Abstract
In approximately 10% of patients with acute myocardial infarction (MI), angiography does not reveal an obstructive coronary stenosis. This is known as myocardial infarction with non-obstructive coronary arteries (MINOCA), which has complex and multifactorial causes. However, this term can be confusing and open to dual interpretation, because MINOCA is also used to describe patients with acute myocardial injury caused by ischemia-related myocardial necrosis. Therefore, with regards to this specific context of MINOCA, the generic term for MINOCA should be replaced with troponin-positive with non-obstructive coronary arteries (TpNOCA). The causes of TpNOCA can be subcategorized into epicardial coronary (causes of MINOCA), myocardial, and extracardiac disorders. Cardiac magnetic resonance imaging can confirm MI and differentiate various myocardial causes, while cardiac computed tomography is useful to diagnose the extracardiac causes.
Keywords: Troponin, CT, MR, Acute myocardial infarction
INTRODUCTION
Elevated cardiac troponin in the serum serves as a sensitive and specific marker for the presence of myocardial injury. A rise and fall in serum cardiac troponin levels within two weeks indicates acute myocardial injury (1). Acute myocardial infarction (AMI) is defined as the presence of acute myocardial injury plus at least one of the following findings: symptoms of acute myocardial ischemia, ischemic electrocardiogram (ECG) changes, newly developed pathologic Q waves, and new loss of viable myocardium or wall motion abnormality (1). However, in approximately 10% of AMI cases, angiography does not reveal obstructive coronary artery disease (CAD). This is known as myocardial infarction with non-obstructive coronary arteries (MINOCA), which has complex and multifactorial pathophysiological mechanisms (2). An early cohort study showed that the prognosis in these patients is not as benign, and the all-cause mortality may be as high as 4.7% at 12 months (3). The term MINOCA is used to create a common nomenclature for these groups that can often be overlooked, and to encourage evaluation of underlying causes and appropriate management strategies (4). Therefore, MINOCA is a “working diagnosis” that necessitated the identification of an underlying cause for a more targeted therapeutic approach.
Definition of Troponin-Positive with Non-Obstructive Coronary Arteries and MINOCA
In general, the term MINOCA is used in an all-encompassing context to include all patients fulfilling the universal criteria for AMI without obstructive CAD and no clinically overt cause of presentation at the time of the coronary angiography (4). However, the term MINOCA is also used in a more specific context to describe patients with acute myocardial injury caused by evidence of ischemia-related myocardial necrosis (3,5). This dual interpretation for the term MINOCA can be confusing, particularly during treatment; therefore, the generic form of the term should be replaced with ‘troponin-positive with non-obstructive coronary arteries (TpNOCA).’ The term TpNOCA refers to all conditions with elevated troponin levels (regardless of underlying etiology) plus non-obstructive coronary arteries on the coronary angiography, whereas MINOCA only refers to acute myocardial injury caused by myocardial ischemia (i.e., AMI caused by coronary plaque rupture or occlusion) and non-obstructive coronary arteries on the coronary angiography (3,6).
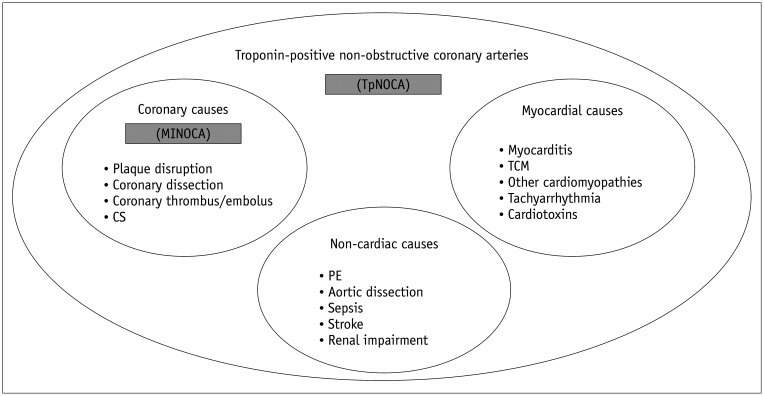
TpNOCA should be subcategorized by epicardial coronary causes (MINOCA), myocardial causes, and extracardiac causes (7). Figure 1 illustrates the definition, various causes, and differentiating features between TpNOCA and MINOCA (3).
Fig. 1. Definition, various causes and differential points of TpNOCA and MINOCA.
CS = coronary spasm, MINOCA = myocardial infarction with non-obstructive coronary arteries, PE = pulmonary embolism, TCM =Takotsubo cardiomyopathy, TpNOCA = troponin-positive non-obstructive coronary arteries
Role of Multimodality Imaging Tools for Evaluation of TpNOCA or MINOCA
When MINOCA is diagnosed during invasive coronary angiography (ICA), the potential causes of MINOCA should be re-evaluated considering the clinical presentations. The ICA may provide coronary etiologies such as coronary thrombi/embolism or dissection. Pharmacologic tests for factors associated with spasm provocation such as ergonovine or acetylcholine can help diagnose coronary artery spasms (7). Performing an ergonovine echocardiography before a coronary angiography is also safe and can be used as a reliable diagnostic screening test for coronary vasospasm in patients with negative treadmill or normal stress myocardial perfusion scan results (8). Furthermore, higher resolution intracoronary imaging at the time of cardiac catheterization with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) may be useful to identify plaque disruption as well as coronary dissection or thrombosis, which may not have been revealed during the ICA (4). Cardiac magnetic resonance imaging (CMR) is known to be a problem-solving tool for the evaluation of MINOCA/TpNOCA because it can confirm or exclude MI (Fig. 2) and can differentiate various myocardial causes such as myocarditis or other cardiomyopathies (9). However, CMR shows normal results in 8–67% of patients with MINOCA/TpNOCA and has limited availability due to requiring a long examination time and being high cost. As an alternative imaging tool, cardiac computed tomography (CT) has the potential ability for evaluating MINOCA/TpNOCA since it can comprehensively analyze the coronary artery anatomy, myocardial perfusion, wall motion of both the ventricles, and extracardiac structures such as pulmonary arteries and the aorta (10). Table 1 summarizes the advantages and limitations of various imaging tools for evaluation of MINOCA/TpNOCA.
