Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS−COV-2), a single-stranded RNA virus, was found to be the causal agent of the disease called coronavirus disease. During December 2019, China informed the World Health Organization (WHO) of an outbreak of cases of pneumonia of unknown etiology, which caused severe-acute respiratory distress. The disease was termed coronavirus disease 2019 (Covid-19). Due to alarming levels of spread and severity, on the 11th of March 2020, the WHO declared the outbreak as a global pandemic. As of September 14, 2020, more than 29 million cases have been reported, with over 900,000 deaths globally. Since the outbreak, although not conclusive, discoveries have been made regarding the understanding of the epidemiology, etiology, clinical features, clinical treatment, and prevention of the disease. SARS−COV-2 has been detected in saliva, respiratory fluids, blood, urine, and faeces. Findings are however controversial regarding its presence in the semen or the testis. Hence, this review aimed to further analyse the literature concerning (i) the effects of previously identified human coronaviruses on male fertility (ii) the impact of Covid-19 on male fertility and (iii) the implication for general health in terms of infection and transmission.
Keywords: SARS−COV-2, Male fertility, SARS−COV, MERS−COV, Covid-19
1. Introduction
Coronaviruses (COVs) are a group of positive single stranded RNA viruses, and they belong to order Nidovirale, family Coronaviridae and sub family Coronavirinae [1,2]. COVs are crown-like in morphology and have up to 26–32 genome kilobases [3]. These viruses have been shown to infect animals (birds), and human, and as such, they have up to four identified genera, namely, the alpha (α)−COV, beta (β)−COV, gamma (γ) −COV and delta (δ)−COV. The α- and β−COvs are the prominent human Coronaviruses (HCOV), while the γ- and δ- COVs are normally seen in birds [4]. Till date, seven COVs (2-α and 5-β) have been identified in human, including HCOV-229E, HCOV−OC43, severe acute respiratory syndrome (SARS−COV), HCOV-NL63, HCOV-HKU1, Middle East respiratory syndrome (MERS−COV) and the most recently discovered severe acute respiratory syndrome-2 (SARS−COV-2).
1.1. Brief overview of the HCOVs
The first human COV to be identified was the HCOV-229E strain, which was isolated from the nasal discharge of patients with upper respiratory tract infection in the 1960s [5]. A year later, Mcintosh et al. reported the presence of HCOV−OC43 from the organ culture of respiratory tract infected patients [6]. Patients infected with both HCOV-229E and HCOV−OC43 presented with common cold symptoms, such as headache, sneezing, malaise, and sore-throat. HCOV−OC43 was later found to possess neurotropism in both in vitro cultured cells and infected mice [7,8]. SARS on the other hand was first reported in 2002, as patients infected with SARS−COV presented with respiratory distress, cough, fever, headache, myalgia, malaise, and chills [9]. In 2003, the SARS epidemic affected about 26 countries with more than 8000 cases and over 700 deaths [10]. The transmission occurs primarily from person to person, and the incubation time ranges from 3 to 14 days, which corresponds to the peak of viral load in the respiratory discharge and stool [10]. While the SARS epidemic was still ongoing, HCOV-NL63 and HCOV-HKU1 were discovered. In 2004, HCOV-NL63 was first isolated from the nasal discharge of a seven-month-old suffering from respiratory tract infection [11]. In 2005, Woo et al. detected a strain of HCOV-HKU1 from a 71-year-old man with pneumonia and bronchiolitis [12]. Another β−HCOV is the MERS−COV which was first reported in Saudi Arabia in 2012. Since the first reveal, about 27 countries have disclosed cases of MERS [13]. About 35 % of the reported patients infected with MERS−COV died. The common MERS symptoms include cough, fever, and shortness of breath and sometimes pneumonia [13].
Seven years after the outbreak of MERS, Wuhan city of China reported the emergence of SARS−COV-2 [14]. In March 2020, the World Health Organization (WHO) declared the outbreak as a global pandemic, given its severity and rapid spread. As of September 14, 2020, more than 29 million cases have been reported, with over 900,000 deaths globally. Symptoms of SARS−COV-2 include fever, dry cough, tiredness, headache and sore throat, amongst others. Since the discovery of SARS−COV-2 and its implication to cause coronavirus disease 2019 (Covid-19), studies have investigated the epidemiology, aetiology, clinical features, clinical treatment, and prevention of the disease. Studies have shown SARS−COV-2 to be present in saliva, respiratory fluids, blood, urine, and faeces. The extent of organ damage outside of the respiratory and cardiovascular system is still clinically unpredictable.
Despite that findings regarding the presence of SARS−COV-2 in the semen or the testis are limited and controversial, concerns relating to transmission through this route should not be overlooked [15]. The reason for this concern is that Ebola virus was reported to remain in the semen samples of patients up to nine months post recovery [[16], [17], [18]]. Similarly, Zika virus was also reported to be sexually transmitted [19].
