Abstract
Papillomaviruses (PVs) are circular double-stranded DNA virus belonging to Papillomaviridae family. During the infection cycle, PVs translate proteins that can influence cell growth and differentiation, leading to epidermal hyperplasia and papillomas (warts) or malignant neoplasms. Canis familiaris papillomaviruses (CPVs) have been associated with different lesions, such as oral and cutaneous papillomatosis, pigmented plaques, and squamous cell carcinomas (SCCs). Here, we report a clinical case of a mixed bred female dog with pigmented plaques induced by CPV16 (Chipapillomavirus 2) that progressed to in situ and invasive SCCs. Gross and histological findings were characterized, and the lesions were mainly observed in ventral abdominal region and medial face of the limbs. In situ hybridization (ISH) revealed strong nuclear hybridization signals in the neoplastic epithelial cells, as well as in the keratinocytes and koilocytes of the pigmented viral plaques. The full genome of the CPV16 recovered directly from the lesions was characterized, and the phylogenetic relationships were determined. The identification of oncoprotein genes (E5, E6, and E7) by high throughput sequencing (HTS) and their expected domains are suggestive of the malignant transformation by CPV16.
Keywords: CPV16, Canine pigmented viral plaques, Oncogenesis, Sequencing, Rolling circle amplification, High-throughput sequencing
Introduction
Papillomaviruses (PVs) are circular double-stranded DNA viruses belonging to the Papillomaviridae family [1]. In the infection cycle, PVs produce proteins that can influence cell growth and differentiation. This stimulates basal cell proliferation and terminal keratinocyte differentiation [2]. Although the majority of PV infections do not develop visible lesions, some PVs can produce hyperplastic papilloma (warts) and induce neoplasia [3].
Canis familiaris papillomaviruses (CPVs) have been associated with different lesions such as oral and cutaneous papillomatosis, pigmented plaques, and squamous cell carcinomas (SCC) [4, 5]. Pigmented plaques typically present well-demarcated black hyperkeratotic plaque with an irregular border, which can result in the development of multiple lesions or progress to invasive SCC. However, CPV-associated malignant lesions are rare, and most cases of SCC have no CPV-induced etiology [3].
To date, 23 types of CPV were reported by the Papillomavirus Episteme (PaVE) database (https://pave.niaid.nih.gov) allocated into three distinct genera. CPV1 and 6 are classified as Lambdapapillomavirus: CPV2, 7, 13, 17, 19, 21, 22, and 23 as Taupapillomavirus, and the Chipapillomavirus genus is composed by the remaining types (CPV3, 4, 5, 8, 9, 10, 11, 12, 14, 15, 16 18, and 20) [6]. The genus Chipapillomavirus is divided in three species: Chipapillomavirus 1, composed by CPV3, 5, 9, 11, and 12; Chipapillomavirus 2, composed by CPV4 and 16; and Chipapillomavirus 3, composed by the types CPV8, 10, 14, and 15. The CPV18 and CPV20 are not assigned in any Chipapillomavirus species at the moment [6].
Here, we describe the features of a CPV16 that induced pigmented viral plaques and SCC in a female dog. The full genome of CPV16 was recovered directly from lesions, and their phylogenetic relationships and genome characterization were determined. In addition, the gross and histological findings were described and the virus was detected in the lesions using in situ hybridization (ISH).
Materials and methods
Case description and sample collection
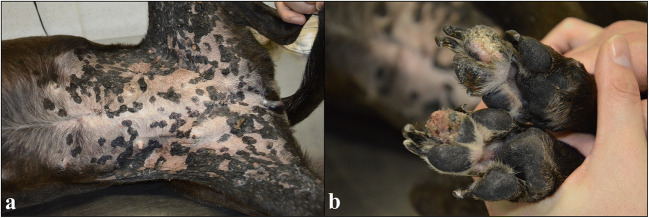
An eight-year-old mixed breed female dog was referred to the Hospital de Clínicas Veterinárias of the Universidade federal do Rio Grande do Sul (HCV-UFRGS), because it had multiple proliferative skin lesions. In the ventral abdominal region and extending to the medial face of the pelvic limbs, there were multiple black, alopecic, and slightly elevated plaques, with irregular to rounded surfaces, measuring 0.5 to 3.0 cm in diameter (Fig. 1a). Among the plaques of the ventral abdominal region, two masses were also observed. The first was firm, white, with an irregular surface and adhered to the adjacent tissues. The second was firm, with a black and alopecic surface, in addition to slightly depressed and ulcerated areas. The mass measurements were 4.0 × 3.0 × 3.0 cm and 2.5 × 2.0 × 2.0 cm, respectively. In the digital, metacarpal, and metatarsal pads of all limbs, multifocal to coalescent ulcerative lesions were observed (Fig. 1b).
