Abstract
Endothelial cells express surface angiotensin-converting enzyme 2 (ACE2), the main receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that promotes the infection of endothelial cells showing activation and damage. Bronchoalveolar lavage fluid from coronavirus disease-2019 (COVID-19) subjects showed a critical imbalance in the renin-angiotensin-aldosterone system with the upregulated expression of ACE2. Recently, intravenous recombinant ACE2 was reported as an effective therapy in severe COVID-19 by blocking the viral entry to target cells. Here, we present a case of a critically ill COVID-19 patient with acute respiratory distress syndrome where circulating ACE2 was first measured to monitor disease prognosis. ACE2 activity increased about 40-fold over the normal range and showed a distinct time course as compared to 2-3-fold higher levels of endothelium biomarkers. Although the level of soluble E-selectin followed the clinical status of our patient similar to ferritin and IL-6 levels, the dramatic rise in serum ACE2 activity may act as an endogenous nonspecific protective mechanism against SARS-CoV-2 infection that preceded the recovery of our patient.
Keywords: COVID-19, ACE2, Endothelial cell, Inflammation, Biomarker
Introduction
Coronavirus disease 2019 (COVID-19) represents a mild course in the majority of cases, but about one-fifth of the patients develop clinical complications, such as acute respiratory distress syndrome (ARDS), severe thrombocytopenia, venous thromboembolism, and a systemic hyperinflammatory response (Huang et al., 2020, Martincic et al., 2020). The latter disorder is accompanied by lymphopenia, decreased interferon-γ expression in CD4+ T cells, and upregulated cytokine levels (e.g., interleukin-6, IL-6) that may lead to the development of a “cytokine storm” in severe COVID-19 (Pedersen and Ho, 2020). Endothelial cells express angiotensin-converting enzyme 2 (ACE2) on their surface, the main receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Hoffmann et al., 2020; Zhang et al., 2020). It has been proposed that intravenous recombinant ACE2 treatment represents an effective therapy in severe COVID-19 cases by blocking the viral entry to the target cells (Zoufaly et al., 2020). ACE2 promotes SARS-CoV-2 infection of endothelial cells, which evokes endothelial activation and damage resulting in the substantial release of von Willebrand factor from Weibel-Palade bodies (Escher et al., 2020) and enhanced serum levels of soluble adhesion molecules (e.g., vascular adhesion molecule-1, VCAM-1) (Tong et al., 2020). In parallel, bronchoalveolar lavage fluid samples from COVID-19 subjects showed a critical imbalance in the renin-angiotensin-aldosterone system with the upregulated expression of ACE2, renin and kallikrein enzymes (Garvin et al., 2020). Nevertheless, interactions among the above variables and their impact on the clinical outcome is poorly understood. Here, we present a case of a critically ill COVID-19 patient with ARDS who was successfully treated with tocilizumab, corticosteroids, intravenous immunoglobulin (IVIG), and concomitant antimicrobial therapies, whereby circulating ACE2 levels and soluble adhesion markers of endothelial cell injury were followed along the clinical course.
Case report
A 69-year-old male patient with hypertension, arrythmia, and benign prostate hyperplasia was admitted to the Department of Infectious Diseases, University Hospital, Debrecen following 5 days of upper respiratory symptoms and fever. Reverse transcription polymerase chain reaction (RT-qPCR) test of a nasopharyngeal swab was positive for SARS-CoV-2. Blood tests on admission showed a white blood cell count of 5.7 G/L with a lymphocyte count of 0.97 G/L, hemoglobin of 111 G/L, C-reactive protein (CRP) of 87.7 mg/L, creatinine of 95 μmol/L, and ferritin of 751 μg/L. Antibiotics (ceftriaxone and azithromycin) with antiviral darunavir were initiated. Three days after admission, following a rapid deterioration of his clinical condition with the development of ARDS, he was transferred to the intensive care unit (ICU). Chest X-ray and CT-scan showed bilateral ground-glass opacities admixed with patchy areas of consolidation in the central and peripheral portions of the lung (Suppl. Figure 1 A and B), thus, invasive ventilation (APRV for 17 days) with 11 courses of prone positioning, sedation, and norepinephrine administration were initiated (Suppl. Figure 1C). At that stage, CRP was moderately elevated (109 mg/L), while the ferritin level was over 2000 μg/L.
