Abstract
Emerging evidence has shown the role of mesenchymal stem cell-derived exosome (MSC-exo) in inducing resistance of cancer cells to chemotherapy. However, it remains unclear whether the change of MSC-exo in response to chemotherapy also contributes to chemoresistance. In this study, we investigated the effect of a standard-of-care chemotherapeutic agent, doxorubicin (Dox), on MSC-exo and its contribution to the development of Dox resistance in breast cancer cells (BCs). We found that the exosome secreted by Dox-treated MSCs (Dt-MSC-exo) induced a higher degree of Dox resistance in BCs when compared with non-treated MSC-exo. By analysis of the MSC-exo-induced transcriptome change in BCs, we identified S100A6, a chemoresistant gene, as a top-ranked gene induced by MSC-exo in BCs, which was further enhanced by Dt-MSC-exo. Furthermore, we found that Dox induced the expression of miR-21-5p in MSCs and MSC-exo, which was required for the expression of S100A6 in BCs. Importantly, silencing of miR-21-5p expression in MSCs and MSC-exo abolished the resistance of BCs to Dox, indicating an exosomal miR-21-5p-regulated S100A6 in chemoresistance. Our study thus uncovered a novel mechanistic insight into the role of MSC-secreted exosome in the development of chemoresistance in the tumor microenvironment.
Keywords: doxorubicin resistance, mesenchymal stem cell exosome, miR-21-5p, S100A6, breast cancer
Graphical Abstract
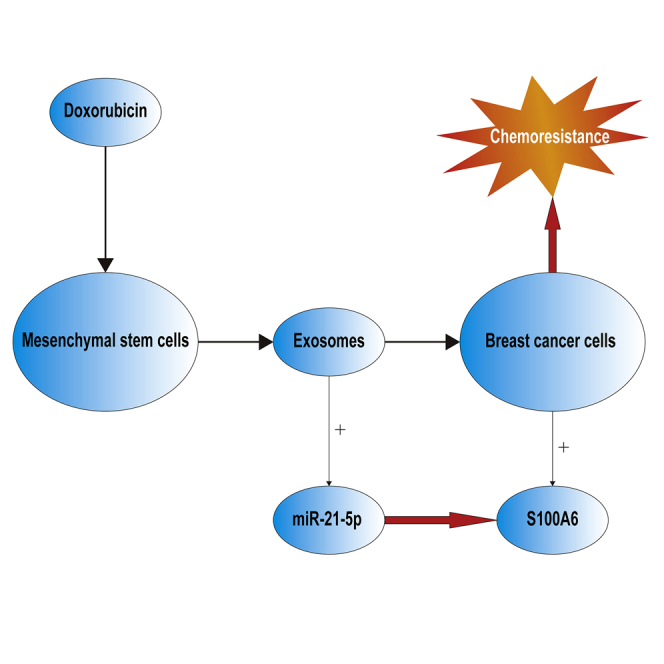
This study uncovered a novel mechanism by which chemotherapy modulates MSC activity to induce resistance through exosome secretion, which advances the understanding of cancer therapy resistance and should be of substantial interest to the cancer research community.
Introduction
Treatment failure due to chemoresistance remains one of the major reasons for breast cancer mortality.1 Mesenchymal stem cells (MSCs) are a unique group of cells capable of self-renewal and multidirectional differentiation, which are also known to play a role in the tumor microenvironment. MSCs have been shown to contribute to tumorigenesis processes, including proliferation, metastasis, and drug resistance in a variety of cancers,2,3 mainly through the secretion of paracrine factors or the cell-cell interaction.4 Recently, growing evidence has shown that MSCs can also generate extracellular vesicles including exosomes (MSC-exo) to promote the tumor growth, angiogenesis, metastasis, and invasion5, 6, 7 through the vesicle transfer of proteins, messenger RNAs (mRNAs), or microRNAs (miRNAs) to the target cancer cells.8,9 Some studies have also demonstrated the role of MSC-exo in drug resistance in cancers such as gastric cancer and multiple myeloma.10,11 Although the tumor microenvironment is well known to be a significant determinant of a tumor’s response to chemotherapy,12,13 it remains unclear whether MSC-exo changes in response to chemotherapy, which is necessary to promote chemoresistance of cancer cells.
In this study, we sought to investigate whether and how doxorubicin (Dox), a standard chemotherapeutic agent in breast cancer treatment, affects MSC-exo. We have demonstrated that Dox treatment induced the expression of miR-21-5p in MSCs and MSC-exo, leading to the induction of S100A6 in the breast cancer cells (BCs). Functional studies validated the role of miR-21-5p-mediated S100A6 expression in chemoresistance both in vitro and in vivo, which supports a novel mechanism by which the tumor microenvironment induces chemoresistance through an MSC-secreted exosome.
Results
The Conditioned Medium of Dox-Treated MSCs Significantly Enhances the Resistance of BCs to Dox
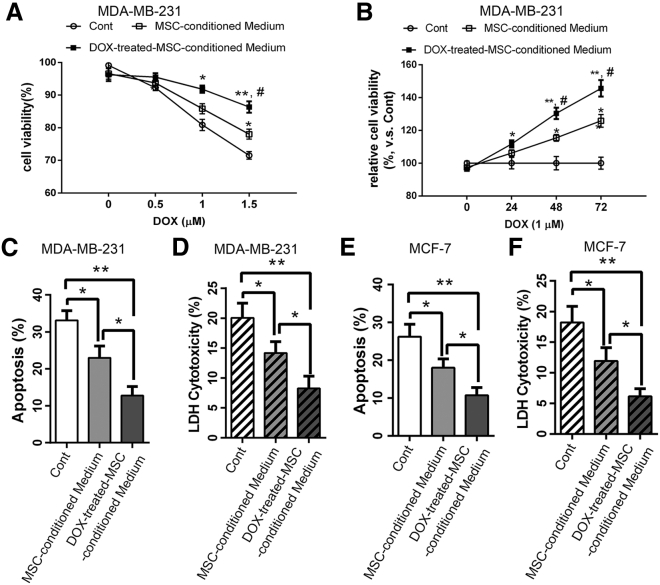
To investigate the paracrine effect of MSCs on BCs, we treated MSCs with 1 μM of Dox for 3 h, followed by a collection of untreated and Dox-treated MSC-conditioned medium. We further used the conditioned medium to treat breast cancer MDA-MB-231 cells in the presence of different concentrations of Dox (0, 0. 5, 1, and 1.5 μM) for 24 h, which was followed by cell viability assessment with a Cell Counting Kit-8 (CCK-8) assay (Figure 1A). The results showed that the Dox-treated MSC-conditioned medium significantly enhanced the cancer cell viability compared with untreated MSC-conditioned medium (Figure 1A). A similar effect was also observed in a time course analysis during 3 days in which Dox-treated MSC-conditioned medium supports a greater level of viability in the presence of 1 μM Dox (Figure 1B).
Figure 1.