Fig. 1.
Gross findings of pigmented viral plaques associated to squamous cell carcinomas in a dog. a In the ventral abdominal region and extending to the medial face of the pelvic limbs, there are multiple black, alopecic, and slightly elevated plaques, with irregular to rounded surfaces, measuring 0.5 to 3.0 cm in diameter. b Multifocal to coalescent ulcerative lesions are seen in the digital pads
Tissue samples of the lesions (three pigmented plaques, the two masses, and one sample of lesions in the pads) were collected. A half of each fragment was fixed in 10% neutral buffered formalin, routinely processed for histopathology and stained by hematoxylin and eosin (H&E). Formalin-fixed tissues were also subjected to ISH. The other half of the samples was stored at − 20 °C and sent to PCR and high throughput sequencing (HTS).
The tissue samples were obtained after being removed for clinical purposes only. The animal and sampling activities described in this study were conducted under accordance with the standards of the Ethics Committee on the Use of Animals (CEUA) of UFRGS under the protocol number 24984. Its attributions and competences are defined according to the provisions of Law 11794/08 and resolutions of the National Council for the Control of Animal Experimentation (CONCEA).
In situ hybridization RNAscope® analysis
Nonradioactive ISH using the RNAscope® technology was performed according to a standardized protocol in order to detect CPV16 specific nucleic acid.
To this end 4 μm sections of formalin-fixed, paraffin-embedded tissue were mount on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and the RNAscope 2.5 HD Reagent kit-RED (cat. 322350, Advanced Cell Diagnostics Srl., Segrate, Milan, Italy) was used according to the manufacturers’ advice. Dewaxed tissue sections were incubated in target retrieval buffer (ACD) maintained at 98 to 104 °C for 15 min. The dry slides were then incubated with protease plus reagent (ACD) for 30 min at 40 °C. As probes RNAscope® Probe—V-CPV16-E6E7 (cat. 434,171) was used. The following negative controls were included to assess the specificity of the obtained signals for CPV16. Consecutive sections of all slides in the study were tested with either an unrelated probe for bovine papillomavirus type 1 (RNAscope® Probe—V-BPV-E, catalog 416,831) or a probe to the bacterial gene diaminopimelate B (RNAscope® Negative Control Probe-DapB, catalog 310,043). As further control to detect Canis lupus familiaris ubiquitin mRNA the RNAscope® Probe—Cl-UBC Positive Control (catalog 409,851) was used. The slides were counterstained with hematoxylin and coverslipped using BioCare EcoMount (ACD).
DNA isolation and PV consensus PCR assay
Total DNA was isolated from the pigmented plaques and abdominal masses using guanidine-isothiocyanate and a silica-based commercial kit (NewGene Preamp, Simbios Biotecnologia, Cachoeirinha, RS, Brazil) [7]. Diagnostic PCR assays were performed with primer pair FAP59/FAP64 that amplifies about 478 bp of L1 gene of papillomaviruses [8] using a bovine papillomavirus 1 (BPV1) isolate 13RO12 (GenBank accession number KP701423) as positive control and ultrapure water as negative control. The PCR products were purified using PureLink™ PCR Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA), according manufacturer’s recommendations. DNA quality and quantity were evaluated spectrophotometrically and fluorometrically, respectively, using NanoDrop Lite (Thermo Fisher Scientific) and Qubit® fluorometer (Invitrogen, Carlsbad, CA, USA). PCR products were sequenced using a Big Dye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) in an ABI Prism 3130 Genetic Analyzer (Applied Biosystems). Similarity analysis was performed using BLAST tool (http://blast.ncbi.nlm.nih.gov) [9].
