Abstract
Elizabethkingia sp. is an opportunistic nosocomially acquired Gram-negative bacterium usually implicated in isolated cases of meningitis, pneumonia, bacteraemia and sepsis. It is a sturdy pathogen, resistant to most of the first-line antibiotics routinely used in laboratories for other Gram-negative pathogens. The current study was planned to assess the demographic profile, clinical picture, sensitivity patterns and species identification of various Elizabethkingia isolates, as well as to follow up cases of infection. All clinical samples of blood, cerebrospinal fluid and respiratory specimens positive for Elizabethkingia during a 2-year period were included in the study. The isolates were first identified with a Vitek-2 GN card system and further confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Follow-up visits of the patients with their demographic records, morbidities and treatment outcomes were also planned and studied. Over a period of 2 years, samples from 27 individuals showed positive growth of Elizabethkingia spp. Among these 27 individuals, 19 were adults and 8 were neonates. Blood samples yielded most isolates (52.6%; n = 10); followed by tracheal aspirate, bronchoalveolar lavage fluid, and cerebrospinal fluid. Eleven out of 27 patients (40.7%) showed concomitant growth of other pathogens along with Elizabethkingia spp.; predominantly Gram-negative organisms. Both species of Elizabethkingia showed 100% susceptibility to drugs such as minocycline and piperacillin-tazobactam. A favourable outcome was seen in 76.9% of the individuals with timely institution of antibiotics and proper diagnosis. Bloodstream infections and meningitis were identified as the most common clinical conditions associated with mortality. Infections due to Elizabethkingia are on the rise in developing countries like India. As a result there is an urgent need to study this pathogen in greater detail to understand its pathogenesis, clinical implications and treatment outcomes, especially in hospital settings such as intensive care units.
Keywords: Bacteraemia, bronchoalveolar lavage, Elizabethkingiasp., meningitis, pneumonia
Highlights
-
•
Elizabethkingia species is an opportunistic nosocomially acquired Gram negative bacteria usually implicated in isolated cases of meningitis, pneumonia, bacteremia, and sepsis.
-
•
It is a sturdy pathogen resistant to most of the first line antibiotics routinely used in the laboratories for other Gram negative pathogens.
-
•
It is implicated in a wide range of infections in both neonates and adults.
-
•
Literature search reveals very scarce studies and case reports on this rare opportunistic pathogen in the healthcare settings and there is a dire need to study this pathogen in greater detail in a developing country like India.
Introduction
Elizabethkingia is a ubiquitous organism that is widely distributed in the environment [1]. The most commonly isolated species is Elizabethkingia meningoseptica, which was formerly known by the names Flavobacterium meningosepticum and Chryseobacterium meningosepticum. It was discovered in 1959 by an American bacteriologist, Elizabeth O. King, and was later identified as the cause of neonatal septicaemia [2]. It is a Gram-negative rod and is non-motile, a non-fermenter, and oxidase and catalase positive. It is implicated in a wide range of infections in both neonates and adults. In neonates, it is known to cause meningitis, pneumonia, bacteraemia and sepsis [3]. The meningitis caused by E. meningoseptica is known to have a mortality rate of c.57%, and hydrocephalus, deafness and developmental delay are seen as late sequelae of the infection. It is pathogenic in both immunocompetent and immunocompromised adult patients, and the infection is usually nosocomially acquired. Pneumonia, endocarditis, postoperative bacteraemia, abdominal infections, bronchitis and meningitis are the most commonly encountered manifestations [3,4]. Some of the common risk factors associated with this infection are long-term immunosuppression with drugs, underlying co-morbidities, prolonged hospital stay, previous use of higher antibiotics (Third generation cephalosporins and carbapenems), indwelling central venous catheter and other invasive devices [4].
Elizabethkingia shows a unique sensitivity pattern and is sensitive to only a few routinely used antibiotics, such as piperacillin-tazobactam and minocycline [5,6]. It shows resistance to most of the routinely used antimicrobials, including aminoglycosides, β-lactam antibiotics, tetracyclines and carbapenems. The mechanism of resistance to most of the β-lactam antibiotics is through production of extended-spectrum β-lactamases and carbapenem hydrolysing metallo-β-lactamases. The SENTRY antimicrobial surveillance programme conducted from 1997 to 2001 showed that quinolones, rifampin, trimethoprim-sulfamethoxazole and piperacillin-tazobactam were the most active agents against E. meningoseptica [5,6].
The other named species of the Elizabethkingia genus, which are less commonly reported in neonatal infections, are Elizabethkingia anophelis, Elizabethkingia endophytica and Elizabethkingia miricola. However, because of the lack of automated species detection methods in the past, many cases reported as E. meningoseptica were actually E. anophelis because these two species are indistinguishable by routine microbiological tests [7]. Infections due to E. endophytica are still questionable and remain unidentified [8].
