Abstract
Objective
Utilize a nationwide database to identify and compare the differences between patient demographics and clinical outcomes for patients undergoing simultaneous bilateral total hip arthroplasty (THA) and unilateral THA.
Methods
A nationwide administrative claims database was utilized; In-hospital, 90-day, and 1-year post-discharge rates of local and systemic complications were collected and compared with multivariate logistic regression.
Results
Incidence of prosthetic joint infection was significantly lower in the bilateral cohort. Length of stay was significantly shorter in the unilateral THA cohort.
Conclusion
Surgeons should consider simultaneous bilateral THA a safe and effective procedure for low risk patients with appropriate comorbidities.
Keywords: Total Hip Arthroplasty, Simultaneous, Unilateral, Outcomes, Length of Stay
1. Introduction
With the incidence of end-stage osteoarthritis expected to increase with the ageing population, total hip arthroplasty (THA) continues to improve the quality of life and help maintain independence in this patient population.1, 2, 3, 4 While THA remains one of the most successful orthopedic surgeries currently performed with a greater than 95% survival at 10 years postoperatively, there remains hesitancy amongst providers when considering a simultaneous bilateral THA (SimBTHA).2, 3, 4, 5 With the growing number of candidates for THA, the incidence of the procedure is predicted to increase to 635,000 procedures annually by 2030.5 This increase should directly translate into an increase in the number of candidates for bilateral THA.
A large proportion of patients who receive unilateral THA eventually require contralateral treatment as forty-two percent of patients with arthritis of the hip have bilateral disease.6, 7, 8 Earlier studies on SimBTHA demonstrated an association with increased blood loss, thromboembolic events, and cardiopulmonary issues.9 However, recent studies suggest SimBTHA can lead to overall reduced length of hospital stay, improved cost effectiveness, less anesthetic, and shorter total surgical time compared to staged procedures.10, 11, 12
With the increase in volume of THA expected and contrasting results regarding safety and outcomes of bilateral verses single THA, this study aimed to utilize a nationwide cohort to compare the differences in rates of local and systemic complications between patients receiving primary unilateral THA and primary SimBTHA. This data can assist physicians on deciding who is an appropriate candidate for SimBTHA and highlight which patients pose an increased odds risk for complications.
2. Methods
Patient records were queried from PearlDiver (PearlDiver Inc, Fort Wayne, IN, USA), a commercially available administrative claims database, using International Classification of Diseases, Ninth Revision and Tenth Revision (ICD-9/ICD-10). This study used the MHip dataset which contains the medical records of two million THAs from 2010 through Q2 of 2018. It is inclusive of all payors. Institutional review board exemption was granted for this study due to the provided data being deidentified and compliant with the Health Insurance Portability and Accountability Act.
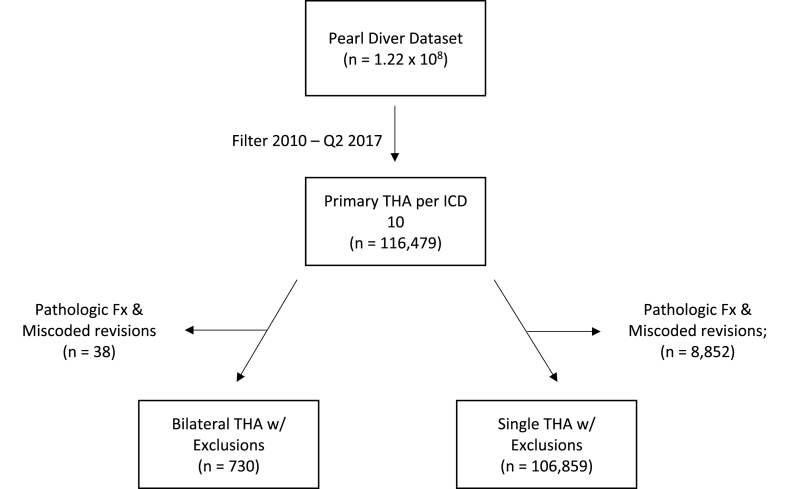
A retrospective cohort design was used to compare patients who received primary bilateral THA versus patients with a single primary THA. Patients were identified by ICD-10 codes rather than Current Procedural Technology (CPT) codes due to the former including temporal data detailing a patient's hospital course and allowing isolation of laterality while the latter does not. Exclusion criteria included patients with a pathologic or traumatic fracture, and those who had revision THA miscoded as a primary THA. Patients were placed into the “SimBTHA” cohort if they received a primary right and left THA simultaneously. “Unilateral THA” cohort patients were identified as having either a primary left or right THA, but not both simultaneously (Fig. 1). The ICD codes defining the study groups are provided in Appendix Table A1.
Fig. 1.
Flow diagram of patients included in study. THA, total hip arthroplasty; Fx, fracture.
Each cohort was queried for basic demographic information, clinical characteristics, and hospital course data such as age, sex, hospital region, body mass index (BMI), length of stay (LOS), 90-day readmission rate, and Charlson comorbidity index (CCI). Regional data were categorized based on the United States Census Bureau classification of Northeast, Midwest, South, and West. Specific comorbidities queried from the database included the presence of a history of diabetes, anemia, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, other cardiac disease, immunocompromised status, liver disease, rheumatoid arthritis, depression, and tobacco use. An immunocompromised status was defined as a patient who had received an antineoplastic drug or immunologic agent in the year before their index procedure. A patient was classified as having “other cardiac disease” if they had a previous diagnosis of ischemic heart disease or coronary heart disease.
The incidences of postoperative joint and systemic complications between the two cohorts were then queried. Postoperative joint complications included prosthetic joint infection (PJI), periprosthetic fracture, hip dislocation, aseptic loosening, and other revision. PJI was defined by procedural codes that indicated a surgical intervention for a deep joint infection to exclude superficial wound complications that would have been included in diagnosis codes for PJI. Other revision was defined as aseptic revisions excluding those performed after periprosthetic fracture or hip dislocations. Each joint complication was examined at 90-days and 1-year postoperatively. The codes used to define postoperative joint complications are provided in Appendix Table A2.
Systemic complications investigated included lower extremity deep vein thrombosis, pulmonary embolism, anemia (post hemorrhagic, iron deficiency from blood loss), acute renal failure, myocardial infarction, cerebrovascular event (stroke, nontraumatic hemorrhage, occlusion of cerebral arteries), respiratory failure, pneumonia, acute mental status change, and urinary tract infection. Incidences of systemic complications were examined during the surgical encounter before discharge, and at 90-days postoperatively. Because the diagnosis of in-hospital anemia could not be specified as preoperative or postoperative, in-hospital transfusion rates were all queried. The codes used to define systemic complications are provided in Appendix Table A3.