The same sample was assessed for canine distemper virus (CDV) using RT-nested PCR. The RNA was isolated using TRIzol LS Reagent (Thermo Fisher Scientific), and the cDNA was synthetized using GoScript™ Reverse Transcriptase (Promega Coorporation, Madison, WI, USA). The nested PCR was performed in order to amplify a partial segment of the CDV nucleocapsid [10, 11]. A commercial vaccine was used as positive control (Vanguard® HTLP 5/CV-L, Zoetis), while ultrapure water was used as negative control.
Multiply-primed rolling-circle amplification and high throughput sequencing
In order to sequence the papillomavirus whole genome present in the SCC, we proceeded with multiply-primed rolling-circle amplification (RCA) to enrich the sample followed by HTS as previously described [12]. RCA was performed according to previously established protocols [13]. Briefly, 100 ng of total DNA (in 2 μL) from tissues were denatured at 95 °C for 5 min and cooled on ice. Twenty-three microliters of a previously prepared solution containing 1.5 mM of each dNTPs (Invitrogen, Carlsbad, CA, USA), 6.2 mM of random exonuclease-resistant hexanucleotides (Thermo Fisher Scientific, Waltham, MA, USA), 2 U of phi29 DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA), and 2.5 μL reaction buffer (50 mM Tris/HCl pH 7.5, 10 mM MgCl2, 10 mM (NH4)2SO4, 4 mM dithiothreitol) were added. The amplification solution was incubated for 18 h at 30 °C, followed by 10 min at 65 °C to inactivate the enzyme. The amplified DNA was electrophoresed in 1% agarose gel and visualized on a UV light source after ethidium bromide staining. RCA products were purified with a commercial kit GFX™ Purification Kit (Amersham Biosciences, Little Chalfont, BUX, UK).
For the preparation of DNA libraries, RCA DNA was tagged and fragmented using the Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA, USA) according to manufacturer instructions. After the amplification step via a limited-cycle PCR program, PCR cleanup was performed with Agencourt AMPure XP beads (Beckman Coulter, Pasadena, CA, USA). The library was sequenced in a MiSeq System (Illumina, San Diego, CA, USA) using a MiSeq Reagent Kit V2 (2 × 150 nt; Illumina, San Diego, CA, USA).
Sequence analysis and phylogenetic inferences
The data were de novo assembled using the SPAdes genome assembler (version 3.6) [14]. Open reading frame (ORF) predictions and genome annotations were performed in Geneious software (version 8.1.4) [15]. Gene and protein comparisons were performed in BLASTN and BLASTP programs [9].
Multiple nucleotide sequence alignments were produced with MUSCLE software [16]. Phylogenetic trees were constructed with MrBayes v3.2.1 [17] using Bayesian analysis coupled with Markov chain Monte Carlo methods of phylogenetic inference. Analysis of the data sets showed the best-fitting evolutionary model to be the 4by4 model, while rate variation across sites was fixed to “invgamma,” which was applied for 100,000 generations, sampling 10 trees every 100 generations. Trees obtained before convergent and stable likelihood values were discarded (i.e., a 5.000-tree burn-in). Finally, a 50% majority rule consensus tree was constructed.