Literature searches reveal very few studies and case reports on this rare opportunistic pathogen in health-care settings and there is an urgent need to study this pathogen in greater detail in a developing country like India. For this reason, we planned this study with a time span of 2 years and included a considerable number of these isolates in clinical specimens. We tried to assess the demographic profile, clinical picture, sensitivity patterns, species identification and follow up of the cases.
Materials and methods
Study setting
This study was an observational study, undertaken for a period of 2 years from June 2018 to June 2020 in our university hospital.
Inclusion criteria
All clinical samples positive for Elizabethkingia during this 2-year period were included in the study. Patients were also categorized according to their various clinical conditions: including ventilator-associated pneumonia, defined as pneumonia occurring more than 48 hours after patients have been intubated and on mechanical ventilation, and bacteraemia, which was diagnosed as per the Pitt bacteraemia score. This score is widely used in intensive care settings and ranges from 0 to 14 points, with a score ≥4 commonly used as an indicator of critical illness and increased mortality. Institutional ethics committee permission was obtained before the study.
Sample processing
Respiratory, cerebrospinal fluid (CSF) and blood samples were collected from patients and sent to the microbiology laboratory for culture and identification of the pathogen. Quantitative culture was performed on MacConkey agar and sheep blood agar for respiratory samples with incubation at 37°C. The cut-off point of 105 CFU/mL was considered significant for tracheal aspirate samples to indicate an infection; and for bronchoalveolar lavage (BAL) samples the cut-off was taken as 104 CFU/mL after 48 hours of incubation. The CSF sample was subjected to direct microscopic examination, and was also cultured on chocolate agar and inoculated in brain–heart infusion broth for subculture to look for fastidious organisms. CSF samples were incubated for 72 hours before reporting the samples as sterile or showing positive growth. Blood cultures were performed with the help of automated blood culture systems (Bact/Alert, bioMérieux, Marcy l’Étoile, France) and were subcultured on MacConkey agar and sheep blood agar after the bottle was flagged positive by this machine. All the culture media used were obtained from HIMEDIA laboratories (Mumbai, India).
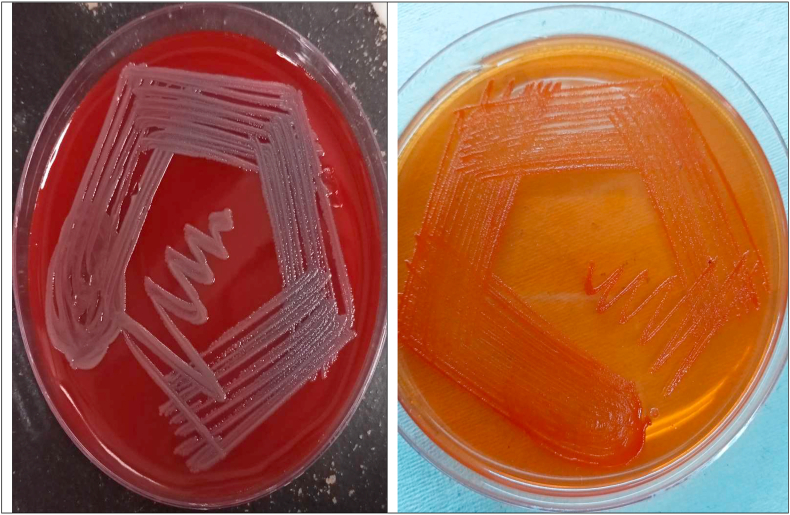
The bacterial isolates were first identified using the routine staining and biochemical tests used in our laboratory [9]. The biochemical reactions for this Gram-negative bacillus revealed the results as follows: catalase-positive, oxidase-positive, non-motile, non-fermenting, mannitol-negative, weakly indole-positive, triple sugar iron, agar-K/K (alkaline/alkaline) and urease-negative. It hydrolysed esculin and gelatin, did not use citrate as the sole source of carbon, and nitrate was not reduced (Fig. 1).
Fig. 1.
Blood agar and MacConkey agar plates showing growth of Elizabethkingia species.
For the automated methods, the identity of bacteria was first tested with a Vitek 2 GN card system (bioMérieux), an automated identification and susceptibility testing system, and finally was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). MALDI-TOF is one of the most advanced techniques in a microbiology laboratory and can identify routine isolates to species level in only a couple of hours. Identification by MALDI-TOF MS was based on a SARAMIS database amended with Elizabethkingia spp. spectra provided to bioMérieux and hence, the results of this updated MALDI-TOF MS were taken as confirmatory and final in the case of disconcordance of results between the two systems.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by the Kirby–Bauer disc diffusion method on Müller–Hinton agar and by Vitek-2 (bioMérieux) system [10]. The MICs of the antibiotics tested by Kirby–Bauer disc diffusion method were interpreted using the CLSI guidelines for other non-Enterobacteriaceae because there are no clear guidelines for sensitivity interpretation of Elizabethkingia isolates [11].