Hence, this review aimed to analyse the literature regarding (i) the effects of previously identified human coronaviruses on male fertility (ii) the impact of Covid-19 on male fertility and (iii) the implication for general health in terms of infection and transmission.
2. Search method
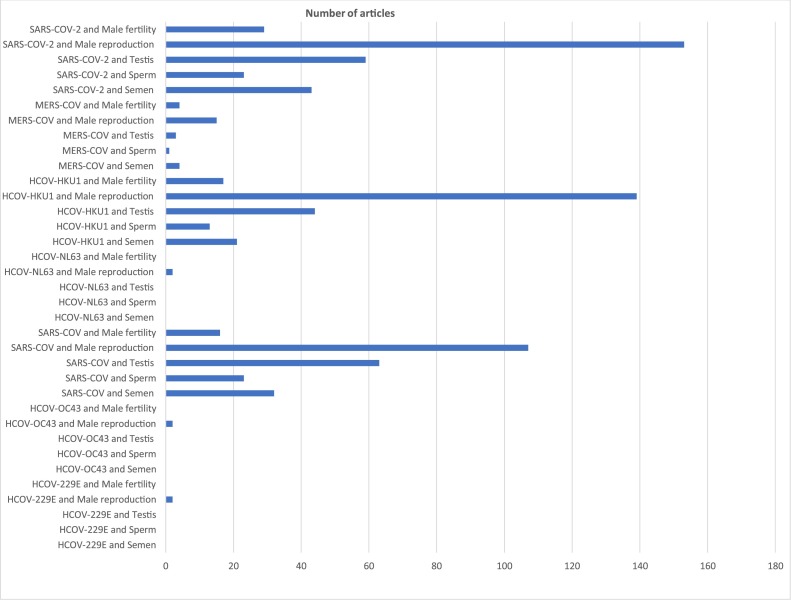
A literature search of studies published on HCOVs until September 2020, was carried out using PubMed. To look at the impact of these HCOVs on male fertility, each HCOV strain was meshed with the following terms, “semen”, “sperm”, “testis”, “male reproduction” and “male fertility”, and searched for on PubMed (e.g, “HCOV-2299E and semen”, “HCOV-229E and sperm”). The search was limited to full length articles published in English. The results of the search terms as of September 18, 2020 are illustrated in Fig. 1 . The vetting process includes:
-
(a)
The mesh terms were searched for. This include “HCOV-2299E and semen”, HCOV-229E and sperm”, HCOV-229E and testis”, HCOV-229E and male reproduction”, and HCOV-229E and male fertility”. The remaining HCOVs followed the same sequence as HCOV-229E.
-
(b)
The titles of articles were screened, and if title conforms with the mesh term, the abstract was read.
-
(c)
If abstract correlates with the aim of the current review, the whole article was read.
-
(d)
Only original articles/studies were included.
Fig. 1.
Summary of search outcome.
Following the literature search, before screening, a total of 822 articles were retrieved, comprising of both original and reviews articles (book chapters included). The results are as follows, total number of articles on HCOV-229E and the five individual mesh terms was 2, HCOV−OC43 = 2, SARS−COV = 248, HCOV-NL63 = 2, HCOV-HKU1 = 234, MERS−COV = 27, SARS−COV-2 = 307. From the results, it is important to note that many of the search outcomes for each mesh-term were mainly covid-19 related articles. After screening the results, only 22 articles met the inclusion criteria, including 11 original articles on SARS−COV-2.
3. What is known about the effects of previously discovered HCOVs on male fertility
The common denominator in the route of transmission/ infection of the previously identified HCOVs (HCOV-229E, HCOV−OC43) is through respiratory droplets and fomites, with SARS−COV and MERS−COV being transmitted through respiratory droplets, fomites and faecal-oral routes [20]. Very limited studies suggested the plausibility of transmission through the semen and what the general health implication is, when present in the testis.
After the isolation of HCOV-229E, it was reported to cause bronchiolitis and mild infection/inflammation of the upper respiratory tract in few patients [21]. The only study that investigated the impact of HCOV-229E on reproduction accessed the maternal vaginal and respiratory mucus during labour and the new-born gastric sample, of mother-child couples. The authors reported the presence of HCOV-229E in three samples and hence suggested the possibility of vertical transmission during infection [22].
HCOV−OC43 is not only present in the upper respiratory tract, and consequently cause the inflammation thereof, it was also implicated to play a role in neurological diseases, as findings have shown that this virus invades the central nervous system [[23], [24], [25]]. Dubé et al. reported that one of the processes involved in the neuropathogenesis of HCOV−OC43 is the neuron-to-neuron propagation of this virus which is aided by axonal transport [26]. Keyaerts et al. showed that HCOV−OC43 infection in new-born mice can be treated with chloroquine acquired transplacentally or through maternal milk [27]. From these findings, the following can be suggested (i) HCOV−OC43 may cause neonatal mortality and (ii) HCOV−OC43 may be vertically transmitted. However, studies on the direct and indirect impact of the virus on male fertility are not available.