Morphine milligram equivalents (MME) (USC-02211, USC-02212, USC-02214, USC-02221, USC-02222, USC-02231, USC-02232) were also queried for both cohorts in order to compare opiate consumption for pain management load between the two cohorts. Patients who received general anesthesia within the 1-year follow-up were excluded to account for opioid use due to additional procedures. The evaluation captured patients who had an opioid claim (a) between discharge and 90-days and (b) a subsequent claim between 90-days and 6-months (c) and a subsequent claim between 6-months and 1-year. Average MME was calculated directly in PearlDiver for each period.
All data analyses were performed using the R statistical software (R Project for Statistical Computing, Vienna, Austria) integrated into PearlDiver with an α level set to 0.05. Demographic and clinical characteristics were compared using chi-square analysis for categorical variables and Welch's t-test for continuous variables. Multivariate logistic regression adjusting for patient sex, age, CCI, BMI, and the presence of the comorbidities tobacco use and diabetes mellitus were used to calculate odds ratios (ORs) with corresponding 95% confidence intervals (CIs) for comparing rates of postoperative complications between the bilateral and unilateral THA cohorts.
3. Results
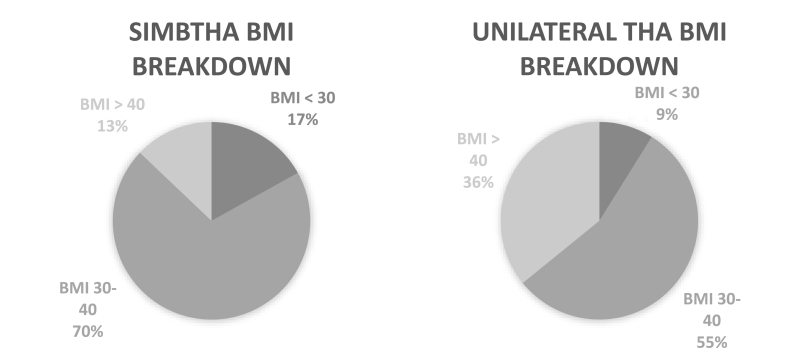
Between 2010 and Q2 of 2018 in the PearlDiver database, 185,123 primary total hip arthroplasty procedures were identified using ICD-10 procedural codes. After adjusting for exclusion criteria and dates for adequate follow up, this number decreased to 107,589, of which 106,859 (99.3%) patients received a primary unilateral THA and 730 (0.7%) patients received a SimBTHA (Fig. 1). Table 1 highlights SimBTHA had a greater proportion of males (Male: 53.3% vs 43.4%, p < .001), in the age range of <65 (75.3% vs 44.0%, p < .001), have a BMI less than 30 (16.9% vs 8.9%, p = .003), as well as a BMI between 30 and 40 (BMI 30–40: 70.2% vs 55.2%, p < .001) (Fig. 2.), and had a lower average burden of comorbidities (CCI: 0.64 vs 1.01, p < .001). Patients in the unilateral cohort had a shorter hospital length-of-stay (LOS: 2.61 vs 6.11, p < .001) and had higher rates of the following comorbidities: Diabetes 33.9% vs 22.1%, p < .001, Tobacco Use 26.0% vs 19.5%, p = .002, Congested Heart Failure 7.7% vs 2.2%, p < .001, Cardiac Disease 28.1% vs 14.7%, p < .001, COPD 26.6% vs 20.8%, p = .007, CKD 10.5% vs 4.4%, p < .001, Pre-operative anemia 28.4% vs 20.5%, p < .001.
Table 1.
Comparison of demographics and clinical characteristics of patients receiving THA.
| Demographic Variable | Bilateral Primary THA (n = 730) | Unilateral Primary THA (n = 106,859) | p |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 341 (46.7) | 60,531 (56.7) | <.001 |
| Male | 389 (53.3) | 46,328 (43.4) | <.001 |
| Age, n (%) | |||
| <65 | 550 (75.3) | 47,011 (44.0) | <.001 |
| 65–79 | 180 (24.7) | 59,940 (56.1) | <.001 |
| ≥80 | 0 (0.0) | 0 (0.0) | <.001 |
| BMIa, n (%) | |||
| <30 | 21 (16.9) | 1643 (8.9) | 0.003 |
| 30–40 | 87 (70.2) | 10,200 (55.2) | <.001 |
| ≥40 | 16 (12.9) | 6620 (35.9) | <.001 |
| CCI, mean ± SD | 0.64 ± 1.36 | 1.01 ± 1.72 | <.001 |
| Specific Comorbidities, n (%) | |||
| Tobacco use | 142 (19.5) | 27,807 (26.0) | 0.002 |
| Rheumatoid Arthritis | 39 (5.3) | 5262 (4.9) | 0.694 |
| Liver Disease | 61 (8.4) | 11,178 (10.5) | 0.105 |
| Congestive Heart Failure | 16 (2.2) | 8201 (7.7) | <.001 |
| Cardiac Disease | 107 (14.7) | 29,969 (28.1) | <.001 |
| COPD | 152 (20.8) | 28,446 (26.6) | 0.007 |
| Chronic Kidney Disease | 32 (4.4) | 11,207 (10.5) | <.001 |
| Diabetes | 161 (22.1) | 36,240 (33.9) | <.001 |
| Pre-operative Anemia | 150 (20.5) | 30,300 (28.4) | <.001 |
| Immunocompromised | 34 (4.7) | 4062 (3.8) | 0.290 |
| Depression | 154 (21.1) | 26,775 (25.1) | 0.058 |
THA, Total Hip Arthroplasty; BMI, Body Mass Index; CCI, Charlson co-morbidity Index.
BMI data were available for 17.0% of bilateral cases and 17.3% of unilateral cases.
Fig. 2.
BMI breakdown of SimBTHA and unilateral cohorts.
BMI, body mass index; THA, total hip arthroplasty.
LOS was significantly shorter in the unilateral THA cohort (LOS 2.61 vs. 6.11, p < .001). For the SimBTHA cohort, MME data was available for 409 (56.0%), 92 (12.6%), and 65 (9.9%) patients out of the original 730 patients at the 90-day, 6-month, and 1-year MME evaluation, respectively. For the unilateral THA cohort MME data was available for 56,341 (52.7%), 17,168 (16.1%), and 12,765 (12.0%) patients out of the original 106,859 patients at the 90-day, 6-month, and 1-year MME evaluation, respectively. There was not a statistically significant difference in MME at the 90-day, 6-month, or 1 year between the two cohorts.
