Abstract
Filamentous fungi have been proved to have a pronounced capability to recover metals from mineral ores. However, the metal recovery yield is reduced due to toxic effects triggered by various heavy metals present in the ore. The current study highlights the fungal adaptations to the toxic effects of metals at higher pulp densities for the enhanced bio-recovery of aluminum from low-grade bauxite. In the previous studies, a drastic decrease in the aluminum dissolution was observed when the bauxite pulp density was increased from 1 to 10% (w/v) due to the high metal toxicity and low tolerance of Aspergillus niger and Penicillium simplicissium to heavy metals. These fungi were adapted in order to increase heavy metal tolerance of these fungal strains and also to get maximum Al dissolution. A novel approach was employed for the adaptation of fungal strains using a liquid growth medium containing 5% bauxite pulp density supplemented with molasses as an energy source. The mycelia of adapted strains were harvested and subsequently cultured in a low-cost oat-agar medium. Batch experiments were performed to compare the aluminum leaching efficiencies in the direct one-step and the direct two-step bioleaching processes. FE-SEM analysis revealed the direct destructive and corrosive action by the bauxite-tolerant strains due to the extension and penetration of the vegetative mycelium filaments into the bauxite matrix. XRD analysis of the bioleached bauxite samples showed a considerable decline in oxide minerals such as corundum and gibbsite. Results showed a high amount of total Al (≥ 98%) was successfully bioleached and solubilized from low-grade bauxite by the adapted fungal strains grown in the presence of 5% pulp density and molasses as a low-cost substrate.
Graphical abstract
Keywords: Bio-recovery, Tolerance, Filamentous fungi, Cane molasses, Aluminum
Introduction
Aluminum (Al) is widely used in the aerospace, architectural construction, and marine and chemical industries, as well as many domestic uses, because it is light, conductive, and corrosion-resistant metal [1]. Primary Al is produced from bauxite ore through Bayer’s process; however, this method is not applicable in the processing of low-grade bauxite because of low alumina extraction and massive red mud production [2].
Bioleaching of metals from the low-grade ores has emerged as an efficient, economical, and eco-friendly process compared to the existing traditional processes such as Bayer’s process and chemical (mineral acid-base) leaching [3–5]. Bioleaching with heterotrophic fungi and their metabolites (organic acids, amino acids and enzymes etc.) has been extensively studied for the extraction of metals [2, 6–8]. It is being considered as the most cost-effective alternative because the use of fungi as a cell factory for the organic acids production has several advantages, including ease in cultivation, high fermentation productivity, agro-industrial by-product utilization as low-cost substrates, and enhancing the organic acids production capability through genetic manipulation, metabolic engineering, and optimizing the culture conditions [9–13].
The filamentous fungi Aspergillus and Penicillium are considered organic acid production cell factories and have shown a pronounced potential for metal bioleaching due to their ability to produce citric, oxalic, and gluconic acids [14–16]. These organic acids are proven metal leaching agents forming chelates/complexes and are successfully used for the recovery of valuable metals [17]. In the literature, few studies are reported on marine-derived fungi for the metal-leaching process [18]. These fungi are being considered as one of the important sources for the production of bioactive compounds in biotechnological applications [19]. Although they have been explored to a much lesser extent, these strains have shown the superiority over their terrestrial counterparts for the production of secondary metabolites [20, 21]. In this context, marine-derived fungi Aspergillus niger and Penicillium simplicissium were used in the present study for the bioleaching of aluminum from the bauxite ore.
Metal leaching by heterotrophic fungi generally involves the combination of the direct impact due to the physical force exerted by the extension of hyphae and an indirect process due to the action of amino acids, organic acids, and other metabolites at low pH. These metabolites liberate hydrogen ions, and the metals are solubilized by their displacement from the ore through hydrogen ions and also due to the formation of soluble metal complexes and chelates [22, 23].
Metal recovery yield is reduced at higher pulp densities due to toxic effects triggered by various heavy metals present in the ore. They exert toxic effects in numerous ways via blocking the functional groups of enzymes [24, 25]. Metal tolerance can be defined as the ability of the microorganism to survive metal toxicity through one or more mechanisms devised in direct response to the metal(s) concerned [3, 26]. The presence of heavy metals causes the induction of physiological and morphological adaptation strategies including the production of intracellular/extracellular enzymes, extracellular sequestration such as chelation and cell wall binding, intracellular physical sequestration of metal by binding to proteins or other ligands to prevent it from damaging the metal-sensitive cellular targets, suppressed influx, enhanced metal efflux, metal binding to cell walls, intracellular sequestration and complexation, and extracellular metal sequestration and precipitation [27–29]. Fungal metal tolerance varies from strain to strain depending on their sources or sites of isolation, their competence to the toxicity of the specific metal, and also its concentration [30, 31]. However, mostly indigenous fungal strains isolated from heavy metal–contaminated sites are extensively studied to get the metal-resistant strains showing significant tolerance for high heavy metal concentrations [32–34].
Several studies have been reported showing the ability of the fungal strains to survive in high concentrations of toxic heavy metals by adaptation or mutation [3, 35, 36]. The adaptation occurs due to phenotypic plasticity and mutation followed by natural selection. Such an approach can be adjusted in order to achieve heavy metal–tolerant strains, for the enhanced bio-recovery of metals [35–37]. According to the literature, successful bioleaching studies on the spent catalyst were carried out using an adapted strain of A. niger that was formerly exposed to Ni, Mo, and Al (single-metal ions and also a mixture of such metal ions) [3, 38].
Fungal strains need some metals in definite concentrations for their metabolic activities, including the production of organic acids, enzymes, and other metabolites. Though, all the metals, either essential or non-essential, will have a tendency to demonstrate toxicity at definite levels [36]. Bioleaching of Al at higher bauxite pulp densities is hampered due to the poor tolerance of the strains to toxic metals, which propose the necessity for the adaptation of the fungal strains which can tolerate high concentrations of metals. In the current study, the adaptation of fungal strain was carried out in order to increase the bioleaching efficiency and also to reduce the inhibitory effects of various toxic metals present in bauxite ore. The fungal strains were adapted using 5% bauxite pulp density in a liquid growth medium containing molasses as a low-cost carbon substrate. Batch experiments were conducted in a direct one-step and direct two-step bioleaching processes using 1% bauxite pulp density. Tolerance training strategies for fungal adaptation to high metal concentrations proved to be more appropriate to obtain an enhanced bio-recovery of aluminum from the low-grade bauxite ore.
