Abstract
Background
Several cases of autism spectrum disorder have been linked to mutations in the SHANK3 gene. Haploinsufficiency of the SHANK3 gene contributes to Phelan-McDermid syndrome, which often presents an autism spectrum disorder phenotype along with moderate to severe intellectual disability. A SHANK3 gene deletion in mice results in elevated excitation of cortical pyramidal neurons that alters signaling to other brain areas. Serotonin 1A receptors are highly expressed on layer 2 cortical neurons and are known to have inhibitory actions. Serotonin 1A receptor agonist treatment in autistic cases with SHANK3 mutations and possibly other cases may restore excitatory and inhibitory balance that attenuates core symptoms.
Methods
A series of experiments investigated the effects of acute tandospirone treatment on spatial learning and self-grooming, subchronic treatment of tandospirone on self-grooming behavior, and the effect of tandospirone infusion into the anterior cingulate on self-grooming behavior.
Results
Only male Shank3B+/− mice exhibited a spatial learning deficit and elevated self-grooming. Acute i.p. injection of tandospirone, 0.01 and 0.06 mg/kg in male Shank3B+/− mice, attenuated a spatial acquisition deficit by improving sensitivity to positive reinforcement and reduced elevated self-grooming behavior. Repeated tandospirone (0.06 mg/kg) treatment attenuated elevated self-grooming behavior in male Shank3B+/− mice. Tandospirone injected into the anterior cingulate/premotor area reduced self-grooming behavior in male Shank3B+/− mice.
Conclusions
These results suggest that stimulation of cortical serotonin 1A receptors may reduce repetitive behaviors and cognitive impairments as observed in autism spectrum disorder, possibly by attenuating an excitation/inhibition imbalance. Further, tandospirone may serve as a treatment in autism spectrum disorder and other disorders associated with SHANK3 mutations.
Keywords: 5-HT1A receptor, tandospirone, autism, repetitive behaviors, learning, Shank3
Significance Statement.
The SHANK3 gene has been implicated in the pathogenesis of several autism cases as well as an haploinsufficiency syndrome, Phelan-McDermid, which commonly manifests an autism phenotype with intellectual disability. Past studies have predominantly used Shank3 homozygous mice to understand pathophysiology, behavior, and potential drug treatments. The current study characterized learning deficits and repetitive motor behaviors in Shank3 heterozygous mice and determined whether the partial 5-HT1A agonist, tandospirone, could alleviate behavioral impairments. Heterozygous Shank3 mice (Shank3B+/−) exhibited a probabilistic learning deficit and elevated self-grooming behavior. Both acute and chronic treatment with tandospirone alleviated the deficits. Direct infusions of tandospirone into the anterior cingulate region, a brain area found altered in autism, also reduced self-grooming behavior in Shank3B+/− mice. The results suggest that treatment with a partial 5-HT1A agonist may reduce both cognitive and repetitive motor behaviors in autism, at least in part, by acting on cortical circuitry.
Introduction
Autism spectrum disorder (ASD) is characterized by restricted interests and repetitive behaviors (RRBs) and social-communicative deficits (Harstad et al., 2015). There is evidence for both environmental (Grabrucker, 2012; Sealey et al., 2016) and genetic factors conferring risk (Lichtenstein et al., 2010; Robinson et al., 2016) for ASD. Accumulating evidence suggests that the SHANK3 gene is a high-risk gene related to ASD. SHANK3 mutations are associated with ASD (Boccuto et al., 2013; Soorya et al., 2013; Sanders et al., 2015) that includes Phelan-McDermid syndrome in which a high percentage of individuals meet criteria for an ASD diagnosis and is associated with haploinsufficiency of the SHANK3 gene (Bill and Geschwind, 2009; Bonaglia et al., 2011).
Because SHANK3 mutations have been implicated in contributing to ASD as well as other disorders (Soorya et al., 2013; Guilmatre et al., 2014; Zhang et al., 2017), several Shank3 mutant mouse lines have been developed to understand underlying neuropathophysiology (Bozdagi et al., 2010; Peca et al., 2011; Lee et al., 2015; Jaramillo et al., 2017; Bey et al, 2018; Drapeau et al., 2018). SHANK3 produces Shank3 proteins, which play a large role in anchoring scaffolding protein complexes to the F-actin cytoskeleton of both presynaptic axon terminals and postsynaptic densities (Bozdagi et al., 2010; Verpelli et al., 2011; Halbedl et al., 2016). The autism phenotype has been hypothesized to occur from an excitation/inhibition imbalance in the brain (Rubenstein and Merzenich, 2003). Studies of Shank3 mutations in mice have revealed altered glutamatergic transmission and synaptic plasticity (Bozdagi et al., 2010; Lee et al., 2015; Speed et al., 2015; Jaramillo et al., 2017; Bey et al., 2018) as well as greater activity of cortical neurons in medial prefrontal cortex and elevated self-grooming behavior (Lee et al., 2015; Wang et al., 2016). Therefore, treatments that reduce excitation of cortical neurons may be effective in reducing RRBs in Shank3 mutant mice.
Serotonin 1A receptors (5-HT1ARs) are expressed in the rodent cortex where activation produces an inhibitory effect on cortical pyramidal neurons (Chalmers and Watson, 1991). Related to RRBs, the partial 5-HT1AR agonist tandospirone reduces marble-burying behavior in Wistar rats (Abe et al., 1998). Other experiments demonstrated that drugs with mixed 5-HT1A/5-HT7R properties also reduce motor stereotypies in mice (Middlemiss and Fozard, 1983; Wood et al., 2000; Canal et al., 2015). Besides stereotyped motor behaviors, understanding the role of 5-HT1ARs on learning in Shank3 mutant mice is important as individuals with SHANK3 mutations show learning impairments (LeBlond et al., 2014; Egger et al., 2016). Spatial discrimination tests with probabilistic reinforcement have been developed to understand reward learning in ASD individuals (Solomon et al., 2011; D’Cruz et al., 2013, 2016) and in mouse models of autism (Amodeo et al., 2012, 2018). The same treatments that reduce stereotyped motor behaviors in rodents also enhance learning (Micheau and Van Marrewijk, 1999; Wolff et al., 2004). Taken together, the findings suggest that stimulation of 5-HT1ARs may improve behaviors in mice comparable with core symptoms in ASD. However, many treatments lack specificity for 5-HT1ARs and/or lead to significant side effects.
The present set of studies determined whether the partial 5-HT1AR agonist tandospirone attenuates elevated self-grooming and a probabilistic learning deficit in Shank3B+/− mice. Experiments included studying the effects of systemic treatment, both acute and chronic, as well as infusions into the anterior cingulate, a frontal cortex area identified where altered Shank3 expression may contribute to autism-like behaviors (Guo et al., 2019). Tandospirone possesses a high affinity for 5-HT1A receptors similar to buspirone but a significantly lower affinity for D2 receptors compared with the more commonly studied buspirone (Hamik et al., 1990). Therefore, tandospirone may be a more specific treatment for 5-HT1A receptors.
Methods
Subjects
Male and female Shank3+/− (HET) mice with a B6 background were acquired from the Jackson Laboratory (Bar Harbor, ME) to serve as founders for a Shank3B mouse colony. A trio (1 male, 2 female) breeding system was employed in a temperature-controlled vivarium. After 15 days, female mice were placed in individual housing (28-cm-wide × 17-cm-long × 12-cm-high plastic cage).
Mice genotypes were derived at a typical Mendelian rate. A 3-mm tail tissue sample was obtained from each pup at approximately day 15 for genotyping (Transnetyx, Memphis, TN). Pups were weaned at 21 days of age and group housed with same sex littermates. All mice were placed in individual housing at 5 weeks of age in preparation for behavioral testing procedures, which began when mice reached 7 weeks of age.