Years after the discovery of HCOV−OC43, SARS−COV was identified in China. In contrast to the mild infection of the upper respiratory tract seen in HCOV-229E and HCOV−OC43 infected patients, SARS−COV is pathogenic and may cause severe inflammation. Studies have shown the wide distribution of SARS−COV in various organs (lung, trachea/bronchus, stomach, small intestine, renal distal tubule, sweat gland, pituitary, pancreas, adrenal gland and cerebrum), and consequently leading to diverse pathologies [28]. Some of the pathological findings reported, include severe degeneration of the renal distal tubule epithelium, hyaline membrane formation, exudation of fibrin fluid and damage to the aveoli of the lungs [29,30]. Regarding the presence and the impact of SARS−COV on male reproduction, findings remain controversial. Ding et al. reported that SARS−COV was not detected in the testis [28], while Xu et al. not only showed its presence in the testis, they also reported its role in orchitis [31]. Their results showed that the testes of patients who died from SARS exhibited germ cell destruction, absence of spermatozoa in the lumen of the seminiferous tubule, thickened basement membrane and leukocyte infiltration. It was suggested that the adverse impact seen may be as a result of (i) high fever that occurs in SARS and (ii) the treatment of SARS with steroids which could affect spermatogenesis [32]. Additionally, Zhao et al. reported that SARS−COV was found in the testicular epithelial cells and Leydig cells using electron microscopy [33]. These studies agreed on the extensive inflammatory response with cytokines release accompanying SARS infections. Hence, it can be suggested that these microvascular changes could provoke obstructive azoospermia on short term and psychological effect of the illness could result in endocrine disturbances.
This collectively suggests that even if SARS−COV is not present in the semen, it can negatively impact spermatogenesis, and ultimately impair male fertility.
During the SARS epidemic, an additional two strains of HCOV were identified (HCOV-NL63 and HCOV-HKU1) [11,12]. HCOV-NL63 was identified as the key pathogen responsible for croup in children [[34], [35], [36]], while Infection with HCOV-HKU1 has been shown to be associated with pneumonia and bronchiolitis [12]. However, no report was found on the impact of both HCOV-NL63 and HCOV-HKU1 on male reproduction.
Seven years after the discovery of HCOV-HKU1, MERS−COV, another HCOV that is known to cause severe upper respiratory tract infection, and other pathological conditions were identified. Studies from different parts of the world have shown the susceptibility of pregnant women to MERS−COV infection [[37], [38], [39], [40]]. In Saudi Arabia, a group of authors reported a still birth in a woman infected with MERS−COV at 5 month of gestation [37], while a woman died after giving birth in the United Arab Emirates [39]. In Korea however, although a 39-year-old pregnant woman was infected with MERS−COV, she delivered and reached full recovery [40]. To the best of our knowledge, no human study linking MERS−COV to male fertility has been reported. However, a recent study conducted by Hemida et al. reported that MERS−COV was detected in the seminal plasma from camels [41].
Looking at the findings that SARS−COV was detected in the testis and the consequent testicular architectural alteration, and its role in orchitis, together with the findings that MERS−COV was found in the seminal plasma of dromedary camels, it is not unreasonable to believe that (i) SARS−COV-2 may also cause testicular dysfunction and (ii) SARS−COV-2 may be plausibly transmitted through the semen.
4. SARS−COV-2 and male fertility: what is known?
In comparison to the other two pathogenic COVs (SARS−COV and MERS−COV), studies on SARS−COV-2 are quite numerous (Fig. 1). This is due to the rapid and harsh spread of the virus, and how this has affected the global mortality and morbidity rates. Many key discoveries have been made with respect to the epidemiology, diagnosis, cause, and treatment of Covid-19. Studies have shown SARS−COV-2 to be present in body fluids and faeces [42]. Findings regarding its presence in the semen and/or the male reproductive tract at large are controversial. This section of the review will highlight original studies that have:
-
(i)
Investigated whether SARS−COV-2 is present in semen
-
(ii)
Examined the semen parameters of acute, mild, severe, and recovered Covid-19 patients
-
(iii)
Evaluated the testis of Covid-19 patients
-
(iv)
Investigated the prostatic secretion of Covid-19 patients.
From the general search outcome (Fig. 1), a total of 307 papers (summation of SARS−COV-2 and the individual mesh terms) were available on PubMed as of September 18, 2020. Of the 307 papers, only 11 studies met the inclusion criteria listed above. These studies are briefly discussed in this section and the summary is illustrated in Table 1 .
Table 1.
