Abstract
Objective
We aimed to evaluate the diagnostic value and prognostic relevance of FDG positron emission tomography/computed tomography (PET-CT) in extrahepatic cholangiocarcinoma patients.
Materials and Methods
This study included 234 extrahepatic cholangiocarcinoma patients who underwent FDG PET-CT between June 2008 and February 2016. The diagnostic performance of FDG PEG-CT was compared to that of contrast-enhanced multidetector row CT (MDCT) and MRI. Independent prognosticators for poor survival were also assessed.
Results
The sensitivity of FDG PET-CT for detecting primary tumor and regional lymph node metastases was lower than that of MDCT or MRI (p < 0.001), whereas the specificity and positive predictive value for detecting regional lymph nodes metastases was significantly better in FDG PET-CT compared to MDCT and MRI (all p < 0.001). There was no significant difference in the diagnostic yield of distant metastases detection among three diagnostic imaging techniques. In a multivariate analysis, maximum standardized uptake values (SUVmax) of the primary tumor (adjusted hazard ratio [HR], 1.75; 95% confidence interval [CI], 1.13–2.69) and of the metastatic lesions ≥ 5 (adjusted HR, 8.10; 95% CI, 1.96–33.5) were independent contributors to poor overall survival in extrahepatic cholangiocarcinoma patients. In a subgroup analysis of 187 patients with periductal infiltrating type of cholangiocarcinoma, an SUVmax of the primary tumor ≥ 5 was associated with an increased risk of regional lymph node (adjusted odds ratio [OR], 1.60; 95% CI, 0.55–4.63) and distant metastases (adjusted OR, 100.57; 95% CI, 3.94–2567.43) at diagnosis as well as with poor overall survival (adjusted HR, 1.81; 95% CI, 1.04–3.15).
Conclusion
FDG PET-CT showed lower sensitivity for detecting primary tumor and regional lymph node involvement than MDCT and MRI. However, the SUVmax of primary tumors and metastatic lesions derived from FDG PET-CT could have significant implications for predicting prognoses in extrahepatic cholangiocarcinoma patients.
Keywords: FDG PET-CT, Maximum standardized uptake value, Extrahepatic cholangiocarcinoma, Survival
INTRODUCTION
Extrahepatic cholangiocarcinoma is a heterogeneous group consisting of common bile duct (CBD) cancer and hilar cholangiocarcinoma. These tumors have a high mortality rate, and surgical resection with appropriate lymph node dissection is advocated as the curative approach for long-term survival (1). Therefore, accurate diagnostic evaluation and staging are critical to provide appropriate indications for surgery and to prevent unnecessary surgical interventions in those in the advanced stages of the disease.
18F-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography/computed tomography (PET-CT) is a noninvasive imaging modality that allows for the in vivo assessment of metabolic activities underlying malignant disease. It has been shown to be more accurate than CT and MRI for the assessment of several primary and metastatic tumors, including colorectal, lung, esophageal, and biliary cancer (2,3,4). By providing additional information for functional properties of the tumor, FDG PET-CT allows for earlier detection of tumors, identification of occult metastatic disease, characterization of indeterminate lesions, assessment of therapeutic responses, and more accurate staging for potential surgery. Several previous studies have assessed the clinical impact of FDG PET-CT in the work-up of patients with primary and recurrent biliary malignancies (5,6,7,8,9). However, few studies have reported the clinical impact and prognostic relevance of FDG PET-CT for patients with extrahepatic cholangiocarcinoma including CBD cancer and hilar cholangiocarcinoma.
The purpose of the current study was to evaluate the diagnostic performance and prognostic relevance of FDG PET-CT and its maximum standardized uptake value (SUVmax) in patients with extrahepatic cholangiocarcinoma.
MATERIALS AND METHODS
Patients
Two hundred thirty-four patients with confirmed diagnoses of extrahepatic cholangiocarcinoma (including hilar and CBD cancer) who underwent FDG PET-CT at Kangbuk Samsung Hospital between June 2008 and February 2016 were retrospectively and consecutively recruited and analyzed in the current study. The primary work-up of the patients with suspected extrahepatic cholangiocarcinoma included contrast-enhanced multidetector row CT (MDCT), trans-abdominal ultrasound with Doppler imaging, and direct cholangiography obtained by endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic biliary drainage. Referrals for MRI and FDG PET-CT were made on a case-by-case basis after discussion at a specialist multi-disciplinary team meeting. MRI was further performed in 147 (62.8%) patients with ambiguous CT results for determining surgical resection and confirming tumor extent as well as the possibility of vascular invasion and intrahepatic metastases (10). FDG PET-CT was performed to characterize indeterminate primary tumor lesions or to establish baseline staging and suitability for surgical resection where there was some ambiguity in defining abnormal-appearing lymph nodes or distal metastatic lesions seen in the initial primary imaging studies. The diagnosis of each case of cholangiocarcinoma was confirmed by histopathological analyses for surgical specimens or endoscopic biopsies or by follow-up radiologic imaging of more than 6 months. We analyzed imaging studies based on nodal stations, not individual lymph nodes (11). We also performed further histology or radiologic evaluations when PET-CT revealed a newly suspected distant metastasis that was not identified in the initial primary imaging studies (7).
This study was conducted in accordance with the principles of the Declaration of Helsinki. Our study protocol obtained the approval of the Ethics Committee of Kangbuk Samsung Hospital (2013-01-065).
MDCT Examination and Interpretation
All CT images were obtained using 64-row (Brilliance 64, Philips Healthcare) or 128-row (iDose, Philips Healthcare) MDCT scanners. CT protocols were based on effective levels of 50–250 mAs, 100 or 120 kVp, 0.625 or 1.25 collimation, 3- to 5-mm thickness reconstruction at 4- to 5-mm intervals, 0.5- or 0.75-second rotation time, and 2 mL/kg intravenous contrast agent injection after a 60- to 70-second delay, administered at a rate of 2 mL/sec. Images were acquired from the diaphragm dome through the pubic symphysis (12).
All CT interpretations were confirmed by a board-certified abdominal radiologist who was aware of the patient's medical history but unaware of the MRI or PET-CT imaging findings. The primary tumor was classified as malignant on MDCT when there was a mass lesion with heterogeneous peripheral enhancement and gradual centripetal enhancement, marked thickening and enhancement of the bile duct wall, or alterations in duct caliber. Lymph nodes were considered positive if the diameter of the short axis was greater than 1 cm. Lesions in the liver not characteristic of a cyst, hemangioma, or abscess were considered suspicious of metastases. In the lung, pulmonary nodules without calcification were deemed suspicious of metastases.
