Abstract
Background
Opioid use during pregnancy is a significant public health issue. The standard of care for treating opioid use disorder during pregnancy includes medications for opioid disorder (MOUD). However, tobacco use often goes unaddressed among pregnant women on MOUD. In 2018, our team received a National Institute on Drug Abuse (NIDA) funded R34 to conduct a three year-randomized trial to test the feasibility of a novel tobacco intervention for pregnant women receiving MOUD.
Aims
The aims of this study are: (1) to determine the impact of the B-EPIC intervention on maternal tobacco use and stage of change; (2) to determine the impact of B-EPIC on tobacco-related maternal and infant health outcomes including gestational age at birth, birthweight, NAS diagnosis and severity, and number of ear and respiratory infections during the first six months; (3) to compare healthcare utilization and costs incurred by pregnant patients that receive the B-EPIC intervention versus TAU.
Methods
We plan to enroll 100 pregnant women on MOUD for this randomized controlled trial (B-EPIC intervention n = 50 and treatment as usual n = 50). A major strength of this study is its wide range of health and economic outcomes assessed on mother, neonate and the infant.
Conclusions
Despite the very high rates of smoking among pregnant women with OUD, there are few tobacco treatment interventions that have been tailored for this high - risk population. The overall goal of this study is to move towards a tobacco treatment standard for pregnant women receiving treatment for OUD.
Keywords: tobacco Treatment, Opioid dependence, Pregnancy
1. Introduction
Opioid use and untreated opioid dependence (Diagnostic and Statistical Manual of Mental Disorder, 4th Edition, DSM 4) during pregnancy is a significant public health issue associated with complications such as intrauterine growth restriction, placental abruption, preterm delivery, cesarean delivery, and stillbirth [1]. Intravenous opioid use increases the risk of infectious diseases such as hepatitis C and endocarditis [2], which complicate pregnancy [3,4]. As opioid use in the U.S. has increased, there has been a dramatic increase in the prevalence of neonatal abstinence syndrome (NAS) [5]. From 2004 to 2014, the total healthcare costs covered by Medicaid for infants with NAS increased from $65.4 million to $462 million [6].
The standard of care for treating opioid use disorder (OUD) during pregnancy includes FDA-approved medication for opioid use disorder (MOUD; also known as medication assisted treatment), including methadone or buprenorphine. [7].According to the Substance Abuse and Mental Health Service Administration (SAMHSA), these medications help pregnant women stop injection drug use and reduce withdrawal and cravings [8]. However, tobacco use often goes unaddressed, which is unfortunate because pregnant women in MOUD programs have high rates of smoking (88–95%) [9,10] and smoking is an independent risk factor for several maternal and infant adverse health outcomes [9]. For instance, smoking during pregnancy can lead to miscarriage [11], premature birth [12], and sudden infant death syndrome [13]. In addition to the general risks, smoking during pregnancy is associated with higher amounts of medication needed to treat NAS, longer duration of treatment, and longer hospital stays [14]. Smoking during pregnancy also has adverse economic consequences for families and the healthcare system. Maternal smoking increases the risk admission to the neonatal intensive care unit (NICU) by 19% [15], which results in significant smoking-attributable hospital costs during the episode of delivery.
Smoking cessation has immense benefits for women and their families over the long-term. Infants born to mothers who smoke during pregnancy are more likely to be readmitted to the hospital during their first year of life [16], more likely to experience asthma requiring medication use, and are more likely to use the emergency room [17]. Further, smoking increases the risk for several women's health issues, including breast cancer [18], osteoporosis [19], and infertility [20]. Smoking cessation is particularly important for women with substance use disorders, because smoking has been associated with an increased risk of relapse [21].
Few tailored tobacco treatment interventions have been tested for pregnant women on MOUD. Across these studies, a small percentage of participants stopped smoking [10,22], indicating the need for a more intense, tailored intervention for this population. One study showed promise using an intervention that included contingency-behavioral incentives [23], but effects of contingency incentives often cease after the intervention period ends. Incentives are difficult to implement in real-world clinical practice as there is no current way to reimburse for them. Thus, there is a critical need to develop clinical care models that incorporate feasible and effective behavioral interventions for tobacco use among pregnant women receiving opioid use disorder treatment.
