Abstract
Introduction
In intensive care unit (ICU), the decision of extubation is a critical time because mortality is particularly high in case of reintubation. To reduce that risk, guidelines recommend to systematically perform a spontaneous breathing trial (SBT) before extubation in order to mimic the postextubation physiological conditions. SBT is usually performed with a T-piece disconnecting the patient from the ventilator or with low levels of pressure-support ventilation (PSV). However, work of breathing is lower during PSV than during T-piece. Consequently, while PSV trial may hasten extubation, it may also increase the risk of reintubation. We hypothesise that, compared with T-piece, SBT performed using PSV may hasten extubation without increasing the risk of reintubation.
Methods and analysis
This study is an investigator-initiated, multicentre randomised controlled trial comparing T-piece vs PSV for SBTs in patients at high risk of reintubation in ICUs. Nine hundred patients will be randomised with a 1:1 ratio in two groups according to the type of SBT. The primary outcome is the number of ventilator-free days at day 28, defined as the number of days alive and without invasive mechanical ventilation between the initial SBT (day 1) and day 28. Secondary outcomes include the number of days between the initial SBT and the first extubation attempt, weaning difficulty, the number of patients extubated after the initial SBT and not reintubated within the following 72 hours, the number of patients extubated within the 7 days following the initial SBT, the number of patients reintubated within the 7 days following extubation, in-ICU length of stay and mortality in ICU, at day 28 and at day 90.
Ethics and dissemination
The study has been approved by the central ethics committee ‘Ile de France V’ (2019-A02151-56) and patients will be included after informed consent. The results will be submitted for publication in peer-reviewed journals.
Trial registration number
Keywords: adult intensive & critical care, respiratory medicine (see thoracic medicine), respiratory physiology
Strengths and limitations of this study.
This large randomised controlled trial may help to establish strong recommendations on daily clinical practice for extubation in intensive care units with a high level of evidence.
Spontaneous breathing trials performed using T-piece or pressure-support ventilation have never been compared in the subset of patients at high risk of reintubation.
A large population of patients considered to be at high risk for reintubation will be included. Patients older than 65 years or those with an underlying chronic cardiac or lung disease are easy to identify in clinical practice and represent nearly half of the patients extubated in intensive care units.
The individual study assignments of the patients will not be masked. Given the characteristics of the two strategies under evaluation, a double-blind trial is not possible.
Introduction
Background and rationale
In intensive care unit (ICU), the decision of extubation is a critical time because mortality is particularly high in case of extubation failure leading to reintubation.1 The overall rate of reintubation after planned extubation is around 10% but may exceed 20% in patients at high risk of extubation failure.1 To reduce that risk, guidelines recommend to systematically perform a spontaneous breathing trial (SBT) before extubation in all patients intubated at least 24 hours in order to mimic the postextubation physiological conditions.2 A standard test for extubation readiness is an SBT with a T-piece disconnecting the patient from the ventilator and providing additional oxygen (T-piece trial). Another widely used trial is performed without disconnecting the patient from the ventilator, using low levels of pressure-support ventilation (PSV trial). In recent large cohort studies, these two types of SBTs were performed with nearly the same frequency.3 4 However, these two trials are not equivalent in terms of patient breathing effort. Physiological studies have shown that work of breathing measured during T-piece was similar to work of breathing after extubation.5 In contrast, work of breathing is markedly lower during PSV trial than during T-piece. Consequently, while PSV trial may potentially hasten extubation, it may also increase the risk of reintubation by underestimating the work of breathing needed after extubation.6
A large randomised controlled trial recently found that the proportion of patients successfully extubated 72 hours after the initial SBT was higher using a PSV trial for 30 min than using a T-piece trial for 2 hours.7 In this study, reintubation rates did not differ using PSV trial or T-piece. These findings confirm that PSV trial is an easier test to pass than T-piece trial, and that it may hasten extubation without an increased risk of reintubation. However, in this study, the proportion of patients with simple weaning was particularly high and patients with weaning difficulties were not monitored up until extubation, thereby limiting the application of these findings to simple weaning. Moreover, reintubation rates were particularly low meaning that the population mainly included patients at low risk of extubation failure.8 The latest American guidelines suggested an initial SBT using PSV rather than T-piece to hasten extubation.2 The strength of this recommendation was only conditional given the moderate certainty of evidence. To improve the level of evidence of daily clinical practice, we have decided to assess whether SBTs performed using PSV may hasten extubation without increasing the risk of reintubation in patients at high risk of extubation failure as compared with T-piece.