MRI Examination and Interpretation
MRI was acquired using a 3T whole-body MR system (Intera Achieva 3T; Philips Healthcare) equipped with a dual-source parallel radiofrequency transmission system and quadrature body coil. Baseline MRI included a T1-weighted turbo field-echo in-phase and opposed-phase sequence, breath-hold multishot T2-weighted imaging (T2WI), and respiratory-triggered heavily T2WI.
For gadoxetic acid-enhanced imaging, unenhanced, arterial phase (20–35 seconds), portal venous phase (60 seconds), late-phase (3 minutes) and 20-minute hepatobiliary phase images were obtained using a T1-weighted 3D turbo-field-echo sequence (T1 high-resolution isotropic volume examination; THRIVE, Philips Healthcare) with 2-mm section thickness. Contrast agent was administered intravenously using a power injector at a rate of 1 mL/sec at a dose of 0.025 mmol/kg body weight, followed by a 20-mL saline flush.
All MRI interpretations were confirmed by a board-certified abdominal radiologist who was aware of the patient's medical history and MDCT findings but unaware of the PET-CT imaging results. The primary tumor was classified as malignant on MRI when there was a well-defined mass centered on the central hepatic ducts showing inhomogeneous and progressive enhancement following gadolinium exposure and prominent intrahepatic duct dilatation distal to the tumor, or circumferential thickening and delayed enhancement of the bile duct wall (13). A short axis diameter greater than 1 cm was used to indicate malignant involvement of the node. Peritoneal thickening and enhancement was considered suspicious of peritoneal metastases, and enhanced lesions in lung or liver were also deemed suspicious of metastases (13).
FDG PET-CT Examination and Interpretation
All patients fasted for at least 6 hours before the 18F-FDG PET-CT examination. Blood glucose levels were determined before 18F-FDG administration and patients were rescheduled if their blood glucose level exceeded 130 mg/dL. A range of 370–555 MBq 18F-FDG was injected intravenously. Scanning began 60 minutes later, after voiding. No intravenous contrast agent was used for the CT scans. Imaging and data acquisition were performed using a Discovery STE combined PET-CT system (General Electric Healthcare). A total of six to eight bed positions were acquired, and the acquisition time per bed position was 2 minutes. PET-CT images were reconstructed using iterative reconstruction with two iterations and 14 subsets, resulting in 47 two-dimensional sections spaced 3.27 mm apart over each axial field-of-view increment of 157 mm. The attenuation-corrected PET images, CT images, and co-registered PET-CT images were interpreted simultaneously by a board-certified nuclear medicine physician with knowledge of the patient's medical history and MDCT and/or MRI findings, on an AW workstation with viewing-dedicated software (General Electric Healthcare). The image interpretation was based on identifying regions with increased FDG uptake on the PET images and the anatomic delineation of all FDG-avid lesions on the co-registered PET-CT images. Tumors were defined as positive for FDG uptake if the radioactivity of the tumor was higher than that of the surrounding organs showing physiologic tracer uptake, such as the liver. The images of each biliary tumor were then assessed semi-quantitatively, by calculating the SUVmax normalized to lean body mass. The SUVmax was calculated for the quantitative analysis of tumor 18F-FDG uptake as follows: SUVmax = C (kBq/mL)/ID (kBq)/body weight (kg), where C represents the tissue activity concentration measured by PET and ID indicates the injected dose. All CT images were viewed separately to identify additional lesions without FDG uptake using soft tissue, lung, and bone window leveling (7).
Data Analysis and Statistical Method
Data are expressed as median (range), mean ± standard deviation (SD), or frequency (%). Sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and the accuracy of FDG PET-CT, MDCT, and MRI in detecting primary tumors, regional lymph node metastases, and distal metastases were calculated and compared using the McNemar test. We further analyzed the diagnostic performance of the three imaging modalities by stratification by lesion size. The area under the receiver operating characteristic curve (AUROC) was also calculated for each imaging modality, to assess differences in diagnostic performance between modalities. Survival time was defined as the time interval from the date of the pathologic or clinically confirmed diagnosis until death or the last follow-up. Overall cumulative survival was analyzed following the Kaplan-Meier method with a log-rank comparison. To explore independent and significant contributors to poor overall survival in patients with extrahepatic cholangiocarcinoma, we performed Cox proportional hazard regression analyses. We further performed a subgroup analysis of 187 patients with the periductal infiltrating type of cholangiocarcinoma to reveal whether primary tumors with higher FDG uptake at the time of diagnosis are associated with a higher risk of regional lymph node metastases and distant metastases at diagnosis and with poor overall survival during the follow-up period, using logistic regression and Cox proportional regression analysis, respectively. Variables with p < 0.10 in a first univariate analysis were included in a subsequent multivariate analysis. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc.), and values of p < 0.05 were deemed to be statistically significant.
RESULTS
Characteristics of the Enrolled Patients
The median age of our patients was 72 years (range, 46–99 years), with a sex distribution of 123 males (52.6%) to 111 females (47.4%, Table 1). One hundred fifty-six patients (66.7%) had CBD cancer and 78 patients (33.3%) had hilar cholangiocarcinoma. According to the morphologic characteristics of the primary tumor, conventional imaging and/or cholangiography showed mass-forming types in 38 patients (16.2%), periductal infiltrating types in 187 (79.9%), and intraductal growing types in nine patients (3.8%). The methods for diagnostic confirmation included pathologic examination for surgically-resected specimens in 123 (52.6%) patients, endobiliary forceps biopsy specimens obtained during ERCP in 93 (39.7%) patients, and follow-up radiologic imaging of more than 6 months in 18 (7.7%) patients. The treatment approaches for the enrolled patients included curative surgical resection such as Whipple's operation, pylorus-preserving pancreaticoduodenectomy, or segmental bile duct resection with partial hepatectomy in 120 (51.3%) patients, explorative laparotomy without tumor resection in four (1.7%) patients, and non-surgical treatments such as biliary drainage by metal or plastic stenting and/or systemic chemotherapy in 110 (47.0%) patients.