Consecutive blood tests indicated gradually increasing serum IL-6 levels reaching a peak of 10,729 ng/L in a “cytokine storm” after 2 days of ICU admission, therefore, anti-IL-6 receptor tocilizumab was empirically administered (Merad and Martin, 2020), and thereafter a remarkable IL-6 decline to 110.4 ng/L was observed (Figure 1 A). In the next 8 days, the patient received intravenous piperacillin/tazobactam (3 × 4 g/0.5 g/day), hydroxychloroquine (1 × 0.5 g/day), and methylprednisolone (80 mg/day) (Suppl. Figure 1C). On the tenth ICU day, renal insufficiency developed with elevated serum urea levels (27.6 mmol/L), which resolved conservatively. Subsequently, IL-6 concentration raised again up to 551 ng/L in the presence of normal CRP (0.6 mg/L) and high ferritin levels (1631 μg/L) at a body temperature of 40 °C and increased norepinephrine demand. Extended spectrum of antibiotic therapy (teicoplanin, meropenem) with antifungal regimens (fluconazole and caspofungin) and a 5-day course of IVIG were commenced. By day 19, improved oxygenation was noticed, and chest CT-scan showed a moderate regression in pulmonary consolidations. Repeated RT-qPCR tests suggested that the patient became negative for SARS-CoV-2. The ventilatory support could be gradually reduced translating to pressure support ventilation, but due to severe ICU-acquired muscle weakness (ICUAW) weaning could not be completed until day 37. The tracheostomy tube was finally removed on day 50 (Suppl. Figure 1C). No neurological complications were revealed. Serum IL-6 level consistently decreased with lowering ferritin values (Figure 1A and B). The patient was fully mobilized and then discharged from ICU in a stable clinical condition after 10 weeks from the onset of symptoms. Blood tests at discharge showed normal IL-6 (9.1 ng/L) and ferritin (211.6 μg/L) concentrations. No thrombotic complication and thrombocytopenia developed, D-dimer was only slightly elevated (between 0.8 and 4.0 mg FEU/L), while platelet count remained moderately decreased (between 102 and 178 G/L) during ICU treatment. Using an automated SARS-CoV-2 IgG test, seropositivity was detected 3 weeks after the onset of disease (data not shown).
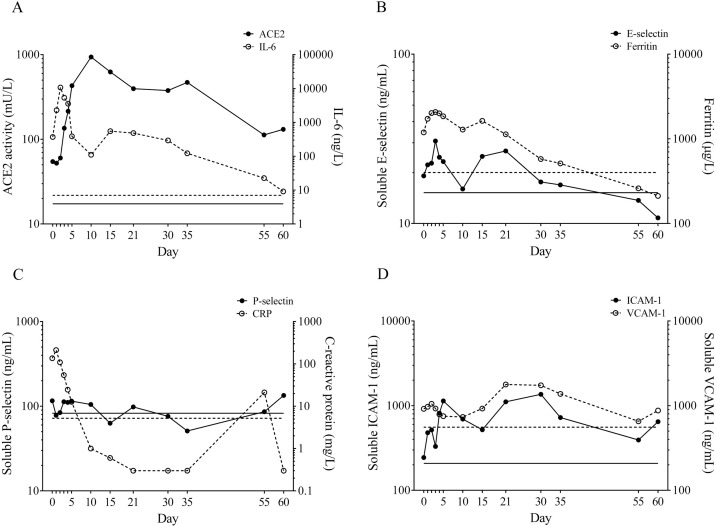
Figure 1.