Results
Pathological data: histopathology and ISH
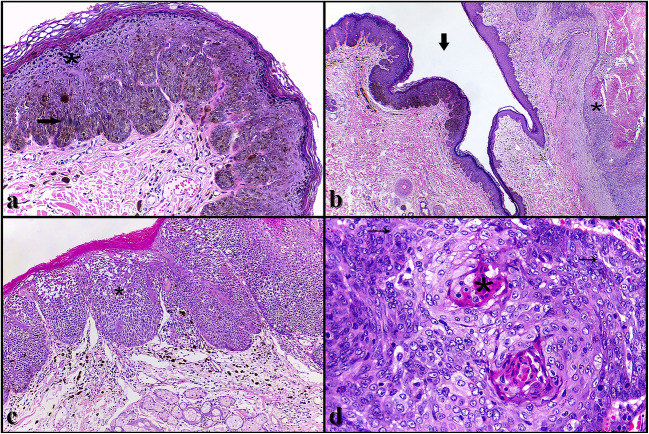
Histologically, the plaque-shaped lesions were characterized by marked multifocal hyperplasia of the epidermis associated with marked parakeratotic hyperkeratosis. There was also an intense increase in the keratohyaline granules (hypergranulosis), and a moderate amount of keratinocytes of the spinous and granular layers presented pale and swollen cytoplasm, with pycnotic nucleus, surrounded by a clear halo (koilocytes). The lower layers of the epidermis showed marked hyperpigmentation, and there was a moderate amount of melanophages in the superficial dermis. In some segments of the lesion, the epidermis showed a “scalloped” configuration. The association of these histological findings characterized a canine pigmented viral plaque (Fig. 2a). In some sections, beyond the pigmented viral plaques, squamous cell carcinomas (SCCs) were also observed. These tumors were characterized by a delimited and non-encapsulated neoplastic proliferation, sometimes extending from the epidermis to the deep dermis (invasive SCC) (Fig. 2b), observed in the two abdominal masses, and sometimes restricted to the epidermis (in situ SCC) (Fig. 2c), observed in the pads and in two of the three plaque-shaped lesions. Neoplasms consisted of polygonal epithelial cells arranged in islands and trabeculae. These cells showed moderate eosinophilic cytoplasm, round to oval nucleus, with finely dotted chromatin and evident nucleoli. There was marked anisocytosis and anisocariosis, macrocariosis, and an average of six mitotic figures per field of greatest magnification (× 400) (Fig. 2d). Amidst the neoplastic cells there was marked proliferation of fibrous connective tissue (desmoplasia), especially in invasive SCCs, besides an accumulation of lamellar eosinophilic material (keratin “pearls”) in the center of the islands (Fig. 2d). Inflammation and necrosis were frequently observed, as well as areas of ulceration of the epidermis, especially in invasive SCCs.
Fig. 2.
Histological findings of pigmented viral plaques associated to squamous cell carcinomas in a dog. a Pigmented viral plaque. The lesion is characterized by marked hyperplasia of the epidermis with hyperkeratosis and hypergranulosis (asterisk). There are also marked hyperpigmentation of the lower layers of the epidermis and moderate amount of melanophages in the superficial dermis (arrow). Hematoxylin and eosin (H&E), × 10. b On the left side of the figure there is a pigmented viral plaque (arrow), and contiguously, a squamous cell carcinoma (SCC) can be observed (asterisk). H&E, × 4. c In situ SCC. This tumor is characterized by a neoplastic proliferation of squamous epithelial cells. The cells are arranged in islands or nests and restricted to the epidermis (asterisk). There is also melanophages in the superficial dermis. H&E, × 20. d Invasive SCC. This tumor is characterized by the invasion of the dermis. The neoplastic cells present a characteristic squamous aspect with marked pleomorphism and accumulation of lamellar eosinophilic material (keratin “pearls”) in the center (asterisk). There are also a high number of mitotic figures (arrows). H&E, × 40
In the ISH technique, a strong nuclear hybridization signal was observed in the neoplastic epithelial cells (Fig. 3), as well as in the keratinocytes of the pigmented viral plaques, predominantly in the stratum spinosum, granular, and corneum, in addition to the koilocytes. No signals were obtained using the bovine papillomavirus specific RNA probes. As positive control a diffuse staining specific for canine tissue was obtained with the CI-UBC positive control probe.
Fig. 3.