Conditioned Medium of Dox-Pretreated MSCs Significantly Enhances the Resistance of BCs to Dox
(A) MDA-MB-231 cells were incubated with normal medium (control [Cont]), MSC-conditioned medium, and DOX-treated MSC-conditioned medium and then treated with various concentrations of Dox (0, 0.5, 1.0, and 1.5 μM) for 24 h. Cell viability was assessed using a CCK-8 assay. (B) To determine the change in viability of the cells with time after exposure to Dox, Dox was first added to the MDA-MB-231 cells to 1 μM, and the CCK8 assay then carried out after 0, 24, 48, and 72 h. (C and E) Annexin V-APC/7-AAD double staining showed the effect of different conditioned media on the percentages of apoptotic BCs (C, MDA-MB-231 cells; E, MCF-7 cells). (D and F) LDH cytotoxicity assay measured the effect of different conditioned media on the release of LDH from damaged BCs (D, MDA-MB-231cells; F, MCF-7 cells). Results are expressed as mean ± SD. Data were analyzed by the one-way analysis of variance (ANOVA). Significant differences between the means as determined by the Tukey post hoc test. ∗∗p < 0.05, ∗∗∗p < 0.001 in (A) and (B); ∗p < 0.05, ∗∗p < 0.001 versus the cells of Cont group; and #p < 0.05 versus the cells treated with MSC-conditioned medium.
We further used flow cytometry and the annexin V-allophycocyanin (APC)/7-aminoactinomycin D (7-AAD) double staining to evaluate the effect of MSC-conditioned medium on the apoptosis and cytotoxicity of MDA-MB-231 cells induced by Dox treatment. The results showed that Dox-induced apoptosis and cytotoxicity were significantly reduced by Dox-MSC-conditioned medium, while to a lesser extent by untreated MSC-conditioned medium (Figures 1C and 1D). A similar effect was also obtained in MCF-7 cells (Figures 1E and 1F). These findings showed that the conditioned medium from MSCs, especially under the Dox treatment, significantly enhance the resistance of BCs to Dox.
Exosomes Secreted from Dox-Treated MSCs Significantly Enhances the Resistance of BCs to Dox
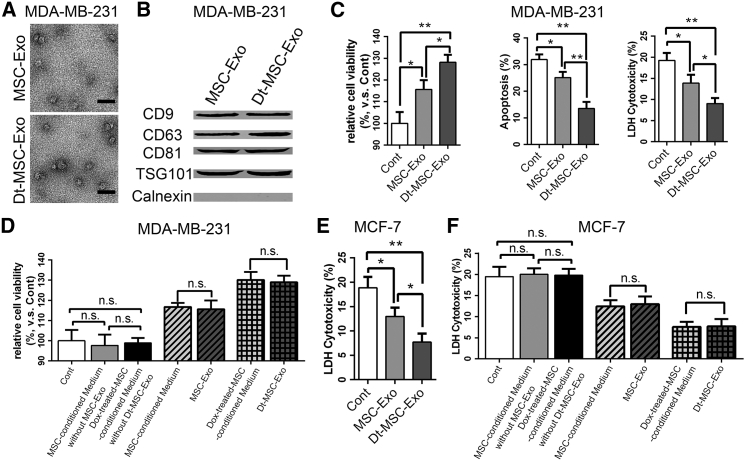
Mounting evidence has suggested a role of tumor-derived exosome in therapy failure.14 To determine whether the Dox-treated and untreated MSC-secreted exosomes (hereafter referred to as Dt-MSC-exo and MSC-exo, respectively) are responsible for the MSC-mediated chemoresistance, we extracted the exosomes from the conditioned medium of MSCs using a series of centrifugation and ultracentrifugation steps. The characteristics of exosomes were evaluated by electron microscopy, which revealed the homogeneous nature of exosomes in morphology, with sizes ranging from 50 to 100 nm (Figure 2A). Western blot further confirmed the abundant expression of the exosome-related proteins, including CD9, CD63, CD81, TSG101, and endoplasmic reticulum protein calnexin (Figure 2B). Taken together, these findings verified that the examined extracellular vesicles we isolated were indeed exosomes.
Figure 2.
Exosomes Secreted by Dox-Pretreated MSCs Can Significantly Enhance BC Resistance to DOX
(A) Transmission electron microscope (TEM) images of exosomes from MSC-conditioned medium and Dox-treated MSC-conditioned medium. Scale bars, 200 nm. (B) Phenotype analysis of protein markers of CD9, CD63, CD81, TSG 101, and calnexin on vesicles by western blot. (C–F) MDA-MB-231 (C and D) and MCF-7 (E and F) cells were treated with MSC-exo and Dt-MSC-exo, and then treated with Dox (1 μM) for 48 h: (C) CCK-8 (C, left; D), annexin V-APC/7-AAD double-staining (C, middle; F), and LDH cytotoxicity (C, right; E) assays detected the viability of the cells, percentage of apoptotic cells, and cell damage. The exosomes were first removed by centrifugation from the supernatant of MSCs and then and the supernatant was added to MDA-MB-231 (D, left) and MCF-7 (F, left) cells: CCK-8 (D) and LDH cytotoxicity (F) assays detected the viability of the cells, vesicular body, and its corresponding conditioned medium on MDA-MB-231 (D, middle, right) and MCF-7 (F, middle, right) cell activity. The data are mean ± SD, averaged from three separate experiments. ∗p < 0.05, ∗∗p < 0.01. n.s., not significant.
To directly evaluate the effects of MSC-exo and Dt-MSC-exo on BCs, we added them to the culture medium of MDA-MB-231 cells, followed by treatment with 1 μM Dox for 48 h. Tests with the CCK-8 assay, flow cytometry apoptotic double staining, and lactate dehydrogenase (LDH) assays showed that Dt-MSC-exo, and MSC-exo to a lesser degree, markedly enhanced the tolerance of MDA-MB-231 cells to Dox (Figure 2C).
To further ascertain the effect of the MSC-secreted exosome, we used centrifugation to remove the exosome from the MSC-conditioned medium, which was further used to treat the MDA-MB-231 cells. The result shows that Dt-MSC-conditioned medium deprived of exosome was unable to induce resistance of MDA-MB231 cells to Dox treatment, as shown by the CCK-8 cell viability assay (Figure 2D). This finding was further verified in MCF-7 cells (Figures 2E and 2F). Collectively, these results verify that the Dox enhances the capacity of MSCs to inducing BC resistance to Dox in an exosome-dependent manner.
Dox-Treated MSC-Exosome Significantly Induces S100A6 Expression in BCs
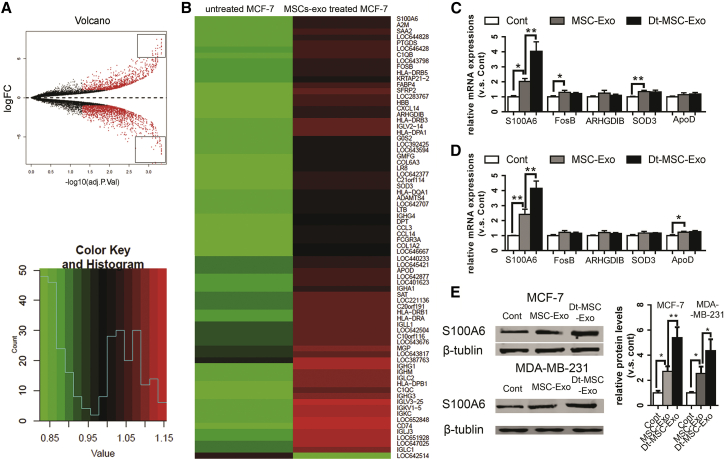
To identify the molecular mechanism by which the MSC-derived exosome induces chemoresistance of BCs, we took advantage of a public database comprising the transcriptome change of MCF-7 cells treated with MSC-exo (GEO: GSE46950). By applying a highly stringent cutoff (>23-fold change), we identified 75 genes whose expression exhibited a significant change in response to MSC-exo treatment.