The analysis of circulating ACE2, IL-6, and endothelial biomarkers. (A) Circulating ACE2 activity with biomarkers of endothelial cell injury were measured in 13 different serum samples in parallel with routinely available laboratory parameters. Compared to IL-6, serum ACE2 activity showed extreme values with a “delay”; (B) both soluble E-selectin and ferritin correlated with IL-6 levels; (C) soluble P-selectin had no significant alteration, while CRP showed an IL-6-like time course; and (D) circulating ICAM-1 and VCAM-1 changed with a time course different from that of other biomarkers. Cut-off values are depicted by lines of identical types without symbols.
In parallel with routinely measured laboratory parameters mentioned above, we investigated the time courses of serum ACE2 activity (Figure 1A) and soluble adhesion markers of endothelial cell injury (Figure 1B–D). Compared to formerly measured serum ACE2 levels of healthy individuals and hypertensives (16.2 ± 0.8 mU/L and 24.8 ± 0.8 mU/L, respectively) (Úri et al., 2016), a two-fold elevated baseline ACE2 activity was measured on the first day of his admission (54.5 mU/L), which was then increased by a factor of 20 reaching an extreme peak (937.4 mU/L) by day 10 that may reflect the shedding of the enzyme from damaged cells. Although both IL-6 and ACE2 peaked at high levels, they did not change in parallel, instead a two-day shift was observed: decrease in IL-6 was accompanied by increase in ACE2 during the resolution of the severe clinical condition (Figure 1A). Based on the normal values of NT-pro-BNP (24.3 ± 19.1 ng/L) measured throughout the study period, cardiological disorders were unlikely to explain the extremely high circulating ACE2 levels. Total LDH activity was sustainedly increased (521 ± 59.6 U/L) and did not correlate with ACE2 activity (r = 0.657 and p = 0.175) (data not shown).
Soluble E-selectin, VCAM-1, ICAM-1, and P-selectin levels were proposed as biomarkers of endothelium dysfunction in cardiovascular disease (Szük et al., 2016). They demonstrated two- to threefold higher serum levels than normal during the course of severe COVID-19 (Figure 1B–D) similar to a recent study (Tong et al., 2020). However, only the level of soluble E-selectin followed the clinical status of our patient and–similarly to ferritin and IL-6 levels–it returned to the normal range by the end of therapy (Figure 1B). In addition, the level of soluble E-selectin showed a strong positive correlation with that of ferritin (r = 0.730 and p = 0.006) (data not shown); therefore, it behaves as an inflammatory marker of endotheliitis described in COVID-19 (Varga et al., 2020).
Discussion and conclusion
Early diagnosis and effective clinical monitoring of COVID-19 are essential to prevent severe consequences or death (Borges do Nascimento et al., 2020). This is the first report on elevated circulating ACE2 and soluble E-selectin levels in severe COVID-19. Importantly, based on a delay in the time course of ACE2 activity as compared to inflammation-dependent biomarkers, we propose that the changes in ACE2 activity do not respond to pro-inflammatory signals in COVID-19. As the viral titers were still high when ACE2 activity peaked with about 40-fold over the normal range, it is intriguing to hypothesize that increased serum ACE2 may represent an endogenous protection against SARS-CoV-2 by blocking its binding sites for the cellular ACE2 receptor. Indeed, recombinant ACE2 was protective in animal studies against SARS-CoV-2 infections (Linsky et al., 2020) and intravenous recombinant ACE2 was beneficial in a severe case of COVID-19 (Zoufaly et al., 2020). However, the clinical value of recombinant ACE2 administration in COVID-19 needs to be confirmed by further clinical studies.
In conclusion, circulating ACE2 and soluble E-selectin levels are elevated as new biomarkers of severe lung disorder with vascular injury in COVID-19, and high serum ACE2 may contribute to the resolution of the disease.
Conflict of interest
There are no competing interests to declare among the authors of this work.
Ethical approval
The study was approved by the Scientific and Research Ethics Committee of the University of Debrecen and the Ministry of Human Capacities under the registration number of 32568-3/2020/EÜIG.
Contributions
BN designed the study, analyzed the data, and wrote the manuscript. ZF and MF performed the laboratory analyses, created the figures and assisted with writing the manuscript. ZS, RS, and IV provided the clinical data of the patient. AT, ZP, JK, EA, and MF contributed to data interpretation and revised the manuscript. All authors discussed the results, commented on the manuscript and approved the final version of the manuscript.