Unless noted, mice received food and water ad libitum throughout an experiment. A 12-hour-light/-dark cycle was employed throughout the study. Only Shank3B+/+ (wild type [WT]) and HET mice were tested. Shank3B−/− (knockout [KO]) were not tested. Animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago.
Sex Differences in Learning and Self-Grooming Behavior
Spatial Learning Apparatus
Training and testing were conducted in a black acrylic maze (76 cm long × 50 cm wide × 30 cm high) containing a start area that was distinguished by a center wall separating the start and choice areas. The choice area contained 2 distinct spatial areas separated by an acrylic piece (30 cm long × 16 cm high) extending from the back wall of the maze. Each spatial location contained a food well near the back wall and unique visual cues attached to their respective back and side walls. A small plastic door (10 cm high × 5 cm wide) was inserted into the center of the wall separating the start and choice areas.
Training
WT and HET mice were food restricted until reaching 85% of their ad libitum body weight. Training sessions began by placing a mouse into the start area. The start door was opened, allowing the mouse to enter the choice area. After consuming a one-half piece of Fruity Pebbles (Post Foods, St. Louis, MO) from each food well, the mouse returned to the start area. This sequence was repeated until 15 minutes had elapsed. Mice achieved training criterion after completing 6 or more trials within a 15-minute session for 2 consecutive days. Mice were trained for 2 to 5 days before testing.
Spatial Learning
All mice received an i.p. injection of sterile water 30 minutes prior to testing. Prior to testing, 1 location was designated the “correct” spatial location and contained a one-half piece of cereal on 80% of trials. On the other 20% of trials, the “incorrect” location was baited with a one-half piece of cereal. On each trial, a mouse was allowed to sample only 1 food well per trial. The first 2 trials always contained food reinforcement in the correct reward area. Between trials, the choice area was cleaned with a 2% quatricide solution to minimize the use of odor cues. Learning criterion was achieved when a mouse chose the correct location on 6 consecutive trials (Amodeo et al., 2017, 2018).
Self-Grooming Apparatus
The self-grooming test was conducted using an empty, clear plastic cage (28 cm wide × 17 cm long × 12 cm high) covered with a clear plastic cage filter top.
Procedure
Different WT and HET mice were tested for self-grooming and then used in the spatial learning test. Mice were placed in the apparatus 20 minutes after receiving an i.p. injection of sterile water. Mice were allowed to freely explore for 20 minutes. The first 10 minutes served as a habituation period. The second 10 minutes served as the test. A trained observer recorded the cumulative amount of time spent grooming all body regions. All materials were cleaned or replaced between tests.
Locomotor activity was measured during the self-grooming test. The apparatus was divided into equal 9.33-cm × 17-cm zones. Locomotor activity was measured as the total number of lines crossed, with a line crossing defined as any instance of all 4 paws of a mouse traveling from one zone to a different zone.
Acute Tandospirone Effect on Spatial Learning
Male WT and HET mice receiving vehicle treatment were used as controls in this experiment. All aspects of training and testing were as described above. Mice received an i.p. injection of sterile water or tandospirone at 0.01 or 0.06 mg/kg 30 minutes prior to testing. An error analysis was conducted to assess whether a deficit was driven by an early or late learning impairment (Solomon et al., 2011). Early learning was defined as all trials of the testing period prior to the first instance of 3 consecutive correct choices. All trials following the first instance of a mouse making 3 consecutive correct choices were defined as late learning. All errors made during late learning were scored as late learning errors.
Learning was further analyzed as a function of trial to trial feedback in a manner comparable with that applied to ASD individuals during probabilistic learning (D’Cruz et al., 2013). The number of trials on which a mouse made an error after receiving positive reinforcement for a correct choice on the previous trial was calculated and termed “win-stay” errors. The number of trials on which a mouse switched its choice to the incorrect spatial location after not receiving positive reinforcement for making a correct choice on the previous trial was calculated and termed “lose-shift” errors.
Acute Tandospirone Treatment on Self-Grooming Behavior
A separate cohort of mice was generated for this experiment as described above. Male WT and HET were tested for self-grooming after receiving treatment of sterile water, 0.01, 0.06, and 0.3 mg/kg tandospirone across 4 sessions following a Latin Square design. Successive tests for a given mouse occurred 1 week following the previous test.
Chronic Tandospirone Treatment on Self-Grooming Behavior
A separate cohort of male WT and HET mice were generated for this experiment as described above. Prior to behavioral testing, mice received either an i.p. injection of sterile water or tandospirone 0.06 mg/kg for 14 consecutive days. This repeated injection procedure was based on a previous study investigating the effects of chronic tandospirone treatment (Uehara et al., 2013). Twenty-four hours after the last injection, each mouse received a self-grooming test. All aspects of testing were as described above.
Tandospirone Injection Into the Anterior Cingulate on Self-Grooming Behavior
A separate cohort of male WT and HET mice were generated for this experiment as described above.
Stereotaxic Surgery
Each mouse (7 weeks of age) received stereotaxic surgery to bilaterally implant cannulae aimed at the anterior cingulate. Before surgery, each mouse received an i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). A 5-mm stainless-steel guide cannulae (Plastics One, Roanoke, VA) was implanted at a 10-degree angle aimed medially. The stereotaxic coordinates for the anterior cingulate were the following: 1.3 mm anterior to bregma, ±1.1 mm lateral, 1.5 mm below the skull. To minimize pain or discomfort, mice received s.c. administration of the anti-inflammatory meloxicam after surgery and for 2 days subsequently.
Microinfusion Procedure
Mice were first placed in a tapered plastic cone (Braintree Scientific Inc.) with a cut-out that allowed guide cannulae to protrude out. Mice were restrained in the plastic cone throughout the duration of the microinfusion procedure. A 33-gauge injection cannula was inserted into each guide cannula. The injection cannula extended 1 mm beyond the guide cannula tip. The injection cannulae were attached to polyethylene tubes (PE-20) connected to separate 10-μL syringes. The syringes were driven by a microinfusion pump with solutions infused in a volume of 0.2 μL per side for 2 minutes. The total volume infused was 0.2 μL per side. The cannulae were left in place for 30 seconds to allow drug diffusion around the injector tip. After removal of the injection cannulae, mice were removed from the plastic cone and left undisturbed in their home cage for 1 minute. Subsequently, mice were placed in the apparatus for testing.
Self-Grooming Procedure
All aspects of testing were as described above. Male WT and HET mice were tested for self-grooming behavior after receiving a microinfusion of sterile water, 1 μg/side and 5 μg/side tandospirone across 3 sessions following a Latin Square design. Successive tests for a given mouse occurred 1 week following the previous test.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism software. In Experiment 1, separate 2-way ANOVAs were conducted to determine if there was a significant difference between genotypes and sex in mice for spatial learning and self-grooming behavior. For all subsequent experiments, a 2-way ANOVA was conducted to determine if there was a significant difference for genotype or treatment in spatial learning or grooming measures. A 3-way ANOVA with repeated measures was conducted for early and late learning errors. Post-hoc Tukey multiple comparisons tests were used to determine significant differences between specific groups.