For joint complications, incidence of PJI at 90-days and 1-year post-discharge was significantly lower in the SimBTHA cohort (PJI 90-day: OR 0.12, 95% CI 0.01–0.52; 1-year: OR 0.18, 95% CI 0.03–0.54). No other significant differences were found between the two cohorts at 90-days postoperatively and 1-year postoperatively Table 2. Rates of systemic complications during the inpatient hospital stay and at 90-days post-discharge were all insignificant between the two cohorts Table 3.
Table 2.
Ninety-day and 1-year comparison of joint complications.
| Joint Complication | Bilateral Primary THA (n = 730) | Primary Unilateral THA (n = 106,859) | ORa (95% CI) |
|---|---|---|---|
| Prosthetic Dislocation | |||
| 90-day | 1 (0.1) | 157 (0.2) | 1.11 (0.06–4.99) |
| 1 yr | 1 (0.1) | 196 (0.2) | 0.84 (0.05–3.74) |
| Prosthetic Joint Infection | |||
| 90-day | 1 (0.1) | 1363 (1.3) | 0.12 (0.01–0.52) |
| 1 yr | 2 (0.3) | 1781 (1.7) | 0.18 (0.03–0.54) |
| Periprosthetic Fracture | |||
| 90-day | 2 (0.3) | 424 (0.4) | 0.85 (0.14–2.65) |
| 1 yr | 2 (0.3) | 587 (0.6) | 0.62 (0.10–1.93) |
| Aseptic Loosening | |||
| 90-day | 1 (0.1) | 272 (0.3) | 0.59 (0.03–2.64) |
| 1 yr | 1 (0.1) | 609 (0.6) | 0.24 (0.01–1.07) |
| Prosthetic Revision | |||
| 90-day | 2 (0.3) | 328 (0.3) | 1.12 (0.18–3.50) |
| 1 yr | 2 (0.3) | 443 (0.4) | 0.79 (0.13–2.47) |
THA, Total Hip Arthroplasty; OR, Odds ratio; CI, confidence interval.
Adjusting for sex, age, BMI, diabetes, tobacco use, and CCI.
Table 3.
In-hospital and ninety-day comparison of systemic complications.
| Systemic Complication | Bilateral Primary THA (n = 730) | Unilateral Primary THA (n = 106,859) | ORa (95% CI) |
|---|---|---|---|
| Deep Vein Thrombosis | |||
| In-hospital | 1 (0.1) | 270 (0.3) | 0.59 (0.04–2.65) |
| 90-day | 9 (1.2) | 1781 (1.7) | 0.86 (0.41–1.57) |
| Altered Mental Status | |||
| In-hospital | 3 (0.4) | 326 (0.3) | 2.61 (0.80–6.17) |
| 90-day | 3 (0.4) | 1026 (1.0) | 0.56 (0.14–1.45) |
| Pulmonary Embolism | |||
| In-hospital | 2 (0.3) | 239 (0.2) | 1.42 (0.23–4.46) |
| 90-day | 5 (0.7) | 1005 (0.9) | 0.87 (0.31–1.88) |
| Anemia | |||
| In-hospital | 235 (32.2) | 22,583 (21.1) | 1.96 (1.67–2.29) |
| 90-day | 84 (11.5) | 7866 (7.4) | 1.99 (1.57–2.49) |
| Acute Renal Failure | |||
| In-hospital | 11 (1.5) | 2370 (2.2) | 0.96 (0.49–1.65) |
| 90-day | 12 (1.6) | 2323 (2.2) | 1.11 (0.59–1.88) |
| Myocardial Infarction | |||
| In-hospital | 0 (0.0) | 231 (0.2) | NA |
| 90-day | 5 (0.7) | 540 (0.5) | 1.93 (0.69–4.21) |
| Cerebrovascular Event | |||
| In-hospital | 1 (0.1) | 895 (0.8) | 0.23 (0.01–1.02) |
| 90-day | 2 (0.3) | 1778 (1.7) | 0.23 (0.04–0.72) |
| Pneumonia | |||
| In-hospital | 0 (0.0) | 442 (0.4) | 0.82 (0.14–2.57) |
| 90-day | 4 (0.6) | 1465 (1.4) | 0.78 (0.31–1.60) |
| Respiratory Failure | |||
| In-hospital | 2 (0.3) | 1021 (1.0) | 0.65 (0.16–1.71) |
| 90-day | 3 (0.4) | 1367 (1.3) | 0.64 (0.20–1.51) |
| Urinary Tract Infection | |||
| In-hospital | 11 (1.5) | 1393 (1.3) | 1.68 (0.86–2.92) |
| 90-day | 21 (2.9) | 4574 (4.3) | 0.96 (0.60–1.45) |
THA, Total Hip Arthroplasty; OR, Odds ratio; CI, confidence interval.
Adjusting for sex, age, BMI, diabetes, tobacco use, and CCI.
4. Discussion
The increasing demand for THA along with the predicted shortage of over 5000 orthopedic surgeons by 2025 in the United States is driving surgeons to be as efficient as possible.13 This present study suggests healthy, younger patients with bilateral osteoarthritis can undergo SimBTHA without significantly increasing their odds risk of systemic and joint complications relative to patients undergoing primary unilateral THA. Bilateral procedures in this study demonstrated a lower incidence of PJI at both 90-days and 1-year (PJI: 90-day OR 0.12; 1-year OR 0.18). At 90-days and 1-year, rates of all other joint complications were comparable for the two cohorts. Furthermore, rates of all systemic complications assessed during the in-hospital stay and at 90-days post-discharge were similar for both patient populations. However, patients in the SimBTHA cohort experienced a significantly longer average length of hospital stay (LOS: 2.61 vs. 6.11, p < .001). Additionally, SimBTHA patients were significantly younger (Age <65: 75.3% vs 44.0%, p = .003), had a lower CCI (CCI: 0.64 vs 1.01, p < .001), and were less likely to be classified as overweight or obese (BMI <30: 16.9% vs 8.9%, p < .001; BMI 30–40: 70.2% vs 55.2%, p < .001).