Objectives
We aim to conduct a prospective multicentre randomised controlled trial comparing two strategies of extubation performing SBT with T-piece or with PSV in patients at high risk of extubation failure. Our hypothesis is that SBTs with PSV may hasten extubation without increasing the risk of reintubation.
Primary objective
To compare the number of invasive ventilator-free days within the 28 days following the initial SBT between a strategy of extubation performing SBT with T-piece or with PSV.
Secondary objectives
To compare between the two groups: (1) the number of ventilator-free days (including intubation and non-invasive ventilation) within the 28 days following the initial SBT, (2) probability of extubation within the 72 hours and within the 7 days following the initial SBT, (3) proportion of patients with simple (≤24 hours), difficult (>24 hours and ≤7 days) or prolonged (>7 days) weaning, (4) proportion of patients extubated after the initial SBT and not reintubated within the following 72 hours, (5) weaning duration between the initial SBT and the first extubation attempt among extubated patients, (6) probability of reintubation within the 72 hours and within the 7 days following extubation, (7) proportion of patients with postextubation respiratory failure within the 7 days following extubation, (8) length of stay in ICU and (9) the mortality in ICU, at day 28 and at day 90.
Methods: participants, interventions and outcomes
Trial design
The TIP-EX study (acronym of T-pIece vs. Pressure-support ventilation for spontaneous breathing trials before EXtubation) is an investigator-initiated, multicentre, randomised, controlled, open-label trial comparing a strategy of extubation in patients at high risk of reintubation in ICUs. Patients will randomly be assigned to one of the two groups performing SBT with T-piece or with pressure-support (PS), with a 1:1 ratio.
The TIP-EX study is taking place in 31 ICUs in France. Patient flow chart is detailed in the figure 1.
Figure 1.
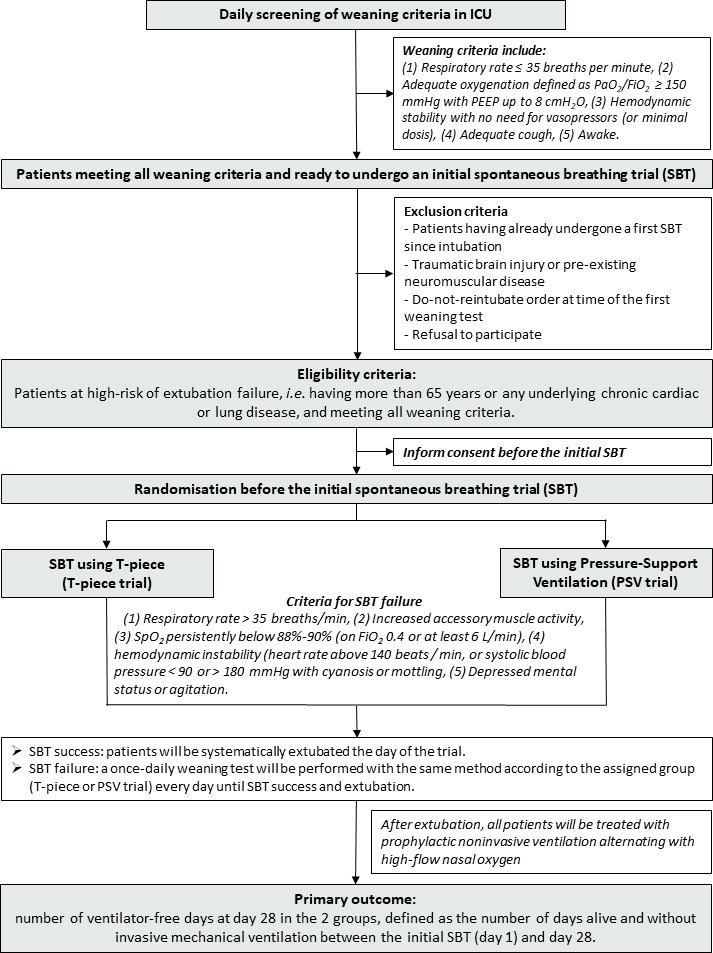
Flow chart of the patients and study design. Fio2, fractional inspired oxygen; ICU, intensive care unit; Pao2, arterial oxygen tension (or pressure; PSV, pressure-support ventilation.