Table 1. Clinicopathologic Characteristics of Patients.
| Characteristics | Values |
|---|---|
| Total No. of patients (%) | 234 (100) |
| Age (years) | |
| Median (range) | 72 (46–99) |
| Sex (%) | |
| Male:Female | 123 (52.6):111 (47.4) |
| Final diagnosis (%) | |
| Common bile duct cancer | 156 (66.7) |
| Hilar cholangiocarcinoma | 78 (33.3) |
| Gross morphologic type (%) | |
| Mass forming | 38 (16.2) |
| Periductal infiltrating | 187 (79.9) |
| Intraductal papillary growing | 9 (3.8) |
| Differentiation (%) | |
| Well differentiated | 56 (23.9) |
| Moderately differentiated | 95 (40.6) |
| Poorly differentiated | 16 (6.8) |
| Could not be assessed | 67 (28.6) |
| Diagnosis based on (%) | |
| Surgical specimen | 123 (52.6) |
| Endoscopic biopsy | 93 (39.7) |
| Follow-up | 18 (7.7) |
| Treatment modality (%) | |
| Curative intent surgery | 120 (51.3) |
| Explorative laparotomy | 4 (1.7) |
| Medical treatment such as palliative chemotherapy or best supportive treatment | 110 (47.0) |
Values are presented as median (range) and number (percentages) unless otherwise indicated.
Diagnostic Performance of FDG PET-CT in Patients with Extrahepatic Cholangiocarcinoma
One hundred eighty-four (78.6%) out of 234 primary tumor lesions were detected on FDG PET-CT, 222 (94.9%) out of 234 primary tumor lesions on MDCT, and 143 (97.4%) out of 147 primary tumor lesions on MRI. Forty-five (19.2%) primary tumor lesions were detected on MDCT but not on FDG PET-CT, and only seven primary tumor lesions (3.0%) were detected on FDG PET-CT but not on MDCT (p < 0.001; McNemar test). Thirty-three (22.4%) primary tumor lesions were detected on MRI but not on FDG PET-CT and only two primary tumor lesions (1.4%) were detected on FDG PET-CT but not on MRI (p < 0.001; McNemar test). Regional lymph node metastases were confirmed in 94 (40.2%) patients by histopathological analyses for surgical specimens or follow-up radiologic imaging. Regional lymph node metastases were detected in 41 out of 94 (43.6%) patients on FDG PET-CT, in 70 out of 94 (74.5%) patients on MDCT, and in 45 out of 58 (77.6%) patients on MRI, and FDG PET-CT showed a lower sensitivity for the detection of regional lymph node metastases than MDCT (p < 0.001) or MRI (p < 0.001) (Table 2). However, FDG PET-CT showed higher specificity and PPV for the detection of regional lymph node metastases (95.0% and 85.4%) than MDCT (72.1% and 64.2%; all p < 0.001) and MRI (69.7% and 62.5%; p = 0.007 and p = 0.007, respectively). Additionally, ROC curve analyses showed that the diagnostic performance of FDG PET-CT (AUROC, 0.656; 95% confidence interval [CI], 0.573–0.732) was not inferior to that of MDCT (AUROC, 0.713; 95% CI, 0.633–0.785; p = 0.210) or MRI (AUROC, 0.736; 95% CI, 0.657–0.805; p = 0.070) in the detection of regional lymph node metastases (Supplementary Fig. 1A). Distant metastases were confirmed in 20 (8.5%) patients by histopathological analysis of surgical specimens or follow-up radiologic imaging, and detected in 17 out of 20 (85.0%) patients on PET-CT, in 16 out of 20 (80.0%) patients on MDCT, and in 12 out of 13 (92.0%) patients on MRI (Table 2); there were no significant differences in the overall values for sensitivity, specificity, PPV, NPV, and detection accuracy among the three diagnostic imaging techniques. The diagnostic performances of FDG PET-CT (AUROC, 0.901; 95% CI, 0.841–0.944), MDCT (AUROC, 0.912; 95% CI, 0.854–0.852), and MRI (AUROC, 0.935; 95% CI, 0.883–0.969) for the detection of distant metastases were also similar in terms of AUROCs (p = 0.889 and p = 0.618 for FDG PET-CT vs. MDCT and FDG PET-CT vs. MRI, respectively; Supplementary Fig. 1B). Similar results were obtained when stratifying into primary tumor sizes of < 25 mm and ≥ 25 mm (Table 2).
Table 2. 18F-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography (FDG PET-CT) Results for Primary Tumor Detection, Positive Regional Lymph Nodes and Distant Metastases Compared to Multi-Detector Row CT (MDCT), and MRI.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Primary tumor | |||||
| FDG PET-CT | 78.6 (184/234) | - | - | - | - |
| MDCT | 94.9 (222/234) | - | - | - | - |
| MRI | 97.4 (143/147) | - | - | - | - |
| p | < 0.001 | ||||
| Lymph node metastases | |||||
| FDG PET-CT | 43.6 (41/94) | 95.0 (133/140) | 85.4 (41/48) | 71.5 (133/186) | 74.4 (174/234) |
| MDCT | 74.5 (70/94) | 72.1 (101/140) | 64.2 (70/109) | 80.8 (101/125) | 73.1 (171/234) |
| MRI | 77.6 (45/58) | 69.7 (62/89) | 62.5 (45/72) | 82.7 (62/75) | 72.8 (107/147) |
| p | < 0.001 | < 0.001 | < 0.001 | NS | NS |
| Distant metastases | |||||
| FDG PET-CT | 85.0 (17/20) | 95.8 (205/214) | 65.4 (17/26) | 98.6 (205/208) | 94.9 (222/234) |
| MDCT | 80.0 (16/20) | 94.9 (203/214) | 59.3 (16/27) | 98.1 (203/207) | 93.6 (219/234) |
| MRI | 92.0 (12/13) | 94.8 (127/134) | 63.2 (12/19) | 99.2 (127/128) | 94.6 (139/147) |
