Highlights
-
•
Systemic mastocytosis and neuroendocrine tumors may present with similar symptoms.
-
•
Co-occurrence of both diseases may be due to activating mutations in KIT.
-
•
Recognition of these distinct diseases is necessary to ensure timely treatment.
Keywords: Neuroendocrine tumor, Pancreas, Systemic mastocytosis, Tryptase, Chromogranin A, Case report
Abstract
Introduction
Systemic mastocystosis, a disorder of clonal mast cell expansion presents with symptoms of flushing, pruritus, musculoskeletal pain, gastrointestinal cramping and vascular instability. Patients with neuroendocrine tumors may present with similar symptoms due to the release of vasoactive mediators in both diseases. We report the co-occurrence of systemic mastocytosis and a neuroendocrine pancreatic tumor for which the patient received disease-specific treatment.
Case presentation
A 58-year-old woman with a history of indolent systemic mastocytosis and a serum tryptase of 51 ng/mL was diagnosed with a solid pancreatic lesion on ultrasound when assessing for organomegaly. Lesional biopsy was consistent with a pancreatic neuroendocrine tumor which was successfully resected.
Discussion
Presenting symptoms such as skin rashes, flushing, fatigue and diarrhea, are similar for systemic mastocytosis and neuroendocrine tumors. The co-occurrence of both diseases has not been previously reported. Activating mutations in KIT, which are a hallmark of systemic mastocytosis, may drive neoplastic proliferation in neuroendocrine tumors. Furthermore, mast cells infiltrating pancreatic tissue may have a trophic effect on the development of pancreatic neuroendocrine tumors.
Conclusion
While challenging to diagnose both diseases presenting with similar symptoms, recognition of these distinct diseases is necessary to ensure timely treatment.
1. Introduction
Systemic mastocytosis is a disease characterized by clonal mast cell expansion which results in increased localization of aberrant mast cells in vascularized tissues. Rare genetic mutations, the most common being the activating KIT c.2447A>T, p.D816V missense, lead to increased mast cell proliferation, survival and activation [1]. These outcomes often are associated with mild to severe signs and symptoms of flushing, pruritus, musculoskeletal pain, gastrointestinal cramping and vascular instability. Due to the higher mast cell burden and reactivity, patients are thus at increased risk of anaphylaxis to venoms, drugs, and to unknown causes [2]. The World Health Organization (WHO) mastocytosis classification distinguishes cutaneous from systemic mastocytosis; the latter being subclassified into indolent systemic mastocytosis (ISM) (most common), smoldering systemic mastocytosis, aggressive systemic mastocytosis, and systemic mastocytosis with an associated hematologic neoplasm [1]. The latter include myeloproliferative and lymphoproliferative disorders including multiple myeloma [3], non-Hodgkin’s lymphoma [4], primary thrombocythemia and polycythemia vera [5]. There is no reported association of clonal mast cell disorders with neuroendocrine tumors. However, as a result of vasoactive mediator release, both mastocytosis and neuroendocrine tumors may present with similar symptoms, (such as fatigue, flushing, abdominal pain and diarrhea) making a dual diagnosis difficult. Here we report a patient with indolent systemic mastocytosis diagnosed with a pancreatic neuroendocrine tumor. The work is reported in line with the SCARE criteria [6].
2. Presentation of case
A 58-year-old woman with a history of ISM and mitral valve prolapse status post repair was diagnosed with a solid pancreatic lesion on ultrasound during an evaluation to assess degree of organomegaly in the setting of systemic mastocytosis. Her initial symptoms attributed to mastocytosis began in her early 20 s when she presented with diffuse pruritic non-blanching macules refractory to oral non-sedating H1 antihistamines. A skin biopsy which revealed a mast cell infiltrate was interpreted as consistent with maculopapular cutaneous mastocytosis (urticaria pigmentosa). The mast cell serum tryptase level which reflects mast cell burden was elevated at 51 ng/mL (reference range <11.4 ng/ml). She was prescribed oral fexofenadine 10 mg daily and ranitidine 150 mg every 12 h with resulting symptomatic control. The initial bone marrow biopsy displayed features consistent with WHO criteria for the diagnosis of systemic mastocytosis including the presence of 10–20% mast cells, focal mast cell aggregates (>15 mast cells), and CD25+ mast cells by immunostaining which had a spindle shape morphology. In addition, the mast cells in a bone marrow aspirate were positive for CD2 and CD25 by flow cytometry. Finally, bone marrow mast cells from were positive for the D816V KIT mutation.