Eligibility criteria
Inclusion criteria
Adult patients intubated more than 24 hours in ICU and at high risk of reintubation will be eligible as soon as possible once they meet all weaning criteria for an initial SBT.
Patients will be considered at high risk of extubation failure according to the following criteria9: patients older than 65 years, or those having any underlying chronic cardiac or lung disease. Underlying chronic cardiac diseases include left ventricular dysfunction (whatever the cause) defined by left ventricular ejection fraction ≤45%, history of cardiogenic pulmonary oedema, documented ischaemic heart disease or permanent atrial fibrillation. Chronic lung diseases include any underlying chronic obstructive pulmonary disease, obesity-hypoventilation syndrome (OHS) or restrictive pulmonary disease. The underlying lung disease will be either documented or highly suspected by the physician in a patient intubated for acute hypercapnic respiratory failure and having (1) a history of smoking with intrinsic positive end-expiratory pressure during mechanical ventilation and/or emphysema on chest X-ray or scanner suggesting underlying chronic obstructive pulmonary disease, (2) obesity (body mass index >30 kg/m2) with alveolar hypoventilation (arterial carbon dioxide tension, PaCO2 >45 mm Hg) suggesting OHS or (3) rib cage deformation suggesting restrictive pulmonary disease.
According to the international conference consensus on weaning,10 patients will be considered as ready for an initial SBT as soon as they meet all the following criteria: a respiratory rate ≤35 breaths per minute, adequate oxygenation defined as pulse oximetry (SpO2 ≥90% with fractional inspired oxygen, FiO2 ≤0.4 or PaO2/FiO2 ≥150 mm Hg with positive end-expiratory pressure ≤8 cmH2O, haemodynamic stability with no need for vasopressors (or minimal dosis ≤0.3 µg/kg/min), adequate cough, patient awake with a Richmond Agitation-Sedation Scale between +1 and −2.11
Exclusion criteria
Patients fulfilling one of the following criteria will not be included: patients having already undergone an initial SBT at any time since intubation, patients admitted for traumatic brain injury or with pre-existing peripheral neuromuscular disease (underlying myopathy or myasthenia gravis), patients with do-not-reintubate order at time of the initial SBT, patients previously included in the study, patients without health insurance coverage, people under protection (pregnant or breastfeeding women, minor patients, subjects with guardianship or under law protection) or refusal to participate.
Intervention
SBTs before extubation
Patients included will be randomised before the initial SBT and assigned to one of the following two groups: (1) In patients assigned to control group all SBTs will be performed using T-piece and (2) In patients assigned to experimental group all SBTs will be performed using PSV with a PS level of 8 cm H2O without positive end-expiratory pressure.
Control group: T-piece trial
The T-piece trial will be performed with a T-piece connected to the patient connection port of the endotracheal tube and providing additional oxygen (≤6 L/min). We will propose to add an oxygen flow rate of 3 L/min (oxygen blend) during the T-piece trial in patients mechanically ventilated with a FiO2 0.3 prior to the T-piece trial and 6 L/min for those mechanically ventilated with a FiO2 0.4.
Interventional group: PSV trial
The PSV trial will be performed without disconnecting the patient from the ventilator, by using a low level of PS (PS of 8 cm H2O) with a FiO2 ≤40% without positive end-expiratory pressure, and without activation of automatic tube compensation mode, while continuously monitoring respiratory rate and tidal volume on the ventilator display.
Duration of treatment and strategy of weaning and extubation
In both groups, the SBT will be performed for around 1 hour according to weaning guidelines.10 In case of SBT success, patients will be systematically extubated the day of the trial. After a successful T-piece trial, patients will be reconnected to the ventilator with prior ventilatory settings for around 1 hour before extubation to avoid exhaustion. A previous study showed that a 1-hour period at rest under mechanical ventilation after SBT trial with T-piece may improve outcome.12 We, therefore, decided to apply this protocol in our interventions.
