Abstract
Introduction
Treatment with anti-PD-1 immunotherapy does not lead to long-lasting clinical responses in approximately 60% of patients with metastatic melanoma. These refractory patients, however, can still respond to treatment with tumour infiltrating lymphocytes (TIL) and interferon-alpha (IFNa). A combination of TIL, pegylated-interferon-alpha (PEG-IFNa) and anti-PD-1 is expected to provide a safe, feasible and effective therapy for patients with metastatic melanoma, who are refractory to standard of care treatment options.
Methods and analysis
Patients are treated in two phases. In phase I, the safety of the combination TIL and anti-PD-1 is assessed (cohort 1) according to CTCAE 4.03 criteria. Subsequently, the safety of cotreatment with PEG-IFNa is tested in cohort 2. The efficacy will be evaluated in the second phase of the trial. Efficacy is evaluated according to RECIST 1.1 and immune-related response criteria. Clinical and immunological parameters will be evaluated for their relation with clinical responsiveness.
Ethics and dissemination
Ethical approval of the trial was obtained from the Central Committee on Research Involving Human Subjects in the Netherlands. The trial results will be shared with the scientific community at (inter)national conferences and by publication in a peer-reviewed journal.
Trial registration number
Keywords: oncology, dermatological tumours, immunology
Strengths and limitations of this study.
This is the first study to investigate the combination of a mild conditioning and supportive regimen for adoptive cell therapy and anti-PD-1.
Study findings could be used to create a prognostic (bio)marker profile in order to select patients who will benefit most from this treatment in future protocols/studies.
Expansion of tumour infiltrating lymphocytes is a time-consuming process, limiting the number of patients treated.
Introduction
Immune checkpoint inhibition has revolutionised the treatment of metastatic melanoma in recent years. Antibodies targeting programmed cell death protein 1 (anti-PD-1) have become the new first-line standard of care immunotherapy treatment in patients with metastatic melanoma. Approximately 60% of treated patients do not have long-lasting responses.1 The presence of sufficient numbers of activated T cells is a requirement for a durable response to anti-PD-1.2 This condition is not always met; consequently, patients may benefit from therapies that provide these T cells, including adoptive cell therapy (ACT).
We use ACT to transfuse ex vivo expanded autologous tumour infiltrating lymphocytes (TIL) to the patients. The most commonly used protocol includes chemotherapy driven lymphodepletion prior to T cell infusion and concomitant administration of high-dose IL-2. This is related to serious toxicity and a long hospitalisation time.3–6 Alternatively, this conditioning and support regimen can be replaced by cotreatment with low-dose IFNa. Treatment with IFNa induces a relatively mild leukopaenia, neutropaenia and lymphopaenia.7 8 The combination of TIL and IFNa resulted in clinical benefit (complete response, partial response or stable disease >6 months) in 20% of patients who were progressive after prior treatment with immune checkpoint inhibition (cytotoxic T-lymphocyte-associated protein 4 antibody, anti-PD-1 or the combination of both).7
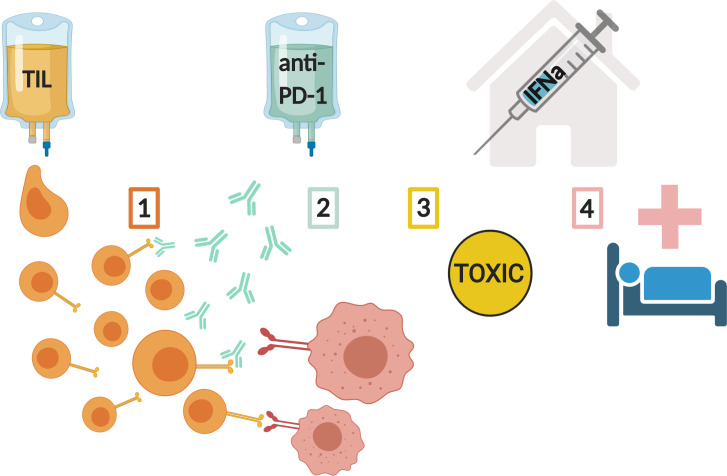
We propose that the combination of ACT, with anti-PD-1 infusions and pegylated-interferon-alpha (PEG-IFNa), is a safe and effective therapy for patients with metastatic melanoma solving four of the most important aspects curtailing the efficacy and feasibility of current immunotherapies (see figure 1).
