Abstract
Acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV) is a major global public health problem. The aim of this study is to determine the prevalence of HIV-1 infection in four municipalities of Pará State (Marabá, Parauapebas, Curionópolis, and Canaã dos Carajás), in northern, Brazil. The municipalities are located in the Carajás Complex iron mining area. The employment opportunities result in extensive migratory flow of people. A total of 4771 serum samples were obtained from 2005 to 2014 and were sent to Evandro Chagas Institute, Belém-Pará, where they were tested by enzyme-linked immunosorbent assay, with reactive samples confirmed by Western blot analysis. The samples were from individuals from 23 Brazilian states and the Federal District, mainly Maranhão (39.53%) and other municipalities of Pará (34.25%). The total positivity rate was 0.48% (23/4771). The rate was 0.47% (14/2975) in males and 0.50% (9/1796) in females. Of these, 0.33% (14/4275) were from asymptomatic individuals whose serum were collected during the serological survey, 1.81% (9/497) were from cases featuring clinical symptoms including fever/diarrhea/jaundice, which were included in febrile, diarrheal, and icteric syndromes analyzed during the study. The findings indicated the presence of HIV-1 infection in the general population studied. The majority of cases (60.9%, 14 of 23 positive cases) were asymptomatic.
Keywords: AIDS, HIV-1, Amazon region, Epidemiology, Migratory flow
Introduction
Human immunodeficiency virus (HIV) is responsible for acquired immunodeficiency syndrome (AIDS). HIV-1 is the most prevalent worldwide, accounting for 95% of all HIV infections. HIV-2 is less prevalent and causes a disease that progresses more slowly with fewer deaths and is found more prevalent in West Africa and European communities with connections to West Africa. HIV-1 and HIV-2 are similar, especially the Gag protein, which has a homology of approximately 60% [1].
Several strategies have been implemented for over 30 years to define the infection trend, the profile of those who are affected, the impact of prevention and control measures, and the detection of cases of asymptomatic infections [2].
Despite this scrutiny, AIDS remains a global public health challenges. It is the fifth most common cause of death among adults, as well as the virus HIV itself, with consequences for individuals, families, and societies [3]. In 10-year study, from 2008 to 2018, Brazil reported a 24.1% decrease in the standardized mortality rate for Brazil, from 5.8 to 4.4 deaths per 100,000 inhabitants [4].
The prevalence of the HIV epidemic in Latin America and the Caribbean region remains largely confined to vulnerable populations, such as men who have sex with men and transgender women. Late initiation of treatment and less-than-ideal compliance to treatment remain major challenges in the treatment of HIV-1 infection, with resulting burden on health programs. Recent studies on the provision of pre-exposure prophylaxis (PrEP) in key populations for the prevention of HIV-1 infection have shown promising results, with the implementation of projects as well as effective adoption of PrEP by various national health systems. In accordance with the guidelines of the World Health Organization (WHO) [3], Brazil offered PrEP in several cities in different regions of the country, with encouraging results during the first year of the program [5].
The present study was performed from 2005 to 2014 in an iron mining area of Carajás Complex and surrounding cities. This region experiences a pronounced migratory flow of people from different parts of the country because of the mining-related employment opportunities. The region was thus an appropriate locality to assess the health-disease situation, as well as the environmental and social changes observed in this locality, including the determination of the prevalence of antibodies to HIV in serum samples. This information is valuable, since this region of Brazil has been poorly informed concerning the spread of the virus. The methodologies used were adequate to obtain data that were representative of the studied population.
Methods
Study populations
A large serological survey involved communities in the vicinity of the mineral complex, environmentally protected areas, was carried out by spontaneous demand. In addition, a syndromic survey was performed in hospitals and other health facilities focused on febrile, diarrheal, and icteric syndromes. A team composed of doctors and field staff was responsible for interviewing and explaining the purpose of the study, as well as obtaining the consent form and the epidemiological data of these individuals. For symptomatic patients, all the presenting symptoms were annotated in a specific file, which was transferred to a common database that was used in the present study. Sample and data collection occurred in different places (every household of various villages), including two hospitals (Parauapebas Municipal Hospital and Yutaka Takeda Hospital) and the Parauapebas Health Post. Blood samples (10 mL) were collected from each person. The obtained serum was stored at − 20 °C until transfer to the Evandro Chagas Institute for immediate analysis. The 4771 blood samples obtained during the serological survey carried out among the residents and/or workers from the four cities were collected from 2005 to 2014. Collection was not done in 2007 and 2011 due to problems with study renewal. A total of 266 individuals had two samples collected in different years or in different months of the same year.