In 2018, our team received a National Institute on Drug Abuse (NIDA) funded R34 to conduct a three year-randomized trial to test the feasibility of a novel tobacco intervention (Behavioral and Enhanced Perinatal Intervention for tobacco Cessation; B-EPIC) for pregnant women receiving comprehensive buprenorphine treatment for their OUD. The purpose of this paper is to present the B-EPIC study design, intervention and intervention framework, and data analysis plan.
2. Methods
2.1. Study aims
The study aims are to:
Aim 1. To determine the impact of the B-EPIC intervention on maternal tobacco use and stage of change [24] during and after pregnancy compared to the tobacco treatment as usual (TAU) control group among women with opioid dependence receiving MOUD.
Hypothesis 1
The B-EPIC group will have a greater percentage of perinatal women who quit smoking, decrease number of cigarettes smoked per day, and/or increase their readiness to quit smoking compared to TAU.
Aim 2. To determine the impact of B-EPIC on tobacco-related maternal and infant health outcomes including gestational age at birth, birthweight, NAS diagnosis and severity, and number of ear and respiratory infections during the first six months.
Hypothesis 2
Women in the B-EPIC intervention will have a gestational period of at least 37 weeks and their infants will experience less severe NAS (e.g, number of days in the neonatal intensive care unit or total mg of morphine needed to treat NAS) and fewer associated childhood illnesses (e.g, frequency of ear and respiratory infections), and increased number of well-child visits compared to TAU.
Aim 3: To compare healthcare utilization and costs incurred by pregnant patients that receive the B-EPIC intervention versus TAU, with estimates of the incremental cost-effectiveness of the B-EPIC intervention.
Hypothesis 3
Infants of participants in B-EPIC will have fewer hospitalizations and hospital days, as well as fewer NICU admissions, emergency department (ED) visits, and sick-child outpatient visits. As a secondary hypothesis, we will investigate whether infants also demonstrate greater adherence to the recommended well-child visits. Reduced healthcare utilization associated with B-EPIC will yield a favorable incremental cost-effectiveness ratio.
2.2. Intervention framework
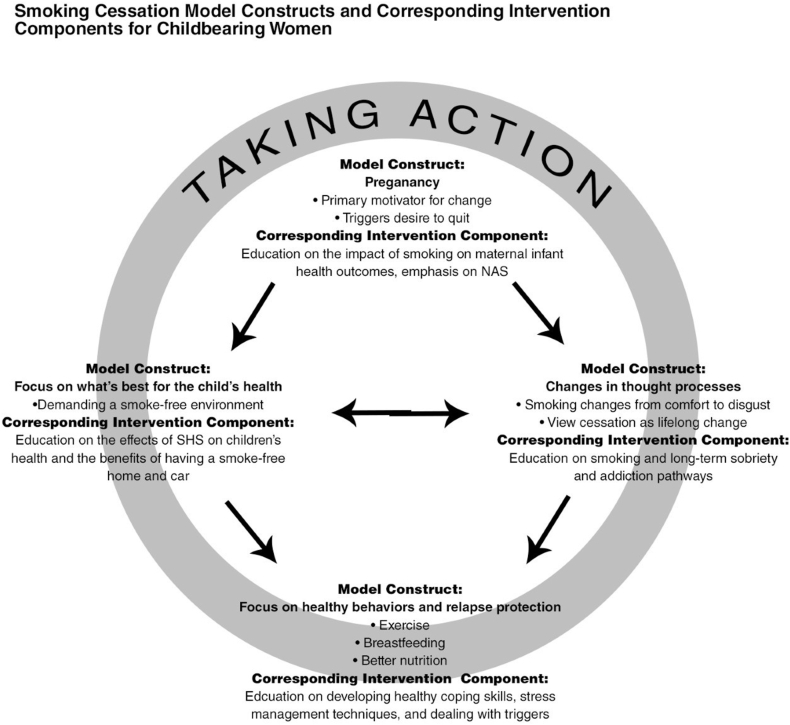
We conducted two qualitative studies that provided the foundation for the theoretical framework used to guide B-EPIC. First, our team conducted a qualitative study to describe factors that contribute to successful maintenance of smoking cessation during the postpartum period among women who quit smoking during pregnancy (n = 11) (although they did not have OUD). Primary motivators and lifestyle characteristics of women who do not relapse to cigarette smoking [25] for at least 6 months were investigated. Women's narratives described the process of postpartum smoking abstinence. Four themes emerged and provide the basis for the holistic theoretical model Smoking Cessation Model for Childbearing Women (Fig. 1): a) pregnancy is the primary motivator for change; b) changes in thought processes that promote quit attempts and help maintain abstinence; c) focus on healthy behaviors, which may serve as healthy coping skills/replacement smoking behaviors and prevention of smoking lapse/relapses; and d) child's health as primary motivator for cessation [25].