Summary of studies illustrating the impact of SARSCOV-2 on male fertility.
| Authors | Sample Type | Sample size | Infection stage | Sample COV-2 Status | Other findings |
|---|---|---|---|---|---|
| Li et al. 2020 | Semen | 15 | Acute infection | Positive (26.7 %) | |
| 23 | Recovered | Positive (8.7 %) | |||
| Holtman et al. 2020 | Semen | 2 | Active | Negative | |
| 18 | Recovered | Negative | ↓semen volume, ↓ sperm concentration, ↓ sperm count, ↓percentage (%) of progressively motile spermatozoa, ↓% of total motility | ||
| Ma et al. 2020 | Semen | 1 | Active | Negative | 33 % of samples showed ↓ sperm motility, ↑DNA fragmentation index ↓ normal sperm morphology, ↓ total sperm count |
| 11 | Recovered | Negative | |||
| Kayaaslan et al. 2020 | Semen | 16 | Acute infection | Negative | |
| Ning et al. 2020 | Semen | 9 | Active | Negative | 2.7 % presented with orchidoptosis |
| 8 | Recovered | Negative | |||
| Guo et al. | Semen | 12 | Active | Negative | |
| 11 | Recovered | Negative | |||
| Paoli et al., 2020 | Semen | 1 | Active | Negative | |
| Pan et al. 2020 | Semen | 34 | Recovered | Negative | 19 % showed scrotal discomfort (viral orchitis) |
| Song et al. 2020 | Semen | 12 | Recovered | Negative | |
| Song et al. 2020 | Testis | 1 | Post-mortem | Negative | |
| Yang et al. 2020 | Testis | 12 | Post-mortem | Positive (8.3 %) | 100 % showed symptoms of Swelling of the Sertoli cells, Vacuolation, Cytoplasmic rarefaction, Detachment from tubular basement membrane, Loos and sloughing of intratubular cell mass, ↓ Leydig cell number, Interstitial oedema, Mild inflammatory infiltrates |
| Zhang et al. 2020 | Prostatic secretion | 10 | Active | Negative | ↑ inflammatory indicators such as: C-reaction protein, erythrocyte sedimentation and ↑ interleukin-6 |
COV-2 = severe acute respiratory syndrome coronavirus 2, ↑ = increase, ↓ = decrease.
Li et al. reported that out of the thirty-eight covid-19 patients that donated semen samples for evaluation (23 recovered, 15 acute stage), six samples were positive for SARS−COV-2, including two from the 23 recovered patients and four from the acutely infected patients [43]. Findings from the study of Holtmann et al. showed that although the semen samples of the twenty recruited covid-19 patients (18 recovered, 2 acute) was void of SARS−COV-2, the semen parameters of patients with moderate infection (from recovered group) were impaired [44]. Ma et al. reported the absence of SARS−COV-2 after accessing the semen samples of twelve covid-19 patients [45]. However, 33 % of the study population had altered semen parameters and higher number of spermatozoa undergoing DNA fragmentation. They further evaluated the sera of another one hundred and nineteen patients for sex-related hormones. They reported that, although there were no statistical significant difference in the serum testosterone and serum follicle stimulating hormone (FSH), the ratio of testosterone to luteinizing hormone (LH) (T:LH) and the ratio of FSH to LH (FSH:LH) were significantly reduced in the sera of covid-19 patients compared to the control group. This suggests that being infected with SARS−COV-2 may alter sex-related hormones. In addition, Kayaaslan et al. reported that the semen samples of sixteen male patients in the acute infection stage were void of SARS−COV-2 [46]. Although, Ning et al. reported the absence of SARS−COV-2 in the semen of 9 active positive covid-19 patients and 8 recovered patients, three patients with severe infection presented with orchidoptosis [47]. Guo et al. also described the absence of SARS−COV-2 in the semen samples of 23 patients (12 active moderate infection and 11 recovered) [48].
Paoli et al. on the other hand, reported that the semen and urine samples of a thirty-one year old covid-19 patient were negative for SARS−COV-2 [49]. This was supported by the findings of Pan et al., who reported the absence of SARS−COV-2 in the semen samples of thirty-four patients in recovery [50]. However, they reported that 19 % of the study population demonstrated scrotal discomfort, which is suggestive of viral orchitis. Song et al. reported the absence of SARS−COV-2 in the semen of twelve patients in their recovery phase and the testis of one patient who died from covid-19 [51].
Additionally, Yang et al. investigated whether SARS−COV-2 is present in the testes and if there is or are any pathological changes in the testis of Covid-19 patients [52]. After accessing the testicular tissues of twelve patients, they reported the absence of SARS−COV-2 in the testis of 90 % of the cases. However, the testes of all the patients exhibited significant seminiferous tubular injury, reduced Leydig cell number, swelling of the Sertoli cells and mild lymphocytic inflammation. Zhang et al. also reported the absence of SARS−COV-2 in the prostatic secretion of ten covid-19 patients [53]. The sera of these patients, however, showed increased levels of inflammatory biomarkers.
