Highlights
-
•
Both osteo- and rheumatoid arthritis increase with age.
-
•
Osteoarthritis is more common in whites, but rheumatoid arthritis is in blacks.
-
•
Obesity and female sex increase risk of risk of both osteo- and rheumatoid arthritis.
-
•
Smoking increases risk of both forms of arthritis in women.
-
•
These two forms of arthritis have many common risk factors.
Keywords: Osteoarthritis, Rheumatoid arthritis, NHANES, BMI, Race/ethnicity, Smoking
Abstract
Osteoarthritis and rheumatoid arthritis are both diseases of joints, but they have very different etiologies. Osteoarthritis is a disease assumed to result from wear and tear over time, whereas rheumatoid arthritis is an autoimmune disease where the body’s immune system attacks joint tissues. Using NHANES data (1999–2015), we have compared the influence of age, sex, ethnicity, body mass index and smoking on these two very different forms of arthritis. Incidence of both increases with age and are more frequent in females than males. There is little apparent difference between osteoarthritis and rheumatoid arthritis in women of normal as comparted to overweight, but both are more frequent in obese women, especially those over the age of 60. While osteoarthritis is more frequent in whites, blacks have more rheumatoid arthritis, and Hispanics show an intermediate prevalence. Smoking significantly increased the incidence of both osteoarthritis and rheumatoid arthritis in women, but increased prevalence of only RA in men. There was no effect of smoking on OA prevalence in males. It is remarkable that two diseases of joints, which have quite different causes, should have so many commonalities. The differences that exist appear to be due to a combination of inflammatory markers and access to health care.
1. Background
Arthritis is a serious and chronic disease that affects about 23% of the adult US population (CDC, 2017a). The most common forms are rheumatoid arthritis (RA) and osteoarthritis (OA). The financial burden on the economy due to the prevalence of RA, OA and other arthritic conditions is high, particularly from work loss, disability and high cost of medical treatment and management of the condition(s). RA is the most common systemic autoimmune disease affecting about 0.5–1.0% of the population (Helmick et al., 2008, Kobayashi et al., 2008). RA is a disease in which the immune system attacks the joints and creates inflammation that causes the tissues that line the joint to thicken. This results in swelling and pain in and around the joints, leading to permanent disability.
The precise pathogenesis of RA is unknown but it is widely believed that both genetic and environmental factors play a role (Kobayashi et al., 2008, Linn-Rasker et al., 2006, Parks et al., 2011). The major risk factors include sex, age, and family history. Women are more likely to develop RA compared to men. The sex ratio is typically around 3:1 for RA prevalence. (Wolfe et al, 1968). Genetic (X-linked) factors and hormonal differences are suspected to play a role (O'Brien et al., 2007, Ostensen, 1999, Vanvollenhoven and Mcguire, 1994, Wilder, 1998). RA most commonly begins between the ages of 40 and 60. Family history is an important risk factor, but it is not clear whether this represents genetic susceptibility or rather an environmental exposure that is common to the family unit. A recent study also found that the risk of rheumatoid factor (RF)-seropositive RA was associated with one of the classic genetic risk factors for immune-mediated diseases, i.e. the shared epitope of HLA–DR, is strongly increased by the presence of smoking (Padyukov et al., 2004).
Osteoarthritis (OA), on the other hand, is a degenerative disease of the joints that occurs when the cartilage or cushion between the joints breaks down leading to pain, swelling and stiffness. OA is the most common chronic condition of the joints, affecting over 30 million US adults. Some risk factors include joint injury or overuse, gender, age, being obese, race and genetics (CDC, 2017). It is highly prevalent among the elderly. Symptoms include pain, swelling, and stiffness. Diagnosis of OA involves the physical examination and range of motion tests of the joints. Confirmation of diagnosis typically is done using joint aspiration, x-ray, or magnetic resonance imaging (MRI). Several studies have found that the risk of developing OA increases with age and/or body mass index (BMI) (Felson et al., 2000, Flugsrud et al., 2002, Sturmer et al., 2000). Women consistently have higher risk of OA compared to men (Lanyon et al., 2003, Prieto-Alhambra et al., 2014). Obesity is also a major risk factor for OA (Powell et al., 2005). Many studies have observed a positive association between BMI and OA (Cooper et al., 1998, Felson et al., 1988, Oliveria et al., 1999, Spector et al., 1994). OA pathogenesis is associated with both excessive joint loading and altered biomechanical patterns together with hormonal and cytokine dysregulation (King et al., 2013). The impact of BMI on both knee and hip OA was assessed in two recent meta-analyses (Jiang et al., 2011, Jiang et al., 2012). Both found a dose-dependent relationship between BMI and the risk of OA. A 5-unit increase in BMI was associated with a 35% increased risk of knee OA and an 11% increased risk of hip OA.
The two forms of arthritis differ in which joints are most affected. RA most commonly affects the small joints of the hands and feet and is usually symmetrical. OA usually affects weight-bearing joints, such as knees and hips, and is commonly asymmetrical. However OA of the hand is common, which may result in confusion with RA (Felson et al., 2000). OA diagnosis is based on patient’s history and physical examinations, with or without radiographic evidence such as x-ray, MRI, and computerized tomography (CT) scan (Taruc-Uy & Lynch, 2013). The most common symptoms of OA are intense pain worsened by movement of extensive use, joint stiffness, and Heberden & Bouchard nodes in the hands. Some physical examination findings in people with OA include effusion, decreased range of motion and crepitus. RA diagnosis is also based on the patient’s history and physical examination indicating synovitis in multiple joints (Gibofsky, 2014), as well as antibodies, anti-CCP and RF. Some misclassification undoubtedly occurs.