Study site/area
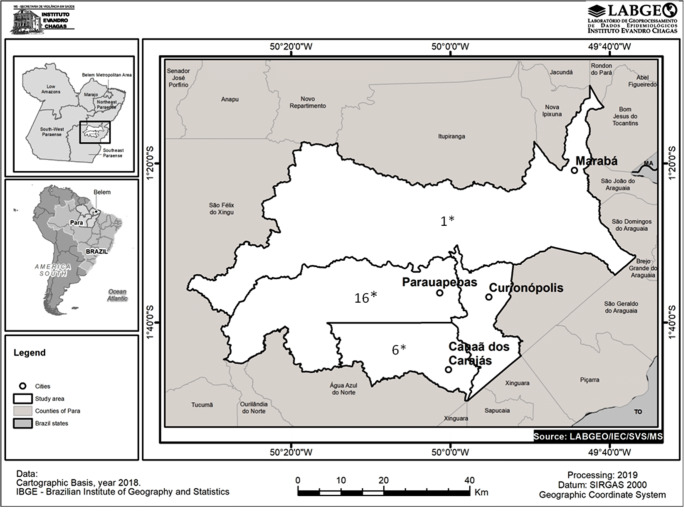
The municipalities in the Carajás Complex included Canaã of Carajás, Curionópolis, Marabá, and Parauapebas (Fig. 1).
Fig. 1.
Map showing the four municipalities in the State of Pará, Brazil, where the iron mining area is located and where research on infection by the human immunodeficiency virus-1 was carried out, in the period 2005–2014. Source: Geoprocessing Laboratory (LABGEO) Evandro Chagas Institute, Secretariat of Health Surveillance, Brazilian Ministry of Health. *Number of positive HIV cases detected in this municipality
The latest census conducted in Canaã of Carajás in 2010 reported a population of 26,716 people, with approximately 36,050 people estimated for 2018, according to the Brazilian Institute of Geography and Statistics (IBGE) [6]. Hence, in 2018, there was a demographic density of 11.45 inhabitants/km2 in a region of 3146.4 km2. Canaã of Carajás is located at latitude 06° 29′51″/south and longitude 49°52′42″/west with an elevation of 286 m. The town’s economy is focused on mineral extraction, mainly copper extraction by the Vale Mining Company through the Serra do Sossego Mining subsidiary. When the company commenced work in the region in 2002, the population of Canaã and other locations increased significantly. Diamonds, bauxite, red nickel, and gold are also present in the soil of the municipality and stimulated other mining activities.
Curionópolis is 2368.7 km2 in size and is located in the microregion of Parauapebas, in the southeastern mesoregion of Pará State. In the latest census of 2010, its population was 18,288 people with a demographic density of 7.72 inhabitants/km2. According to IBGE, its estimated population was 18,014 in 2018. During the 1980s, it housed the Serra Pelada district, the largest open-pit mine in the world. The district is located at latitude 6°3′58″/south and longitude 49°33′40″/west at an altitude of 182 m.
Marabá is located in the southeastern part of Pará. It is the 4th most populous municipality in the state, with 233,669 inhabitants in the last census and 279,349 inhabitants according to the IBGE estimate of 2018, with an area of 15,128.058 km2 and a demographic density of 15.45 inhabitants/km2. Marabá is situated at 84 m altitude, south latitude 05°22′12″/south, and longitude 49°7′1″/west. It is the main socioeconomic center of southeastern Pará and one of the most dynamic municipalities in Brazil.
The municipality of Parauapebas, belonging to the Southeast Paraense mesoregion, extends over 6957.3 km2, with 153,908 inhabitants in the 2010 census and an estimated 202,882 inhabitants in 2018. The population density was 29.16 inhabitants/km2 in 2018. Parauapebas is located at 168 m altitude, latitude 6°04′15″/south, and longitude 49°54′15″/west. It is 719 km from the capital of Belém. Serra of Carajás is in this municipality. It is the largest mineral mining province on Earth. Iron mining activity is mainly carried out at the Carajás Iron Mine in Vale. Manganese and gold are also extracted.
Serology
The blood samples obtained during the serological survey were initially analyzed for the presence of anti-HIV antibodies and p24 antigen using the Vironostika™ HIV Ag/Ac commercial enzyme immunoassay kit (BIOMÈRIEUX, France) and the included positive and negative controls. Reactivity was confirmed by Western blot using INNO-LIA™ HIVI/II Score (INNOGENETICS, Belgium) including the HIV-1 group O and HIV-2. Both tests were performed according to the manufacturer’s recommendations.
Statistical methods
The G-Test, a maximum likelihood statistical significance test, was performed to compare the prevalence rates observed in each Brazilian region. The G-Test was also used to assess the statistical relevance of the age-related prevalence of HIV infection in males and females, to analyze the infection rate obtained in asymptomatic and symptomatic individuals affected by HIV, and to compare the positivity rate by gender in relation to the associated febrile, diarrheal, and icteric symptoms. The Chi-square test was used to evaluate the formal education level of the enrolled subjects in relation to the prevalence of HIV-1 infection. Finally, the binomial test was applied to compare the HIV rates observed according to gender. The statistical analyses were performed by BioEstat 5.3 [7] software (https://www.playonlinux.com/en/app-2666-BioEstat_53_freeware_software_for_Biostatistical_analysis.html). A p value ≤ 0.05 indicated statistical significance.