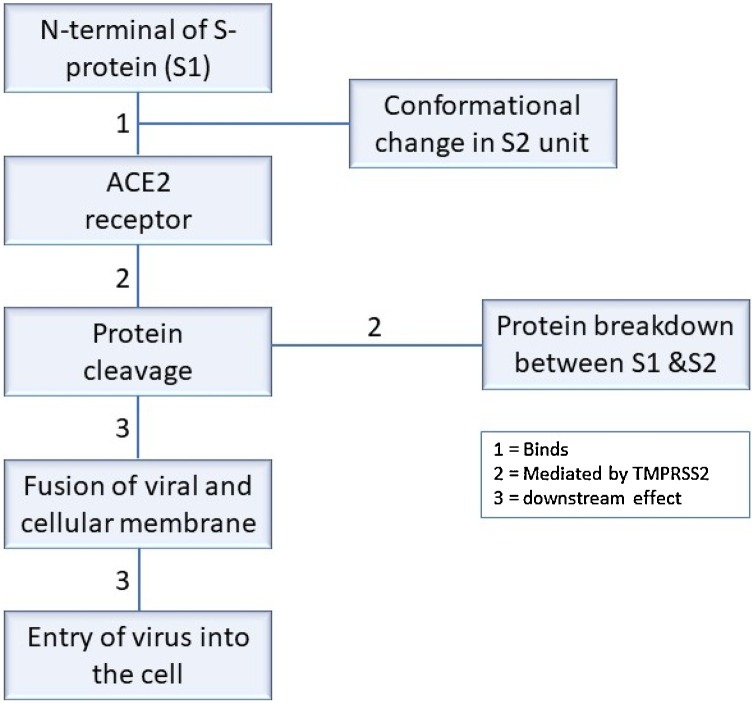
From the studies available, SARS−COV-2 has exhibited relevant characteristic factors supporting the views that COVID survivors might develop sexual and reproductive health issues on the long run. This is partly because the protein that enables SARS−COV-2 entry into the cell, angiotensin−COnverting enzyme 2 (ACE 2), is widely distributed in the testis, including spermatogonia, the Leydig and Sertoli cells. Hikemet et al., reported that amongst over 150 investigated cell types, which correspond to human tissues and organs, the expression of ACE 2 was higher in some tissues/organs, including the male reproductive organs [54]. Viral entry requires binding of spike (S) glycoproteins of SARS−COV-2 to the host receptor. Upon entry, the host proteases, such as transmembrane serine protease 2 (TMPRSS2) cleaves the viral S protein to stimulate a conformational change, to allow fusion of the viral and host cell membranes [55,56]. Represented in Fig. 2 is the summary of SARS−COV-2 entry into the cell. Zhen et al. also reported the distribution of ACE 2 in the testes, and that it was highly expressed in the testes of infertile men compared to the control [57]. This suggests that SAR−COV-2 may cause male reproductive disorder and that men with reproductive issues may be more susceptible to infection.
Fig. 2.
Summary of SARS−COV-2 entry into the cell. The spike (S) proteins have two subunits, namely S1 and S2 subunits. Binding of S1 leads to a conformational change in S2, hence the primary function of S1 is to bind, while that of S2 is to fuse. For viral entry, the N-terminal of S (S1) binds ACE2 receptor. Binding of S1 to ACE2 leads to a conformational change in S2. During the conformational change, viral protein cleavage occurs, which is mediated by receptor transmembrane protease serine 2 (TMPRSS2). Following protein cleavage, fusion between viral and cellular membrane occurs, which subsequently result in the entry of the virus into the cell, thereby releasing its content.
On the other hand, the proportion of mortality by sex is higher in men. Hence, SARS-CoV-2 causes a more severe outcome in men, which is a disadvantage compared to women [58]. It is, therefore, vital to determine the factors involved in these differences.
5. Conclusion
Bringing all the findings together, although most of the studies reported the absence of SARS−COV-2 in the semen and prostatic secretions, as well as testicular tissues, which may reduce the possibility of transmission through this route, it is evident that (i) there is testicular injury and inflammatory infiltration (ii) viral orchitis may occur, as patients experienced scrotal discomfort (iii) there is altered semen parameters and (iv) the number of spermatozoa with DNA fragmentation is increased. These results collectively suggest that infection with SARS−COV-2 may lead to potential fertility issues.
5.1. Future recommendations
The question to be asked at this point is how these adverse effects are exerted. Although the mechanism (s) involved is largely unknown, studies have implicated the main receptor ACE 2 to which SARS−COV-2 binds to play a role. Studies have shown that the ACE 2 receptor is widely distributed in the testis, including the Leydig and Sertoli cells. Hence, studies investigating the mechanisms / pathways should be developed.