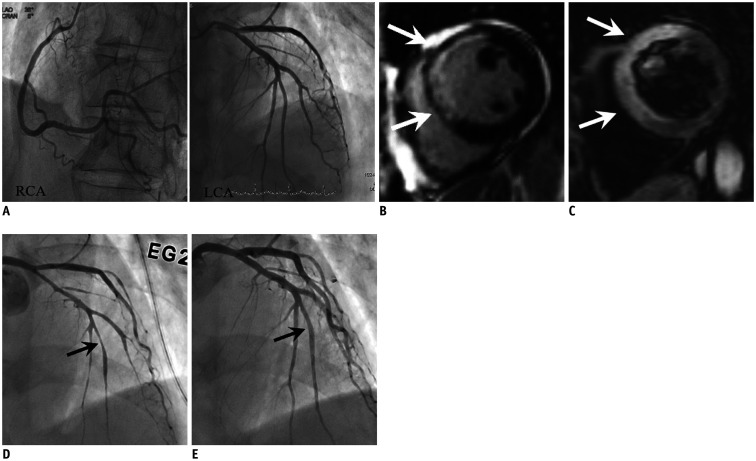
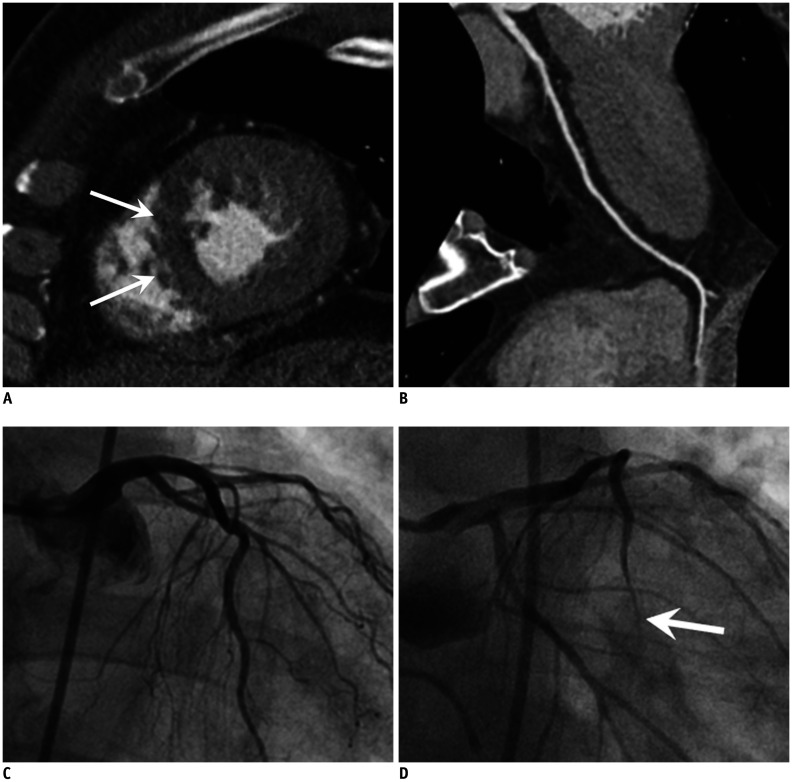
Fig. 2. AMI due to CS in 74-year-old male patient with cardiac arrest after chest pain, elevated troponin (TnI: 4.54 ng/mL) and ECG change (ST elevation in V1–4).
A. Invasive angiograms (RCA and LCA) show no evidence of obstructive coronary artery although increased troponin and ECG abnormality. B, C. Cardiac MR images, which performed due to continuous chest pain, show enhancement at LAD territory (transmural extent 75%) on late gadolinium enhancement (arrows) (B) and hyperintense edema at same territory on T2-weighted image (arrows) (C), indicating AMI of LAD territory. D, E. Thus, invasive coronary angiograms were performed again with ergonovine provocation test and revealed significant stenosis at distal LAD (arrow) (D), which relieved after infusion of nitroglycerin (arrow) (E). AMI = acute myocardial infarction, ECG = electrocardiogram, LAD = left anterior descendingartery, LCA = left coronary artery, RCA = right coronary artery
Table 1. Comparison of Imaging Tools for Evaluation of MINOCA/TpNOCA.