The patient established care at the National Institutes of Health Clinical Center at age 48 and was followed annually under protocol 02-I-0277. At her routine follow up visit at age 58, she reported generalized fatigue, chronic intermittent pruritis, occasional flushing, intermittent gastroesophageal reflux and bilateral hand, hip and ankle arthralgias. In view of her diagnosis of ISM and these debilitating symptoms, she was considered for enrollment a randomized clinical trial utilizing a monoclonal antibody for the treatment of ISM. A part of the screening process, an abdominal ultrasound was performed to assess for hepatosplenomegaly prior to trial enrollment. Unexpectedly, the ultrasound identified a predominantly solid lesion associated with the pancreatic neck that measured 3.4 × 4.0 × 2.8 cm (Fig. 1A) which was confirmed on endoscopic ultrasound (Fig. 1B). CT imaging re-demonstrated the pancreatic mass as a large solid or cystic hyper-enhancing mass within the neck of the pancreas measuring 3.9 cm × 3.7 cm (Fig. 1C). There was no evidence of distal pancreatic ductal dilation. However, the mass appeared to abut the gastroduodenal artery and superior mesenteric vein. At the time of this finding, serum alkaline phosphatase (56 U/L), aspartate aminotransferase (15 U/L), alanine aminotransferase (14 U/L) and total and direct bilirubin were within reference range. Serum tryptase was elevated at 36 ng/ml. Serum Chromogranin A (a biomarker for neuroendocrine tumors) was within normal range at 47 ng/ml (ref. <93 ng/ml). Needle biopsy of the pancreatic lesion was consistent with a grade 2 well-differentiated neuroendocrine tumor. Additionally, the lesion was positive on Gallium-68 DOTATATE scan consistent with a neuroendocrine tumor.
Fig. 1.
Ultrasound and CT imaging of mass. A. Predominately solid mass (arrow) associated with the pancreatic neck/body measuring 3.4 × 4.0 × 2.8 cm with no clear pancreatic ductal dilatation. B. Neck of pancreas mass on Endoscopic Ultrasound (EUS). C. CT showing solid or cystic mass within the neck of the pancreas measuring 3.9 cm × 3.7 cm, hyperenhancing relative to the background pancreatic parenchyma. No evidence of distal pancreatic ductal dilatation.
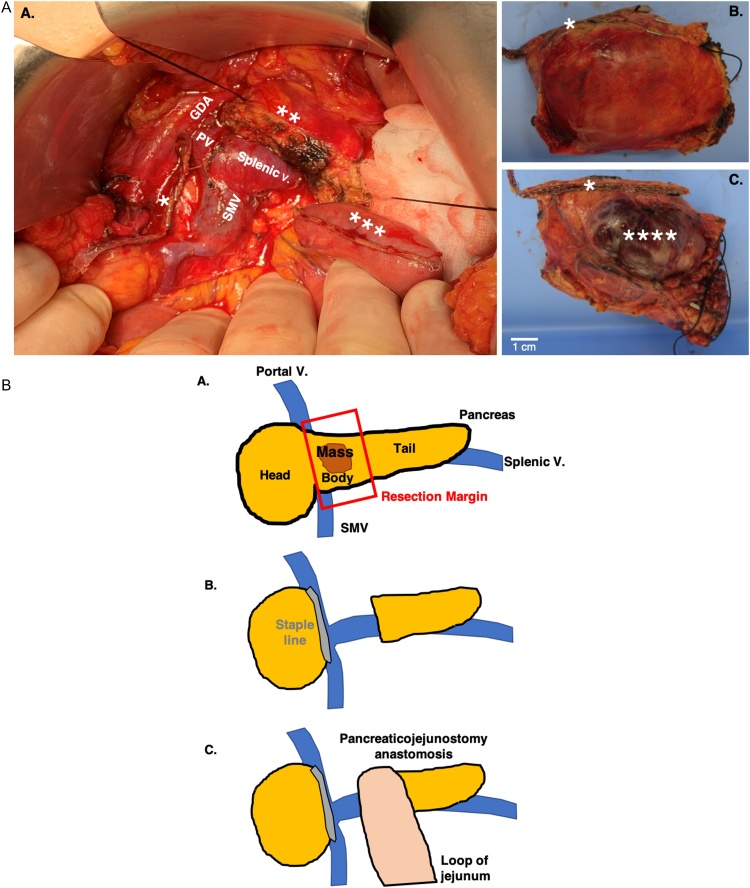