Among the 75 candidate genes shown in Figures 3A and 3B, S100A6,15 FosB,16 ARHGDIB,17 SOD3,18 and ApoD19 have been previously implicated in promoting cell survival. Further RT-PCR validation showed that only the top-ranked S100A6 showed corresponding induction in BCs in response to exosome treatment (Figure 3C). Of notice, Dt-MSC-exo, compared with the untreated MSC-exo, induced a higher level of S100A6 expression in both MCF-7 and MDA-MB-231 cells, while the other top candidate genes failed to respond to Dt-MSC-exo (Figures 3C and 3D). The quantitative real-time RT-PCR results were further validated by western blot (Figure 3E).
Figure 3.
Screening for Genes Affected by Exosomes Secreted by Dox-Pretreated MSCs and Identifying the Increase of S100A6 Expression in BCs
(A and B) Differentially expressed proliferation-related genes in untreated MCF-7 cells and MSC-exo-treated MCF-7 cells are displayed in a volcano plot (A) and heatmap (B). Quantitative PCR analysis of exosome-treated BCs. (C and D) MCF-7 (C) and MDA-MB-231 (D) cells treated with MSC-exo and Dt-MSC-exo. The relative levels of S100A6, FOSB, ARHGDIB, SOD3, and ApoD in the Cont, MSC-exo, and Dt-MSC-Exo. (E) Western blot analysis. Images are representative of three independent experiments. Data are expressed as the mean ± SD of the relative values from three independent experiments. ∗p < 0.05, ∗∗p < 0.01.
S100A6 Upregulation by Dt-MSC-exo Confers Increased Cell Viability and Dox Resistance
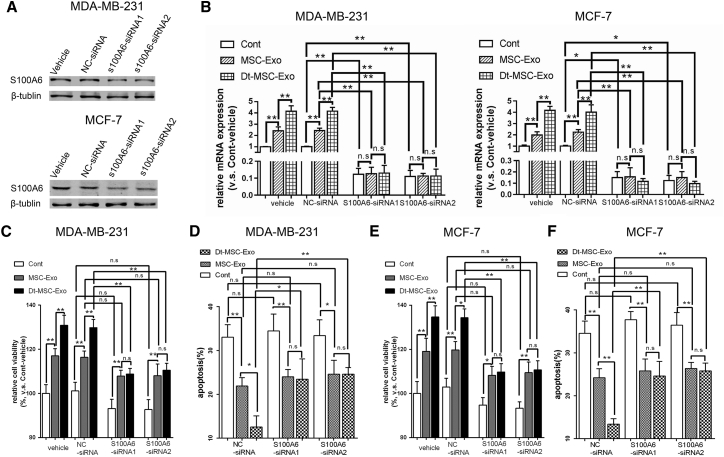
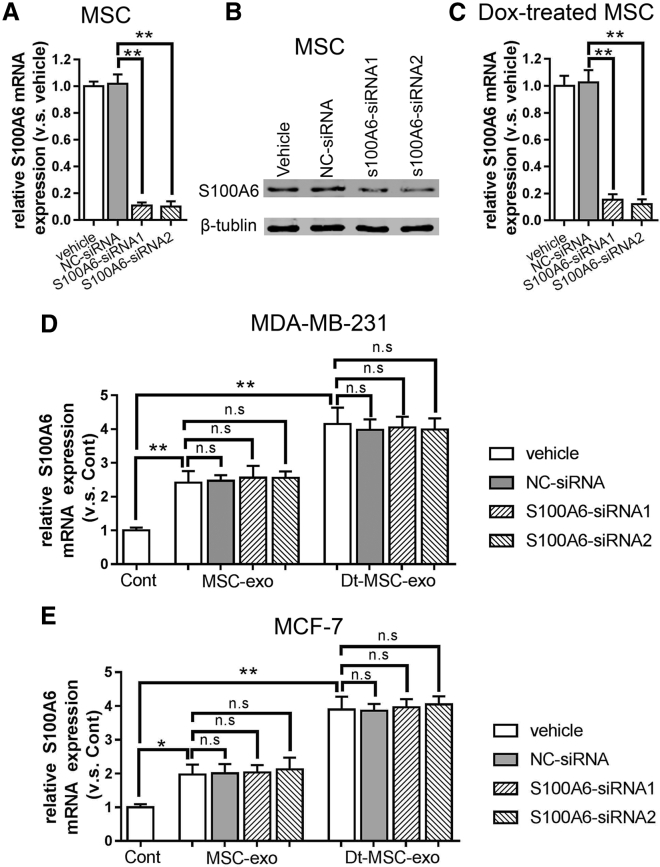
Previous studies have reported an anti-apoptotic effect of S100A6 in breast cancer.20 Next, we performed S100A6 knockdown to evaluate the functional role of S100A6 in MSC-exo-induced chemoresistance. Quantitative real-time RT-PCR and western blot analyses confirmed that small interfering RNA (siRNA)-mediated S100A6 knockdown has successfully abolished the increased S100A6 expression by MSC-exo or Dt-MSC-exo (Figures 4A and 4B). As expected, S100A6 knockdown in MCF-7 and MDA-MB-231 cells abolished the Dt-MSC-exo-induced resistance to Dox (Figures 4C and 4E) and promoted an apoptotic response to Dox (Figures 4D and 4F).
Figure 4.
Downregulation of S100A6 in BCs Lessens the Induction of Dox Tolerance by Dt-MSC-exo
MDA-MB-231 cells and MCF-7 cells were transfected with S100A6 siRNAs and negative control siRNA (nc-siRNA). (A and B) The protein (A) and mRNA (B) expression of S100A6 in MDA-MB-231 cells and MCF-7 cells after treatment with Dt-MSC-exo and MSC-exo (B). (C–F) Analysis by CCK8 viability (C and E) and apoptotic double-staining (D and F) assays showing no significant difference between Dt-MSC-exo and MSC-exo in the tolerance of BCs to Dox. Compared with nc-siRNA, treatment with S100A6 siRNAs resulted in more apoptotic cells in MSC-exo and Dt-MSC-exo. (C and D) MDA-MB-231 cells; (E and F) MCF-7 cells. The data are mean ± SD, averaged from three separate experiments. ∗p < 0.05, ∗∗p < 0.01. n.s., not significant.
To test the possibility that MSC-exosome-derived S100A6 plays a role in causing resistance to BCs, we performed S100A6 knockdown in MSCs and Dox-treated MSCs and collected its exosome for incubation with BCs. Although S100A6-siRNA significantly inhibited the expression of S100A6 in MSCs (Figures 5A–5C), BCs treated with MSC-exo or Dt-MSC-exo showed no significant difference in S100A6 expression (Figures 5D and 5E). This result has excluded a possible role of the exosomal source of S100A6 mRNA that could be transmissible to cancer cells.
Figure 5.
Downregulation of S100A6 in MSCs Has No Significant Effect on the Enhancement of S100A6 by MSC-exo and Dt-MSC-exo in BCs
(A–C) The mRNA (A and C) and protein (B) expression of S100A6 in MSCs (A and B) and Dox-treated MSCs (C) after treatment with S100A6-siRNA. (D and E) Downregulation of S100A6 in MSCs: relative expression of S100A6 in MSC-exo and Dt-MSC-exo treated with S100A6-siRNA in MDA-MB-231 (D) and MCF-7 (E) cells. The data are mean ± SD, averaged from three separate experiments. ∗p < 0.05, ∗∗p < 0.01. n.s., not significant.