Fig. 1.
Model guiding the B-EPIC intervention based on qualitative data collection
Second, we conducted a qualitative study among pregnant women with opioid dependence (DSM 4) in an MOUD clinic who were being treated with buprenorphine (n = 22), to assess attitudes toward smoking cessation, facilitators and barriers to smoking cessation, and past experiences with smoking cessation attempts [26]. The women reported: a) a desire to stop smoking for their health and the health of their baby, which was similar to the previous study and provided further support for the idea that smoking cessation interventions would be welcome by pregnant patients receiving MOUD; b) complex barriers to cessation, including the use of tobacco products to deal with chronic stress, which fits into the need to develop healthy coping and relapse prevention skills for stress; c) periodic nicotine abstinence in tobacco-free rehabilitation facilities and in prison, which supports that abstinence is feasible; and d) desire for intensive support for tobacco cessation, which suggested that the intervention would need regular follow-up and opportunities for supplemental help [22]. Lessons learned from these narrative accounts have been integrated into the Smoking Cessation Model [21] to inform the B-EPIC intervention, as shown in Fig 2.
3. Study design
3.1. Study setting and sample
The goal is to enroll and randomize 100 pregnant women receiving MOUD treatment with buprenorphine for OUD. The study recruitment is ongoing at two sites in Lexington, Kentucky. Recruitment began at University of Kentucky Healthcare's (UKHC) buprenorphine treatment program for pregnant women with OUD, and later expanded to include Baptist Health Lexington's (BHL) similar prenatal buprenorphine MOUD program.
3.2. Study eligibility
Planned eligibility for this study included: 1) current diagnosis of opioid dependence (DSM 4) with participation in the UKHC integrated prenatal care and addiction treatment program with buprenorphine pharmacotherapy; 2) pregnant and less than 24 weeks gestation; 3) age 18–49 years old; 4) diagnosis of current tobacco use disorder; and 5) ability to read or write in English. Women are excluded if they have current prisoner status, diagnosed current severe mental illness, or alcohol or sedative/hypnotic dependence that requires medical intervention. Given recruitment challenges, two modifications were made to the eligibility criteria: 1) recruitment was expanded to include participants in the Baptist Health Lexington integrated prenatal care and addiction treatment program with buprenorphine pharmacotherapy, and 2) participants of gestational age up to 32 weeks.
4. Procedure
4.1. Recruitment
There are two methods of recruitment: 1) study flyers are posted in highly visible areas in the two MOUD clinics for pregnant/postpartum women and 2) in-person invitation by a research nurse. At an initial prenatal visit in the buprenorphine clinic, clinic staff determines patient's smoking status. For all confirmed smokers, clinic staff assess their willingness to receive study information from a research nurse. If the patient is agreeable, a research nurse explains the purpose and procedures of the study, and determines initial eligibility for participation. Women who are interested in participating and meet eligibility requirements are then taken through the informed consent process by a research nurse prior to initiating any study procedures.
4.2. Randomization
Eligible participants are randomly assigned to one of two groups: TAU tobacco treatment (control group) or B-EPIC group (tobacco intervention) or. Participants are randomized to study group using SAS PLAN (SAS Institute, Inc) that incorporates a stratification procedure based on age (30+ verse less than 30). All participants continue to receive opioid dependence treatment with buprenorphine integrated with prenatal care, regardless of treatment assignment.
4.3. Tobacco treatment as usual (TAU)
Women enrolled in the TAU control group receive standard of care from their healthcare provider, which is the American College of Obstetrics and Gynecology (ACOG) recommended 5 A's approach [27] (see Table 2). This standard takes approximately 5–15 min and is offered by the healthcare provider at each prenatal and postpartum appointment.
Table 2.