Figure 1.
Resolving four of the most important aspects curtailing the efficacy and feasibility of current immunotherapies: (1) providing tumour-reactive TIL; (2) alleviating immune checkpoint inhibition; (3) reducing toxicity of ACT treatment; (4) Minimalising hospitalisation and patient burden. ACT, adoptive cell therapy; IFNa, interferon-alpha; TIL, tumour infiltrating lymphocytes.
Insufficient number of TIL
The magnitude of T cell infiltration in the tumour has a predictive value with respect to the natural history of primary cancers. It was shown that a greater density of tumour antigen-restricted CD8+ T cells in metastatic melanomas is associated with a better antitumour response in patients following anti-PD-1 treatment.2 ACT delivers high numbers of activated TIL to patients. Patients with low levels of activated T cells may benefit from treatments that deliver these T cells.
Inhibition of T cell effector function
Upregulated expression of PD-1 ligand (PD-L1) by tumour cells or tumour-infiltrating myeloid cells is one of the major mechanisms underlying immune escape. PD-L1 can bind to PD-1 on T cells and subsequently trigger inhibitory signalling downstream of the T cell receptor, blocking effector functions and reducing T cell killing capacity.9 We showed that a substantial percentage of the infused TIL in ACT express one or more coinhibitory molecules, including PD-1. These data suggest that the full capacity of transfused T cells to control tumour cell growth may be hampered due to checkpoint inhibition.7 Hence, the combination of TIL with anti-PD-1 may increase the tumour-reactivity of ACT.
Toxicity of chemotherapy and high-dose IL-2
Toxicities related to the most commonly used ACT protocol10 need to be resolved to push ACT more to the forefront of melanoma care.11 12 These toxicities are predominantly related to the conditioning regimen, used to create lymphopaenia (chemotherapy) and the high dose of IL-2 that is given to patients as a supportive regimen for the infused T cells.13–15 The conditioning is believed to create space for the infused T cells as well as to allow their homeostatic proliferation by elimination of the cellular sinks for endogenous cytokines.3 4 16 17
IFNa has been shown to result in a discernible but mild and transient leucopaenia7 8 18 19 and is routinely used in allogeneic stem cell transplantation to support donor lymphocyte infusions.20 We have observed a much lower number of adverse events when IFNa is used as conditioning and supportive regimens when compared with trials using high dose IL-2 with chemotherapy and TIL.7 8
Long-term hospitalisation
The previously described commonly used ACT protocol requires hospitalisation for 3–4 weeks, due to the side effects of treatment with lymphodepleting chemotherapy and high-dose IL-2. As a consequence of the use of our far less toxic protocol, treatment does not require any hospitalisation. Both the TIL and anti-PD-1 are given at the outpatient clinic, while PEG-IFNa subcutaneous injections are administered by patients themselves at home.
Methods
Study design
The ACTME study is an investigator initiated, single-centre phase I/II clinical trial for patients with progressive, unresectable stage III or stage IV melanoma who are refractory to standard of care treatment options. The trial is conducted in the Leiden University Medical Center, the Netherlands.
Eligibility and screening
Potential participants are screened by the principle investigator or one of the associate investigators, according to the eligibility criteria in box 1. Those patients found to be potentially eligible undergo baseline viral tests prior to biopsy or resection of a metastatic lesion for TIL culture.
Box 1. Eligibility criteria.
Inclusion criteria
≥18 years old and histologically proven unresectable (or residual) regional metastatic cutaneous melanoma.
Eastern Cooperative Oncology Group (ECOG) performance status ≤1.
Treated with standard treatment options (anti-PD-1, cytotoxic T-lymphocyte-associated protein 4 antibody, ±BRAF/MEK-inhibition) and experiencing progressive disease according to RECIST 1.1.
Within 2 weeks prior to study: haemoglobin ≥6.0 mmol/L, creatinine clearance ≥60 min/mL, aspartate transaminase and alanine aminotransferase≤5× the normal upper limit, lactate dehydrogenase ≤2× the normal upper limit.
Viral tests: no antibodies against human immunodeficiency viruses type 1/2, human T-lymphotropic virus, treponema pallidum, hepatitis B virus, and hepatitis C virus.