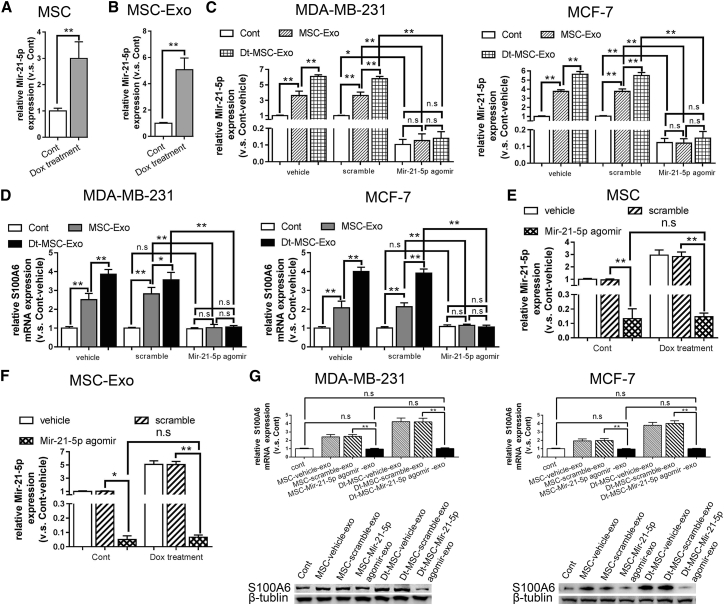
Dox-Induced miR-21-5p Expression in MSCs and MSC-exo Is Required for the S100A6 Induction in BCs
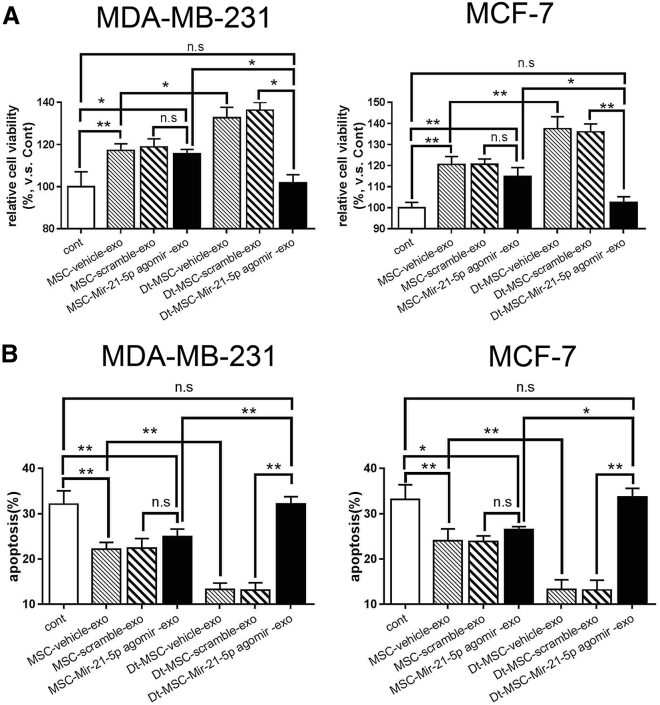
It has been previously shown that miR-21-5p can induce the expression of S100A6.21 We next investigated whether MSC-exosomal miR-21-5p is responsible for the induction of S100A6 in cancer cells. Indeed, we show that miR-21-5p was present in both MSCs and MSC-exo, which was significantly induced by Dox treatment (Figures 6A and 6B). Transfection of MDA-MB-231 and MCF-7 cells with a miR-21-5p antagomir abrogated the increased expression of miR-21-5p by MSC-exo and Dt-MSC-exo (Figure 6C), resulting in the abolishment of S100A6 induction by MSC-exo or Dt-MSC-exo (Figure 6D). Importantly, miR-21-5p antagomir also inhibited the expression of miR-21-5p in MSCs and MSC-exo (Figures 6E and 6F). As such, miR-21-5p antagomir-treated MSC-exo was unable to induce mRNA and protein expression of S100A6 in BCs (Figure 6G). Consequently, miR-21-5p antagomir-treated Dt-MSC-exo was unable to improve the BC viability (Figure 7A) or inhibit the Dox-induced apoptosis (Figure 7B). To investigate whether Dox can induce expression of miR-21-5p or S100A6 in BCs, we treated MCF-7 cells and MDA-MB-231 cells in the presence of different concentrations of Dox (0, 0. 5, 1, and 1.5 μM) for 12 h and in a time-dependent manner (0, 6, 12, and 18 h) with 1 μM of Dox. Quantitative real-time RT-PCR and western blot analyses showed that Dox treatment in BCs had no significant effect on the enhancement of miR-21-5p and S100A6 (Figure S1). This observation suggests a transmittable effect of miR-21-5p from exosome to BCs and indicates that inhibition of miR-21-5p expression in Dt-MSC-exo could significantly alleviate the Dox resistance.
Figure 6.
MSC-exo and Dt-MSC-exo Promote the Expression of S100A6 in BCs by Delivering miR-21-5p
(A and B) Relative expression of miR-21-5p in MSCs (A) and MSC-exo (B). The Dox-treated group displayed a higher content than did the control group. (C and D) MDA-MB-231 cells and MCF-7 cells were transfected with miR-21-5p antagomir or negative control scrambled RNA. Relative expression of miR-21-5p (C) and S100A6 (D) in MDA-MB-231 cells (left) and MCF-7 cells (right) treated with miR-21-5p antagomir. Two independent experiments showed similar results. (E and F) Reduced miR-21-5p expression in MSCs (E) and MSC-exo (F). (G) The mRNA and protein expression of S100A6 in MSC-exo and Dt-MSC-exo measured after treatment with miR-21-5p antagomir. Two independent experiments showed similar results. The data are mean ± SD, averaged from three separate experiments. ∗p < 0.05, ∗∗p < 0.01. n.s., not significant.
Figure 7.
Downregulation of miR-21-5p in MSC-exo Inhibited the Promotion of Tolerance of BCs to Dox
(A and B) Cell viability was assessed using a CCK-8 assay (A), and apoptosis was assessed using the apoptotic double-staining assay (B). The data show means ± SD (n = 3). ∗p < 0.05, ∗∗p < 0.01, compared with control. n.s., not significant.
MSC-exo Delivers miR-21-5p, Leading to S100A6 Expression and Dox Resistance In Vivo
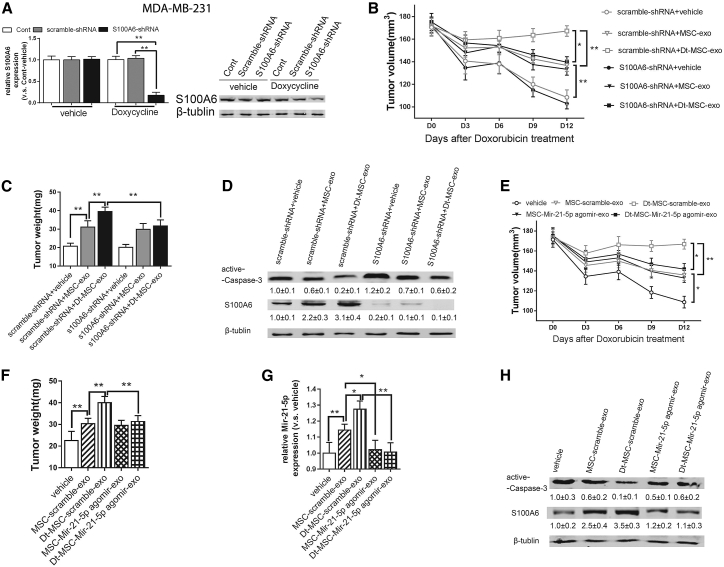
We next used an MDA-MB-231 tumor xenograft model to validate the in vivo capacity of the MSC-exo in regulating the tumor growth response to Dox. To this end, we silenced the S100A6 in MDA-MB-231 cells (Figure 8A) for xenograft tumor formation and injected MSC-exo or Dt-MSC-exo into the xenograft tumor. The result shows that the application of MSC-exo and Dt-MSC-exo promoted tumor growth in response to Dox treatment, while S100A6 knockdown inhibited the MSC-exo-induced tumor growth (Figures 8B and 8C). Caspase-3, known as the death enzyme, has an important role in the controlled execution of programmed cell death.22 Correspondingly, western blot shows that tumors treated with MSC-exo showed reduced active caspase-3 expression, while tumor with S100A6 knockdown showed increased expression of active caspase-3 (Figure 8D).