| Modalities | Advantages | Limitations |
|---|---|---|
| ICA | • Detection of obstructive CAD • May detect coronary dissection or thrombus • Can detect vasospastic angina using stress agent (acetylcholine or ergonovine) |
• Invasiveness • Radiation • Contrast related complication • Pharmacological complication |
| Echocardiography | • Lack of exposure to ionizing radiation • Providing both anatomic and functional assessment of heart • Can detect vasospastic angina using stress agent (acetylcholine or ergonovine) |
• Operator dependent • Long duration of testing • Echo-window dependent • Pharmacological complication |
| CMR | • Confirm or exclude MI • Evaluation of various myocardial causes • Can assess risk stratification and expect the prognosis |
• High cost • Long procedure time • Breath holding is required • Normal CMR in 8–67% patients with MINOCA |
| IVUS/OCT | • Excellent spatial resolution • Highly detect coronary causes (plaque disruption or emboli, etc.) |
• Invasiveness |
| CT | • Detection of obstructive CAD • May detect some coronary causes (coronary dissection or thrombus, etc.), although less spatial resolution that IVUS/OCT • May detect some myocardial causes (TCM, hypertrophic cardiomyopathy, etc.), although less diagnostic than CMR • Well detect extracardiac causes of TpNOCA |
• High radiation dose • Contrast toxicity |
CAD = coronary artery disease, CMR = cardiac magnetic resonance imaging, CT = computed tomography, ICA = invasive coronary angiography, IVUS = intravascular ultrasound, MI = myocardial infarction, MINOCA = myocardial infarction with non-obstructive coronary arteries, OCT = optical coherence tomography, TCM = Takotsubo cardiomyopathy, TpNOCA = troponin-positive with non-obstructive coronary arteries
Causes and Pathophysiology of MINOCA/TpNOCA
The causes of MINOCA/TpNOCA are various and heterogenous. In this article, we subcategorized the causes of MINOCA/TpNOCA into three parts: coronary, myocardial, or extra-cardiac causes. Table 2 summarizes the various causes of elevated troponin levels and mechanisms causing MINOCA/TpNOCA.
Table 2. Various Causes of Elevated Troponin and Mechanisms of MINOCA/TpNOCA.
| Etiologies | Mechanism of Troponin Release | Diagnostic Modality |
|---|---|---|
| Coronary causes (MINOCA) | ||
| Coronary plaque disruption | • Myonecrosis with plaque disruption (plaque rupture, ulceration, or erosion) is mediated by thrombosis, thromboembolism or vasospasm • Spontaneous thrombolysis is endogenous protective mechanism against thrombus formation |
• OCT/IVUS • CMR (confirms MI and edema) |
| Coronary embolism | • Myocardial hypoperfusion caused by corresponding coronary embolism | • OCT/IVUS • ICA • Sometimes CT |
| Coronary dissection | • Isolated dissection at coronary artery, leads to myocardial ischemia | • OCT/IVUS • ICA • CT |
| Coronary spasm | • Endothelial injury or ruptured vulnerable plaque cause myocardial injury | • OCT/IVUS • ICA with provocation test • Echocardiography (provocation test) • Sometimes CT |
| Myocardial causes | ||
| Myocarditis or inflammatory cardiomyopathy | • Inflammation causing myocardial injury (viral, bacterial or granulomatous inflammation) | • CMR • PET (particularly, cardiac sarcoidosis) |
| Takotsubo cardiomyopathy | • Myocardial stunning caused by increased stress hormones • Multivessel spasm • Plaque disruption |
• CMR • Sometimes CT |
| Hypertrophic cardiomyopathy | • Myocardial injury caused by interstitial fibrosis or hypoperfusion | • CMR • Sometimes CT |
| Dilated cardiomyopathy | • Myocardial injury resulting from left ventricle strain/overload | • CMR • Sometimes CT |
| Infiltrative cardiomyopathy | • Myocardial injury by amyloidosis, etc. | • CMR |
| Extra-cardiac causes | ||
| Pulmonary embolism | • Myocardial injury resulting from right ventricle strain/overload • Hypoxemia due to perfusion-ventilation mismatch • Hypoperfusion as consequence of low output and reduced coronary blood flow |
• CT • V/Q scan |
| Aortic dissection | • Extended flap from aortic dissection to coronary artery or decreased perfusion leads to myocardial ischemia | • CT |
| Stroke | • Decreased myocardial perfusion after stroke • Associated with increased prevalence of coronary artery diseases |
• Brain MRI • Myocardial SPECT • CMR |
| Sepsis | • Myocardial ischemia which caused by systemic hypoxemia from respiratory failure, hypotension or microcirculatory dysfunction | • Serum inflammatory marker • Myocardial SPECT • CMR |
SPECT = single photon emission CT, V/Q scan = ventilation-perfusion scan
Coronary Disorders (MINOCA)
Coronary Artery Plaque Disruption
Coronary artery plaque disruption has reported in approximately 40% of patients with MINOCA (11). The term of disruption encompasses pathologic findings of plaque rupture, ulceration, or erosion (4). Myocardial injury in MINOCA with plaque disruption is mediated by thrombosis or superimposed vasospasm. One theory explaining non-obstructive coronary stenosis in patients with MINOCA is spontaneous thrombolysis. This process may operate as an endogenous protective mechanism in order to salvage the myocardium (12). However, coronary angiographers may only identify a non-obstructive coronary artery stenosis at the culprit lesion as a result of substantial thrombolysis and/or distal embolization (13). In other words, only a non-obstructive coronary stenosis can remain at the culprit lesion due to spontaneous thrombolysis, even though the critical stenosis might have already been present during the initial rupture of the vulnerable plaque. If there is any clinical suspicion of thrombosis, higher resolution intracoronary imaging modalities such as IVUS or OCT might be the best downstream test, because it can precisely assess the atheromatous fibrous cap integrity (Fig. 3). CMR shows well-defined AMI findings on late gadolinium-enhancement (LGE) imaging as well as edema on T2-weighted imaging at the vascular territory or in a small area of the LGE. This may suggest embolization of the atherothrombotic debris from the disruption site (11). Statin and antiplatelet agents should be prescribed in these patients in order to prevent subsequent cardiac events (12).