Results
Sex Differences in Learning and Self-Grooming Behavior
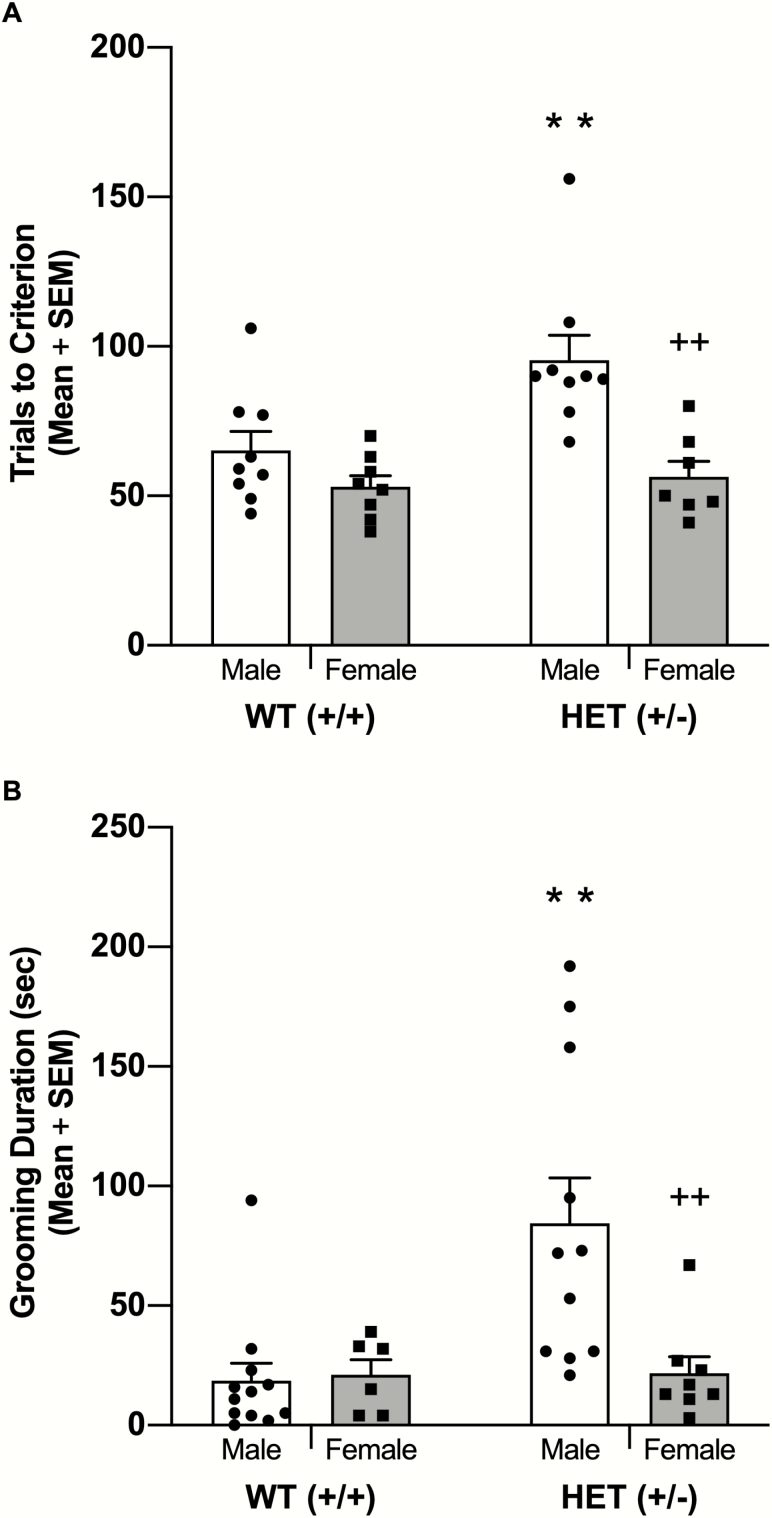
The initial study examined male and female Shank3B HET mice on spatial learning with probabilistic reinforcement. Male HET mice required approximately 100 trials to achieve criterion while other groups required 60 to 65 trials (see Figure 1A). A 2-way ANOVA revealed significant main effects for sex (F1,29 = 15.67, P < .001) and genotype (F1,29 = 6.76, P = .015) as well as a significant sex × genotype interaction (F1,29 = 4.29, P = .047). Post-hoc comparisons revealed that male HET mice required significantly more trials to criterion compared with that of all other groups (P < .01).
Figure 1.
(A) Male+/− (HET) mice required significantly greater number of trials to criterion on a spatial discrimination with probabilistic reinforcement compared with that of wildtype (WT) and female HET mice. (B) Male HET mice exhibited elevated grooming behavior compared with that of WT and female HET mice. **P < .01 vs WT, ++P < .01 vs male HET mice.
The self-grooming results are shown in Figure 1B. There were significant main effects for sex (F1,33 = 5.06, P = .031) and genotype (F1,33 = 6.18, P = .018) as well as a significant sex × genotype interaction (F1,33 = 5.97, P = .020). Post-hoc comparisons revealed that male HET mice spent significantly more time grooming compared with that of all other groups (P < .05).
Tandospirone Effects on Spatial Learning
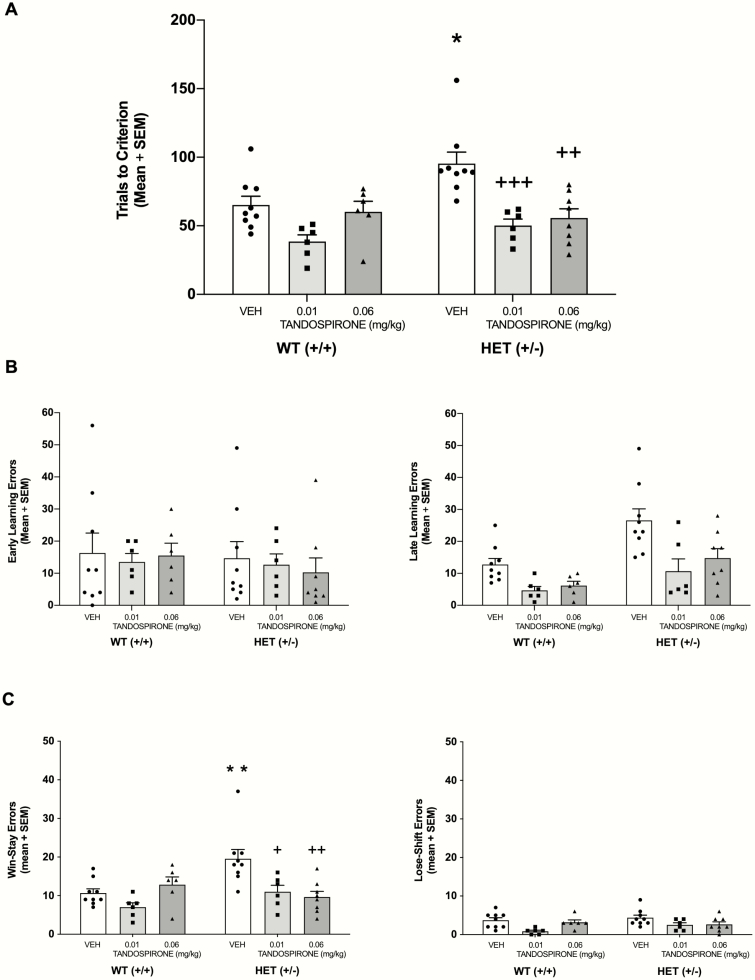
Because only male HET mice exhibited a spatial learning deficit, tandospirone was examined in only male WT and HET mice (see Figure 2). The analysis revealed significant main effects for genotype (F1,38 = 4.61, P = .038) and treatment (F2,38 = 13.81, P < .001) as well as a significant genotype × treatment interaction (F2,38 = 3.29, P = .048). A post-hoc test indicated that HET mice were significantly impaired in spatial learning compared with that of WT mice (P < .05). Administration of 0.01 and 0.06 mg/kg tandospirone significantly reduced trials to criterion in HET mice compared with that of vehicle-treated HET mice (P < .001 and P < .01, respectively) and to a level that did not significantly differ from than that of vehicle-treated WT mice (P > .05).