ACOG's 5 A's treatment as usual.
|
4.4. B-EPIC intervention
Women enrolled in the intervention group will receive TAU plus B-EPIC, which includes four core components: 1) individualized tobacco treatment plus supplemental counseling providing intensive support, 2) change in maternal thought process and adoption of healthy behavior, in accordance with the findings from our previous qualitative work [25,26], 3) biomarker validation and feedback [28], and 4) pharmacotherapy targeted at tobacco cessation as needed. See Table 3 for a summary of the study visits and the components of each visit for the TAU and B-EPIC groups. The initial assessment for this intervention takes 60 min, with follow-up sessions typically lasting 15–20 min. All sessions occur prior to or after pre-scheduled perinatal appointments.
Table 3.
Summary of study visits.
| Time points | B-EPIC -Taking Action | Treatment as Usual | Outcomes | Incentives |
|---|---|---|---|---|
| Baseline (up to 32 weeks) |
|
ACOG recommended 5A's conducted by healthcare provider |
|
$20 gift card |
| 3rd trimester (28–36.6 weeks) |  |
 |
|
$20 gift card |
| After Delivery of Baby (2–8.6 weeks) |
|
$40 gift card | ||
| After Delivery of Baby (20–28.6 weeks) |
|
$40 gift card |
Note: American College of Obstetricians and Gynecologists (ACOG); Certified Tobacco Treatment Specialist (CTTS); if all four study visits are completed, participant receives an additional $20 gift card; Survey covers demographics, prenatal history, self-report tobacco use, nicotine dependence, secondhand smoke exposures, self-recall substance use, recovery capital, perceived stress, adverse childhood events, anxiety, and depression.
The intervention will be led by a research nurse who is certified as a certified tobacco treatment specialist (CTTS). A CTTS is a professional who possesses the skills, knowledge and training to provide effective, evidence-based interventions for tobacco dependence across a range of intensities. CTTS's are trained in core competencies for the delivery of tobacco dependence treatment consistent with established evidence (see Table 1 for CTTS core competencies). Assessment, motivational counseling, and treatment planning (for both cessation and relapse), which are tailored to the unique needs of tobacco users, are core skills used in the delivery of CTTS services. Based on the CTTS assessment, intervention materials are tailored based on each woman's individual reasons for being interested in smoking cessation (Table 4). Further, in accordance with the model guiding this study, CTTS's are trained to empower women to adopt a smoke-free home environment to support their cessation efforts and to avoid secondhand smoke exposure [25].
Table 1.
Certified tobacco Treatment Specialists Core Competencies.
|
Table 4.
B-EPIC intervention topicsa.
|
Information presented to participants is tailored based on their personal reasons for wanting to stop smoking.
Perinatal Pharmacotherapy: According to the U.S. Preventive Services Task Force, nicotine replacement therapy has not been sufficiently evaluated for safety or efficacy during pregnancy [29]. ACOG recommends that nicotine replacement therapy be used during pregnancy only after a discussion of the risks of both continuing to smoke and of using nicotine replacement therapy, under close medical supervision, and with patients who express a strong desire to quit smoking [27]. The CTTS provides clear information about the pharmacotherapy options that are available (e.g, benefits, contraindications, and side effects) and will communicate with the participant's obstetrician about participant's interest in pharmacotherapy, if desired by the participant.
5. Measures
A major strength of this study is its wide range of health and economic outcomes assessed on mother, neonate and the infant.
5.1. Primary outcomes
The primary outcomes for Aim 1 are smoking cessation, decrease in number of cigarettes smoked per day, and readiness to quit smoking. Smoking cessation is defined as a urine cotinine assay <100 ng/mL. Cotinine is a metabolite of nicotine and has a half-life of approximately 9 h in pregnant women [30]. A commercial urine assay, NicAlert ®, with cut-off limits of urine cotinine levels is a valid assay to measures urine cotinine levels and to verify conventional cigarette use. Cigarettes per day is measured via self-report on a survey. Readiness to quit is measured using a ladder scale with ten options to indicate an individual's readiness to quit smoking [24].