Patient follow up
Culture, sensitivity and other study parameters were kept in a computer database along with patient profile. Information on underlying diseases and other co-morbidities during the episode, type of infection, use of invasive procedures, duration of hospital stay and outcomes was obtained and analysed. Follow-up visits of the patient were recorded from the outpatient department for the treatment outcomes.
Statistical analysis
Statistical tests were performed using Social Sciences software for Windows Version 14.0 (SPSS Inc., Chicago, IL, USA) for descriptive statistics. Categorical data were described using numbers and percentages. Values of p < 0.05 were taken as significant.
Results
Over a period of 2 years, Elizabethkingia spp. were isolated from 27 patients (19 adults and eight neonates). The adults comprised 15 men (78.9%) and 4 (21.0%) women; among the neonates five were male and three were females. The average age of the adult patients was 67.2 years and the range varied from 38 to 89 years. Approximately 68% of the patients belonged to the lower strata for socio-economic status and were from rural areas.
Co-morbidities were associated with all 19 adult cases of Elizabethkingia infection. Hypertension and diabetes mellitus were present in 31.5% (n = 6), haematological malignancies in 21% (n = 4), other malignancies (e.g. ovarian, gallbladder) in 15.7% (n = 3), lung diseases and chronic obstructive pulmonary disease in 15.7 % (n = 3) and one case (5.2%) each of renal failure, liver failure/cirrhosis and complicated urinary tract infection. Among the eight neonates, two were diagnosed with anaemia and neonatal jaundice, four were premature and the other two were normal (Table 1, Table 2).
Table 1.
Clinical details and outcomes of adult patients (n = 19) with Elizabethkingia isolates
| No. | Age (yr)/Sex | Clinical diagnosis | Underlying illness/co-morbidities | Sample taken/collected | Elizabethkingia spp. Isolated (by MALDI-TOF) | Duration of hospital stay (days) | Days from admission to develop-ment of infection | Antibiotics institute/changed for Elizabethkingia infection | Outcome/follow up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 47/M | Sepsis | AML | Blood | meningoseptica | 34 | 22 | Minocycline, piperacillin-tazobactam | Recovered |
| 2 | 53/M | Sepsis | UTI with strictures, stones in ureter | Blood | meningoseptica | 42 | 19 | Minocycline, piperacillin-tazobactam | Recovered |
| 3 | 62/F | Sepsis | Gallbladder cancer | Blood | meningoseptica | 26 | 16 | Minocycline, piperacillin-tazobactam | Recovered |
| 4 | 38/M | LRTI | ALL | BAL | meningoseptica | 10 | 18 | Minocycline, piperacillin-tazobactam, vancomycin | Recovered |
| 5 | 75/M | Intracerebral haemorrhage, meningitis | HTN | CSF | anophelis | 29 | 23 | Minocycline, piperacillin-tazobactam | Recovered |
| 6 | 68/M | Sepsis | COPD | BAL | meningoseptica | 31 | 14 | Minocycline, piperacillin-tazobactam, cotrimoxazole | Left against medical advice |
| 7 | 56/M | Pneumothorax, VAP | COPD | BAL | anophelis | 28 | 25 | Minocycline, piperacillin-tazobactam, vancomycin | Recovered |
| 8 | 63/M | Sepsis | Liver failure with cirrhosis | Blood | meningoseptica | 39 | 17 | Minocycline, piperacillin-tazobactam, ticarcillin-clavulunate | Expired |
| 9 | 41/F | Sepsis, meningitis | ALL | Blood, CSF | anophelis | 16 | 19 | Minocycline, piperacillin-tazobactam, vancomycin | Recovered |
| 10 | 87/M | LRTI | DM, HTN | Tracheal aspirate | meningoseptica | 44 | 16 | Minocycline, piperacillin-tazobactam | Recovered |
| 11 | 61/M | LRTI, VAP | COPD | BAL, tracheal aspirate | meningoseptica | 36 | 11 | Minocycline, piperacillin-tazobactam | Recovered |
| 12 | 89/M | Inter-trochanteric fracture of femur | DM, HTN | Blood | anophelis | 25 | 13 | Minocycline, piperacillin-tazobactam, cefoperazone-sulbactam, vancomycin | Recovered |
| 13 | 65/F | Pneumothorax with sepsis | Ovarian cancer | BAL, blood | meningoseptica | 29 | 18 | Minocycline, piperacillin-tazobactam | Recovered |
| 14 | 86/M | LRTI | DM, HTN | Tracheal aspirate | meningoseptica | 33 | 16 | Minocycline, vancomycin | Recovered |
| 15 | 66/M | Sepsis | Renal failure | Blood | anophelis | 19 | 19 | Minocycline, piperacillin-tazobactam | Recovered |
| 16 | 49/F | Myocardial ischaemia | HTN, DM | Blood | meningoseptica | 22 | 13 | Minocycline, piperacillin-tazobactam | Expired |
| 17 | 79/M | Stroke with intracerebral haemorrhage | HTN | CSF | anophelis | 42 | 24 | Minocycline, piperacillin-tazobactam, cefoperazone-sulbactam, vancomycin | Expired |
| 18 | 73/M | Sepsis | Lung cancer | Blood | meningoseptica | 45 | 18 | Minocycline, piperacillin-tazobactam | Recovered |
| 19 | 62/M | LRTI | NHL | BAL | meningoseptica | 12 | 10 | Minocycline, piperacillin-tazobactam | Recovered |
Abbreviations: ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; CSF, cerebrospinal fluid; DM, diabetes mellitus; F, female; HTN, hypertension; LRTI, lower respiratory tract infection; M, male; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; NHL, non-Hodgkin lymphoma; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Table 2.
