + 0b | |
| Kidney | 0 | 0a | 4.00 × 101 + 0b | |
| Liver | 0 | 0 | 0 | |
| 7 days | Spleen | 0 | 0 | 0 |
| Gut | 0 | 0 | 1.00 | |
| Kidney | 0 | 0 | 1.95 | |
| Liver | 0 | 0 | 0 | |
Original stock concentration used to enrich artemia for 3 days feeding were 2.00 × 105, 2.00 × 108, and 2.00 × 1011 PFUmL−1
Superscript lower case letters denote significant differences (P < 0.05) between phage concentrations and the ETP-1 plaque numbers in tissues at a particular time point
Hence, we postulate that continuous ETP-1-enriched artemia delivery may be a choice to maintain the persistency of phages in the absence of host bacteria. In agreement with Christiansen et al. [8], continuous administration of ETP-1-enriched artemia (1011 PFUmL−1) for 10 days resulted constant abundance and dispersion of phages with varying levels in all the tissues tested (Table 3, Fig. 2, Sup. Table 2) compared to the results of which, ETP-1-enriched artemia feeding was interrupted after 3 days. The persistency was maintained, but it was less than the day 1 for each tissues. The highest average ETP-1 counts observed were in the gut (7.62 × 107 + 5.20 × 105) and kidney (1.32 × 107 + 5.20 × 105) at day 1 (P < 0.05), spleen (5.31 × 105 + 1.43 × 105) and gut (2.49 × 105 + 3.03 × 104) at day 4 (P > 0.05), and gut (1.33 × 106 + 6.00 × 104) and liver (1.32 × 105 + 5.95 × 104 mg−1tissues) at day 7 (P < 0.05) (Fig. 2, Sup. Table 2). At day 10, all tissues, except kidney (1.93 × 104 + 1.24 × 103 PFUmg−1 tissues) showed phage abundance as more than 105 PFUmg−1 tissues (spleen; 1.62 × 106 PFUmg−1 tissues, gut; 2.32 × 105 PFUmg−1 tissues; liver; 1.12 × 105 + 5.54 × 103 PFUmg−1 tissues), and values were significantly different (P < 0.05) when compared to that of the spleen tissue. This high abundance of ETP-1 at early stages suggests that continuous feeding of phage-enriched artemia could be a suitable prophylactic treatment measure for protection of fish against early infection of E. tarda. Comparable to our findings, FpV-9 phages when administered by three modes (bath, oral intubation, and phage-coated fish feed pellets) to juvenile rainbow trout, the phage was efficiently and rapidly spread in to all the tested tissues [8]. As Christiansen et al. [8] suggested, we could anticipate that artimia-enriched phages also might penetrate the barrier of the intestine and could be absorbed into the circulatory system, and during that stage, some might lost by other means. Moreover, individual variation among same sampling points could be due to unequal ETP-1 consumption by individual fish, thereby the phage adsorption, distribution, and persistence might have changed.
Table 3.
Qualitative analysis of phage dispersion and abundance upon oral feeding of ETP-1-enriched artemia to zebrafish for continuous 10 days
| Sampling time (days) | Oral feeding (ETP-1-enriched artemia) | ||||
|---|---|---|---|---|---|
| Spleen | Gut | Kidney | Liver | Water | |
| 1 | ++ | +++++++++ | ++++++++ | ++ | ++++ |
| 4 | ++++ | ++++++ | +++++ | ++++ | ++++++ |
| 7 | +++ | +++++++ | +++++ | ++++++ | +++++++ |
| 10 | +++++ | ++++++ | +++++ | ++++++ | +++++++ |
A “+” sign shows the presence of phages in the tissues. “−”sign indicates the absence of phages
Phages were examined by spotting 5 μL of sample on to the E. tarda host strain in triplicate. If one or more of the triplicate showed phages, the sample was considered as positive
Number of “+” indicates the tenfold value per mL for water or 10-fold value per dissolved whole tissue volume (457.5 μL)
Stock concentration used to enrich artemia for 1–10 days feeding: 2.00 × 1011 PFU mL−1; the highest enrichment level obtained after 4 h: 3.00 × 109 PFUmL−1
Fig. 2.