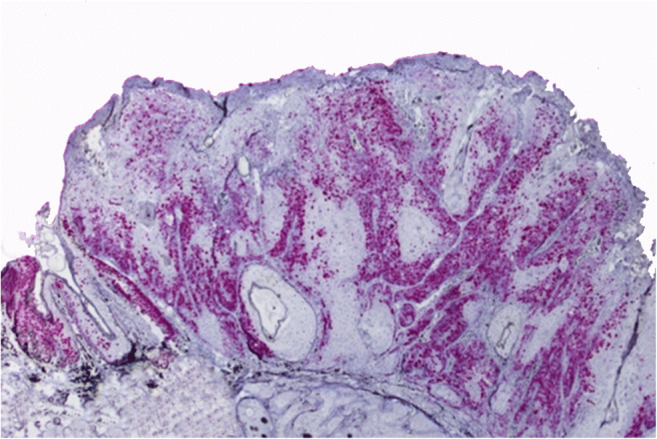
In situ hybridization (ISH) findings of pigmented viral plaques associated to squamous cell carcinomas in a dog. ISH assay, SCC. There is a marked hybridization signal in the neoplastic epithelial cells (red color). ISH, × 10
HTS, CPV16 genomic characterization, and phylogenetic inferences
The CPV was obtained directly from the lesions by PCR using primer pairs FAP59/FAP64 [8]. The HTS generated 153,620 paired-end high-quality reads (average read length of 140 nt). De novo assembling in SPAdes 3.6 software revealed a circular contig of 7796 nt, sharing 99.7% nucleotide identity with CPV16 strain Chana detected in 2011 in the USA (GenBank accession number NC_026640) [18].
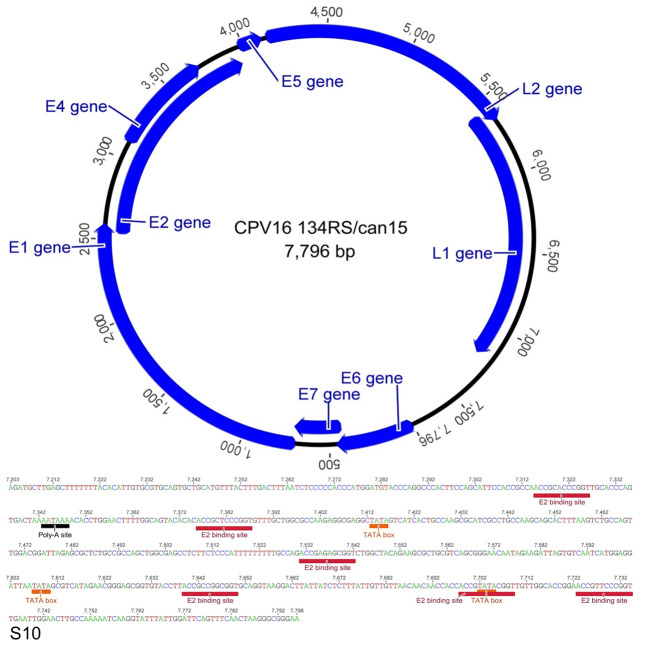
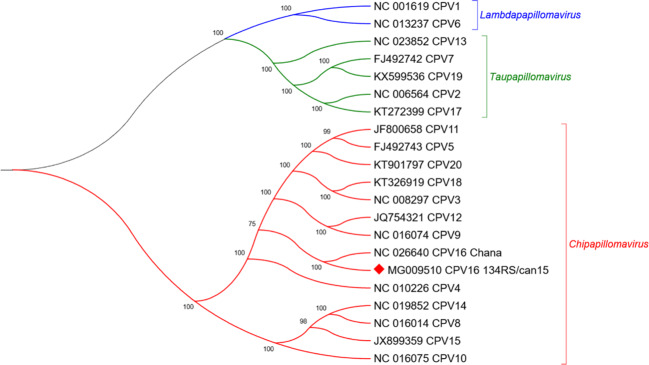
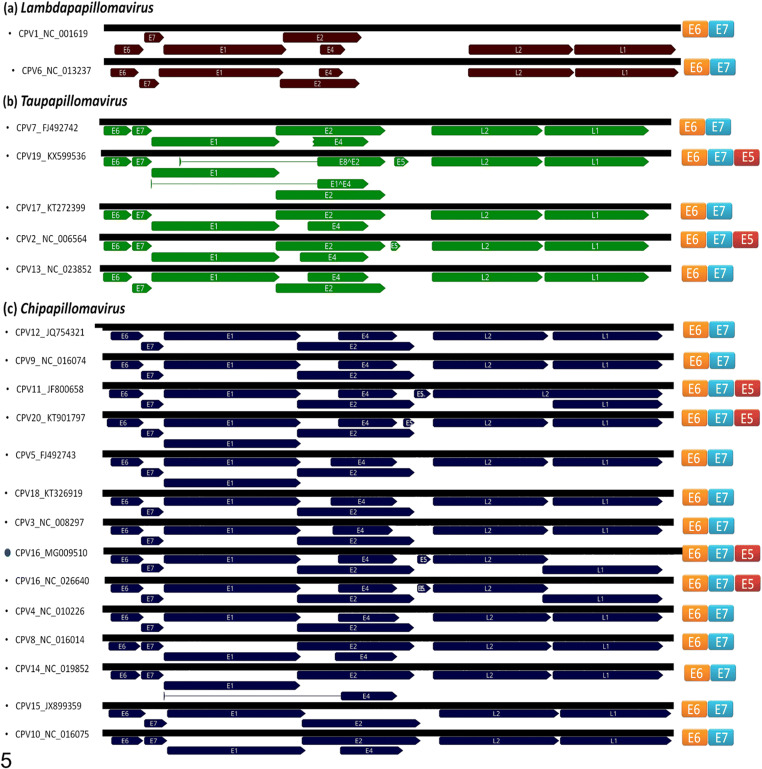
The complete genome size of CPV16 (named 134RS/can15) has a GC content of 50.6% and encodes eight proteins: six early genes E1, E2, E4, E5, E6, and E7 and two late genes L1 and L2 (Fig. 4). The phylogenetic tree was reconstructed with optimized alignments based on the nucleotide sequence of the L1 gene containing 17 sequences retrieved from GenBank database using Bayesian analysis [17]. Using a set of genus-representative sequences of CPV, CPV16 134RS/can15 strain grouped within the Chipapillomavirus genus, closest to the Chana strain (Fig. 5), which was also detected from pigmented skin plaques that progressed to SCC in a female Basenji dog [18]. Similar observation occurred in other Basenji dogs [5, 19]. The CPV16 134RS/can15 was deposited in GenBank under accession number MG009510.
Fig. 4.
Genomic features of the CPV16 134RS/can15. Identification of putative ORFs was made with the aid of ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder), and figure drawn was performed in Geneious software, 8.1.4 version.8 Features of the CPV16 134RS/can15 non-coding region. Colored boxes (red) display the genomic locations of the E2 binding site (ACCN5GGT), polyadenylation site (AATAAA) (black), and TATA box (TATAAA) (orange)
Fig. 5.
Phylogenetic analysis of the complete L1 nucleotide of canine papillomavirus. L1 nucleotide sequence was compared to those of 17 canine papillomaviruses retrieved from GenBank. Posterior probability values are indicated above the branches. CPV16 134RS/can15 is labeled with a red diamond. Evolutionary analyses were conducted in MrBayes (v3.2.1)
In order to correlate the presence or absence of the oncoprotein complex (E5, E6, and E7) with malignant potential, the complete genomes of reference sequences were aligned (Fig. 6). The comparison revealed that the oncoprotein complex is absent in the Chipapillomavirus strains linked to malignant transformation (CPV9, CPV12). However, CPV16 strains Chana and 134RS/can15 contain this complex equally arranged in the genome. Moreover, the process of pathological transformation showed similarities between both cases.
Fig. 6.