| p | NS | NS | NS | NS | NS |
| Primary tumor size < 25 mm | |||||
| Primary tumor | |||||
| FDG PET-CT | 78.1 (75/96) | - | - | - | - |
| MDCT | 91.7 (88/96) | - | - | - | - |
| MRI | 96.4 (53/55) | - | - | - | - |
| p | < 0.001 | ||||
| Lymph node metastases | |||||
| FDG PET-CT | 35.7 (10/28) | 95.6 (65/68) | 76.9 (10/13) | 78.3 (65/83) | 78.1 (75/96) |
| MDCT | 67.9 (19/28) | 75.0 (51/68) | 52.8 (19/36) | 85.0 (51/60) | 72.9 (70/96) |
| MRI | 80.0 (12/15) | 65.0 (26/40) | 46.2 (12/26) | 89.7 (26/29) | 69.1 (38/50) |
| p | < 0.05 | < 0.001 | NS | NS | NS |
| Distant metastases | |||||
| FDG PET-CT | 100.0 (2/2) | 96.8 (91/94) | 40.0 (2/5) | 100.0 (91/91) | 96.9 (93/96) |
| MDCT | 100.0 (2/2) | 95.7 (90/94) | 33.3 (2/6) | 100.0 (90/90) | 95.8 (92/96) |
| MRI | 100.0 (2/2) | 92.5 (49/53) | 33.3 (2/6) | 100.0 (49/49) | 92.7 (51/55) |
| p | NS | NS | NS | NS | NS |
| Primary tumor size ≥ 25 mm | |||||
| Primary tumor | |||||
| FDG PET-CT | 79.0 (109/138) | - | - | - | - |
| MDCT | 97.1 (134/138) | - | - | - | - |
| MRI | 97.8 (90/92) | - | - | - | - |
| p | < 0.001 | ||||
| Lymph node metastases | |||||
| FDG PET-CT | 47.0 (31/66) | 94.4 (68/72) | 88.6 (31/35) | 60.0 (68/103) | 71.7 (99/138) |
| MDCT | 77.3 (51/66) | 69.4 (50/72) | 69.9 (51/73) | 76.9 (50/65) | 73.2 (101/138) |
| MRI | 76.7 (33/43) | 73.5 (36/49) | 71.7 (33/46) | 78.3 (36/46) | 75.0 (69/92) |
| p | < 0.05 | < 0.001 | < 0.05 | < 0.05 | NS |
| Distant metastases | |||||
| FDG PET-CT | 83.3 (15/18) | 95.0 (114/120) | 71.4 (15/21) | 97.4 (114/117) | 93.5 (129/138) |
| MDCT | 77.8 (14/18) | 96.6 (113/117) | 77.8 (14/18) | 96.6 (113/117) | 94.1 (127/135) |
| MRI | 90.9 (10/11) | 96.3 (78/81) | 76.9 (10/13) | 98.7 (78/79) | 95.7 (88/92) |
| p | NS | NS | NS | NS | NS |
Values are presented as percentages (number) unless otherwise indicated. NPV = negative predictive value, NS = not significant, PPV = positive predictive value
Prognostic Implications of SUVmax for Primary Tumor and Metastatic Lesions in Patients with Extrahepatic Cholangiocarcinoma
The mean ± SD SUVmax of the primary tumor for all enrolled patients was 4.3 ± 3.5. SUVmax did not significantly differ between patient groups with CBD cancer and hilar cholangiocarcinoma, but significant differences were observed in relation to sex, gross morphologic characteristics of the primary tumor, differentiation, tumor size, regional lymph node and distant organ metastases, and American Joint Committee on Cancer (AJCC) staging (Table 3).
Table 3. Correlation of Maximum Standardized Uptake Value (SUVmax) of Primary Tumor and Clinic-Pathological Factors in Patients with Extrahepatic Cholangiocarcinoma.
| No. of Patients | SUVmax (Mean ± SD) | P | |
|---|---|---|---|
| Age (years) | 0.212 | ||
| ≥ 65 | 171 | 4.4 ± 3.7 | |
| < 65 | 63 | 3.8 ± 2.8 | |
| Sex | 0.005 | ||
| Male | 123 | 3.6 ± 2.8 | |
| Female | 111 | 4.9 ± 4.1 | |
| Diagnosis | 0.166 | ||
| Common bile duct cancer | 156 | 4.0 ± 3.2 | |
| Hilar cholangiocarcinoma | 78 | 4.7 ± 4.2 | |
| Gross morphologic type | 0.009 | ||
| Mass forming | 38 | 5.8 ± 4.2 | |
| Periductal infiltrating | 187 | 3.9 ± 3.3 | |
| Intraductal papillary growing | 9 | 4.3 ± 2.7 | |
| Primary tumor size (mm) | 0.001 | ||
| ≥ 25 | 138 | 4.8 ± 4.1 | |
| < 25 | 96 | 3.4 ± 2.3 | |
| Histologic differentiation | 0.002 | ||
| Well differentiated | 56 | 3.5 ± 2.7 | |
| Moderately differentiated | 95 | 4.0 ± 3.1 | |
| Poorly differentiated | 16 | 6.8 ± 4.2 | |
| Lymph node metastasis | 0.001 | ||
| N0 | 140 | 3.5 ± 2.6 | |
| N1 | 94 | 5.3 ± 4.4 | |
| Distant organ metastasis | < 0.001 | ||
| M0 | 214 | 4.0 ± 3.3 | |
| M1 | 20 | 7.1 ± 4.4 | |
| AJCC tumor staging | < 0.001 | ||
| IA | 22 | 3.9 ± 2.9 | |
| IB | 49 | 3.2 ± 2.2 | |
| IIA | 58 | 3.5 ± 2.6 | |
| IIB | 62 | 4.7 ± 3.7 | |
| III | 22 | 5.0 ± 5.4 | |
| IV | 21 | 7.0 ± 4.4 |
AJCC = American Joint Committee on Cancer, SD = standard deviation
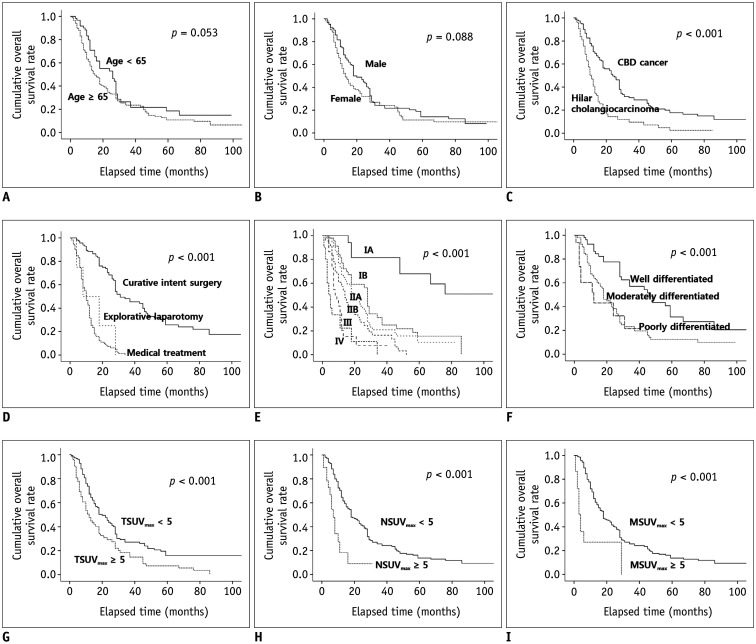
With a median follow-up period of 13.5 months (interquartile range [IQR], 7.8–28.0 months; maximum, 123 months), 170 (72.6%) cases of death from extrahepatic cholangiocarcinoma were identified. The cumulative overall survival rate was significantly higher in patients with CBD cancer (compared to hilar cholangiocarcinoma), curative-intent resection, lower AJCC staging, well-differentiated histology, SUVmax of the primary tumor < 5, SUVmax of the regional lymph nodes < 5, and SUVmax of the metastatic lesions < 5 (Fig. 1). In the multivariate analysis adjusted for potential confounders, hilar cholangiocarcinoma, advanced histology, advanced AJCC tumor staging, non-curative intent treatment modalities, SUVmax of the primary tumor ≥ 5, and SUVmax of the metastatic lesions ≥ 5 were independent and significant contributors to poor overall survival in patients with extrahepatic cholangiocarcinoma (Table 4).