Quantitative analysis of ETP-1 phage distribution in different tissues after ETP-1-enriched artemia fed to zebrafish continuously for 10 days. Phage were detected by dot assay using the host strain E. tarda. Total of 3 fish were sampled at each time point. Error bars represent the + SD of the means of triplicate experiments. ***P < 0.001, *P < 0.05
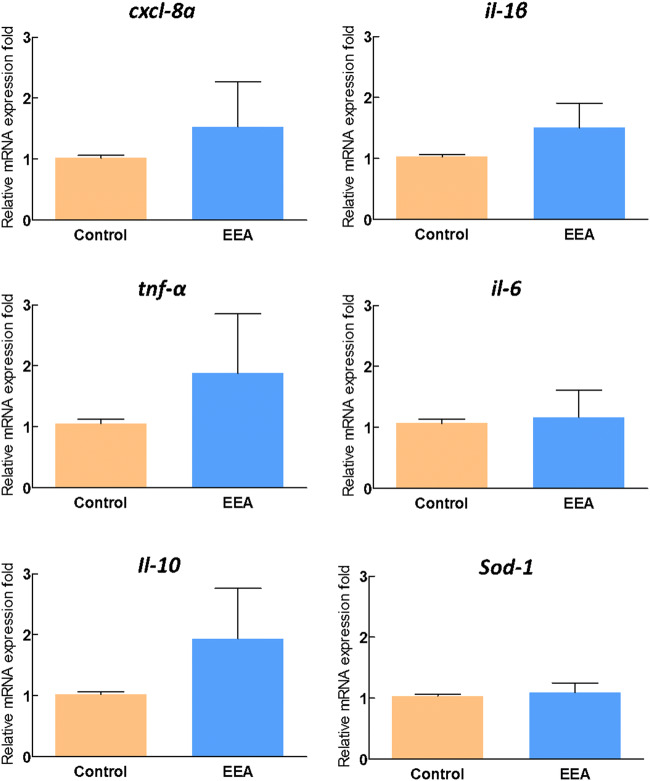
One of the factors that have been reported for the cause of phage decay was phage interacts with host innate or adaptive immune system or production of phage-neutralization antibodies [8, 13]. Thus, we tested the expression of 6 immune genes related to inflammatory and antioxidants in zebrafish gut to find out the adaptation and safety of continuous ETP-1-enriched artemia administration. Our results established that expression of most of the genes at 7 days post administration of ETP-1-enriched artemia was at the basal level or <2-fold with no statistically significant (P > 0.05) compared to control (Fig. 3). Accordance with our previous studies [23, 24], the results suggest that though the phage treatment induced the expression of a few cytokine genes at day 7, no adverse immune responses have been detected. Furthermore, histopathological changes in ETP-1-enriched artemia-fed zebrafish and healthy fish were compared to understand the effects of phage administration at the tissue level. Figure 4 shows the histopathology of SM/control (Fig. 4a-1, a-1′), ETP-1-enriched artemia-fed (Fig. 4b-2, b-2′) gut sections after 7 days post administration. The results indicated that there is neither any adverse pathophysiological changes in the tissues due to phage internalization to the gut tissues, nor adverse differences with control samples. Both immune gene expression and histopathology data imply the safety aspects for the use of the ETP-1-enriched artemia at the 1011 PFUmL−1.
Fig. 3.
Immune gene expression analysis of zebrafish gut after 7 days of ETP-1-enriched artemia administration. Relative mRNA expression levels of cxcl-8a, il-1β, tnf-α, il-6, il-10, and sod-1. Experiments were conducted in triplicate for control and phage administration, and 3 animals were pooled for each replicate group. Error bars represent the + SE of the means. Relative mRNA expression fold change for a particular candidate gene of SM buffer-enriched ETP-1-fed fish at day 7 = 1. Control; SM buffer-immersed artemia, EEA; ETP-1-enriched artemia
Fig. 4.