Additionally, since a small but growing and significant percentage of Covid-19 patients, many of whom initially experienced a mild initial illness, are suffering lasting effects, the complete impact of “long Covid” on male reproduction has not been established. For the time being, male partners of couples planning to fall pregnant, should heed to the policy statements and guidelines from the various reproductive societies (e.g. International Federation of Fertility Societies, European Society of Human Reproduction and Embryology, Spanish Fertility Society, American Society for Reproductive Medicine). Thus, precautionary measurements should be taken by candidates suspected to be infected, and also by those previously infected with SARS-CoV-2, even after the signs and symptoms have resolved. Until enough evidence on the impact of the virus on gametes and embryos have been established, cryopreservation should be encouraged.
Funding
This project was not funded by any organizational body.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
References
- 1.Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and sources of endemic human coronaviruses. Adv. Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/mmbr.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;3527:60286–60289. doi: 10.1016/s0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saif L.J. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? OIE Rev. Sci. Tech. 2004;23:643–660. doi: 10.20506/rst.23.2.1513. [DOI] [PubMed] [Google Scholar]
- 5.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. U.S.A. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbour N., Côté G., Lachance C., Tardieu M., Cashman N.R., Talbot P.J. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J. Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacomy H., Fragoso G., Almazan G., Mushynski W.E., Talbot P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349:335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C., Chan K.H., Tsang D.N.C., Yung R.W.H., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . 2020. Severe Acute Respiratory Syndrome Coronavirus-outbreak News.www.who.int/ith/diseases/sars/en/ [Google Scholar]
- 11.Van Der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J.M., Wolthers K.C., Wertheim-Van Dillen P.M.E., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo P.C., Lau S.K., Chu C., Chan K., Tsoi H., Huang Y., Wong B.H. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. LK- http://huji-primo.hosted.exlibrisgroup.com/openurl/972HUJI/972HUJI_SP?sid=EMBASE&sid=EMBASE&issn=0022538X&id=doi:10.1128%2FJVI.79.2.884-895.2005&atitle=Characterization+and+complete+genome+sequence+of+a+novel+coronavirus%2C+coronavirus+HKU1%2C+from+patients+with+pneumonia&stitle=J.+Virol.&title=Journal+of+Virology&volume=79&issue=2&spage=884&epage=895&aulast=Woo&aufirst=Patrick+C.+Y.&auinit=P.C.Y.&aufull=Woo+P.C.Y.&coden=JOVIA&isbn=&pages=884-895&date=2005&aui. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 2020. Middle-East-Respiratory-Syndrome-Coronavirus-(Mers-Cov)www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov) [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maya W.D. Cardona, Du Plessis S.S., Velilla P.A. SARS-CoV-2 and the testis: similarity with other viruses and routes of infection. Reprod. Biomed. Online. 2020;40:763–764. doi: 10.1016/j.rbmo.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deen G.F., Broutet N., Xu W., Knust B., Sesay F.R., McDonald S.L.R., Ervin E., Marrinan J.E., Gaillard P., Habib N., Liu H., Liu W., Thorson A.E., Yamba F., Massaquoi T.A., James F., Ariyarajah A., Ross C., Bernstein K., Coursier A., Klena J., Carino M., Wurie A.H., Zhang Y., Dumbuya M.S., Abad N., Idriss B., Wi T., Bennett S.D., Davies T., Ebrahim F.K., Meites E., Naidoo D., Smith S.J., Ongpin P., Malik T., Banerjee A., Erickson B.R., Liu Y., Liu Y., Xu K., Brault A., Durski K.N., Winter J., Sealy T., Nichol S.T., Lamunu M., Bangura J., Landoulsi S., Jambai A., Morgan O., Wu G., Liang M., Su Q., Lan Y., Hao Y., Formenty P., Ströher U., Sahr F. Ebola RNA persistence in semen of ebola virus disease survivors — final report. N. Engl. J. Med. 2017;377:1428–1437. doi: 10.1056/NEJMoa1511410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardona-Maya W.D., Hernandez P.A.V., Henao D.E. Male ebola survivors: do not forget to use a condom! Reprod. Sci. 2019;26:1326. doi: 10.1177/1933719114563733. [DOI] [PubMed] [Google Scholar]
- 18.Maya W.D. Cardona, Du Plessis S.S., Velilla P.A. Semen as virus reservoir? J. Assist. Reprod. Genet. 2016;33:1255–1256. doi: 10.1007/s10815-016-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy M. Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ. 2016;352:i720. doi: 10.1136/bmj.i720. [DOI] [PubMed] [Google Scholar]