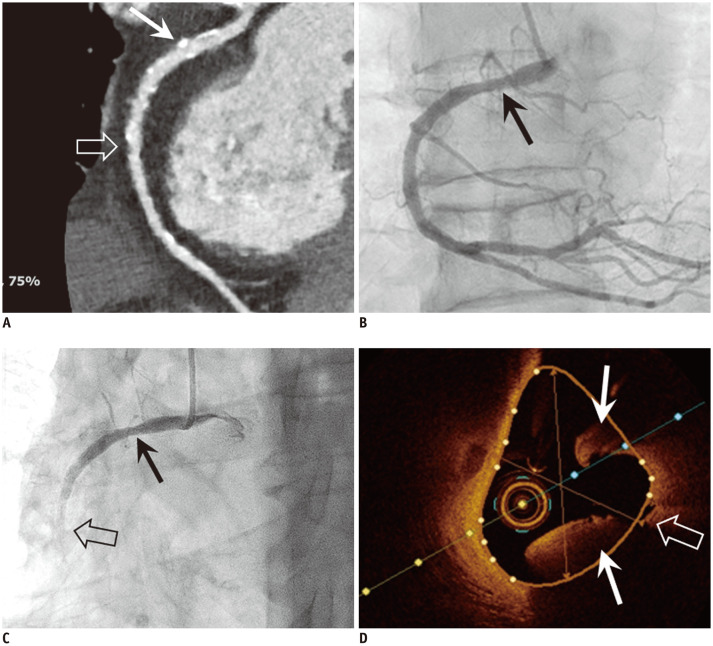
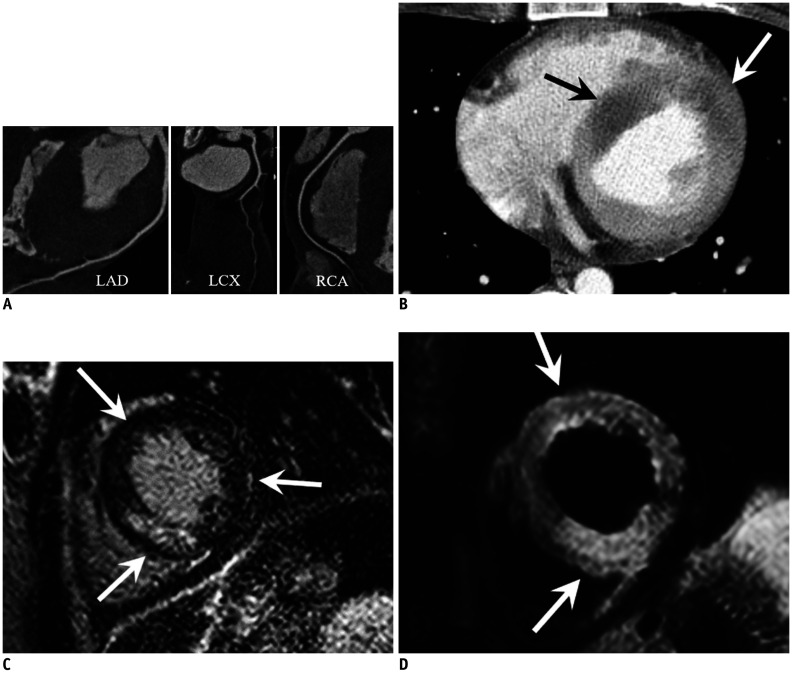
Fig. 3. Coronary artery plaque rupture in 79-year-old male patient with acute chest pain and elevated troponin.
A. Curved multiplanar reformatted CT image of RCA showed mild stenosis due to mixed (open arrow) and calcified plaques (arrow), but no significant stenosis that can explain elevated troponin. B, C. Invasive coronary angiography was done for identification of cause of elevated troponin. Initial invasive coronary angiogram (B) showed insignificant stenosis of proximal RCA (arrow). However, patient complained of acute chest pain and ECG showed ST elevation at inferior leads during angiogram. After that, invasive coronary angiogram (C) showed sluggish flow of contrast media and stenosis of mid RCA (open arrow) was aggravated, while RCA stenosis was maintained (arrow). D. Optical coherence tomogram clearly showed plaque rupture with thrombus formation (arrows) at fissuring (open arrow) of mid RCA. CT = computed tomography
Coronary Thromboembolism
Myocardial hypoperfusion caused by coronary embolism may increase troponin levels. The source of the coronary embolism can be intracardiac or paradoxical (Fig. 4) (14). Causes of coronary embolization include hereditary or acquired thrombophilia syndrome (e.g., protein C or S deficiency, antiphospholipid syndrome, factor V Leiden thrombophilia, and myeloproliferative disease), hypercoagulable status (e.g., atrial fibrillation and valvular heart disease), and emboli from coronary arterial thrombotic and non-thrombotic sources (valvular vegetations, cardiac tumor such as myxoma, calcified valve, and iatrogenic air emboli) (4,14). Therefore, thrombophilia testing should be the first diagnostic step. IVUS or OCT may be useful to identify the coronary emboli which may not have been appreciated during angiography. Echocardiography or CT can assess the embolic source in the cardiac chamber, and the presence of both venous thrombus and right-to-left shunt (patent foramen ovale, atrial septal defect or coronary artery fistula); although it is difficult to assess the intracoronary thrombi (15).