Histopathology of ETP-1-enriched artemia-fed zebrafish gut after 7 days. (a1) Standard H&E stained SM buffer/control-enriched artemia-fed zebrafish gut section at magnification under 200 × and (a1) 400 ×. (b1) Standard H&E stained ETP-1-enriched artemia fed gut section at magnification under 200 × and (b1′) 400 ×. To determine the pathophysiological changes between SM/control; SM buffer enriched and EEA; ETP-1-enriched artemia fed gut, average of three gut tissues were examined
In summary, we successfully developed a phage delivery method to zebrafish by oral administration of live artemia nauplii enriched with phage ETP-1. We presume that this is the first report showing that phage could be incorporated with artemia nauplii as efficient oral delivery method of phage. We investigated that the optimum time period of the ETP-1 enrichment at 25 °C was as 4 h, and it is important to deliver ETP-1-enriched artemia continuously to the fish for effective phage dispersion and persistency in fish tissues. Our feeding experiment results indicate that oral feeding of ETP-1-enriched artemia did not show any adverse effect to the fish up to 7 days. The data obtained from this study provided useful information for development of effective phage therapy against E. tarda infection in aquatic animals (larvae or adult), and this technique could be considered as a promising phage delivery method for the aquaculture. However, in phage therapy efficacy against E. tarda in concern, some additional factors such as production of bacterial toxins and phage endotoxins need to be concerned before launching as a potential antibacterial alternative.
Electronic supplementary material
(DOCX 27 kb)
Funding information
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the government of Korea (MSIT) (2017010990).
Compliance with ethical standards
Zebrafish experiments were conducted in accordance with the institutional animal care guidelines and supervision of committees of Chungnam National University (CNU-00866).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jehee Lee, Email: jehee@jejunu.ac.kr.
Mahanama De Zoysa, Email: mahanama@cnu.ac.kr.
References
- 1.Santos L, Fernando R. Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents. 2018;52:135–143. doi: 10.1016/j.ijantimicag.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Zhang L, Tiu L, Wang HH. Characterization of antibiotic resistance in commensal bacteria from an aquaculture ecosystem. Front Microbiol. 2015;6:article 914. doi: 10.3389/fmicb.2015.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Principi N, Silvestri E, Esposito S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front Pharmacol. 2019;10:article 513. doi: 10.3389/fphar.2019.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doss J, Culbertson K, Hahn D, Camacho J, Barekzi N. A review of phage therapy against bacterial pathogens of aquatic and terrestrial organisms. Viruses. 2017;9(50):1–10. doi: 10.3390/v9030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domingo-Calap P, Delgado-Martínez J. Bacteriophages: protagonists of a post-antibiotic era. Antibiotics. 2018;7:E66. doi: 10.3390/antibiotics7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culot A, Grosset N, Gautier M. Overcoming the challenges of phage therapy for industrial aquaculture: a review. Aquaculture. 2019;513:734423. doi: 10.1016/j.aquaculture.2019.734423. [DOI] [Google Scholar]
- 7.Richards GP. Bacteriophage remediation of bacterial pathogens in aquaculture: a review of the technology. Bacteriophage. 2014;4:1–12. doi: 10.4161/21597081.2014.975540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen RH, Dalsgaard I, Middelboe M, Lauritsen AH, Madsen L. Detection and quantification of Flavobacterium psychrophilum-specific bacteriophages in vivo in rainbow trout upon oral administration: implications for disease control in aquaculture. Appl Environ Microb. 2014;80(24):7683–7693. doi: 10.1128/AEM.02386-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SC, Nakai T. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis Aquat Org. 2003;53:33–39. doi: 10.3354/dao053033. [DOI] [PubMed] [Google Scholar]