Fig. 1. Cumulative overall survival rates according to baseline characteristics.
(A) Age, (B) Sex, (C) Diagnosis, (D) Treatment modality, (E) AJCC tumor staging, (F) Histologic differentiation, (G) TSUVmax, (H) NSUVmax, and (I) MSUVmax. Cumulative overall survival rate was significantly higher in patients with CBD cancer, curative-intent surgery, lower AJCC staging, well-differentiated histology, SUVmax of primary tumor < 5, SUVmax of regional lymph nodes < 5, and SUVmax of metastatic lesions < 5 (all p < 0.001).
AJCC = American Joint Committee on Cancer, CBD = common bile duct, MSUVmax = SUVmax of metastatic lesions, NSUVmax = SUVmax of regional lymph nodes, TSUVmax =SUVmax of primary tumor
Table 4. Independent and Significant Clinicopathologic Factors of Poor Overall Survival for Patients with Extrahepatic Cholangiocarcinoma.
| Variables | Crude HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|
| Age (years) | ||||
| < 65 | 1 (Reference) | 1 (Reference) | ||
| ≥ 65 | 1.40 (0.99–1.98) | 0.061 | 1.32 (0.82–2.14) | 0.252 |
| Male | 0.78 (0.57–1.05) | 0.097 | 1.15 (0.75–1.77) | 0.513 |
| Diagnosis | ||||
| Common bile duct cancer | 1 (Reference) | 1 (Reference) | ||
| Hilar cholangiocarcinoma | 2.42 (1.74–3.35) | < 0.001 | 1.90 (1.09–3.30) | 0.023 |
| Histologic differentiation | ||||
| Well differentiated | 1 (Reference) | 1 (Reference) | ||
| Moderately differentiated | 2.35 (1.52–3.65) | < 0.001 | 2.29 (1.39–3.79) | 0.001 |
| Poorly differentiated | 3.27 (1.58–6.80) | 0.001 | 1.40 (0.56–3.52) | 0.474 |
| AJCC tumor staging | ||||
| IA | 1 (Reference) | 1 (Reference) | ||
| IB | 3.74 (1.63–8.60) | 0.002 | 2.77 (1.15–6.68) | 0.023 |
| IIA | 4.99 (2.20–11.30) | < 0.001 | 3.48 (1.45–8.36) | 0.005 |
| IIB | 7.71 (3.37–17.64) | < 0.001 | 4.09 (1.61–10.41) | 0.003 |
| III | 14.31 (5.76–35.55) | < 0.001 | 2.71 (0.82–8.97) | 0.103 |
| IV | 23.14 (9.14–58.58) | < 0.001 | 1.30 (0.29–5.87) | 0.731 |
| Treatment modality | ||||
| Curative intent surgery | 1 (Reference) | 1 (Reference) | ||
| Explorative laparotomy | 4.31 (1.55–12.01) | 0.005 | 3.29 (1.12–9.60) | 0.030 |
| Medical treatment | 6.84 (4.70–9.95) | < 0.001 | 9.59 (4.92–18.71) | < 0.001 |
| SUVmax of primary tumor | ||||
| < 5 | 1 (Reference) | 1 (Reference) | ||
| ≥ 5 | 1.72 (1.26–2.35) | 0.001 | 1.75 (1.13–2.69) | 0.012 |
| SUVmax of regional lymph nodes | ||||
| < 5 | 1 (Reference) | 1 (Reference) | ||
| ≥ 5 | 3.32 (1.89–5.81) | < 0.001 | 1.76 (0.71–4.37) | 0.225 |
| SUVmax of distant metastases | ||||
| < 5 | 1 (Reference) | 1 (Reference) | ||
| ≥ 5 | 4.99 (2.67–9.31) | < 0.001 | 8.10 (1.96–33.50) | 0.004 |
CI = confidence interval, HR = hazard ratio, SUVmax = maximal standardized uptake value
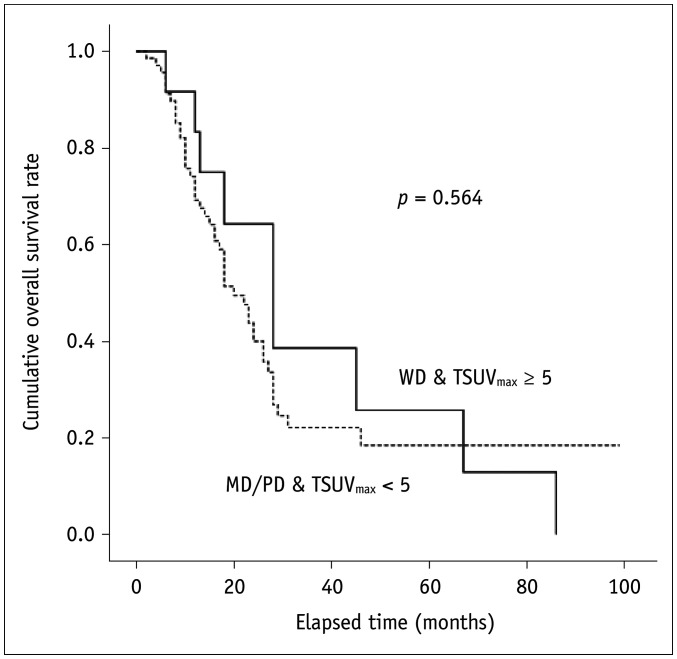
The comparison of the cumulative overall survival rates between patients with well-differentiated histology and an SUVmax of the primary tumor ≥ 5 and those with moderately or poorly differentiated histology and an SUVmax of the primary tumor < 5 is shown in Figure 2. There was no significant difference in cumulative overall survival rates between the two groups (p = 0.564).