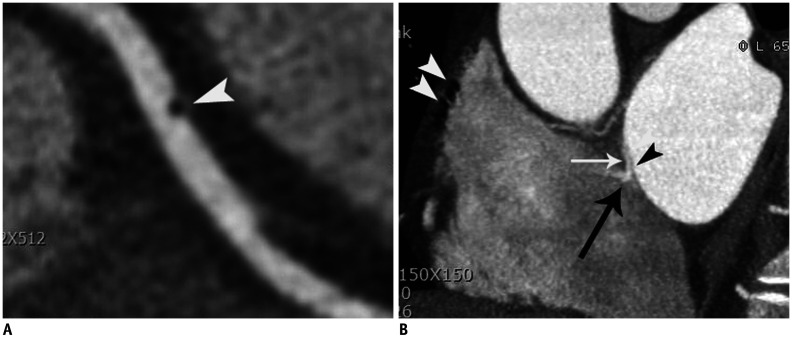
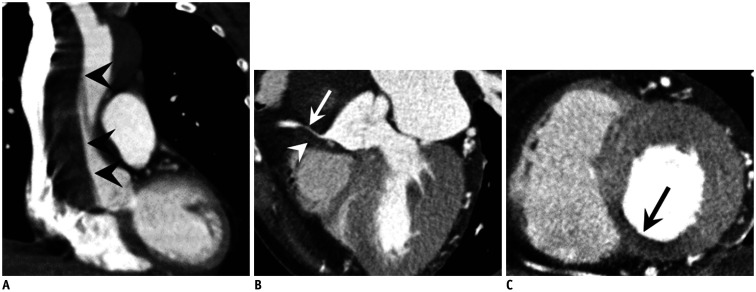
Fig. 4. Coronary thromboembolism in 43-year-old male patient with atypical chest pain who was confirmed as coronary embolism.
A. Curved multiplanar reformatted CT image showed air bubble (arrowhead) in middle RCA. B. Air bubble passed through patent foramen ovale (space between septum primum [black arrowhead] and secondary septum [white arrow]) with contrast jet flow (black arrow). Note that multiple air-bubbles (white arrowheads) are demonstrated in right atrium. Air-bubbles are introduced into right atrium when contrast materials were flushed into right antecubital vein.
Coronary Artery Dissection
Spontaneous coronary artery dissection (SCAD) is a rare entity mainly affecting young women without typical coronary atherosclerotic risk factors (16). Intracoronary imaging is crucial for the diagnosis of SCAD. Fibromuscular dysplasia is most frequently associated disease in patients with SCAD. Aneurysm, a beaded appearance of the involved artery, stenosis, or dissection have been observed in the extra-coronary artery in patients with SCAD. Thus, some authors recommended performing a brain MRI to exclude the possibility of an intracranial artery aneurysm (17).
Coronary Spasm
Coronary spasm (CS) is transient and reversible vasoconstriction of the major epicardial coronary artery, which evoking myocardial ischemia termed as vasospastic angina. The usual clinical presentation of vasospastic angina is recurrent episodes of rest angina that respond promptly to short-acting nitrates, particularly in patients with ECG changes or troponin elevation with circadian patterns (during night or early morning) (18). Thus, ergonovine or acetylcholine provocative spasm tests should be the first down-stream test in patients with clinical presentation indicative of epicardial CS. In the provocation test, a coronary artery diameter reduction of at least 75% is the diagnostic cut-off for epicardial CS accompanied by ischemic symptoms and ECG changes. Although the most frequent form of epicardial CS is a focal spasm in a specific coronary segment, multifocal or multi-vessel forms of epicardial CS can also occur (13). A previous OCT study indicated that plaque rupture, erosion, and thrombus are more frequently identified in patients with spasm-induced acute coronary syndrome (ACS) compared with those with chronic stable variant angina (19). Spasm-induced ACS can be caused by endothelial injury (i.e., plaque rupture, erosion or intraluminal thrombus), with the morphology similar to that of the ruptured vulnerable plaque, while chronic stable variant angina may be caused by smooth muscle hyper-reactivity without any underlying endothelial injury (i.e., absence of the characteristic features of vulnerable plaque, intimal hyperplasia without typical lipid core) (18,19,20). Compared to OCT, CT has limited ability to identify the culprit plaques causing spasm-induced ACS. Kang et al. (21) reported that critical stenosis with negative remodeling without a definite plaque is a typical CT finding of epicardial CS. However, a recent study indicated that coronary CT had low sensitivity in terms of identifying epicardial CS (22). Thus, it should be stressed that myocardial perfusion defects in the subendocardial portion of the corresponding arterial territory may only be a clue for the presence of MINOCA, regardless of the presence of a wall motion abnormality (Fig. 5).
Fig. 5. CS in 65-year-old male patient with acute chest pain and elevated troponin.
A, B. Cardiac CT with short axis view of LV in systolic phase shows hypokinesia and subendocardial hypoperfusion at apical septal wall of LV myocardium (arrows). However, curved multiplanar reformatted image of LAD artery (B) shows no significant stenosis. C, D. Invasive angiograms initially demonstrated no obstructive stenosis. However, the significant stenosis is noted at distal LAD (arrow) (D) after ergonovine provocation test, confirming the CS. LV = left ventricle
Myocardial Disorders
Myocarditis
Typical manifestations of myocarditis include antecedent viral illness, fever, pleuritic chest pain, cardiac failure, or wall motion abnormality of the left and/or right ventricle. With typical clinical presentations supported by an increased level of C-reactive protein and ST elevation on the ECG change, the diagnosis of myocarditis may be straightforward. However, diagnosis of myocarditis is often difficult due to a significant overlap with ACS findings. Although endomyocardial biopsy still represents the gold standard for the diagnosis of myocarditis, according to the recently revised 2018 Lake Louise criteria (23) for the diagnosis of myocarditis, major criteria of myocarditis include: 1) at least T2-based myocardial edema (increase of myocardial T2 relaxation time or an increased signal intensity on T2-weighted images) and 2) at least one T1-based criterion (increased myocardial T1, extracellular volume or LGE) (Fig. 6). Typical LGE patterns of myocarditis include mid-wall or epicardial enhancement. The simultaneous presence of two major criteria on the CMR increases the specificity of diagnosing myocarditis compared with the presence of only one major criteria (23). In the new criteria for myocarditis, pericardial effusion and wall motion abnormality are supportive criteria for the diagnosis of myocarditis. Recently, several reports showed that a delayed cardiac CT, which is performed 10–15 minutes after administration of contrast media, can be an alternative method for myocarditis, particularly in patients with contraindications to CMR (24,25).