In case of SBT failure, a once-daily SBT will be performed with the same method according to the assigned group (T-piece or PSV trial) every day as long as weaning criteria are met until SBT success and extubation. SBT failure will be defined according to the usual criteria from the international conference consensus on weaning,10 as development during the trial of any of the following events: (1) respiratory rate >35 breaths/min, (2) increased accessory muscle activity, (3) SpO2 persistently below 90% (or below 88% in case of underlying chronic lung disease) on FiO2 ≥0.4 or at least 6 L/min of oxygen, (4) haemodynamic instability defined as heart rate persistently above 140 beats/min or systolic blood pressure <90 or>180 mm Hg, with signs of hypoperfusion (appearance of cyanosis or mottling) and (5) depressed mental status or agitation.
All patients will be followed until day 28 after the initial SBT. In the event of extubation failure and reintubation, weaning will then be performed with the same method according to the assigned group (T-piece or PSV trial). After extubation, prophylactic use of non-invasive ventilation alternating with high-flow nasal oxygen between non-invasive ventilation sessions will be recommended in all patients for at least 48 hours according to the results of our previous study.13 14
Outcomes
Primary outcome
The primary outcome is the number of ventilator-free days at day 28, defined as the number of days alive and without invasive mechanical ventilation (intubation or tracheostomy) between the initial SBT (day 1) and day 28.
Secondary outcomes
Secondary outcome variables include the following:
The number of days alive and without mechanical ventilation (including intubation and non-invasive ventilation) between the initial SBT (day 1) and day 28.
The number of patients extubated within the 72 hours and within the 7 days following the initial SBT.
The number of patients extubated after simple (less than 24 hours), difficult (between 24 hours and 7 days) or prolonged (more than 7 days) weaning.
The number of patients extubated after the initial SBT and not reintubated within the following 72 hours.
The number of days between the initial SBT and the first extubation attempt.
The number of patients reintubated within the 72 hours and within the 7 days following extubation.
The number of patients with postextubation respiratory failure within the 7 days following extubation.
Length of stay in ICU.
Mortality in ICU, at day 28 and at day 90.
Criteria for postextubation respiratory failure
An episode of postextubation respiratory failure will be defined by the presence of at least two criteria among the following: a respiratory rate above 25 breaths per minute, clinical signs suggesting respiratory distress with increased accessory muscle activity, respiratory acidosis defined as pH <7.35 units and PaCO2 >45 mm Hg, hypoxaemia defined as a need for FiO2 at 50% or more to maintain SpO2 level at least 92% or a PaO2/FiO2 ratio <150 mm Hg.
Criteria for reintubation
To ensure the consistency of indications across sites and reduce the risk of delayed intubation patients will be immediately reintubated if at least one of the following criteria is fulfilled: severe respiratory failure, haemodynamic failure defined by a vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg with signs of hypoperfusion and serum lactate level greater than 2 mmol/L, neurological failure (altered consciousness with Glasgow Coma Scale below 12), cardiac or respiratory arrest.
Severe respiratory failure leading to reintubation will be defined by the presence of at least two criteria among the following: a respiratory rate above 35 breaths per minute, clinical signs suggesting respiratory distress with increased accessory muscle activity, respiratory acidosis defined as pH <7.25 units and PaCO2 >45 mm Hg, hypoxaemia defined as a need for FiO2 at 80% or to maintain SpO2 level at least 92% or a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2)<100 mm Hg.
Sample size
We determined that enrolment of 900 patients would provide a power of 80% to show an absolute prolonged duration of mechanical ventilation by 2 days (number of ventilator-free days reduced by 2 days) using the T-piece trial as compared with the PSV trial at a two-sided alpha level of 0.05.
Expected number of patients to be included in the study: statistical justification
We calculated the number of patients to include based on results of our previous cohort.15 In this study, the number of ventilator-free days at day 28 among the 150 patients at high risk for reintubation was 23 days (±9) in mean, and all patients were extubated following a strategy of weaning using PSV trials. In the present study, a number of patients will never be extubated, that is, either died or still under mechanical ventilation at day 28, and thus with no ventilator-free days. According to a recent large cohort study,4 these patients will represent around 2% –3% of patients. Thus, we calculated that the number of ventilator-free days at day 28 would be 22 days (±10) days using a PSV trial. In keeping with these results, we determined that enrolment of 786 patients would provide a power of 80% to show absolute prolonged duration of mechanical ventilation by 2 days (number of ventilator-free days reduced by 2 days) using the T-piece trial as compared with the PSV trial at a two-sided alpha level of 0.05. However, given the non-normal distribution of ventilator-free days in this population, the number of patients needed to be included was increased by 1.045 times in each group in order to compare the two groups with non-parametric tests.16 Therefore, we estimated that 820 patients will be needed. To ensure analysing at least 820 patients for primary and secondary outcomes taking into account of patients with withdrawal of consent or lost to follow-up, we decided to increase the number of inclusions by 10%, that is, 900 patients in total (450 patients per group).