Figure 2.
The beneficial effect of tandospirone treatment on spatial acquisition with probabilistic reinforcement in male+/− (HET) mice. (A) Vehicle-treated HET mice required an increased number of trials to criterion compared with that of wildtype (WT) mice. Tandospirone at 0.01 and 0.06 mg/kg significantly reduced the number of trials to criterion in HET mice. (B) HET mice exhibited an increase in late, but not early, learning errors that was attenuated by tandospirone. (C) HET mice had significantly greater number of Win-Stay errors that was attenuated by tandospirone. *P < .05 vs WT VEH, **P < .01 vs WT VEH, +++P < .001 vs HET VEH, ++P < .01 vs HET VEH, +P < .05 vs HET VEH.
Analysis of early and late learning errors indicated all groups exhibited a comparable level of errors early in learning. Late in learning, vehicle-treated HET mice exhibited an increase in errors that was decreased by tandospirone treatment (see Figure 2B). This is reflected by a significant genotype × error interaction (F1,37 = 5.62, P = .023). However, tandospirone also decreased late learning errors in WT mice, leading to an overall main effect for treatment (F2,37 = 5.33, P = .009). There were no other significant main effects or interactions. Thus, male HET mice could initially learn the spatial discrimination at a similar rate as WT mice but were impaired in the later phase of learning that was attenuated by tandospirone.
Analysis of win-stay and lose-shift errors during the late phase of learning revealed that HET mice exhibited an increase in win-stay errors compared with that of WT mice, which was reversed by tandospirone treatment (see Figure 2C). There were significant main effects of genotype, (F1,38 = 4.83, P = .034) and treatment (F2,38 = 6.29, P = .004). Additionally, there was a significant genotype × treatment interaction (F2,38 = 6.22, P = .005). A post-hoc test indicated that vehicle-treated HET mice committed a significantly greater number of win-stay errors than vehicle-treated WT mice (P < .01). In addition, HET mice that received tandospirone treatment at a dose of either 0.01 or 0.06 mg/kg committed significantly fewer win-stay errors than vehicle-treated HET mice (P < .05 and P < .01, respectively) and to a level that was not significantly different from that of vehicle-treated WT mice (P > .05). For lose-shift errors, WT and HET mice made a similar amount of errors. Tandospirone, particularly at the low dose, appeared to reduce lose-shift errors in both WT and HET mice. The analysis revealed there was no significant effect for genotype (F1,38 = 1.21, P = .278), but there was a significant main effect for treatment (F2,38 = 6.30, P = .004). There was no significant genotype × treatment interaction (F2, 38 = 1.26, P = .30). Thus, tandospirone treatment attenuated the learning deficit in male HET mice by improving sensitivity to positive reinforcement late in learning.
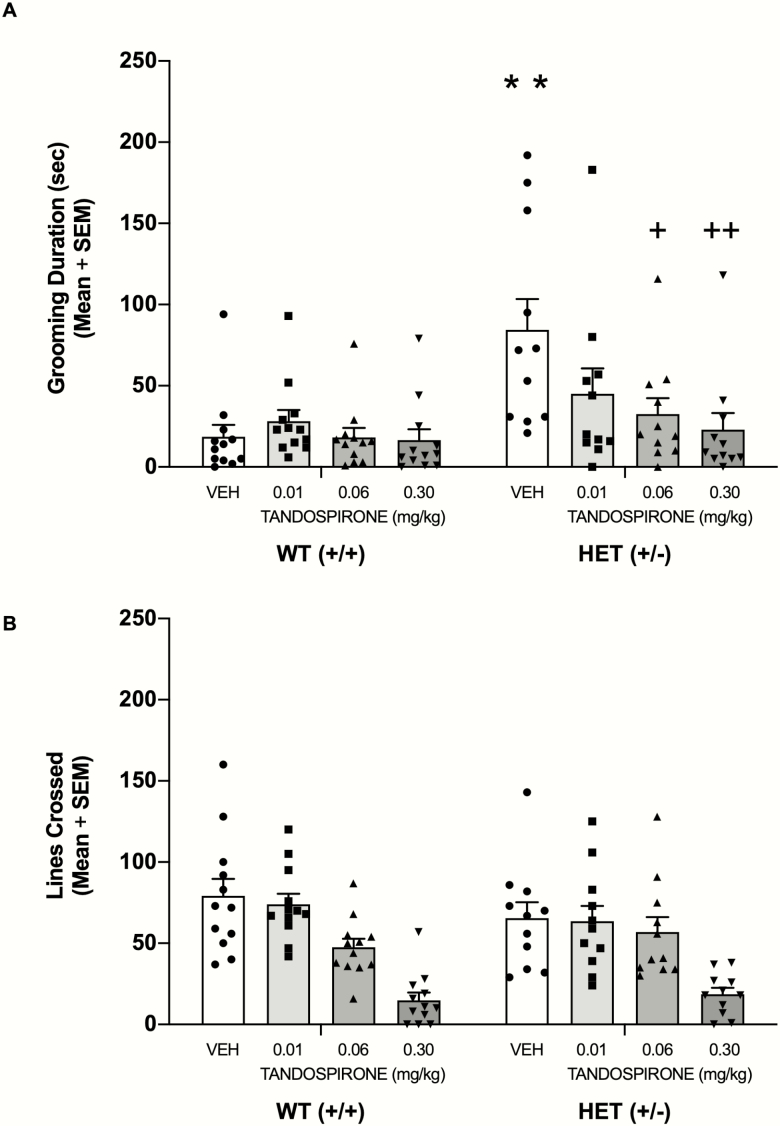
Acute Tandospirone Effects on Self-Grooming and Locomotion
Male HET mice groomed for approximately 90 seconds compared with 20 seconds for WT mice. Tandospirone, in a dose-dependent manner, reduced self-grooming behavior in male HET mice (see Figure 3A). The analysis indicated a significant main effect of genotype (F1,84 = 11.53, P = .001) and a significant main effect of treatment (F3,84 = 3.37, P = .022). The analysis also indicated that there was a significant genotype × treatment interaction (F3,84 = 3.14, P = .029). A post-hoc test revealed that vehicle-treated HET mice had significantly greater grooming behavior than vehicle-treated WT mice (P < .01). Tandospirone treatment at the 0.06- and 0.3-mg/kg doses in HET mice significantly reduced self-grooming compared with that of vehicle-treated HET mice (P < .05 and P < .01, respectively) and to a level that was not significantly different from that of vehicle-treated WT mice (P > .05). In contrast, tandospirone treatment at 0.01 mg/kg in HET mice did not significantly decrease self-grooming behavior compared with that of vehicle-treated HET mice (P > .05). Moreover, tandospirone at all doses tested in WT mice did not significantly affect self-grooming behavior compared with that of vehicle-treated WT (P > .05).
Figure 3.
Tandospirone, in a dose-dependent manner, alleviates elevated self-grooming behavior in male+/− (HET) mice. (A) Tandospirone significantly reduced grooming behavior in HET mice at doses of 0.06 and 0.3 mg/kg, but not 0.01 mg/kg. (B) Tandospirone at the 0.30-mg/kg dose reduced the number of line crossings in both wildtype (WT) and HET mice. **P < .01 vs WT VEH, ++P < .01 vs HET VEH, +P < .05 vs HET VEH.
Acute treatment with tandospirone, particularly at the highest dose, reduced locomotor activity in both genotypes (see Figure 3B). There was no significant effect for genotype (F1,84 = 0.08, P = .78), but there was a significant effect for treatment (F3, 84 = 16.81, P < .0001). The genotype × treatment interaction was not significant (F3, 84 = 1.04, P = .38).