Fig. 6. Myocarditis in 23-year-old female with acute chest pain, elevated troponin (TnI: 7.6 ng/mL) and ST elevation at V1–4 on ECG.
A. Curved multiplanar reformatted CT images of three vessels showed no significant stenosis. B. Delayed cardiac CT image obtained 90 seconds after contrast injection shows multifocal hypoperfusion on LV wall (arrows). C, D. Cardiac MR images revealed multifocal patchy enhancement on LV, enhancement is randomly distributed and mainly located at mesodermal or subepicardial area (arrows) (C). T2-weighted image shows hyperintense edema at similar area which corresponding to enhancement (arrows) (D), suggesting acute myocarditis. LCX = left circumflex artery
Takotsubo Cardiomyopathy
Takotsubo cardiomyopathy (TCM) commonly occurs in postmenopausal women with emotional or physical stress. Its clinical presentation is characterized by acute, reversible heart failure associated with myocardial stunning, in the absence of occlusive CAD. The pathophysiological mechanisms for TCM have been hypothesized, including plaque disruption, multivessel spasm, or catecholamine hypersensitivity (26).
The revised Mayo clinic diagnostic criteria include: 1) Transient hypokinesis, akinesia, or dyskinesia of the left ventricular mid segments with or without apical involvement; the regional wall motion abnormalities extend beyond a single epicardial vascular distribution; a stressful trigger is often, but not always present. 2) The absence of obstructive CAD or angiographic evidence of acute plaque rupture (although it is recognized that obstructive CAD may pre-date the Takotsubo event in some cases). 3) New ECG abnormalities (either ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin levels. 4) The absence of pheochromocytoma and myocarditis (27). CMR can be used to establish the diagnosis based on a typical pattern of wall motion abnormality and edema, which resolves in 2–3 months without any myocardial scarring and with complete functional recovery (28). The faint LGE consistent with wall motion abnormalities is occasionally observed, which does not indicate myocardial necrosis but is also known as edema-related LGE (29). CT also can detect typical wall motion abnormalities without obstructive CAD (Fig. 7).
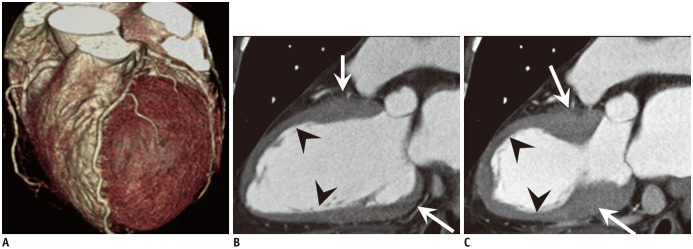
Fig. 7. TCM in 63-year-old female with acute chest pain and elevated troponin (TnI: 1.15 ng/mL).
A. Volume rendering cardiac CT image shows no significant stenosis. B, C. Two-chamber view of diastolic phase (B) and systolic phase (C) show apical ballooning (arrowheads) with akinesia compared to hypercontractile basal wall (arrows), indicating TCM.
Other Cardiomyopathies
In a study by Dastidar et al. (9) assessing a total of 388 consecutive patients with MINOCA undergoing CMR, patients with various cardiomyopathies had the highest mortality, followed by patients with MI and myocarditis. Among the cardiomyopathies, TCM was the most common (43%), followed by dilated cardiomyopathy (29%), hypertrophic cardiomyopathy (18%), and infiltrative cardiomyopathy (amyloidosis, 4%).
CMR with LGE can differentiate various causes of cardiomyopathies; however, the diagnostic yield of CMR varies from 30% to 90% (9,30,31). CT also can assess the morphology of the left ventricle (hypertrophy or dilated chamber), and recent studies showed that delayed iodine enhancement on the cardiac CT may be helpful because it correlated with LGE on the MRI (25).
Extra-Cardiac Disorders
Extra-cardiac TpNOCA disorders include pulmonary embolism (PE), aortic dissection, sepsis, stroke, and end-stage renal failures (4).
Although the reason for elevated troponin levels in the PE is still unclear, acute right ventricular strain secondary to increased pulmonary artery resistance may cause elevated troponin levels in the PE. Other theories for elevated troponin levels in the PE include hypoxemia due to perfusion-ventilation mismatch, hypoperfusion as a consequence of low output and reduced coronary blood flow, or paradoxical embolism from systemic veins to the coronary arteries, usually via a patent foramen ovale (6). The clinical pretest probability such as the Wells PE score and D-dimer is helpful for the diagnosis of PE (32). CT can be used to clearly diagnose acute PE with a high accuracy, and a central filling defect within a vessel surrounded by contrast material is a direct sign of PE (Fig. 8) (33). Sometimes, pulmonary infarction, which is consequence of acute PE, manifests as wedge-shaped, peripheral opacity with a central ground glass and a rim of consolidation.
