Abstract
Objective
The objective of our study was to explore the perspectives of patients and general practitioners (GPs) regarding interventions to increase initiation of cholesterol lowering medication (or statins), including a proposed laboratory-based facilitated relay intervention.
Design
Qualitative descriptive study using interviews and focus groups for data collection, and thematic analysis for data analysis.
Setting
Primary care providers and patients in Calgary, Alberta, Canada.
Participants
17 GPs with primarily community-based, non-academic practices with at least 1 year of practice experience participated in semistructured interviews. 14 patients at high risk of cardiovascular disease participated in focus groups.
Main outcome measures
Exploration of strategies that might be used to enhance the prescription of, and adherence to statin therapy for patients with statin-indicated conditions.
Results
GPs proposed a variety of interventions to improve statin prescription, including electronic record audit solutions, GP directed education, and patient-oriented campaigns. Patients expressed that they may benefit from being provided access to their laboratory test results, as well as targeted education. Both parties provided positive feedback on the proposed laboratory-based facilitated relay intervention, while pointing out areas for improvement. Notably, GPs were concerned that the patient-directed component of the intervention might jeopardise therapeutic relationships, and patients were concerned about accidental disclosure of personal health information. Important considerations for the design of facilitated relay messaging should include brevity, simplicity and the provision of contact information for inquiries.
Conclusions
GPs and patients described several suggestions for increasing statin initiation and welcomed the proposal of a laboratory-based facilitated relay strategy. These findings support further testing of this intervention which may enhance GPs’ ability to successfully engage patients in cardiovascular risk reduction through statin therapy.
Keywords: qualitative research, general medicine (see internal medicine), quality in health care, cardiology, preventive medicine
Strengths and limitations of this study.
This is a qualitative study, with relatively few participants—therefore, we cannot say definitively if the views represented here represent those of all patients and prescribers.
We sampled physician participants to the point of saturation, which means that we are confident the views represented here span the breadth of those held by physicians.
The patient sample we recruited may not be representative of the broader population, as many of them had previously stated an interest in quality improvement and research—and this group was not sampled to saturation.
Given the context-dependent nature of qualitative data, the applicability of these findings to other settings is not certain.
By collecting qualitative data through open-ended questions, we were able to record detailed accounts and opinions.
Introduction
Vascular disease, including coronary artery disease, peripheral artery disease and cerebrovascular disease, remains among the leading causes of mortality worldwide.1 A class of medications, 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, commonly known as statins, have proven to be effective for lowering the risk of vascular events.2 Individuals who have previously had vascular disease (ie, secondary prevention) derive a greater absolute risk reduction from statins than those who have never had vascular disease (ie, primary prevention).3 There are some individuals who have never had vascular disease, such as those with diabetes or chronic kidney disease, who have also been shown in randomised controlled trials to benefit from therapy.4–6 Despite over 30 years of clinical use, efficacy, safety and cost-effectiveness data,7 8 only 23%–55% of individuals who would benefit take this medication and fewer than half of individuals are treated to target cholesterol levels.7 9–11 There is substantial unwanted variability in dyslipidaemia management, and health system intervention is required to promote equitable treatment.12 13 The lack of statin treatment for patients with indicated conditions results in significant excess morbidity and mortality. In Canada, specifically, if all patients with indications for statins were treated, this would result in nearly 40 000 averted cardiovascular events annually.14 In the USA, 13% of cardiovascular deaths could be prevented with statin adherence among patients at high cardiovascular risk.15
Physicians and patients face numerous barriers when it comes to prescribing and adhering to statin therapy, from the providers perspective this includes lack of knowledge, conflicting clinical guidelines, and a lack of systems to identify patients who should be taking statins.16 On the other hand, patients often experience or fear side effects, or are simply averse to taking additional medications.16 Furthermore, patients that face social disadvantages such as low income, lack of health insurance, and minority race are more likely to not use statins.17 A large US-based survey found that side effects were common and that many former statin users were unsatisfied with the explanation provided by their prescriber about the importance of the medication.18 Providers need resources to help them provide this counselling to patients and to arm them with strategies to mitigate common statin side effects, like muscle aches.19
There are clearly many challenges that lead to the observed clinical treatment gap for patients who have indications for statin treatment. However, some studies have shown that such treatment gaps, in related conditions like hypertension, can be closed using quality improvement strategies.20–22 Integrated quality improvement strategies that target both patients and healthcare providers are more likely to achieve quality indicators than strategies which only target one aspect in isolation.21 One such strategy is facilitated relay. Facilitated relay is a quality improvement strategy whereby information about individual patients is sent directly to healthcare providers through a means other than the usual clinical encounter.23 Despite the establishment and promotion of facilitated relay and other quality improvement strategies, there remain significant treatment gaps in hypertension24 and other chronic conditions.25 Furthermore, while facilitated relay has been shown to be effective in improving a number of cardiovascular risk factors,21 26 it remains among the least commonly used quality improvement strategies27 and has not been explored in the management of dyslipidaemia.
For an intervention to have the potential to yield maximum impact, it is important to qualitatively seek the input of key stakeholders prior to the application of any intervention.28 This allows for the development of a higher quality intervention, rather than one that relies on physician feedback alone.29 As such, the objective of our study was to explore the perspectives of both patients and general practitioners’ (GPs) regarding interventions to increase cholesterol lowering medication (or statin) prescription, including specific feedback on a proposed laboratory-based facilitated relay intervention.
Methods
Study design
We conducted a qualitative descriptive study28 to explore patients’ and GPs’ perspectives on interventions to increase initiation of statins for cardiovascular risk reduction and treatment of high cholesterol in those at high cardiovascular risk. In addition to generic thoughts on potential hypothetical interventions, we specifically sought directed feedback and perceptions on the acceptability of the proposed facilitated relay intervention from both patients and GPs.30 We used the consolidated criteria for reporting qualitative research (COREQ) as the reporting framework for this study.31
Proposed intervention
We drew from behaviour change theory to develop a facilitated relay intervention to increase statin prescriptions32–34 (figure 1). Our proposed intervention partners with our province’s single unified laboratory system to identify individuals who have elevated cholesterol levels, statin-indicated conditions, and who are not currently filling prescriptions for statins. Our lab system has access to province-wide administrative databases, including laboratory, pharmacy dispensation and hospitalisation data. For every elevated low density lipoprotein (LDL)-cholesterol level, the lab would have an algorithm that would check the patients’ records for evidence of statin-indicated conditions (administrative markers of myocardial infarction, stroke, diabetes or chronic kidney disease), and would then identify if the patient has recently filled a statin prescription. This is possible because of province-wide, linkable databases. For patients who are not filling statins, but who should be, their GP (who had ordered the cholesterol level) and the patient, would then each receive a letter outlining the indication for treatment and the potential to benefit from statin therapy. The patient letter will encourage them to speak to their GP, and the GP letter will encourage them to make an appointment to discuss directly with the patient—both with the objective to initiate or renew statin prescriptions. We felt that it was important to include patients in the facilitated relay to empower them in discussions with their GP and to enable shared decision-making,35 which has been demonstrated to improve adherence with statins.36
Figure 1.