Smoking is a well-known risk factor for various diseases including RA (Baka et al., 2009). The risk of RA is increased among smokers compared to non-smokers and lifelong cigarette smoking is positively associated with the risk of RA even among smokers with low lifelong exposure (Di Giuseppe et al, 2014). Recent studies also show that the overall age- and sex-adjusted prevalence of RA appears to have increased between 1995 and 2007 from 0.62% to 0.72% (Myasoedova et al., 2010). RA poses significant clinical as well as economic burden. People with RA have reduced life expectancy because of the comorbid conditions associated with RA such as cardiovascular diseases, osteoporosis, and lung disease compared to those without RA (Goodson et al., 2005).
The evidence on associations between smoking and OA is less clear. Smoking has been reported to have a protective association with OA in some studies (Hui et al., 2011, Leung et al., 2014). In the Framingham Osteoarthritis Study, a study of elderly members of the Framingham Heart Study, cohort, the occurrence of knee OA in 1983 – 1985 was evaluated in relation to the smoking status at the first Framingham examination, 36 years earlier. After adjusting for various confounders including age, sex, weight, knee injury history, physical activity, alcohol consumption and weight change after the first examination, a protective effect of smoking against OA remained (Felson et al., 1989). A recent cross-sectional study in a Chinese population of 3,789 subjects also observed a negative association between cigarette smoking and radiographic knee OA, even after controlling for potential confounders (Zhang et al., 2015). The protective effect of smoking on OA is widely observed in cross-sectional studies.
However, no such association has been found in most cohort studies. In the Clearwater Osteoarthritis Study, a cohort of 2,505 men and women aged 40 years or older, adjusted point estimates ranged from 0.60 to 1.48 suggesting no significant association between smoking and OA (Wilder et al., 2003). Similarly, a review of current literature observed a protective effect in cross-sectional studies but not in longitudinal studies. The cross-sectional findings have been attributed to residual confounding (Dube et al., 2016). Increased BMI, obesity, and bone injuries due to sporting activities have all been associated with OA. Hence, the lower BMI and low physical sporting activities may be the reason for the reduced risk of OA in smokers. However, this explanation has not been supported after adjustment for BMI and amount of physical activity (Hui et al., 2011).
These equivocal associations infer that the protective effect is a false-negative. Similarly, the inhibitory role of smoking was only observed among current smokers and not ever-smokers or ex-smokers, implying that smoking and OA do not display a dose-response relationship, further suggesting no causal relationship between smoking and OA (Hui et al., 2011). The review of evidence of publications on the effect of smoking on OA by Felson et al. (2000) suggests adjusting for BMI as a confounder only shows the direct effect of smoking but does not highlight the effect of smoking through BMI. Therefore, BMI should be assessed as a mediator or effect modifier.
The main objectives of this study are to utilize the NHANES data set to examine the association of selected risk factors, such as age, race/ethnicity, BMI, sex, and smoking, with the risk of developing RA and/or OA. Since the etiology of RA and OA differ, there may be significant differences in effects of these risk factors on the outcomes. Very few studies have compared the risk of RA or OA across race/ethnicity in the United States. Significant differences in genetic factors influencing susceptibility, outcome, or severity of disease may exist across race/ethnicity. A higher RA prevalence has been reported in Native American populations while a lower prevalence has been reported in some Asian populations (Silman & Pearson, 2002). Furthermore, the association between smoking and OA reported in the literature is inconsistent. Use of NHANES, as a national random survey of Americans with oversampling of several ethnic groups offers the possibility of obtaining a broad understanding of those factors which influence the development of these two major forms of arthritis.
2. Materials & methods
Data from the National Health and Nutrition Examination Survey (NHANES) collected between 1999 and 2016 were used. Designed to assess the health and nutritional status of non-institutionalized civilians in the US, this continuous, cross-sectional survey utilizes interviews and physical examinations to estimate prevalence of health behaviors and diseases. There are many publications using NHANES data for study of dietary data (Ahluwalia et al., 2016), symptoms, tests and diagnosis of various diseases (Koru-Sengul et al., 2011), as well as prevalence and trends of various types of arthritis in the US (Park et al., 2018). Interview data focused on self-reported arthritis, smoking, and sociodemographic information; supplemented with physical measurements of height and weight were used to address the research questions. NHANES data is publicly available on the web, and therefore IRB review and approval are not required for its use.
The study sample included NHANES participants, ages 20 and older, who provided a definitive answer to the question “has a doctor or other health professional ever told you that you have arthritis?” Participants who answered “don’t know” or refused to answer were excluded. For participants who reported arthritis, a subsequent question asked, “Which type of arthritis was it?” The responses to this question were categorized as follows: RA, OA, psoriatic arthritis, other arthritis, don’t know the type of arthritis, and refused to answer the question.
Sociodemographic characteristics extracted from NHANES for analyses included age, sex, living in poverty, and race/ethnicity. All information was self-reported. Living in poverty is a variable calculated by NHANES based on household income and household occupants. Three categories of income were used: Below twice the official level of poverty, between twice and three-time the level of poverty and above three-time the level of poverty. Four race/ethnicity classes were identified as non-Hispanic white, non-Hispanic black, Hispanic and other.
Smoking history was assessed by answer to the question “Have you smoked at least 100 cigarettes in your entire life?”