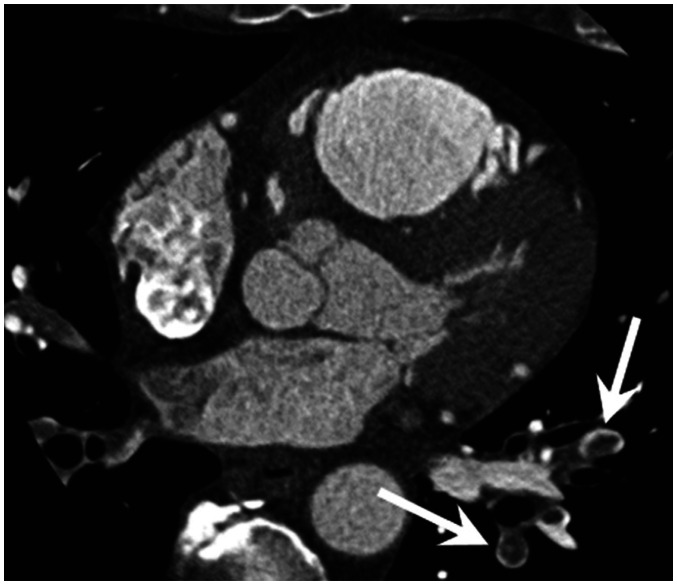
Fig. 8. PE in 49-year-old male with acute chest pain with elevated troponin (TnI: 4.17 ng/mL).
Cardiac CT image shows central filling defect within vessel surrounded by contrast material in left subsegmental pulmonary arteries (arrows), which is direct sign of PE.
An aortic dissection may also cause elevated troponin due to extension of the intimo-medial flap to the coronary artery or decreased myocardial flow leading to damaged myocardial cells. CT is known as the gold standard for diagnosis of an aortic dissection because it can clearly identify the intimo-medial flap and its extension into coronary artery and other branches such as aortic vessels or splanchnic vessels (Fig. 9).
Fig. 9. Aortic dissection in 61-year-old female with acute chest and back pain, and elevated troponin (TnI: 7.14 ng/mL).
A–C. Cardiac CT images show type A aortic dissection (arrowheads in A is dissection flap) involving proximal RCA which consists of contrast filled true lumen (arrow) (B) and hypodense thrombus filled false lumen (arrowhead) (B). Thus, systolic phase short axis view shows hypoperfusion at inferior wall (arrow) (C) of LV caused by myocardial ischemia.
Sepsis, stroke, or end-stage renal failures are causes of MI secondary to ischemia due to an increased oxygen demand or decreased supply (type II) compared to coronary artery occlusion due to plaque rupture (type I). A meta-analysis of 20 studies including 3278 patients with sepsis found that the prevalence of elevated troponin was 43% (34). Myocardial dysfunction during sepsis has been theorized to be caused by global myocardial ischemia, which is caused by systemic hypoxemia due to respiratory failure, hypotension, or microcirculatory dysfunction. This results in the release of troponin from damaged myocardial cells (6). In a meta-analysis of 15 studies including 2901 patients with acute stroke, 18% of the patients had elevated troponin. Although the causes of increased troponin levels in stoke patients have not been elucidated, decreased myocardial perfusion after stroke, or its association with an increased prevalence of CADs have been theorized to cause elevated troponin levels in stroke patients (35). Another mechanism is cardiac abnormalities which are secondary to increased or disturbed sympathetic activity provoked by acute stoke (36).
CONCLUSION
The causes of troponin elevation in MINOCA/TpNOCA are various and heterogeneous. In clinical practice, troponin-positive patients are often assessed by coronary angiography as a standard downstream test. As a problem-solving tool, CMR could be recommended for the evaluation of myocardial causes, while CT is helpful for the detection of extracardiac causes of TpNOCA. Thus, radiologists should understand the pathophysiological mechanism of elevated troponin levels, and be familiar with CT and MR findings for the various causes of MINOCA/TpNOCA.
Footnotes
Bronze award for an education exhibit at the 2019 KCR Annual Meeting (SE 03 CV-02).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 2.Stensaeth KH, Fossum E, Hoffmann P, Mangschau A, Klow NE. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int J Cardiovasc Imaging. 2011;27:355–365. doi: 10.1007/s10554-010-9671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation. 2017;135:1490–1493. doi: 10.1161/CIRCULATIONAHA.117.027666. [DOI] [PubMed] [Google Scholar]
- 4.Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 5.Pasupathy S, Tavella R, Beltrame JF. The what, when, who, why, how and where of myocardial infarction with non-obstructive coronary arteries (MINOCA) Circ J. 2016;80:11–16. doi: 10.1253/circj.CJ-15-1096. [DOI] [PubMed] [Google Scholar]
- 6.Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32:404–411. doi: 10.1093/eurheartj/ehq456. [DOI] [PubMed] [Google Scholar]
- 7.Scalone G, Niccoli G, Crea F. Editor's choice- pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care. 2019;8:54–62. doi: 10.1177/2048872618782414. [DOI] [PubMed] [Google Scholar]
- 8.Song JK, Lee SJ, Kang DH, Cheong SS, Hong MK, Kim JJ, et al. Ergonovine echocardiography as a screening test for diagnosis of vasospastic angina before coronary angiography. J Am Coll Cardiol. 1996;27:1156–1161. doi: 10.1016/0735-1097(95)00590-0. [DOI] [PubMed] [Google Scholar]
- 9.Dastidar AG, Baritussio A, De Garate E, Drobni Z, Biglino G, Singhal P, et al. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging. 2019;12:1973–1982. doi: 10.1016/j.jcmg.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Yoo SM, Chun EJ, Lee HY, Min D, White CS. Computed tomography diagnosis of nonspecific acute chest pain in the emergency department: from typical acute coronary syndrome to various unusual mimics. J Thorac Imaging. 2017;32:26–35. doi: 10.1097/RTI.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. doi: 10.1161/CIRCULATIONAHA.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal SN, Feit F, Mancini GB, Wood D, Patel R, Pena-Sing I, et al. Characteristics of plaque disruption by intravascular ultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am Heart J. 2014;167:715–722. doi: 10.1016/j.ahj.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–481. doi: 10.1093/eurheartj/ehu469. [DOI] [PubMed] [Google Scholar]