Ethical considerations
This project was approved by the Ethics and Research Committee (CEP) of Evandro Chagas Institute (IEC), Health Surveillance Secretariat (SVS), and Ministry of Health (MS) under protocol numbers 0014/04 and 002/09.
Results
A total of 4771 samples collected during the 10 years from 2005 to 2014 were analyzed for the presence of anti-HIV-1 antibody and the p24 antigen. The samples were obtained from 4505 individuals from 23 Brazilian states and the Federal District who migrated to work and resided in the municipalities located around the Carajás Complex. These individuals included 266 who provided two samples. All 266 individuals were HIV-negative.
The overall positivity rate was 0.48% (23/4771). The samples were analyzed according to the individuals’ place of origin. Four states experienced a greater influx of people: Maranhão, (n = 1781, 39.53%); other municipalities of Pará (n = 1543, 34.25%); Piauí (n = 219, 4.86%); and Goiás (n = 195, 4.33%). The highest HIV positivity rate was verified in Alagoas and Mato Grosso (14.29%, 1/7 each) (Table 1).
Table 1.
Distribution of individuals with serum samples collected in municipalities of southeast Pará State, Brazil from 2005 to 2014, according to the state of origin and the results of HIV-1 serology test
| States and federal district | Tested individualsa | Positive samples | |||
|---|---|---|---|---|---|
| Region | No | % | No (23) | % Posb in relation individuals tested | |
| Acre | 4 | 0.09 | - | - | |
| Amazonas | 3 | 0.07 | - | - | |
| North | Roraima | 6 | 0.13 | - | |
| Amapá | 5 | 0.11 | - | - | |
| Pará (other municipalities) | 1.543 | 34.25 | 2 | 0.13 | |
| Tocantins | 162 | 3.60 | - | - | |
| Maranhão | 1.781 | 39.53 | 14 | 0.79 | |
| Piauí | 219 | 4.86 | 2 | 0.91 | |
| Ceará | 103 | 2.29 | - | - | |
| Rio Grande do Norte | 9 | 0.20 | - | - | |
| Northeast | Paraíba | 29 | 0.64 | - | - |
| Pernambuco | 44 | 0.98 | - | - | |
| Alagoas | 7 | 0.16 | 1 | 14.29 | |
| Sergipe | 5 | 0.11 | - | - | |
| Bahia | 98 | 2.18 | |||
| Goiás | 195 | 4.33 | 3 | 1.54 | |
| Distrito Federal | 9 | 0.20 | - | - | |
| Center-West | Mato Grosso | 7 | 0.16 | 1 | 14.29 |
| Mato Grosso do Sul | 4 | 0.09 | - | - | |
| Minas Gerais | 129 | 2.86 | - | - | |
| Southeast | Espírito Santo | 24 | 0.53 | - | - |
| Rio de Janeiro | 10 | 0.22 | - | - | |
| São Paulo | 33 | 0.73 | - | - | |
| Paraná | 22 | 0.49 | - | - | |
| South | Santa Catarina | 1 | 0.02 | - | - |
| Rio Grande do Sul | 6 | 0.13 | - | - | |
| Uninformed | 47 | 1.04 | - | - | |
| Total | 4,505 | 100 | 23 | - | |
aA total of 4771 serum samples was collected from 4505 individuals. Two samples were collected from 266 individuals in different years or in different months of the same year
bPos denotes positive. Significant prevalence was verified when the north (G = 6.95; p = 0.008) and northeast (G = 4.14; p = 0.04) regions were compared with the others regions
Statistical analyses of the positive cases by geographic region of Brazil revealed a significant prevalence in the north (G = 6.95; p = 0.008) and northeast (G = 4.14; p = 0.04) regions compared with the prevalence in the other regions.
Table 2 presents the results of the distribution of the HIV-1 positivity rate by gender and year of collection. A similar rate of positivity was observed in females (37.6%, 1796/4771) and males (62.4%, 2975/4771), with values of 0.50% (9/1796) and 0.47% (14/2975), respectively (binomial test: power (0.05) = 0.01; p = 0.88). Regarding the collection period, HIV case registration was highest in 2014, with a positivity rate of 2.18% (7/321). In 2006, the seven reported cases were all male (three symptomatic and four asymptomatic). This gender corresponded to 78.9% of the total samples obtained in 2006. In 2009, only one female case (asymptomatic) was observed. In 2010, five cases (one symptomatic in men and four asymptomatic, involving two males and two females). In 2012, there were two cases (one asymptomatic woman and one symptomatic man). In 2014, the first two symptomatic cases were found in women, two more in men, and three asymptomatic (two women and one man), suggesting greater exposure of women to HIV.
Table 2.