Clinical details and outcome of neonates (n = 8) with Elizabethkingia isolates
| No. | Age (days)/Sex | Clinical diagnosis | Underlying illness/co-morbidities | Sample taken/collected | Elizabethkingia spp. isolated (by MALDI-TOF) | Duration of hospital stay (days) | Days from admission to develop-ment of infection | Antibiotics instituted/changed for Elizabethkingia infection | Outcome/follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6/F | Meningitis | Anaemia, jaundice | Blood, CSF | meningoseptica | 25 | 11 | Minocycline, piperacillin-tazobactam | Expired |
| 2 | 9/M | Meningitis | None | Blood, CSF | meningoseptica | 29 | 9 | Minocycline, piperacillin-tazobactam | Recovered |
| 3 | 12/M | Sepsis | Premature | Blood, CSF | anophelis | 34 | 15 | Minocycline, piperacillin-tazobactam | Recovered |
| 4 | 7/M | Meningitis | Anaemia, jaundice | Blood, CSF | anophelis | 16 | 17 | Minocycline, piperacillin-tazobactam, vancomycin | Recovered |
| 5 | 21/M | Meningitis | Premature | Blood, CSF, | meningoseptica | 42 | 8 | Minocycline, vancomycin | Recovered |
| 6 | 8/F | Sepsis | Premature | Blood, CSF | meningoseptica | 19 | 10 | Minocycline, piperacillin-tazobactam | Expired |
| 7 | 19/F | Meningitis | None | Blood, CSF, | anophelis | 11 | 13 | Minocycline, piperacillin-tazobactam,vancomycin | Recovered |
| 8 | 24/M | Meningitis | Premature | Blood, CSF | meningoseptica | 28 | 21 | Minocycline, piperacillin-tazobactam | Expired |
Abbreviations: CSF, cerebrospinal fluid; F, female; M, male; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Regarding sample categorization, most isolates were cultured from blood (52.6%; n = 10), followed by tracheal aspirate and BAL fluid (42.1%; n = 8) and the remainder from CSF (15.8%; n = 3) (Table 1, Table 2). Subsequently, bacteraemia and sepsis were finally diagnosed in 47.3% (n = 9), ventilator-associated pneumonia and lower respiratory tract infection with pneumothorax in 36.8% (n = 7), meningitis with intracerebral haemorrhage in 15.7% (n = 3) and a few presented with fractures and myocardial ischaemia. All the patients with underlying haematological malignancies developed the infection after the induction dose of chemotherapy. Seven patients required mechanical ventilation after clinical deterioration in their symptoms and nine had a history of central or peripheral line insertion at the time of development of sepsis/bacteraemia. Diabetes as a co-morbidity was seen in 89% of the Elizabethkingia-positive patients with bloodstream infections and hypertension was found in 67% of the individuals with ventilator-associated pneumonia and colonization by Elizabethkingia sp. Among the eight neonates, six had neonatal meningitis whereas the other two had neonatal sepsis. Blood and CSF were collected from all the neonates (100%) and positive growth was seen in all the samples with a good clinical correlation. The average duration of hospital stay was 28 days (range 10–45 days) and the mean time of development of infection after hospitalization was 16.2 days.
Eleven out of 27 patients (40.7%) showed concomitant growth of other pathogens along with Elizabethkingia spp. Gram-negative bacilli were the predominant isolates, followed by Gram-positive cocci in a minority of cases. Further speciation was performed on a VITEK-2 GN card system and was confirmed by MALDI-TOF MS. It revealed E. meningoseptica in most cases (66.7%; n = 18), followed by E. anophelis (33.3%; n = 9). Both VITEK 2 and MALDI-TOF MS were able to identify all 18 cases of E. meningoseptica, whereas VITEK 2 misidentified all nine E. anophelis isolates, which were correctly identified by MALDI-TOF MS. Detailed species identification and scores have been listed in Table 3. Elizabethkingia meningoseptica was isolated from 12 blood samples, five BAL samples, three tracheal aspirates and five CSF samples; whereas E. anophelis was isolated from six blood samples, six CSF samples and one BAL sample. Detailed samplewise categorization of Elizabethkingia isolates in positive patients is given in Table 1, Table 2. No isolates were identified of E. miricola or E. endophytica.
Table 3.