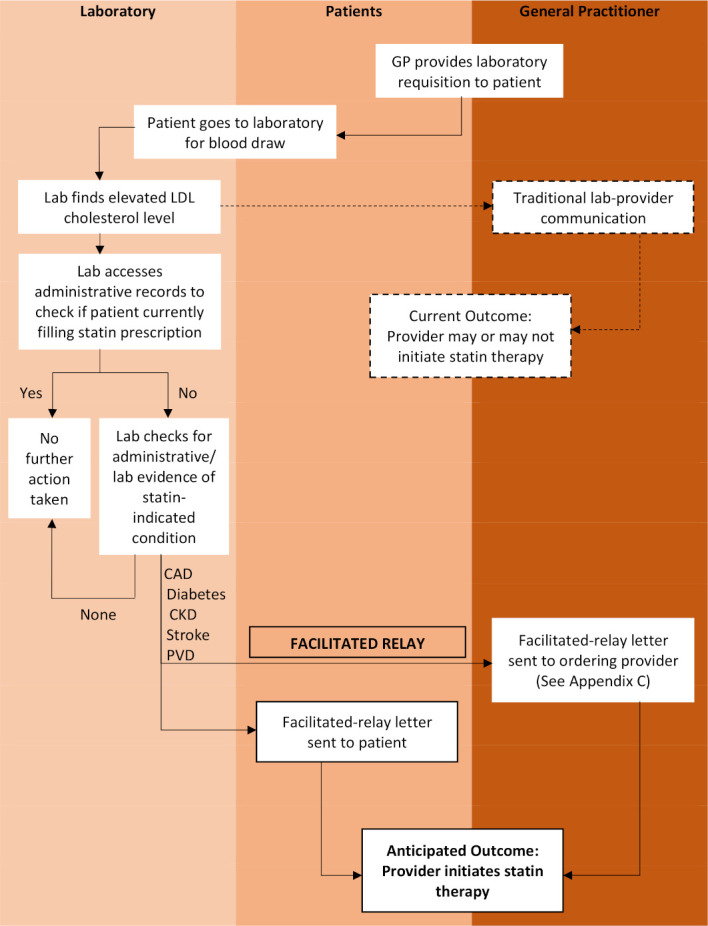
Laboratory-based facilitated-relay intervention. Dashed lines: traditional interface between lab and ordering provider. CAD, coronary artery disease; CKD, chronic kidney disease; GP, general practitioner; LDL, low density lipoprotein; PVD, peripheral vascular disease.
Participant recruitment
General practitioners
We recruited GPs to participate in individual interviews, using a snowball sampling approach. First, we asked key stakeholders in areas of primary care, endocrinology, nephrology and cardiology affiliated with the university medical centre, to recommend community-based (non-academic) GPs to participate in the study. Individuals were then contacted by telephone and email with a formal invitation to participate. GPs who met the following criteria were enrolled: (1) currently practising in community general practice settings; and (2) at least 1 year of experience working as a GP in independent practice. We sampled participants purposively based on several key demographic characteristics in order to achieve representation across a range of ages, genders and practice types.
Patients
We recruited patients who would qualify as recipients of the proposed intervention. Specifically, we were interested in recruiting those at high risk of cardiovascular disease, who self-reported a prior history of high cholesterol, preferably with coexisting vascular disease (myocardial infarction, stroke or peripheral vascular disease), diabetes or chronic kidney disease. Using a convenience sampling approach, we invited patients who were part of an established advisory panel and previously agreed to be contacted about research opportunities for study participation.37 38 In addition, patients were recruited using poster advertisements placed throughout the academic health sciences centre and in various clinical care areas where care is provided to patients with diabetes, heart disease and kidney disease.
Data collection
Data were collected from September 2018 to November 2018 using both qualitative semistructured interviews (with GPs) and focus groups (with patients). We chose focus groups for patients as rich personal disclosures are more likely to occur in this setting than in individual interviews.39 However, we purposely used individually scheduled interviews to offset potential aversion to focus groups by community-based GPs due to their competing clinical demands. Furthermore, we wanted to recruit from both urban and rural locales which is more challenging to do in a focus group.
Question guides
Both focus groups and interviews were informed by question guides (see online supplemental appendix A and B) which were developed based on a review of the literature40 41 and discussion with the research team. These were designed so that they initially asked study participants what they thought would be effective strategies or interventions to improve statin uptake (ie, prescribing, patient use and adherence). After they had given their unprompted views, participants were then given a brief explanation of facilitated relay, the specifics of the proposed intervention (figure 1), and they were shown a copy of the proposed intervention letter for GPs (see online supplemental appendix C). After briefing participants on the principles and practices of facilitated relay and showing them our preliminary documents for the intervention, we asked them to provide feedback on this proposed intervention.
bmjopen-2020-038469supp001.pdf (82.2KB, pdf)
bmjopen-2020-038469supp002.pdf (620KB, pdf)
Provider interviews
All interviews were conducted in-person (in clinician offices) or via telephone, by a female trained research assistant (RCWL) with oversight by experienced study team members (DJTC and SB). Physician interviews were continued until the point of theoretical saturation when no new information emerged from the interviews.42 Because the research objective was relatively focused, interviews were brief and lasted approximately 30–45 min.
Patient focus groups
None of the study team were acquainted with or involved in the clinical care of the patients who participated. We convened two focus groups in our academic medical centre which each lasted approximately 90 min. No one but researchers (including one facilitator and two field-note takers) and participants were present. Focus group facilitators tried to ensure that there were no dominant members and provided all participants with equal opportunity to voice their opinions.