Measures of height, weight, and waist circumference were obtained during the physical examination. Body mass index (BMI) was computed by NHANES. The standard cut points were used for BMI: <18.5 kg/m2, 18.5 to 24.9 kg/m2, 25 to 29.9 kg/m2, and ≥30 kg/m2, for underweight, normal weight, overweight, and obese, respectively.
All analyses were performed with SAS statistical software (version 9.4, SAS Inc., Cary, NC, USA). Differences in covariate distributions between those with RA, OA and those free of disease were compared using Wald and Pearson tests for continuous and categorical variables respectively, taking survey weights into account. Continuous variables were log-transformed, if needed, to achieve normality prior to testing the differences in means. A survey-weighted logistic regression was carried out to evaluate the aims stated above. Significant variables (set at a significance of 0.05) revealed by bivariate analysis were further analyzed by multivariate analysis.
Analysis of NHANES de-identified data does not require Institutional Review Board (IRB) approval or informed consent by subjects.
3. Results
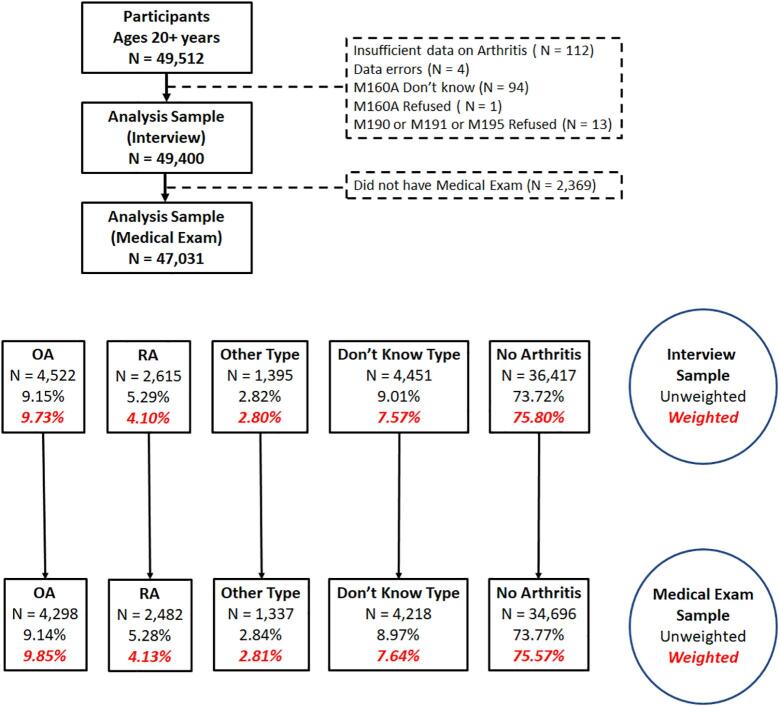
Fig. 1 shows the flow diagram for this study. We limited analysis to individuals 20 years and older and excluded those who did not have a medical exam because this provided BMI data. Respondents were asked if they had arthritis, and if so, was it OA, RA, psoriatic arthritis (PA), other arthritis, and whether they did not know what kind. While it is likely that most who reported that they did not know what kind had OA, we excluded these from further consideration because our goal was a comparison of risks for confirmed OA and RA. We also lumped those with PA in with “other” because those numbers were small. Over the full period there were 47,031 persons who had the medical exam and were included in our study. Of these there were 4,298 reported cases of OA, 2,482 reported case of RA, 1,337 individuals with other types of arthritis, and 4,218 individuals who did not know what type of arthritis they had. A total of 34,696 persons reported that they did not suffer from arthritis. Table 1 gives the information available in NHANES for all available years on self-reported arthritis.
Fig. 1.
The flow diagram for this study.
Table 1.
Unadjusted and adjusted odds ratios between sociodemographic and behavioral characteristics and rheumatoid arthritis (RA).
| Covariates | Bivariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% confidence interval | p-value | Adjusted Odds Ratio | 95% confidence interval | p-value | |||
| Sex | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.55 | 1.43 | 1.69 | <0.0001 | 1.84 | 1.67 | 2.02 | <0.0001 |
| Ages 60 + years | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.91 | 1.71 | 2.13 | <0.0001 | 1.98 | 1.74 | 2.25 | <0.0001 |
| White* | 1 | 1 | ||||||
| Black* | 1.87 | 1.64 | 2.13 | <0.0001 | 1.68 | 1.45 | 1.95 | <0.0001 |
| Hispanic | 1.16 | 1.01 | 1.32 | 0.0350 | 0.99 | 0.85 | 1.16 | 0.9205 |
| Ages 50–59 years | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.55 | 1.28 | 1.87 | <0.0001 | 1.59 | 1.29 | 1.95 | <0.0001 |
| White* | 1 | 1 | ||||||
| Black* | 1.45 | 1.16 | 1.81 | 0.0011 | 1.33 | 1.05 | 1.69 | 0.0193 |
| Hispanic | 0.93 | 0.73 | 1.18 | 0.5434 | 0.83 | 0.64 | 1.07 | 0.1535 |
| Ages 20–49 years | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.85 | 1.53 | 2.24 | <0.0001 | 1.74 | 1.43 | 2.12 | <0.0001 |
| White* | 1 | 1 | ||||||
| Black* | 0.99 | 0.80 | 1.22 | 0.9094 | 0.85 | 0.68 | 1.06 | 0.1516 |
| Hispanic | 0.62 | 0.49 | 0.77 | <0.0001 | 0.51 | 0.40 | 0.64 | <0.0001 |
| Race/Ethnicity | ||||||||
| White* | 1 | 1 | ||||||
| Black* | 1.36 | 1.24 | 1.50 | <0.0001 | 0.85 | 0.68 | 1.07 | 0.1576 |
| Hispanic | 0.83 | 0.75 | 0.92 | 0.0003 | 0.51 | 0.40 | 0.64 | <0.0001 |
| Household economic status | ||||||||
| 300% + of poverty line | 0.80 | 0.70 | 0.92 | 0.0020 | 0.86 | 0.74 | 1.00 | 0.0442 |
| 200% to 299% of poverty line | 1 | 1 | ||||||
| < 200% of poverty line | 1.44 | 1.27 | 1.64 | <0.0001 | 1.57 | 1.37 | 1.80 | <0.0001 |
| Smoking history | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.56 | 1.44 | 1.69 | <0.0001 | 1.52 | 1.38 | 1.68 | <0.0001 |
| Body mass index | ||||||||
| Healthy or underweight | 1 | 1 | ||||||
| Overweight | 1.19 | 1.06 | 1.34 | 0.0037 | 1.13 | 0.99 | 1.28 | 0.0687 |
| Obese | 1.90 | 1.71 | 2.12 | <0.0001 | 1.89 | 1.68 | 2.13 | <0.0001 |
*Non-Hispanic White and non-Hispanic Black.