- 20.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradburne A.F., Bynoe M.L., Tyrrell D.A.J. Effects of a “New” human respiratory virus in volunteers. Br. Med. J. 1967;3:767–769. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagneur A., Dirson E., Audebert S., Vallet S., Quillien M.C.L., Baron R., Laurent Y., Collet M., Sizun J., Oger E., Payan C. Vertical transmission of human coronavirus. Prospective pilot study. Pathol. Biol. 2007;55:525–530. doi: 10.1016/j.patbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drosten C., Günther S., Preiser W., Van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 24.Kuiken T., Fouchier R.A.M., Schutten M., Rimmelzwaan G.F., Van Amerongen G., Van Riel D., Laman J.D., De Jong T., Van Doornum G., Lim W., Ling A.E., Chan P.K.S., Tam J.S., Zambon M.C., Gopal R., Drosten C., Van Der Werf S., Escriou N., Manuguerra J.C., Stöhr K., Peiris J.S.M., Osterhaus A.D.M.E. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Peñaranda S., Bankamp B., Maher K., hsin Chen M., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C.T., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Günther S., Osterhaus A.D.H.E., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science (80-.) 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 26.Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-Neuron propagation of human coronavirus OC43. J. Virol. 2018;92:1–21. doi: 10.1128/jvi.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis virus transmission pathways. J. Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse G.M.K., To K.F., Chan P.K.S., Lo A.W.I., Ng K.C., Wu A., Lee N., Wong H.C., Mak S.M., Chan K.F., Hui D.S.C., Sung J.J.Y., Ng H.K. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J. Clin. Pathol. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y., Zou W., Zhan J., Wang S., Xie Z., Zhuang H., Wu B., Zhong H., Shao H., Fang W., Gao D., Pei F., Li X., He Z., Xu D., Shi X., Anderson V.M., Leong A.S.Y. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Qi L., Chi X., Yang J., Wei X., Gong E., Peh S., Gu J. Orchitis: A complication of severe acute respiratory syndrome (SARS) Biol. Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui-Bao G., Ming-Han T., Yan-Qiang H.U., Qing-Su G., Renshan G.E., Hardy M.P. Glucocorticoid induces apoptosis in rat Leydig cells. Endocrinology. 2002;143:130–138. doi: 10.1210/en.143.1.130. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J.M., Zhou G.D., Sun Y.L., Wang S.S., Yang J.F., Meng E.H., Pan D., Li W.S., Zhou X.S., Wang Y.D., Lu J.Y., Li N., Wang D.W., Zhou B.C., Zhang T.H. CliN.iCal pathology and pathogenesis of severe acute respiratory syndrome. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:217–221. [PubMed] [Google Scholar]
- 34.Lau S.K.P., Woo P.C.Y., Yip C.C.Y., Tse H., Tsoi H.W., Cheng V.C.C., Lee P., Tang B.S.F., Cheung C.H.Y., Lee R.A., So L.Y., Lau Y.L., Chan K.H., Yuen K.Y. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J. Clin. Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L.M., Peiris J.S.M. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi E.H., Lee H.J., Kim S.J., Eun B.W., Kim N.H., Lee J.A., Lee J.H., Song E.K., Kim S.H., Park J.Y., Sung J.Y. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin. Infect. Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Hameed F., Wahla A.S., Siddiqui S., Ghabashi A., Al-Shomrani M., Al-Thaqafi A., Tashkandi Y. Characteristics and outcomes of middle east respiratory syndrome coronavirus patients admitted to an intensive care unit in Jeddah, Saudi Arabia. J. Intensive Care Med. 2014;31:344–348. doi: 10.1177/0885066615579858. [DOI] [PubMed] [Google Scholar]
- 38.Choi W.S., Kang C.I., Kim Y., Choi J.P., Joh J.S., Shin H.S., Kim G., Peck K.R., Chung D.R., Kim H.O., Song S.H., Kim Y.R., Sohn K.M., Jung Y., Bang J.H., Kim N.J., Lee K.S., Jeong H.W., Rhee J.Y., Kim E.S., Woo H., Oh W.S., Huh K., Lee Y.H., Song J.Y., Lee J., Lee C.S., Kim B.N., Choi Y.H., Jeong S.J., Lee J.S., Yoon J.H., Wi Y.M., Joung M.K., Park S.Y., Lee S.H., Jung S.I., Kim S.W., Lee J.H., Lee H., Ki H.K., Kim Y.S. Clinical presentation and outcomes of middle east respiratory syndrome in the Republic of Korea. Infect. Chemother. 2016;48:118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik A., El Masry K.M., Ravi M., Sayed F. Middle east respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg. Infect. Dis. 2016;22:515–517. doi: 10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong S.Y., Sung S.I., Sung J.H., Ahn S.Y., Kang E.S., Chang Y.S., Park W.S., Kim J.H. MERS-CoV infection in a pregnant woman in Korea. J. Korean Med. Sci. 2017;32:1717–1720. doi: 10.3346/jkms.2017.32.