Interviews and focus group proceedings were digitally audio-recorded and transcribed verbatim by a professional transcriptionist. Field notes were recorded to inform data analysis. All data were anonymised and stored securely. Signed informed consent was received from each study participant. Gift cards were provided to all participants. Ethics approval was granted from the University’s Health Research Ethics Board.
Data analysis
Analysis was completed using conventional qualitative content analysis,43 a method of interpreting interview data with the goal of describing the phenomenon of interest. Transcripts for the initial three interviews were reviewed by three team members (DJTC, RCWL and SB), with the objective of inductively establishing a preliminary coding template that was used for subsequent data analysis. All transcripts were then analysed by two reviewers (DJTC and RCWL). Codes were generated from the interview data and systematically applied to identify themes and patterns. The process was iterative, reflexive and interactive as continual data collection and analysis shaped each other. For example, code titles or definitions identified based on earlier interviews were modified according to the data collected during subsequent interviews. The team met together to review the coding to elicit discussion about the coding strategy and attempted to achieve consensus to resolve coding discrepancies. NVivo V.12 (Doncaster, Australia) qualitative data analysis software was used to facilitate the coding process.
Patient and public involvement
Patient partners and family members from the Libin Cardiovascular Institute’s established patient and family member advisory group44 voiced that prevention was one of their top research priorities for cardiovascular health. This work is related to prevention of cardiovascular disease. Patients were included in focus groups.
Results
In total, we eventually reached out to 27 GPs to invite them to participate, 4 declined to participate, 4 did not respond to the invitation, 19 were scheduled for interviews, with 2 cancelling. We reached saturation after having completed 17 individual GP interviews (table 1A). The majority were women (88%) with 65% having graduated from medical school within the last 10 years. All GPs spent more than 50% of their time in clinical practice, most were in urban centres within primary care networks (PCNs). PCNs are networks of GPs that share interdisciplinary resources to enhance the delivery of primary care within geographical regions45; they are associated with improved chronic disease care and outcomes.46
Table 1A.
Descriptive statistics for general practitioners (n=17)
| Physician characteristics | Total (%) |
| Age (years) | |
| <40 | 13 (76) |
| 40–60 | 4 (24) |
| Gender | |
| Man | 2 (12) |
| Woman | 15 (88) |
| Years of primary care practice | |
| <10 | 14 (83) |
| 10–20 | 3 (18) |
| Years since medical school graduation | |
| <10 | 11 (65) |
| ≥10 | 6 (35) |
| Primary care network membership | |
| Yes | 15 (88) |
| No | 2 (12) |
| Location of primary care practice | |
| Urban | 13 (76) |
| Rural | 4 (24) |
| Focused practice interest | |
| Yes* | 9 (53) |
| No | 8 (47) |
| Clinical practice last 12 months | |
| Estimated number of patients at high CVD risk | |
| <20 | 1 (6) |
| 20–99 | 7 (41) |
| ≥100 | 9 (53) |
| Use of endocrinology consultation services | |
| Yes | 5 (29) |
| No | 12 (71) |
| Use of cardiology consultation services | |
| Yes | 10 (59) |
| No | 7 (41) |
| Use of nephrology consultation services | |
| Yes | 3 (18) |
| No | 14 (82) |
| Proportion of patients in their practice who would be considered high risk on the basis of cardiovascular risk factors (n=14) | Mean: 32% |
| Range 10%–75% | |
| Proportion of high-risk patients in their practice who have a current LDL-level on file (n=9) | Mean: 82% |
| Range 70%–90% | |
*Focused practice, or special interest types: care of the elderly (n=2), emergency medicine (n=1), urgent care (n=1), refugee medicine (n=1), obstetrics (n=2), indigenous health (n=2), lactation (n=1).
CVD, cardiovascular disease; LDL, low density lipoprotein.
Our patient focus groups had eight and six participants, respectively (table 1B). There was a range of ages represented among patients, with a similar number of men and women. Nearly all had a GP and were also followed by medical specialist(s). The conditions represented in our patient group were diabetes, history of myocardial infarction and elevated cholesterol level; none reported a history of stroke, chronic kidney disease or peripheral arterial disease.
Table 1B.
Descriptive statistics for patient participants based on self-report (n=14)
| Patient characteristics | Total (%) |
| Age (years) | |
| <40 | 2 (15) |
| 40–60 | 5 (39) |
| >60 | 6 (46) |
| Gender | |
| Men | 6 (46) |
| Women | 7 (54) |
| Chronic condition qualifying as ‘high CVD risk’ | |
| High cholesterol only | 3 (23) |
| Diabetes only | 6 (46) |
| Myocardial infarct (MI) only | 1 (8) |
| Diabetes and MI | 3 (23) |
| Has a primary care provider | |
| Yes | 12 (92) |
| No | 1 (8) |
| Followed by a medical specialist | |
| Yes | 10 (77) |
| No | 3 (23) |
| Self-reported awareness of high cholesterol levels | |
| Yes | 11 (85) |
| No | 2 (15) |
| Current use of statin medication | |
| Yes | 6 (46) |
| If not, had spoken with physicians about statins | 3 (23) |
| If not, had not spoken with physicians about statins | 4 (31) |
One participant did not complete a demographic questionnaire.
CVD, cardiovascular disease.
General suggestions for potential interventions
Several themes arose regarding interventions to improve statin initiation during the unprompted portion of the interviews (table 2). GPs described that statin prescribing may be improved by: (1) enhancing aspects of physician education to promote appropriate statin prescribing; and (2) implementation of support tools to help physicians in decision-making and identification of patients for whom statins are indicated. In addition, patients suggested that having access to their own laboratory results may enable them to be more effective self-advocates.
Table 2.