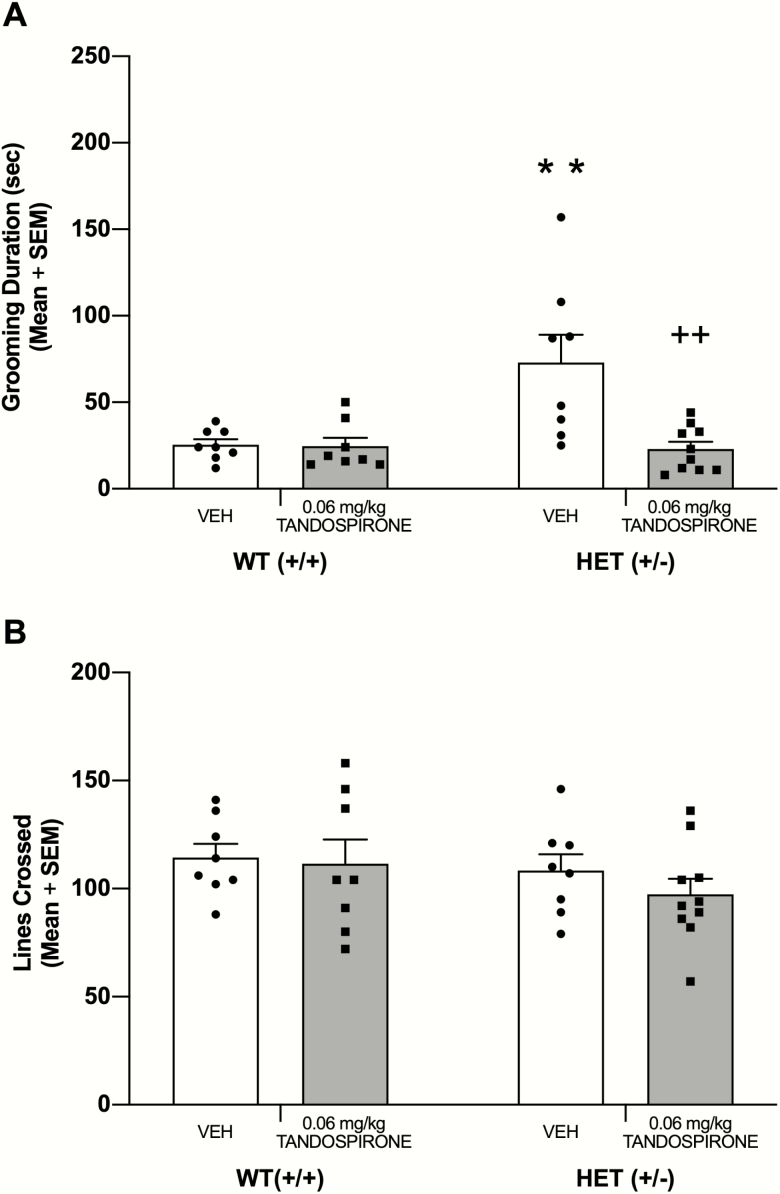
Chronic Tandospirone Treatment on Self-Grooming Behavior
The effects of tandospirone treatment administered for 14 days was examined on self-grooming behavior in male HET and WT mice (see Figure 4A). The 0.06-mg/kg dose was chosen because acute administration reduced self-grooming without affecting locomotor activity. Prior to chronic treatment, all mice received a self-grooming test because HET mice exhibited a bimodal level of self-grooming in the acute treatment experiment (see supplementary Figure 1). To ensure that HET mice in the vehicle and tandospirone groups had similar levels of self-grooming behavior prior to treatment, mice were pseudo-randomly assigned to a treatment group. The vehicle-treated group had a mean grooming duration of 65.38 seconds ± 17.58 SEM, and the tandospirone-treated group had a mean grooming duration of 62.00 seconds ± 13.15 SEM.
Figure 4.
Repeated tandospirone treatment attenuated elevated self-grooming behavior in male+/− (HET) mice. (A) Fourteen days of tandospirone injections significantly reduced grooming behavior in HET mice when tested 24 hours after the last injection. (B) Repeated tandospirone treatment did not affect number of lines crossed during self-grooming test. **P < .01 vs wildtype (WT) VEH, ++P < .01 vs HET VEH.
After 2 weeks of treatment, all mice were again tested for self-grooming behavior. There was a significant main effect of genotype (F1,30 = 7.49, P = .01), a significant main effect of treatment (F1,30 = 9.28, P = .005), and a significant genotype × treatment interaction (F1,30 = 8.48, P = .007). Post-hoc tests revealed that vehicle-treated HET mice had significantly greater grooming behavior than vehicle-treated WT mice (P < .01). Chronic administration of tandospirone at 0.06 mg/kg in HET mice significantly reduced self-grooming behavior compared with that of vehicle-treated HET mice (P < .01) and to a level not significantly different from that of vehicle-treated WT mice (P > .05). Chronic tandospirone treatment in WT mice did not significantly affect self-grooming behavior compared with that of vehicle-treated WT mice (P > .05).
Chronic treatment with tandospirone (0.06 mg/kg) did not affect locomotor activity during the self-grooming test (see Figure 4B). There was no significant effect for genotype (F1,30 = 1.48, P = .23), nor was there was a significant effect for treatment (F1, 30 = 0.70, P = .41). There also was no significant genotype × treatment interaction (F6, 84 = 1.20, P = .32).
Tandospirone Injection Into the Anterior Cingulate on Self-Grooming Behavior
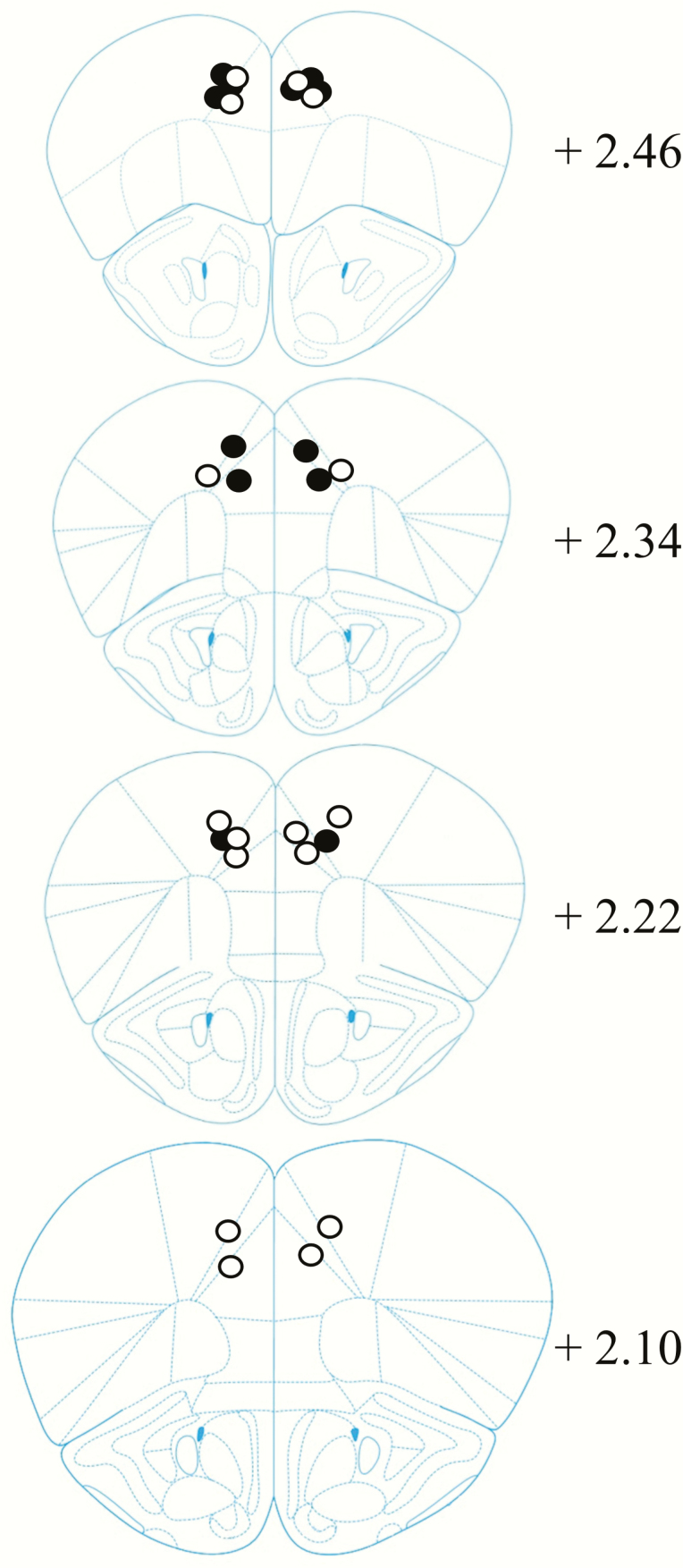
Histological analysis indicated that cannula placements were principally located in the rostral anterior cingulate at the level of forceps minor of the corpus callosum (Figure 5). Some mice had a cannula placement in the premotor area juxtaposed to the anterior cingulate.