Material and methods
Mineral ore and fungal strains
Bauxite ore, collected from Alcoa, SP, Brazil, was pulverized to 180 μm and sterilized by autoclaving at 121 °C for 15 min. The Ascomycota fungi A. niger and P. simplicissimum were isolated from marine algae Padina sp. collected at the São Sebastião beach, located at the Northern coast of São Paulo state, Brazil [20].
Fungal strains were identified by conventional and molecular approaches. Fungal macromorphology was studied using colony observation through a stereomicroscope (Leica MZ6, Wetzlar, Germany) and micromorphology examination via a light microscope (Leica DM LS, Wetzlar, Germany) using wet mounts stained with Cotton Blue. Molecular identification was performed by sequencing the 28S rDNA (D1/D2 region) and/or ITS1-5.8S-ITS2 rDNA regions coupled with phylogenetic analyses and identification sequences for each fungal strain [39].
Culture media for spore inoculum and organic acids production
For the comparison purpose, two different agar media were tested for the better fungal spore production at low cost including commercial potato dextrose agar and cost-effective oat-agar medium in triplicate. The solid media were prepared and were autoclaved at 121 °C for 15 min. The cultured fungi on the solid medium were incubated at 28 °C for 5 days. The mature spores were harvested and dispensed in a saline solution to be counted using the Neubauer counting chamber. The composition of oat-agar solid media was: oat 30 g, agar 15 g, H2O 1 L, and pH 5.
Cane molasses was used as a cost-effective carbon source for the organic acids production. Raw molasses were obtained from Zillo Lorenzetti S.A Groupo Zilor, Macatuba city of Sao Paulo State, Brazil, and the suspended impurities were removed through filtration. The initial total sugar content of the crude molasses was 60% (52% sucrose and 8% reducing sugars), and it was adjusted to 15 g L−1 (total sugar concentration) by diluting it with tap water. The medium for organic acids production contained (g L−1) molasses 100, NH4Cl 4.0, KH2PO4 1.0, MgSO4·7H2O 0.25. The pH of the medium was adjusted to 4.5 with HCl (0.1 mol L−1) [40]. This liquid medium was autoclaved at 121 °C for 15 min.
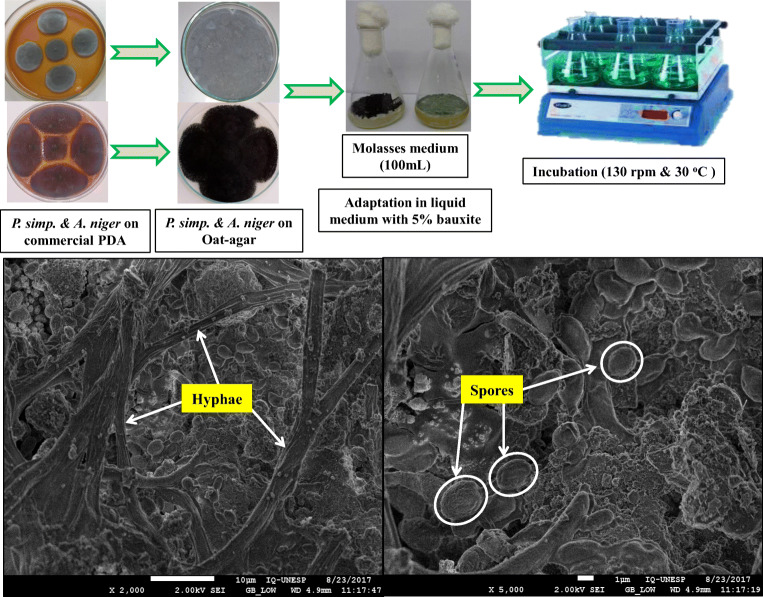
Adaptation of the fungal strains with bauxite
Firstly, the adaptation of fungi at different concentrations of bauxite pulp densities (1–10%) (w/v) was evaluated. However, no germination of the fungal spores was observed when the bauxite pulp density was augmented beyond 5% (w/v), probably due to the very toxic inhibitory effects caused by high concentrations of toxic heavy metals.
For the adaptation process, fungal growth tests were achieved over a series of repeated (three times) sub-culturing using 100-mL sterilized molasses medium with 5% (w/v) bauxite pulp density inoculated with fungal spores (1 × 107 spores/mL). The fungal spores with sterilized bauxite sample were incubated in an orbital shaker-incubator under previously established optimal conditions, such as temperature 30 °C for 15 days with an agitation speed of 130 rpm. The fungal spores were germinated in the form of filamentous mycelium, and after 10 days, the spores were germinated using the mycelium biomass as a solid growth medium. The mature spores were harvested and cultured in the oat-agar solid medium which was further used for the bioleaching process.
Bioleaching experiments in shake flasks
Bioleaching experiments were performed using 100-mL molasses medium dispensed in 250-mL Erlenmeyer flask containing 1% (w/v) bauxite pulp density and inoculated with 1-mL (1 × 107 spores) spore suspension. The same set of experiments was repeated using 5% bauxite pulp density. Two different bioleaching approaches were established to achieve the highest aluminum recovery. Thus, the batch experiments were conducted in a direct one-step and direct two-step bioleaching processes using 1% bauxite pulp density. For comparison, control sterile flasks were mounted in the absence of fungal spores. All the experiments were performed in duplicates by monitoring the pH and the bioleached Al concentration continuously until the last day of the experiment.
Direct one-step (D1) bioleaching
The molasses medium, previously autoclaved at 121 °C for 15 min, was inoculated with fungal spores (1 × 107 spores), and 1% (w/v) sterilized bauxite sample was added to it. The fungus with sterilized bauxite sample was incubated in an orbital shaker-incubator (C25KC incubator-shaker, New Brunswick Scientific, Edison, N.J., USA) at a rotation speed of 130 rpm for 10 days and at 30 °C.