Table S1 gives the information available in NHANES for all available years on self-reported arthritis (see supplementary document). Fig. S1 shows the prevalence of OA (A) and RA (B) by sex. OA is more common than RA, but both increase with age and are more common in women. There is a clearly greater increase in OA with age than is the case for RA. Fig. S2 shows the associations with degree of obesity for OA in females (A) and males (B) and RA in females (C) and males (D). Obesity is associated with a higher prevalence of both OA and RA in both men and women, although there were only small differences for both OA and RA between persons that were overweight as compared to those with a BMI of less than 25. Associations with degree of poverty are shown in Fig. S3. For OA there are only minor differences up until age 60 in both females (A) and males (B), but then rates increase in those of the lowest socio-economic group. For RA, the differences are greater and are apparent even at age 30 years. Prevalence of OA and RA are a function of ethnicity is shown in Fig. S4. There are greater differences between OA and RA here. Both female (A) and male (B) OA is much more common in non-Hispanic whites, while both female (C) and male (D) RA is much more common in non-Hispanic blacks. Hispanics show little difference with blacks in rates of OA but are intermediate with the other racial groups for RA. Fig. S5 shows the effects of smoking. Prevalence of both OA and RA is greater in women smokers than non-smokers, and smokers also have an elevated rate of RA among men. However, there was little or no difference in prevalence of OA among men.
In the bivariate and multivariate analyses, females had an increased risk of RA compared to males (1.55; 95% CI: 1.43 – 1.69 and 1.84; 95% CI: 1.67 – 2.02 respectively) (Table 1). Stratified by age, the increased risk persisted in females compared to males. Being non-Hispanic black and over 50 years was also statistically significantly associated with increased risk of RA; while no statistically significant risk was observed in non-Hispanic blacks between 20 and 49 years old (adjusted OR: 0.85; 95% CI: 0.68, 1.07). Lower socio-economic status (SES) was significantly associated with increased risk of RA in this population. Smoking and being overweight or obese were also significant risk factors for RA.
Table 2 shows both unadjusted and adjusted odds ratios between risk factors and osteoarthritis (OA). Similar risk factors and associations to the RA results were observed in the OA bivariate and multivariate analyses. Being female was significantly associated with an increased risk of OA compared to males in all stratified age groups. Non-Hispanic Blacks who were over 50 years old were at an increased risk for OA. Smoking and being obese were also statistically significantly associated with an increased risk of OA. The risk for OA was not statistically significant by household economic status, although the risk was higher in those in the higher SES category.
Table 2.
Unadjusted and adjusted odds ratios between sociodemographic and behavioral characteristics and osteoarthritis (OA).
| Covariates | Bivariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% confidence interval | p-value | Adjusted Odds Ratio | 95% confidence interval | p-value | |||
| Sex | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.89 | 1.77 | 2.02 | <0.0001 | 1.52 | 1.28 | 1.81 | <0.0001 |
| Ages 60 + years | ||||||||
| Male | 1 | 1 | ||||||
| Female | 2.55 | 2.33 | 2.78 | <0.0001 | 2.71 | 2.45 | 2.99 | <0.0001 |
| White* | 1 | 1 | ||||||
| Black* | 0.55 | 0.47 | 0.64 | <0.0001 | 0.47 | 0.41 | 0.54 | <0.0001 |
| Hispanic | 0.42 | 0.36 | 0.48 | <0.0001 | 0.38 | 0.33 | 0.43 | <0.0001 |
| Ages 50–59 years | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.93 | 1.63 | 2.27 | <0.0001 | 2.12 | 1.78 | 2.54 | <0.0001 |
| White* | 1 | 1 | ||||||
| Black* | 0.56 | 0.43 | 0.73 | <0.0001 | 0.53 | 0.42 | 0.66 | <0.0001 |
| Hispanic | 0.40 | 0.30 | 0.52 | <0.0001 | 0.38 | 0.30 | 0.48 | <0.0001 |
| Ages 20–49 years | ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.54 | 1.31 | 1.82 | <0.0001 | 1.52 | 1.28 | 1.81 | <0.0001 |
| White* | 1 | 1 | ||||||
| Black* | 0.57 | 0.44 | 0.74 | <0.0001 | 0.51 | 0.40 | 0.63 | <0.0001 |
| Hispanic | 0.31 | 0.23 | 0.40 | <0.0001 | 0.32 | 0.25 | 0.41 | <0.0001 |
| Race/Ethnicity | ||||||||
| White* | 1 | 1 | ||||||
| Black* | 0.44 | 0.40 | 0.48 | <0.0001 | 0.49 | 0.44 | 0.54 | <0.0001 |
| Hispanic | 0.33 | 0.30 | 0.36 | <0.0001 | 0.37 | 0.33 | 0.41 | <0.0001 |
| Household economic status | ||||||||
| 300% + of poverty line | 1.00 | 0.91 | 1.10 | 0.9895 | 1.03 | 0.92 | 1.16 | 0.5686 |
| 200% to 299% of poverty line | 1 | 1 | ||||||
| < 200% of poverty line | 0.87 | 0.79 | 0.95 | 0.0035 | 1.05 | 0.94 | 1.17 | 0.4159 |
| Smoking history | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.40 | 1.32 | 1.50 | <0.0001 | 1.37 | 1.26 | 1.48 | <0.0001 |
| Body mass index | ||||||||
| Healthy or underweight | 1 | 1 | ||||||
| Overweight | 1.33 | 1.22 | 1.46 | <0.0001 | 1.33 | 1.20 | 1.48 | <0.0001 |
| Obese | 1.93 | 1.77 | 2.10 | <0.0001 | 2.21 | 2.00 | 2.44 | <0.0001 |
*Non-Hispanic White and non-Hispanic Black