An inherent limitation in any administrative claims database study is the accuracy of the findings depends on the accuracy of codes in the database, which are subject to human error. Additionally, clinical data such as duration of surgery, blood loss, implant information, radiographic images, functional outcomes scores, and patient satisfaction could not be queried from the database such that this is limited to the identification of comorbidities and complications to the binary presence or absence of the factor. The use of ICD-10 codes drastically reduced the number of THAs in this study compared to the total amount of THAs performed during the time period studied; however, limiting the definition of THA to only ICD-10 codes allowed for greater precision as it details laterality and allowed for assessment of LOS. While confounders were reduced with the use of multivariate logistic regression, it is possible other confounders influenced the data.
It is important to note SimBTHA patient's demographics tended to be young males with a low CCI which aligns with recent studies.7,12,14 After adjusting for these factors, the present study found SimBTHA still had fewer PJIs at 90-days and at 1-year post-discharge. In 2015, Stavrakis et al. performed a 15-year review of 202,986 patients receiving THA of which 1.1% were SimBTHA and compared the outcomes versus unilateral; they reported no significant difference in PJIs.15 The results of both studies support the notion patients without notable preexisting conditions can undergo bilateral THA if indicated without an increased risk of PJI.
The present study does not, however, indicate SimBTHA is comparably as safe to unilateral THA across all ranges of patient demographics. Fewer patients in the SimBTHA cohort had a BMI >40 and a large majority were <65 years old when compared to the unilateral THA cohort. In a study analyzing outcomes of SimBTHA, Weinstein et al. demonstrated a higher risk of postoperative complications in patients >75 years old when compared younger patients.16 Moreover, this study emphasizes the importance of optimizing preoperative management and coordination between the surgeon and the patients primary care provider to reduce patient preoperative comorbidities and refine patient selection for bilateral versus unilateral THA accordingly.17,18
Furthermore, the present study found the LOS for SimBTHA was significantly longer than the unilateral cohort. Numerous studies align with this, showing SimBTHA having an extended LOS, however, recent reports have shown no association with increased LOS.14,15,19 While not evaluated in the current study, a common argument in support of SimBTHA is a shorter LOS when compared to a two-stage procedure. Parvizi demonstrated SimBTHA length of stay was significantly shorter than the two-staged THA.11 This improved efficiency can reduce the time patients spend in hospitals, which could reduce medical complications and healthcare spending.
The United States has a significant portion of patients using opioids and it has been documented opioid use can impact patient outcomes and morbidity following orthopedic procedures.20, 21, 22, 23, 24 Pivec et al. evaluated an opioid naïve cohort compared to patients on opioids prior to THA and reported the patients in the opiate cohort received significantly higher total daily opioid doses as inpatients and had longer hospital stays.21 Weick et al. demonstrated opioid naïve patients had significantly lower revision rates at 1 year and readmissions at 30-days postoperatively.24 With a majority of staged THAs occurring between 3 and 6 months apart,10,11 this could place patients at an increased risk of prolonged opiate use which has also been shown to increase dependence and abuse.21, 22, 23
5. Conclusion
Surgeons should consider simultaneous bilateral total hip arthroplasty a safe and effective procedure for low risk patients with appropriate comorbidities. The identification of these patients and optimizing preoperative management could improve efficiencies and reduce recovery to one surgical event.
Author contributions
Travis Flick: writing, data curation, formal analysis, investigation. Sione Ofa: Data curation, formal analysis. Akshar Patel: writing, investigation. Bailey Ross: writing. Fernando L. Sanchez: Writing, validation. William Sherman: Supervision, writing, validation, formal anlaysis.
Funding
Authors declare they have not received grant support or research funding and do not have any proprietary interests in the materials described in this article.
Declaration of competing interest
The Authors declare that there is no conflict of interest.
The authors received no financial support for the research, authorship, and/or publication of this article.
Appendix A.
Table A1Codes used to evaluate for total hip arthroplasty
| Primary THA Codes | |||
|---|---|---|---|
| ICD-10-P-0SR9019 | ICD-10-P-0SRA039 | ICD-10-P-0SRB0JZ | ICD-10-P-0SRR0JZ |