Distribution of the 4771 serum samples collected in municipalities of southeast Pará State, Brazil, according to gender and year, in relation to the HIV-1 serology test results, from 2005 to 2014
| Sex/year | 2005 | 2006 | 2008 | 2009 | 2010 | 2012 | 2013 | 2014 | Total |
|---|---|---|---|---|---|---|---|---|---|
| pos/test | pos/test | pos/test | pos/test | pos/test | pos/test | pos/test | pos/test | pos/test/% | |
| Fem | 1/213 | -/260 | -/16 | 1/144 | 2/559 | 1/202 | -/248 | 4/154 | 9/1796/0.50 |
| Male | 0/234 | 7/973 | -/93 | -/140 | 3/912 | 1/173 | -/283 | 3/167 | 14/2975/0.47 |
| Total | 1/447 | 7/1,233 | -/109 | 1/284 | 5/1,471 | 2/375 | -/531 | 7/321 | 23/4771/0.48 |
| % | 0.22 | 0.57 | - | 0.35 | 0.34 | 0.53 | - | 2.18 |
pos positive, test tested, Fem female, % percentage, “-” zero
The age of the study patients ranged from < 1 to 99 years (average 29.7 years, median 28 years). When the 23 HIV-1-positive samples were related to the age group, a greater rate of positivity was evident in males aged 40–49 years (0.96%; G = 1.20; p = 0.27) and in the females aged 20–29 years (1.36%; G = 3.87; p = 0.04). Considering the overall population, the highest prevalence was observed in the people 40–49 years of age (0.80%; G = 0.77; p = 0.38) (Table 3).
Table 3.
Distribution of the 4771 serum samples collected in municipalities of southeast Pará State, Brazil according to age and gender, in relation to the HIV-1 serology test results, from 2005 to 2014
| Age group | Pos/male | % | Pos/Fem | % | Pos/total | % | G/p |
|---|---|---|---|---|---|---|---|
| 0–9 | -/273 | - | -/295 | - | -/568 | - | |
| 10–19 | 1/456 | 0.22 | -/385 | - | 1/841 | 0.12 | |
| 20–29 | 1/808 | 0.12 | 5/368 | 1.36 | 6/1176 | 0.50 | |
| 30–39 | 4/572 | 0.70 | 2/310 | 0.64 | 6/882 | 0.68 | |
| 40–49 | 4/415 | 0.96 | 1/207 | 0.48 | 5/622 | 0.80 | 0.77/0.38 |
| ≥ 50 | 4/443 | 0.90 | 1/230 | 0.43 | 5/673 | 0.74 | |
| No information | 0/8 | - | 0/1 | - | 0/9 | - | |
| Total | 14/2975 | 0.47 | 9/1796 | 0.50 | 23/4771 | 0.48 |
Pos positive, Male male, Fem female, % percentage, “-” zero
In relation to education, subjects ranged from ‘Illiterates’ to ‘Higher education degree holders’. HIV-1-positive cases were detected at all education levels, with a higher percentage in the elementary school certificate holders (0.58%) (Table 4). Statistical analyses comparing each level of schooling revealed no significance in the prevalence of HIV.
Table 4.
Distribution of the serum samples collected in municipalities of southeast Pará State, Brazil, according to educational status and gender, in relation to the HIV-1 serology test results, from 2005 to 2014
| Schooling | Sex | Total/ Pos/ % | p | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| Total | P Pos | % | Total | Pos | % | |||
| Illiterate | 139 | – | – | 103 | 1 | 0.97 | 242/ 1/ 0.41 | 0.82 |
| Elementary school | 1593 | 9 | 0.56 | 984 | 6 | 0.61 | 2577/ 15/ 0.58 | 0.60 |
| High school | 859 | 3 | 0.35 | 389 | 2 | 0.51 | 1248/ 5/ 0.40 | 0.65 |
| Higher education | 109 | 1 | 0.92 | 90 | – | – | 199/ 1/ 0.50 | 0.20 |
| No information | 114 | 1 | 0.88 | 80 | – | – | 194/ 1/ 0.51 | |
| Totala | 2814 | 14 | 0.50 | 1646 | 9 | 0.55 | 4460/ 23/ 0.52 | |
Legend: Pos positive, Male male, Fem female, %- percentage, − zero
aIn this total, 311 small children (162 male and 149 female) who were not in school were excluded. Also, the cases without information (194) were not included in the p analysis
Distribution of the samples by gender and the presence of any clinical symptom, such as fever, diarrhea, and jaundice, which were evaluated based on the febrile, diarrheal, or icteric syndromes, revealed a greater percentage for symptomatic cases (1.81%) than asymptomatic (0.33%) with pronounced statistical significance (G = 11.42; p = 0.0007) (Table 5).
Table 5.