Fig. 2. Similar cumulative overall survival rates between patients with WD histology and TSUVmax ≥ 5, and those with MD/PD histology and TSUVmax < 5 (p = 0.056).
MD = moderately differentiated, PD = poorly differentiated, WD = well differentiated
In a subgroup analysis of 187 patients with the periductal infiltrating type, patients with an SUVmax of the primary tumor ≥ 5 had a significantly higher incidence of regional lymph node metastases (54% vs. 38%; p = 0.049) and distant metastases (18% vs. 2.9%; p < 0.001) at the time of diagnosis, and a significantly lower cumulative incidence of overall survival (p = 0.002) than those with an SUVmax of the primary tumor < 5. In the multivariate analysis, after adjusting for confounding factors, an SUVmax of the primary tumor ≥ 5 was significantly associated with an increased risk of distant metastases at diagnosis (adjusted odds ratio [OR], 100.57; 95% CI, 3.94–2567.43) and a higher mortality rate (adjusted hazard ratio [HR], 1.81; 95% CI, 1.04–3.15) during the follow-up period. We also observed a tendency for an increased risk of lymph node metastases at diagnosis in patients with an SUVmax of the primary tumor ≥ 5 (adjusted OR, 1.60; 95% CI, 0.55–4.63) (Table 5).
Table 5. Risk Factors for Lymph Node Metastasis and Distant Metastasis at Time of Diagnosis, and Mortality during Follow-Up Period, among 187 Patients with Periductal Infiltrating Type.
| Variables | Lymph Node Metastasis | Distant Metastasis | Death | |||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| SUVmax of primary tumor | ||||||
| < 5 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| ≥ 5 | 1.92 (1.00–3.69) | 1.60 (0.55–4.63) | 7.30 (2.14–24.94) | 100.57 (3.94–2567.43) | 1.75 (1.21–2.52) | 1.81 (1.04–3.15) |
| Primary tumor size (cm) | ||||||
| < 2.5 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | - | - |
| ≥ 2.5 | 2.20 (1.20–4.01) | 1.27 (0.52–3.10) | 10.45 (1.33–82.14) | 6.03 (0.24–153.92) | ||
| Age (years) | ||||||
| < 65 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | |
| ≥ 65 | 2.23 (1.10–4.50) | 4.52 (1.56–13.06) | 1.20 (0.32–4.55) | 1.42 (0.95–2.12) | 1.58 (0.90–2.77) | |
| Male | 0.60 (0.33–1.07) | 0.57 (0.24–1.36) | 0.37 (0.11–1.25) | 0.69 (0.49–0.96) | 0.83 (0.51–1.35) | |
| Diagnosis | ||||||
| Common bile duct cancer | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Hilar cholangiocarcinoma | 2.93 (1.58–5.43) | 3.61 (1.44–9.06) | 4.39 (1.30–14.84) | 31.31 (2.01–487.21) | 2.10 (1.47–2.99) | 1.58 (0.88–2.81) |
| Histologic differentiation | ||||||
| Well differentiated | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Moderately differentiated | 4.18 (1.56–11.19) | 4.90 (1.63–14.69) | 0.97 (0.09–11.09) | 0.15 (0.01–3.74) | 2.58 (1.52–4.40) | 3.77 (1.94–7.33) |
| Poorly differentiated | 33.58 (2.98–188.75) | 33.88 (4.85–236.67) | 18.00 (1.88–172.23) | 1.09 (0.24–153.92) | 4.32 (1.96–9.50) | 3.15 (1.24–8.03) |
| AJCC tumor staging | - | - | - | - | ||
| IA | 1 (Reference) | 1 (Reference) | ||||
| IB | 3.52 (1.35–9.22) | 4.59 (1.54–13.71) | ||||
| IIA | 4.33 (1.68–11.20) | 4.70 (1.54–14.38) | ||||
| IIB | 6.88 (2.63–17.98) | 6.25 (2.02–19.30) | ||||
| III | 15.76 (5.53–44.90) | 3.95 (1.00–15.66) | ||||
| IV | 14.16 (4.70–42.63) | 4.71 (0.96–23.19) | ||||
| Treatment modality | - | - | - | - | ||
| Curative intent surgery | 1 (Reference) | 1 (Reference) | ||||
| Explorative laparotomy | 4.05 (1.44–11.42) | 2.69 (0.90–8.03) | ||||
| Medical treatment | 5.96 (3.95–9.00) | 7.38 (3.54–15.39) | ||||
OR = odds ratio
DISCUSSION
In the current study of 234 patients with confirmed extrahepatic cholangiocarcinoma (including CBD cancer and hilar cholangiocarcinoma), we found that the diagnostic performance of FDG PET-CT for the detection of primary tumors is inadequate compared to MDCT and MRI. In a previous study, FDG PET-CT also showed no diagnostic advantage over CT and MRI/MRCP for detecting primary tumors of suspected and potentially-operable cholangiocarcinoma (7). Additionally, the subgroup analyses of the same study showed significantly higher detection rates of MRI/MRCP for primary tumors in patients with extrahepatic cholangiocarcinoma, compared to FDG PET-CT (7). The role of radiologic diagnosis in patients with extrahepatic cholangiocarcinoma is to characterize the primary tumor lesions and determine their resectability. Surgical resection, especially surgery achieving R0 resection, is the only proven curative treatment with an obvious survival benefit for patients with extrahepatic cholangiocarcinoma. FDG PET-CT showed significantly higher specificity and PPV (but lower sensitivity) for the detection of regional lymph node involvement than MDCT and MRI for the same lesions. This means that the diagnostic performance of FDG PET-CT for regional lymph node involvement, which is a very important preoperative prognostic factor for overall survival in patients with extrahepatic cholangiocarcinoma after curative intent surgery, is similar to the diagnostic performance of ERCP with brush cytology or endobiliary forceps biopsy, which often has high specificity but an average sensitivity of only 50 percent (14, 15). Similar results of significantly higher specificity, similar or low sensitivity, and higher or similar accuracy for the diagnosis of regional lymph node metastases in patients with cholangiocarcinoma were reported in previous studies (7,16).