Exclusion criteria
Patients with brain metastases who are neurologically unstable and/or use dexamethasone.
Patients with active autoimmune disease requiring immunosuppressive drugs and patients with severe autoimmune AEs following immune checkpoint inhibition therapy not related to on-target toxicity (ie, vitiligo).
Use of systemic chronic steroid therapy (≥10 mg/day prednisone or equivalent) or any immunosuppressive therapy within 14 days prior to start of study treatment. Topical, inhaled, nasal, ophthalmic steroids and adrenal replacement therapy are allowed.
Other malignancy within 2 years prior to entry into the study, except for treated non-melanoma skin cancer and in situ cervical carcinoma.
Pregnancy or breastfeeding.
Known allergy to penicillin or streptomycin (used during the culturing of TIL).
Study objectives
The primary objective is to evaluate the safety and toxicity of ACT with anti-PD-1, followed by evaluating the safety and toxicity of anti-PD-1, ACT plus PEG-IFNa, according to CTCAE 4.03 criteria.
Furthermore, the disease control rate (stable disease >6 months and partial or complete response) is evaluated according to the RECIST 1.1 criteria and immune-related response criteria (irRC). Clinical response is evaluated by overall survival (OS) and progression-free survival (PFS).21 22 The potential mechanisms of action of the different treatment compounds are studied and the ACT infusion product is characterised. Finally, potential correlations between the clinical response and hypothesis related immune parameters are analysed to establish a possible prognostic biomarker profile.
Study phases
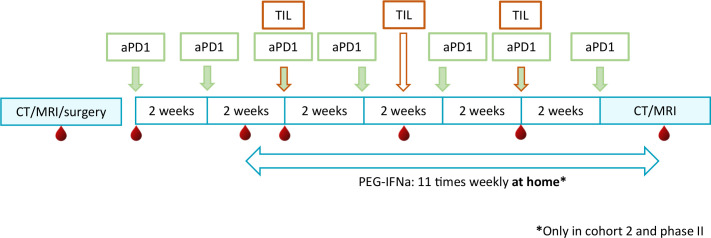
The phase I part of our trial consists of two cohorts. In the first cohort, the weekly subcutaneous injections with PEG-IFNa are omitted. If the treatment with ACT and anti-PD-1 (nivolumab) is considered safe, the subcutaneous PEG-IFNa injections are added in cohort 2 (see figure 2).
Figure 2.
Study design of ACTME trial. Blood and serum are collected at indicated time-points (red blood drop). In cohort 1, treatment with PEG-IFNa is omitted. In cohort 2 and phase II, pegylated-IFNa is added to the treatment with aPD1 and TIL. aPD1, anti-PD-1; IFNa, interferon-alpha; PEG-IFNa, pegylated-interferon-alpha; TIL, tumour infiltrating lymphocytes.
In the phase II part of the study, the patients are treated similarly to cohort 2 of the phase I part of the trial. A second cycle of PEG-IFNa, nivolumab and ACT can be added at the discretion of the treating physician, unless disease progression or complete regression of all metastases is observed during treatment evaluation at week 13. The second cycle has to be initiated within 1 month after completion of the first treatment cycle.
Treatment regimen
Nivolumab is given as 2-weekly infusions at the dose of 3 mg/kg. Patients receive two infusions before the first TILs are given.
One week prior to the first TIL infusion, patients in cohort 2 and phase II start with weekly subcutaneous injections of PEG-IFNa, 1 µg/kg/week (maximum 90 µg/week). The injections are continued for 11 weeks in total (see figure 2 and online supplemental table 1).
bmjopen-2020-044036supp001.pdf (66.3KB, pdf)
The dose, frequency and route of administration of the TIL is similar to our previously published protocols.7 8 We use a fixed 4-week TIL culturing period. Furthermore, based on our previous findings, we implemented a TIL dose range of 2.5–7.5×108 T cells per infusion, as this was feasible in this fixed time period and because responses to treatment were distributed among all TIL dose cohorts (1–2.5×108, 2.5–5 ×108 and 7.5–10×108) in our previous study.7 Per treatment cycle, three TIL infusions are administered with a 3-week interval. Based on the safety data from our previous trial and data from the first patients treated in the ACTME trial, hospital admission for 24 hours following the first TIL infusion is no longer required.