Comparison of Elizabethkingia species identified by MALDI-TOF MS system and the Vitek 2 GN card system
| No. of Elizabethkingia isolates | MALDI-TOF MSa (no. of isolates) | Identification score of MALDI TOF MS | Vitek 2 with GN card system (no. of isolates) | Identification score of Vitek 2 system |
|---|---|---|---|---|
| E. meningoseptica (n = 18) | E. meningoseptica (n = 18) | 100% | E. meningoseptica (n = 18) | 100% |
| E. anophelis (n = 9) | E. anophelis (n = 9) | 100% | E. meningoseptica (n = 8) Chryseobacterium indologenes (n = 1) | 0% |
Identification was based on a SARAMIS database amended with Elizabethkingia spp. spectra provided to bioMérieux.
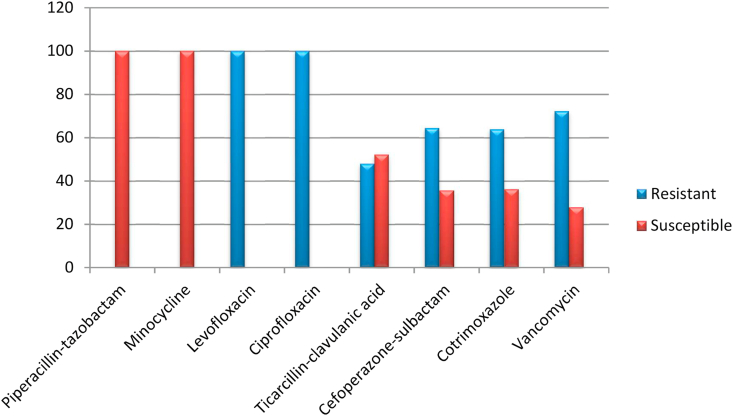
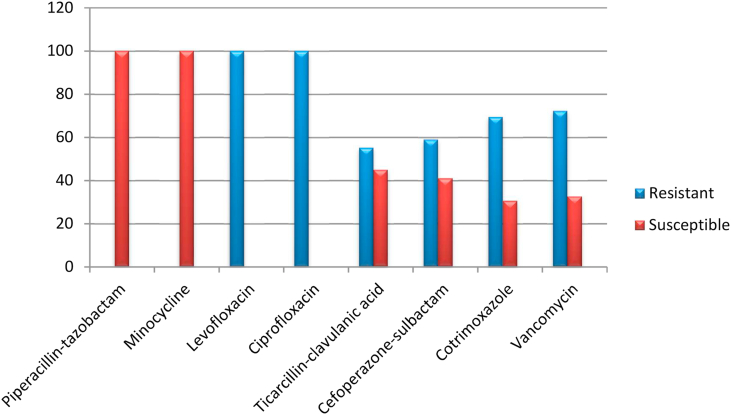
The antibiotic sensitivity testing of these isolates revealed in vitro resistance to the fluoroquinolone antibiotics ciprofloxacin and levofloxacin, which are used to treat Gram-negative pathogens. All the isolates of Elizabethkingia showed almost 100% resistance to levofloxacin and ciprofloxacin. The overall susceptibilities of the Elizabethkingia isolates to cotrimoxazole, cefoperazone-sulbactam and ticarcillin-clavulanic acid were found to be 33.4%, 38.3% and 48.5%, respectively. Drugs such as minocycline and piperacillin-tazobactam showed 100% susceptibility in both the species of Elizabethkingia. Detailed antibiotic susceptibilities of E. meningoseptica and E. anophelis isolates are listed in Fig. 2, Fig. 3. Vancomycin was also tested by E-test and showed 27.8 % sensitivity in E. meningoseptica and 32.6% in E. anophelis.
Fig. 2.
Antibiotic sensitivity profile of Elizabethkingia meningoseptica isolates.
Fig. 3.
Antibiotic sensitivity profile of Elizabethkingia anophelis isolates.
Six of the 26 patients died during their hospital stay; the other 20 were discharged in good health with advice for routine follow up in the outpatient department depending on the medical condition. One adult patient left the hospital against medical advice, so the follow-up report could not be assessed. Hence, favourable outcome was seen in 76.9% of the individuals with timely institution of antibiotics and proper diagnosis.
Discussion
Infection with Elizabethkingia spp. is an uncommon phenomenon; however, they are clinically significant because the pathogen shows intrinsic resistance to a wide range of antibiotics used routinely in hospital settings to treat Gram-negative pathogens. A variety of cases have been reported with Elizabethkingia spp. and these include pneumonia, meningitis, catheter-related bloodstream infections, biliary sepsis, osteomyelitis and keratitis [12] Elizabethkingia meningoseptica is said to survive in chlorine-treated municipal water supplies, so it often colonizes sink basins and taps inside hospital settings. These act as reservoirs inside hospital environments and colonize the patients via contaminated medical devices involving fluids, such as respirators, intubation tubes, mist tents and humidifiers [12]. In neonates the colonization of incubators, ice chests and syringes has been documented in some studies [13]. As highlighted by various previous studies, E. anophelis was often misidentified as E. meningoseptica, so remained under-reported in most clinical studies. Elizabethkingia anopheles was first discovered in Anopheles mosquito gut, hence, its name—anophelis. However, it was later shown that mosquitoes were unlikely to be the route of transmission in E. anopheles-associated infections. More detailed research and work further clarified the point and speculated that contaminated hospital environments, like infected catheters and intravenous infusions and fluids acted as reservoirs for such infections [14].