The oncogene comparison using complete genome sequences alignment. The E5 oncogene is present in two CPV19, CPV2 (Taupapillomavirus), and in CPV11, CP16 and CPV20 (Chipapillomavirusgenus). CPV16 strains Chana and 134RS/can15 present this complex of oncoproteins equally arranged in the genome
Discussion
The gross and histological findings allowed the diagnosis of multiple pigmented viral plaques in addition to in situ and invasive SCCs in a dog. Based on molecular findings and the IHS technique, these lesions were associated with the CPV16. Pigmented viral plaques are skin lesions induced by different types of CPVs [20, 21], generally seen in the ventral abdominal region and medial face of the limbs [3, 20, 21], similar to what was observed in the dog of this report. These lesions can affect dogs of different age groups; however, similar to what was reported in this case, they have a higher incidence in dogs between 6 and 8 years old [20].
Regarding to racial predisposition, some breeds, such as Pug, Miniature Schnauzer, and Miniature Daschund, are more predisposed to the development of pigmented viral plaques [3, 20]. Dogs of other breeds can also be affected; however, there is often an association with immunosuppressive factors [21] that were not observed in the present report. Contributing factors that cannot be disregarded are iatrogenic immunosuppression, breed susceptibility, systemic diseases, senility, and genetic mutations, among others. In a study describing the occurrence of papillomavirus infection in transplanted dogs with X-linked severe combined immunodeficiency (XSCID), CPV2 was linked with a papilloma lesion that progressed to metastatic SCC [22].
In dogs, pigmented viral plaques and papillomas associated with the PV can undergo malignant transformation and progress to in situ or invasive SCCs; however, carcinomas are rarely associated with PV [5, 21]. Similar to what was described by Luff and collaborators [5], the dog of this report had pigmented viral plaques, associated with in situ and invasive SCCs. In addition, in the IHS technique, a strong hybridization signal could be observed in the nucleus of neoplastic cells, in koilocytes, and in keratinocytes of the viral plaques, predominantly from the spinosum, granular, and corneum layers. It was also possible to identify CPV16, for both viral plaques and carcinomas, which possibly allows the association of the PV with lesions.
According to recent reports, pigmented plaques that affect canines have been associated with closely related types of Chipapillomavirus [3]. Recently, Luff and collaborators [19] identified four sites of viral integration into the host genome, when analyzing the full genome of CPV16 Chana strain, which has 99.7% nucleotide identity with CPV16 134RS/can15 strain described here. That was the first description of integration of a canine papillomavirus into the host genome, raising the possibility that CPV16 may be a potential canine high-risk papillomavirus type.
In cattle, the Deltapapillomavirus genus comprises the high-risk mucosal types that can cause urinary bladder cancer in their natural hosts. The set of all three oncoproteins E5, E6, and E7 present in the Deltapapillomavirus and its distribution in the genome appear to be essential for the progression of malignancy [23]. Similarly, we identified the putative oncoproteins E5, E6, and E7 by the genomic characterization besides the pathological analysis. The presence of the oncoprotein genes in the tissue suggests that the oncoproteins could be interfering in the cellular cycle. Therefore, the replication of the oncogenic virus in the tissue strongly suggests the malignant transformation conducted by CPV16. The putative E6 of CPV16 134RS/can15 strain has two zinc-finger domains (CX2CX29CX2C) that are critical for the transformation activity of the viral oncoproteins, including the product of the pRB gene [24]. Moreover, the putative E7 contains one zinc-finger domain, as well as the conserved binding site for the retinoblastoma protein (LXCXE) that is closely linked to malignant potential and cell transformation [25, 26].
Conclusions
In this work, we reported a case of a mixed-breed female dog that presented pigmented viral plaques, in situ and invasive SCCs, related to CPV infection. CPV16 was identified, and a phylogenetic inference was build, identifying and comparing the oncoprotein genes of the CPV16 strain. Also, the study highlights the importance of CPV-induced oncogenesis and expands the current knowledge of the genetic background of the Papillomaviridae family.