4. Discussion
Despite the very different mechanisms leading to the development of OA and RA, we find using the NHANES dataset that many of the risk factors are common to both forms of arthritis. Prevalence of both increases with age, although it rises more steeply at older age for OA as would be expected due to wear and tear. Both occur more frequently in women than men. However, the sex difference we find using NHANES data is not nearly as large as literature reports of a three-fold difference (Wolfe et al., 1968). The reasons for this are unclear. One possibility is that the random survey used by NHANES provides a more accurate distribution than seen in a hospital or clinic-based study, which often reflects greater use of medical care by women than by men. Nevertheless, we do find rates in women to be higher than men.
The basis for this is likely the known sex differences in inflammatory markers, such a C-reactive protein (CRP). CRP is commonly used as an indicator of systemic inflammation and is higher in women than men even after accounting for obesity (Khera et al., 2009). Both OA (Azamar-Llamas et al., 2017) and RA (MacDonald et al., 2009) are clearly associated with inflammation of the joints, mediated by adipokines and other pro-inflammatory cytokines.
It is not surprising that obesity is a major risk factor for OA, which we confirm for both women and men. Elevated weight is expected to put more demands on joints, but this is not the only known factor, as inflammatory markers also play a major role with OA (Azamar-Llamas et al., 2017). Obesity is equally important in prevalence of RA (Albrecht et al., 2016), and this is apparent even at young ages in our data, as shown in Fig. S3. Obesity is associated with elevations in CRP and other inflammatory markers (George et al., 2017) and this is almost certainly the reason that both OA and RA are elevated in prevalence among obese individuals. Valentine et al. (2009) have shown that the association between central obesity and CRP is stronger in women than men, but the percent fat is the strongest predictor of systemic inflammation in men
5. The role of socio-economic status
For OA, the prevalence rates were somewhat higher among more affluent persons, while for RA rates were higher in the lowest socioeconomic group. The reasons for these differences are not clear, and they may reflect factors beyond just risk of arthritis. More affluent individuals are more likely to access medical care, but poor people are more often dependent upon use of the small joints in the hands for their livelihood. The elevated prevalence of RA among the poor may reflect the degree of disability and how it influences employment, leading to increased diagnosis. There has not been much study of associations between rates of arthritis and socioeconomic status, although Brennan-Olsen et al. (2017) reported that all forms of arthritis were elevated among people with lower education.
The most striking differences between OA and RA that we observe in the NHANES data are for race/ethnicity. For OA, prevalence is much greater among non-Hispanic whites for both females and males, with little differences between US Hispanics and blacks. Similar observations have been reported by Deshpande et al. (2016) using data from the National Health Interview Survey. In contrast, for RA, rates are much higher among blacks for both females and males, and lowest among whites. Others have seen this result and have suggested that African Americans may have enriched single nucleotide polymorphisms that promote RA relative to whites (Hughes et al., 2010). In addition, blacks may be less likely than whites to get early treatment, leading to more severe disease (McBurney and Via, 2012). This factor should not, however, alter the prevalence. There has been little or no attention to relative prevalence of RA in Hispanics. Our observations suggest that rates among Hispanics are intermediate between those of blacks and whites.
6. Smoking and arthritis
NHANES data show that smoking increases prevalence of both OA and RA in females and RA in males. The effects of smoking on RA have been previous reported (Di Giuseppe et al., 2014, Anderson et al., 2016). There is also a large literature showing that air pollution increases the risk of RA (Gan et al., 2013, Hart et al., 2013, De Roos et al., 2014, Chang et al., 2016). Anderson et al. (2016) suggest that smoking and air pollution act via common mechanisms by inducing pro-inflammatory triggers in the joints.
The literature on the effects of smoking on OA is less consistent, with some reporting protective associations (Felson et al., 1989, Zhang et al., 2008) and some finding no effect (Wilder et al., 2003, Haugen et al., 2017). Our results indicate no evidence of a protective effect. We find a clear increased prevalence of OA in female smokers, which is consistent with the general hypothesis that pro-inflammatory factors are common between RA and OA. But we do not have a good explanation as to why this positive association was seen only in women. We are not aware of previous studies that has looked at sex differences in prevalence of OA in relation to smoking.