Infections due to Elizabethkingia species are on the rise in different parts of the world. This is indicated by a recent report from Taiwan that reported a number of cases of E. meningoseptica [6]. Our study also reported a significant number of patients with infections caused by both E. meningoseptica and E. anophelis.
Elizabethkingia were isolated from a total of 27 individuals, 19 adults and eight neonates. Our study showed a strong preference for extremes of age as the average age of the adult patients was 67.2 years (range 38–89 years). This was supported by a study conducted in Central Taiwan, where the mean age of patients was 72.2 years [15]. Male patients showed a higher rate of infection (78.9%) compared with female patients, which was also seen in other similar studies [15].
Most cultures that were positive for Elizabethkingia isolates in our study were from blood samples (52.6%), followed by tracheal aspirates and BAL fluid, then CSF. This finding showed perfect correlation with a similar study by Chang et al., who identified blood as the predominant specimen for the isolation of Elizabethkingia species (48.7%) followed by sputum (41%) [15]. In another study conducted at AIIMS in New Delhi, the authors recorded maximum positivity in BAL samples (70%), followed by blood and CSF [16].
Elizabethkingia species are known to cause meningitis, sepsis, bacteraemia, lower respiratory tract infection, pneumonia, pneumothorax, endocarditis, cellulitis, endophthalmitis, keratitis, wound infection after bone fractures and urinary tract infections [[17], [18], [19]]. In our study, bacteraemia and sepsis were diagnosed most often (47.3%), followed by ventilator-associated pneumonia and lower respiratory tract infection with pneumothorax, meningitis and a few wound infections after bone fractures.
In our study, 40.7% of the patients showed concomitant growth of other pathogens along with Elizabethkingia spp. with Gram-negative bacilli being the predominant pathogens. This is supported by a similar finding from a study conducted by Venkatesh et al., which recorded Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii and Proteus mirabilis as common species [20]. Hence, accurate identification is of utmost importance in understanding the exact pathogenesis and clinical implications of Elizabethkingia spp. in cultured specimens. Automated culture and identification systems like VITEK and MALDI-TOF play a crucial role in discriminating the Elizabethkingia isolates from these concomitantly growing organisms.
The average duration of hospital stay in our study was 28 days (range 10–45 days). Our findings were consistent with other similar studies by Lin et al., where the mean duration of hospital stay was 32 days (range 13–99 days) [1] and by Moore et al., where the mean duration of hospital stay was 17 days (range 4–35 days) [21].
Infection by Elizabethkingia spp. is usually associated with the presence of a central venous line infection, inappropriate use of antibiotics, prolonged hospital stay, or a prolonged course of chemotherapy [16,22]. Seven patients in our study required mechanical ventilation after clinical deterioration in their symptoms and nine patients had a history of central or peripheral line insertion at the time of development of sepsis/bacteraemia. Induction of chemotherapy was also present in patients with haematological malignancies [22]. This fact is also supported by a survey conducted by Rastogi et al., wherein all the patients were on broad-spectrum antibiotics before developing the Elizabethkingia infection [16].
Elizabethkingia is a sturdy pathogen showing resistance to a wide variety of drugs, including most of the β-lactam antibiotics including carbapenems and aztreonam, the aminoglycoside group of drugs and chloramphenicol. A previous literature search indicates the susceptibility of Elizabethkingia to cotrimoxazole, fluoroquinolones, minocycline, ticarcillin-clavulunate, piperacillin and piperacillin-tazobactam [[23], [24], [25], [26], [27]]. This widespread resistance to a variety of β-lactams is due to its production of metallo-β-lactamases coded by BlaB and Bla (GOB) genes, conferring the ability to degrade most of the β-lactam antibiotics [25]. Minocycline and piperacillin-tazobactam proved to be the most effective drugs in our study, showing 100% sensitivity. Many past reports have also studied minocycline and piperacillin as 100% effective towards Elizabethkingia infection [15,27]. Vancomycin was also tested in our study by E-test strip, showing almost 30% sensitivity in the Elizabethkingia isolates, and was started as therapy in a large number of patients (Table 1). There are reports of successful outcomes on initiation of vancomycin as therapy for Elizabethkingia infections [[28], [29], [30]]. The current study did not notice any significant difference in antibiotic sensitivities among the two isolated species of Elizabethkingia.