Distribution of the 4771 serum samples collected in municipalities of southeast Pará State, Brazil according to gender and the presence of clinical symptoms (febrile, diarrheal, or icteric syndrome) in relation to the HIV-1 serology test results, from 2005 to 2014
| Health condition | Sex | Total/ Pos/ % | |||||
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Total | Pos | % | Total | Pos | % | ||
| Asymptomatic | 2686 | 7 | 0.26 | 1588 | 7 | 0.44 | 4275/14/0.33 |
| Symptomatic | 290 | 7 | 2.41 | 207 | 2 | 0.97 | 497/9/1.81 |
| Febrilea | 203 | 4 | 2.0 | 135 | 2 | 1.5 | 338/6/1.8 |
| Diarrheala | 16 | 2 | 12.5 | 9 | - | - | 25/2/8.0 |
| Icterica | 27 | 1 | 3.7 | 14 | - | - | 41/1/2.43 |
| Total | 2976 | 14 | 0.47 | 1795 | 9 | 0.50 | 4771/23/0.48 |
Pos positive, % percentage, “-” zero
aThese were the three syndromes studied during the project. Of 164 cases, we had no access to medical records
The G-test was used to compare the positivity rate by gender in relation to the three syndromes. The lack of statistical relevance between the variables suggested that the prevalence of infection by gender was not associated with the febrile (G = 0.07; p = 0.93), diarrheal (G = 0.12; p = 0.72), and icteric (G = 0.10; p = 0.74) syndromes. Among clinical indicators and symptoms, diarrhea showed higher positivity, but without significance (G = 1.27; p = 0.25).
The municipality of the residence, location of approach, health status, and year of collection were factors analyzed for the 23 HIV-1 seropositive individuals. All positive cases were from three municipalities: Parauapebas (16/3027 = 0.53%), Canaã of Carajás (6/1015 = 0.59%), and Marabá (1/52 = 1.92%). All symptomatic cases were collected in the hospital. Of note, two asymptomatic cases were from a couple (Table 6).
Table 6.
Distribution of the 23 HIV-1 seropositivity, collected in municipalities of southeast Pará State, Brazil, according to their origin, health status, place of approach, and year of collection, 2005 to 2014
| Sample ID | Sex | Age | Municipality of residence | Location of approach | Year of collection | Origin | Health condition |
|---|---|---|---|---|---|---|---|
| 00558-A | M | 53 | Parauapebas | Hospital | 2006 | GO | Symptomatic |
| 00639-A | M | 41 | Parauapebas | Hospital | 2006 | MA | Symptomatic |
| 00662-A | M | 28 | Parauapebas | Hospital | 2006 | AL | Symptomatic |
| 04547-A | M | 53 | Parauapebas | Hospital | 2010 | PI | Symptomatic |
| 05171-A | M | 55 | Parauapebas | Hospital | 2012 | MA | Symptomatic |
| 05943-D | F | 25 | Parauapebas | Hospital | 2014 | MA | Symptomatic |
| 05946-A | M | 49 | Parauapebas | Hospital | 2014 | MA | Symptomatic |
| 05967-A | M | 38 | Parauapebas | Hospital | 2014 | MA | Symptomatic |
| 05987-B | F | 35 | Parauapebas | Hospital | 2014 | MA | Symptomatic |
| 01064-A | M | 35 | Parauapebas | Job Vacancy | 2006 | MA | Asymptomatic |
| 01092-A | M | 34 | Parauapebas | Job Vacancy | 2006 | MA | Asymptomatic |
| 01144-A | M | 42 | Parauapebas | Job Vacancy | 2006 | MA | Asymptomatic |
| 00047-B | F | 34 | Parauapebas | V Sansão | 2005 | PI | Asymptomatic |
| 00039-D | F | 20 | Parauapebas | V Sansão | 2009 | MA | Asymptomatic |
| 04751-A | F | 23 | Parauapebas | Accom S Barb | 2010 | MA | Asymptomatic |
| 04271-A | M | 50 | Parauapebas | Accom Odebr | 2010 | GO | Asymptomatic |
| 00378-A | M | 40 | Marabá | Proj Salobo | 2006 | MA | Asymptomatic |
| 04604-Aa | M | 42 | Canaã dos Carajás | V B.Jesus | 2010 | MA | Asymptomatic |
| 04604-Ba | F | 28 | Canaã dos Carajás | V B.Jesus | 2010 | PA | Asymptomatic |
| 04641-D | F | 40 | Canaã dos Carajás | V B.Jesus | 2014 | MA | Asymptomatic |
| 04650-F | M | 16 | Canaã dos Carajás | V B.Jesus | 2014 | PA | Asymptomatic |
| 06053-A | F | 52 | Canaã dos Carajás | V B.Jesus | 2014 | GO | Asymptomatic |
| 05129-B | F | 24 | Canaã dos Carajás | V Planalto | 2012 | MT | Asymptomatic |
Accom S Barb Accommodation Santa Bárbara, V Sansão Village Sansão, Accom Odebr Accommodation Odebrech, Proj. Salobo Project Salobo, V B Jesus Village Bom Jesus, V Planalto Village Planalto, MA Maranhão, PI Piauí, GO Goiás, AL Alagoas, PA Pará, MT Mato Grosso
aCases that came from husband and wife
Discussion
Although Alagoas and Mato Grosso demonstrated the highest HIV positivity rate (both 14.29%) of the 23 Brazilian states and the Federal District, the number of samples collected was very small (n = 14). The samples number may have influenced this result. For this reason, we believe that Goiás (1.54%), Piauí (0.91%), and Maranhão (0.79%) can be considered the locales with the highest HIV-positive rates.