Study endpoints
Primary and secondary outcome measures are obtained through standardised clinical notes, CT scans and MRI. Furthermore, the treating physician records in the standardised clinical notes any observed treatment-related adverse events during the course of treatment and follow-up.
Scans to determine response are made at baseline and after 13 weeks.
Follow-up
If patients have stable disease, partial response or complete response, repeat evaluations are performed every 12 weeks during the first 2 years after start of treatment. Thereafter, patients receive radiological evaluations every 4–6 months until at least 5 years after start of treatment. Patient follow-up is performed for at least 5 years or until disease progression or death.
Outcome measures
Safety and toxicity of anti-PD-1, ACT plus PEG-IFNa are recorded according to the CTCAE 4.03 criteria. Toxicity grade 3 or less and serious adverse events related to treatment but not resulting in treatment termination are considered acceptable for continuation of the study.
Disease control rate is reported according to the RECIST 1.1 criteria and irRC, clinical response to treatment is defined as stabilisation of disease >6 months, partial response or complete response. Survival is calculated from start of treatment to either progression (PFS), death (OS) or date of final analysis.
To study the potential underlying mechanisms of action of the different treatment compounds and to establish a possible prognostic biomarker profile, we collect blood samples at the indicated time points before, during and after treatment (see figure 2 and online supplemental table 1). Furthermore, the potential prognostic value of type of resistance (primary versus secondary) on prior immune checkpoint inhibition will be analysed in patients treated with the combination of anti-PD-1, ACT plus PEG-IFNa.
Changes in the number and phenotype of circulating immune cells
The measurement of absolute numbers of leukocytes, neutrophils and lymphocytes is determined by differential blood counts performed by the CKHL (central clinical and haematological laboratory) of the LUMC on the blood samples. The duration and level of leukopaenia, neutropaenia and lymphopaenia is monitored in the subsequent blood samples.
The percentage and composition of circulating immune cells may strongly affect response to immunotherapy.23 To assess the impact of our treatments on these parameters, we use four sets of up to 11 cell surface markers to identify subsets of dendritic cells, macrophages, myeloid-derived suppressor cells, to evaluate the expression of costimulatory and coinhibitory molecules on T cells and regulatory T cells by flow cytometry, according to standard operating procedures and as was published by our group.7 24 25
Reactivity of TIL against autologous cell lines
The reactivity of TIL to autologous tumour cells will be assessed using either a tumour cell line established from the surgery specimen or very small cryopreserved tumour fragments as stimulator cells. The frequency of activated T cells is determined by flow cytometry using the activation marker CD137 in combination with CD3, CD4, CD8, as published by us and others before.7 8 26 The supernatants of these tumour stimulated TIL cultures are used to determine specific production of IFN-γ, TNFα, IL-10, IL-5, IL-4 and IL-2 by a flow cytometer based cytokine bead array (human Th1/Th2 kit, BD) according to the manufacturer’s instructions and reported earlier.7 8 25
Serum/plasma markers of persistence
Lymphodepleting conditioning regimens are thought to support the persistence of infused T cells by increasing the serum/plasma levels of homeostatic cytokines IL-7 and IL-15.4 The effect of PEG-IFNa on the serum levels of IL-7 and IL-15 collected at the indicated time points will be tested by ELISA (see figure 2 and online supplemental table 1).
Immunohistochemistry
A small piece of the initially removed tumour is embedded in paraffin and will be analysed for the expression of PD-L1 and for the presence of the four-parameter signature of responsiveness, previously published by our group. These parameters include numbers of CD8+ T cells, the ratio between galectin-9+ DCs/DC-like macrophages and between M1/M2 macrophages as well as galectin-3 expression intensity.27
After the first treatment-cycle, surgery or a biopsy of another metastasis is performed to culture more TIL and to compare biological and immunological markers before and after treatment, both in phases I and II, when possible.