Figure 5.
Cannula tip placements in the anterior cingulate and premotor areas of the mouse frontal cortex for mice included in the behavioral analyses. Mouse brain sections adapted from The Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin 2001). Black circle, cannula placements for wildtype (WT) mice; white circle, cannula placements for male+/− (HET) mice.
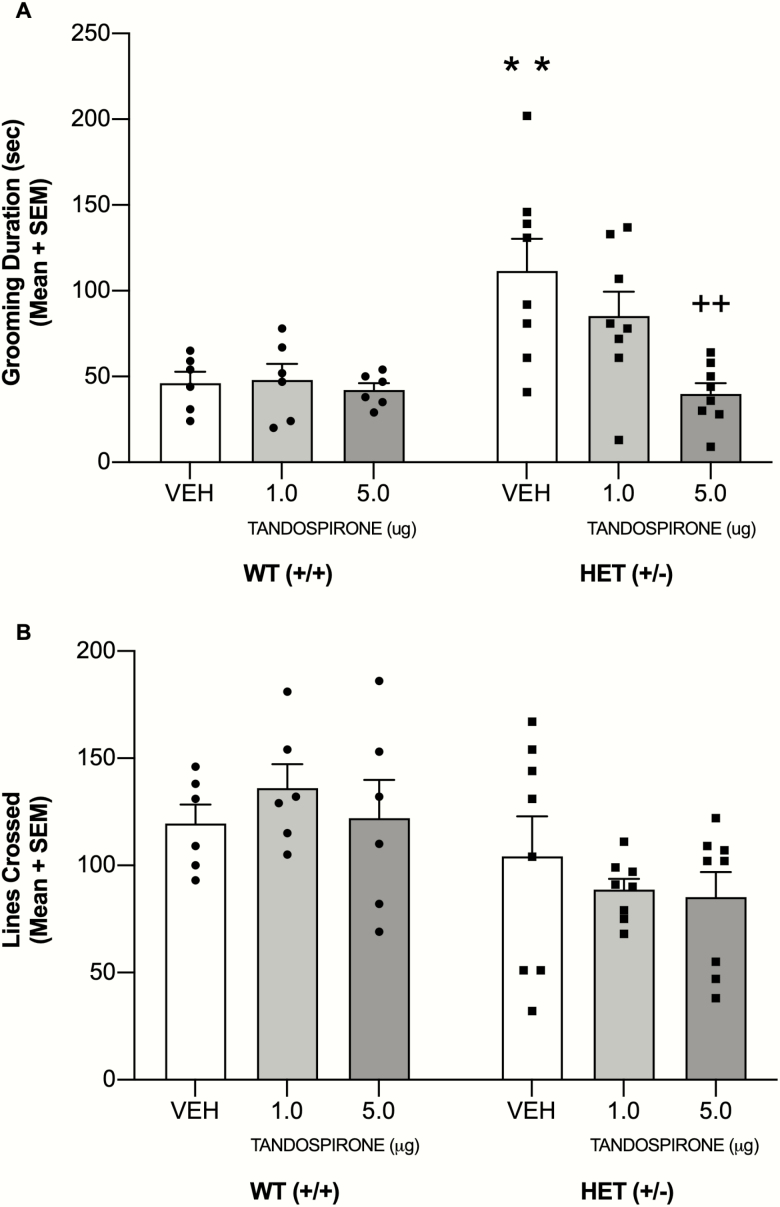
Analysis of grooming behavior following injections into the anterior cingulate revealed there was a significant main effect of genotype (F1,36 = 11.07, P = .002), a significant main effect of treatment (F2,36 = 4.92, P = .013), and a significant genotype × treatment interaction (F2,36 = 3.81, P = .032) (see Figure 6). Post-hoc tests revealed that vehicle-treated HET mice had significantly greater grooming behavior than vehicle-treated WT mice (P < .01). Tandospirone 1 μg in HET mice did not affect self-grooming behavior compared with that of vehicle-treated HET mice (P > .05). In contrast, tandospirone 5 μg in HET mice significantly reduced grooming behavior compared with that of vehicle-treated HET mice (P < .01) and to a level that was not significantly different from that of vehicle-treated WT mice (P > .05). Anterior cingulate infusion of tandospirone in WT mice did not significantly affect self-grooming compared with that of vehicle treatment (P > .05).
Figure 6.
(A) Tandospirone infusion into the anterior cingulate, in a dose-dependent manner, reduced self-grooming behavior in male+/− (HET) mice. (B) HET mice exhibited reduced line crossings compared with that of wildtype (WT) mice. Tandospirone did not affect line crossings in WT or HET mice. **P < .01 vs WT VEH, ++P < .01 vs HET VEH.
Analysis of locomotor activity during self-grooming revealed a significant effect for genotype (F1,36 = 9.12, P = .005), reflecting that HET mice exhibited fewer line crossings compared with that of WT mice across all treatments. There was no significant effect for treatment (F2, 36 = 0.27, P = .77) or significant genotype × treatment interaction (F2, 36 = 0.74, P = .48).
Discussion
The results indicate that male HET mice, but not female HET mice, exhibit a spatial learning deficit when reinforcement contingencies are probabilistic. Male HET mice showed a similar learning rate as WT mice early in acquisition but were impaired later in learning. This acquisition impairment is comparable with that observed in a visual discrimination test using Shank3B HET mice (Copping et al., 2017). The present study extends those past findings by using a probabilistic learning test, as used with ASD individuals (Solomon et al., 2011; D’Cruz et al., 2013), and revealing HET mice display a learning deficit due to reduced positive reinforcement sensitivity. These results are consistent with a study in ASD individuals showing impaired probabilistic learning resulting from a limited ability to use positive feedback for learning (Solomon et al., 2011). Thus, the male Shank3B HET mouse may model some of the learning deficits observed in ASD individuals.
Tandospirone treatment at both doses tested rescued the learning deficit in male HET mice. Further, increased win-stay errors late in learning were alleviated by tandospirone treatment. That tandospirone reduced win-stay errors in male HET mice suggests that activation of 5-HT1ARs is sufficient to increase reward sensitivity and facilitate learning when outcomes are probabilistic. Future studies that explore whether tandospirone may also alleviate a probabilistic reversal learning deficit in HET mice and whether female HET mice exhibit a deficit in probabilistic reversal learning that can be treated with tandospirone will be important to better understand the role of 5-HT1ARs in cognition related to ASD.