| ICD-10-P-0SR901A | ICD-10-P-0SRA03A | ICD-10-P-0SRB0KZ | ICD-10-P-0SRR0KZ |
| ICD-10-P-0SR901Z | ICD-10-P-0SRA03Z | ICD-10-P-0SRE009 | ICD-10-P-0SRS019 |
| ICD-10-P-0SR9029 | ICD-10-P-0SRA07Z | ICD-10-P-0SRE00A | ICD-10-P-0SRS01A |
| ICD-10-P-0SR902A | ICD-10-P-0SRA0J9 | ICD-10-P-0SRE00Z | ICD-10-P-0SRS01Z |
| ICD-10-P-0SR902Z | ICD-10-P-0SRA0JA | ICD-10-P-0SRE019 | ICD-10-P-0SRS039 |
| ICD-10-P-0SR9039 | ICD-10-P-0SRA0JZ | ICD-10-P-0SRE01A | ICD-10-P-0SRS03A |
| ICD-10-P-0SR903A | ICD-10-P-0SRA0KZ | ICD-10-P-0SRE01Z | ICD-10-P-0SRS03Z |
| ICD-10-P-0SR903Z | ICD-10-P-0SRB019 | ICD-10-P-0SRE039 | ICD-10-P-0SRS0J9 |
| ICD-10-P-0SR9049 | ICD-10-P-0SRB01A | ICD-10-P-0SRE03A | ICD-10-P-0SRS0JA |
| ICD-10-P-0SR904A | ICD-10-P-0SRB01Z | ICD-10-P-0SRE03Z | ICD-10-P-0SRS0JZ |
| ICD-10-P-0SR904Z | ICD-10-P-0SRB029 | ICD-10-P-0SRE0J9 | ICD-10-P-0SRS0KZ |
| ICD-10-P-0SR907Z | ICD-10-P-0SRB02A | ICD-10-P-0SRE0JA | |
| ICD-10-P-0SR90J9 | ICD-10-P-0SRB02Z | ICD-10-P-0SRE0JZ | |
| ICD-10-P-0SR90JA | ICD-10-P-0SRB039 | ICD-10-P-0SRR019 | |
| ICD-10-P-0SR90JZ | ICD-10-P-0SRB03A | ICD-10-P-0SRR01A | |
| ICD-10-P-0SR90KZ | ICD-10-P-0SRB03Z | ICD-10-P-0SRR01Z | |
| ICD-10-P-0SRA009 | ICD-10-P-0SRB049 | ICD-10-P-0SRR039 | |
| ICD-10-P-0SRA00A | ICD-10-P-0SRB04A | ICD-10-P-0SRR03A | |
| ICD-10-P-0SRA00Z | ICD-10-P-0SRB04Z | ICD-10-P-0SRR03Z | |
| ICD-10-P-0SRA019 | ICD-10-P-0SRB07Z | ICD-10-P-0SRR07Z | |
| ICD-10-P-0SRA01A | ICD-10-P-0SRB0J9 | ICD-10-P-0SRR0J9 | |
| ICD-10-P-0SRA01Z | ICD-10-P-0SRB0JA | ICD-10-P-0SRR0JA | |
| Exclusion Codes for Hip | |||
| ICD-9-D-82021 | ICD-10-D-S72141C | ICD-10-D-S72001B | ICD-10-D-M84559A |
| ICD-9-D-82011 | ICD-10-D-S72064A | ICD-10-D-S72146B | ICD-10-D-M84559D |
| ICD-9-D-82020 | ICD-10-D-S72043C | ICD-10-D-S72012B | ICD-10-D-M84559G |
| ICD-9-D-8209 | ICD-10-D-S72142B | ICD-10-D-S72002B | ICD-10-D-M84559K |
| ICD-9-D-82031 | ICD-10-D-S72051B | ICD-10-D-S72101E | ICD-10-D-M84559S |
| ICD-9-D-82013 | ICD-10-D-S72044B | ICD-10-D-S72012C | ICD-10-D-M84659A |
| ICD-9-D-82030 | ICD-10-D-S72142C | ICD-10-D-S72009B | ICD-10-D-M84659D |
| ICD-9-D-82010 | ICD-10-D-S72052B | ICD-10-D-S72051A | ICD-10-D-M84659G |
| ICD-9-D-82019 | ICD-10-D-S72046B | ICD-10-D-S72019B | ICD-10-D-M84659K |
| ICD-9-D-82012 | ICD-10-D-S72143B | ICD-10-D-S72002C | ICD-10-D-M84659P |
| ICD-9-D-82032 | ICD-10-D-S72061B | ICD-10-D-S72101J | ICD-10-D-M84659S |
| ICD-9-D-73314 | ICD-10-D-S72091B | ICD-10-D-S72052A | |
| ICD-10-D-S72009C | ICD-10-D-S72143C | ICD-10-D-S72001C | |
| ICD-10-D-S72062A | ICD-10-D-S72063A | ICD-10-D-S72102B | |
| ICD-10-D-S72041B | ICD-10-D-S72091C | ICD-10-D-S72061A | |
| ICD-10-D-S72109B | ICD-10-D-S72144B | ICD-10-D-M84459A | |
| ICD-10-D-S72059A | ICD-10-D-S72092B | ICD-10-D-M84459D | |
| ICD-10-D-S72042B | ICD-10-D-S72066A | ICD-10-D-M84459G | |
| ICD-10-D-S72141B | ICD-10-D-S72101B | ICD-10-D-M84459K | |
| ICD-10-D-S72065A | ICD-10-D-S72145B | ICD-10-D-M84459P | |
| ICD-10-D-S72043B | ICD-10-D-S72011B | ICD-10-D-M84459S | |
Table A2.
Codes used to evaluate for Hip joint complications
| Joint Infection | |||
|---|---|---|---|
| ICD-9-D-99667 | ICD-10-D-T8451XS | ICD-10-D-T8453XA | ICD-10-D-T8454XD |
| ICD-9-D-99666 | ICD-10-D-T8452XA | ICD-10-D-T8453XD | ICD-10-D-T8454XS |
| ICD-10-D-T8451XA | ICD-10-D-T8452XD | ICD-10-D-T8453XS | |
| ICD-10-D-T8451XD | ICD-10-D-T8452XS | ICD-10-D-T8454XA | |
| Periprosthetic Fracture | |||
| ICD-9-D-99644 | ICD-10-D-M9711XA | ICD-10-D-M9712XD | ICD-10-D-T84042S |
| ICD-9-D-99644 | ICD-10-D-M9711XD | ICD-10-D-M9712XS | ICD-10-D-T84043A |
| ICD-10-D-M9701XA | ICD-10-D-M9711XS | ICD-10-D-T84042A | ICD-10-D-T84043D |
| ICD-10-D-M9702XA | ICD-10-D-M9712XA | ICD-10-D-T84042D | ICD-10-D-T84043S |
| Aseptic Loosening | |||
| ICD-9-D-99641 | ICD-10-D-T84030S | ICD-10-D-T84032A | ICD-10-D-T84033D |
| ICD-9-D-99641 | ICD-10-D-T84031A | ICD-10-D-T84032D | ICD-10-D-T84033S |
| ICD-10-D-T84030A | ICD-10-D-T84031D | ICD-10-D-T84032S | |
| ICD-10-D-T84030D | ICD-10-D-T84031S | ICD-10-D-T84033A | |
| Prosthetic Dislocation | |||
| ICD-9-P-7975 | ICD-10-P-0SS93ZZ | ICD-10-P-0SSB44Z | ICD-10-P-0SSCXZZ |
| ICD-9-P-7985 | ICD-10-P-0SS944Z | ICD-10-P-0SSBX4Z | ICD-10-P-0SSD04Z |
| ICD-9-P-7976 | ICD-10-P-0SS9X4Z | ICD-10-P-0SSBX5Z | ICD-10-P-0SSD0ZZ |
| ICD-9-P-7986 | ICD-10-P-0SS9XZZ | ICD-10-P-0SSBXZZ | ICD-10-P-0SSDX5Z |
| ICD-10-P-0SS904Z | ICD-10-P-0SSB04Z | ICD-10-P-0SSC04Z | ICD-10-P-0SSDXZZ |
| ICD-10-P-0SS905Z | ICD-10-P-0SSB0ZZ | ICD-10-P-0SSC0ZZ | |
| ICD-10-P-0SS90ZZ | ICD-10-P-0SSB34Z | ICD-10-P-0SSC3ZZ | |
| ICD-10-P-0SS934Z | ICD-10-P-0SSB3ZZ | ICD-10-P-0SSC4ZZ | |
| Prosthetic Revision | |||
| ICD-9-P-0070 | ICD-10-P-0SW909Z | ICD-10-P-0SWA0JZ | ICD-10-P-0SWB3JZ |
| ICD-9-P-0071 | ICD-10-P-0SW90BZ | ICD-10-P-0SWAXJZ | ICD-10-P-0SWBXJZ |
| ICD-9-P-0072 | ICD-10-P-0SW90JZ | ICD-10-P-0SWB04Z | |
| ICD-9-P-0073 | ICD-10-P-0SW93JZ | ICD-10-P-0SWB08Z | |
| ICD-10-P-0SW904Z | ICD-10-P-0SW9X8Z | ICD-10-P-0SWB09Z | |
| ICD-10-P-0SW908Z | ICD-10-P-0SW9XJZ | ICD-10-P-0SWB0JZ | |
Table A3.