- 14.Lee HY, Yoo SM. A case of paradoxical air embolism in the coronary artery through a patent foramen ovale demonstrated by coronary CT angiography. Clin Imaging. 2013;37:167–169. doi: 10.1016/j.clinimag.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Jin KN, Chun EJ, Choi SI, Ko SM, Han MK, Bae HJ, et al. Cardioembolic origin in patients with embolic stroke: spectrum of imaging findings on cardiac MDCT. AJR Am J Roentgenol. 2010;195:W38–W44. doi: 10.2214/AJR.09.3218. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Kaadan MI, Duran JM, Ponzini F, Mishra S, Tsiaras SV, et al. Risk factors, imaging findings, and sex differences in spontaneous coronary artery dissection. Am J Cardiol. 2019;123:1783–1787. doi: 10.1016/j.amjcard.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal V, Kim ESH. Spontaneous coronary artery dissection: cardiac manifestations of vascular disease. Prog Cardiovasc Dis. 2018;60:629–634. doi: 10.1016/j.pcad.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Lerman A, Kwon TG, Lerman LO. Morphological characteristics of coronary arteries in patients with vasospastic angina: another form of atherosclerosis? JACC Cardiovasc Imaging. 2015;8:1068–1070. doi: 10.1016/j.jcmg.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Park HC, Shin JH, Jeong WK, Choi SI, Kim SG. Comparison of morphologic findings obtained by optical coherence tomography in acute coronary syndrome caused by vasospasm and chronic stable variant angina. Int J Cardiovasc Imaging. 2015;31:229–237. doi: 10.1007/s10554-014-0543-4. [DOI] [PubMed] [Google Scholar]
- 20.Shin ES, Ann SH, Singh GB, Lim KH, Yoon HJ, Hur SH, et al. OCT-defined morphological characteristics of coronary artery spasm sites in vasospastic angina. JACC Cardiovasc Imaging. 2015;8:1059–1067. doi: 10.1016/j.jcmg.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Kang KM, Choi SI, Chun EJ, Kim JA, Youn TJ, Choi DJ. Coronary vasospastic angina: assessment by multidetector CT coronary angiography. Korean J Radiol. 2012;13:27–33. doi: 10.3348/kjr.2012.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Kim HK, Park EA, Park JB, Lee SP, Lee W, et al. Coronary computed tomography angiography for the diagnosis of vasospastic angina: comparison with invasive coronary angiography and ergonovine provocation test. Korean J Radiol. 2019;20:719–728. doi: 10.3348/kjr.2018.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 24.Bouleti C, Baudry G, Iung B, Arangalage D, Abtan J, Ducrocq G, et al. Usefulness of late iodine enhancement on spectral CT in acute myocarditis. JACC Cardiovasc Imaging. 2017;10:826–827. doi: 10.1016/j.jcmg.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Im DJ, Youn JC, Chang S, Suh YJ, Hong YJ, et al. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: a prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2017;33:577–584. doi: 10.1007/s10554-016-1024-8. [DOI] [PubMed] [Google Scholar]
- 26.Dastidar AG, Rodrigues JC, Ahmed N, Baritussio A, Bucciarelli-Ducci C. The role of cardiac MRI in patients with troponin-positive chest pain and unobstructed coronary arteries. Curr Cardiovasc Imaging Rep. 2015;8:28. doi: 10.1007/s12410-015-9345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhavan M, Rihal CS, Lerman A, Prasad A. Acute heart failure in apical ballooning syndrome (Tako Tsubo/stress cardiomyopathy): clinical correlates and Mayo clinic risk score. J Am Coll Cardiol. 2011;57:1400–1401. doi: 10.1016/j.jacc.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Perazzolo Marra M, Zorzi A, Corbetti F, De Lazzari M, Migliore F, Tona F, et al. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens' ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm. 2013;10:70–77. doi: 10.1016/j.hrthm.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Avegliano G, Huguet M, Costabel JP, Ronderos R, Bijnens B, Kuschnir P, et al. Morphologic pattern of late gadolinium enhancement in Takotsubo cardiomyopathy detected by early cardiovascular magnetic resonance. Clin Cardiol. 2011;34:178–182. doi: 10.1002/clc.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoudi M, Harden S, Abid N, Peebles C, Nicholas Z, Jones T, et al. Troponin-positive chest pain with unobstructed coronary arteries: definitive differential diagnosis using cardiac MRI. Br J Radiol. 2012;85:e461–e466. doi: 10.1259/bjr/90663866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312–1318. doi: 10.1136/hrt.2010.204818. [DOI] [PubMed] [Google Scholar]
- 32.Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi: 10.1016/S0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 33.Moore AJE, Wachsmann J, Chamarthy MR, Panjikaran L, Tanabe Y, Rajiah P. Imaging of acute pulmonary embolism: an update. Cardiovasc Diagn Ther. 2018;8:225–243. doi: 10.21037/cdt.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim W, Qushmaq I, Devereaux PJ, Heels-Ansdell D, Lauzier F, Ismaila AS, et al. Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med. 2006;166:2446–2454. doi: 10.1001/archinte.166.22.2446. [DOI] [PubMed] [Google Scholar]
- 35.Jensen JK, Kristensen SR, Bak S, Atar D, Hoilund-Carlsen PF, Mickley H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol. 2007;99:108–112. doi: 10.1016/j.amjcard.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Masuda T, Sato K, Yamamoto S, Matsuyama N, Shimohama T, Matsunaga A, et al. Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke. 2002;33:1671–1676. doi: 10.1161/01.str.0000016327.74392.02. [DOI] [PubMed] [Google Scholar]