Direct two-step (D2) bioleaching
Spores of A. niger and P. simplicissimum were first cultured in the sterilized molasses medium in the absence of bauxite and incubated at 30 °C in an orbital shaker-incubator at 130 rpm. After 3 days, a sudden decrease in pH was observed due to the large production of organic acids (logarithmic stage), the sterilized bauxite (1%) was added to the culture, and the incubation was sustained until the 10th day.
Determination of bioleached aluminum
The concentration of the aluminum in the leachate was determined by flame atomic absorption spectroscopy (Agilent Technologies, 200-Series AA) at a wavelength of 309.3 nm with a background correction.
Characterization of the bauxite ore
High-resolution field emission scanning electron microscope (FE-SEM; JEOL model 7500F) was used to examine the surface morphology of raw and the bioleached bauxite samples. An energy-dispersive X-ray (EDX) spectrometer (Thermo Scientific, model Ultra Dry) was used for the mineralogical composition. X-ray diffraction (XRD) technique was carried out using SIEMENS model D5000, DIFFRAC PLUS XRD Commander, to determine the mineralogical composition of the bauxite sample. Fourier transform infrared (FTIR) absorption spectrometer (VERTEX 70 with DLaTGS detector, Bruker, Germany) was used to record the infrared absorption spectra of raw and various bioleached bauxite samples. Attenuated total reflection (ATR) is a random sample system practiced in combination with infrared spectroscopy which permits samples to be examined directly in the solid or liquid state without further preparation. The spectra were recorded between 4000 and 400 cm−1.
Results and discussion
Production of spores on solid media
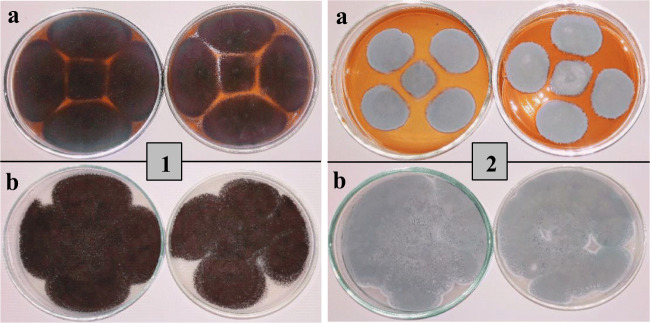
The production of fungal spores was tested on two different solid media including oat-agar and commercially available potato dextrose agar. According to the results, the oat-agar medium gave higher spore production with the characteristic color of the fungal colony. In the case of A. niger, about 5.52 × 108 spores/mL was produced in oat-agar and 3.45 × 108 spores/mL using potato dextrose agar medium. The numbers of P. simplicissimum spores counted on oat-agar medium reached 5.02 × 108 spores while on potato dextrose agar reached 1.95 × 108 spores. The spores produced on oat-agar medium were more than sufficient and covered the whole solid media surface as shown in Fig. 1. Moreover, oat-agar medium is cost-effective, simple, and easy to prepare without any addition of glucose, sucrose, or dextrose as compared to commercially available potato dextrose agar. The produced fungal spores on oat-agar medium were further applied for the production of organic acids using liquid medium augmented with molasses as a carbon substrate.
Fig. 1.
Pictorial presentation of (1) A. niger and (2) P. simplicissimum spore production using a potato dextrose agar and b oat-agar medium
Bioleaching studies with adapted fungal strains
Effect of aluminum dissolution
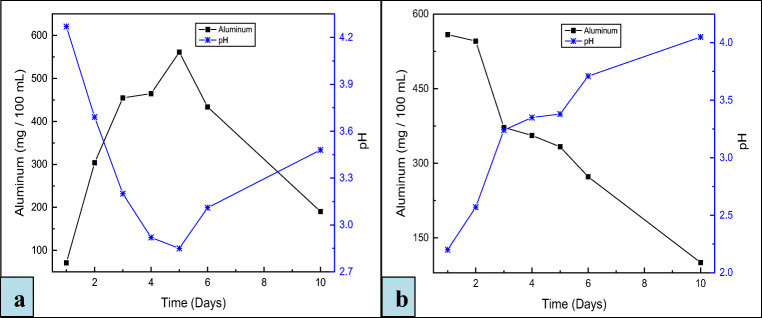
The fungal strains were adapted to increase the Al dissolution and to adapt the fungal strains to the toxic effects of the metals present in the bauxite. Due to the tolerance training of A. niger, comparatively, the bio-recovery of Al reached 561 mg/100 mL in the direct one-step and 558 mg/100 mL in the direct two-step bioleaching process respectively as depicted in Fig. 2a and b. It can be noted that the Al dissolution is higher in the direct one-step process from the direct two-step bioleaching process. Moreover, the dry biomass weight in the direct one-step was (1.63 g/100 mL) much more than in the direct two-step process (1.11 g/100 mL). It can be assumed that under this adaptive behavior, A. niger developed a continuously strong tolerance with increasing metal concentrations [36]. Therefore, we observed higher Al dissolution using the adapted strain compared to non-adapted strains [18]. According to the best of our knowledge, no study has yet been reported in the literature using a liquid medium for the adaptation of the fungal strains. The liquid medium ensures the regular contact between the fungal biomass and the released toxic metals from the bauxite. Metals are capable of exerting detrimental effects by means of their strong coordinating capabilities. These toxic effects involve the blockage of biologically important functional groups and denaturation of enzymes. Such effects may possibly be inhibited through complexation and ore precipitation of the heavy metals [41]. According to Baker and Walker [42], the inhibition processes become significantly more effective at the higher metal concentration which supports the better tolerance development under this condition.
Fig. 2.