General suggestions by general practitioners (GPs) and patients to increase initiation of statins
| Providers | Treatment of specific subpopulations | Patients with chronic kidney disease: |
| “I struggle with the GFRs [glomerular filtration rate] – knowing when it would be safe, when it wouldn’t be safe. I do get confused as to the dosing based on GFR.” (GP-05) | ||
| Patients who previously experienced side effects with statin(s): | ||
| “I have one strategy but if somebody is still like ‘no, it’s completely not tolerable for me’ then I don’t know what the next step is after that.” (GP-13) | ||
| Elderly patients: | ||
| “…getting some better understanding about the elderly. Are there any contraindications to starting on statin therapy? Is there one statin that may be more beneficial than another?” (GP-10) | ||
| Patients with hypertriglyceridaemia: | ||
| “I always find it hard to know what to do with triglycerides… more education around how to manage those [patients].” (GP-15) | ||
| Treatment to targets* | “Most people in my office are confused about what we are doing in terms of treating to the target of 2 mmol/L, because the cardiologist is still sending consults about that, but then we have these family medicine evidence-based groups saying that targets don’t matter”. (GP-02) | |
| “I know the TOP [Towards Optimized Practice] guidelines don’t necessarily correlate with CCS [Canadian Cardiovascular Society] guidelines, so there are several schools of thought”. (GP-09) | ||
| “There’s no real way to unify the guidelines, but to have an education session on why they’re different and how to approach it so maybe you’ll break down patient populations that fit better with one guideline vs another”. (GP-08) | ||
| Preferred modality of education | “we have a lot of drug reps [representatives] coming to town, so it would be great to have more [education] that was not pharma, absolutely”. (GP-04) | |
| EMR-based tools | “One thing that would be helpful for me is if there was some automatic flag that came when I saw a patient that would alert to the fact that their treatment is not optimized for their conditions”. (GP-06) | |
| Patients | Laboratory results | “I would like to get a copy, in addition to the doctor. I can do with it what I want” (Pt-09) |
| “It gets you questioning things so that you can come back to your doctor and say ‘I saw these numbers, what does that mean? What do I need to do?’”(Pt-02) | ||
| Enhanced education | “What if somebody was going regularly to a lab, and a clinician sort of goes: ‘How are you doing on this?’”. (Pt-08) |
*Specialist guidelines, the 2016 Canadian Cardiovascular Society guideline52 advocates that patients at high risk (based on risk calculators) or those with ‘statin-indicated conditions’ (defined as diabetes, chronic kidney disease or pre-existing vascular disease be treated with statin therapy to achieve a target LDL-c level of < 2.0 mmol/L. GP guidelines, the 2015 TOP Alberta Guideline61 encourages GPs to treat high-risk patients with moderate-to-high intensity statins and should not repeat lipid levels, or attempt to treat to a fixed target.
EMR, electronic medical record; LDL, low density lipoprotein.
GP education
Nearly all GPs highlighted that there are general areas of knowledge that could be bolstered in order to enhance statin prescription. One of the main content areas in which they sought enhanced education related to the treatment of specific patient subpopulations, in particular those with chronic kidney disease, patients who have had prior statin intolerance/side-effects, elderly patients and those with other concurrent lipid disorders (ie, hypertriglyceridaemia).
Whether providers should be treating patients to a specific cholesterol level was a major source of confusion. They frequently referenced receiving conflicting advice, including a contradiction in clinical practice guidelines,47 some of which advocate for a ‘fire and forget’ approach,8 48 while Canadian7 and European49 specialist guidelines recommend a ‘treat-to-target’ approach.7
Regarding the modality of education sessions, most preferred in-person education sessions delivered at their clinics and delivered by someone who did not have clear conflicts of interest with pharmaceutical companies. Many GPs also suggested the use of handouts, tools or algorithms to simplify their decision-making process.
GP tools
In addition to education, several GPs suggested that the use of automated tools would facilitate their prescribing of statins. Most felt that they would benefit from optimising the use of their electronic medical records (EMRs) to ‘flag’ individuals who were at high cardiovascular risk or had elevated cholesterol levels. Other GPs spoke of wishing for a ‘running list’ of eligible patients, while some mentioned using an employee or contractor designated as a panel manager to perform these tasks.
Patient results and information
Many patients independently indicated that they would like to have access to their lipid test results, without needing to rely on this being conveyed to them by their GP. Some patients also suggested that providing them with their own results might reduce the frequency of unnecessary follow-up visits; and as a result, alleviate related financial burden on the healthcare system. Doing so was also thought to help foster patient engagement with their GP.
Patients also felt that having greater access to information about cholesterol and treatment might facilitate more patients being on statin therapy. Suggestions were made to deliver this through enhanced patient-facing materials (ie, brochures), as well as pharmacists or lab technicians who were able to discuss results and treatment options. Further information about patient education, shared decision-making and clinical decision support tools are described in our other report from this work.16
Feedback on the proposed facilitated relay intervention
Emerging themes regarding our proposed intervention were organised into four major categories: (1) general feedback and impression; (2) suggested changes; (3) intervention details; and (4) workflow processing considerations.
General feedback and impression
GPs responded to the proposed intervention with strongly positive feedback (table 3), which included stating that they found the information to be helpful and direct. They generally felt that the letter was written in a clear fashion and with a respectful tone. Several mentioned that the information provided them with reassurance and credibility in making recommendations to their patients.
Table 3.