The patient subsequently underwent resection of mass via central pancreatectomy with pancreticojejunostomy reconstruction for remnant distal pancreas (Fig. 2a and 2b [pictural diagram]). This operative approach was elected over a distal pancreatectomy to preserve healthy pancreatic tail parenchyma and reduce the risk of post-operative pancreatic endocrine insufficiency. Patient tolerated the procedure well, and abdominal drains were placed at the end of the operation per routine practice. Her serum chromogranin A remained unchanged at 43 ng/ml at 7 weeks post operatively. She reported having decreased flushing postoperatively although serum tryptase remained elevated at 45.4 ng/dL.
Fig. 2.
Macroscopic appearance of neuroendocrine tumor.
A. After surgical removal, ink was applied to the surface of the resected specimen to delineate surgical margins prior to sectioning. After removal of the surgical staples and sutures, the specimen was bisected to reveal a solid, multinodular tumor surrounded by a rim of dense connected tissue. B. Pictural diagram of resection and construction of pancreatojejunostomy anastomosis.
Histopathology of the resected mass showed a unifocal, 4 × 3.7 × 2.5 cm tumor with 3 mitoses/10 high power field, Ki67 proliferative index of 3%, and well-differentiated monomorphic neuroendocrine histology consistent with a WHO G2 neuroendocrine tumor. One lymph node was extracted with resection specimen and was free of tumor, although lymphovasular invasion was present in primary specimen. Resection margins were free of tumor. The final pathologic stage was pT2N0M0. Tumor cells were positive for chromogranin A and synaptophysin (a synaptic vesicle protein used as a biomarker for neural and neuroendocrine tissue) on immunohistochemical staining. Immunostains for CD25+ cells (interleukin-2 receptor alpha chain, present on aberrant mast cells) positive for mast cell tryptase were present in both the tumor and the adjacent unaffected pancreas (Fig. 3, Fig. 4). Genomic interrogation using TruSight Oncology 500 gene panel revealed a tumor mutational burden of 2.4 mutations/megabase, microsatellite stable (MS-S), and a likely pathogenic frameshift mutation in SMARCD1 gene (involved in chromatin stability), although interestingly mutations commonly observed in sporadic PNET (MEN1, DAXX, ATRX, PTEN, TSC2) or familial PNET (MEN1, VHL1, NF1) were not detected.