Variation of pH during the Al dissolution as a function of time using adapted A. niger during a direct one-step and b direct two-step bioleaching process. Each point represents the mean value of the duplicates. Standard errors are within ± 6 mg/100 mL Al dissolution and ± 0.07 pH units
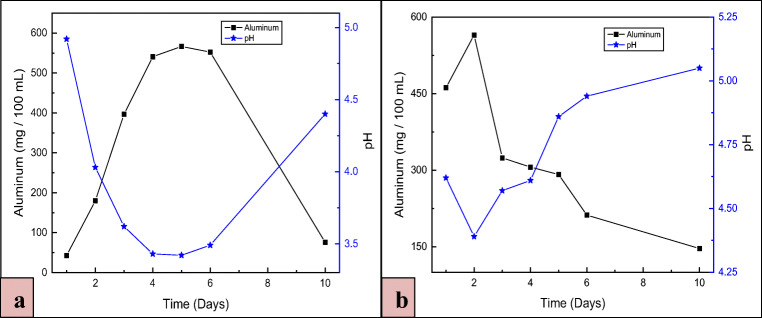
The adapted strain of P. simplicissimum showed a remarkable increase in the Al dissolution due to the increased tolerance to the metal toxicity. On the 5th day, Al dissolution reached 566.52 mg/100 mL in the direct one-step process when the pH was at the lowest (3.42) and in the direct two-step process, it reached 564.72 mg/100 mL on the 2nd day of incubation at a pH of 4.39 as demonstrated in Fig. 3 a and b. It is evident from the direct one-step process that a strong negative correlation exists between the pH and Al dissolution, indicating that as the pH of the medium decreases due to the production of the biogenic acids, amino acids, and enzymes, the Al dissolution increases. The decrease in the pH of the medium during the fungi growth is attributed to four processes [14]: (1) the release of protons through the proton-translocating plasma membrane ATPase, (2) the exchange of protons during the absorption of nutrients, (3) the secretion of organic acids, and (4) the production of carbon dioxide by the respiratory activity of the fungus. Comparatively, in the direct one-step process, the dry biomass weight was (1.421 g/100 mL) higher than in the direct two-step process (1.02 g/100 mL).
Fig. 3.
Variation of pH and Al dissolution as a function of time with adapted P. simplicissimum during a direct one-step and b direct two-step bioleaching process. Each point represents the mean value of the duplicates. Standard errors are within ± 8 mg/100 mL Al dissolution and ± 0.08 pH units
During the course of time, Al dissolution was decreased continuously in the direct two-step process, simply because of the alkaline nature of bauxite and or the precipitation of the Al complexes due to the pH increase. No aluminum oxalate crystals were detected by XRD analyses because of its presence in the amorphous precipitate [43]. The production of alkaline metabolites after the 5th day of cultivation indicates that the dissimilation of amino acids and ammonia release were enhanced significantly [44].
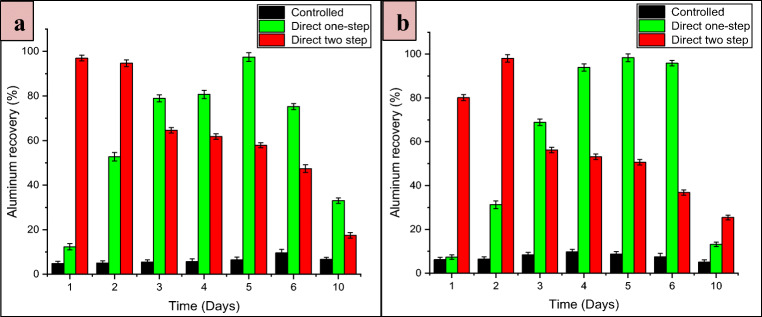
The bio-recovery of Al as a function of time during two different direct bioleaching processes is depicted in Fig. 4. For A. niger, the bio-recovery of Al during the direct one-step process reached 97.47% on the 5th day and in the direct two-step process, it attained 97.00% on the 1st day of incubation as shown in Fig. 4a. For the P. simplicissimum, in the direct one-step process, Al dissolution reached its maximum (98.35%) on the 5th day of incubation, and in the direct two-step process, it attained the value of 98.04% on the 2nd day of incubation as demonstrated in Fig. 4b. High Al dissolution by the adapted fungi can be attributed to the high tolerance and the adaptability of these fungal strains to the bauxite ore sample at the given pulp density.
Fig. 4.
Bio-recovery of Al as a function of time using adapted strains of a A. niger and b P. simplicissimum in control and two different bioleaching conditions
FE-SEM analysis of bioleached bauxite
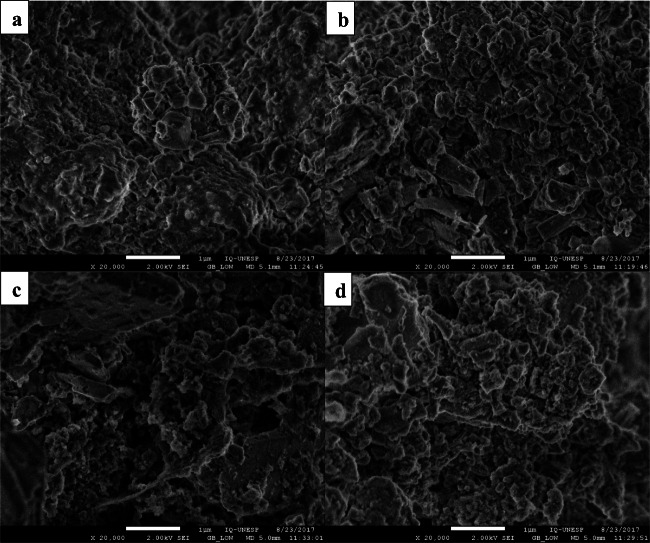
The SEM micrographs of bioleached bauxite with adapted A. niger after the direct one-step and direct two-step bioleaching process are shown in Fig. 5 a and b. Comparatively, the direct one-step process gave the maximum Al recovery due to the combined actions of fungal mycelia and the produced organic acids. The corrosive chemical reactions due to the produced organic acids, strong chelating/complex formation properties, physical forces exerted by fungal hyphae, and reduced pH are all the suitable factors that lead to the maximum eroding and weathering of bauxite sample during the direct bioleaching process. FE-SEM micrographs of bioleached bauxite with adapted P. simplicissimum are shown in Fig. 5 c and d.
Fig. 5.