It is important to highlight that the information from the present study did not allow us to assess the exact location of infection. Thus, we could not determine if infections occurred in the municipalities investigated or in the state of origin of the individuals. However, all individuals with positive serology have lived for at least 1 year in the places that we investigated, with the exception of one case with a 6 month period of residence. This is a limitation of the study.
Between 2008 and 2018, there was an increase in the standardized mortality rate for Brazil, with increases of 26.0% and 2.8% in the north and northeast regions, respectively. Other regions experienced decreases of 43.8% in the southeast, 41.5% in the south, and 26. 4% in relation to the midwest. The analysis of mortality by Federative Unit revealed higher coefficients of death per 100,000 inhabitants in Pará (7.6), Roraima (7.6), Amazonas (6.9), and Maranhão (5.4) compared with the national value of 4.4 [4].
During the 10 years of study, the highest positive rate was obtained in 2014 (2.18%). This value was statistically significant (binomial test: power (0.05) = 0.75; p < 0.0001) when compared with the other years studied. The sole HIV-positive case in 2005 involved an asymptomatic woman from Piauí.
Regarding sex, the 4771 serum samples were more often from men (62.4%) than women (37.6%). However, the overall HIV prevalence in both genders (0.47% male and 0.50% female) was similar and low. The increasing prevalence presented was similar to those described elsewhere in Brazil [8].
At the beginning of the epidemic in Brazil, a much larger number of infected men were observed than women. Over time, this gap narrowed. However, more recently, the gender difference is increasing with the growing number of positive cases in men [9]. The finding also highlights the importance of the early diagnosis of HIV-1 infection. One of WHO goals is that 90% of an infected population should become aware of their status. Presently of the 23 positive cases, 13 (56%) were asymptomatic, and so likely did not know their serological status. Early diagnosis allows immediate initiation of therapy, which can break the transmission cycle and improve a patient’s immune reconstitution [10, 11]. Many of the cases had no symptoms or displayed nonspecific symptoms, such as febrile and diarrheal syndromes which can be confused with other diseases.
Concerning the age-related distribution, the youngest HIV-infected individual was a 16-year-old male. He was asymptomatic. The oldest was a 55-year-old male. He was symptomatic. The highest HIV positivity rate was found in females aged 20–29 years (1.4%) and males > 40 years of age. The increased frequency of positive cases for those > 20 years of age was expected considering that sexual activity begins or becomes more intense during that period, which also favors the risk of infection.
The number of young people (0–19 years of age) who were tested comprised 29.5% of the total number of individuals. However, their positivity rate of 0.07% (1/1409) was markedly lower than the rates described in the other age groups. Infection was also observed in men > 50 years of age. This has also been observed by the Brazil Ministry of Health [12, 13], and likely involves several factors, such as increased life expectancy, improved quality of life, and extent of sexual activity in these age groups due to new medicines used to treat erectile dysfunction. Young people aged 15–24 years account for approximately one third of new HIV infections. In some areas, young women can be disproportionately impacted [10].
Higher positivity rates, especially in men > 40 years of age, may be associated with the natural constitution of the populations of the evaluated municipalities. Men of this age are more likely to migrate in search of employment and at risk of exposure to HIV-1. Residence is often in villages that are close to the large mining enterprises of the region. They are made up of leaders of families who are directly linked to the local economic activities. Another study conducted in the state of Pará on the Marajó archipelago [14], a region characterized by precarious social situation, reported an HIV positivity rate of 0.64% (12/1877) in the samples of individuals aged 13–72 years, including 83.33% of women. The findings highlight the direct influences of the study model, migration destination, demography, and socioeconomic profile on the dynamics of infection.
Moreover, of the ten symptomatic cases, seven occurred in men and three in women, representing a male/female ratio of 2.3. This gender ratio is similar to the ratio of 2.6 observed in Brazil in 2017, excluding HIV cases in pregnant women (i.e., 26 males for every 10 women) [9].
According to the educational status, the number of samples collected in the illiterate (242) and higher education (199) groups was low compared with those of the other groups. Only one positive case was observed, which produced a similar rate of positivity to those of the other groups. A slightly higher rate of positivity was found in those with elementary education, which included 57.8% of the people in this study. It is possible that the low level of education directly influenced the population’s vulnerability to HIV infection, due to lack of knowledge of how the transmission of the virus occurs.