Mortality due to Elizabethkingia infections ranges from 21% to 52% as described in previous studies [6]. In a study by Venkatesh et al., the mortality was 30.8%, and 69.2% of the patients recovered from their infection [20]. Another study, by Lin et al. estimated mortality rate to be around 41% [31]. Our study showed favourable outcome in 76.9% of the cases and mortality in the remaining 23.1%. On stratifying the mortality according to the clinical condition of the patients, most mortality was due to bloodstream infections (96%) among the adult population and via meningitis (89%)in the paediatric group of patients.
Conclusion
Elizabethkingia is emerging as an important cause of nosocomially acquired bacteraemia/sepsis and meningitis in developing countries like India. Multiple risk factors, including indwelling catheters, venous line insertion, irrational and prolonged use of broad-spectrum antibiotics, pre-existing co-morbidities, have led to increasing numbers of cases. Microbiologists as well as clinicians have to play an active and enthusiastic role for the timely identification and treatment of this multidrug-resistant pathogen. Very few studies and isolated case reports have been published on infections caused by this organism. Hence, the current study of a large number of isolates over a 2-year period is an effort to study and assess the clinical as well as demographic profiles of patients with infections caused by various Elizabethkingia isolates. Detailed studies and research work are required to understand the pathogenesis of this opportunistic pathogen as well as its impact in health-care settings.
Funding source
No funding was received.
Conflict of interest
The authors have stated that there are no conflicts of interest in relation to this article.
Ethical considerations
Informed consent was obtained from all the patients and their legal guardians (in the case of minors) regarding the publication of images and clinical information in the journal. They were informed of the confidentiality of the data, but that anonymity could not be guaranteed.
Authors' contributions
SwS and CS contributed to the conceptualization, methodology and original manuscript drafting. CS also performed the software analyses. SSP and SaS were responsible for data curation, visualization and investigation. UGwrote the final draft of the manuscript, and was responsible for reviewing and editing.
References
- 1.Lin P.Y., Chu C., Su L.H., Huang C.T., Chang W.Y., Chiu C.H. Clinical and microbiological analysis of bloodstream infections caused by Chryseobacterium meningosepticum in nonneonatal patients. J Clin Microbiol. 2004;7:3353–3355. doi: 10.1128/JCM.42.7.3353-3355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King E.O. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959;31:241–247. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- 3.Ozkalay N., Anil M., Agus N., Helvaci M., Sirti S. Community-acquired meningitis and sepsis caused by Chryseobacterium meningosepticum in a patient diagnosed with thalassemia major. J Clin Microbiol. 2006;44:3037–3039. doi: 10.1128/JCM.00588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceyhan M., Celik M. Elizabethkingia meningosepticum(Chryseobacterium meningosepticum) infections in children. Int J Pediatr. 2011;2011:215237. doi: 10.1155/2011/215237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirby J.T., Sader H.S., Walsh T.R., Jones R.N. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp.: report from the SENTRY antimicrobial surveillance program (1997–2001) J Clin Microbiol. 2004;42:445–448. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu M.S., Liao C.H., Huang Y.T., Liu C.Y., Yang C.J., Kao K.L. Clinical features, antimicrobial susceptibilities, and outcomes of Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteremia at a medical center in Taiwan, 1999–2006. Eur J Clin Microbiol Infect Dis. 2011;30:1271–1278. doi: 10.1007/s10096-011-1223-0. [DOI] [PubMed] [Google Scholar]
- 7.Breurec S., Criscuolo A., Diancourt L., Vandenbogaert M., Passet V., Caro V. Genomic epidemiology and global diversity of the emerging bacterial pathogen Elizabethkingia anophelis. Sci Rep. 2016;6:30379. doi: 10.1038/srep30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doijad S., Ghosh H., Glaeser S., Kampfer P., Chakraborty T. Taxonomic reassessment of the genus Elizabethkingia using whole-genome sequencing: Elizabethkingia endophytica Kampfer P, Busse HJ, MacInroy JA, Glaeser SP. 2015 is a later subjective synonym of Elizabethkingia anophelis Kampfer P,Matthews H, Glaeser SP, Martin K, Lodders N, Faye I. 2011. Int J Syst Evol Microbiol. 2016;66:4555–4559. doi: 10.1099/ijsem.0.001390. [DOI] [PubMed] [Google Scholar]
- 9.Collee J.G., Mackie T.J., McCartney J.E. Mackie & McCartney Practical medical microbiology. 14th ed. Churchill Livingstone; New York: 1996. Processing of samples. [Google Scholar]