High-resolution field emission scanning electron microscope (FE-SEM) micrographs of bioleached bauxite with adapted A. niger a direct one-step and b direct two-step and P. simplicissimum c direct one-step and d direct two-step bioleaching process
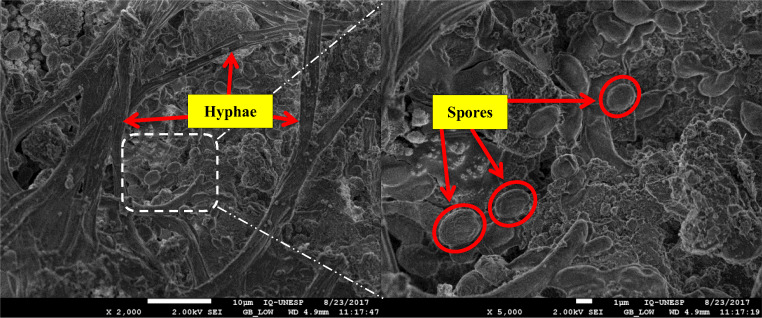
On close inspection, SEM examination of the bioleached bauxite with adapted A. niger attack showed spores and hyphae (vegetative mycelium filaments) of the fungus as depicted in the Fig. 6. According to Broderick and Greenshields [45], the average diameter size of A. niger spore is 3–5 μm, and the average length of hyphae filaments is 50 μm which is clearly evident in Fig. 6. This fact, in addition to organic acid production, suggested a combination of the direct and indirect mechanism of action on the bauxite by an adapted strain of A. niger. Significant differences can be observed in the micromorphology of bioleached bauxite samples by the combined actions of fungal mycelium in combination with the action of produced organic acids, enzymes, and amino acids. Moreover, the produced organic acids are proven chelating agents which also reduce the pH of the medium. The lower pH helps in the solubilization of Al, and thus, the bauxite sample suffers a drastic change in its surface morphology [46].
Fig. 6.
High-resolution field emission scanning electron microscope (FE-SEM) micrographs of bioleached bauxite with adapted A. niger in the direct two-step bioleaching process showing vegetative filaments and spores
EDX analysis of bioleached bauxite with the adapted strains
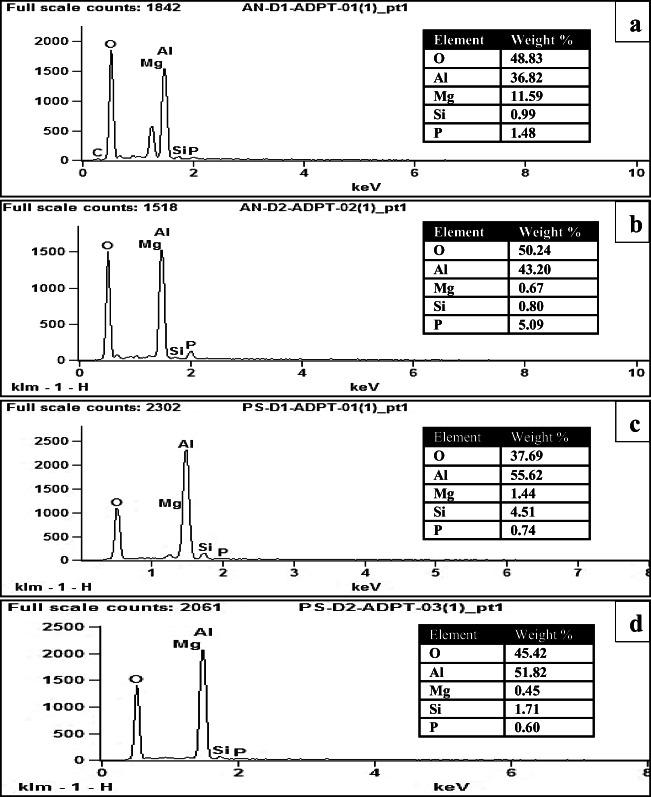
The energy-dispersive X-ray (EDX) analysis of bioleached bauxite sample showed only the presence of O, Mg, Al, Si, and P. All the other elements including Fe, Na, Cl, K, and Ca were absent, assuming that they were leached completely from the matrix [47, 48]. The EDX spectrum of the bioleached bauxite sample with adapted A. niger after direct one-step and direct two-step process is shown in Fig. 7 a and b. We consider the results relevant mainly seeing the data scarcity about fungi for the metal-leaching process. However, a limitation of this study concerns performing the experiments in duplicates. On the other hand, we have evidence of the standard deviation, and the experimental errors of the duplicates were at minimum. Thereby, further studies would be useful to enlarge the understanding of enhanced bio-recovery of aluminum from low-grade bauxite using adapted fungal strains.
Fig. 7.
Energy dispersive X-ray (EDX) spectrum of bioleached bauxite with adapted A. niger after a direct one-step, b direct two-step and with adapted P. simplicissimum, c direct one-step and d direct two-step bioleaching process
The adaptation of the P. simplicissimum to the higher bauxite pulp densities has a positive direct effect on its tolerance. Several elements that were left intact in the bauxite sample after the bioleaching process with unadapted strains were found to be leached out completely with the adapted strains. This could be attributed to the high tolerance and adaptation of the fungal strain to the high concentrations of the metals liberated from the bauxite sample during the bioleaching process. According to Valix and Loon [36], iron in high concentrations has toxic effects on P. simplicissimum, but with the fungal adaptation, this problem was overcome by mutation or precipitation of the iron complexes, and the strain developed a continuously strong tolerance with increasing metal concentration up to 2000 ppm. In the present study, the organic acids produced by P. simplicissimum are proven chelating agents and also have the ability to form stable metal complexes [49]. Moreover, the fungal biomass can also act as a sink to actively accumulate or passively immobilize the free Al+3 ions into microbial cell wall via biosorption [50]. The EDX spectrum of bioleached bauxite with adapted P. simplicissimum is depicted in the Fig. 7 c and d.
FTIR analyses of raw and bioleached bauxite samples with adapted strains
The FTIR spectra of original, controlled, and bioleached bauxite samples are shown in Fig. 8 a; however, almost no differences were observed in these spectra. The spectral bands appearing at 3400–3600 cm−1 are owing to hydroxyl groups (Al–O–H) stretching of gibbsite and kaolinite [51]. The bands at 1629–1641 cm−1 can be associated to the Al–O–H bending vibrations. The bands detected at 979, 1043, 1028, 1029, and 1031 cm−1 are caused by Al–O–H deformation vibrations and are characteristic of gibbsite present in the bauxite ore [52]. The bands near 450 cm−1 are attributed to the stretching vibrations (Al–Si–O) of aluminosilicates [53].