Similar to spatial learning, the self-grooming results indicate that male, but not female, HET mice exhibit elevated self-grooming behavior. Thus, for both learning and grooming, there was a sex difference. For individuals with haploinsufficiency of the SHANK3 gene, there does not appear to be a sex difference in learning and RRBs (Soorya et al., 2013; Zwanenberg et al., 2016). One possibility is that the sex difference observed in the present study is related to the deletion site in Shank3B mice, and a more extensive deletion or different deletion site may lead to a similar phenotype in female and male HET mice.
Increased self-grooming behavior in male HET mice was bimodal, with approximately one-half of the mice exhibiting grooming around 120 seconds while the other one-half had a grooming duration of 30 seconds (see supplementary Data). The increased grooming behavior in male HET mice is consistent with a study demonstrating Shank3BE13 heterozygous mice also show elevated grooming behavior (Jaramillo et al., 2017). In contrast, another experiment found that HET mice did not display elevated grooming behavior (Drapeau et al., 2014). The difference in behavioral results may be related to the deletion site within the SHANK3 gene. HET mice used by Drapeau et al., (2014) were generated with a deletion of the ankyrin repeat domain (exons 4–9), whereas HET mice in the present experiment possessed a deletion of the PDZ domain (exons 13–16). Six intragenic promoters have been identified in SHANK3, and transcripts for both promoters existing between the ankyrin and PDZ domains continue to be expressed in Shank3e4-9 KO mice (Wang et al., 2011). Thus, deletion of exons 4 to 9 in HET mice could have produced Shank3 proteins with an intact PDZ domain, which is expected to be absent in the HET mice used in the present experiment. By extension, this difference in self-grooming behavior between HET mouse models seems to specifically implicate a relationship between the Shank3 PDZ domain and stereotyped motor behavior. Consistent with this idea, Jaramillo et al., (2017) found disrupting the Shank3 PDZ domain by inserting a transcriptional stop cassette prior to exon 13 led to male Shank3E13 heterozygous mice displaying elevated self-grooming behavior similar to the male HET mice in the present study.
The 2 highest doses of tandospirone tested with acute injection reduced grooming behavior in the male HET group. Although tandospirone at 0.3 mg/kg reduced grooming behavior, this dose also significantly decreased locomotor activity, indicating this higher dose had a more general effect on motor activity. In contrast, the 0.06-mg/kg dose decreased grooming without affecting locomotor activity. Moreover, acute treatment also tended to reduce grooming behavior even in male HET mice who were “low groomers.” Although this was not significant, this was likely due to the low grooming values under the vehicle condition. Further, chronic treatment with tandospirone at 0.06 mg/kg was also effective in reducing self-grooming in male HET mice without affecting locomotor activity. Taken together with the findings on probabilistic learning, the results indicate that tandospirone treatment can alleviate both a learning impairment and an elevated stereotyped motor behavior without affecting general activity levels.
Systemic tandospirone administration improving learning and reducing grooming behavior in male HET mice raises the issue of what neural circuitry may be affected by stimulating 5-HT1ARs to rescue the phenotype. Recent findings indicate that conditional KO of Shank3 in the anterior cingulate region of mice leads to social interaction deficits, while restoration of SHANK3 in the anterior cingulate region rescues the deficit (Guo et al., 2019). Based on these recent findings, we determined whether direct infusions of tandospirone into the anterior cingulate affected self-grooming behavior. The results indicate that tandospirone into the anterior cingulate, in a dose-dependent manner, reduced elevated self-grooming in HET mice without affecting locomotor activity. Thus, the anterior cingulate may be one region in which stimulating 5-HT1ARs may alter cortical signaling to reduce RRBs.
At a neural systems level, one possibility is that atypical neuronal signaling originating in 1 or more areas of cortex, that is, anterior cingulate, preferentially projects to the basal ganglia direct pathway with greater than typical excitatory drive (Lei et al., 2004). If learning deficits and RRBs in ASD are generated as a product of relatively greater activation of the direct vs indirect pathway of the basal ganglia as previously suggested (Wang et al., 2011; Shepherd, 2013), then tandospirone may rescue the phenotype in Shank3 HET mice by reducing cortical excitatory drive of the striatum (De Almeida et al., 2008). Such a mechanism would be consistent with the findings of Peixoto et al. (2016), who demonstrated that mini excitatory postsynaptic currents of striatal medium spiny neurons in Shank3B KO mice can be reduced by chemogenetic inhibition of layer 5 cortical pyramidal neurons. Because 5-HT1ARs are primarily expressed on pyramidal neurons in cortical layers 2, 3, and 6, tandospirone might reduce elevated cortico-striatal glutamatergic transmission by reducing excitation of layer 2 pyramidal neurons on target layer 5a neurons (Anderson et al., 2010). Layer 5a pyramidal neurons project exclusively to the striatum (Lei et al., 2004; Reiner et al., 2010). This proposed effect of tandospirone could produce a downstream effect of restoring balance of excitation and inhibition in the basal ganglia by minimizing glutamatergic input onto direct pathway MSNs.
The ability of tandospirone to alleviate both a stereotyped motor behavior and a learning deficit without affecting motor behavior more broadly suggests the drug may be effective in treating a range of RRBs while having limited side effects. Future studies testing the effects of tandospirone on a wider range of learning and stereotyped motor behaviors in this mouse model can help determine this. If tandospirone is able to improve core symptoms with minimal side effects will be important because the sedative effect of risperidone that accompanies its usefulness in alleviating some symptoms of ASD is problematic to the extent that it often becomes the reason individuals stop treatment (Lemmon et al., 2011). There is no evidence from the present experiments to suggest that a similar undesirable effect is induced by tandospirone even with chronic treatment. On the contrary, the findings from the present experiments demonstrate that treatment with tandospirone effectively and selectively reduces self-grooming behavior and a probabilistic learning deficit in Shank3B HET mice. Thus, this treatment may facilitate daily living in some ASD individuals, such as those that have a SHANK3 mutation. More broadly, the present findings suggest that 5-HT1AR agonism could be a viable treatment approach for individuals with ASD or other disorders associated with a SHANK3 mutation that express RRBs or cognitive deficits.
Supplementary Material
Acknowledgments
We are grateful for all the technical assistance that Pamela Teneqexhi provided in the breeding and genotyping of mice.
This research was supported by National Institutes of Health (NIH) grant HD084953.
Statement of Interest
None.