Positive and negative feedback on facilitated relay intervention from general practitioners (GPs) and patients
| General practitioners | Patients | ||
| Positive | |||
| Composition | “Overall I thought it was worded quite well and was very clear” (GP-08) | Provides structure to interaction | “My doctor would be okay with that. It gives them a little checklist of things to talk about”. (Pt-05) |
| “I think it’s appropriate, it didn’t take me very long to get through” (GP-16) | |||
| Tone | “it’s written in a way that doesn’t make you feel stupid, I guess” (GP-11) |
Enhances communication |
“I think that’s good ‘cause these doctors, some guys don’t communicate”. (Pt-13) |
| “it’s good because [it’s] not telling you to do this [start statin therapy], but telling you to have a conversation”. (GP-17) | |||
| Credibility | “it gives family physicians more confidence to do those things and know the specialists are behind them in that recommendation” (GP-02) | Increases doctor accountability | “I think it keeps them [doctors] honest as well. They should actually be proactive in terms of having that information already, but that’s not always the case. So I don’t have a problem with a patient having all their information at their disposal”. (Pt-14) |
| “there’s so much information for people to sift through… if you can get valid information that’s corroborated and consistent, that’s helpful” (GP-15) | |||
| Direct | “it’s a good idea… it tells you what to do, which is great. You don’t have to look up the guideline every time” (GP-04) | Increases patient accountability | “If [patients] are encouraged to work with their doctor to monitor your numbers, you have a bit of control as well as the doctor… like working together”. (Pt-03) |
| “it’s just one of those extra little reminders that takes the brain power out of the work you have to do day-to-day” (GP-06) | |||
| Information | “[side effects] are what people hear about in the news a lot, so it’s very helpful to have some numbers around it, and strategies to address that” (GP-09) | Provides peace of mind | “It gives me a little peace of mind in that we’ve talked about all of the things that are important and that should be covered… that we haven’t left anything out”. (Pt-05) |
| “All the suggestions that you made are excellent. I’m reading through this and I’m like ‘oh yeah, I didn’t realize this’ and ‘this is something I can do for some of my patients’’’ (GP-12) | |||
| Negative | |||
| Increased workload | “I would caution against anything that causes more documents or more paperwork… there’s already so much” (GP-16) | Privacy concerns | “You know what, my doctor isn’t going to send it out to me, anyway. It’s going to go on to a receptionist, who might pass it on to somebody else in the office, so there’s no guarantee of privacy there” (Pt-05) |
| “Privacy is always an issue. I mean it’s like, the less information that’s out there about you, the better off you are, period. I don’t care what it is” (Pt-07) | |||
| Disclosing new diagnoses | “my concern is that they get this information from a letter… my preference would be that it came straight to me” (GP-01) | Difficulty interpreting results | “Some people might know all the numbers and everything else, I don’t. You give me a bunch of numbers, it means nothing to me. So unless the doctor explained it to me… I’d rather talk to my doctor” (Pt-07) |
| Therapeutic relationship | “If the patient gets a letter that’s like ‘you need to be on a statin’ and we already had a conversation that they didn’t need a statin. That could cause some issues in the therapeutic relationship”. (GP-04) | Provoking anxieties | “There are people who are coming down with every disease known to man, so for someone like that, that kind of information would just send them off the deep-end, right?” (Pt-05) |
| Logistical concerns | “What if a person gets a check from a walk-in clinic? My concern is then is that walk-in clinic docs are just going to ignore this letter” (MD-05) | Lack of engagement | “You mentioned mail outs and things like that… have they proven to be effective, though, ‘cause how many people read them? How many people understand them? I don’t think there would be a lot of point in it, ‘cause I don’t think people pay that much attention” (Pt-09) |
| “If it goes to the patient, sometimes you get lots of mail and they may just discard it” (MD-10) | Sense of intimidation | “Some will [say] ‘I can’t talk to my doctor like that’. There will be some people who might be intimidated to initiate that conversation” (Pt-03) | |
However, GPs also voiced some questions and potential concerns after hearing about our proposed intervention. These concerns included whether the introduction of a facilitated relay intervention might increase their workload, lead to possible disclosure to patients of new diagnoses of conditions that qualified them as high risk (ie, diabetes) and pose a threat to their therapeutic relationships with patients. In addition, logistical issues around how the letter would be best delivered to ordering providers and patients were raised as concerns.
Patients generally felt that bringing their facilitated relay letter to a scheduled appointment would be positive in their relationship by providing structure to the follow-up encounter, holding GPs to account and enhancing patient–provider communication. Even though most were generally positive, some patients expressed concern about the facilitated relay intervention, including the possibility for privacy breaches and increasing patient anxieties.
Suggested information to remove or add
We asked GPs specifically what they would like to see changed in the preliminary materials shown. Almost unanimously, they suggested that the letter would be more appreciated if it the two-page document was shortened to fit on one page. Several participants suggested removing the references, mention of clinical studies and guideline citations to make it more reader-friendly. There was also a preference voiced for revising the introductory paragraphs to have direct relevance to individual patient(s):
I’m going to read it for sure, but then when you start to read it, people might put it down and say ‘oh this is a study intervention’, [but] if you have the first thing at the very top: ‘you know this person has been identified as being at risk’—then it’s about the patient rather than being about the studies. (GP-16)
A few GPs voiced opinions that specific additions could be made to improve the letter’s utility. These suggestions included adding: information about health behaviour change (“the whole picture, as opposed to just medication” (GP-04)); adding contact information for a specialist; and details about how/why a particular individual was flagged as eligible for the facilitated relay intervention: “It would be helpful if I got a name, condition and then the statin-indicated condition, and where the condition was pulled from”. (GP-01)
Patient feedback was notable for also suggesting that the intervention provide contact information, in case they have further questions about interpreting their results: “back that up with a helpline for somebody that doesn’t know what the [results] mean” (Pt-10). Similar to physicians, patients expressed a strong preference for brevity: “If I have to go through 14 pages of information to figure out what that means, I’m sorry, I don’t have time for that” (Pt-07). However, numerous patients also stressed the importance of not only providing results or diagnoses, but also giving some basic education and an action plan to follow.
Intervention details
In addition to general feedback, we also explicitly asked GPs whether they would prefer to receive information about their patient in the form of facilitated relay (individual letter for each patient identified) or ‘audit and feedback’ (summary report including a group of their patient panel). A summary list or report (audit and feedback) was preferred by roughly 2/3 of the GPs interviewed. Regarding receiving letters for each patient, participants stated:
this is going to get tiresome very quickly (GP-05)
Am I going to get this letter 20 times? I’m probably just going to read it once (GP-03)
[a list would] decrease paper burden, decrease the chance of it getting misplaced (GP-13)
While the audit and feedback approach was more popular, some GPs were clearly in favour of facilitated relay: “I can’t even think of the amount of work it would take to do it patient-specific. I’d love it. Sure go for it, if you have the means to do it, then why not?” (GP-10)
We also asked pointedly about how providers would feel about receiving a follow-up reminder from the study team, if patients had not filled the prescription as recommended in the initial letter. The response was split with roughly half of the GPs stating that a reminder would not be necessary. Those who felt a reminder would be acceptable generally agreed that a 6-month window should be sufficient to ascertain whether or not the patient would have started on therapy: “There are people that have a three month wait list time, you may have to pick an interval more like six months to appeal to the masses…”. (GP-13)
Most patients felt that they would benefit from receiving a follow-up reminder. After considerable discussion among the groups, consensus was achieved that follow-up should not happen prior to 4 months, and possibly even as long as 6 months after the initial contact. One participant stated: “close enough that I vaguely remember that I meant to do something with that, but not a few weeks later, [so] it’s not irritating”. (GP-17)
We also asked patients if they had a preference for who had signed the letter. Most felt that having letters come from a local specialist in cardiology or endocrinology would be preferable to having them signed by a GP.