Fig. 8.
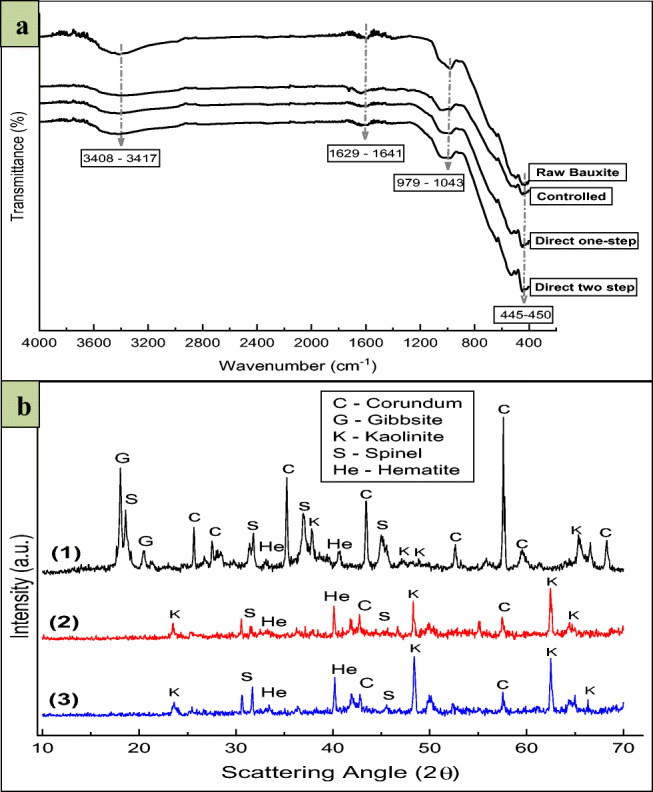
a Fourier transform infrared (FTIR) spectra of raw, controlled, and various bioleached bauxite samples and b X-ray diffraction (XRD) pattern of (1) raw bauxite sample, (2) after direct one-step and (3) after direct two-step bioleaching process
XRD analyses of the raw and bioleached bauxite samples
The X-ray diffraction (XRD) technique was applied to examine the mineralogical composition of the raw and bioleached bauxite samples. According to the XRD data, raw bauxite ore contains oxide minerals including corundum (Al2O3), gibbsite (Al2O3·3H2O), hematite (Fe2O3), spinel (MgAl2O4), and silicate minerals such as kaolinite (Al2Si2O5(OH)4). Interestingly, only the peaks for spinel, kaolinite, and hematite are visible, and most of the peaks for corundum and gibbsite were absent in the bioleached samples, confirming the maximal bio-recovery and enhanced dissolution of Al in such forms. The diffractograms of the bioleached bauxite treated in the direct one-step and direct two-step process are different from each other. Since higher Al solubilization capacity was observed in the direct one-step process as compared to the direct two-step process owing to more corrosion and weathering of the bauxite sample. The XRD patterns of the bauxite ore samples are depicted in the Fig. 8b.
Conclusions
In the bioleaching process, the metal recovery yield is reduced due to toxic effects triggered by various heavy metals present in the ore. However, the inhibitory effects of various toxic metals present in bauxite ore at higher pulp densities can be minimized by the tolerance training of the fungal strains. In this study, marine-derived fungi A. niger and P. simplicissimum were adapted using a liquid growth medium supplemented with molasses containing 5% bauxite pulp density for the enhanced bio-recovery of aluminum from low-grade bauxite. The mycelia of adapted strains were harvested and subsequently cultured in a low-cost oat-agar medium. Aluminum bioleaching efficiency in the direct one-step and direct two-step process supplemented with cost-effective molasses medium and 1% bauxite pulp density was considerably enhanced using the adapted fungal strains. According to the results obtained, more than 98% of the total Al was bioleached in the direct two-step bioleaching process using the adapted fungal strains. FE-SEM analyses of the bioleached bauxite sample confirmed the presence of the fungal spores and mycelium on the weathered and corroded bauxite surface. XRD analyses revealed the diminishment of oxide minerals (corundum and gibbsite) in the bioleached samples, and the EDX spectrum disclosed the complete absence of several elements including Fe, Na, Cl, K, and Ca after the bioleaching process. In conclusion, marine-derived A. niger and P. simplicissimum demonstrated an improved capability to withstand the metal toxic effects and to bioleached maximum Al from low-grade bauxite after an induced-tolerance training.