FDG PET-CT has been reported to be more valuable than CT or MRI/MRCP for the detection of unsuspected distant metastases of potentially-operable cholangiocarcinoma (5,6,7,8,9). The demonstrated advantages of FDG PET-CT in the preoperative staging of cholangiocarcinoma, especially for detecting unsuspected distant metastases on CT and/or MRI/MRCP, also have a significant clinical impact on the management of cholangiocarcinoma. However, in the current study, the additional use of FDG PET-CT did not yield a significantly higher detection rate of unsuspected distant metastases in patients with extrahepatic cholangiocarcinoma. The ability of MRI to detect small and occult liver metastatic foci appeared to be superior to conventional MDCT and FDG PET-CT. We speculate that the difference between MRI and FDG PET-CT explains why FDG PET-CT, which covers a greater area of the body, showed a similar diagnostic performance for the detection of distant metastases compared. Indeed, of the 20 confirmed cases of distant metastases, the three cases that were not detected on FDG PET-CT had metastases only in the liver (n = 17/20, sensitivity of 85%), while all other cases of extrahepatic metastases including lung and bone were detected on FDG PET-CT (n = 11/11). On the other hand, MRI did not reveal one case of lung metastasis (n = 12/13, sensitivity of 92%) and was not performed in the five cases of distant metastases involving extrahepatic metastases, resulting in a higher sensitivity of MRI than FDG PET-CT (Supplementary Table 1). The enhanced ability of FDG PET-CT to detect extra-abdominal metastatic lesions was counterbalanced by its low resolution and resulting low sensitivity for the detection of occult liver metastatic lesions. The small number of cases of distant metastases may also be an explanation for these results, and further studies with larger sample sizes are needed to verify our findings.
FDG PET-CT measured the SUVmax, a semiquantitative simplified measurement of the metabolism rate of tissue deoxyglucose. A few studies have reported on the evaluation of the prognostic value of the primary tumor SUVmax in bile duct and gallbladder cancer, and most of these reports included only a small number of patients (17,18,19). In the current study, the mean ± SD SUVmax of the primary tumor showed significant differences in relation to multiple clinicopathologic variables that seemed to be associated with prognosis in patients with extrahepatic cholangiocarcinoma. We set the cutoff value of SUVmax to 5.0, and found that patients with an SUVmax above the cutoff value in the primary tumor, regional lymph node, and metastatic lesions had a significantly poorer survival rate than those with an SUVmax below the cutoff value. Previous studies (17,18,19) reported that a higher SUVmax is independently associated with poor overall survival in patients with extrahepatic cholangiocarcinoma (18) and cholangiocarcinoma/gallbladder cancer (17,19). In the current study, the SUVmax of the primary tumor and that of metastatic lesions were identified as independent and significant prognostic factors in multivariate analyses, and might thus be considered possible biologic markers for predicting clinical outcomes of patients with extrahepatic cholangiocarcinoma. In addition, patients with well-differentiated histology and an SUVmax of the primary tumor ≥ 5 had cumulative overall survival rates similar to those with a moderately or poorly differentiated histology and an SUVmax of the primary tumor < 5. These findings suggest that extrahepatic cholangiocarcinomas with a high FDG uptake are more likely to be aggressive, even if histologic findings are well differentiated. Furthermore, in a subgroup analysis of 187 patients with the periductal infiltrating type, the SUVmax of the primary tumor was significantly associated with the presence of regional lymph node and distant metastases at the time of diagnosis, resulting in shortened overall survival times. It was also identified as an independent determinant for the existing regional lymph node and distant metastases at diagnosis, and for a poor prognosis in patients with the periductal infiltrating type of extrahepatic cholangiocarcinoma. This finding also suggests that the periductal infiltrating type of extrahepatic cholangiocarcinoma with high FDG uptake is more aggressive, and that clinicians may have to adopt a more aggressive pre-surgical treatment and post-operative surveillance approach.
The current study has several limitations. First, this was a retrospective study and our cohort was recruited from a single referral center in Korea. Therefore, some degree of selection bias might have occurred, and generalizations from our results should be made with caution. Second, all FDG PET-CT images were analyzed by one highly experienced interpreter at our institution, which may have affected the reliability of the results. Third, for the evaluation of nodal staging, node-by-node correlations between MDCT/or MRI and FDG PET-CT were not assessed, because we deemed it impossible to derive precise correlations between individually sampled and mapped lymph nodes from imaging studies (11). Fourth, data on volumetric PET parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were not available, and SUVmax was defined as the highest SUV among all metastatic lesions; the SUVmax of metastatic lesions in our study may thus not represent the actual metabolic activity of metastatic lesions. However, unlike SUVmax, volumetric measurements of FDG PET-CT are greatly affected by the use of different segmentation methods, and the optimal segmentation method for the measurement of these values has not yet been established (20). Lastly, our data include a large proportion of the periductal infiltrating type of extrahepatic cholangiocarcinoma (79.9%), and the 18F-FDG uptake of periductal infiltrating cholangiocarcinoma has been reported to be underestimated due to tumor geometry and partial volume effects, resulting in relatively low diagnostic yields of FDG PET-CT (21,22). However, in the clinical practice, periductal infiltrating cholangiocarcinoma is the most common type of extrahepatic cholangiocarcinoma, as it was in our study. Demonstrating that FDG PET-CT has a lower sensitivity for the detection of primary tumor lesions than MDCT or MRI may thus in itself be meaningful, as it reflects the diagnostic accuracy of FDG PET-CT in a real cohort of patients with extrahepatic cholangiocarcinoma.