Workflow processing considerations (GPs only)
To each GP we asked specific details about how our intervention letter would be received in their offices and what would happen on receipt. The majority stated that such a letter would be opened and processed by their front-desk staff. One participant clarified that the information on the envelope would determine who opened it: “if it’s addressed to me then it will come to me, if it has a patient name for me, then it goes through our document people [who file it]”. (GP-09)
Once the letter has been opened, different offices employed a variety of different processes. In many practices, it would be given directly to the GP; while in others it would be scanned directly into a patient’s file in an EMR, yet in others, the hardcopy would be filed in a patient’s chart.
In terms of the preferred delivery modality, most GPs felt that electronic delivery directly via the EMR platform would be the preferred method of receiving the intervention. However, a number still felt that conventional delivery via paper mail or fax would be preferable. Even those who expressed a preference for conventional delivery, many elaborated that such letters would often be scanned into a patient’s electronic file: “if it was to come by mail or fax, then they have to scan it onto the computer” (GP-11). A few GPs described systems which can do this process automatically: “our office works with a new web system, so everything that comes in via the fax actually goes directly into the computer and they then allocate to the patient”. (GP-11)
Discussion
While statins have a more limited role in certain populations (low risk and those with limited life expectancy),50 51 they are important for the prevention of cardiovascular disease in patients who have previous vascular disease and in those with diabetes and kidney disease.4–6 52 In this study, both GPs and patients acknowledged that there is the potential to improve the prescription and use of statin therapy among those at high risk for cardiovascular disease. In unprompted questions, GPs acknowledged that there was a need for improved physician education on this topic, and that tools to help identify and track patients would be helpful. Patients also suggested that directly receiving laboratory test results and information on treatment options may result in better medical care, generally supporting our hypothesis that facilitating shared decision making was a key element of a novel intervention. When shown the proposed intervention, both groups were strongly supportive of the facilitated relay intervention. While there were clear benefits to the intervention, some potential downsides were raised by both GPs and patients. In general, all recipients would prefer letters to be succinct, yet contain high-yield information and provide contact information where clarification could be sought.
Many interventions have been attempted to address the problem of statin underuse. A number of patient-centred approaches have been tried with varying success.22 While active forms of education, like cognitive education and behavioural counselling seem to work,53 more passive forms of education are often unsuccessful at changing behaviour, as in a recent trial where the intervention comprised of a mail and phone education strategy to encourage patients to take prescribed medication had no impact on adherence.54 Others have found that multifaceted interventions focusing on enhancing care provision through team-based care may be effective at increasing statin adherence.55
However, when trying to target the problem of low statin prescribing, interventions directed only at patients are not likely to work. An alternate approach is to facilitate GPs ability to identify and prescribe statins, to those in whom they are appropriate,56 through audit and feedback or facilitated relay. An educational audit and feedback intervention regarding dyslipidaemia treatment in Italian primary care practices was shown to increase adherence to statins by approximately 10%.57 Improved communication and shared decision-making, which are explicit goals of facilitated relay interventions, can improve patient adherence.58 While these and other studies have reviewed the clinical efficacy of quality improvement strategies,21 few have used detailed qualitative methods as we have done. One large qualitative study interviewed audit and feedback experts to generate hypotheses about the various factors that may contribute to the efficacy of such interventions.59 Others have used qualitative methods to highlight the barriers physicians face in encouraging adherence,60 but ours is unique in using such methods to design and develop an intervention to address these challenges. Finally, we also appreciate that as much as there is underuse of statins, there is also overuse in certain groups, for example, in people with short life expectancy. Perhaps interventions to increase initiation may also include a component that conveys statin benefits are measured in years rather than months.
The fact that participants suggested elements of our facilitated relay intervention in the unprompted portion of the interviews lends credibility and face validity to the proposed intervention. However, it is notable that while GPs felt they would benefit from having internal systems to monitor patients’ records, none independently suggested a strategy mediated by an independent third party (such as facilitated relay or audit and feedback), as we have proposed. Investigators who wish to implement facilitated relay interventions to enhance adherence to medical therapies can use the findings of this study to help develop interventions that are more likely to be acceptable to both GPs and patients. One of the main findings is to ensure that any information provided is brief and high yield, containing patient identifiers early to capture GP’s attention. Such interventions can be strengthened by incorporating education on controversial or little-known topics. Patients strongly preferred any correspondence to also contain direct suggestions or an action plan. Workflow and processing of these letters need to be considered and interventions designed to be as minimally disruptive to clinical practice as possible—with most physicians preferring that it be embedded directly within the EMR; yet in healthcare settings (like ours) where there is marked heterogeneity in the use and type of EMRs, this may not be possible.
There are limitations to this study. First, as in most qualitative studies, the number of participants was relatively small. This concern over sample size is mitigated by the fact that physician interviews proceeded until the point of saturation. Patient data were not collected in this manner, and these themes may not be fully saturated and we appreciate this as a limitation. Furthermore, the patient sample we recruited may not be representative of the broader population, as many of them had previously stated an interest in quality improvement and research and therefore may be attuned to the importance of preventive therapies more than other members of the general public. Second, given the context-dependent nature of qualitative data, the applicability of these findings to other settings is not certain. Yet physicians in most settings face similar problems (ie, time constraints, patient complexity and comorbidities and patient resistance to medical therapies) in numerous facets of medical care; therefore, it is conceivable that the findings of this study would apply to interactions between patients and GPs in other clinical settings. Due to time constraints of participants and researchers, member checking was not undertaken in this study. Finally, it is important to note that feedback was sought specifically about the proposed intervention. However, given the details reported, we feel that these findings are likely to be helpful to others proposing similar quality improvement interventions. One of the major strengths of this study is the depth and richness of the qualitative data that were collected. By asking questions in an open-ended manner, we were able to record detailed accounts and opinions. Another strength of this work is the fact that we sought patient input into the development of this intervention, rather than relying on physician feedback alone.
Statin therapy has been demonstrated to effectively lower cholesterol and reduce the risk of cardiovascular events and death in individuals at high risk of cardiovascular disease. Despite this, they remain underused. There are patient, provider and system factors that contribute to statin underuse. Facilitated relay interventions hold promise as a potential method to address this important care gap. Our study sought perspectives of both healthcare providers and patients, which will be incorporated into intervention design to maximise acceptability. The findings from this qualitative data will be used to improve the likelihood of success and achieve the desired clinical impact. The insights about these interventions are also likely to be of interest to many researchers and clinicians who are considering and designing provider-facing and/or patient-facing interventions to improve the uptake of preventive medications.