A limitation of the study is that all data are self-reported. There is also the lack of statistical power to carry out multi-way interactions with potential interacting variables. However, it is unlikely that these interactions would be informative. Our overall results indicate many common risk factors for OA and RA, which is consistent with the hypothesis that inflammatory markers such as CRP are important in both forms of arthritis. Both increase with age and obesity. Yet there are some differences in relation to level of poverty, race/ethnicity, and smoking. These may reflect behavioral, occupational, and genetic factors.
The results should be interpreted with consideration of the study’s limitations. NHANES data is obtained through multistage random sampling of the US civilian, noninstitutionalized population. The response proportion for NHANES has decreased in recent years raising concern about potential nonresponse bias. Unfortunately, even NHANES researchers have had difficulty determining the potential magnitude of bias related to selection and information bias (https://www.cdc.gov/nchs/data/bsc/bscpress_fakhouri_january_2018.pdf). A study comparing arthritis prevalence derived using multiple surveys found that some differences in arthritis prevalence exist, however the estimates were within about three percent (Murphy et al., 2017). Measures of association likely are impacted by misclassification. For example, underestimates of arthritis among younger adults may occur because they have not been diagnosed. Comparing surveys found measures of association between race/ethnicity and arthritis being consistent. Another limitation of the study is that all data are self-reported. Self-report of arthritis is thought to underestimate the true prevalence (Sachs et al., 2005). While we lacked statistical power to carry out multi-way interactions with potential interacting variables, we did assess three-way interactions routinely and noted when they appeared to add important information; given the findings it is unlikely that more complex interactions would be informative. Confounding was assessed through adjustment of key demographic factors using logistic regression; there was remarkably minimal confounding once interactions with age and sex were included in the graphs and statistical models. Our overall results indicate many common risk factors for OA and RA, which is consistent with the hypothesis that inflammatory markers such as CRP are important in both forms of arthritis. Both increase with age and obesity. Yet there are some differences in relation to level of poverty, race/ethnicity and smoking. These may reflect behavioral, occupations and genetic factors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CRediT authorship contribution statement
Azad Mohammed: Data curation, Formal analysis, Writing - original draft, Investigation. Taraf Alshamarri: Validation. Temilayo Adeyeye: Software, Validation. Victoria Lazariu: Data curation, Formal analysis. Louise-Anne McNutt: Methodology, Supervision. David O. Carpenter: Supervision, Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101242.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahluwalia N., Dwyer J., Terry A., Moshfegh A., Johnson C. Updae on NHANES dietary data: Focus on collection, release, analytical considerations, an duses to inform public policy. Adv. Nutr. 2016;7:121–134. doi: 10.3945/an.115.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht K., Richter A., Callhoff J., Huscher D., Schett G., Strangfeld A., Zink A. Body mass index distribution in rheumatoid arthritis: a collaborative analysis from three large German rheumatoid arthritis databases. Arthritis Res. Therapy. 2016;18(1):149. doi: 10.1186/s13075-016-1043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Meyer P.W.A., Ally M.M., Tikly M. Smoking and air pollution as pro-inflammatory triggers for the development of rheumatoid arthritis. Nicotin Tobacco Res. 2016;18:1556–1565. doi: 10.1093/ntr/ntw030. [DOI] [PubMed] [Google Scholar]
- Azamar-Llamas D, Hernandez-Molina G, Ramos-Avalos B and Furazawa-Carballeda J (2017) adipokine contribution to the pathogenesis of osteoarthritis. Mediators of Inflammation https://doi.org/101155/2017/5468023. [DOI] [PMC free article] [PubMed]
- Baka, Z., Buzas, E., & Nagy, G. (2009). Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Research & Therapy, 11(4). doi:ARTN 23810.1186/ar2751. [DOI] [PMC free article] [PubMed]
- Brennan-Olsen S.L., Cook S., Leech M.T., Bowe S.J., Kowai P., Naidoo N., Ackerman L.N., Page R.S. Prevalence of arthritis according to age, sex and socioeconomic status in six low and middle income countries: analysis of data from the World Health organization study on global AGEing and adult health (SAGE) Wave 1. Musculoskelatal Disorders. 2017;18:271. doi: 10.1186/s12891-017-1624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017a, December 27, 2017). Arthritis. Retrieved from https://www.cdc.gov/chronicdisease/resources/publications/aag/arthritis.htm.
- Centers for Disease Control and Prevention. (2017b). Osteoarthritis Fact Sheet. Retrieved from https://www.cdc.gov/arthritis/basics/osteoarthritis.htm.