The primary outcomes for Aim 2 are gestational age at birth, birthweight, NAS diagnosis and severity, and number of ear and respiratory infections during the first 5–6 months of age. All these data will be collected via medical record review or by linking to Kentucky Medicaid claims data. Gestational age at birth is defined as age in weeks on delivery day, based on estimated weeks at first ultrasound. Birthweight is defined as the first infant weight on day of delivery in grams. NAS severity is determined based on the modified Finnegan Scale [31]. The International Statistical Classification of Diseases and Related Health Problems (typically abbreviated as ICD) diagnostic codes will be collected from well-child and sick visits and hospital admissions and re-admissions to determine ear and respiratory infections (e.g, tinnitus unspecified is code 388.30).
The primary outcomes for Aim 3 are hospitalizations, hospital days, NICU days, ED visits and sick-child outpatient visits for infants. These data will be collected primarily from Kentucky Medicaid claims data, with supplementary information from the medical record (for non-Medicaid study participants).
5.2. Data analysis
Descriptive analysis, including means, standard deviations and frequency distribution summarize all study outcomes. Baseline characteristics between the intervention group and the control, and between those who complete the study and those who drop out will be examined for potential covariates. These comparisons will be done using t-sample t-tests, Mann-Whitney U tests, or chi-square tests of association, and significant outcomes will be used as covariates in the following analyses. Primary outcomes to be compared between treatment groups will include tobacco indicators, such as smoking and illicit drug use status (abstinence via urine cotinine) smoking frequency and readiness to quit (cigarettes per day and stages of change), and maternal-child measures (gestational age of birth, birthweight, NAS, NAS severity, early childhood respiratory and ear infections). The collected variables will include not only these primary outcomes and covariates, but also feasibility indicators, such as average number of sessions completed and satisfaction ratings of the intervention by participants randomized to the intervention group. We will also make group comparisons between other variables that may affect maternal-child outcomes, including second hand smoke (SHS) exposure, infant feeding status at discharge (breastfeeding vs. formula), and number of people smoking in the home. The continuous outcomes for the first two study aims will be analyzed with a two-factor (group x time) repeated measures mixed model. Generalized estimating equation (GEE), analyses with an exchangeable correlation structure will assess for significant differences between groups and across time for categorical and dichotomous outcomes (e.g., preterm birth, NAS) using the SAS GENMOD procedure. The main effects of group and time and the interaction between them will be included in each model, and covariates will be added to control for group differences. As appropriate, post-hoc pairwise comparisons will be calculated using Fisher's least significant difference procedure for mixed models and using contrast statements for GEE models. All analyses will be conducted using SAS 9.4 (SAS Institute, Inc) and considered significant at p ≤ 0.05.
For Aim 3, the economic analysis will combine outcomes data from Aim 2 (gestational age, birthweight) with cost data (direct and indirect program costs, infant health services utilization) to assess incremental cost effectiveness from a health system/payer perspective. Direct program costs will include personnel time directly associated with intervention delivery, including logged staff time and travel, but also pro rata portions of supervision and office costs and any other costs that may be associated with care delivery (e.g. printed materials). Indirect program costs will include personnel time, materials, or facilities associated with general program development and operations. This will be derived from grant budgets and information supplied by community partners. Health services utilization (e.g. physician office visit, emergency department visit, hospitalization) will be costed out using average payment rates for these services, by age, gender and year (source: Medical Expenditure Panel Survey). Any facility usage (square feet) will be converted to an imputed rent amount, using local rental rates (per square foot) for office properties in the same zip code. This offers a conservative (high) estimate of the cost of space and represents the opportunity cost of the space. Personnel time will be converted into monetary costs using actual wage rates available through grant budgets or Bureau of Labor Statistics (BLS) area wage surveys. To facilitate translation of our results to other settings while maintaining privacy, our publications will summarize labor cost data by category (direct program, indirect program, participant) and subcategory (e.g. faculty, staff, participant), and include information on both time (in hours) and average hourly wage rates.
The within-trial, incremental cost effectiveness ratio will be computed as the ratio of the (1) difference in costs (B-EPIC group minus TAU group) and (2) the difference in clinical outcomes. Separate estimates will be generated for each outcome measure (gestational age, birthweight). We will also conduct sensitivity analyses where we dichotomize outcomes (preterm, low birth weight). All estimates of costs will be adjusted for inflation and transformed to net present value using an annual discount rate. The results of this exploratory economic analysis will provide critical preliminary data that will be used to estimate the statistical power for a larger future study.