Other factors such as sexual choice, early onset of sexual activity, number of partners, drug use, alcohol use, and relationships with sex workers, which are very common in these areas, also increase the risk of HIV infection. These aspects were not investigated in this study. Studies conducted in other municipalities in the northern region [14] that had a high incidence of HIV found high percentages of uneducated individuals. The prevalence of HIV in the area could be associated with lack of knowledge about sexually transmitted infections, which includes engaging in sexual activity without the use of a condom. The lack of information is probably associated with the absence of public campaigns to educate people about sexually transmitted infections. Information disseminated in schools and public campaigns could increase public knowledge on the modes of infection and prevention of HIV. Such information is needed, given the increase of HIV cases in Brazil, from 7.8% in 2007 to 25.7% in 2017 [8].
At the time of enrollment, each topic received a health evaluation by a physician, with emphasis on the febrile, diarrheal, and icteric syndromes. The HIV positivity rate of 1.81% obtained in the symptomatic group was statistically significant (p = 0.0007) in relation to the rate of 0.33% in asymptomatic individuals. These data indicate that treatment might be less successful in symptomatic patients with the late diagnosis of HIV when compared with those who are still asymptomatic. HIV testing has become easier with time, allowing more individuals to know their HIV status.
Currently, approximately one-in-four people with HIV do not know they are infected, even with all the global efforts made over the past decades [15]. Global data have revealed a correlation between the aforementioned syndromes and HIV. Presently, one of the three syndromes was observed in all ten symptomatic patients. Febrile syndrome was found in six people, including 4/203 (2.0%) males and 2/135 (1.5%) females. Diarrheal and icteric syndromes were found only in men at percentages of 12.5% (2/16) and 3.7% (1/27), respectively. No statistical differences were observed between the two genders.
De Munter et al. [16] reported that in patients with HIV, fever is often caused by opportunistic conditions, especially in the early years of the infection. The authors also described that little is known about the diagnosis spectrum and outcome of febrile episodes in patients with good access to antiretroviral therapy. The same authors found 220 febrile episodes in 146 patients. Of these, 25.9% had CD4 count < 200/mm3 and 78.6% were receiving antiretroviral therapy. Multiple episodes were observed in 44 patients with a diagnosis established in 91.8% of the episodes. Respiratory tract infections, viral syndromes, and abdominal infections were implicated in 82.3% of these cases. In 6.4% of the cases, the patient died 6 months after the onset of fever, probably due to advanced age and low CD4+ T level. The data indicate that HIV-positive patients with access to antiretroviral therapy have the same chance of developing fever as the general population, whereas those with HIV and low CD4 count remain at risk of fever due to opportunistic conditions and death.
Similarly, Lambertucci et al. [17] observed that a fever of unknown origin is associated with HIV infections, a fact that has been rarely addressed in the literature. In addition, the authors noted the high correlation between fever and AIDS, which is associated with opportunistic infections, fever induced by HIV (viremia), neoplasm, and drug reactions. However, in daily clinical routine, fever is a frequent problem and may occur in 8.2–21% of AIDS patients.
The 23 HIV seropositive individuals detected in this study lived in three municipalities, Parauapebas, Canaã de Carajás, and Marabá. However, came from different states, with the largest number from Maranhão (n = 14, 60.9%). Of the 16 cases found in Parauapebas, nine (52.9%) symptomatic patients were identified at the hospital. The other seven subjects, who were all asymptomatic, were enrolled at different locations including job sites, dormitory, residences, and Village of Sansão. In Canaã of Carajás, five asymptomatic cases originated from Vila Bom Jesus (three in 2010 and two in 2014) and one asymptomatic individual came from Vila Planalto. Only one asymptomatic case was found in Marabá. Of note, two of the cases from Vila Bom Jesus were a husband and wife aged 42 and 28 years, respectively. Both their serum samples were collected in 2010.
Another important factor is the interiorization of HIV infection. There is a tendency for the number of cases outside the major urban centers and capitals to increase, a scenario that did not occur at the beginning of the epidemic in Brazil . [8, 10]. In the present study, cases were found in small villages in municipalities located far from Belém the capital of Pará. The finding indicates that even individuals who live in these places are exposed to risk factors that directly influence their vulnerability to HIV. This is especially true in places with intense land/air migratory activity involving many people, as well as socio-demographic and economic impacts by mining-related activities.
Brazil recorded a continuous increase in the number of AIDS cases, with 606,936 cases for males and 319,682 for females from 1980 to June 2018. Data obtained anywhere in the country are relevant [18]. Especially, the data from this study are significant because they involved a mining area to which people migrated from different regions. These people may have been infected prior to migration or became infected in their new place of residence. This type of research is poorly described in the literature. Typically, studies involve individuals from groups at risk of HIV infection or patients hospitalized with AIDS.
Acknowledgments
We are grateful to Sr. Lindomar Vasconcelos and the entire field staff that helped with the collection of serum samples and clinical data used in the present study. We thank the valuable technical support provided by Celina Freitas and Dielle Teixeira; as well as the Geoprocessing Laboratory (LABGEO), especially to the technician Clístenes Pamplona Catete, for the elaboration of the mapping of the study region, and Sr. Afonso Alves for spreadsheet. Special thanks to all the persons who participated in this study.