Supplementary Material
Footnotes
Twitter: @Rachelle9307, @Sonia_ButaliaMD
Contributors: DJTC, RCWL-K, KMB, TA, HQ, AAL, GC, ML, CN and SB collaborated to develop the research question and methods. The study design was conceived by DJTC and SB. DJTC wrote the first draft of the study protocol. Data collection and analysis were completed by DJTC, RCWL-K and SB. KMB, TA, HQ, AAL, GC, ML and CN contributed to the interpretation and contextualisation of study findings. The first draft of the manuscript was written by DJTC. RCWL-K, KMB, TA, HQ, AAL, GC, ML, CN and SB contributed substantively to further revisions of the manuscript and have consented to the publication of this version.
Funding: Funding for this project was provided by a research grant to Sonia Butalia from Diabetes Canada.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. Given that qualitative data are not deidentified and tell individuals’ personal stories, data cannot be shared beyond the scope of this project, as per our research ethics board.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.World Health Organization The top 10 causes of death, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 2.Silverman MG, Ference BA, Im K, et al. . Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–97. 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 3.Byrne P, Cullinan J, Smith SM. Statins for primary prevention of cardiovascular disease. BMJ 2019;367:l5674. 10.1136/bmj.l5674 [DOI] [PubMed] [Google Scholar]
- 4.Colhoun HM, Betteridge DJ, Durrington PN, et al. . Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96. 10.1016/S0140-6736(04)16895-5 [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Landray MJ, Reith C, et al. . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. 10.1016/S0140-6736(11)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins R, Armitage J, Parish S, et al. . MRC/BHF heart protection study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–16. 10.1016/s0140-6736(03)13636-7 [DOI] [PubMed] [Google Scholar]
- 7.Anderson TJ, Grégoire J, Pearson GJ, et al. . 2016 Canadian cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–82. 10.1016/j.cjca.2016.07.510 [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Stone NJ. 2018 American heart association/American college of cardiology/multisociety guideline on the management of blood cholesterol-secondary prevention. JAMA Cardiol 2019;4:589–91. 10.1001/jamacardio.2019.0911 [DOI] [PubMed] [Google Scholar]
- 9.Butalia S, Lewin AM, Simpson SH, et al. . Sex-based disparities in cardioprotective medication use in adults with diabetes. Diabetol Metab Syndr 2014;6:117. 10.1186/1758-5996-6-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson TJ, Grégoire J, Hegele RA, et al. . 2012 update of the Canadian cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–67. 10.1016/j.cjca.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 11.Johansen ME, Green LA, Sen A, et al. . Cardiovascular risk and statin use in the United States. Ann Fam Med 2014;12:215–23. 10.1370/afm.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamble JM, Butalia S. Medical practice variations in diabetes mellitus : Johnson A, Stukel T, Medical practice variations. health services research. Springer, 2015: 1–40. [Google Scholar]
- 13.Tu JV, Chu A, Maclagan L, et al. . Regional variations in ambulatory care and incidence of cardiovascular events. CMAJ 2017;189:E494–501. 10.1503/cmaj.160823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennessy DA, Tanuseputro P, Tuna M, et al. . Population health impact of statin treatment in Canada. Health Rep 2016;27:20–8. [PubMed] [Google Scholar]
- 15.Yang Q, Zhong Y, Gillespie C, et al. . Assessing potential population impact of statin treatment for primary prevention of atherosclerotic cardiovascular diseases in the USA: population-based modelling study. BMJ Open 2017;7:e011684. 10.1136/bmjopen-2016-011684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butalia S, Lee-Krueger RCW, McBrien KA, et al. . Barriers and facilitators to using statins: a qualitative study with patients and family physicians. CJC Open 2020. 10.1016/j.cjco.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroff P, Gamboa CM, Durant RW, et al. . Vulnerabilities to health disparities and statin use in the REGARDS (reasons for geographic and racial differences in stroke) study. J Am Heart Assoc 2017;6:1. 10.1161/JAHA.116.005449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JD, Brinton EA, Ito MK, et al. . Understanding statin use in America and gaps in patient education (usage): an Internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15. 10.1016/j.jacl.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Jacobson TA, Khan A, Maki KC, et al. . Provider recommendations for patient-reported muscle symptoms on statin therapy: insights from the understanding statin use in America and gaps in patient education survey. J Clin Lipidol 2018;12:78–88. 10.1016/j.jacl.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 20.Worrall G, Chaulk P, Freake D. The effects of clinical practice guidelines on patient outcomes in primary care: a systematic review. CMAJ 1997;156:1705–12. [PMC free article] [PubMed] [Google Scholar]
- 21.Tricco AC, Ivers NM, Grimshaw JM, et al. . Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–61. 10.1016/S0140-6736(12)60480-2 [DOI] [PubMed] [Google Scholar]
- 22.Rash JA, Campbell DJT, Tonelli M, et al. . A systematic review of interventions to improve adherence to statin medication: what do we know about what works? Prev Med 2016;90:155–69. 10.1016/j.ypmed.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 23.Walsh J, McDonald KM, Shojania KG, et al. . Closing the quality gap: a critical analysis of quality improvement strategies (vol 3: hypertension care. Rockville, MD: AHRQ Technical Reviews, 2005. [PubMed] [Google Scholar]
- 24.Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension 2017;70:736–42. 10.1161/HYPERTENSIONAHA.117.09801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardine MJ, Kasiske B, Adu D, et al. . Closing the gap between evidence and practice in chronic kidney disease. Kidney Int Suppl 2017;7:114–21. 10.1016/j.kisu.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh JME, McDonald KM, Shojania KG, et al. . Quality improvement strategies for hypertension management: a systematic review. Med Care 2006;44:646–57. 10.1097/01.mlr.0000220260.30768.32 [DOI] [PubMed] [Google Scholar]
- 27.Campbell DJT, Sargious P, Lewanczuk R, et al. . Use of chronic disease management programs for diabetes: in Alberta's primary care networks. Can Fam Physician 2013;59:e86–92. [PMC free article] [PubMed] [Google Scholar]