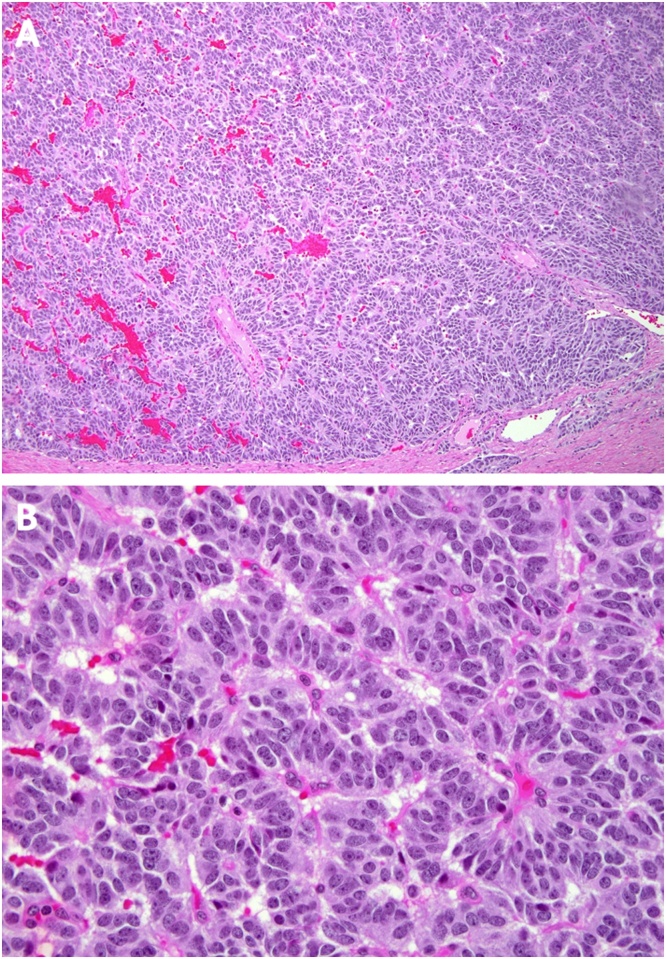
Fig. 3.
Histology of pancreatic neuroendocrine tumor.
A. Upper panel (10× original magnification) revealed a well circumscribed and monotonous neuroendocrine neoplasia arranged in nests and cords of tumor cells separated by a delicate fibrovascular stroma. B. Lower panel (20× original magnification) reveals nests of neuroendocrine tumor cells characterized by round to slightly elongated nuclei, finely granular (salt-and-pepper chromatin pattern, inconspicuous nucleoli, and a scant to moderate amount of pale eosinophilic cytoplasm. (hematoxylin and eosin stain).
Fig. 4.
Immunohistochemical staining of pancreatic neuroendocrine tumor.
CD25 (A and B), Chromogranin (C), Synaptophysin (D), and Mast cell tryptase (E and F). Tumor cells are positive for chromogranin A and synaptophysin. Interstitial mast cells are weakly positive for CD25 and strongly positive for mast cell Tryptase.
3. Discussion
Skin rashes, flushing, fatigue and diarrhea are common findings in patients with neuroendocrine tumors. These findings are also common presenting signs and symptoms in systemic mastocytosis. We are not aware of another similar case of ISM associated with a pancreatic neuroendocrine tumor. There is a case report in abstract form of a pancreatic neuroendocrine tumor in a male with tachycardia, diaphoresis and hives who had an elevated serum tryptase [7]. This patient was described as having mast cell activation syndrome, although it is unclear as to whether he had a workup to formally diagnose mastocytosis by WHO criteria.
Treatment of systemic mastocytosis depends on disease severity. Patients with aggressive systemic mastocytosis may be treated cytoreductive therapies that target KIT or considered for a matched allogeneic stem cell transplant [1]. Similar to mastocytosis, activating KIT mutations may drive neoplastic proliferation in neuroendocrine tumors [8]. Targeted inhibition of KIT mutations with imatinib led to an almost complete response in a metastatic neuroendocrine tumor [9]. Increased serum interleukin (IL-)6 levels have been reported in both systemic mastocytosis and in gastrointestinal and pancreatic neuroendocrine tumors [10,11]. It is unclear as to whether mast cells infiltrating pancreatic tissue have a trophic effect on the development of pancreatic neuroendocrine tumors.