Recruitment
The expected initial duration of patient enrolment is 2 years, starting in January 2020.
End of 2018: national grant award.
2019: approval by an independent ethics committee.
2020–2021: inclusion of patients (the first participant was enrolled the 31 January 2020).
2021–2022: end of inclusions, monitoring of participating centres and queries to investigators; blind review to determine protocol violation, to define intention-to-treat and per-protocol analysis populations; new queries to investigators, cleaning and closure of the database.
2022–2023: data analysis, writing of the manuscript and submission for publication.
Methods: assignment of intervention, data collection, management and analysis
Allocation and sequence intervention
After obtaining consent from the patient or his/her relative, all inclusion/exclusion criteria will be verified by the investigator before randomisation. Before the initial SBT the investigator will randomise patients to determine the type of trial allocated (T-piece or PSV trial). Randomisation will be stratified on centre and carried out by connecting to the electronic case report form (e-CRF) website https://chu-poitiers.hugo-online.fr/CSOnline/ after fulfilling the ‘randomisation’ page including all the criteria for eligibility.
Data collection and management
Data will be collected on an e-CRF by a trained investigator or research assistant at each centre. Patient follow-up and data collected are detailed in the study flow chart (table 1).
Table 1.
Study flow chart
| Procedures and assessments | From inclusion to the initial SBT | From the initial SBT to extubation | From the initial SBT to day 28 | Until ICU discharge and day 90 |
| Inclusion and non-inclusion criteria | X | |||
| Information and consent | X | X | ||
| Randomisation | X | |||
| Characteristics of the patient* | X | |||
| Characteristics of the initial SBT† | X | |||
| Characteristics at time of extubation‡ | X | |||
| Characteristics after extubation§ | X | |||
| Vital status | X |
*Characteristics of the patient include age, gender, height, weight, severity score indicated by the Simplified Acute Physiological Score II and the Sepsis-related Organ Failure Assessment score, underlying chronic cardiac or respiratory disease, date and reason for admission/ intubation, duration of intubation prior to the initial SBT, ventilatory settings and blood gases before the initial SBT.
†Characteristics of the SBT include duration, type and settings of the initial SBT, vital parameters at the end of the initial SBT, and criteria for SBT failure.
‡Characteristics at time of extubation include duration of weaning between the initial SBT and extubation, the number of SBTs attempts before extubation, classification according to the weaning difficulty, administrations of steroids before extubation, qualitative assessment of cough strength and amount of secretions at time of extubation.
§Characteristics after extubation include the use and duration of non-invasive ventilation and high-flow nasal oxygen after extubation (as well prophylactic use as rescue therapy to treat postextubation respiratory failure), criteria for postextubation respiratory failure, criteria for reintubation, need for reintubation, number of days of mechanical ventilation (invasive and non-invasive), tracheostomy and death.
ICU, intensive care unit; SBT, spontaneous breathing trial.
Statistical methods
All the analyses will be performed by the study statistician according to a predefined statistical analysis plan and using statistical software (SAS V.9.4; SAS Institute). A two-tailed p<0.05 will be considered as indicating statistical significance.
Descriptive analysis of patient groups at baseline
The analysis will be performed on an intention-to-treat basis after validation by a blind review committee of the inclusion/exclusion criteria for each patient. The continuous variables will be summarised with the classic parameters of descriptive analysis (median, IQRs and extreme values or mean and SD), while indicating the number of missing data. The category variables will be presented in the form of absolute frequency and percentage in each modality. Eligibility criteria will be verified on the basis of the data recorded in the case reports. Wrongly included subjects as well as those lost to follow-up will be described. Deviations from the protocol will be described and analysed on a case-by-case basis.
Analysis pertaining to the main criteria of evaluation
The number of ventilator-free days at day 28, defined as the number of days alive and without invasive mechanical ventilation (intubation or tracheostomy) between the initial SBT (day 1) and day 28, will be compared between the two groups by means of the Student’s t-test or the Mann-Whitney U test as appropriate. A two-tailed p<0.05 will be considered as indicating statistical significance.