Figure 8.
In Vivo Antitumor Activity of MSC-exo and Dt-MSC-exo
(A) MDA-MB-231 cells were stably transfected with Dox-conditioned S100A6-shRNA or its control scramble-shRNA, and the mRNA and protein expression levels of S100A6 in the control group and the Dox-induced group were measured. (B–D) Downregulation of S100A6 in BC cells: tumor volumes (B) and weight (C) of the xenografts in each group are shown. The measurements were initiated at day 12 post-injection. (D) Protein expression of S100A6 and active-caspase 3. (E–H) Downregulation of miR-21-5p content in MSC-exo: tumor volume (E), weight (F), and expression levels of miR-21-5p are shown. (H) Protein expression of S100A6 and active-caspase 3. Data are expressed as the mean ± SD of the relative values from three independent experiments. ∗p < 0. 05, ∗∗p < 0. 01.
To evaluate the exosomal effect of miR-21-5p in vivo, MSCs were treated with miR-21-5p antagomir, followed by isolation of exosomes used to treat the xenograft tumors. The results showed that the miR-21-5p antagomir-treated Dt-MSC-exo markedly inhibited xenograft tumor growth (Figures 8E–8G). Western blot also confirmed the reduction of S100A6 by miR-21-5p antagomir and increased the expression of active caspase-3 (Figure 8H). Taken together, these data demonstrated that Dt-MSC-exo could significantly enhance BC resistance to Dox through exosomal miR-21-5p-mediated S100A6 expression.
Discussion
The importance of the microenvironment in tumor development and progression is widely recognized. The tumor microenvironment is composed of both tumor cells and stromal cells. MSCs, one of the pivotal components of the tumor microenvironment, is a focus of research on the progression of tumors.23,24 Notably, evidence suggests that MSCs release cytokines and growth factors that influence the behavior of tumors in a paracrine-dependent manner.25, 26, 27 Previous studies have demonstrated that tumor progression, enhancement of metastatic potential, chemoresistance, and resistance to radiotherapy may be attributed to MSCs.28, 29, 30 For instance, head and neck squamous carcinoma cells are resistant to paclitaxel when co-cultured with bone marrow-derived MSCs.31 MSCs can also utilize autophagy to recycle macromolecules and synthesize antiapoptotic factors to facilitate growth and survival of surrounding tumor cells.32 In colorectal carcinoma, NRG1 released by MSCs activates the phosphatidylinositol 3-kinase (PI3K)/AKT pathway to stimulate the growth of tumor cells.33 Platinum-based chemotherapy in breast cancer also induces MSCs to secrete unique fatty acids that confer chemoresistance.34 Adipose-derived MSCs enhanced BCRP protein expression and secreted interleukin (IL)-8, leading to reduced Dox sensitivity in triple-negative breast cancer.35 In this study, we investigated how Dox, a common chemotherapeutic agent in breast cancer treatment, affects the role of MSC-exo in the development of Dox resistance by BCs.
In our present study, we found that MSC-exo induced resistance in BCs to Dox. However, exosomes secreted by Dox-treated MSCs resulted in greater resistance of BCs to Dox than did those from untreated MSCs. We found that MSC-exo augmented the level of S100A6, a chemoresistance gene, in the BCs, whereas Dt-MSC-exo led to a greater increase in S100A6 level in the BCs than in MSC-exo. Our study also showed that Dox augmented miR-21-5p expression in MSCs and the increase of the miR-21-5p level in MSC-exo.
miR-21-5p is an important oncogenic miRNA that was recently reported to be highly upregulated in multiple tumors.36, 37, 38 The molecular mechanism of miR-21-5p action has been well characterized. miR-21-5p promotes tumor growth and metastasis by targeting several tumor suppressors, including PTEN, PDCD4, Bcl2, and tissue inhibitor of metalloproteinase 3 (TIMP3) in breast cancer, suggesting that miR-21-5p may be involved in regulating angiogenesis.36,39,40 S100A6 has been shown to be overexpressed in many human tumors, including pancreatic cancer,41, 42, 43 melanocytic tumors,44,45 colorectal cancer,46,47 and breast cancer.20 Previous studies have shown that S100A6 can promote the proliferation and migration of MCF-7 BCs and inhibit its apoptosis. Therefore, miR-21-5p and S100A6 are involved in regulating those genes that cause apoptosis in many diseases. Studies have shown that miR-21-5p promotes the expression of S100A6.21 In the present study, we showed that silencing miR-21-5p in MSCs eliminated the significant difference between MSC-exo and Dt-MSC-exo in their ability to elevate S100A6 level and promote Dox resistance in BCs. These results suggested that Dox can enhance MSC-exo-induced Dox resistance of BCs by increasing S100A6 content in BCs as a result of promoting the elevation of the miR-21-5p level in MSC-exo. Other potential miR-21 target genes also may be responsible for the effects of Dt-MSC-exo on breast cancer chemo-resistance, which need further in-depth study. Thus, the expression of miR-21-5p in MSCs is a prominent factor in oncology therapies employing Dox-treated MSCs. Note that in addition to overexpression in MSCs, previous studies have demonstrated that miR-21-5p is upregulated in numerous types of cancer and has been associated with cellular proliferation, migration, invasion, and apoptosis, which are the main processes underlying cancer pathogenesis.48,49 This suggests that when studying the use of MSCs for tumor therapy, it may be insufficient to consider only miR-21-5p expression in MSCs; the effect of pretreatment of MSCs with chemotherapy drugs on the tumor microenvironment also should be considered.
Growing evidence suggests that MSC-exo transfers proteins, mRNA, and miRNA to recipient cells, where these molecules exert various effects on the growth, metastasis, and drug response of different tumor cells. It has been reported that MSC-exo induces drug resistance in different tumor cells. MSC-exo triggered the activation of calcium/calmodulin-dependent protein kinases (CaM-Ks) and Raf/ mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) kinase cascade in gastric cancer cells.10 MSC-exo not only increases multiple myeloma cell growth but also induce resistance to bortezomib, a proteasome inhibitor. MSC-exo inhibits the reduction of Bcl-2 expression caused by bortezomib and reduces the cleavage of caspase-9, caspase-3, and PARP.11 In some studies, MSC-exo has been found to mediate drug efflux and the transfer of drug resistance to recipient cells, by transferring protein (MRP2, ATP7A, and ATP7B) and miRNAs (miR-100, miR-222, miR-30a, and miR-17). The miRNAs transferred to receptor cells can change the cell cycle and affect cell apoptosis, thus reducing the susceptibility to drugs. Consistent with previous results, this study demonstrated that MSC-exo was sufficient to confer Dox resistance in BCs both in vivo and in vitro and that Dt-MSC-exo resulted in a higher level of resistance to Dox in BCs than in MSC-exo. Therefore, the finding that Dt-MSC-exo increases chemotherapy resistance could be utilized to predict whether Dox should be used for treatment. Further studies are needed to explore whether MSC-exo treated with other chemotherapy drugs will also change the tumor microenvironment and lead to chemoresistance.