- Chang K.H., Hsu C.C., Muo C.H., Hsu C.Y., Liu H.C., Kao C.H., Chen C.Y., Chang M.Y., Hsu Y.C. Air polution exposure increases the risk of rheumatoid arthritis: a longitudinal and nationwide study. Environ. Int. 2016;94:495–499. doi: 10.1016/j.envint.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Cooper C., Inskip H., Croft P., Campbell L., Smith G., McLaren M., Coggon D. Individual risk factors for hip osteoarthritis: obesity, hip injury and physical activity. Am. J. Epidemiol. 1998;147(6):516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- De Roos A.J., Koehoorn M., Tamburic L., Davies H.W., Brauer M. Proximity to traffic, ambient air pollution and community noise in relation to incident rheumatoid arthritis. Environ. Health Perspect. 2014;112:1075–1080. doi: 10.1289/ehp.1307413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande B.R., Katz J.N., Solomon D.H., Yelin E.G., Hunter D.J. The number of persons with symptomatic knee osteroarthritis in the United Stats: impact of race/ethnicity, age, sex and obesity. Arthritis Care Res. 2016;68:1743–1750. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giuseppe D., Discacciati A., Orsini N., Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res. Therapy. 2014;16(2):R61. doi: 10.1186/ar4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C.E., Liu S.H., Driban J.B., McAlindon T.E., Eaton C.B., Lapane K.L. The relationship between smoking and knee osteoarthritis in the Osteoarthritis Initiative. Osteoarthritis Cartil. 2016;24(3):465–472. doi: 10.1016/j.joca.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D.T., Anderson J.J., Naimark A., Hannan M.T., Kannel W.B., Meenan R.F. Does smoking protect against osteoarthritis? Arthritis Rheum. 1989;32(2):166–172. doi: 10.1002/anr.1780320209. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Anderson J.J., Naimark A., Walker A.M., Meenan R.F. Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med. 1988;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Lawrence R.C., Dieppe P.A., Hirsch R., Helmick C.G., Jordan J.M., Fries J.F. Osteoarthritis: New Insights. Part 1: the disease and its risk factors. Ann. Intern. Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- Flugsrud G.B., Nordsletten L., Espehaug B., Havelin L.I., Meyer H.E. Risk factors for total hip replacement due to primary osteoarthritis – a cohort study in 50,034 persons. Arthritis Rheum. 2002;46(3):675–682. doi: 10.1002/art.10115. [DOI] [PubMed] [Google Scholar]
- Gan R.Y., Deane K.D., Zerbe G.O., Demoruelle K., Weisman M.H. Relatinship between air pollution and positivity of RA-related autoantibodies in individuals without established RA: a report on SERA. Ann. Rheum. Dis. 2013;72 doi: 10.1136/annrheumdis-2012-202949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M.D., Giles J.T., Katz P.P., England B.R., Mikuls T.R., Michaud K., Sauer B.C. Impact of obesity and adiposity on inflammatory markers in patients with rheumatoid arthritis. Arthritis Care Res. 2017;69(12):1789–1798. doi: 10.1002/acr.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibofsky A. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am. J. Managed Care. 2014;20(7):S128–S135. [PubMed] [Google Scholar]
- Goodson N., Marks J., Lunt M., Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann. Rheum. Dis. 2005;64(11):1595–1601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J.E., Kallberg H., Laden F., Costenbader K.H., Yanosky J.D. Ambient air pollution exposures and risk of rheumatoid arthritis in the Nurses' Health Study. Arthritis Care Res. 2013;65:1190–1196. doi: 10.1002/acr.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen I.K., Magnusson K., Turkiewicz A., Englund M. The prevalence, incidence and progression of hand osteoarthritis in relation to body mass index, smoking and alcohol consumption. J. Rheumatol. 2017;44:1402–1409. doi: 10.3899/jrheum.170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmick C.G., Felson D.T., Lawrence R.C., Gabriel S., Hirsch R., Kwoh C.K., Workgrp N.A.D. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- Hughes L.B., Reynolds R.J., Brown E.E., Kelley J.M., Thomson B. Most common SNPs associated with rheumatoid arthritis in subjects of European ancestry confer risk of rheumatoid arthritis in African-Americans. Arthritis Rheum. 2010;62:3547–3553. doi: 10.1002/art.27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui M., Doherty M., Zhang W.Y. Does smoking protect against osteoarthritis? Meta-analysis of observational studies. Ann. Rheum. Dis. 2011;70(7):1231–1237. doi: 10.1136/ard.2010.142323. [DOI] [PubMed] [Google Scholar]
- Jiang L.Y., Rong J.S., Wang Y.C., Hu F.L., Bao C.D., Li X., Zhao Y.S. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78(2):150–155. doi: 10.1016/j.jbspin.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Jiang L.Y., Tian W.J., Wang Y.C., Rong J.S., Bao C.D., Liu Y.P., Wang C.X. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79(3):291–297. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Khera A., Vega G.L., Das S.R., McGuire Ayers C, Grundy S.M., de Lemos J.A. Sex differences in the relationship between C-reactive protein and body fat. J. Clin. Endocrinol. Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.K., March L., Anandacoomarasamy A. Obesity & osteoarthritis. Indian J. Med. Res. 2013;138:185–193. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Okamoto H., Iwamoto T., Toyama Y., Tomatsu T., Yamanaka H., Momohara S. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology. 2008;47(9):1317–1322. doi: 10.1093/rheumatology/ken259. [DOI] [PubMed] [Google Scholar]