Analysis pertaining to the secondary criteria of evaluation
Extubation success and reintubation rates at the various predefined times, difficulty of weaning (simple, difficult or prolonged), postextubation respiratory failure rates and mortality will be compared between the two groups by means of the χ2 test (or Fisher’s exact test).
Weaning duration and lengths of stay will be compared between the two treatment groups by means of the Student’s t-test or the Mann-Whitney U test as appropriate.
Kaplan-Meier curves will be plotted to assess the probability of extubation from the initial SBT to the following 72 hours and to the following 7 days, the probability of reintubation from extubation to the following 72 hours and to the following 7 days, and the probability of death between the initial SBT until day 90, and will be compared by means of the log-rank test.
The variables associated with extubation success and reintubation with a p<0.20 will be assessed by means of a multivariate logistic regression analysis or Cox proportional hazard regression analysis using a backward-selection procedure as appropriate.
The final model will include variables significantly associated with intubation with a p<0.05 and will be expressed using adjusted relative risk and OR or HR with 95% CI.
Predetermined subgroup analysis
Patients with prolonged duration of mechanical ventilation may have weaning difficulties and an increased risk of reintubation.4 15 Therefore, subgroups analyses will be performed for primary and secondary outcomes according to the duration of mechanical ventilation (>7 versus ≤7 days) prior to the initial SBT after an interaction test carried out to detect heterogeneity of treatment effect between patients with a prior duration of mechanical ventilation of more than 7 days and the others.
Data monitoring
The trial will be overseen by a steering committee regarding the progression and monitoring of the study at Réseau Européen Ventilation Artificielle Network meetings every 6 months.
Research assistants from the coordinating centre will regularly monitor all the centres on site to check adherence to the protocol and the accuracy of the recorded data. After being trained to conduct the protocol and to fulfil the e-CRF, an investigator at each centre will be responsible for daily patient screening, enrolling patients in the study, ensuring adherence to the protocol and completing the electronic case report form. Although the individual study assignments of the patients cannot be masked, the coordinating centre and all the investigators will remain unaware of the study group outcomes until the database will be locked.
Ethics and dissemination
The study has been approved by the central ethics committee (Ethics Committee Ile de France V, Paris, France) with the registration number 2019-A02151-56 (07 October 2019).
Consent or assent
The patient will be included after having provided a written informed consent to the investigator according to the decision of the central ethics committee. If the patient is not able to understand the information given, he/she can be included if the same procedure is completed with a next of kin. After the patient’s recovery, he/she will be asked if he/she agrees to continue the trial.
Confidentiality
Data will be handled according to French law. Coding subjects will be done by recording the first letter of the name and forename, accompanied by a single study identifier indicating the order of subject inclusion, in order to store anonymised data in the e-CRF. The sponsor will ensure that each study participant has given his/her consent for access to his/her personal data that is strictly required for quality control of the study. All original records will be archived at trial sites for 15 years
Declaration of interest
The TIP-EX study is an investigator-initiated trial supported by the French Ministry of Health with funds obtained in 2018 from a national hospital clinical research programme (Programme Hospitalier de Recherche Clinique National 2018). The study is promoted by the University Hospital of Poitiers.
Access to data
All investigators will have access to the final data set. Investigators will make available the documents and individual data strictly required for monitoring, quality control and audit of the study to persons having access to them, in accordance with the statutory and regulatory provisions in place (articles L.1121–3 and R.5121–13 of the French Public Health Code).
Dissemination policy
Findings will be published in peer-reviewed journals and presented at national and international meetings. Communications, reports and publication of the results of the study will be placed under the responsibility of the principal investigator-coordinator of the study and the executive committee. Rules of publication will follow the international recommendations according to The Uniform Requirements for Manuscripts (ICMJE, April 2010).
Patient and public Involvement
Patients and public are not involved in the study
Discussion
According to the physiological results,5 PSV trial is an easier test than T-piece trial and may potentially increase the risk of reintubation by underestimating the work of breathing needed after extubation.6 However, no study has demonstrated an increased reintubation rate using PSV trial as compared with T-trial.