5.3. Sample size
Sample size for this project was premised with a power of 0.8, alpha = 0.05 and medium effect sizes. These assumptions were based upon previous studies conducted with similar experimental designs and outcomes. With at least 30 mother/baby dyads completing the study per group and an alpha level of 0.05, the power of the repeated measures analysis of variance F test to detect a significant main for group will be 79%, assuming a medium effect size, while the power of the F tests to detect a significant main effect of time or group by time interaction will be at least 95%. In a preliminary study of this population by our group (EMPOWR), we found that 14% of pregnant women decreased their cigarette consumption between intake (typically during the second trimester) and the third trimester. While smoking cessation is the ultimate goal of this intervention, prior research has demonstrated that cutting cigarette consumption below 8 cigarettes per day is a risk reduction strategy for preventing low birthweight, so we will consider outcomes of both successful cessation as well as reduction in consumption for this project. Analysis of these power estimates indicates that 100 subjects (50 per group) completing the proposed study will be sufficient to detect significant main effects of treatment group.
6. Discussion
This study has several innovative features. First, the B-EPIC intervention is based on a novel theoretical framework developed by the Principal Investigators based on previous qualitative work among pregnant and postpartum tobacco and opioid dependent women, including those receiving buprenorphine treatment. Second, this feasibility study has the potential to lead to a larger, multi-site trial intervention, which if successful, would have the capacity to shift clinical practice paradigms to give specific emphasis on tobacco use rather than letting it fall into general group discussions on illicit substance use and psychosocial issues. Current standard of care does not prioritize tobacco treatment for pregnant women with OUD. Due to time constraints on clinicians, and the complex issues facing many women with substance use disorders, tobacco often does not receive priority of attention. Third, we are conducting a comprehensive evaluation of this major public health issue, including a wide-range of maternal, neonatal, and infant health outcomes, as well as associated healthcare costs and cost effectiveness of the B-EPIC intervention.
There is a high likelihood of adoption, scalability and sustainability of B-EPIC in clinical practice settings for two primary reasons. First, The B-EPIC intervention was designed to be integrated into programs providing comprehensive treatment for OUD, including buprenorphine, and across the country, there are a growing number of buprenorphine providers (e.g., over 111,000 providers who are waivered to provide buprenorphine) [32]. Second, the intervention relies on CTTS, who are providing billable services that are reimbursed from private and public insurers, and there are CTTS training programs available across the country in-person and via virtual platforms [33].
Limitations. This study also has several limitations. First, the validity of the FTND may vary across sex, gender and race/ethnicity [34,35]. Additionally, some healthcare providers have concerns that using urine cotinine to validate smoking status may their clinical relationship with their patients [36]. However, pregnant and postpartum women who smoked before or during pregnancy have reported that point-of-care cotinine testing had benefits, including encouraging conversations around tobacco cessation [36]. Further, in order to rigorously evaluate the impact of the intervention on smoking outcomes, it is necessary to verify smoking status. Previous research indicates that more than 25% of pregnant and postpartum women did not disclose tobacco use but had a positive urine cotinine screening [37].
7. Conclusion
Smoking during pregnancy is a serious maternal child health issue. Despite the very high rates of smoking among pregnant women with OUD, there are few tobacco treatment interventions that have been tailored for this high - risk population. The overall goal of this study is to move towards a tobacco treatment standard for pregnant women receiving treatment for OUD.
Declaration of competing interest
1) We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
2) We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
3) We confirm that neither the entire paper nor any of its content has been submitted, published, or accepted by another journal. The paper will not be submitted elsewhere if accepted for publication in the Journal.
4) We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
5) We confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
6) We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Acknowledgements
This study was funded by the National Institute on Drug Abuse NIDA R34DA046005-02.