Sample size calculation
Phase I
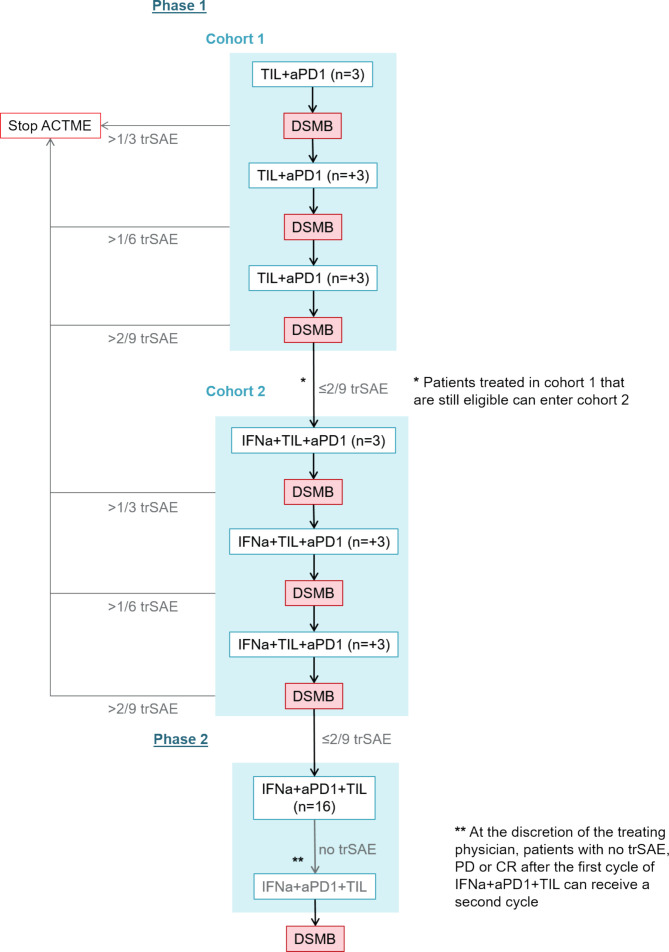
The toxicity of TIL in combination with anti-PD-1, with and without PEG-IFNa, is assessed after the treatment of 9 patients in both groups (see figure 3). The number of patients is based on a set probability of treatment related serious adverse events (trSAE) of less than 35% and was calculated using R 3.4.4 GUI statistical software for a binominal distribution. With the stopping rules as shown in figure 3, the probability is 75% per cohort that accrual stops if the true toxicity is 35%.
Figure 3.
Number of patients treated per cohort and in the two study phases and data safety monitoring during ACTME trial. aPD1, anti-PD-1 treatment; DSMB; Data Safety Monitoring Board; IFNa, peginterferon-alpha2a; TIL, tumour infiltrating lymphocytes; trSAE, treatment-related serious adverse event.
A data safety monitoring board is installed to review the safety after the treatment of each three patients (see figure 3). After completing cohort 2, an interim analysis is performed to assess the efficacy of the combination treatment. The trial will be stopped when less than two patients experience disease control after treating nine patients with PEG-IFNa TIL and anti-PD-1.
Phase II
The main objective of the second stage of this phase I/II study is to assess efficacy of the combination of TIL, anti-PD-1 and PEG-IFNa in patients with metastatic cutaneous melanoma as determined by response rate according to RECIST 1.1.
The sample size is based on Fleming’s design for single-stage phase II trials and A’Hern’s adaptation of the Fleming design.28 29 Patients eligible for this phase I/II clinical trial are refractory to the standard treatment lines. Therefore, a response rate of less than 10% (P0) would not be sufficiently large enough to warrant further investigation. A response rate of 30% (P1) or more would indicate that the combination of anti-PD-1, TIL and PEG-IFNa may be tested in a phase III setting.
Using a one-sided α of 5% and 80% power (β), this requires a total of 25 patients in our study (α=0.05, β=0.20, P0=10%, P1=30%). If 6 or more out of the 25 patients have a response, then there is evidence to proceed to phase III at the end of the study. Calculated with PASS, this gives the following output showing that the actual alpha and beta are within our predefined confines:
| P0 | P1 | Alpha | Beta | Cut-off; R+1 | N | Actual alpha | Actual beta |
| 0.1 | 0.3 | 0.05 | 0.2 | 6 | 25 | 0.033 | 0.193 |
Data analysis plan
The primary focus of the data analysis is to determine the safety of anti-PD-1 and TIL in cohort 1. If two or less patients experience a trSAE, cohort 2 will start. In cohort 2, the primary focus is to determine the safety of anti-PD-1, TIL and PEG-IFNa. If 2 or less patients experience a trSAE, phase II starts. Only patients who completed all three TIL infusions will be included in the analyses.