Recently, a large randomised controlled trial including 1153 patients found that the proportion of patients successfully extubated 72 hours after the initial SBT was higher using a PSV trial for 30 min than using a T-piece trial for 2 hours.7 However, the proportion of patients with simple weaning (ie, patients extubated after the initial SBT) was particularly high in this study (nearly 90%) whereas usual rates in the literature are closer to 60%–70%.3 Moreover, patients with difficult weaning (ie, those who failed the initial SBT) were not monitored up until extubation, thereby limiting application of these findings to simple weaning, and not taking into account patients with weaning difficulties.3 4 Lastly, reintubation rates were particularly low (around 11%) meaning that the population mainly included patients at low risk of extubation failure.8 Although an easy test using PSV trial may hasten extubation, inclusion of patients at low risk with reintubation rates around 10% or less might not enable to detect an increased risk of extubation failure. Therefore, to avoid underpowering the study and so as be able to detect the risk, we decided to focus on patients at high risk of extubation failure and to include patients with weaning difficulties. In this population at high risk of reintubation a recent post host analysis from a large randomised controlled trial showed that execution of an initial SBT using PSV significantly increased the proportion of patients successfully extubated within the following 72 hours as compared with T-piece.17 However, a large prospective clinical trial is needed to confirm these findings in this population before being in a position to apply this weaning strategy to all ICU patients.
To assess as primary outcome the duration of weaning on the one the hand and the risk of reintubation on the other hand, we decided to assess the number of ventilator-free days at day 28. This criterion has the advantage of evaluating the two end-points (duration of weaning and risk of reintubation) with one and the same criterion. In previous studies, primary outcome was the number of patients extubated after the initial SBT and not reintubated at 48 hours or 72 hours.7 18 19 Although this outcome has weaknesses (too early and focusing only on simple weaning), we will also assess the number of patients extubated after the initial SBT and not reintubated within the following 72 hours, in order to compare our results to previous studies. Lastly, as performing a T-piece or PSV trial may influence the success of the initial SBT and duration between the initial SBT and successful extubation, we will compare the proportion of patients with simple, difficult and prolonged weaning according to type of SBT. Simple weaning includes patients extubated within the first 24 hours after the initial SBT, difficult weaning includes patients extubated between 24 hours and 7 days after the initial SBT, and prolonged weaning includes patients extubated more than 7 days after the initial SBT.4
No risk is expected with these two SBTs, as both of them are routinely and daily performed in the clinical practice of participating centres. Type of SBT may modify only the physician’s decision of extubation, and no other treatment will be added or modified.
In conclusion, the TIP-EX trial is an investigator-initiated pragmatic randomised controlled trial empowered to test the hypothesis that SBTs performed using PSV may hasten extubation without increasing the risk of reintubation in patients at high risk of extubation failure as compared with T-piece. These two strategies have never been compared in patients at high risk of reintubation, and therefore, this large trial may help to establish strong recommendations with a high level of evidence on a daily clinical practice for extubation in ICUs.
Supplementary Material
Footnotes
Twitter: @Quenot
Contributors: AWT, RC and J-PF in collaboration with all authors and the REVA network (Réseau Européen Ventilation Artificielle) designed the study and wrote the manuscript together. SR provided substantial contributions to the conception and design of the study, and wrote the statistical analysis plan and estimated the sample size with AWT. AWT, RC, AG, SE, DC, LD, AR, CG, GL, J-PQ, BL, GP, NT, GP, GL, JR, GB, JD, M-AN, AR, AD, NS, J-PM, JB, AL, LAA, QL, JD, EV, M-AA, CL, MD, RR, SR, J-PF contributed for drafting the work, revising it critically for important intellectual content and approved the final version of the manuscript. All authors give their agreement to be accountable for all aspects of the work, and ensure the accuracy and integrity of any part of the work.
Funding: This research will be conducted with the support of the 'Programme Hospitalier de Recherche Clinique National 2018' from the French Ministry of Health (PHRC 2018, grant number 18-007). The study sponsor is the University Hospital of Poitiers, Poitiers, France.