Funding information
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Finance Code 001, and Propesq/UFRGS.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Villiers E-M, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32:7–15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Munday JS, Thomson NA, Luff JA. Papillomaviruses in dogs and cats. Vet J. 2017;225:23–31. doi: 10.1016/j.tvjl.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Munday JS, O’Connor KI, Smits B. Development of multiple pigmented viral plaques and squamous cell carcinomas in a dog infected by a novel papillomavirus. Vet Dermatol. 2011;22:104–110. doi: 10.1111/j.1365-3164.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 5.Luff J, Rowland P, Mader M, Orr C, Yuan H. Two canine papillomaviruses associated with metastatic squamous cell carcinoma in two related Basenji dogs. Vet Pathol. 2016;53:1160–1163. doi: 10.1177/0300985816630795. [DOI] [PubMed] [Google Scholar]
- 6.Van Doorslaer K, Tan Q, Xirasagar S, et al. The papillomavirus episteme: a central resource for papillomavirus sequence data and analysis. Nucleic Acids Res. 2013;41:D571–D578. doi: 10.1093/nar/gks984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boom R, Sol CJ, Salimans MM, et al. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1556/AMicr.58.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forslund O, Antonsson A, Nordin P, Stenquist B, Göran Hansson B. Abroad range of human papilomavírus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol. 1999;80:2437–2443. doi: 10.1099/0022-1317-80-9-2437. [DOI] [PubMed] [Google Scholar]
- 9.Ye J, McGinnis S, Madden TL. BLAST: improvements for better sequence analysis. Nucleic Acids Res. 2006;34:W6–W9. doi: 10.1093/nar/gkl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilho JG, Brandão PE, Carnieli P, et al. Molecular analysis of the N gene of canine distemper virus in dogs in Brazil. Arq Bras Med Vet Zootec. 2007;59:654–659. doi: 10.1590/S0102-09352007000300016. [DOI] [Google Scholar]
- 11.Frisk AL, König M, Moritz A, Baumgärtner W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J Clin Microbiol. 1999;37:3634–3643. doi: 10.1128/JCM.37.11.3634-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva FRC, Cibulski SP, Daudt C, et al. Novel bovine papillomavirus type discovered by rolling-circle amplification coupled with next-generation sequencing. PLoS One. 2016;11:e0162345. doi: 10.1371/journal.pone.0162345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dezen D, Rijsewijk FAM, Teixeira TF, Holz CL, Cibulski SP, Franco AC, Dellagostin OA, Roehe PM. Multiply-primed rolling-circle amplification (MPRCA) of PCV2 genomes: applications on detection, sequencing and virus isolation. Res Vet Sci. 2010;88:436–440. doi: 10.1016/j.rvsc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 18.Luff J, Mader M, Britton M, Fass J, Rowland P, Orr C, Schlegel R, Yuan H. Complete genome sequence of canine papillomavirus type 16. Genome Announc. 2015;3:e00404–e00415. doi: 10.1128/genomeA.00404-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luff J, Mader M, Rowland P, Britton M, Fass J, Yuan H. Viral genome integration of canine papillomavirus 16. Papillomavirus Res. 2019;7:88–96. doi: 10.1016/j.pvr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldschmidt MH, Goldschmidt KH. Epithelial and melanocytic tumors of the skin. In: Meuten D, editor. Tumors of domestic animals. 5. Iowa: Wiley Blackwell, Ames; 2017. pp. 88–141. [Google Scholar]
- 21.Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol. 2010;47:254–264. doi: 10.1177/0300985809358604. [DOI] [PubMed] [Google Scholar]
- 22.Goldschmidt MH, Kennedy JS, Kennedy DR, Yuan H, Holt DE, Casal ML, Traas AM, Mauldin EA, Moore PF, Henthorn PS, Hartnett BJ, Weinberg KI, Schlegel R, Felsburg PJ. Severe papillomavirus infection progressing to metastatic squamous cell carcinoma in bone marrow-transplanted X-linked SCID dogs. J Virol. 2006;80:6621–6628. doi: 10.1128/JVI.02571-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daudt C, Da Silva FRC, Lunardi M, et al. Papillomaviruses in ruminants : An update. Transbound Emerg Dis. 2018;65:1381–1395. doi: 10.1111/tbed.12868. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 25.Münger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 26.Dahiya A, Gavin MR, Luo RX, Dean DC. Role of the LXCXE binding site in Rb function. Mol Cell Biol. 2000;20:6799–6805. doi: 10.1128/MCB.20.18.6799-6805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]