Funding
This research was supported by Vale do Rio Doce Company and Evandro Chagas Institute (IEC), and the payment of English review was done by the Virology Pós-Graduation Program of IEC.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nyamweya S, Hegedus A, Jaye A, Rowland-Jones S, Flanagan KL, Macallan DC. Comparing HIV-1 and HIV-2 infection: lessons for viral munopathogenesis. Rev Med Virol. 2013;23:221–240. doi: 10.1002/rmv.1739. [DOI] [PubMed] [Google Scholar]
- 2.Castro SS, Scatena LM, Miranzi Neto A, Camargo FC, Nunes AA. (2018). HIV/AIDS case definition criteria and association between sociodemographic and clinical aspects of the disease reported in the state of Minas Gerais from 2007 to 2016. Rev Soc Bras Med Trop 51: 4 Uberaba [DOI] [PubMed]
- 3.World Health Organization (WHO) (2016) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection [PubMed]
- 4.Boletim Epidemiológico HIV/Aids Secretaria de Vigilância em Saúde | M.S Número Especial | Dez. 2019
- 5.Luz PM, Veloso VG, Grinsztejn B. The HIV epidemic in Latin America accomplishments and challenges on treatment and prevention. Curr Opinion in HIV and AIDS. 2019;14(5):366–373. doi: 10.1097/COH.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Instituto Brasileiro de Geografia e Estatística (IBGE), acesso em 17-10-2019, https://cidades.ibge.gov.br/brasil/pa/canaa-dos-carajas/curionopolis/maraba
- 7.Ayres M, Ayres Junior M, Ayres DL, Santos AS (2007) BioEstat. 5.0. Aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém, p 364
- 8.Brasil . V49 #53. 2018. Ministério da Saúde (MS). Boletim Epidemiológico Secretaria de Vigilância em Saúde. [Google Scholar]
- 9.Mangal TD, Pascom ARP, Vesga JF, Meireles MV, Benzaken AS, Hallett TB. Estimating HIV incidence from surveillance data indicates a second wave of infections in Brazil. Epidemics. 2019;27:77–85. doi: 10.1016/j.epidem.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herout S, Mandorfer M, Breitenecker F, Reiberger T, Grabmeier-Pfistershammer K, Rieger A, Aichelburg MC. Impact of early initiation of antiretroviral therapy in patients with acute HIV infection in Vienna, Austria. PLoS One. 2016;11(4):e0152910. doi: 10.1371/journal.pone.0152910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laanani M, Ghosn J, Essat A, MeLard A, Seng R, Gousset M, Panjo H, et al. Agence Nationale de recherché Sur le Stda PRIMO cohort study group impact of the timing of initiation of Atiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 1. 2015;60(11):1715–1721. doi: 10.1093/cid/civ171. [DOI] [PubMed] [Google Scholar]
- 12.Caro-Vega Y, Belaunzarán-Zamudio PF, Crabtree-Ramirez B, Shepherd BE, Mejia F, Giganti MJ, Paterson P, et al. Trends in proportion of older HIV-infected people in care in Latin América and the Caribbean: a growing challenge. Epidemiol Infect. 2018;146(10):1308–1311. doi: 10.1017/S0950268818001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos AFM, Assis M. Vulnerabilidade das idosas ao HIV/AIDS: despertar das políticas públicas e profissionais de saúde no contexto da atenção integral: revisão de literatura. Rev Bras Geriatr Gerontol. 2011;4(1):147–157. doi: 10.1590/S1809-98232011000100015. [DOI] [Google Scholar]
- 14.Vallinoto ACR, Aguiar S, Sá KG, Freitas FB, Ferreira G, Lima SS. Prevalence and risk behaviour for human immunodeficiency virus 1 infection in Marajó Island, northern Brazil. Ann Hum Biol. 2016;43(4):397–404. doi: 10.1080/03014460.2016.1196244. [DOI] [PubMed] [Google Scholar]
- 15.Henry J Kaiser Family Foundation (KFF) access 07 15 2019. Available from https://www.kff.org/global-health-policy/fact-sheet/the-global-hivaids-epidemic/
- 16.De Munter P, Derdelinckx R, Peetermans WE, Vanderschueren S, Van WE. Clinical presentation, and causes febrile episodes of outcome in a prospective cohort of HIV infected patients. Infect Dis (Lond) 2017;49(1):65–70. doi: 10.1080/23744235.2016.1216655. [DOI] [PubMed] [Google Scholar]
- 17.Lambertucci JR, Avila RE, Voieta I. Fever of unknown origin in adults. J Braz Soc Trop Med. 2005;38(6):507–513. doi: 10.1590/S0037-86822005000600012. [DOI] [PubMed] [Google Scholar]
- 18.UNAIDS. AIDSinfo. 2017 [cited August 2017]. Available from: http://aidsinfo.unaids.org