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemida M.G., Waheed M., Ali A.M., Alnaeem A. Detection of the Middle East respiratory syndrome coronavirus in dromedary camel’s seminal plasma in Saudi Arabia 2015–2017. Transbound. Emerg. Dis. 2020:1–6. doi: 10.1111/tbed.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohseni A.H., Taghinezhad-S S., Xu Z., Xu Z., Fu X. Body fluids may contribute to human-to-human transmission of severe acute respiratory syndrome coronavirus 2: evidence and practical experience. Chin. Med. (U.K) 2020;15:7–10. doi: 10.1186/s13020-020-00337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holtmann N., Philippos E., Marcel A., Cornelius D., Dunja B.-B., Ortwin A., Steffen K.J.-, Petra B.A. Assessment of SARS-CoV-2 in human semen - a cohort study Holtmann. Fertil. Steril. 2020 doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma L., Xie W., Li D., Shi L., Ye G., Mao Y., Xiong Y., Sun H., Zheng F., Chen Z., Qin J., Lyu J., Zhang Y., Zhang M. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J. Med. Virol. 2020:0–2. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kayaaslan B., Korukluoglu G., Hasanoglu I., Kalem A.K., Eser F., Akinci E., Guner R. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol. Int. 2020:678–683. doi: 10.1159/000510531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ning J., Li W., Ruan Y., Xia Y., Wu X., Hu K., Ding X., Wu X., Yu L., Zhou J., Mao Z., Xu W., Yu W., Cheng F. 2020. Effects of 2019 Novel Coronavirus on Male Reproductive System: A Retrospective Study, Preprints. [DOI] [Google Scholar]
- 48.Guo L., Zhao S., Li W., Wang Y., Li L., Jiang S., Ren W., Yuan Q., Zhang F., Kong F., Lei J., Yuan M. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2020;00:1–6. doi: 10.1111/andr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paoli D., Pallotti F., Colangelo S., Basilico F., Mazzuti L., Turriziani O., Antonelli G., Lenzi A., Lombardo F. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J. Endocrinol. Invest. 2020 doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan F., Xiao X., Guo J., Song Y., Li H., Patel D., Spivak A., Alukal J., Zhang X., Xiong C., Li P., Hotaling J. No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertil. Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song C., Wang Y., Li W., Hu B., Chen G., Xia P., Wang W., Li C., Diao F., Hu Z., Yang X., Yao B., Liu Y. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol. Reprod. 2020 doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang M., Chen S., Huang B., Zhong J., Su H., Chen Y., Cao Q., Ma L., He J., Li X.-F., Li X., Zhou J.-J., Fan J., Luo D.-J., Chang X.-N., Arkun K., Zhou M., Nie X. Pathological findings in the testes of COVID-19 patients: clinical implications. Infections. 2020:1–6. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S., Wang X., Zhang H., Xu A., Fei G., Jiang X., Tu J., Quf G., Xug X., Li Y. The absence of coronavirus in expressed prostatic secretion in COVID-19 patients in Wuhan city. Reprod. Toxicol. 2020;96:90–94. doi: 10.1016/j.reprotox.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (80-.) 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanley K.E., Thomas E., Leaver M., Wells D. Coronavirus disease (COVID-19) and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil. Steril. 2020 doi: 10.1016/j.fertnstert.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen Q., Xiao X., Aierken A., Yue W., Wu X., Liao M., Hua J. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J. Cell. Mol. Med. 2020:1–6. doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi T., Ellingson M.K., Wong P., Israelow B., Lucas C., Klein J., Silva J., Mao T., Oh J.E., Tokuyama M., Lu P., Venkataraman A., Park A., Liu F., Meir A., Sun J., Wang E.Y., Casanovas-Massana A., Wyllie A.L., Vogels C.B.F., Earnest R., Lapidus S., Ott I.M., Moore A.J., Anastasio K., Askenase M.H., Batsu M., Beatty H., Bermejo S., Bickerton S., Brower K., Bucklin M.L., Cahill S., Campbell M., Cao Y., Courchaine E., Datta R., DeIuliis G., Geng B., Glick L., Handoko R., Kalinich C., Khoury-Hanold W., Kim D., Knaggs L., Kuang M., Kudo E., Lim J., Linehan M., Lu-Culligan A., Malik A.A., Martin A., Matos I., McDonald D., Minasyan M., Mohanty S., Muenker M.C., Naushad N., Nelson A., Nouws J., Nunez-Smith M., Obaid A., Ott I., Park H.J., Peng X., Petrone M., Prophet S., Rahming H., Rice T., Rose K.A., Sewanan L., Sharma L., Shepard D., Silva E., Simonov M., Smolgovsky M., Song E., Sonnert N., Strong Y., Todeasa C., Valdez J., Velazquez S., Vijayakumar P., Wang H., Watkins A., White E.B., Yang Y., Shaw A., Fournier J.B., Odio C.D., Farhadian S., Dela Cruz C., Grubaugh N.D., Schulz W.L., Ring A.M., Ko A.I., Omer S.B., Iwasaki A. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020 doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]