In conclusion, FDG PET-CT showed lower sensitivity in the detection of primary tumors and regional lymph node involvement than MDCT and MRI. Additionally, FDG PET-CT did not show a significantly higher detection rate for unsuspected hepatic metastases in patients with extrahepatic cholangiocarcinoma than MDCT or MRI. However, the SUVmax of the primary tumors and metastatic lesions detected on FDG PET-CT could have implications for the overall survival of such patients with extrahepatic cholangiocarcinoma. The SUVmax of the primary tumors may also allow to predict regional lymph node and distant metastases and poor prognoses in patients with the periductal infiltrating type of extrahepatic cholangiocarcinoma.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2019.0891.
AUROC curve for each imaging modality for (A) regional lymph node involvement and (B) distant metastases. Overall diagnostic performance of PET-CT was not inferior compared to that of MDCT and MRI in detection of regional lymph node metastases (A, p = 0.210 and p = 0.070) and distant metastases (B, p = 0.734 and p = 0.618). AUROC = area under the receiver operating characteristic curve, MDCT = multi-detector row computed tomography, PET-CT = positron emission tomography/computed tomography
Detection of Distant Metastatic Lesions according to Imaging Modalities
References
- 1.Alberts SR, Gores GJ, Kim GP, Roberts LR, Kendrick ML, Rosen CB, et al. Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin Proc. 2007;82:628–637. doi: 10.4065/82.5.628. [DOI] [PubMed] [Google Scholar]
- 2.Keidar Z, Haim N, Guralnik L, Wollner M, Bar-Shalom R, Ben-Nun A, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med. 2004;45:1640–1646. [PubMed] [Google Scholar]
- 3.Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, et al. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33:779–784. doi: 10.1007/s00259-006-0072-z. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics. 2007;27:1635–1652. doi: 10.1148/rg.276065742. [DOI] [PubMed] [Google Scholar]
- 5.Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560–566. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- 6.Albazaz R, Patel CN, Chowdhury FU, Scarsbrook AF. Clinical impact of FDG PET-CT on management decisions for patients with primary biliary tumours. Insights Imaging. 2013;4:691–700. doi: 10.1007/s13244-013-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–1151. doi: 10.1111/j.1572-0241.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- 8.Moon CM, Bang S, Chung JB, Park SW, Song SY, Yun M, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol. 2008;23:759–765. doi: 10.1111/j.1440-1746.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Kuehl H, Grabellus F, Müller SP, Radunz S, Antoch G, et al. Preoperative assessment of hilar cholangiocarcinoma by dual-modality PET/CT. J Surg Oncol. 2008;98:438–443. doi: 10.1002/jso.21136. [DOI] [PubMed] [Google Scholar]
- 10.Olthof SC, Othman A, Clasen S, Schraml C, Nikolaou K, Bongers M. Imaging of Cholangiocarcinoma. Visc Med. 2016;32:402–410. doi: 10.1159/000453009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa H, Ikuma H, Asakura-Yokoe K, Uesaka K. Preoperative staging of biliary carcinoma using 18F-fluorodeoxyglucose PET: prospective comparison with PET+CT, MDCT and histopathology. Eur Radiol. 2008;18:2841–2847. doi: 10.1007/s00330-008-1062-2. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Kim HJ, Park HW, Kwon HJ, Lee SY, Kook SH, et al. Impact of high-grade obstruction on outcomes in patients with appendiceal inflammatory masses managed by nonoperative treatment. Ann Surg Treat Res. 2017;92:429–435. doi: 10.4174/astr.2017.92.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderveen KA, Hussain HK. Magnetic resonance imaging of cholangiocarcinoma. Cancer Imaging. 2004;4:104–115. doi: 10.1102/1470-7330.2004.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siqueira E, Schoen RE, Silverman W, Martin J, Rabinovitz M, Weissfeld JL, et al. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002;56:40–47. doi: 10.1067/mge.2002.125105. [DOI] [PubMed] [Google Scholar]
- 16.Kato T, Tsukamoto E, Kuge Y, Katoh C, Nambu T, Nobuta A, et al. Clinical role of (18)F-FDG PET for initial staging of patients with extrahepatic bile duct cancer. Eur J Nucl Med Mol Imaging. 2002;29:1047–1054. doi: 10.1007/s00259-002-0852-z. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa H, Ikuma H, Asakura K, Uesaka K. Prognostic importance of standardized uptake value on F-18 fluorodeoxyglucose-positron emission tomography in biliary tract carcinoma. J Surg Oncol. 2009;100:494–499. doi: 10.1002/jso.21356. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Narita M, et al. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography in patients with extrahepatic bile duct cancer. J Hepatobiliary Pancreat Sci. 2011;18:39–46. doi: 10.1007/s00534-010-0293-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Kim HJ, Yim SH, Shin DS, Yu JH, Ju DY, et al. Primary tumor maximum standardized uptake value measured on 18F-fluorodeoxyglucose positron emission tomography-computed tomography is a prognostic value for survival in bile duct and gallbladder cancer. Korean J Gastroenterol. 2013;62:227–233. doi: 10.4166/kjg.2013.62.4.227. [DOI] [PubMed] [Google Scholar]
- 20.Im HJ, Bradshaw T, Solaiyappan M, Cho SY. Current methods to define metabolic tumor volume in positron emission tomography: which one is better? Nucl Med Mol Imaging. 2018;52:5–15. doi: 10.1007/s13139-017-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon CM, Bang S, Chung JB. The role of (18) F-fluorodeoxyglucose positron emission tomography in the diagnosis, staging, and follow-up of cholangiocarcinoma. Surg Oncol. 2011;20:e10–e17. doi: 10.1016/j.suronc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Yi HK, Park YJ, Bae JH, Lee JK, Lee KH, Choi SH, et al. Inverse prognostic relationships of 18F-FDG PET/CT metabolic parameters in patients with distal bile duct cancer undergoing curative surgery. Nucl Med Mol Imaging. 2018;52:334–341. doi: 10.1007/s13139-018-0542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AUROC curve for each imaging modality for (A) regional lymph node involvement and (B) distant metastases. Overall diagnostic performance of PET-CT was not inferior compared to that of MDCT and MRI in detection of regional lymph node metastases (A, p = 0.210 and p = 0.070) and distant metastases (B, p = 0.734 and p = 0.618). AUROC = area under the receiver operating characteristic curve, MDCT = multi-detector row computed tomography, PET-CT = positron emission tomography/computed tomography
Detection of Distant Metastatic Lesions according to Imaging Modalities