- 10.Nakai T, Park SC. Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol. 2002;153:13–18. doi: 10.1016/S0923-2508(01)01280-3. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Li S, Han S, Wang D, Zhao J, Xu L, Liu H, Lu T. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture. 2020;523:735193. doi: 10.1016/j.aquaculture.2020.735193. [DOI] [Google Scholar]
- 12.Kim JH, Gomez DK, Nakai T, Park SC. Isolation and identification of bacteriophages infecting ayu Plecoglossus altivelis altivelis specific Flavobacterium psychrophilum. Vet Microbiol. 2010;140:109–115. doi: 10.1016/j.vetmic.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Madsen L, Bertelsen SK, Dalsgaard I, Middelboe M. Dispersal and survival of Flavobacterium psychrophilum phages in vivo in rainbow trout and in vitro under laboratory conditions: implications for their use in phage therapy. Appl Environ Microbiol. 2013;79:4853–4861. doi: 10.1128/AEM.00509-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorgeloos P, Dhert P, Candreva P. Use of the brine shrimp, Artemia spp in marine fish larviculture. Aquaculture. 2001;200:147–159. doi: 10.1016/S0044-8486(01)00698-6. [DOI] [Google Scholar]
- 15.Azimirad M, Meshkini S. Determination of the optimal enrichment Artemia franciscana with a synbiotic combination of probiotics Pediococcus acidilactici and prebiotic fructooligosaccharide. Vet Res Forum. 2017;8(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- 16.Chair M, Gapasin RSJ, Dehasque M, Sorgeloos P. Vaccination of European sea bass fry through bioencapsulation of Artemia nauplii. Aquac Int. 1994;2:254–261. doi: 10.1007/BF00123437. [DOI] [Google Scholar]
- 17.Immanuel G. Bioencapsulation of brine shrimp Artemia nauplii with probionts and their resistance against Vibrio pathogens. J Fish Aquat Sci. 2016;11:323–330. doi: 10.3923/jfas.2016.323.330. [DOI] [Google Scholar]
- 18.Hamsah W, Alimuddin YM, Zairin M Jr (2017) The nutritional value of Artemia sp. enriched with the probiotic Pseudoalteromonas piscicida and the prebiotic mannan-oligosaccharide. AACL Bioflux 10:8–17
- 19.Touraki M, Niopas I, Ladoukakis E, Karagiannis V (2010) Efficacy of flumequine administered by bath or through medicated nauplii of Artemia fransiscana (L.) in the treatment of vibriosis in sea bass larvae:146–152. 10.1016/j.aquaculture.2010.05.033 [DOI] [PubMed]
- 20.Oliveira J, Castilho F, Cunha A, Pereira MJ. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquac Int. 2012;20:879–910. doi: 10.1007/s10499-012-9515-7. [DOI] [Google Scholar]
- 21.Miernikiewicz P, Dąbrowska K, Piotrowicz A, Owczarek B, Wojas-Turek J, Kicielinska J et al (2013) T4 phage and its head surface proteins do not stimulate inflammatory mediator production. 8:e71036. 10.1371/journal.pone.0071036 [DOI] [PMC free article] [PubMed]
- 22.Park SB, Aoki T, Jung TS. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Vet Res. 2012;43:67. doi: 10.1186/1297-9716-43-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikapitiya C, Chandrarahna HPSU, Dananjaya SHS, De Zoysa M, Lee J. Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio) Biologicals. 2020;63:14–23. doi: 10.1016/j.biologicals.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Chandrarathna HPSU, Nikapitiya C, Dananjaya SHS, De Silva BCJ, Heo GJ, De Zoysa M, Lee J. Isolation and characterization of phage AHP-1 and its combined effect with chloramphenicol to control Aeromonas hydrophila. Braz J Microbiol. 2019;51:409–416. doi: 10.1007/s42770-019-00178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2-∆∆CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Park SC, Shimamura I, Fukunaga M, Kori KI, Nakai T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol. 2000;66:1416–1422. doi: 10.1128/AEM.66.4.1416-1422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 27 kb)