In conclusion, our study demonstrated that MSC-exo induces resistance of BCs to Dox and that Dt-MSC-exo can lead to a greater resistance of BCs to Dox than MSC-exo. Dox enhances MSC-exo-induced Dox resistance of BCs by increasing the S100A6 content of BCs as a result of promoting an increase in the level of mir-21-5p level in MSC-exo. Most importantly, silencing miR-21-5p in MSCs eliminated the significant differences between MSC-exo and Dt-MSC-exo in inducing the elevation of the S100A6 level of BCs and the development of Dox resistance of BCs in vitro and in vivo. This finding provides a novel understanding of the role of Dox-treated MSCs in the tumor microenvironment and may aid in the development of novel therapeutic strategies to circumvent Dox-treated MSCs-related drug resistance in patients with breast cancer.
Materials and Methods
Cell Culture and Culture Conditions
Human bone marrow-derived MSCs were isolated and cultured as described previously.50 MDA-MB-231 cells and MCF-7 cells were obtained from ATCC (Manassas, VA, USA). All cells were cultured in complete DMEM containing 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 mg/mL) at 37°C in humidified air with 5% CO2. Dox was purchased from Selleck Chemicals (USA). To collect Dox-treated MSC-conditioned medium, MSCs were treated with 1 μM Dox for 3 h, after which the culture medium was replaced with fresh DMEM/F-12. After a 24-h incubation, Dox-treated MSC-conditioned medium was collected and filtered through a 0.22-μm filter. The MSC-conditioned medium was obtained by collection through 0.22-μm filtration of the supernatant media from MSCs treated without Dox. BCs were cultured with a control medium, MSC-conditioned medium, and Dox-treated MSC-conditioned medium.
Exosomes Extraction and Purification
The MSC-conditioned medium consisted of DMEM supplemented with 10% FBS from which bovine exosomes and protein aggregates had been removed by ultracentrifugation at 100,000 × g for 16 h at 4°C. When the MSCs reached 80%–90% confluence, they were cultured in the conditioned medium for 48 h, and then the supernatants containing exosomes were harvested. The exosomes were purified by a modification of the procedure of differential centrifugation and purification on a sucrose cushion described by Qu et al.51 In brief, the supernatants were centrifuged for 20 min at 2,000 × g to separate cellular debris. The clarified supernatant was then concentrated through centrifugation twice for 30 min at 1,000 × g using a 100-kDa molecular weight cutoff (MWCO) hollow-fiber membrane (Millipore, Bedford, MA, USA). The concentrated supernatant was collected and diluted in PBS. The diluted supernatant was transferred to an ultracentrifuge tube, underlaid with a 30% sucrose/D2O cushion (density 1.210 g/cm3) and followed by ultracentrifugation at 100,000 × g for 1 h at 4°C. Gradient fractions were collected from the bottom of the tube and washed three times with PBS by centrifugation at 1,000 × g for 30 min in a 100-kDa MWCO Ultrafree-15 capsule. Finally, the protein content of the concentrated exosomes was determined using a bicinchoninic acid (BCA) protein assay kit. CD9, CD63, CD81, TSG101, and calnexin (Abcam, Cambridge, UK) were measured by western blot. The aliquots were passed through 0.22-μm microcentrifuge filters and stored at −80°C.
In Vivo Study
Sixty BALB/c 6-week-old female nude mice were purchased from Shanghai SLAC Laboratory Animal (Shanghai, P.R. China). MDA-MB-231 cells were stably transfected with S100A6-shRNA or scramble-shRNA (negative control group). MDA-MB-231 cells (2 × 106) stably expressing S100A6-shRNA or scramble-shRNA were subcutaneously injected into the right axilla of the mice and were randomly divided into three groups (n = 5) each. Mice were administered Dox (4 mg/kg; intravenously [i.v.]) every 3 days when tumors reached a volume of 150–200 mm3. The mice carrying xenograft tumor of MDA-MB-231 cells infected with S100A6-shRNA were divided into three groups (5 mice/group) randomly. Three groups respectively received an intratumoral injection containing (1) PBS (100 mg/mL), (2) MSC-exo (100 mg/mL), and (3) Dt-MSC-exo (100 mg/mL). Similarly, mice carrying a xenograft tumor of MDA-MB-231 cells infected with scramble-shRNA were also divided into three groups (5 mice/group) randomly and given the same treatment as for the S100A6-shRNA group. The mice were examined every 2 days and sacrificed at 12 days after Dox treatment. The tumor tissue was excised from mice, weighed, and the tumor volumes were calculated by the modified ellipsoidal formula V = ½(length × width2).
miR-21-5p Antagomir Intervention
The miR-21-5p antagomir (RiboBio Guangzhou, P.R. China) was used to antagonize the miR-21-5p expression specifically. Cells were treated with 120 nM miR-21-5p antagomir for 24 h to achieve sufficient miR-21-5p inhibition (verified by quantitative real-time RT-PCR as described below). Scrambled RNA was used as a negative control. Cells were changed with fresh medium supplemented with 120 nM miR-21-5p antagomir and cultured for 24 h. The medium was collected for exosome enrichment. The miR-21-5p expression was analyzed by a Hairpin-it miRNA qPCR quantitation kit.
Stable shRNA Transfection
Lentiviral vector (LV)-shRNA vectors targeting human S100A6 were purchased from Shanghai GenePharma (Shanghai, P.R. China) and were used for stable shRNA transfection. For lentiviral infection, MDA-MB-231 cells and MCF-7 cells were plated on six-well tissue culture plates at a density of 2 × 105 cells and transfected the next day with lentiviral particles in the presence of 5 μg/mL Polybrene (Sigma). The transfected clones were selected using 6 μg/mL puromycin (Invitrogen) and were then harvested via trypsinization within cloning rings. A scrambled non-specific shRNA was transfected in parallel with the S100A6 shRNA as control experiments. S100A6 expression was then verified via immunofluorescence and western blot analyses.
Transfection of S100A6-Targeting siRNA
Three siRNAs for silencing human S100A6 (si-S100A6) and a negative control siRNA (si-NC) were obtained from RiboBio (Guangzhou, P.R. China). The sequences of the human S100A6-specific siRNA were 5′-GCGAAUGUGCGUUGUGUAATT-3′ (siRNA-1), 5′-GUGGCCAUCUUCCACAAGUTT-3′ (siRNA-2), and 5′-CCUCUCUGAGUCAAAUCCATT-3′ (siRNA-3); the sequence 5′-UUC UCCGAA CGU GUC ACG UTT-3′ served as the negative control (scrambled non-specific siRNA). Cells were transfected with si-S100A6 or si-NC using Lipofectamine RNAiMAX according to the manufacturer’s instructions (Invitrogen).
Quantitative Real-Time RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). The concentration and purity of RNA were determined by measuring the absorbance at 260 nm and the absorbance ratio of 260/280 nm in a Take3 micro-volume plate (BioTek, USA). Subsequently, quantitative real-time RT-PCR was performed using SYBR Green master mix (Invitrogen) on the StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). The method to quantify mRNA and miRNA was performed as described previously.52 Expression of mRNA was normalized to endogenous GAPDH mRNA levels.