Codes used to evaluate for systemic complications
| Acute Renal Failure | |||
|---|---|---|---|
| ICD-9-D-5845 | ICD-9-D-58081 | ICD-10-D-N179 | ICD-10-D-N004 |
| ICD-9-D-5846 | ICD-9-D-58089 | ICD-10-D-N19 | ICD-10-D-N005 |
| ICD-9-D-5847 | ICD-9-D-5809 | ICD-10-D-N990 | ICD-10-D-N006 |
| ICD-9-D-5848 | ICD-10-D-N170 | ICD-10-D-N000 | ICD-10-D-N007 |
| ICD-9-D-5849 | ICD-10-D-N171 | ICD-10-D-N001 | ICD-10-D-N008 |
| ICD-9-D-5800 | ICD-10-D-N172 | ICD-10-D-N002 | ICD-10-D-N009 |
| ICD-9-D-5804 | ICD-10-D-N178 | ICD-10-D-N003 | |
| Anemia | |||
| ICD-9-D-2851 | ICD-9-D-2800 | ICD-10-D-D500 | ICD-10-D-D62 |
| Altered Mental Status | |||
| ICD-9-D-78097 | ICD-10-D-R4182 | ||
| Cerebrovascular Event | |||
| ICD-9-D-430 | ICD-10-D-I610 | ICD-10-D-I6320 | ICD-10-D-I63442 |
| ICD-9-D-431 | ICD-10-D-I611 | ICD-10-D-I6329 | ICD-10-D-I63443 |
| ICD-9-D-4320 | ICD-10-D-I612 | ICD-10-D-I658 | ICD-10-D-I63449 |
| ICD-9-D-4321 | ICD-10-D-I613 | ICD-10-D-I659 | ICD-10-D-I6349 |
| ICD-9-D-4329 | ICD-10-D-I614 | ICD-10-D-I6501 | ICD-10-D-I6350 |
| ICD-9-D-4359 | ICD-10-D-I615 | ICD-10-D-I6502 | ICD-10-D-I63511 |
| ICD-9-D-4358 | ICD-10-D-I616 | ICD-10-D-I6503 | ICD-10-D-I63512 |
| ICD-9-D-43300 | ICD-10-D-I618 | ICD-10-D-I6509 | ICD-10-D-I63513 |
| ICD-9-D-43301 | ICD-10-D-I619 | ICD-10-D-I6521 | ICD-10-D-I63519 |
| ICD-9-D-43310 | ICD-10-D-I6200 | ICD-10-D-I6522 | ICD-10-D-I63521 |
| ICD-9-D-43311 | ICD-10-D-I6201 | ICD-10-D-I6523 | ICD-10-D-I63522 |
| ICD-9-D-43320 | ICD-10-D-I6202 | ICD-10-D-I6529 | ICD-10-D-I63523 |
| ICD-9-D-43321 | ICD-10-D-I6203 | ICD-10-D-G458 | ICD-10-D-I63529 |
| ICD-9-D-43330 | ICD-10-D-I629 | ICD-10-D-G459 | ICD-10-D-I63531 |
| ICD-9-D-43331 | ICD-10-D-I6302 | ICD-10-D-I6330 | ICD-10-D-I63532 |
| ICD-9-D-43380 | ICD-10-D-I6312 | ICD-10-D-I63311 | ICD-10-D-I63533 |
| ICD-9-D-43381 | ICD-10-D-I6322 | ICD-10-D-I63312 | ICD-10-D-I63539 |
| ICD-9-D-43390 | ICD-10-D-I651 | ICD-10-D-I63313 | ICD-10-D-I63541 |
| ICD-9-D-43391 | ICD-10-D-I63031 | ICD-10-D-I63319 | ICD-10-D-I63542 |
| ICD-9-D-43400 | ICD-10-D-I63032 | ICD-10-D-I63321 | ICD-10-D-I63543 |
| ICD-9-D-43401 | ICD-10-D-I63033 | ICD-10-D-I63322 | ICD-10-D-I63549 |
| ICD-9-D-43410 | ICD-10-D-I63039 | ICD-10-D-I63323 | ICD-10-D-I6359 |
| ICD-9-D-43411 | ICD-10-D-I63131 | ICD-10-D-I63329 | ICD-10-D-I636 |
| ICD-9-D-43490 | ICD-10-D-I63132 | ICD-10-D-I63331 | ICD-10-D-I638 |
| ICD-9-D-43491 | ICD-10-D-I63133 | ICD-10-D-I63332 | ICD-10-D-I639 |
| ICD-10-D-I6000 | ICD-10-D-I63139 | ICD-10-D-I63333 | ICD-10-D-I6601 |
| ICD-10-D-I6001 | ICD-10-D-I63231 | ICD-10-D-I63339 | ICD-10-D-I6602 |
| ICD-10-D-I6002 | ICD-10-D-I63232 | ICD-10-D-I63341 | ICD-10-D-I6603 |
| ICD-10-D-I6010 | ICD-10-D-I63233 | ICD-10-D-I63342 | ICD-10-D-I6609 |
| ICD-10-D-I6011 | ICD-10-D-I63239 | ICD-10-D-I63343 | ICD-10-D-I6611 |
| ICD-10-D-I6012 | ICD-10-D-I63011 | ICD-10-D-I63349 | ICD-10-D-I6612 |
| ICD-10-D-I602 | ICD-10-D-I63012 | ICD-10-D-I6339 | ICD-10-D-I6613 |
| ICD-10-D-I6020 | ICD-10-D-I63013 | ICD-10-D-I6340 | ICD-10-D-I6619 |
| ICD-10-D-I6021 | ICD-10-D-I63019 | ICD-10-D-I63411 | ICD-10-D-I6621 |
| ICD-10-D-I6022 | ICD-10-D-I63111 | ICD-10-D-I63412 | ICD-10-D-I6622 |
| ICD-10-D-I6030 | ICD-10-D-I63112 | ICD-10-D-I63413 | ICD-10-D-I6623 |
| ICD-10-D-I6031 | ICD-10-D-I63113 | ICD-10-D-I63419 | ICD-10-D-I6629 |
| ICD-10-D-I6032 | ICD-10-D-I63119 | ICD-10-D-I63421 | ICD-10-D-I668 |
| ICD-10-D-I604 | ICD-10-D-I63211 | ICD-10-D-I63422 | ICD-10-D-I669 |
| ICD-10-D-I6050 | ICD-10-D-I63212 | ICD-10-D-I63423 | |
| ICD-10-D-I6051 | ICD-10-D-I63213 | ICD-10-D-I63429 | |
| ICD-10-D-I6052 | ICD-10-D-I63219 | ICD-10-D-I63431 | |
| ICD-10-D-I606 | ICD-10-D-I6300 | ICD-10-D-I63432 | |
| ICD-10-D-I607 | ICD-10-D-I6309 | ICD-10-D-I63433 | |
| ICD-10-D-I608 | ICD-10-D-I6310 | ICD-10-D-I63439 | |
| ICD-10-D-I609 | ICD-10-D-I6319 | ICD-10-D-I63441 | |
| Deep Vein Thrombosis | |||
| ICD-9-D-45340 | ICD-10-D-I82403 | ICD-10-D-I824Z9 | ICD-10-D-I825Z1 |
| ICD-9-D-45341 | ICD-10-D-I82409 | ICD-10-D-I82501 | ICD-10-D-I825Z2 |
| ICD-9-D-45342 | ICD-10-D-I82491 | ICD-10-D-I82502 | ICD-10-D-I825Z3 |
| ICD-9-D-45111 | ICD-10-D-I82492 | ICD-10-D-I82503 | ICD-10-D-I825Z9 |