Competing interests: AWT reports financial support (payment for lectures and travel expenses coverage to attend scientific meetings) by Fisher & Paykel, Covidien, Maquet—Getinge, GE Healthcare. J-PF reports consulting fees from Fisher & Paykel and SOS oxygène.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Thille AW, Richard J-CM, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med 2013;187:1294–302. 10.1164/rccm.201208-1523CI [DOI] [PubMed] [Google Scholar]
- 2.Schmidt GA, Girard TD, Kress JP, et al. Official executive summary of an American thoracic Society/American College of chest physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. Am J Respir Crit Care Med 2017;195:115–9. 10.1164/rccm.201610-2076ST [DOI] [PubMed] [Google Scholar]
- 3.Peñuelas O, Frutos-Vivar F, Fernández C, et al. Characteristics and outcomes of ventilated patients according to time to liberation from mechanical ventilation. Am J Respir Crit Care Med 2011;184:430–7. 10.1164/rccm.201011-1887OC [DOI] [PubMed] [Google Scholar]
- 4.Béduneau G, Pham T, Schortgen F, et al. Epidemiology of weaning outcome according to a new definition. The wind study. Am J Respir Crit Care Med 2017;195:772–83. 10.1164/rccm.201602-0320OC [DOI] [PubMed] [Google Scholar]
- 5.Sklar MC, Burns K, Rittayamai N, et al. Effort to breathe with various spontaneous breathing trial techniques. A physiologic meta-analysis. Am J Respir Crit Care Med 2017;195:1477–85. 10.1164/rccm.201607-1338OC [DOI] [PubMed] [Google Scholar]
- 6.Tobin MJ. Extubation and the myth of "minimal ventilator settings". Am J Respir Crit Care Med 2012;185:349–50. 10.1164/rccm.201201-0050ED [DOI] [PubMed] [Google Scholar]
- 7.Subirà C, Hernández G, Vázquez A, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA 2019;321:2175–82. 10.1001/jama.2019.7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernández G, Vaquero C, González P, et al. Effect of Postextubation high-flow nasal cannula vs conventional oxygen therapy on Reintubation in low-risk patients: a randomized clinical trial. JAMA 2016;315:1354–61. 10.1001/jama.2016.2711 [DOI] [PubMed] [Google Scholar]
- 9.Thille AW, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011;39:2612–8. 10.1097/CCM.0b013e3182282a5a [DOI] [PubMed] [Google Scholar]
- 10.Boles J-M, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033–56. 10.1183/09031936.00010206 [DOI] [PubMed] [Google Scholar]
- 11.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation scale (RASS). JAMA 2003;289:2983–91. 10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez MM, González-Castro A, Magret M, et al. Reconnection to mechanical ventilation for 1 h after a successful spontaneous breathing trial reduces reintubation in critically ill patients: a multicenter randomized controlled trial. Intensive Care Med 2017;43:1660–7. 10.1007/s00134-017-4911-0 [DOI] [PubMed] [Google Scholar]
- 13.Thille AW, Muller G, Gacouin A, et al. Effect of Postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on Reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA 2019;322:1465–75. 10.1001/jama.2019.14901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thille AW, Muller G, Gacouin A, et al. High-Flow nasal cannula oxygen therapy alone or with non-invasive ventilation during the weaning period after extubation in ICU: the prospective randomised controlled HIGH-WEAN protocol. BMJ Open 2018;8:e023772. 10.1136/bmjopen-2018-023772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thille AW, Boissier F, Ben Ghezala H, et al. Risk factors for and prediction by caregivers of extubation failure in ICU patients: a prospective study. Crit Care Med 2015;43:613–20. 10.1097/CCM.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 16.Machin D, Campbell MJ, Tan SB, et al. Sample size tables for clinical studies. 3rd Edn, 2009. [Google Scholar]
- 17.Thille AW, Coudroy R, Nay M-A, et al. Pressure-Support Ventilation vs T-Piece During Spontaneous Breathing Trials Before Extubation Among Patients at High Risk of Extubation Failure: A Post-Hoc Analysis of a Clinical Trial. Chest 2020;158:1446–55. 10.1016/j.chest.2020.04.053 [DOI] [PubMed] [Google Scholar]
- 18.Esteban A, Alía I, Gordo F, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish lung failure Collaborative group. Am J Respir Crit Care Med 1997;156:459–65. 10.1164/ajrccm.156.2.9610109 [DOI] [PubMed] [Google Scholar]
- 19.Esteban A, Alía I, Tobin MJ, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish lung failure Collaborative group. Am J Respir Crit Care Med 1999;159:512–8. 10.1164/ajrccm.159.2.9803106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