Western Blot
Exosomes were directly used for protein analysis. The protein concentration of cells and exosomes was determined using a protein assay kit (Bio-Rad), and samples were separated on SDS polyacrylamide gels for western blot analysis. Anti-S1000A6 (ab181975) and anti-active caspase-3 (ab2302) antibodies were purchased from Abcam (Cambridge, UK), and anti-β-tublin antibody was purchased from Sigma-Aldrich. β-tublin was used as an internal loading control.
Cell Viability/Cytotoxicity Assay
Cell viability was assessed by CCK-8 assays (Dojindo Laboratories, Japan) according to the manufacturer’s recommendations. Cytotoxicity was assessed by LDH leakage (Dojindo Laboratories, Japan) according to the recommendations of the manufacturer.
Apoptosis Assay
An annexin V/propidium iodide (PI) apoptosis detection kit (BD Biosciences) was used to evaluate apoptosis according to the manufacturer’s instructions. MDA-MB-231 cells and MCF-7 cells cultured with MSC-exo (50 μg/ mL) and Dt-MSC-exo (50 μg/mL) for 60 h were harvested. Cells were incubated with 5 μL of annexin V-fluorescein isothiocyanate (FITC) for 15 min. Subsequently, PI staining was performed. Cell apoptosis was detected by flow cytometry (LSR II, BD Biosciences).
Statistical Analysis
Statistical analysis was performed by GraphPad Prism (v5.01; GraphPad, La Jolla, CA, USA). All experimental data in vitro were obtained from at least three independent experiments and expressed as the mean ± standard deviation. A Student’s t test was used to compare experimental and relative control groups. Multiple comparisons were performed using one-way analysis of variance followed by the Tukey post hoc test. p <0.05 was considered to indicate a statistically significant difference.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant nos. 81960477, 81760478, 81602315, 81660424, and 81560468); the China Postdoctoral Science Foundation (grant no. 2017M623295XB); the Natural Science Foundation of Guangxi (grant nos. 1598012-42 and 2016GXNSFBA380139); and by the Guangxi Medical University Training Program for Distinguished Young Scholars.
Author Contributions
Y.Liu and Q.Y. designed the total project. T.L., Q.L., Y.J., L.N., and J.T. performed the in vitro experiments. A.T., L.D., W.Z., and W.X. performed the in vivo experiments. Y.Liu supervised the study and wrote the manuscript. Y.Lu and Q.Y. revised the paper. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2020.10.008.
Contributor Information
Yongkui Lu, Email: luyongkui616@126.com.
Qiang Yu, Email: yuq@gis.a-star.edu.sg.
Yan Liu, Email: liuyansunny@163.com.
Supplemental Information
References
- 1.Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Ridge S.M., Sullivan F.J., Glynn S.A. Mesenchymal stem cells: key players in cancer progression. Mol. Cancer. 2017;16:31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y., Du L., Lin L., Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat. Rev. Drug Discov. 2017;16:35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 4.Konala V.B., Mamidi M.K., Bhonde R., Das A.K., Pochampally R., Pal R. The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cukierman E., Bassi D.E. The mesenchymal tumor microenvironment: a drug-resistant niche. Cell Adhes. Migr. 2012;6:285–296. doi: 10.4161/cam.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W., Huang L., Li Y., Qian H., Shan X., Yan Y., Mao F., Wu X., Xu W.R. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10:3198–3207. doi: 10.4161/cc.10.18.17638. [DOI] [PubMed] [Google Scholar]
- 7.Zhu W., Huang L., Li Y., Zhang X., Gu J., Yan Y., Xu X., Wang M., Qian H., Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315:28–37. doi: 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Lai R.C., Yeo R.W., Lim S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Tu H., Yang Y., Fang L., Wu Q., Li J. Mesenchymal stem cell-derived extracellular vesicles: roles in tumor growth, progression, and drug resistance. Stem Cells Int. 2017;2017:1758139. doi: 10.1155/2017/1758139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji R., Zhang B., Zhang X., Xue J., Yuan X., Yan Y., Wang M., Zhu W., Qian H., Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J., Hendrix A., Hernot S., Lemaire M., De Bruyne E., Van Valckenborgh E., Lahoutte T., De Wever O., Vanderkerken K., Menu E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124:555–566. doi: 10.1182/blood-2014-03-562439. [DOI] [PubMed] [Google Scholar]
- 12.Chien J., Kuang R., Landen C., Shridhar V. Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front. Oncol. 2013;3:251. doi: 10.3389/fonc.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao Y., Keller E.T., Garfield D.H., Shen K., Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azmi A.S., Bao B., Sarkar F.H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leśniak W., Wilanowski T., Filipek A. S100A6—focus on recent developments. Biol. Chem. 2017;398:1087–1094. doi: 10.1515/hsz-2017-0125. [DOI] [PubMed] [Google Scholar]
- 16.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 17.Solár P., Sytkowski A.J. Differentially expressed genes associated with cisplatin resistance in human ovarian adenocarcinoma cell line A2780. Cancer Lett. 2011;309:11–18. doi: 10.1016/j.canlet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Laatikainen L.E., Incoronato M., Castellone M.D., Laurila J.P., Santoro M., Laukkanen M.O. SOD3 decreases ischemic injury derived apoptosis through phosphorylation of Erk1/2, Akt, and FoxO3a. PLoS ONE. 2011;6:e24456. doi: 10.1371/journal.pone.0024456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najyb O., Do Carmo S., Alikashani A., Rassart E. Apolipoprotein D overexpression protects against kainate-induced neurotoxicity in mice. Mol. Neurobiol. 2017;54:3948–3963. doi: 10.1007/s12035-016-9920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cross S.S., Hamdy F.C., Deloulme J.C., Rehman I. Expression of S100 proteins in normal human tissues and common cancers using tissue microarrays: S100A6, S100A8, S100A9 and S100A11 are all overexpressed in common cancers. Histopathology. 2005;46:256–269. doi: 10.1111/j.1365-2559.2005.02097.x. [DOI] [PubMed] [Google Scholar]
- 21.Juskeviciute E., Dippold R.P., Antony A.N., Swarup A., Vadigepalli R., Hoek J.B. Inhibition of miR-21 rescues liver regeneration after partial hepatectomy in ethanol-fed rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G794–G806. doi: 10.1152/ajpgi.00292.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawa P., Hell K., Giroux A., Grimm E., Han Y., Nicholson D.W., Xanthoudakis S. Catalytic activity of caspase-3 is required for its degradation: stabilization of the active complex by synthetic inhibitors. Cell Death Differ. 2004;11:439–447. doi: 10.1038/sj.cdd.4401360. [DOI] [PubMed] [Google Scholar]
- 23.El-Khattouti A., Sheehan N.T., Monico J., Drummond H.A., Haikel Y., Brodell R.T., Megahed M., Hassan M. CD133+ melanoma subpopulation acquired resistance to caffeic acid phenethyl ester-induced apoptosis is attributed to the elevated expression of ABCB5: significance for melanoma treatment. Cancer Lett. 2015;357:83–104. doi: 10.1016/j.canlet.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z., Wang S., Zhao R.C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scherzad A., Steber M., Gehrke T., Rak K., Froelich K., Schendzielorz P., Hagen R., Kleinsasser N., Hackenberg S. Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. Int. J. Oncol. 2015;47:391–397. doi: 10.3892/ijo.2015.3009. [DOI] [PubMed] [Google Scholar]