In phase II, the primary focus of the data analysis is to determine the efficacy of anti-PD-1, TIL and PEG-IFNa. With a one-sided α of 5% and 80% power (β), 6 or more out of the 25 patients have to respond to treatment.
Descriptive statistics are used to summarise patient baseline characteristics at start of study treatment. Survival from start of treatment to progression and death is estimated according to Kaplan-Meier’s method using SPSS V.25.
Paired analyses between FACS data from peripheral blood mononuclear cells (PBMC) of patients before start of anti-PD-1, at the moment of start of PEG-IFNa, at time of the first TIL infusion and after the first treatment cycle are compared using Cytosplore V.2.1.5, R V.3.4.4 and using R-package Cytofast.30
Furthermore, paired and independent analyses are performed on the data generated by FACS analysis on both the T cell products and the PBMC’s by GraphPad Prism V.7.00 for Windows and SPSS V.25. A D’Agostino & Pearson omnibus K2 test are performed to determine whether data are normally distributed within groups. To compare paired data following a normal distribution, a paired t-test is used; when the assumption of normality is violated, a Wilcoxon signed rank test is performed. For unpaired data following a normal distribution, a unpaired t-test is used; when the assumption of normality is violated, a Mann-Whitney U test is performed.
Ethics and dissemination
Results from our trial could increase the efficacy of ACT by overcoming four of the previously described most important aspects curtailing the efficacy and feasibility of current immunotherapies. Our outcomes will therefore be communicated to the community of oncologists treating patients with ACT during (inter)national scientific conferences, and by publication of the results in an open-access peer-reviewed international journal, the Dutch Oncology up-to-date-magazine and via the website of the Dutch Melanoma Foundation.
All patients have to give written informed consent to a member of the study team before inclusion in the ACTME study. This study is conducted according to the principles of the Declaration of Helsinki (Declaration of Helsinki, 64th WMA General Assembly, Fortaleza, Brazil, October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO). The protocol is approved by the Central Committee on Research Involving Human Subjects in the Netherlands and has been prospectively registered in the U.S. National Library of Medicine (NCT03638375).
An electronic case report form is made using Castor Electronic Data Capture, where all data on patient eligibility, treatment cycles and clinical parameters will be collected by trained staff-members of the Medical Oncology Department. The clinical trial will be monitored approximately twice a year by an independent monitor.
Patient and public involvement
Patients were involved in the design of the protocol. Patient representatives from the Dutch Melanoma Foundation will be invited to identify the key messages that need to be disseminated.
Discussion
Current research has shown that immunotherapy with immune checkpoint inhibition is not sufficient for approximately 60% of patients. New combinations have to be implemented to overcome the mechanisms hampering current standard of care treatment options. In this phase I/II trial, we tackle the four most important aspects curtailing the efficacy and feasibility of current immunotherapies. We hypothesise that anti-PD-1 in combination with TIL and PEG-IFNa provides and maintains more activated tumour-reactive T cells, thereby improving clinical outcome while hospitalisation is not required due to the acceptable toxicity profile.
We hope to complete the enrolment of the trial by mid-2023, with a 14-week follow-up first data expected by the end of 2023.
Supplementary Material
Acknowledgments
Figure 1 was created with BioRender.com.
Footnotes
Contributors: MKvdK, EMEV, EK and SHvdB designed the clinical study. G-JL performed surgery and provided the resected tumour. PMM performed QP and QC checks of the production process and of the infusion product. ICFMR, MAJ, FMS and EK treated the patients. MKvdK, EMEV, MV, LdB and CEvdM produced the infusion product and performed all analyses. MKvdK designed the patient database. MKvdK, ICFMR, MAJ, SvdB collected data in the patient database. MMvdK wrote the original manuscript and all other authors reviewed and edited the final manuscript.