Funding information
This research was supported by The World Academy of Science (TWAS), Italy, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (Process no. 190112/2013-1).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gándara MJF (2013) Aluminium: the metal of choice. Mater Technol 3:261–265. http://mit.imt.si/Revija/izvodi/mit133/gandara.pdf Accessed 23 January 2018
- 2.Vakilchap F, Mousavi SM, Shojaosadati SA. Role of Aspergillus niger in recovery enhancement of valuable metals from produced red mud in Bayer process. Bioresour Technol. 2016;218:991–998. doi: 10.1016/j.biortech.2016.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Santhiya D, Ting YP. Use of adapted Aspergillus niger in the bioleaching of spent refinery processing catalyst. J Biotechnol. 2006;121:62–74. doi: 10.1016/j.jbiotec.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Rohwerder T, Gehrke T, Kinzler K, Sand W. Bioleaching review part A. Appl. Microb Biotechnol. 2003;63:239–248. doi: 10.1007/s00253-003-1448-7. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Naik Deshavath N, Goud VV, Dasu VV. Bioleaching of Al from spent fluid catalytic cracking catalyst using Aspergillus species. Biotechnol Rep. 2019;23:e00349. doi: 10.1016/J.BTRE.2019.E00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amiri F, Yaghmaei S, Mousavi SM. Bioleaching of tungsten-rich spent hydrocracking catalyst using Penicillium simplicissimum. Bioresour Technol. 2011;102:1567–1573. doi: 10.1016/j.biortech.2010.08.087. [DOI] [PubMed] [Google Scholar]
- 7.Qu Y, Li H, Tian W, Wang X, Wang X, Jia X, Shi B, Song G, Tang Y. Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner Eng. 2015;81:1–4. doi: 10.1016/j.mineng.2015.07.022. [DOI] [Google Scholar]
- 8.Olson GJ, Brierley JA, Brierley CL. Bioleaching review part B: progress in bioleaching: applications of microbial processes by the minerals industries. Appl Microbiol Biotechnol. 2003;63:249–257. doi: 10.1007/s00253-003-1404-6. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Lübeck M, Lübeck PS. Aspergillus as a versatile cell factory for organic acid production. Fungal Biol Rev. 2017;31:33–49. doi: 10.1016/j.fbr.2016.11.001. [DOI] [Google Scholar]
- 10.Papagianni M. Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnol Adv. 2007;25:244–263. doi: 10.1016/j.biotechadv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Amato A, Becci A, Beolchini F. Citric acid bioproduction: the technological innovation change. Crit Rev Biotechnol. 2020;40:199–212. doi: 10.1080/07388551.2019.1709799. [DOI] [PubMed] [Google Scholar]
- 12.Show PL, Oladele KO, Siew QY, Aziz Zakry FA, Lan JC-W, Ling TC. Overview of citric acid production from Aspergillus niger. Front Life Sci. 2015;8:271–283. doi: 10.1080/21553769.2015.1033653. [DOI] [Google Scholar]
- 13.Hu W, Li WJ, Yang HQ, Chen JH. Current strategies and future prospects for enhancing microbial production of citric acid. Appl Microbiol Biotechnol. 2019;103:201–209. doi: 10.1007/s00253-018-9491-6. [DOI] [PubMed] [Google Scholar]
- 14.Burgstaller W, Schinner F. Leaching of metals with fungi. J Biotechnol. 1993;27:91–116. doi: 10.1016/0168-1656(93)90101-R. [DOI] [Google Scholar]
- 15.Ren W-X, Li P-J, Geng Y, Li X-J. Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J Hazard Mater. 2009;167:164–169. doi: 10.1016/j.jhazmat.2008.12.104. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell A, Harvey LM, McNeil B. The roles of the alternative NADH dehydrogenases during oxidative stress in cultures of the filamentous fungus Aspergillus niger. Fungal Biol. 2011;115:359–369. doi: 10.1016/j.funbio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Thompson VS, Gupta M, Jin H, Vahidi E, Yim M, Jindra MA, Nguyen V, Fujita Y, Sutherland JW, Jiao Y, Reed DW. Techno-economic and life cycle analysis for bioleaching rare-earth elements from waste materials. ACS Sustain Chem Eng. 2018;6:1602–1609. doi: 10.1021/acssuschemeng.7b02771. [DOI] [Google Scholar]
- 18.S.S. Shah, M.C. Palmieri, S.R.P. Sponchiado, D. Bevilaqua, Hydrometallurgy. (2020) 105368. 10.1016/j.hydromet.2020.105368, Environmentally sustainable and cost-effective bioleaching of aluminum from low-grade bauxite ore using marine-derived Aspergillus niger
- 19.Lee YM, Kim MJ, Li H, Zhang P, Bao B, Lee KJ, Jung JH. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar Biotechnol. 2013;15:499–519. doi: 10.1007/s10126-013-9506-3. [DOI] [PubMed] [Google Scholar]
- 20.de Vita-Marques AM, Lira SP, Berlinck RGS, Seleghim MHR, Sponchiado SRP, Tauk-Tornisielo SM, Barata M, Pessoa C, de Moraes MO, Cavalcanti BC, Nascimento GGF, de Souza AO, Galetti FCS, Silva CL, Silva M, Pimenta EF, Thiemann O, Passarini MRZ, Sette LD. A multi-screening approach for marine-derived fungal metabolites and the isolation of cyclodepsipeptides from Beauveria felina. Quim Nova. 2008;31:1099–1103. doi: 10.1590/S0100-40422008000500030. [DOI] [Google Scholar]
- 21.Lee S, Park MS, Lim YW. Diversity of marine-derived Aspergillus from tidal mudflats and sea sand in Korea. Mycobiology. 2016;44:237–247. doi: 10.5941/MYCO.2016.44.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anahid S, Yaghmaei S, Ghobadinejad Z. Heavy metal tolerance of fungi. Sci Iran. 2011;18:502–508. doi: 10.1016/j.scient.2011.05.015. [DOI] [Google Scholar]
- 23.Pinzari F, Cuadros J, Napoli R, Canfora L, Baussà Bardají D. Routes of phlogopite weathering by three fungal strains. Fungal Biol. 2016;120:1582–1599. doi: 10.1016/j.funbio.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Gadd GM, Griffiths AJ. Microorganisms and heavy metal toxicity. Microb Ecol. 1977;4:303–317. doi: 10.1007/BF02013274. [DOI] [PubMed] [Google Scholar]
- 25.Gadd GM. Interactions of fungi with toxic metals, in: the genus Aspergillus. Boston: Springer US; 1994. Interactions of Fungi with Toxic Metals; pp. 361–374. [Google Scholar]