Acknowledgments
We thank the patient for participating in this research. BN is a recipient of the Lajos Szodoray Grant and an OTKA Bridging Fund (Faculty of Medicine, University of Debrecen). Z.F. is supported by the ÚNKP-20-4-II-DE-197 New National Excellence Program of The Ministry for Innovation and Technology. This work was funded by the GINOP-2.3.2-15-2016-00043 (the project is cofinanced by the European Union and the European Regional Development Fund) and EFOP-3.6.2-16-2017-00006 (the project is cofinanced by the European Union and the European Social Fund) projects, grants from the National Research, Development and Innovation Office (FK 128809 (to MF) and K 132623 (to AT). The research was financed by the Thematic Excellence Program of the Ministry for Innovation and Technology in Hungary (ED_18-1-2019-0028), within the framework of the thematic program of the University of Debrecen. No funding source had any role in the writing of the manuscript or the decision to submit.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.11.184.
Appendix A. Supplementary data
The following is Supplementary data to this article:
Radiological findings and the time course of clinical status and ICU treatment of a critically ill COVID-19 patient who suffered from severe pneumonia. (A, B) Representative chest X-ray and coronal CT images demonstrate bilateral ground-glass opacities admixed with patchy areas of consolidation in the central and peripheral portions of the lung. (C) A timeline depicting the main issues of the clinical status and interventions of a COVID-19 patient. Details are described in the text. Abbreviations: ARDS: acute respiratory distress syndrome, AKI: acute renal insufficiency, ICUAW: intensive care unit-acquired muscle weakness, and IVIG: intravenous immunoglobulin.
References
- Borges do Nascimento I.J., von Groote T.C., O’Mathúna D.P., Abdulazeem H.M., Henderson C., Jayarajah U., et al. Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: a systematic review and series of meta-analyses. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin M.R., Alvarez C., Miller J.I., Prates E.T., Walker A.M., Amos B.K., et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;9 doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsky T.W., Vergara R., Codina N., Nelson J.W., Walker M.J., Su W., et al. De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2. Science. 2020 doi: 10.1126/science.abe0075. (Epub ahead) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martincic Z., Skopec B., Rener K., Mavric M., Vovko T., Jereb M., et al. Severe immune thrombocytopenia in a critically ill COVID-19 patient. Int J Infect Dis. 2020;99:269–271. doi: 10.1016/j.ijid.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szük T., Fejes Z., Debreceni I.B., Kerényi A., Édes I., Kappelmayer J., et al. Integrity(®) bare-metal coronary stent-induced platelet and endothelial cell activation results in a higher risk of restenosis compared to Xience(®) everolimus-eluting stents in stable angina patients. Platelets. 2016;27:410–419. doi: 10.3109/09537104.2015.1112368. [DOI] [PubMed] [Google Scholar]
- Tong M., Jiang Y., Xia D., Xiong Y., Zheng Q., Chen F., et al. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis. 2020;222:894–898. doi: 10.1093/infdis/jiaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Úri K., Fagyas M., Kertész A., Borbély A., Jenei C., Bene O., et al. Circulating ACE2 activity correlates with cardiovascular disease development. J Renin Angio Aldo Syst. 2016;17 doi: 10.1177/1470320316668435. 1470320316668435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30418-5. (Epub ahead) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Radiological findings and the time course of clinical status and ICU treatment of a critically ill COVID-19 patient who suffered from severe pneumonia. (A, B) Representative chest X-ray and coronal CT images demonstrate bilateral ground-glass opacities admixed with patchy areas of consolidation in the central and peripheral portions of the lung. (C) A timeline depicting the main issues of the clinical status and interventions of a COVID-19 patient. Details are described in the text. Abbreviations: ARDS: acute respiratory distress syndrome, AKI: acute renal insufficiency, ICUAW: intensive care unit-acquired muscle weakness, and IVIG: intravenous immunoglobulin.