| ICD-9-D-45119 | ICD-10-D-I82493 | ICD-10-D-I82509 | |
| ICD-9-D-45389 | ICD-10-D-I82499 | ICD-10-D-I82591 | |
| ICD-9-D-4539 | ICD-10-D-I824Y1 | ICD-10-D-I82592 | |
| ICD-9-D-4512 | ICD-10-D-I824Y2 | ICD-10-D-I82593 | |
| ICD-9-D-45350 | ICD-10-D-I824Y3 | ICD-10-D-I82599 | |
| ICD-9-D-45351 | ICD-10-D-I824Y9 | ICD-10-D-I825Y1 | |
| ICD-9-D-45352 | ICD-10-D-I824Z1 | ICD-10-D-I825Y2 | |
| ICD-10-D-I82401 | ICD-10-D-I824Z2 | ICD-10-D-I825Y3 | |
| ICD-10-D-I82402 | ICD-10-D-I824Z3 | ICD-10-D-I825Y9 | |
| Myocardial Infarction | |||
| ICD-9-D-41000 | ICD-9-D-41041 | ICD-9-D-41072 | ICD-10-D-I2121 |
| ICD-9-D-41001 | ICD-9-D-41042 | ICD-9-D-41060 | ICD-10-D-I229 |
| ICD-9-D-41002 | ICD-9-D-41050 | ICD-9-D-41061 | ICD-10-D-I2101 |
| ICD-9-D-41010 | ICD-9-D-41051 | ICD-9-D-41062 | ICD-10-D-I221 |
| ICD-9-D-41011 | ICD-9-D-41052 | ICD-10-D-I214 | ICD-10-D-I220 |
| ICD-9-D-41012 | ICD-9-D-41080 | ICD-10-D-I213 | ICD-10-D-I228 |
| ICD-9-D-41020 | ICD-9-D-41081 | ICD-10-D-I2119 | |
| ICD-9-D-41021 | ICD-9-D-41082 | ICD-10-D-I2109 | |
| ICD-9-D-41022 | ICD-9-D-41090 | ICD-10-D-I2129 | |
| ICD-9-D-41030 | ICD-9-D-41091 | ICD-10-D-I240 | |
| ICD-9-D-41031 | ICD-9-D-41092 | ICD-10-D-I2111 | |
| ICD-9-D-41032 | ICD-9-D-41070 | ICD-10-D-I2102 | |
| ICD-9-D-41040 | ICD-9-D-41071 | ICD-10-D-I222 | |
| Pneumonia | |||
| ICD-9-D-413 | ICD-9-D-48232 | ICD-9-D-4831 | ICD-10-D-J150 |
| ICD-9-D-4800 | ICD-9-D-48239 | ICD-9-D-4838 | ICD-10-D-J1289 |
| ICD-9-D-4801 | ICD-9-D-48240 | ICD-9-D-4841 | ICD-10-D-J09X1 |
| ICD-9-D-4802 | ICD-9-D-48241 | ICD-9-D-485 | ICD-10-D-J851 |
| ICD-9-D-4803 | ICD-9-D-48242 | ICD-9-D-486 | ICD-10-D-J1001 |
| ICD-9-D-4808 | ICD-9-D-48249 | ICD-9-D-4870 | ICD-10-D-J1108 |
| ICD-9-D-4809 | ICD-9-D-48281 | ICD-9-D-99731 | ICD-10-D-J153 |
| ICD-9-D-481 | ICD-9-D-48282 | ICD-9-D-99732 | ICD-10-D-J122 |
| ICD-9-D-4820 | ICD-9-D-48283 | ICD-10-D-J189 | ICD-10-D-J1281 |
| ICD-9-D-4821 | ICD-9-D-48284 | ICD-10-D-J188 | |
| ICD-9-D-4822 | ICD-9-D-48289 | ICD-10-D-J180 | |
| ICD-9-D-48230 | ICD-9-D-4829 | ICD-10-D-J151 | |
| ICD-9-D-48231 | ICD-9-D-4830 | ICD-10-D-J157 | |
| Pulmonary Embolism | |||
| ICD-9-D-41511 | ICD-9-D-41519 | ICD-10-D-I2609 | ICD-10-D-I2782 |
| ICD-9-D-41513 | ICD-9-D-4162 | ICD-10-D-I2699 | |
| Respiratory Failure | |||
| ICD-9-D-51853 | ICD-9-D-51882 | ICD-10-D-J9611 | ICD-10-D-J9612 |
| ICD-9-D-51851 | ICD-10-D-J9601 | ICD-10-D-J9602 | ICD-10-D-J9692 |
| ICD-9-D-51883 | ICD-10-D-J9600 | ICD-10-D-J9620 | ICD-10-D-J95822 |
| ICD-9-D-51884 | ICD-10-D-J9690 | ICD-10-D-J9622 | ICD-10-D-J952 |
| ICD-9-D-51881 | ICD-10-D-J9621 | ICD-10-D-J9691 | ICD-10-D-J953 |
| ICD-9-D-51852 | ICD-10-D-J9610 | ICD-10-D-J95821 | |
| Urinary Tract Infection | |||
| ICD-9-D-5990 | ICD-10-D-N390 | ||
References
- 1.Schwartz A.J., Chang Y.H., Bozic K.J. Evidence of pent-up demand for total hip and total knee arthroplasty at age 65. J Arthroplasty. 2019;34:194–200. doi: 10.1016/j.arth.2018.09.087. 2018/10/28. [DOI] [PubMed] [Google Scholar]
- 2.Learmonth I.D., Young C., Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. 2007/10/30. [DOI] [PubMed] [Google Scholar]
- 3.Laupacis A., Bourne R., Rorabeck C. The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am. 1993;75:1619–1626. doi: 10.2106/00004623-199311000-00006. 1993/11/01. [DOI] [PubMed] [Google Scholar]
- 4.Berry D.J., Harmsen W.S., Cabanela M.E. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177. doi: 10.2106/00004623-200202000-00002. 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 5.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–1460. doi: 10.2106/JBJS.17.01617. 2018/09/05. [DOI] [PubMed] [Google Scholar]
- 6.Ritter M.A., Carr K., Herbst S.A. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11:242–246. doi: 10.1016/s0883-5403(96)80073-8. 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 7.Aghayev E., Beck A., Staub L.P. Simultaneous bilateral hip replacement reveals superior outcome and fewer complications than two-stage procedures: a prospective study including 1819 patients and 5801 follow-ups from a total joint replacement registry. BMC Muscoskel Disord. 2010;11:245. doi: 10.1186/1471-2474-11-245. 2010/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepper S., Hochberg M.C. Factors associated with hip osteoarthritis: data from the first national health and nutrition examination survey (NHANES-I) Am J Epidemiol. 1993;137:1081–1088. doi: 10.1093/oxfordjournals.aje.a116611. 1993/05/15. [DOI] [PubMed] [Google Scholar]