- 10.Ling T.K.W., Tam P.C., Liu Z.K., Cheng A.F.B. Evaluation of VITEK 2 rapid identification and susceptibility testing system against gram-negative clinical isolates. J Clin Microbiol. 2001;39:2964–2966. doi: 10.1128/JCM.39.8.2964-2966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2017. Performance standards for antimicrobial susceptibility testing 27th edition. CLSI supplement M100. [Google Scholar]
- 12.Du Moulin G.C. Airway colonization by Flavobacterium in an intensive care unit. J Clin Microbiol. 1979;10:155–160. doi: 10.1128/jcm.10.2.155-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoque S.N., Graham J., Kaufmann M.E., Tabaqchali S. Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J Hosp Infect. 2001;47:188–192. doi: 10.1053/jhin.2000.0908. [DOI] [PubMed] [Google Scholar]
- 14.Lau S., Chow W., Foo C., Curreem S., Lo G.C., Teng J.L. Elizabethkingia anopheles bacteremia is associated with clinically significant infections and high mortality. Sci Rep. 2016;6:26045. doi: 10.1038/srep26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Y.C., Lo H.H., Hsieh H.Y., Chang S.M. Identification and epidemiological relatedness of clinical Elizabethkingia meningoseptica isolates from central Taiwan. J Microbiol Immunol Infect. 2014;47:318–323. doi: 10.1016/j.jmii.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi N., Mathur P., Bindra A., Goyal K., Sokhal N., Kumar S. Infections due to Elizabethkingia meningoseptica in critically injures trauma patients: a seven-year study. J Hosp Infect. 2016;92:30–32. doi: 10.1016/j.jhin.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Connell P.P., Wickremasinghe S., Devi U., Waters M.J., Allen P.J. Self-induced Elizabethkingia meningoseptica endophthalmitis: a case report. J Med Case Rep. 2011;5:303. doi: 10.1186/1752-1947-5-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagiya H., Ogawa H., Takahashi Y., Hasegawa K., Iwamuro M., Otsuka F. A nephrostomy-associated urinary tract infection caused by Elizabethkingia meningoseptica. Intern Med. 2015;54:3233–3236. doi: 10.2169/internalmedicine.54.4998. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y.S., Chun J.W., Koh J.W. Keratitis with Elizabethkingia meningoseptica occurring after contact lens wear: a case report. Korean J Ophthalmol. 2013;27:133–136. doi: 10.3341/kjo.2013.27.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesh V., Hegde A., Radhakrishna M. Clinical features and antimicrobial susceptibilities of elizabethkingia meningoseptica – an emerging pathogen from a tertiary care hospital in Mangalore. Asian J Pharm Clin Res. 2018;11(11):110–113. [Google Scholar]
- 21.Moore L.S., Owens D.S., Jepson A., Turton J.F., Ashworth S., Donaldson H. Waterborne Elizabethkingia meningoseptica in adult critical care. Emerg Infect Dis. 2016;22:9–17. doi: 10.3201/eid2201.150139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin P.Y., Chen H.L., Huang C.T., Su L.H., Chiu C.H. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteraemia. Int J Antimicrob Agents. 2010;36:436–440. doi: 10.1016/j.ijantimicag.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 23.Tak V., Mathur P., Varghese P., Misra M.C. Elizabethkingia meningoseptica: an emerging pathogen causing meningitis in a hospitalized adult trauma patient. Indian J Med Microbiol. 2013;31:293–295. doi: 10.4103/0255-0857.115653. [DOI] [PubMed] [Google Scholar]
- 24.Ratnamani M.S., Rao R. Elizabethkingia meningoseptica: emerging nosocomial pathogen in bedside hemodialysis patients. Indian J Crit Care Med. 2013;17:304–307. doi: 10.4103/0972-5229.120323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez L.J., Vila A.J. Carbapenem resistance in Elizabethkingia meningoseptica is mediated by metallo-β-lactamase BlaB. Antimicrob Agents Chemother. 2012;4:1686–1692. doi: 10.1128/AAC.05835-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira G.H., Garcia D.O., Abboud C.S., Barbosa V.L., Silva P.S. Nosocomial infections caused by Elizabethkingia meningoseptica: an emergent pathogen. Braz J Infect Dis. 2013;17:606–609. doi: 10.1016/j.bjid.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maraki S., Scoulica E., Manoura A., Papageorgiou N., Giannakopoulou C., Galanakis E. A Chryseobacterium meningosepticum colonization outbreak in a neonatal intensive care unit. Eur J Clin Microbiol Infect Dis. 2009;28:1415–1419. doi: 10.1007/s10096-009-0797-2. [DOI] [PubMed] [Google Scholar]
- 28.Di Pentima M.C., Mason E.O., Jr., Kaplan S.L. In vitro antibiotic synergy against Flavobacterium meningosepticum: implications for therapeutic options. Clin Infect Dis. 1998;26:1169–1176. doi: 10.1086/520309. [DOI] [PubMed] [Google Scholar]
- 29.Du Moulin G.C. Airway colonization by Flavobacterium in an intensive care unit. J Clin Microbiol. 1979;10:155–160. doi: 10.1128/jcm.10.2.155-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser S.L., Jorgensen J.H. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother. 1997;41:2738–2741. doi: 10.1128/aac.41.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y.T., Chiu C.H., Chan Y.J., Lin M.L., Yu K.W., Wang F.D. Clinical and microbiological analysis of Elizabethkingia meningoseptica bacteremia in adult patients in Taiwan. Scand J Infect Dis. 2009;41:628–634. doi: 10.1080/00365540903089476. [DOI] [PubMed] [Google Scholar]