References
- 1.Maeda A., Bateman B.T., Clancy C.R., Creanga A.A., Leffert L.R. Opioid Abuse and dependence during Pregnancy Temporal trends and obstetrical outcomes. J. Am. Soc. Anesthesiol. 2014;121(6):1158–1165. doi: 10.1097/ALN.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 2.Kuehn B. Opioid use disorder during pregnancy. J. Am. Med. Assoc. 2018;320(12):1232. doi: 10.1001/jama.2018.13546. [DOI] [PubMed] [Google Scholar]
- 3.Ko J.Y., Haight S.C., Schillie S.F., Bohm M.K., Dietz P.M. National trends in hepatitis C infection by opioid use disorder status among pregnant women at delivery hospitalization—United States, 2000–2015. MMWR (Morb. Mortal. Wkly. Rep.) 2019;68(39):833. doi: 10.15585/mmwr.mm6839a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelson P.K., Bernstein S.N. Management of the cardiovascular complications of substance use disorders during pregnancy. Curr. Treat. Options Cardiovasc. Med. 2019;21(11):73. doi: 10.1007/s11936-019-0777-5. [DOI] [PubMed] [Google Scholar]
- 5.McQueen K., Murphy-Oikonen J. Neonatal abstinence syndrome. N. Engl. J. Med. 2016;375(25):2468–2479. doi: 10.1056/NEJMra1600879. [DOI] [PubMed] [Google Scholar]
- 6.Winkelman T.N., Villapiano N., Kozhimannil K.B., Davis M.M., Patrick S.W. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid: 2004–2014. Pediatrics. 2018;141(4) doi: 10.1542/peds.2017-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists . 2017. Opioid Use Disorder and Opioid Use Disorder in Pregnancy. [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration . 2018. Clinical Guidance For Treating Pregnant And Parenting Women With Opioid Use Disorder And Their Infants Rockville, MD. [Google Scholar]
- 9.Chisolm M.S., Fitzsimons H., Leoutsakos J.-M.S. A comparison of cigarette smoking profiles in opioid-dependent pregnant patients receiving methadone or buprenorphine. Nicotine Tob. Res. 2013;15(7):1297–1304. doi: 10.1093/ntr/nts274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akerman S.C., Brunette M.F., Green A.I., Goodman D.J., Blunt H.B., Heil S.H. Treating tobacco use disorder in pregnant women in medication-assisted treatment for an opioid use disorder: a systematic review. J. Subst. Abuse Treat. 2015;52:40–47. doi: 10.1016/j.jsat.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pineles B.L., Park E., Samet J.M. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am. J. Epidemiol. 2014;179(7):807–823. doi: 10.1093/aje/kwt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlin S., Gunnerbeck A., Wikström A.-K., Cnattingius S., Edstedt Bonamy A.-K. Maternal tobacco use and extremely premature birth – a population-based cohort study. BJOG An Int. J. Obstet. Gynaecol. 2016;123(12):1938–1946. doi: 10.1111/1471-0528.14213. [DOI] [PubMed] [Google Scholar]
- 13.Anderson T.M., Ferres J.M.L., Ren S.Y. Maternal smoking before and during pregnancy and the risk of sudden unexpected infant death. Pediatrics. 2019;143(4) doi: 10.1542/peds.2018-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones H.E., Heil S.H., Tuten M. Cigarette smoking in opioid-dependent pregnant women: neonatal and maternal outcomes. Drug Alcohol Depend. 2013;131(3):271–277. doi: 10.1016/j.drugalcdep.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Räisänen S., Sankilampi U., Gissler M. Smoking cessation in the first trimester reduces most obstetric risks, but not the risks of major congenital anomalies and admission to neonatal care: a population-based cohort study of 1 164 953 singleton pregnancies in Finland. J. Epidemiol. Community Health. 2014;68(2):159–164. doi: 10.1136/jech-2013-202991. [DOI] [PubMed] [Google Scholar]
- 16.Ralser E., Mueller W., Haberland C. Rehospitalization in the first 2 years of life in children born preterm. Acta Paediatr. 2012;101(1):e1–e5. doi: 10.1111/j.1651-2227.2011.02404.x. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland F.D., Li Y.-F., Peters J.M. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am. J. Respir. Crit. Care Med. 2001;163(2):429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 18.Andersen Z.J., Jørgensen J.T., Grøn R., Brauner E.V., Lynge E. Active smoking and risk of breast cancer in a Danish nurse cohort study. BMC Canc. 2017;17(1):556. doi: 10.1186/s12885-017-3546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bijelic R., Milicevic S., Balaban J. Risk factors for osteoporosis in postmenopausal women. Med. Arch. 2017;71(1):25–28. doi: 10.5455/medarh.2017.71.25-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarokhani M., Veisani Y., Mohamadi A. Association between cigarette smoking behavior and infertility in women: a case-control study. Biomed. Res. Ther. 2017;4(10):1705–1715. [Google Scholar]