- 28.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health 2000;23:334–40. [DOI] [PubMed] [Google Scholar]
- 29.Pope C, van Royen P, Baker R. Qualitative methods in research on healthcare quality. Qual Saf Health Care 2002;11:148–52. 10.1136/qhc.11.2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayala GX, Elder JP. Qualitative methods to ensure acceptability of behavioral and social interventions to the target population. J Public Health Dent 2011;71:S69–79. 10.1111/j.1752-7325.2011.00241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 32.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7:37. 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michie S, Johnston M, Francis J, et al. . From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008;57:660–80. 10.1111/j.1464-0597.2008.00341.x [DOI] [Google Scholar]
- 35.Barrett B, Ricco J, Wallace M, et al. . Communicating statin evidence to support shared decision-making. BMC Fam Pract 2016;17:41. 10.1186/s12875-016-0436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weymiller AJ, Montori VM, Jones LA, et al. . Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med 2007;167:1076–82. 10.1001/archinte.167.10.1076 [DOI] [PubMed] [Google Scholar]
- 37.Santana M, Zelinsky S, Ahmed S, et al. . Working together with patients and clinician researchers to improve cardiovascular health. Ottawa, ON: SPOR Summit, 2018. [Google Scholar]
- 38.Santana M, Zelinsky S, Ahmed S, et al. . Working together: Co-designing priorities for patient-oriented cardiovascular research by patients, clinicians, and researchers. Halifax, NS: Canadian Association for Health Services and Policy Research (CAHSPR), 2019. [Google Scholar]
- 39.Guest G, Namey E, Taylor J, et al. . Comparing focus groups and individual interviews: findings from a randomized study. Int J Soc Res Methodol 2017;20:693–708. 10.1080/13645579.2017.1281601 [DOI] [Google Scholar]
- 40.Kedward J, Dakin L. A qualitative study of barriers to the use of statins and the implementation of coronary heart disease prevention in primary care. Br J Gen Pract 2003;53:684–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep 2013;15:291. 10.1007/s11883-012-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker JL. The use of saturation in qualitative research. Can J Cardiovasc Nurs 2012;22:37–46. [PubMed] [Google Scholar]
- 43.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 44.Santana M-J, Zelinsky S, Ahmed S, et al. . Patients, clinicians and researchers working together to improve cardiovascular health: a qualitative study of barriers and priorities for patient-oriented research. BMJ Open 2020;10:e031187. 10.1136/bmjopen-2019-031187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drew P, Jones B, Norton D. Team effectiveness in primary care networks in Alberta. Healthc Q 2010;13:33–8. 10.12927/hcq.2010.21813 [DOI] [PubMed] [Google Scholar]
- 46.Manns BJ, Tonelli M, Zhang J, et al. . Enrolment in primary care networks: impact on outcomes and processes of care for patients with diabetes. CMAJ 2012;184:E144–52. 10.1503/cmaj.110755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tibrewala A, Jivan A, Oetgen WJ, et al. . A comparative analysis of current lipid treatment guidelines: nothing stands still. J Am Coll Cardiol 2018;71:794–9. 10.1016/j.jacc.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 48.Toward Optimized Practice (TOP) Cardiovascular Disease Risk Working Group Prevention and management of cardiovascular disease risk in primary care clinical practice guideline, 2015. Available: http://www.topalbertadoctors.org/download/1655/Lipid%20Pathway%20CPG.pdf?_20150916105016
- 49.Catapano AL, Graham I, De Backer G, et al. . 2016 ESC/EAS guidelines for the management of dyslipidaemias. Rev Esp Cardiol 2017;70:115. 10.1016/j.rec.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 50.Kristensen M, Christensen PM, Hallas J. The effect of statins on average survival in randomised trials, an analysis of end point postponement. BMJ Open 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abramson JD, Rosenberg HG, Jewell N, et al. . Should people at low risk of cardiovascular disease take a statin? BMJ 2013;347:f6123. 10.1136/bmj.f6123 [DOI] [PubMed] [Google Scholar]
- 52.Anderson TJ, Grégoire J, Pearson GJ, et al. . 2016 Canadian cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–82. 10.1016/j.cjca.2016.07.510 [DOI] [PubMed] [Google Scholar]
- 53.Jörntén-Karlsson M, Pintat S, Molloy-Bland M, et al. . Patient-centered interventions to improve adherence to statins: a narrative synthesis of systematically identified studies. Drugs 2016;76:1447–65. 10.1007/s40265-016-0640-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivers NM, Schwalm J-D, Bouck Z, et al. . Interventions supporting long term adherence and decreasing cardiovascular events after myocardial infarction (island): pragmatic randomised controlled trial. BMJ 2020;369:m1731. 10.1136/bmj.m1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Driel ML, Morledge MD, Ulep R, et al. . Interventions to improve adherence to lipid-lowering medication. Cochrane Database Syst Rev 2016;12:CD004371. 10.1002/14651858.CD004371.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaya H, Beton O, Yilmaz MB. How to increase level of patients’ awareness regarding the importance of statins despite the influence of the media? Int J Cardiol 2016;207:164. 10.1016/j.ijcard.2016.01.096 [DOI] [PubMed] [Google Scholar]
- 57.Casula M, Tragni E, Piccinelli R, et al. . A simple informative intervention in primary care increases statin adherence. Eur J Clin Pharmacol 2016;72:227–34. 10.1007/s00228-015-1975-z [DOI] [PubMed] [Google Scholar]
- 58.Wilson SR, Strub P, Buist AS, et al. . Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010;181:566–77. 10.1164/rccm.200906-0907OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colquhoun HL, Carroll K, Eva KW, et al. . Advancing the literature on designing audit and feedback interventions: identifying theory-informed hypotheses. Implement Sci 2017;12:117. 10.1186/s13012-017-0646-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kvarnström K, Airaksinen M, Liira H. Barriers and facilitators to medication adherence: a qualitative study with general practitioners. BMJ Open 2018;8:e015332. 10.1136/bmjopen-2016-015332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toward Optimized Practice Cardiovascular Disease Risk Working Group Prevention and management of cardiovascular disease risk in primary care clinical practice guideline Edmonton. Toward Optimized Practice, 2015. https://top.albertadoctors.org/CPGs/Lists/CPGDocumentList/CVD-Risk-CPG.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038469supp001.pdf (82.2KB, pdf)
bmjopen-2020-038469supp002.pdf (620KB, pdf)