- 26.Li W., Zhou Y., Yang J., Zhang X., Zhang H., Zhang T., Zhao S., Zheng P., Huo J., Wu H. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J. Exp. Clin. Cancer Res. 2015;34:52. doi: 10.1186/s13046-015-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ooi Y.Y., Dheen S.T., Tay S.S. Paracrine effects of mesenchymal stem cells-conditioned medium on microglial cytokines expression and nitric oxide production. Neuroimmunomodulation. 2015;22:233–242. doi: 10.1159/000365483. [DOI] [PubMed] [Google Scholar]
- 28.Velletri T., Xie N., Wang Y., Huang Y., Yang Q., Chen X., Chen Q., Shou P., Gan Y., Cao G. p53 functional abnormality in mesenchymal stem cells promotes osteosarcoma development. Cell Death Dis. 2016;7:e2015. doi: 10.1038/cddis.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou K., Xia M., Tang B., Yang D., Liu N., Tang D., Xie H., Wang X., Zhu H., Liu C., Zuo C. Isolation and comparison of mesenchymal stem cell-like cells derived from human gastric cancer tissues and corresponding ovarian metastases. Mol. Med. Rep. 2016;13:1788–1794. doi: 10.3892/mmr.2015.4735. [DOI] [PubMed] [Google Scholar]
- 30.Hendijani F., Javanmard ShH., Rafiee L., Sadeghi-Aliabadi H. Effect of human Wharton’s jelly mesenchymal stem cell secretome on proliferation, apoptosis and drug resistance of lung cancer cells. Res. Pharm. Sci. 2015;10:134–142. [PMC free article] [PubMed] [Google Scholar]
- 31.Scherzed A., Hackenberg S., Froelich K., Kessler M., Koehler C., Hagen R., Radeloff A., Friehs G., Kleinsasser N. BMSC enhance the survival of paclitaxel treated squamous cell carcinoma cells in vitro. Cancer Biol. Ther. 2011;11:349–357. doi: 10.4161/cbt.11.3.14179. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez C.G., Penfornis P., Oskowitz A.Z., Boonjindasup A.G., Cai D.Z., Dhule S.S., Rowan B.G., Kelekar A., Krause D.S., Pochampally R.R. Activation of autophagy in mesenchymal stem cells provides tumor stromal support. Carcinogenesis. 2011;32:964–972. doi: 10.1093/carcin/bgr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Boeck A., Pauwels P., Hensen K., Rummens J.L., Westbroek W., Hendrix A., Maynard D., Denys H., Lambein K., Braems G. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut. 2013;62:550–560. doi: 10.1136/gutjnl-2011-301393. [DOI] [PubMed] [Google Scholar]
- 34.Roodhart J.M., Daenen L.G., Stigter E.C., Prins H.J., Gerrits J., Houthuijzen J.M., Gerritsen M.G., Schipper H.S., Backer M.J., van Amersfoort M. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell. 2011;20:370–383. doi: 10.1016/j.ccr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Chen D.R., Lu D.Y., Lin H.Y., Yeh W.L. Mesenchymal stem cell-induced doxorubicin resistance in triple negative breast cancer. BioMed Res. Int. 2014;2014:532161. doi: 10.1155/2014/532161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song B., Wang C., Liu J., Wang X., Lv L., Wei L., Xie L., Zheng Y., Song X. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J. Exp. Clin. Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S., Liang Z., Xu L., Zou F. MicroRNA-21: a ubiquitously expressed pro-survival factor in cancer and other diseases. Mol. Cell. Biochem. 2012;360:147–158. doi: 10.1007/s11010-011-1052-6. [DOI] [PubMed] [Google Scholar]
- 38.Bonci D. MicroRNA-21 as therapeutic target in cancer and cardiovascular disease. Recent Pat. Cardiovasc. Drug Discov. 2010;5:156–161. doi: 10.2174/157489010793351962. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Chaerkady R., Beer M.A., Mendell J.T., Pandey A. Identification of miR-21 targets in breast cancer cells using a quantitative proteomic approach. Proteomics. 2009;9:1374–1384. doi: 10.1002/pmic.200800551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wickramasinghe N.S., Manavalan T.T., Dougherty S.M., Riggs K.A., Li Y., Klinge C.M. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logsdon C.D., Simeone D.M., Binkley C., Arumugam T., Greenson J.K., Giordano T.J., Misek D.E., Kuick R., Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 42.Crnogorac-Jurcevic T., Missiaglia E., Blaveri E., Gangeswaran R., Jones M., Terris B., Costello E., Neoptolemos J.P., Lemoine N.R. Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J. Pathol. 2003;201:63–74. doi: 10.1002/path.1418. [DOI] [PubMed] [Google Scholar]
- 43.Shekouh A.R., Thompson C.C., Prime W., Campbell F., Hamlett J., Herrington C.S., Lemoine N.R., Crnogorac-Jurcevic T., Buechler M.W., Friess H. Application of laser capture microdissection combined with two-dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics. 2003;3:1988–2001. doi: 10.1002/pmic.200300466. [DOI] [PubMed] [Google Scholar]
- 44.Maelandsmo G.M., Flørenes V.A., Mellingsaeter T., Hovig E., Kerbel R.S., Fodstad O. Differential expression patterns of S100A2, S100A4 and S100A6 during progression of human malignant melanoma. Int. J. Cancer. 1997;74:464–469. doi: 10.1002/(sici)1097-0215(19970822)74:4<464::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Ribe A., McNutt N.S. S100A6 protein expression is different in Spitz nevi and melanomas. Mod. Pathol. 2003;16:505–511. doi: 10.1097/01.MP.0000071128.67149.FD. [DOI] [PubMed] [Google Scholar]
- 46.Stulik J., Osterreicher J., Koupilova K., Knizek J., Bures J., Jandik P., Langr F., Dedic K., Schafer B.W., Heizmann C.W. Differential expression of the Ca2+ binding S100A6 protein in normal, preneoplastic and neoplastic colon mucosa. Eur. J. Cancer. 2000;36:1050–1059. doi: 10.1016/s0959-8049(00)00043-5. [DOI] [PubMed] [Google Scholar]
- 47.Komatsu K., Murata K., Kameyama M., Ayaki M., Mukai M., Ishiguro S., Miyoshi J., Tatsuta M., Inoue M., Nakamura H. Expression of S100A6 and S100A4 in matched samples of human colorectal mucosa, primary colorectal adenocarcinomas and liver metastases. Oncology. 2002;63:192–200. doi: 10.1159/000063812. [DOI] [PubMed] [Google Scholar]
- 48.Makiguchi T., Yamada M., Yoshioka Y., Sugiura H., Koarai A., Chiba S., Fujino N., Tojo Y., Ota C., Kubo H. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir. Res. 2016;17:110. doi: 10.1186/s12931-016-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han Y., Xu G.X., Lu H., Yu D.H., Ren Y., Wang L., Huang X.H., Hou W.J., Wei Z.H., Chen Y.P. Dysregulation of miRNA-21 and their potential as biomarkers for the diagnosis of cervical cancer. Int. J. Clin. Exp. Pathol. 2015;8:7131–7139. [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W., Zhang X., Qian H., Zhu W., Sun X., Hu J., Zhou H., Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp. Biol. Med. (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 51.Qu J.L., Qu X.J., Zhao M.F., Teng Y.E., Zhang Y., Hou K.Z., Jiang Y.H., Yang X.H., Liu Y.P. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig. Liver Dis. 2009;41:875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Zhao X., Liu L., Liu D., Fan H., Wang Y., Hu Y., Hou Y. Progesterone enhances immunoregulatory activity of human mesenchymal stem cells via PGE2 and IL-6. Am. J. Reprod. Immunol. 2012;68:290–300. doi: 10.1111/j.1600-0897.2012.01163.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.