- 9.Macaulay W., Salvati E.A., Sculco T.P. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10:217–221. doi: 10.5435/00124635-200205000-00008. 2002/06/04. [DOI] [PubMed] [Google Scholar]
- 10.Shih C.H., Ho W.B. One-stage versus two-stage bilateral autophor ceramic total hip arthroplasty. Clin Orthop Relat Res. 1985:141–145. 1985/03/01. [PubMed] [Google Scholar]
- 11.Parvizi J., Tarity T.D., Sheikh E. Bilateral total hip arthroplasty: one-stage versus two-stage procedures. Clin Orthop Relat Res. 2006;453:137–141. doi: 10.1097/01.blo.0000246529.14135.2b. 2007/02/22. [DOI] [PubMed] [Google Scholar]
- 12.Tsiridis E., Pavlou G., Charity J. The safety and efficacy of bilateral simultaneous total hip replacement: an analysis of 2063 cases. J Bone Joint Surg Br. 2008;90:1005–1012. doi: 10.1302/0301-620X.90B8.20552. 2008/08/02. [DOI] [PubMed] [Google Scholar]
- 13.National and Regional Projections of Supply and Demand for Surgical Specialty Practitioners: 2013-2025. US Department of Health and Human Services Health Resources and Services Administration Bureau of Health Workforce National Center for Health Workforce Analysis; 2016. [Google Scholar]
- 14.Berend M.E., Ritter M.A., Harty L.D. Simultaneous bilateral versus unilateral total hip arthroplasty an outcomes analysis. J Arthroplasty. 2005;20:421–426. doi: 10.1016/j.arth.2004.09.062. 2005/08/30. [DOI] [PubMed] [Google Scholar]
- 15.Stavrakis A.I., SooHoo N.F., Lieberman J.R. Bilateral total hip arthroplasty has similar complication rates to unilateral total hip arthroplasty. J Arthroplasty. 2015;30:1211–1214. doi: 10.1016/j.arth.2015.02.015. 2015/03/05. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein M.A., Keggi J.M., Zatorski L.E. One-stage bilateral total hip arthroplasty in patients > or = 75 years. Orthopedics. 2002;25:153–156. doi: 10.3928/0147-7447-20020201-19. 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 17.Grosso M.J., Courtney P.M., Kerr J.M. Surgeons' preoperative work burden has increased before total joint arthroplasty: a survey of AAHKS members. J Arthroplasty. 2020;35:1453–1457. doi: 10.1016/j.arth.2020.01.079. 2020/02/15. [DOI] [PubMed] [Google Scholar]
- 18.Feng J.E., Novikov D., Anoushiravani A.A. Team Approach: perioperative optimization for total joint arthroplasty. JBJS Rev. 2018;6 doi: 10.2106/JBJS.RVW.17.00147. e4. 2018/10/10. [DOI] [PubMed] [Google Scholar]
- 19.Parvizi J., Pour A.E., Peak E.L. One-stage bilateral total hip arthroplasty compared with unilateral total hip arthroplasty: a prospective study. J Arthroplasty. 2006;21:26–31. doi: 10.1016/j.arth.2006.04.013. 2006/09/05. [DOI] [PubMed] [Google Scholar]
- 20.Bedard N.A., Pugely A.J., Dowdle S.B. Opioid use following total hip arthroplasty: trends and risk factors for prolonged use. J Arthroplasty. 2017;32:3675–3679. doi: 10.1016/j.arth.2017.08.010. 2017/09/18. [DOI] [PubMed] [Google Scholar]
- 21.Pivec R., Issa K., Naziri Q. Opioid use prior to total hip arthroplasty leads to worse clinical outcomes. Int Orthop. 2014;38:1159–1165. doi: 10.1007/s00264-014-2298-x. 2014/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sing D.C., Barry J.J., Cheah J.W. Long-acting opioid use independently predicts perioperative complication in total joint arthroplasty. J Arthroplasty. 2016;31:170–174. doi: 10.1016/j.arth.2016.02.068. e171. 2016/07/28. [DOI] [PubMed] [Google Scholar]
- 23.Menendez M.E., Ring D., Bateman B.T. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473:2402–2412. doi: 10.1007/s11999-015-4173-5. 2015/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weick J., Bawa H., Dirschl D.R. Preoperative opioid use is associated with higher readmission and revision rates in total knee and total hip arthroplasty. J Bone Joint Surg Am. 2018;100:1171–1176. doi: 10.2106/JBJS.17.01414. 2018/07/19. [DOI] [PubMed] [Google Scholar]