- 26.Zafar S, Aqil F, Ahmad I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol. 2007;98:2557–2561. doi: 10.1016/J.BIORTECH.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Gadd GM. Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res. 2007;111:3–49. doi: 10.1016/j.mycres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Vala KA, Sutariya V. Trivalent arsenic tolerance and accumulation in two facultative marine Fungi, Jundishapur. J Microbiol. 2012;5:542–545. doi: 10.5812/jjm.3383. [DOI] [Google Scholar]
- 29.Fomina MA, Alexander IJ, Colpaert JV, Gadd GM. Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biol Biochem. 2005;37:851–866. doi: 10.1016/J.SOILBIO.2004.10.013. [DOI] [Google Scholar]
- 30.Ruta L, Paraschivescu C, Matache M, Avramescu S, Farcasanu IC. Removing heavy metals from synthetic effluents using “kamikaze” Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol. 2010;85:763–771. doi: 10.1007/s00253-009-2266-3. [DOI] [PubMed] [Google Scholar]
- 31.Rangel DEN, Finlay RD, Hallsworth JE, Dadachova E, Gadd GM. Fungal strategies for dealing with environment- and agriculture-induced stresses. Fungal Biol. 2018;122:602–612. doi: 10.1016/j.funbio.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Ceci A, Maggi O, Pinzari F, Persiani AM. Growth responses to and accumulation of vanadium in agricultural soil fungi. Appl Soil Ecol. 2012;58:1–11. doi: 10.1016/J.APSOIL.2012.02.022. [DOI] [Google Scholar]
- 33.Iskandar NL, Zainudin NAIM, Tan SG. Tolerance and biosorption of copper (Cu) and lead (Pb) by filamentous fungi isolated from a freshwater ecosystem. J Environ Sci. 2011;23:824–830. doi: 10.1016/S1001-0742(10)60475-5. [DOI] [PubMed] [Google Scholar]
- 34.Costa FS, Macedo MWFS, Araújo ACM, Rodrigues CA, Kuramae EE, de Barros Alcanfor SK, Pessoa-Filho M, Barreto CC. Assessing nickel tolerance of bacteria isolated from serpentine soils. Braz J Microbiol. 2019;50:705–713. doi: 10.1007/s42770-019-00111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le L, Tang J, Ryan D, Valix M. Bioleaching nickel laterite ores using multi-metal tolerant Aspergillus foetidus organism. Miner Eng. 2006;19:1259–1265. doi: 10.1016/J.MINENG.2006.02.006. [DOI] [Google Scholar]
- 36.Valix M, Loon L. Adaptive tolerance behaviour of fungi in heavy metals. Miner Eng. 2003;16:193–198. doi: 10.1016/S0892-6875(03)00004-9. [DOI] [Google Scholar]
- 37.El-Gendy MMAA, El-Bondkly AMA. Evaluation and enhancement of heavy metals bioremediation in aqueous solutions by Nocardiopsis sp. MORSY1948, and Nocardia sp. MORSY2014. Braz J Microbiol. 2016;47:571–586. doi: 10.1016/j.bjm.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amiri F, Yaghmaei S, Mousavi SM, Sheibani S. Recovery of metals from spent refinery hydrocracking catalyst using adapted Aspergillus niger. Hydrometallurgy. 2011;109:65–71. doi: 10.1016/j.hydromet.2011.05.008. [DOI] [Google Scholar]
- 39.Kossuga MH, Romminger S, Xavier C, Milanetto MC, do Valle MZ, Pimenta EF, Morais RP, de Carvalho E, Mizuno CM, Coradello LFC, de M. Barroso V, Vacondio B, Javaroti DCD, Seleghim MHR, Cavalcanti BC, Pessoa C, Moraes MO, Lima BA, Gonçalves R, Bonugli-Santos RC, Sette LD, Berlinck RGS. Evaluating methods for the isolation of marine-derived fungal strains and production of bioactive secondary metabolites. Brazilian J Pharmacogn. 2012;22:257–267. doi: 10.1590/S0102-695X2011005000222. [DOI] [Google Scholar]
- 40.Wang J. Improvement of citric acid production by Aspergillus niger with addition of phytate to beet molasses. Bioresour Technol. 1998;65:243–245. doi: 10.1016/S0960-8524(98)00026-1. [DOI] [Google Scholar]
- 41.Gadd G. Metals and microorganisms: a problem of definition. FEMS Microbiol Lett. 1992;100:197–203. doi: 10.1016/0378-1097(92)90209-7. [DOI] [PubMed] [Google Scholar]
- 42.Baker AJM, Walker PL. Physiological responses of plants to heavy metals and the quantification of tolerance and toxicity. Chem Speciat Bioavailab. 1989;1:7–17. doi: 10.1080/09542299.1989.11083102. [DOI] [Google Scholar]
- 43.Lide DR. CRC handbook of chemistry and physics. Boca Raton: CRC Press; 1993. [Google Scholar]
- 44.Aziza M, Amrane A. Diauxic growth of Geotrichum candidum and Penicillium camembertii on amino acids and glucose. Braz J Chem Eng. 2012;29:203–210. doi: 10.1590/S0104-66322012000200001. [DOI] [Google Scholar]
- 45.Broderick A, Greenshields R. Sporulation of Aspergillus niger and Aspergillus ochraceus in continuous submerged liquid culture. J Gen Microbiol. 1981;126:193–202. [Google Scholar]
- 46.Harley AD, Gilkes RJ. Factors influencing the release of plant nutrient elements from silicate rock powders: a geochemical overview. Nutr Cycl Agroecosyst. 2000;56:11–36. doi: 10.1023/A:1009859309453. [DOI] [Google Scholar]
- 47.Abhilash BD, Pandey KA. Natarajan, microbiology for minerals, metals, materials and the environment. 1. Boca Raton: CRC Press; 2015. [Google Scholar]
- 48.Bosshard PP, Bachofen R, Brandl H. Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ Sci Technol. 1996;30:3066–3070. doi: 10.1021/es960151v. [DOI] [Google Scholar]
- 49.Qu Y, Lian B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour Technol. 2013;136:16–23. doi: 10.1016/j.biortech.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 50.Tassist A, Lounici H, Abdi N, Mameri N. Equilibrium, kinetic and thermodynamic studies on aluminum biosorption by a mycelial biomass (Streptomyces rimosus) J Hazard Mater. 2010;183:35–43. doi: 10.1016/J.JHAZMAT.2010.06.078. [DOI] [PubMed] [Google Scholar]
- 51.Kloprogge JT, Ruan HD, Frost RL. Thermal decomposition of bauxite minerals: infrared emission spectroscopy of gibbsite, boehmite and diaspore. J Mater Sci. 2002;37:1121–1129. doi: 10.1023/A:1014303119055. [DOI] [Google Scholar]
- 52.F.A.N.G. Silva, J.A. Sampaio, F.M.S. Garrido, M.E. Medeiros, Study on the Characterization of Marginal Bauxite from Pará/Brazil, in: Light Met. 2011, Springer International Publishing, Cham, 2011: pp. 13–18. 10.1007/978-3-319-48160-9_2
- 53.Ruan H, Frost R, Kloprogge J, Duong L. Infrared spectroscopy of goethite dehydroxylation. II. Effect of aluminium substitution on the behaviour of hydroxyl units, Spectrochim. Acta Part A Mol Biomol Spectrosc. 2002;58:479–491. doi: 10.1016/S1386-1425(01)00556-X. [DOI] [PubMed] [Google Scholar]