- Koru-Sengul T., Clark J.D., Ocasio M.A., Wanner A., Fleming L.E., Lee D.J. Utilization of the National Health and Nutrition Examination (NHANES) Survey for symptoms, tests, and diagnosis of chronic respiratory diseases and assessment of second hand smoke exposure. Epidemiology (Sunnyvale) 2011;1(2) doi: 10.4172/2161-1165.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyon P., Muir K., Doherty S., Doherty M. Age and sex differences in hip joint space among asymptomatic subjects without structural change – implications for epidemiologic studies. Arthritis Rheum. 2003;48(4):1041–1046. doi: 10.1002/art.10886. [DOI] [PubMed] [Google Scholar]
- Leung Y.Y., Ang L.W., Thumboo J., Wang R., Yuan J.M., Koh W.P. Cigarette smoking and risk of total knee replacement for severe osteoarthritis among Chinese in Singapore – the Singapore Chinese health study. Osteoarthrit. Cartil. 2014;22(6):764–770. doi: 10.1016/j.joca.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn-Rasker S.P., van der Helm-van Mil A.H.M., van Gaalen F.A., Kloppenburg M., de Vries R.R.P., le Cessie S., Huizinga T.W.J. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann. Rheum. Diseases. 2006;65(3):366–371. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney C.A., Via E.R. Racial and ethnic disparities in rheumatoid arthritis. Curr. Rheum. Rep. 2012;14:463–471. doi: 10.1007/s11926-012-0276-0. [DOI] [PubMed] [Google Scholar]
- Myasoedova E., Crowson C.S., Kremers H.M., Therneau T.M., Gabriel S.E. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B.T., Tamai K., He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L.B., Cisternas M.G., Greenlund K.J., Giles W., Hannan C., Helmick C.G. Defining arthritis for public health surveillance: methods and estimates in four US poplation health surveys. Arth. Care Res. 2017;69(3):356–367. doi: 10.1002/acr.22943. [DOI] [PubMed] [Google Scholar]
- O'Brien S.M., Fitzgerald P., Scully P., Landers A.M.T., Scott L.V., Dinan T.G. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. NeuroImmunoModulation. 2007;14(2):84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- Oliveria, S. A., Felson, D. T., Cirillo, P. A., Reed, J. I., & Walker, A. M. (1999). Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology, 10(2), 161-166. doi:Doi 10.1097/00001648-199903000-00013. [PubMed]
- Ostensen M. Sex hormones and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Neuroendocrine Immune Basis Rheumatic Dis. 1999;876:131–144. doi: 10.1111/j.1749-6632.1999.tb07630.x. [DOI] [PubMed] [Google Scholar]
- Padyukov L., Silva C., Stolt P., Alfredsson L., Klareskog L., Rheumatoid E.I. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50(10):3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- Park J., Mendy A., Vieira E.R. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. AJPH. 2018;108:256–258. doi: 10.2105/AJPH.2017.304179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks C.G., Walitt B.T., Pettinger M., Chen J.C., de Roos A.J., Hunt J., Howard B.V. Insecticide use and risk of rheumatoid arthritis and systemic lupus erythematosus in the women's health initiative observational study. Arthritis Care Res. 2011;63(2):184–194. doi: 10.1002/acr.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A., Teichtahl A.J., Wluka A.E., Cicuttini F.M. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Brit. J. Sports Med. 2005;39(1):4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Alhambra D., Judge A., Javaid M.K., Cooper C., Diez-Perez A., Arden N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014;73(9):1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J.J., Harrold L.R., Helmick C.G., Gurwitz J.H., Emani S., Yood R.A. Validation of a surveillance case definition for arthritis. J. Rheumatol. 2005;32:340–347. [PubMed] [Google Scholar]
- Silman, A. J., & Pearson, J. E. (2002). Epidemiology and genetics of rheumatoid arthritis. Arthritis Res Ther 4, S265-S272. doi:Pmid 12110146 10.1186/Ar578. [DOI] [PMC free article] [PubMed]
- Spector T.D., Hart D.J., Doyle D.V. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general-population – the effect of obesity. Ann. Rheum. Dis. 1994;53(9):565–568. doi: 10.1136/Ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmer, T., Gunther, K. P., & Brenner, H. (2000). Obesity, overweight and patterns of osteoarthritis: The Ulm Osteoarthritis Study. J Clin Epidem 53(3), 307-313. doi:Doi 10.1016/S0895-4356(99)00162-6. [DOI] [PubMed]
- Taruc-Uy, R. L., & Lynch, S. A. (2013). Diagnosis and treatment of osteoarthritis. Prim Care, 40(4), 821-836, vii. doi:10.1016/j.pop.2013.08.003. [DOI] [PubMed]
- Valentine R.J., Vieira V.J., Woods J.A., Evans E.M. Stronger relationship between central adipositiy and c-reactive protein in older women than men. Menapause. 2009;16(1):84–89. doi: 10.1097/gme.0b013e31817fcb8f. [DOI] [PubMed] [Google Scholar]
- Vanvollenhoven R.F., Mcguire J.L. Estrogen, progesterone, and testosterone – can they be used to treat autoimmune-diseases. Cleveland Clinic J. Med. 1994;61(4):276–284. doi: 10.3949/ccjm.61.4.276. [DOI] [PubMed] [Google Scholar]
- Wilder F.V., Hall B.J., Barrett J.P. Smoking and osteoarthritis: is there an association? The Clearwater Osteoarthritis Study. Osteoarth. Cartilage. 2003;11(1):29–35. doi: 10.1053/joca.2002.0857. [DOI] [PubMed] [Google Scholar]
- Wilder, R. L. (1998). Hormones, pregnancy, and autoimmune diseases. Ann New York Acad Sci 840(1), 45-50. doi:DOI 10.1111/j.1749-6632.1998.tb09547. [DOI] [PubMed]
- Wolfe A.M., Kellgren J.H., Masi A.T. The epidemiology of rheumatoid arthritis: a review. II. Incidence and diagnostic criteria. Bull. Rheum. Dis. 1968;19(3):524–529. [PubMed] [Google Scholar]
- Zhang W., Moskowitz R.W., Nuki G., Abramson S., Altman R.D., Arden N., Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthrit. Cartil. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zeng C., Li H., Yang T., Deng Z.H., Yang Y., Lei G.H. Relationship between cigarette smoking and radiographic knee osteoarthritis in Chinese population: a cross-sectional study. Rheumat. Int. 2015;35(7):1211–1217. doi: 10.1007/s00296-014-3202-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.