The preferred operative approaches to resect lesions located at the ends of the pancreas are well established, with pancreaticoduodenectomy recommended for pancreatic head and neck lesions and distal pancreatectomy for lesions in the tail. Central pancreatic lesions, however, present a unique challenge. Pancreaticoduodenectomy or distal pancreatectomy can entail removal of excess healthy pancreatic tissue which may subsequently predispose patients to post-op pancreatic endocrine insufficiency. The central pancreatectomy technique employed in this case has been proposed to help preserve pancreatic tissue and prevent this complication. While this technique does involve an additional pancreatico-jejunostomy anastomosis compared to distal pancreatectomy – which can increase chances of complications such as anastomotic leaks or pancreatic fistula formation – a recent meta-analysis revealed this technique to be associated with significantly lower incidence of new-onset and worsening diabetes mellitus compared to distal pancreatectomy [12]. Given our patient’s overall good functional status and expected longevity, the central pancreatectomy was chosen to minimize risk of future endocrine complications while obtaining effective oncological resection.
4. Conclusion
Due to similarities in clinical signs and symptoms and available therapeutic approaches between mastocytosis and a neuroendocrine tumor, although co-occurrence appears rare, the presence of one diagnosis does not necessary exclude the presence of the other.
Declaration of Competing Interest
No conflicts of Interest.
Funding
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH).
Ethical approval
Ethical approval was obtained from our institution.
Consent
The patient was consented on admission and enrolment in NIH protocol 02-I-0277.
Author contribution
Keith Sacco – writing the paper, data collection and interpretation, final manuscript review.
Monica Passi. – clinical care, data collection and interpretation, final manuscript review.
Chyi-Chia R. Lee – pathologic evaluation, final manuscript review.
Tahsin M. Khan – clinical care, data collection and interpretation, final manuscript review.
Jonathan M. Hernandez – clinical care, data collection and interpretation, final manuscript review.
Dean D. Metcalfe – clinical care, data collection and interpretation, final manuscript review.
Hirsh Komarow – clinical care, case report concept, writing the paper, data collection and interpretation, final manuscript review.
Registration of research studies
N/A.
Guarantor
Hirsh Komarow, MD.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). We appreciate the contributions of Chyi-Chia R. Lee, M.D. for providing histopathological slides and description and Dean D. Metcalfe, M.D., M.S. for his critical review and edits.
References
- 1.Valent P., Akin C., Metcalfe D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulen T. Risk factor analysis of anaphylactic reactions in patients with systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 2017;5(5):1248–1255. doi: 10.1016/j.jaip.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Prabahran A.A., Juneja S.K. Systemic mastocytosis with concurrent multiple myeloma. Blood. 2018;131(13):1494. doi: 10.1182/blood-2017-12-824318. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal M.F. Systemic mastocytosis in association with small lymphocytic lymphoma. Am. J. Case Rep. 2017;18:1053–1057. doi: 10.12659/AJCR.905759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagan J.W., Jr. Systemic mastocytosis in a patient with polycythemia vera treated with radioactive phosphorus. Blood. 1977;49(4):563–571. [PubMed] [Google Scholar]
- 6.Agha R.A. The SCARE 2018 statement: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Khan F., Ledford D.K., Weston E., Sher M.R. An usual presentation of mast cell activation syndrome and pancreatic neuroendocrine tumor (PanNET) J. Allergy Clin. Immunol. 2019;143:AB181. [Google Scholar]
- 8.Vijayvergia N. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a fox chase cancer center pilot study. Br. J. Cancer. 2016;115(5):564–570. doi: 10.1038/bjc.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins J. Successful imatinib therapy for neuroendocrine carcinoma with activating Kit mutation: a case study. J. Natl. Compr. Cancer. Netw. 2014;12(6):847–852. doi: 10.6004/jnccn.2014.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkovic M.C. IL-6-174 C/G polymorphism in the gastroenteropancreatic neuroendocrine tumors (GEP-NETs) Exp. Mol. Pathol. 2007;83(3):474–479. doi: 10.1016/j.yexmp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Brockow K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol. Allergy Clin. North Am. 2014;34(2):283–295. doi: 10.1016/j.iac.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Dragomir M.P. Central pancreatectomy: a comprehensive, up-to-date meta-analysis. Langenbecks Arch. Surg. 2019;404(8):945–958. doi: 10.1007/s00423-019-01829-3. [DOI] [PubMed] [Google Scholar]