- 21.Weinberger A.H., Platt J., Esan H., Galea S., Erlich D., Goodwin R.D. Cigarette smoking is associated with increased risk of substance use disorder relapse: a nationally representative, prospective longitudinal investigation. J. Clin. Psychiatr. 2017;78(2):e152–e160. doi: 10.4088/JCP.15m10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallin-Bennett A., Rademacher K., Dye H., Elswick A., Ashford K., Goodin A. Perinatal navigator approach to smoking cessation for women with prevalent opioid dependence. West. J. Nurs. Res. 2019;41(8):1103–1120. doi: 10.1177/0193945918825381. [DOI] [PubMed] [Google Scholar]
- 23.Tuten M., Fitzsimons H., Chisolm M.S., Nuzzo P.A., Jones H.E. Contingent incentives reduce cigarette smoking among pregnant, methadone-maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction. 2012;107(10):1868–1877. doi: 10.1111/j.1360-0443.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudreaux E.D., Sullivan A., Abar B., Bernstein S.L., Ginde A.A., Camargo C.A. Motivation rulers for smoking cessation: a prospective observational examination of construct and predictive validity. Addiction Sci. Clin. Pract. 2012;7(1):8. doi: 10.1186/1940-0640-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashford K., Hahn E., Hall L., Peden A.R., Rayens M.K. Postpartum smoking abstinence and smoke-free environments. Health Promot. Pract. 2011;12(1):126–134. doi: 10.1177/1524839909353727. [DOI] [PubMed] [Google Scholar]
- 26.Fallin A., Miller A., Ashford K. Smoking among pregnant women in outpatient treatment for opioid dependence: a qualitative inquiry. Nicotine Tob. Res. 2016;18(8):1727–1732. doi: 10.1093/ntr/ntw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists Smoking cessation during pregnancy. Committee opinion No. 721 web site. 2017. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Smoking-Cessation-During-Pregnancy Published.
- 28.Borrelli B., Endrighi R., Hammond S.K., Dunsiger S. Smokers who are unmotivated to quit and have a child with asthma are more likely to quit with intensive motivational interviewing and repeated biomarker feedback. J. Consult. Clin. Psychol. 2017;85(11):1019. doi: 10.1037/ccp0000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S Preventive Services Task Force Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions. 2015. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1 Updated September 2015. Accessed 24 Jan, 2020. [DOI] [PubMed]
- 30.Dempsey D., Jacob P., 3rd, Benowitz N.L. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J. Pharmacol. Exp. Therapeut. 2002;301(2):594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 31.Maguire D., Cline G.J., Parnell L., Tai C.-Y. Validation of the Finnegan neonatal abstinence syndrome tool–short form. Adv. Neonatal Care. 2013;13(6):430–437. doi: 10.1097/ANC.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 32.Substance Abuse and Mental Health Services Administration . 2020. National Waiver Titles.https://www.samhsa.gov/medication-assisted-treatment/practitioner-program-data/certified-practitioners Accessed 2020, 14 Jan. [Google Scholar]
- 33.Council for Tobacco Treatment Training Programs Tobacco treatment specialist training workshop calendar. 2020. https://ctttp.org/calendar-of-trainings/ Accessed 1 Mar, 2020.
- 34.Kim S.S., Fang H., DiFranza J., Ziedonis D.M., Ma G.X. Gender differences in the fagerström test for nicotine dependence in Korean Americans. J. Smok. Cessat. 2012;7(1):31–36. doi: 10.1017/jsc.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson L., Greaves L., Jategaonkar N., Bell K., Pederson A., Tungohan E. Rethinking an assessment of nicotine dependence: a sex, gender and diversity analysis of the Fagerstrom test for nicotine dependence. J. Smok. Cessat. 2007;2(2):59. [Google Scholar]
- 36.Bobb-Semple A.A., Williams A.F., Boggs M.E., Gold K.J. Prenatal point-of-care tobacco screening and clinical relationships. Ann. Fam. Med. 2018;16(6):507–514. doi: 10.1370/afm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashford K., Wiggins A., Rayens E., Assef S., Fallin A., Rayens M.K. Perinatal biochemical confirmation of smoking status by trimester. Nicotine Tob. Res. 2017;19(5):631–635. doi: 10.1093/ntr/ntw332. [DOI] [PMC free article] [PubMed] [Google Scholar]