Funding: Bristol-Myers Squibb supplies the nivolumab (anti-PD-1).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 2.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005;23:2346–57. 10.1200/JCO.2005.00.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008;26:5233–9. 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550–7. 10.1158/1078-0432.CCR-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988;319:1676–80. 10.1056/NEJM198812223192527 [DOI] [PubMed] [Google Scholar]
- 7.Verdegaal E, van der Kooij MK, Visser M, et al. Low-dose interferon-alpha preconditioning and adoptive cell therapy in patients with metastatic melanoma refractory to standard (immune) therapies: a phase I/II study. J Immunother Cancer 2020;8:e000166. 10.1136/jitc-2019-000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdegaal EME, Visser M, Ramwadhdoebé TH, et al. Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-alpha. Cancer Immunol Immunother 2011;60:953–63. 10.1007/s00262-011-1004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008;8:299–308. 10.1038/nrc2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sim GC, Chacon J, Haymaker C, et al. Tumor-infiltrating lymphocyte therapy for melanoma: rationale and issues for further clinical development. BioDrugs 2014;28:421–37. 10.1007/s40259-014-0097-y [DOI] [PubMed] [Google Scholar]
- 12.Rohaan MW, van den Berg JH, Kvistborg P, et al. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer 2018;6:102. 10.1186/s40425-018-0391-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JC. Toxicities associated with adoptive T-cell transfer for cancer. Cancer J 2015;21:506–9. 10.1097/PPO.0000000000000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innamarato P, Kodumudi K, Asby S, et al. Reactive myelopoiesis triggered by Lymphodepleting chemotherapy limits the efficacy of adoptive T cell therapy. Mol Ther 2020;28:2252–70. 10.1016/j.ymthe.2020.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarnaik A, Khushalani NI, Chesney JA, et al. Long-term follow up of lifileucel (LN-144) cryopreserved autologous tumor infiltrating lymphocyte therapy in patients with advanced melanoma progressed on multiple prior therapies. J Clin Oncol 2020;38(15_suppl):10006. [Google Scholar]
- 16.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 2005;115:1616–26. 10.1172/JCI24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 2010;16:2646–55. 10.1158/1078-0432.CCR-10-0041 [DOI] [PubMed] [Google Scholar]
- 18.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–82. 10.1056/NEJMoa020047 [DOI] [PubMed] [Google Scholar]
- 19.Kartal ED, Alpat SN, Ozgunes I, et al. Adverse effects of high-dose interferon-alpha-2a treatment for chronic hepatitis B. Adv Ther 2007;24:963–71. 10.1007/BF02877700 [DOI] [PubMed] [Google Scholar]
- 20.Posthuma EFM, Marijt EWAF, Barge RMY, et al. Alpha-interferon with very-low-dose donor lymphocyte infusion for hematologic or cytogenetic relapse of chronic myeloid leukemia induces rapid and durable complete remissions and is associated with acceptable graft-versus-host disease. Biol Blood Marrow Transplant 2004;10:204–12. 10.1016/j.bbmt.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST Committee. Eur J Cancer 2016;62:132–7. 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med 2018;24:144–53. 10.1038/nm.4466 [DOI] [PubMed] [Google Scholar]
- 24.Santegoets SJAM, Dijkgraaf EM, Battaglia A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol Immunother 2015;64:1271–86. 10.1007/s00262-015-1729-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welters MJ, van der Sluis TC, van Meir H, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 2016;8:334ra52. 10.1126/scitranslmed.aad8307 [DOI] [PubMed] [Google Scholar]
- 26.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003;281:65–78. 10.1016/S0022-1759(03)00265-5 [DOI] [PubMed] [Google Scholar]
- 27.Melief SM, Visconti VV, Visser M, et al. Long-Term survival and clinical benefit from adoptive T-cell transfer in stage IV melanoma patients is determined by a Four-parameter tumor immune signature. Cancer Immunol Res 2017;5:170–9. 10.1158/2326-6066.CIR-16-0288 [DOI] [PubMed] [Google Scholar]
- 28.A'Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med 2001;20:859–66. 10.1002/sim.721 [DOI] [PubMed] [Google Scholar]
- 29.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics 1982;38:143–51. 10.2307/2530297 [DOI] [PubMed] [Google Scholar]
- 30.Beyrend G, Stam K, Höllt T, et al. Cytofast: A workflow for visual and quantitative analysis of flow and mass cytometry data to discover immune signatures and correlations. Comput Struct Biotechnol J 2018;16:435–42. 10.1016/j.csbj.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044036supp001.pdf (66.3KB, pdf)