References
- Abe M, Nakai H, Tabata R, Saito K, Egawa M (1998) Effect of 5-[3-[((2S)-1,4-benzodioxan-2-ylmethyl)amino]propoxy]-1,3-benzodioxole HCl (MKC-242), a novel 5-HT1A-receptor agonist, on aggressive behavior and marble burying behavior in mice. Jpn J Pharmacol 76:297–304. [DOI] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME (2012) Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res 227:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Rivera E, Cook EH Jr, Sweeney JA, Ragozzino ME (2017) 5HT2A receptor blockade in dorsomedial striatum reduces repetitive behaviors in BTBR mice. Genes Brain Behav 16:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Cuevas L, Dunn JT, Sweeney JA, Ragozzino ME (2018) The adenosine A2A receptor agonist, CGS 21 680, attenuates a probabilistic reversal learning deficit and elevated grooming behavior in BTBR mice. Autism Res 11:223–233. [DOI] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T, Shepherd GM (2010) Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci 13:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey AL, Wang X, Yan H, Kim N, Passman RL, Yang Y, Cao X, Towers AJ, Hulbert SW, Duffney LJ, Gaidis E, Rodriguiz RM, Wetsel WC, Yin HH, Jiang YH (2018) Brain region-specific disruption of Shank3 in mice reveals a dissociation for cortical and striatal circuits in autism-related behaviors. Transl Psychiatry 8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH (2009) Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev 19:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L, Lauri M, Sarasua SM, Skinner CD, Buccella D, Dwivedi A, Orteschi D, Collins JS, Zollino M, Visconti P, Dupont B, Tiziano D, Schroer RJ, Neri G, Stevenson RE, Gurrieri F, Schwartz CE (2013) Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur J Hum Genet 21:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, et al. (2011) Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. Plos Genet 7:e1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD (2010) Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Felsing DE, Liu Y, Zhu W, Wood JT, Perry CK, Vemula R, Booth RG (2015) An orally active phenylaminotetralin-chemotype serotonin 5-HT7 and 5-HT1A receptor partial agonist that corrects motor stereotypy in mouse models. ACS Chem Neurosci 6:1259–1270. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Watson SJ (1991) Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain–a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res 561:51–60. [DOI] [PubMed] [Google Scholar]
- Copping NA, Berg EL, Foley GM, Schaffler MD, Onaga BL, Buscher N, Silverman JL, Yang M (2017) Touchscreen learning deficits and normal social approach behavior in the Shank3B model of Phelan-McDermid syndrome and autism. Neuroscience 345:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA (2013) Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology 27:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz AM, Mosconi MW, Ragozzino ME, Cook EH, Sweeney JA (2016) Alterations in the functional neural circuitry supporting flexible choice behavior in autism spectrum disorders. Transl Psychiatry 6:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida J, Mengod G (2008) Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochem 107:488–496. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Dorr NP, Elder GA, Buxbaum JD (2014) Absence of strong strain effects in behavioral analyses of Shank3-deficient mice. Dis Model Mech 7:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Riad M, Kajiwara Y, Buxbaum JD (2018) Behavioral phenotyping of an improved mouse model of Phelan-McDermid syndrome with a complete deletion of the Shank3 gene. eNeuro 5:ENEURO.0046-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger JI, Zwanenburg RJ, van Ravenswaaij-Arts CM, Kleefstra T, Verhoeven WM (2016) Neuropsychological phenotype and psychopathology in seven adult patients with Phelan-McDermid syndrome: implications for treatment strategy. Genes Brain Behav 15:395–404. [DOI] [PubMed] [Google Scholar]
- Grabrucker AM. (2012) Environmental factors in autism. Front Psychiatry 3:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmatre A, Huguet G, Delorme R, Bourgeron T (2014) The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 74:113–122. [DOI] [PubMed] [Google Scholar]
- Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, Yao H, Yang J, Liu H, Liu Y, Jia F, Qi C, Lynn-Jones T, Hu H, Fu Z, Feng G, Wang W, Wu S (2019) Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci 22:1223–1234. [DOI] [PubMed] [Google Scholar]
- Halbedl S, Schoen M, Feiler MS, Boeckers TM, Schmeisser MJ (2016) Shank3 is localized in axons and presynaptic specializations of developing hippocampal neurons and involved in the modulation of NMDA receptor levels at axon terminals. J Neurochem 137:26–32. [DOI] [PubMed] [Google Scholar]
- Hamik A, Oksenberg D, Fischette C, Peroutka SJ (1990) Analysis of tandospirone (SM-3997) interactions with neurotransmitter receptor binding sites. Biol Psychiatry 28:99–109. [DOI] [PubMed] [Google Scholar]
- Harstad EB, Fogler J, Sideridis G, Weas S, Mauras C, Barbaresi WJ (2015) Comparing diagnostic outcomes of autism spectrum disorder using DSM-IV-TR and DSM-5 Criteria. J Autism Dev Disord 45:1437–1450. [DOI] [PubMed] [Google Scholar]
- Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Escamilla CO, Weaver TP, Liu S, Filonova I, Powell CM (2017) Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res 10:42–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, et al. (2014) Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. Plos Genet 10:e1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Chung C, Ha S, Lee D, Kim DY, Kim H, Kim E (2015) Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front Cell Neurosci 9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A (2004) Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci 24:8289–8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon ME, Gregas M, Jeste SS (2011) Risperidone use in autism spectrum disorders: a retrospective review of a clinic-referred patient population. J Child Neurol 26:428–432. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H (2010) The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry 167:1357–1363. [DOI] [PubMed] [Google Scholar]
- Micheau J, Van Marrewijk B (1999) Stimulation of 5-HT1A receptors by systemic or medial septum injection induces anxiogenic-like effects and facilitates acquisition of a spatial discrimination task in mice. Prog Neuropsychopharmacol Biol Psychiatry 23:1113–1133. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Fozard JR (1983) 8-Hydroxy-2-(di-n-propylamino)-tetralin discriminates between subtypes of the 5-HT1 recognition site. Eur J Pharmacol 90:151–153. [DOI] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G (2011) Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RT, Wang W, Croney DM, Kozorovitskiy Y, Sabatini BL (2016) Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B(-/-) mice. Nat Neurosci 19:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Hart NM, Lei W, Deng Y (2010) Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front Neuroanat 4:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, et al. ; iPSYCH-SSI-Broad Autism Group (2016) Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet 48:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, et al. ; Autism Sequencing Consortium (2015) Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87:1215–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey LA, Hughes BW, Sriskanda AN, Guest JR, Gibson AD, Johnson-Williams L, Pace DG, Bagasra O (2016) Environmental factors in the development of autism spectrum disorders. Environ Int 88:288–298. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG. (2013) Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Smith AC, Frank MJ, Ly S, Carter CS (2011) Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res 4:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, Frank Y, Wang AT, Cai G, Parkhomenko E, Halpern D, Grodberg D, Angarita B, Willner JP, Yang A, Canitano R, Chaplin W, Betancur C, Buxbaum JD (2013) Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Kouser M, Xuan Z, Reimers JM, Ochoa CF, Gupta N, Liu S, Powell CM (2015) Autism-associated insertion mutation (InsG) of Shank3 Exon 21 causes impaired synaptic transmission and behavioral deficits. J Neurosci 35:9648–9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Matsuoka T, Itoh H, Sumiyoshi T (2013) Chronic treatment with tandospirone, a 5-HT(1A) receptor partial agonist, suppresses footshock stress-induced lactate production in the prefrontal cortex of rats. Pharmacol Biochem Behav 113:1–6. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, Di Stefano B, Mantegazza R, Broccoli V, Böckers TM, Dityatev A, Sala C (2011) Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem 286:34839–34850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH (2011) Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet 20:3093–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. (2016) Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat Commun 7:11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff M, Costet P, Gross C, Hen R, Segu L, Buhot MC (2004) Age-dependent effects of serotonin-1A receptor gene deletion in spatial learning abilities in mice. Brain Res Mol Brain Res 130:39–48. [DOI] [PubMed] [Google Scholar]
- Wood M, Chaubey M, Atkinson P, Thomas DR (2000) Antagonist activity of meta-chlorophenylpiperazine and partial agonist activity of 8-OH-DPAT at the 5-HT(7) receptor. Eur J Pharmacol 396:1–8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao B, Xiong Y, Zheng F, Xu X, Yang Y, Hu Y, Wang X (2017) Expression of SHANK3 in the temporal neocortex of patients with intractable temporal epilepsy and epilepsy rat models. Cell Mol Neurobiol 37:857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanenburg RJ, Ruiter SA, van den Heuvel ER, Flapper BC, Van Ravenswaaij-Arts CM (2016) Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.